Cu2ZnSnS4 Nanoparticles as an Efficient Photocatalyst for the Degradation of Diclofenac in Water
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
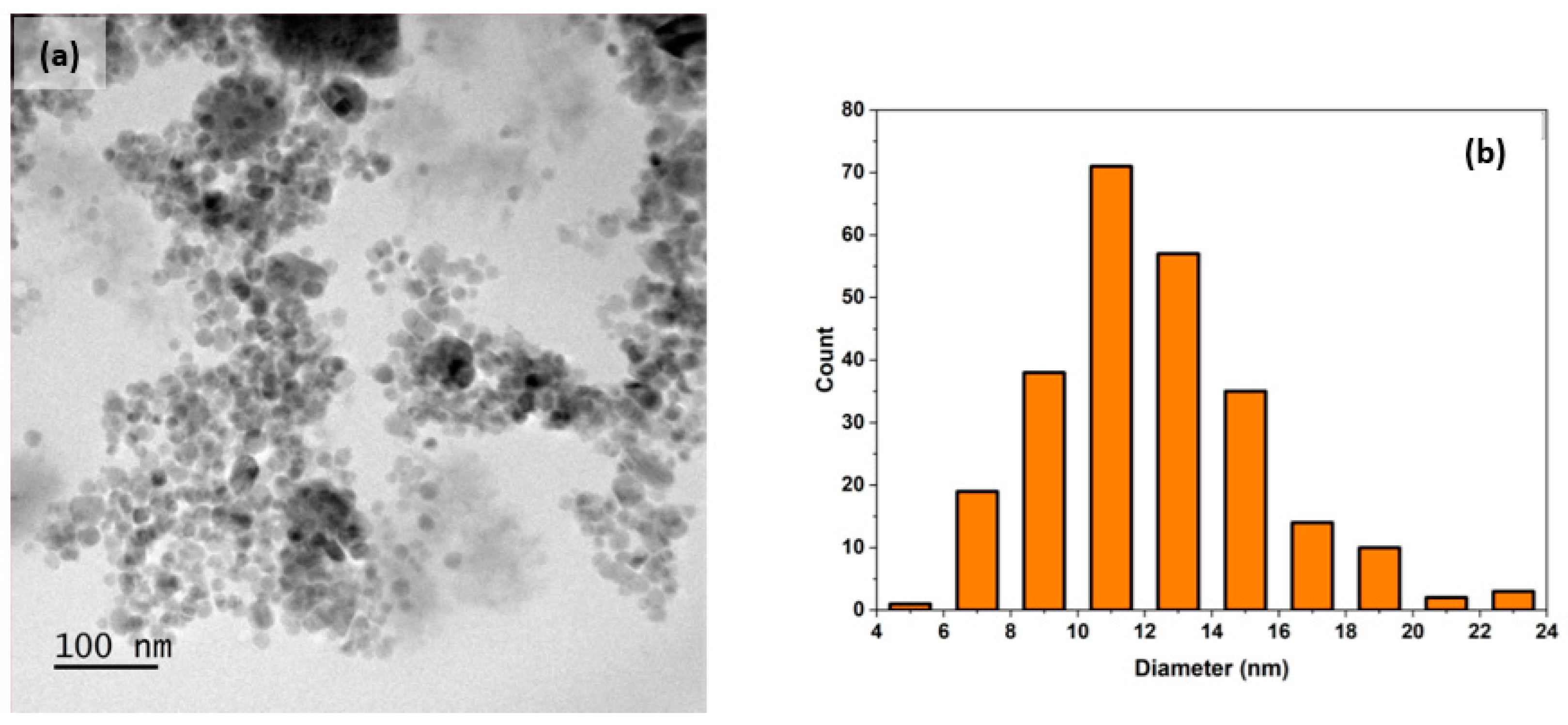
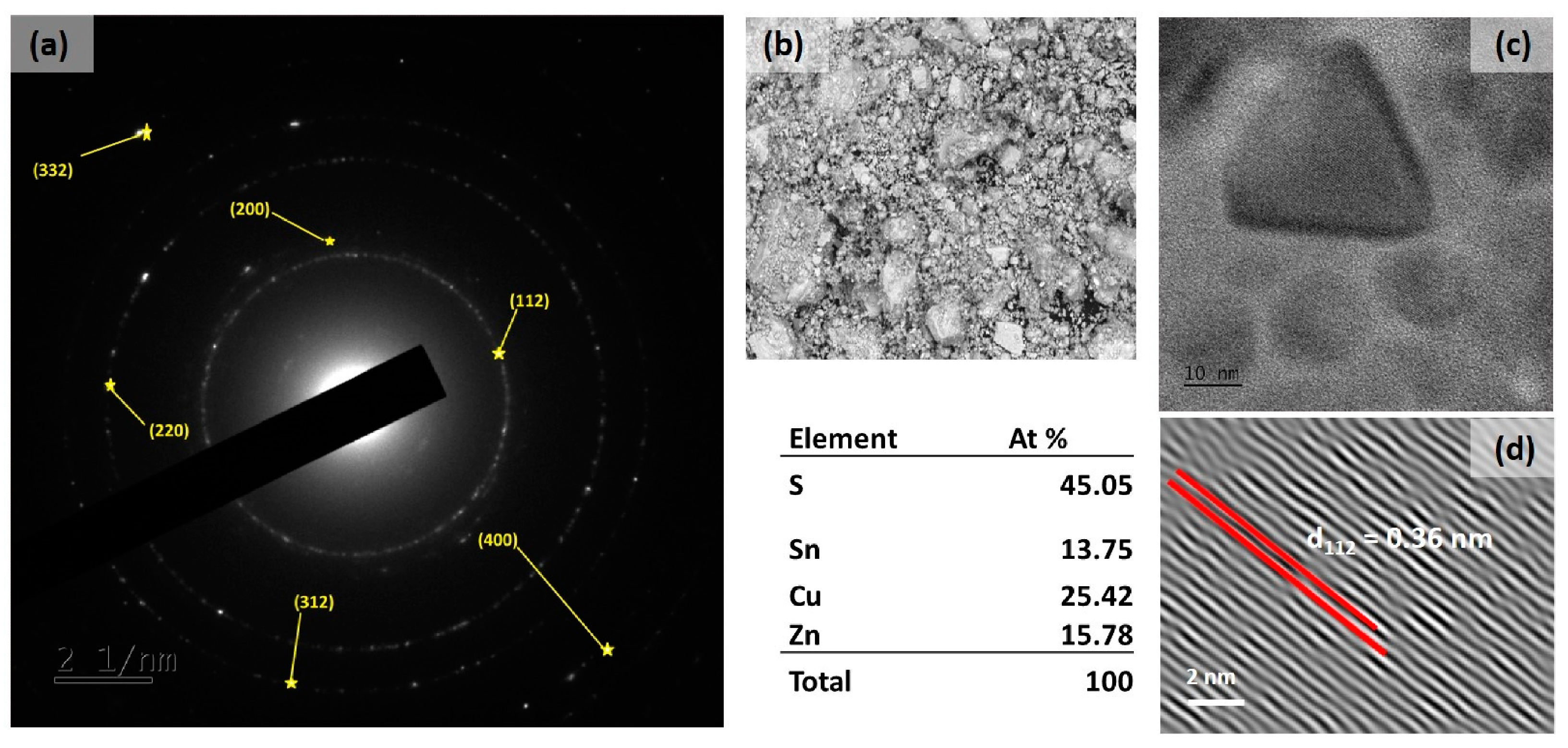
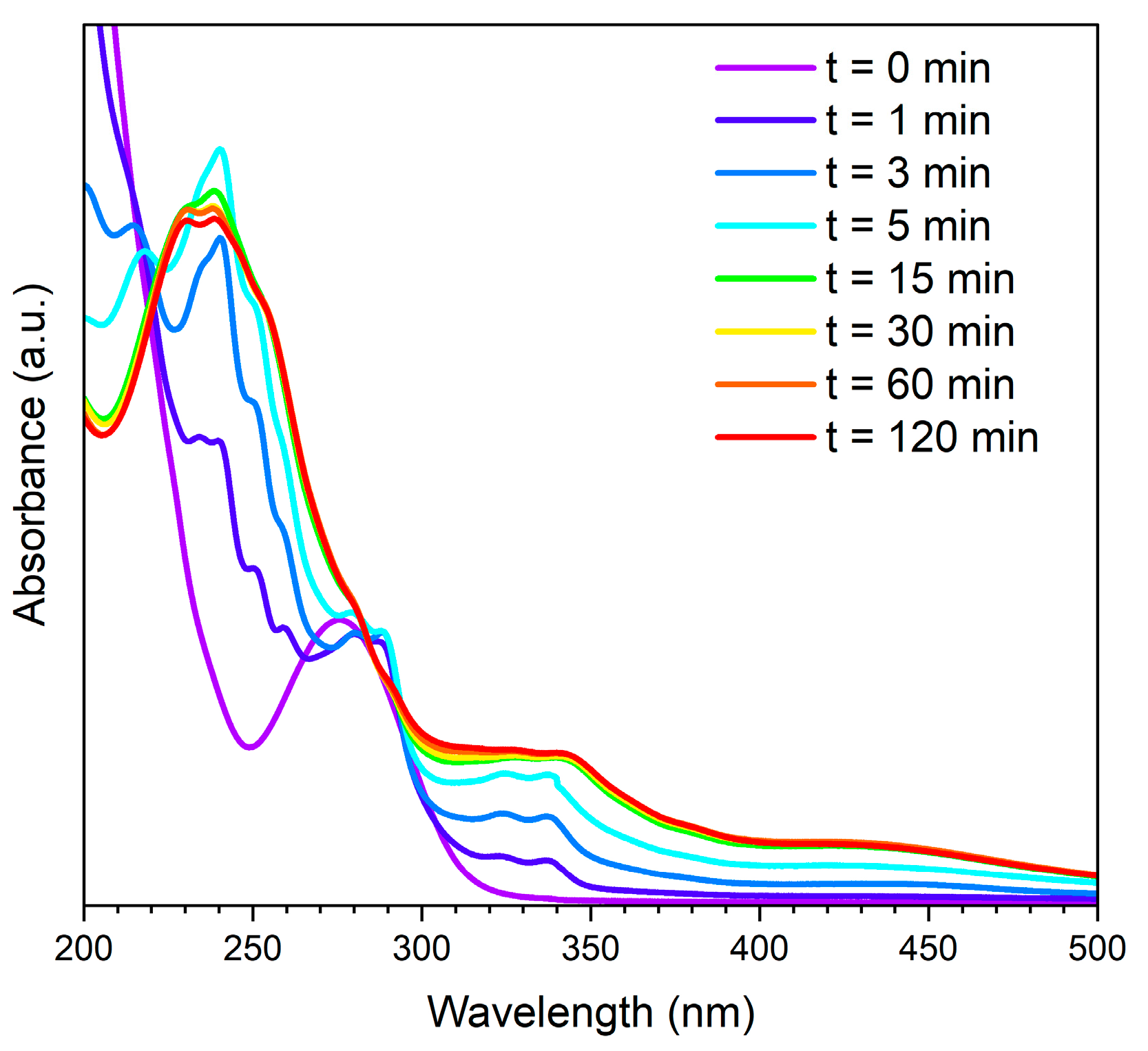
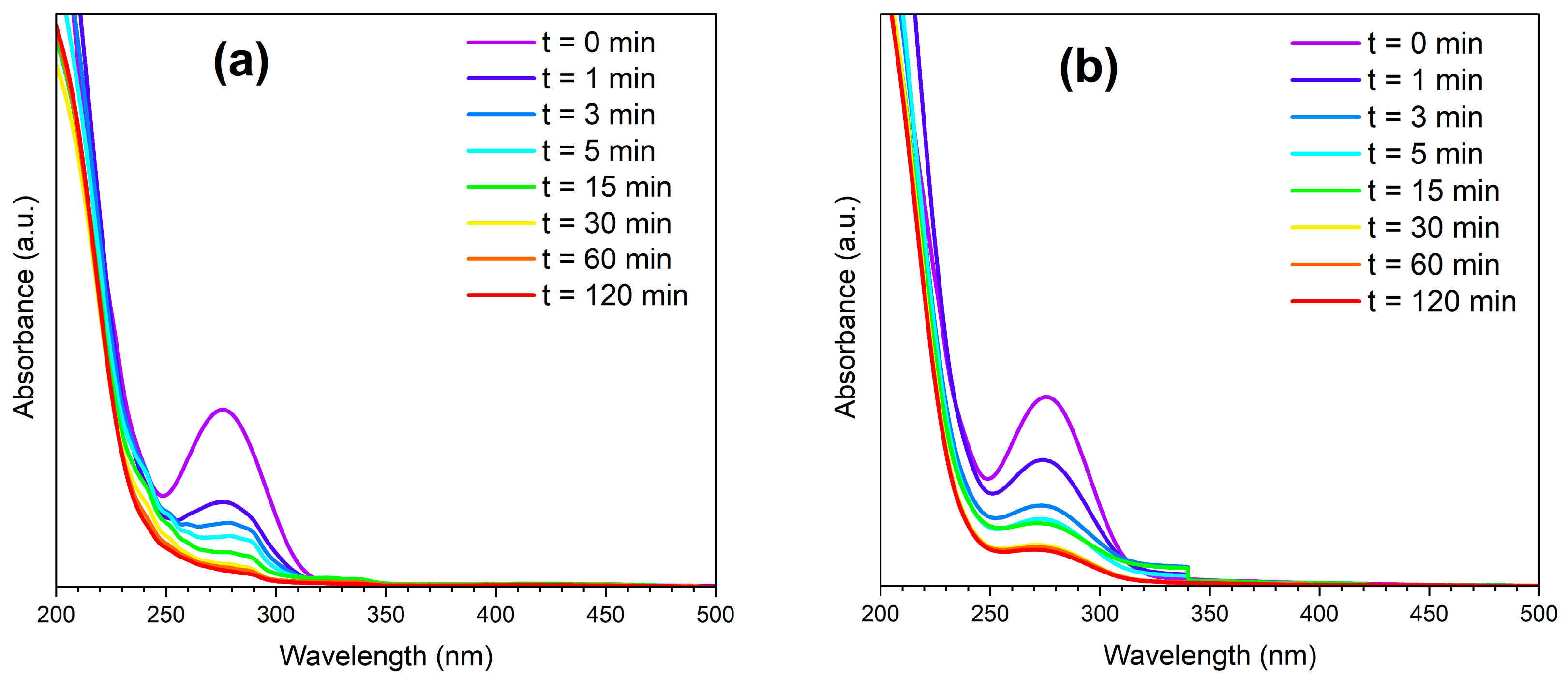
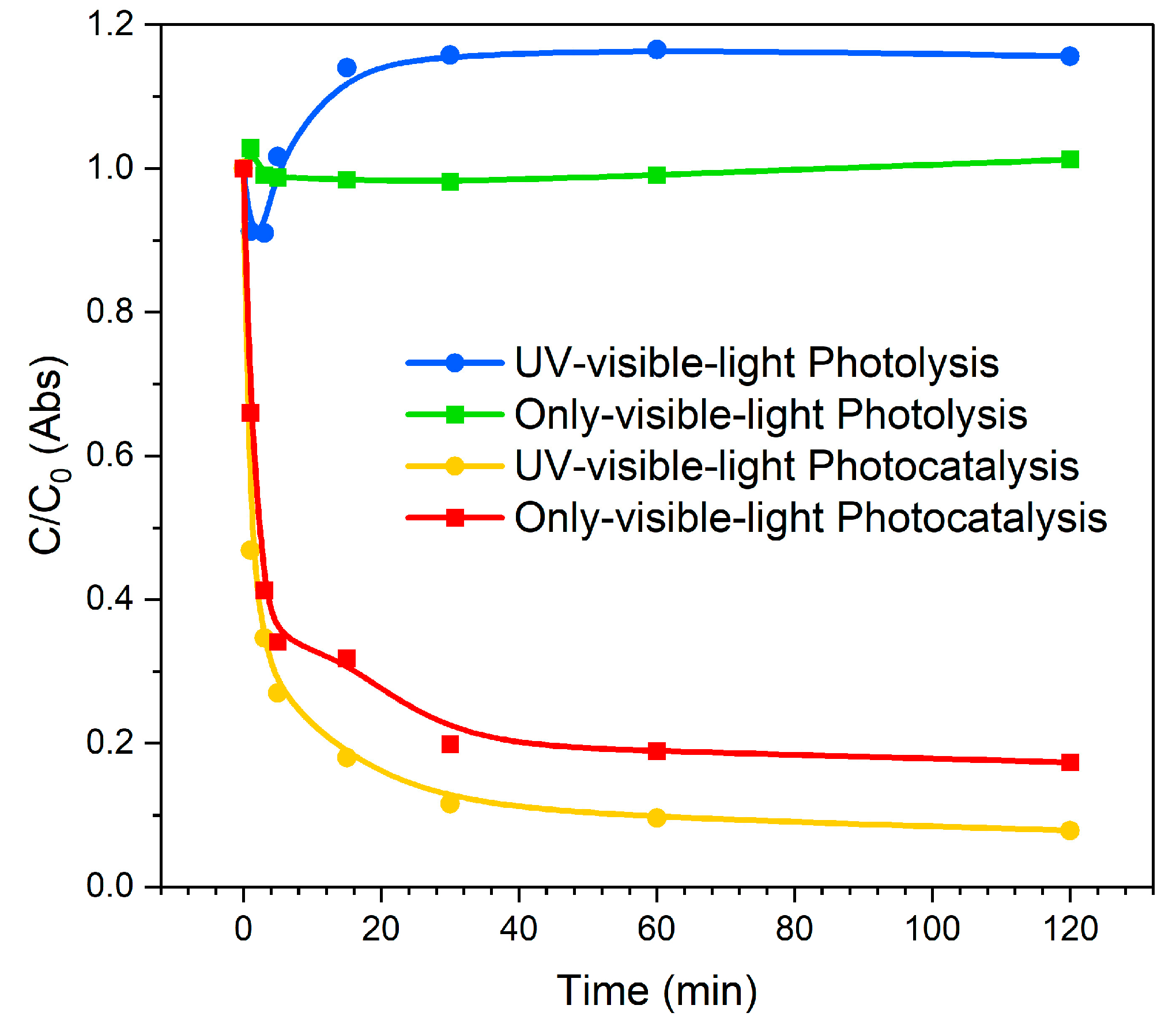
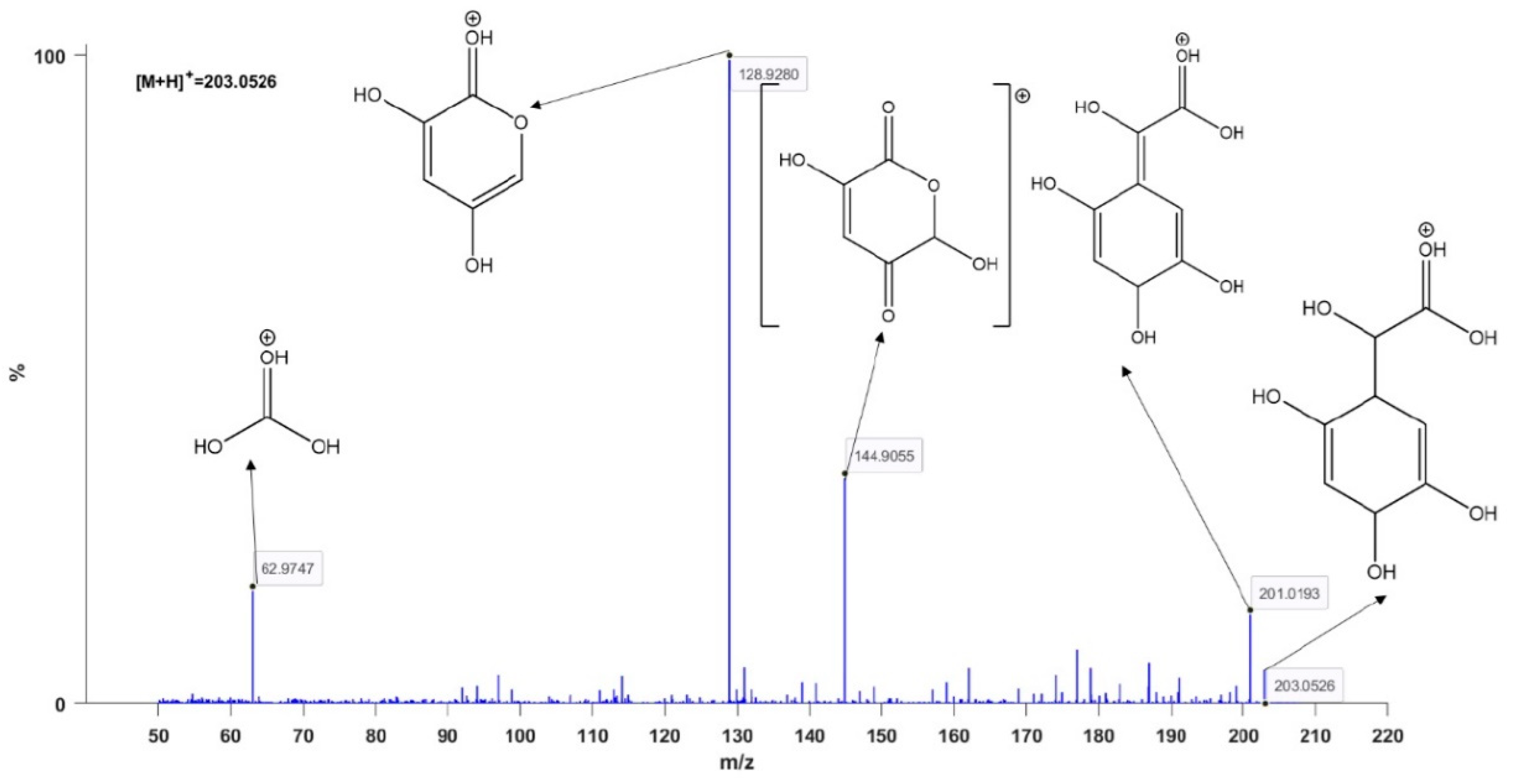
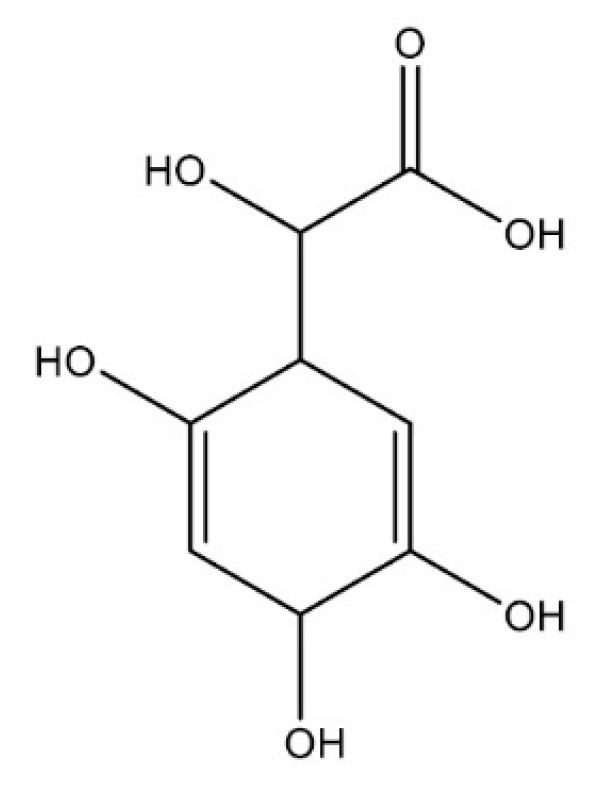
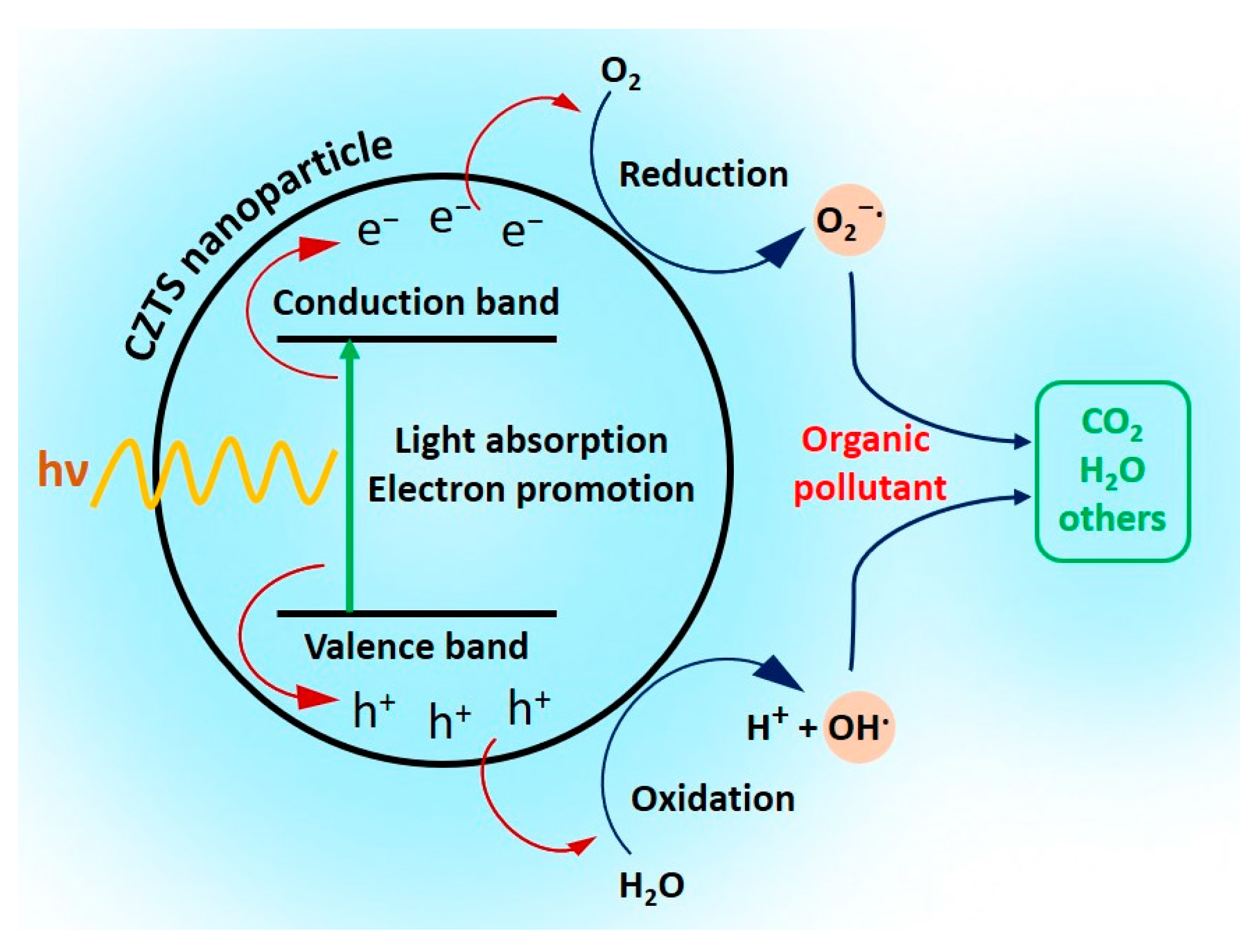
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gosetti, F.; Bottaro, M.; Gianotti, V.; Mazzucco, E.; Frascarolo, P.; Zampieri, D.; Oliveri, C.; Viarengo, A.; Gennaro, M.C. Sun Light Degradation of 4-Chloroaniline in Waters and Its Effect on Toxicity. A High Performance Liquid Chromatography—Diode Array—Tandem Mass Spectrometry Study. Environ. Pollut. 2010, 158, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Gosetti, F.; Belay, M.H.; Marengo, E.; Robotti, E. Development and Validation of a UHPLC-MS/MS Method for the Identification of Irinotecan Photodegradation Products in Water Samples. Environ. Pollut. 2020, 256, 113370. [Google Scholar] [CrossRef] [PubMed]
- Qutob, M.; Alshehri, S.; Shakeel, F.; Alam, P.; Rafatullah, M. Insight into Photodegradation of Diclofenac: Mechanism, Efficiency, Role of Parameters, Toxicity Assessment and Catalyst Stability. Rev. Environ. Contam. Toxicol. 2023, 261, 27. [Google Scholar] [CrossRef]
- Lin, C.-H.; Rohilla, J.; Kuo, H.-H.; Chen, C.-Y.; Mark Chang, T.-F.; Sone, M.; Ingole, P.P.; Lo, Y.-C.; Hsu, Y.-J. Density-Functional Theory Studies on Photocatalysis and Photoelectrocatalysis: Challenges and Opportunities. Sol. RRL 2024, 8, 2300948. [Google Scholar] [CrossRef]
- Fang, M.-J.; Tsao, C.-W.; Hsu, Y.-J. Semiconductor Nanoheterostructures for Photoconversion Applications. J. Phys. D Appl. Phys. 2020, 53, 143001. [Google Scholar] [CrossRef]
- Murgolo, S.; Moreira, I.S.; Piccirillo, C.; Castro, P.M.L.; Ventrella, G.; Cocozza, C.; Mascolo, G. Photocatalytic Degradation of Diclofenac by Hydroxyapatite–TiO2 Composite Material: Identification of Transformation Products and Assessment of Toxicity. Materials 2018, 11, 1779. [Google Scholar] [CrossRef]
- Lara-Pérez, C.; Leyva, E.; Zermeño, B.; Osorio, I.; Montalvo, C.; Moctezuma, E. Photocatalytic Degradation of Diclofenac Sodium Salt: Adsorption and Reaction Kinetic Studies. Environ. Earth Sci. 2020, 79, 277. [Google Scholar] [CrossRef]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Photocatalytic Degradation of Diclofenac Using TiO2–SnO2 Mixed Oxide Catalysts. Environ. Technol. 2019, 40, 929–941. [Google Scholar] [CrossRef]
- Aghababaei, N.; Abdouss, M.; Hosseini-Monfared, H.; Ghanbari, F. Photocatalytic Degradation of Diclofenac Using a Novel Double Z-Scheme Catalyst (O-g-C3N4/ZnO/TiO2@halloysite Nanotubes): Degradation Mechanism, Identification of by-Products and Environmental Implementation. J. Water Process Eng. 2023, 53, 103702. [Google Scholar] [CrossRef]
- Rizzo, L.; Meric, S.; Kassinos, D.; Guida, M.; Russo, F.; Belgiorno, V. Degradation of Diclofenac by TiO2 Photocatalysis: UV Absorbance Kinetics and Process Evaluation through a Set of Toxicity Bioassays. Water Res. 2009, 43, 979–988. [Google Scholar] [CrossRef]
- Moctezuma, E.; Leyva, E.; Lara-Pérez, C.; Noriega, S.; Martínez-Richa, A. TiO2 Photocatalytic Degradation of Diclofenac: Intermediates and Total Reaction Mechanism. Top Catal. 2020, 63, 601–615. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Silveira, J.E.; Carbajo, J.; Zazo, J.A.; Casas, J.A.; Fernandes, A.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Diclofenac Photodegradation with the Perovskites BaFeyTi1-yO3 as Catalysts. Environ. Sci. Pollut. Res. 2021, 28, 23822–23832. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yan, C.; Li, J.; Suryawanshi, M.P.; Kim, J.; Green, M.A.; Hao, X. Kesterite Solar Cells: Insights into Current Strategies and Challenges. Adv. Sci. 2021, 8, 2004313. [Google Scholar] [CrossRef] [PubMed]
- Tseberlidis, G.; Trifiletti, V.; Le Donne, A.; Frioni, L.; Acciarri, M.; Binetti, S. Kesterite Solar-Cells by Drop-Casting of Inorganic Sol–Gel Inks. Sol. Energy 2020, 208, 532–538. [Google Scholar] [CrossRef]
- Maalouf, A.; Okoroafor, T.; Jehl, Z.; Babu, V.; Resalati, S. A Comprehensive Review on Life Cycle Assessment of Commercial and Emerging Thin-Film Solar Cell Systems. Renew. Sustain. Energy Rev. 2023, 186, 113652. [Google Scholar] [CrossRef]
- Sivaraj, S.; Rathanasamy, R.; Kaliyannan, G.V.; Panchal, H.; Jawad Alrubaie, A.; Musa Jaber, M.; Said, Z.; Memon, S. A Comprehensive Review on Current Performance, Challenges and Progress in Thin-Film Solar Cells. Energies 2022, 15, 8688. [Google Scholar] [CrossRef]
- Hou, X.; Li, Y.; Yan, J.-J.; Wang, C.-W. Highly Efficient Photocatalysis of P-Type Cu2ZnSnS4 under Visible-Light Illumination. Mater. Res. Bull. 2014, 60, 628–633. [Google Scholar] [CrossRef]
- Phaltane, S.A.; Vanalakar, S.A.; Bhat, T.S.; Patil, P.S.; Sartale, S.D.; Kadam, L.D. Photocatalytic Degradation of Methylene Blue by Hydrothermally Synthesized CZTS Nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 8186–8191. [Google Scholar] [CrossRef]
- Apostolopoulou, A.; Mahajan, S.; Sharma, R.; Stathatos, E. Novel Development of Nanocrystalline Kesterite Cu2ZnSnS4 Thin Film with High Photocatalytic Activity under Visible Light Illumination. J. Phys. Chem. Solids 2018, 112, 37–42. [Google Scholar] [CrossRef]
- Semalti, P.; Sharma, V.; Sharma, S.N. A Novel Method of Water Remediation of Organic Pollutants and Industrial Wastes by Solution- Route Processed CZTS Nanocrystals. J. Mater. 2021, 7, 904–919. [Google Scholar] [CrossRef]
- Hou, Z.; Li, Y.; Liu, J.; Shen, H.; Huo, X. The Visible Light-Driven Highly Efficient Photocatalytic Properties of Cu2ZnSnS4 Nanoparticles Synthesized by a Hydrothermal Method. New J. Chem. 2021, 45, 1743–1752. [Google Scholar] [CrossRef]
- Alirezazadeh, F.; Alimohammadi, E.; Sheibani, S.; Rashchi, F. A Comparative Study on the Photocatalytic Activity and Formation Mechanism of Nanostructured Cu2ZnSnS4 Prepared by Thermal and Mechano-Thermal Methods. Mater. Chem. Phys. 2022, 292, 126856. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Y.; Zhang, J.; Liang, N.; Long, L.; Liu, J. Insight into the Growth Mechanism of Mixed Phase CZTS and the Photocatalytic Performance. Nanomaterials 2022, 12, 1439. [Google Scholar] [CrossRef] [PubMed]
- Tseberlidis, G.; Hasan Husien, A.; Riva, S.; Frioni, L.; Le Donne, A.; Acciarri, M.; Binetti, S. Semi-Transparent Cu2ZnSnS4 Solar Cells by Drop-Casting of Sol-Gel Ink. Sol. Energy 2021, 224, 134–141. [Google Scholar] [CrossRef]
- Trifiletti, V.; Tseberlidis, G.; Colombo, M.; Spinardi, A.; Luong, S.; Danilson, M.; Grossberg, M.; Fenwick, O.; Binetti, S. Growth and Characterization of Cu2Zn1−xFexSnS4 Thin Films for Photovoltaic Applications. Materials 2020, 13, 1471. [Google Scholar] [CrossRef]
- Tseberlidis, G.; Trifiletti, V.; Vitiello, E.; Husien, A.H.; Frioni, L.; Da Lisca, M.; Alvarez, J.; Acciarri, M.; Binetti, S.O. Band-Gap Tuning Induced by Germanium Introduction in Solution-Processed Kesterite Thin Films. ACS Omega 2022, 7, 23445–23456. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Espindola-Rodriguez, M.; Sylla, D.; Sánchez, Y.; Oliva, F.; Grini, S.; Neuschitzer, M.; Vines, L.; Izquierdo-Roca, V.; Saucedo, E.; Placidi, M. Bifacial Kesterite Solar Cells on FTO Substrates. ACS Sustain. Chem. Eng. 2017, 5, 11516–11524. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, D.; Zhang, N.; Zhao, M.; Yu, X.; Liu, P.; Wei, Y.; Ren, G. Achieving 11.95% Efficient Cu2ZnSnSe4 Solar Cells Fabricated by Sputtering a Cu–Zn–Sn–Se Quaternary Compound Target with a Selenization Process. J. Mater. Chem. A Mater. 2019, 7, 9948–9957. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Taghipour, N.; Peng, L.; Konstantatos, G. Ag-Refined Kesterite in Superstrate Solar Cell Configuration with 9.7% Power Conversion Efficiency. Adv. Funct. Mater. 2022, 32, 2205948. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Konstantatos, G. Highly Efficient, Ultrathin, Cd-Free Kesterite Solar Cells in Superstrate Configuration Enabled by Band Level Tuning via Ag Incorporation. Nano Energy 2022, 94, 106898. [Google Scholar] [CrossRef]
- Trifiletti, V.; Frioni, L.; Tseberlidis, G.; Vitiello, E.; Danilson, M.; Grossberg, M.; Acciarri, M.; Binetti, S.; Marchionna, S. Manganese-Substituted Kesterite Thin-Films for Earth-Abundant Photovoltaic Applications. Sol. Energy Mater. Sol. Cells 2023, 254, 112247. [Google Scholar] [CrossRef]
- Butrichi, F.; Trifiletti, V.; Tseberlidis, G.; Colombo, B.E.G.; Taglietti, F.; Rancan, M.; Armelao, L.; Binetti, S. Wet Synthesis of Cu2MnSnS4 Thin Films for Photovoltaics: Oxidation Control and CdS Impact on Device Performances. Sol. Energy Mater. Sol. Cells 2024, 272, 112924. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, J.; Liu, Y.; Yang, T.; Xu, Z. Surfactant-Tuned Phase Structure and Morphologies of Cu2ZnSnS4 Hierarchical Microstructures and Their Visible-Light Photocatalytic Activities. Nanoscale Res. Lett. 2017, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Sampath, M.; Sankarasubramanian, K.; Archana, J.; Hayakawa, Y.; Ramamurthi, K.; Sethuraman, K. Structural, Optical and Photocatalytic Properties of Spray Deposited Cu2ZnSnS4 Thin Films with Various S/(Cu+Zn+Sn) Ratio. Mater. Sci. Semicond. Process. 2018, 87, 54–64. [Google Scholar] [CrossRef]
- Burhanuz Zaman, M.; Mir, R.A.; Poolla, R. Growth and Properties of Solvothermally Derived Highly Crystalline Cu2ZnSnS4 Nanoparticles for Photocatalytic and Electrocatalytic Applications. Int. J. Hydrogen Energy 2019, 44, 23023–23033. [Google Scholar] [CrossRef]
- Wei, Q.-B.; Xu, P.; Ren, X.-P.; Fu, F. Flower-like Cu2ZnSnS4 Architectures Synthetize and Their Visible-Light Catalytic Properties. J. Alloys Compd. 2019, 770, 424–432. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Feng, K.; Huang, D.; Wang, K.; Li, Y.; Jiang, F. Kesterite Cu2ZnSnS4 Thin-Film Solar Water-Splitting Photovoltaics for Solar Seawater Desalination. Cell Rep. Phys. Sci. 2021, 2, 100468. [Google Scholar] [CrossRef]
- Ros, C.; Andreu, T.; Giraldo, S.; Izquierdo-Roca, V.; Saucedo, E.; Morante, J.R. Turning Earth Abundant Kesterite-Based Solar Cells Into Efficient Protected Water-Splitting Photocathodes. ACS Appl. Mater. Interfaces 2018, 10, 13425–13433. [Google Scholar] [CrossRef]
- Oueslati, S.; Pilvet, M.; Grossberg, M.; Kauk-Kuusik, M.; Krustok, J.; Meissner, D. Kesterite Monograins for Solar Cells and Water Splitting Applications. Thin Solid Films 2021, 739, 138981. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. The Photocatalytic Degradation of Sodium Diclofenac in Different Water Matrices Using G-C3N4 Nanosheets: A Study of the Intermediate by-Products and Mechanism. J. Environ. Chem. Eng. 2021, 9, 105827. [Google Scholar] [CrossRef]
- Wang, J.-H.; Kong, F.; Liu, B.-F.; Zhuo, S.-N.; Ren, N.-Q.; Ren, H.-Y. Enhanced Photocatalytic Degradation of Diclofenac by UiO-66/MgAl-LDH: Excellent Performances and Mechanisms. Environ. Sci. Nano 2024, 11, 3286–3293. [Google Scholar] [CrossRef]
- Awang, H.; Peppel, T.; Strunk, J. Photocatalytic Degradation of Diclofenac by Nitrogen-Doped Carbon Quantum Dot-Graphitic Carbon Nitride (CNQD). Catalysts 2023, 13, 735. [Google Scholar] [CrossRef]
- Khanzada, L.S.; Levchuk, I.; Hou, Y.; Azimi, H.; Osvet, A.; Ahmad, R.; Brandl, M.; Herre, P.; Distaso, M.; Hock, R.; et al. Effective Ligand Engineering of the Cu2ZnSnS4 Nanocrystal Surface for Increasing Hole Transport Efficiency in Perovskite Solar Cells. Adv. Funct. Mater. 2016, 26, 8300–8306. [Google Scholar] [CrossRef]
- Steinhagen, C.; Panthani, M.G.; Akhavan, V.; Goodfellow, B.; Koo, B.; Korgel, B.A. Synthesis of Cu2ZnSnS4 Nanocrystals for Use in Low-Cost Photovoltaics. J. Am. Chem. Soc. 2009, 131, 12554–12555. [Google Scholar] [CrossRef]
- Podsiadlo, S.; Bialoglowski, M.; Fadaghi, M.; Gebicki, W.; Jastrzebski, C.; Zero, E.; Trzybinski, D.; Wozniak, K. Synthesis of Magnetic Doped Kesterite Single Crystals. Cryst. Res. Technol. 2015, 50, 690–694. [Google Scholar] [CrossRef]
- Ito, K. Copper Zinc Tin Sulfide-Based Thin Film Solar Cells; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Musa, K.A.K.; Eriksson, L.A. Photodegradation Mechanism of the Common Non-Steroid Anti-Inflammatory Drug Diclofenac and Its Carbazole Photoproduct. Phys. Chem. Chem. Phys. 2009, 11, 4601–4610. [Google Scholar] [CrossRef]
- Aparici-Espert, I.; Miranda, M.A.; Lhiaubet-Vallet, V. Sunscreen-Based Photocages for Topical Drugs: A Photophysical and Photochemical Study of A Diclofenac-Avobenzone Dyad. Molecules 2018, 23, 673. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of Pharmaceutical Compounds in Urban Wastewater: Removal, Mass Load and Environmental Risk after a Secondary Treatment—A Review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and Its Transformation Products: Environmental Occurrence and Toxicity—A Review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseberlidis, G.; Trifiletti, V.; Husien, A.H.; L’Altrella, A.; Binetti, S.; Gosetti, F. Cu2ZnSnS4 Nanoparticles as an Efficient Photocatalyst for the Degradation of Diclofenac in Water. Appl. Sci. 2024, 14, 9923. https://doi.org/10.3390/app14219923
Tseberlidis G, Trifiletti V, Husien AH, L’Altrella A, Binetti S, Gosetti F. Cu2ZnSnS4 Nanoparticles as an Efficient Photocatalyst for the Degradation of Diclofenac in Water. Applied Sciences. 2024; 14(21):9923. https://doi.org/10.3390/app14219923
Chicago/Turabian StyleTseberlidis, Giorgio, Vanira Trifiletti, Amin Hasan Husien, Andrea L’Altrella, Simona Binetti, and Fabio Gosetti. 2024. "Cu2ZnSnS4 Nanoparticles as an Efficient Photocatalyst for the Degradation of Diclofenac in Water" Applied Sciences 14, no. 21: 9923. https://doi.org/10.3390/app14219923
APA StyleTseberlidis, G., Trifiletti, V., Husien, A. H., L’Altrella, A., Binetti, S., & Gosetti, F. (2024). Cu2ZnSnS4 Nanoparticles as an Efficient Photocatalyst for the Degradation of Diclofenac in Water. Applied Sciences, 14(21), 9923. https://doi.org/10.3390/app14219923








