Experimental Investigations on Repair and Permeability Reduction for Single Sandstone Fracture Using a Mixed CaCO3 and Fe(OH)3 Precipitate
Abstract
1. Introduction
2. Testing Program
2.1. Preparation of Rock Specimen
2.2. Preparation of Aqueous Solution
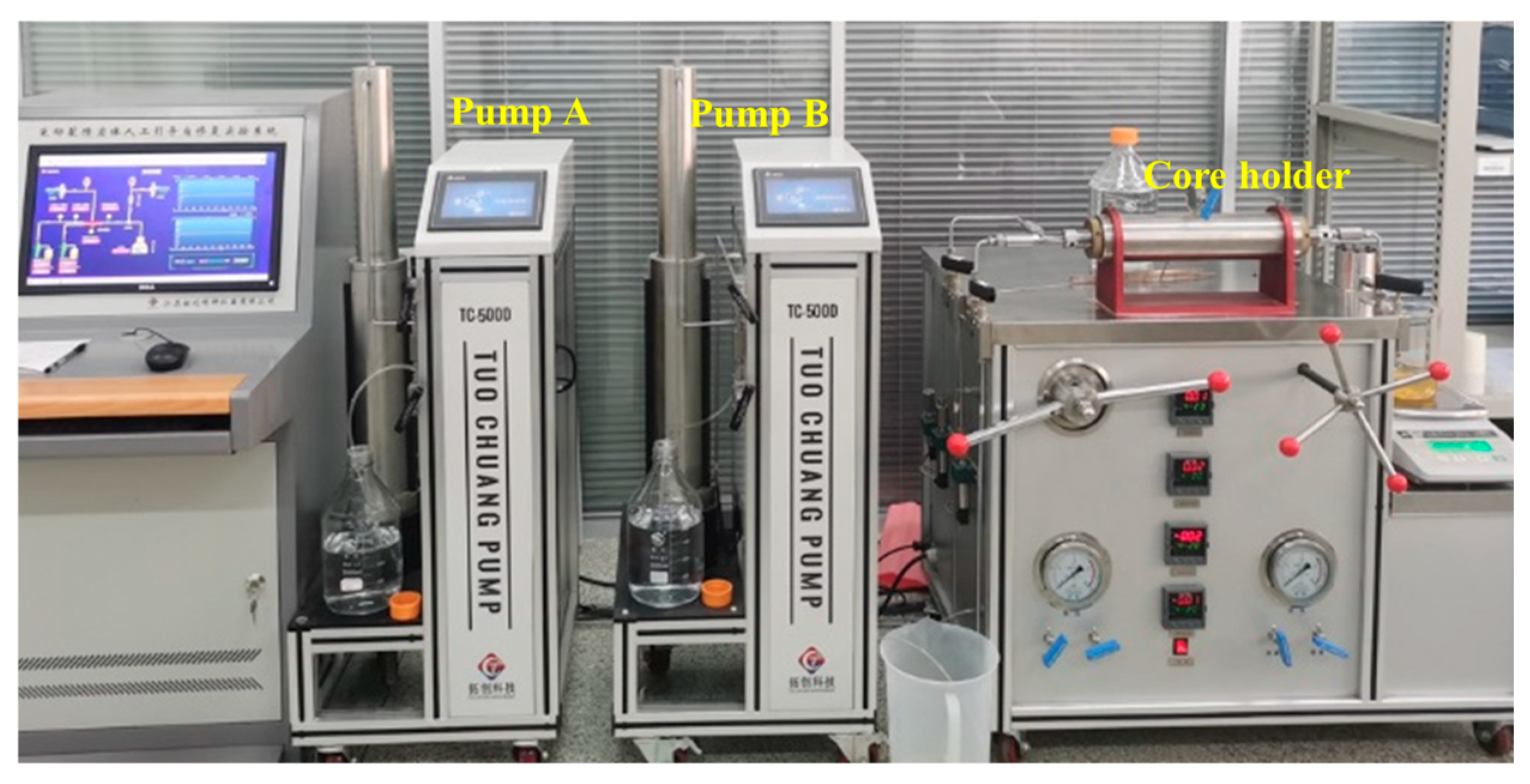
2.3. Experiment Process
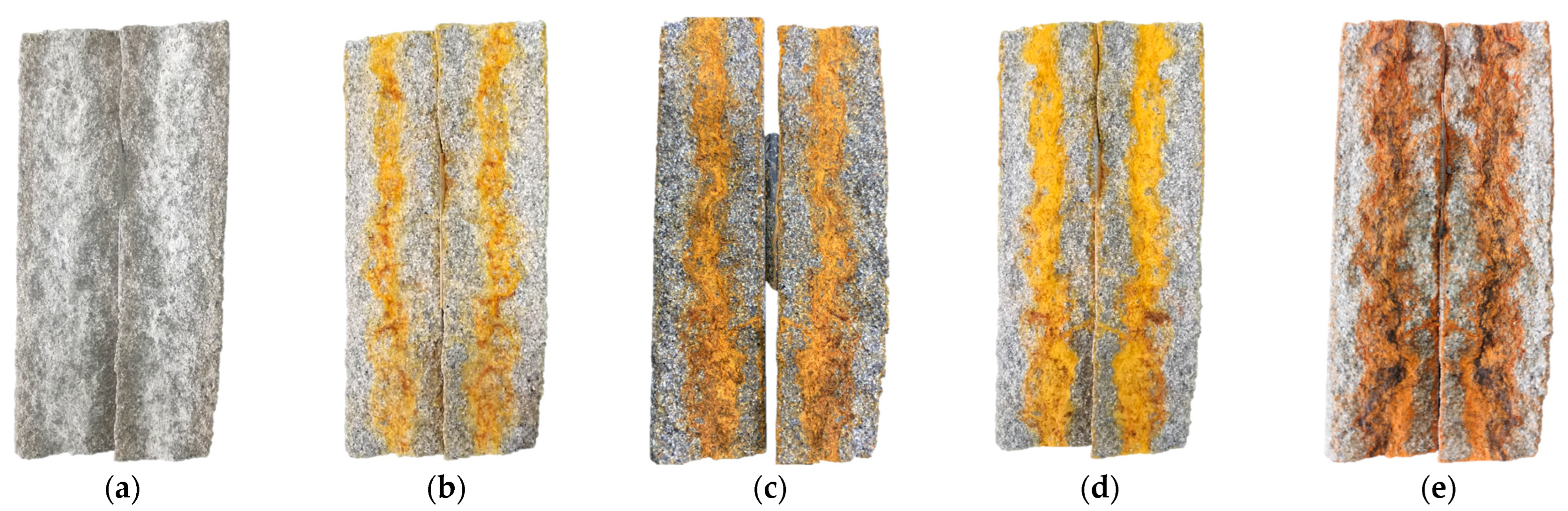
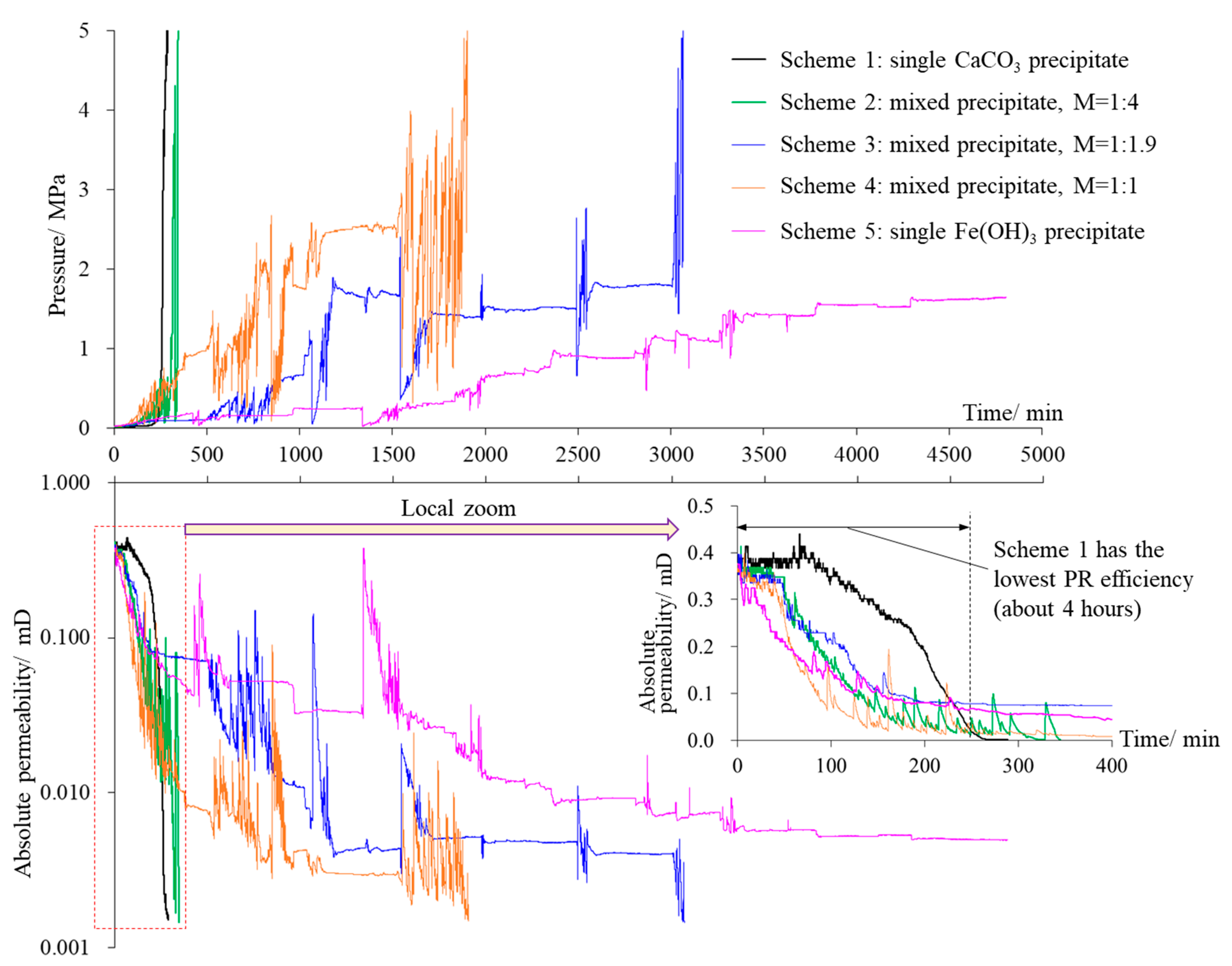
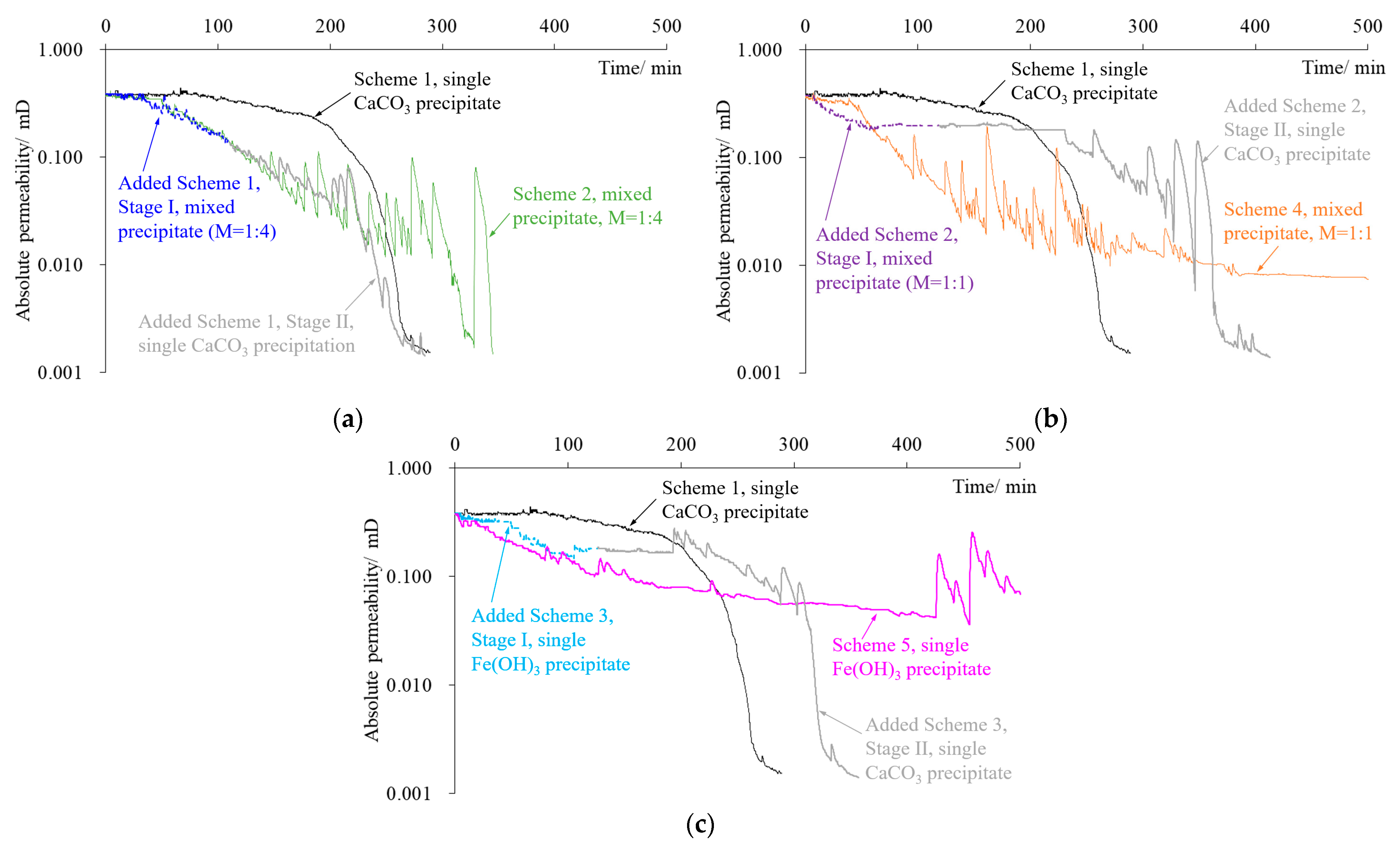
3. PR Characteristics of Rock Fracture Repaired by Precipitate
4. Discussion
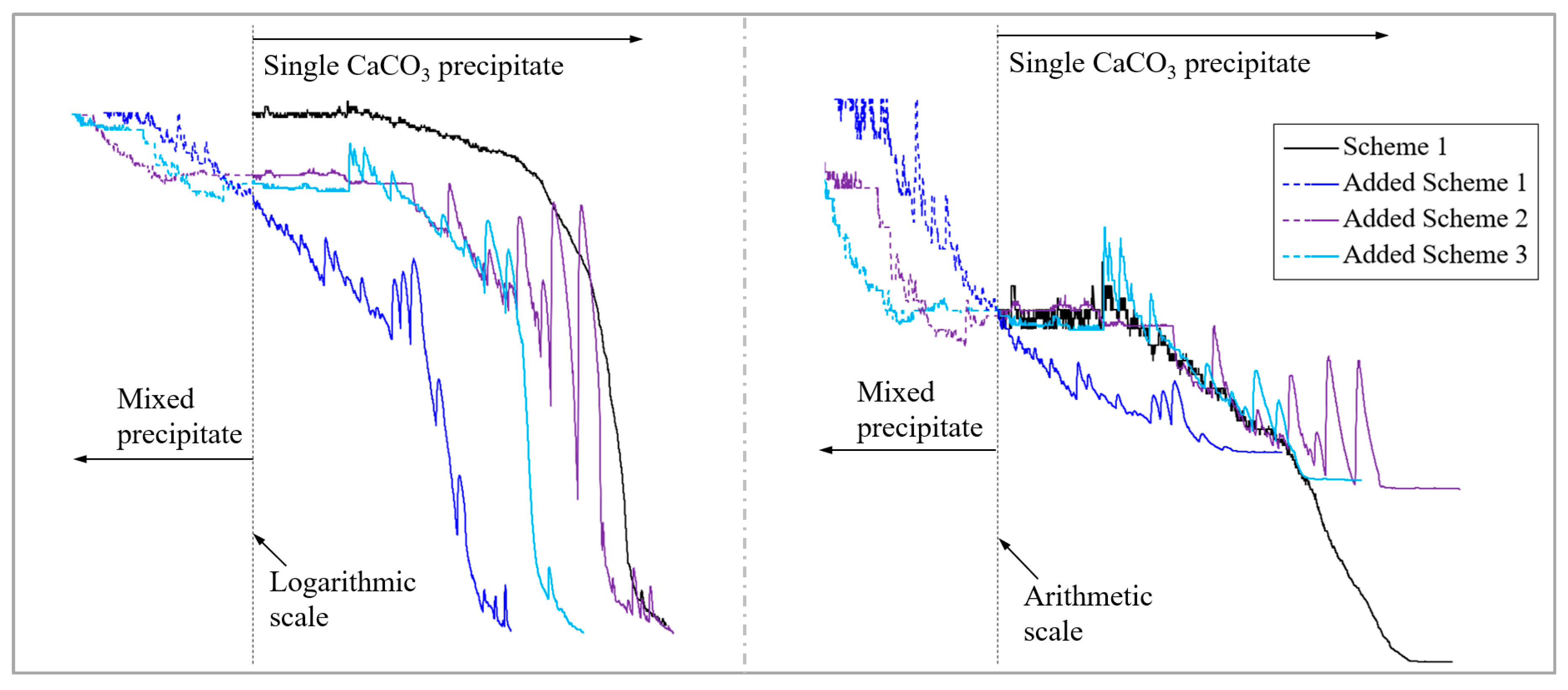
4.1. The Encapsulation of CaCO3 Crystals by Fe(OH)3 Colloid Is the Main Reason for the Decrease in Fracture Repair Efficiency Caused by the Mixed Precipitate
4.2. Fe(OH)3 Precipitate Can Significantly Improve the Efficiency of PR in the Initial Stage of the Experiment
4.3. Adopting a Mixed Fe(OH)3 and CaCO3 Precipitate with a Molar Ratio Close to 1:4 in the Early Stage and a Single CaCO3 Precipitate in the Later Stage Can Achieve Better Repair Efficiency
5. Conclusions
- (1)
- This study conducted repair and PR experiments using a single CaCO3/Fe(OH)3 precipitate or a mixed precipitate with different molar ratios of the two on the same fracture. The Fe(OH)3 colloid encapsulated CaCO3 crystals in a mixed precipitate, reducing the overall structural stability of the mixed precipitate and restricting repair and PR efficiency. The Fe(OH)3 precipitate had better PR efficiency in the initial stage of the experiment. Therefore, a new fracture repair experiment using a mixed precipitate and a single CaCO3 precipitate in two stages was conducted to obtain a reasonable scheme with excellent fracture repair efficiency.
- (2)
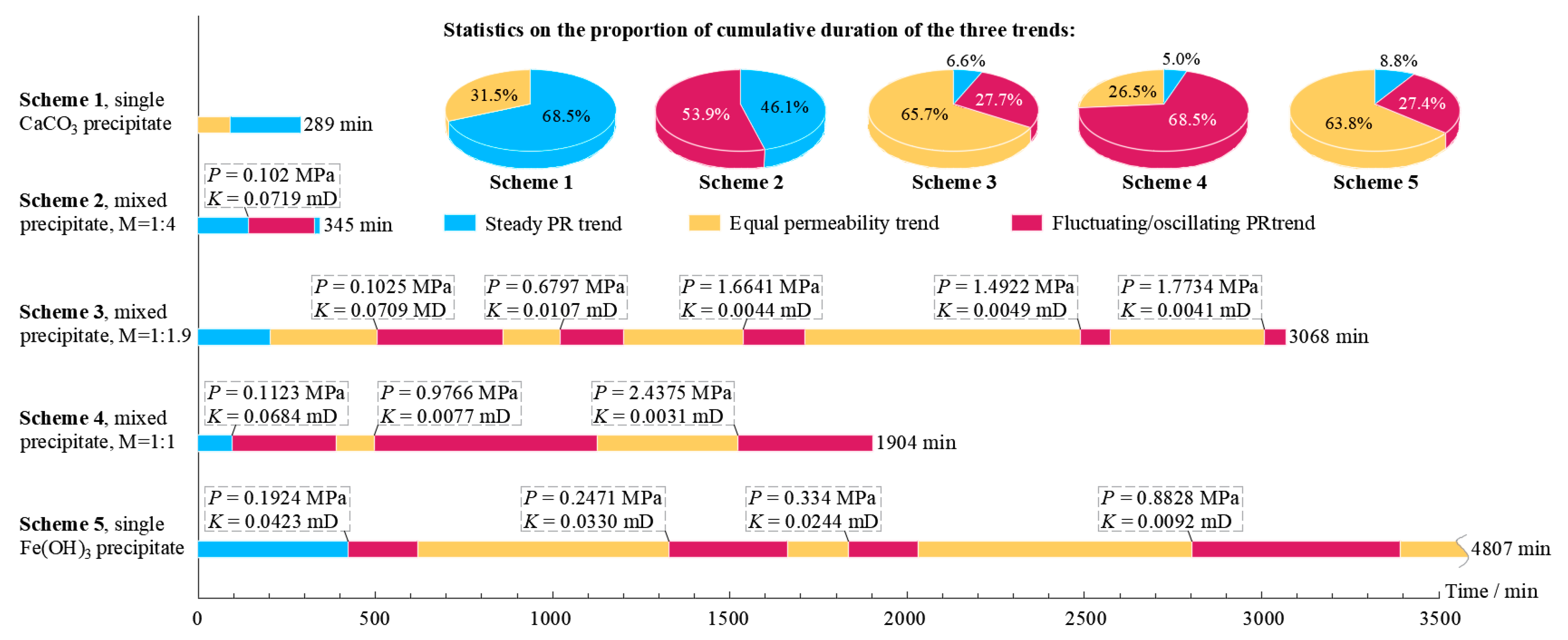
- In the experiment, the fractured rock specimen’s PR curves showed different trend characteristics due to the difference in the adsorption and consolidation degree of the precipitates in the fractures. There were three main trends: a steady PR trend, a flat permeability trend, and a fluctuating/oscillating PR trend. The PR trend caused by the single CaCO3 precipitate was stable and fast, while those caused by the mixed precipitate and single Fe(OH)3 precipitate tended to frequently fluctuate/oscillate and persist for a long period. Instability in precipitate structures caused by hydraulic erosion is the main reason for this phenomenon. Under our experimental conditions, the strength of the mixed precipitate against hydraulic erosion was about 0.1 MPa, while that of the Fe(OH)3 precipitate was about 0.2 MPa.
- (3)
- Within the first 4 h of the initial stage, the repair efficiency of the single CaCO3 precipitate on fractures was always the lowest among the schemes. Further experiments showed that a mixed Fe(OH)3 and CaCO3 precipitate with a molar ratio of about 1:4 in the early stage (the duration should be more than 2 h) and a single CaCO3 precipitate in the later stage should be the relatively optimal scheme for WCF repair.
- (4)
- The PR law of chemical precipitates in repairing WCFs is a complex problem under the comprehensive influence of multiple factors. This study only examined a sandstone sample under specific fracture development conditions. For other lithological rock samples with different fracture development characteristics, these PR laws require further study. The time boundary setting for using different precipitates to repair fractures in stages will also be the focus of subsequent research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karan, S.K.; Samadder, S.R.; Singh, V. Groundwater vulnerability assessment in degraded coal mining areas using the AHP-Modified DRASTIC model. Land Degrad. Dev. 2018, 29, 2351–2365. [Google Scholar] [CrossRef]
- Arkoc, O.; Ucar, S.; Ozcan, C. Assessment of impact of coal mining on ground and surface waters in Tozakli coal field, Kirklareli, northeast of Thrace, Turkey. Environ. Earth Sci. 2016, 75, 514. [Google Scholar] [CrossRef]
- Zhang, J.C.; Shen, B.H. Coal mining under aquifers in China: A case study. Int. J. Rock Mech. Min. Sci. 2004, 41, 629–639. [Google Scholar] [CrossRef]
- Gao, X.C.; Liu, S.; Ma, T.F.; Zhao, C.; Zhang, X.C.; Xia, H.; Yin, J.H. A prediction method for height of water flowing fractured zone based on sparrow search Algorithm-Elman neural network in northwest mining area. Appl. Sci. 2023, 13, 1162. [Google Scholar] [CrossRef]
- Ju, J.F.; Li, Q.S.; Xu, J.L.; Cao, Z.G. Progress of ecological remediation technology of mining-damaged aquifer. Coal Sci. Technol. 2020, 48, 102–108. [Google Scholar]
- Han, G.S.; Dai, S.Z.; Zhang, J.Q.; Gao, Y.; Liu, Y.M. Study on the workability and early mechanical properties of carbon nanotube-coated fly ash modified cement-based grouting materials. Constr. Build. Mater. 2024, 439, 137327. [Google Scholar] [CrossRef]
- Feng, L.; Huang, F.; Wang, G.H.; Huang, T.; Qian, Z.Z. Experimental investigation on pneumatic pre-fracturing grouting in low-permeability soil. Tunn. Undergr. Space Technol. 2023, 131, 104798. [Google Scholar] [CrossRef]
- Lee, H.B.; Oh, T.M.; Park, E.S.; Lee, J.W.; Kim, H.M. Factors affecting waterproof efficiency of grouting in single rock fracture. Geomech. Eng. 2017, 12, 771–783. [Google Scholar] [CrossRef]
- El Tani, M. Grouting rock fractures with cement grout. Rock Mech. Rock Eng. 2012, 45, 547–561. [Google Scholar] [CrossRef]
- Salimian, M.H.; Baghbanan, A.; Hashemolhosseini, H.; Dehghanipoodeh, M.; Norouzi, S. Effect of grouting on shear behavior of rock joint. Int. J. Rock Mech. Min. Sci. 2017, 98, 159–166. [Google Scholar] [CrossRef]
- Liu, S.Q.; Fei, Y.; Xu, Y.C.; Huang, L.; Guo, W.Y. Full-floor grouting reinforcement for working faces with large mining heights and high water pressure: A case study in China. Mine Water Environ. 2020, 39, 268–279. [Google Scholar] [CrossRef]
- Duan, J.H.; Mu, W.Q.; Li, L.C.; Han, Y.C.; Zhang, Y.S.; Ren, B. Slurry flow mechanism with time-varying viscosity in regional long-hole grouting for floor reinforcement. Phys. Fluids 2024, 36, 083115. [Google Scholar] [CrossRef]
- Ju, J.F.; Xu, J.L.; Yang, J. Experimental study on the flow behavior of grout used in horizontal directional drilling borehole grouting to seal mining induced overburden fracture. Geofluids 2021, 2021, 8823902. [Google Scholar] [CrossRef]
- Faramarzi, L.; Rasti, A.; Abtahi, S.M. An experimental study of the effect of cement and chemical grouting on the improvement of the mechanical and hydraulic properties of alluvial formations. Constr. Build. Mater. 2016, 126, 32–43. [Google Scholar] [CrossRef]
- Wang, Y.K.; Han, M.S. Optimal design of slope reinforcement by a new developed polymer micro anti-slide pile in case of emergency and disaster relief. Nat. Hazards 2022, 112, 899–917. [Google Scholar] [CrossRef]
- Wang, P.C.; Li, S.C.; Li, J.L.; Zhou, H.Y.; Ma, P.F.; Tian, Y.; Yuan, C.; Feng, X.D. Seepage behavior and mechanical properties of two kinds of polyurethane/ water glass in combined grouting experiment. Tunn. Undergr. Space Technol. 2023, 136, 105092. [Google Scholar] [CrossRef]
- Cao, C.M.; Feng, Z.Q. Development and application of low viscosity urea-formaldehyde resin grouting material. J. China Coal Soc. 2009, 34, 482–486. [Google Scholar]
- Qian, C.X.; Wang, A.H.; Wang, X. Advances of soil improvement with bio-grouting. Rock Soil Mech. 2015, 36, 1537–1548. [Google Scholar]
- Ju, J.F.; Li, Q.S.; Xu, J.L.; Wang, X.Z.; Lou, J.F. Self-healing effect of water-conducting fractures due to water-rock interactions in undermined rock strata and its mechanisms. Bull. Eng. Geol. Environ. 2020, 79, 287–297. [Google Scholar] [CrossRef]
- Yin, S.H.; Shao, Y.J.; Wu, A.X.; Wang, S.Y.; Li, G.C. The effect of ferrous ions on hydraulic conductivity in fine tailings. Eng. Geol. 2019, 206, 105243. [Google Scholar] [CrossRef]
- van Beek, C.G.E.M. Well-screen and well-head clogging by hydrous ferric oxides. Hydrogeol. J. 2018, 26, 2919–2932. [Google Scholar] [CrossRef]
- Ju, J.F.; Li, Q.S.; Xu, J.L.; Li, J.J.; Cao, Z.G. Experimental study on water permeability decrease character due to restoration function of chemical precipitation on holes or fractures in mining failure rock mass. Coal Sci. Technol. 2020, 48, 89–96. [Google Scholar]
- Ju, J.F.; Li, Q.S.; Xu, J.L.; Li, J.J. Permeability reduction test of CaCO3/Fe(OH)3 repairing single fractured rock specimens. J. Min. Saf. Eng. 2024, 41, 151–159. [Google Scholar]
- Ju, J.F.; Li, Q.S.; Xu, J.L.; Cao, Z.G.; Tian, Y. Experimental investigations on the effect of CaCO3 precipitation on permeability reduction in single brown sandstone fracture. Rock Mech. Rock Eng. 2023, 56, 6647–6666. [Google Scholar] [CrossRef]
- Du, C.; Zuo, R.; Chen, M.H.; Wang, J.S.; Liu, X.; Liu, L.; Lin, Y.H. Influence of colloidal Fe(OH)3 on the adsorption characteristics of strontium in porous media from a candidate high-level radioactive waste geological disposal site. Environ. Pollut. 2020, 260, 113997. [Google Scholar] [CrossRef]

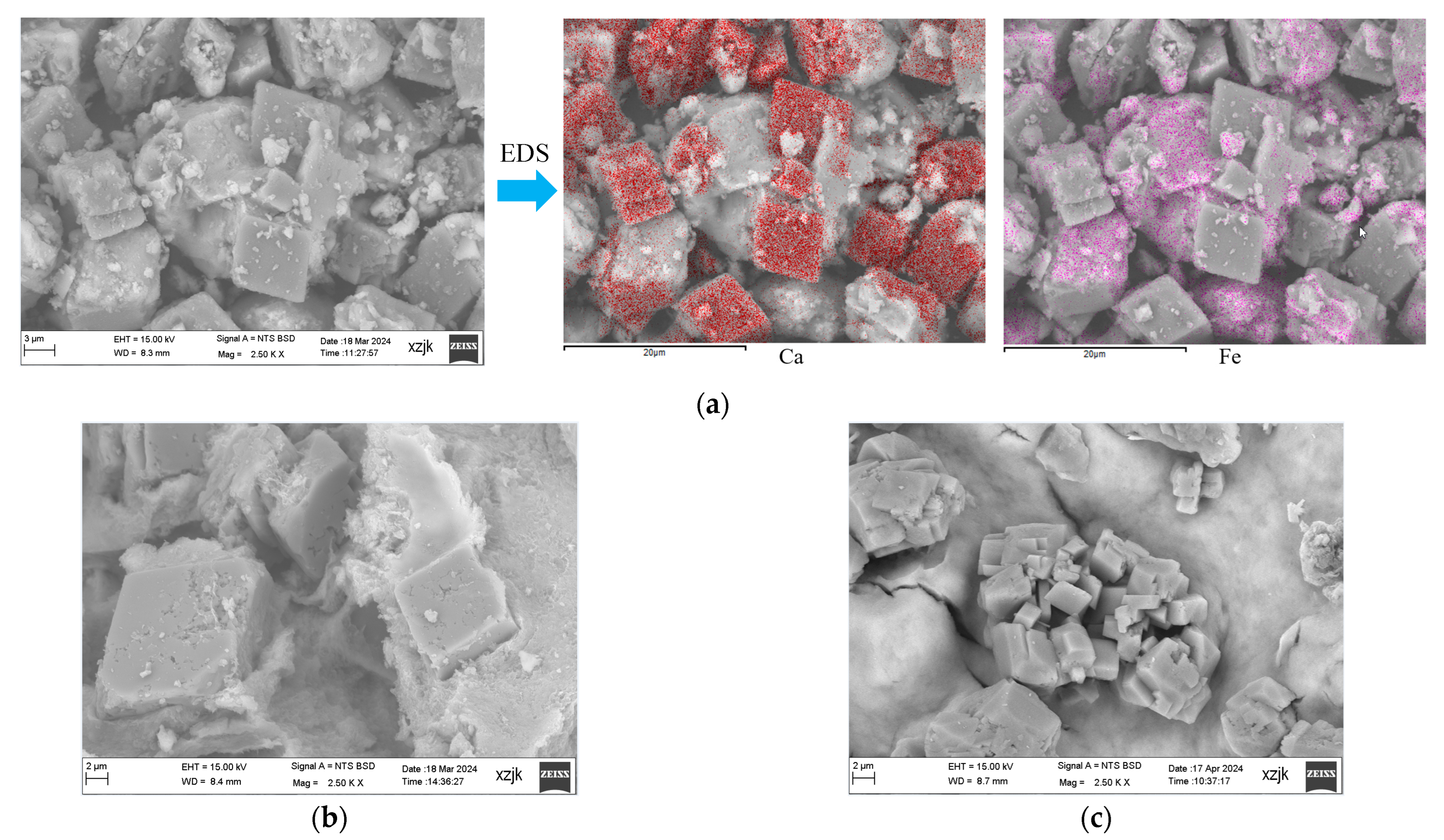
| Water Solution | Simulated Groundwater (Na2CO3 Solution) | Repair Reagent | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Scheme 1 | Scheme 2 | Scheme 3 | Scheme 4 | Scheme 5 | |||||
| Single CaCl2 Solution | FeCl2 + CaCl2 Solution | FeCl2 + CaCl2 Solution | FeCl2 + CaCl2 Solution | Single FeCl2 Solution | |||||
| Ion concentration (mg/L) | CO32− | Ca2+ | Fe2+ | Ca2+ | Fe2+ | Ca2+ | Fe2+ | Ca2+ | Fe2+ |
| 500 | 333.3 | 93.4 | 266.4 | 163.5 | 216.5 | 233.3 | 166.5 | 466.6 | |
| Molar ratio of Fe to Ca | M = 1:4 | M = 1:1.9 | M = 1:1 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, J.; Li, Q.; Wang, C.; Fan, Y. Experimental Investigations on Repair and Permeability Reduction for Single Sandstone Fracture Using a Mixed CaCO3 and Fe(OH)3 Precipitate. Appl. Sci. 2024, 14, 10617. https://doi.org/10.3390/app142210617
Ju J, Li Q, Wang C, Fan Y. Experimental Investigations on Repair and Permeability Reduction for Single Sandstone Fracture Using a Mixed CaCO3 and Fe(OH)3 Precipitate. Applied Sciences. 2024; 14(22):10617. https://doi.org/10.3390/app142210617
Chicago/Turabian StyleJu, Jinfeng, Quansheng Li, Chenyu Wang, and Yanan Fan. 2024. "Experimental Investigations on Repair and Permeability Reduction for Single Sandstone Fracture Using a Mixed CaCO3 and Fe(OH)3 Precipitate" Applied Sciences 14, no. 22: 10617. https://doi.org/10.3390/app142210617
APA StyleJu, J., Li, Q., Wang, C., & Fan, Y. (2024). Experimental Investigations on Repair and Permeability Reduction for Single Sandstone Fracture Using a Mixed CaCO3 and Fe(OH)3 Precipitate. Applied Sciences, 14(22), 10617. https://doi.org/10.3390/app142210617






