Abstract
Diabetes Mellitus (DM) is identified as an important condition that leads to implant complications, and the apico-coronal position and the prosthetic abutment seem to interfere with peri-implant tissue behavior This study aimed at assessing the effect on peri-implant parameters after implant loading of two different methodologies in type two diabetics patients (T2DM) who received implant muco-retained overdentures. Twenty-two mandibular edentulous T2DM received two implants: Test group: prosthetic abutment of a diameter smaller than the platform of the implant and a morse taper subcrestal implant level; Control group: prosthetic abutment of diameter identical to the platform and hexagonal external implant placed at crestal bone level. Clinical, inflammatory, and tomographic evaluations were performed at baseline (after prosthesis installation), and twelve and twenty-four months after implant loading. Test implants presented inferior probing depth and clinical attachment level compared to control at baseline and 12 months (p < 0.05). Test implants presented reduced levels of pro-inflammatory cytokines at 24 months compared to the control implants (p < 0.05). The control group had more changes in bone parameters from baseline to the first and second years (p < 0.05). The test group resulted in reduced bone remodeling and better clinical conditions and positively modulated peri-implant immunoinflammatory molecules. Trial identification UTN code: U1111-1239-3638.
1. Introduction
Diabetes mellitus (DM) is a varied group of metabolic conditions with hyperglycemia in common [1], as a consequence of insulin function defects. It is estimated that 425 million adults have diabetes worldwide [2].
Given the elevated prevalence of type two diabetes (T2DM) in the world population and the high rates of dental loss in these patients [3,4], it is important to study rehabilitation therapies for tooth replacement in people with diabetes, with the aim of adequately reestablishing the masticatory function, aesthetics, and quality of life of these individuals, especially those who are completely edentulous.
The use of overdentures in diabetic patients is a good option for treatment due to the good results regarding quality of life and masticatory function when compared to conventional total dentures [5]. Conversely, diabetic patients have elevated peri-implant diseases [6] and impairment of bone repair around implants [7].
Usually, the crestal region is the locale of the initial collapse of the implant–tissue interface, which may fail osseointegrated implants [8]. In this context, studies have shown that the use of prosthetic abutments with the platform switch concept seems to have significant potential for minimizing the reabsorption of the peri-implant bone crest [9,10,11]. Furthermore, the implant connection type may influence peri-implant bone changes, as augmented bone loss around implants has been related to external hexagonal connections. On the other hand, the morse taper connection promotes a lower concentration and better distribution of stresses/strains in bone tissue around implants when compared with external hexagonal connections [12,13,14,15]. Additional theories have been suggested to elucidate the higher peri-implant bone remodeling in external hexagonal dental implants, including the potential role of the micro gap in the implant–abutment interface regarding bacterial contamination of the connection’s internal portion, resulting in the establishment of peri-implant biological width and, consequently, in bone resorption [15,16,17].
Thus, considering the beneficial aspects of the morse taper implant combined with the platform-switching concept [9], it would be useful to determine whether this therapeutic approach could be considered a better alternative than the hexagonal external implant with the prosthetic component of a diameter equal to the platform in the rehabilitation of T2DM patients with dental implants, since they may be more susceptible than non-diabetic patients to peri-implant changes regarding marginal bone remodeling and local changes in host immune mediators response [18].
Therefore, this study aims at evaluating the effect of two different approaches in diabetic patients on clinical and tomographic parameters and to analyze their effects on peri-implant local biomarkers during rehabilitation using mandibular overdentures.
2. Materials and Methods
2.1. Study Design
The study design is a prospective, split-mouth, randomized controlled trial to evaluate the impact on the clinical, tomographic, and immunoinflammatory peri-implant parameters after implant loading of two different approaches in T2DM rehabilitated with implant muco-retained overdentures.
This trial was approved by the ethics committee of Paulista University (Protocol 077310/2014).
Trial identification UTN code: U1111-1239-3638.
2.2. Population Screening
The population of this study was enlisted from a pool of patients referred by the University.
As inclusion criteria, the age of the patients was stated between 50 and 80 years, and all of them should have completely edentulous jaws; extractions needed to have taken place at least six months before treatment. The patients needed to present the diagnosis of T2DM, made by a physician, and at least five years of diagnosis. Patients were also under use of oral hypoglycemic or insulin. Regarding exclusion criteria, it can be listed as: smoking or ex-smokers; pregnancy; lactation; systemic conditions affecting bone metabolism; the use of drugs such as anti-inflammatory, bisphosphonate, and immunosuppressive medicine; patients with bone graft needed previously or along with the implant surgery; and regenerative procedures conducted in the past in the area selected for implant placement. When critical complications related to T2DM were present, patients were excluded.
2.3. Sample Size Calculation
The sample size was calculated using α = 0.05 and 80% power. The minimum clinically significant value (δ) considered was 0.6 mm [19] with bone-level change assessed after 1 year of loading chosen as the primary outcome variable. For the variability (σ = SD), a value of 0.8 mm was employed. It was established that a sample of at least 16 patients would be necessary. Bearing in mind that patients could drop off during the experiment, 22 patients were included in the trial. The primary variable, bone-level change assessed after 1 year of loading, reached 0.87 power in this study (SPSS 21, IBM, Armonk, NY, USA).
2.4. Experimental Groups
Following the split-mouth design, each patient received two dental implants that were randomly allocated from the following groups (computer-generated list):
- Test group: morse taper (MT) dental implant surface blasted with TiO2, placed 2 mm subcrestal with an abutment diameter smaller than the implant platform.
- Control group: external hexagonal (EH) dental implant placed at the level of the crestal bone with an abutment diameter equal to that of the implant platform.
2.5. Fasting Plasma Glucose and Glycated Hemoglobin Monitoring
Fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c) were measured at baseline and 12- and 24-month follow-ups, employing the glucose oxidase method (milligrams per deciliter), and HbA1c (percentage) was evaluated using high-performance liquid chromatography. The same laboratory (University Clinical Analysis Laboratory) performed all analyses.
2.6. Treatment Protocol
The same operator (FRC) performed all surgeries. As all patients presented an adequate prosthesis with correct occlusion and dimensions, the prostheses were duplicated to create an acrylic resin guide. The installed implants presented a diameter of 3.5 mm and surface blasted with TiO2 (Implacil de Bortoli, São Paulo, SP, Brazil), in the interforaminal region of the mandible. After anesthesia, a mucoperiosteal flap was performed, and the surgery was done following the manufacturer’s instructions. Suture consisted of interrupted surgical stitches using nylon suture 5.0 (Ethicon J&J Medical Devices, Raritan, NJ, USA). Amoxicillin (2 g, 1 h before the procedure), postoperative sodic-dipyrone (500 mg, 6/6 h for 48 h), and 0.12% chlorhexidine mouthwash (12/12 h for 1 week) were prescribed.
A two-stage protocol was adopted, and the prostheses were fixed in place four months after implant placement. Before fixation, the patients used a much-supported prosthesis that was appropriately adjusted and supported by soft resin. The prosthetic retention system used was the O’ring (Implacil de Bortoli, São Paulo, SP, Brazil), and the components were captured directly in the patient’s mouth [19].
Seven and 14 days after fixation, the individuals returned for occlusal adjustments and to check how they had adapted to the prostheses. These were made entirely of acrylic resin with no metallic reinforcement, and the patients’ antagonist mandibles were edentulous and rehabilitated with muco-supported prostheses. All parameters were assessed at baseline (immediately after prosthesis installation) and then reassessed 12- and 24-months following rehabilitation.
2.7. Reassessment Evaluations
Besides the time points used to monitor the variables defined in the investigation, reassessment visits occurred twice in the first 30 days following implant placement and then they occurred quarterly during the time of follow-up. At each visit, any healing problems or implant failures were recorded. When required, individuals received prophylactic procedures such as peri-implant scaling and polishing, and oral hygiene orientation to assure accurate plaque control.
2.8. Clinical Examination
Clinical measurement was performed by the same examiner (BG), calibrated, and blinded. For this, 15 non-study persons with dental implants were selected, and the peri-implant probing depth of all the patients was measured twice in 24 h. The reproducibility found was 93%.
Clinical parameters were measured in dental implants at four sites at baseline and at the 12- and 24-month follow-ups, employing a North Carolina/Colorvue probe (Hu-Friedy, Chicago, IL, USA): (1) Modified Plaque Index (MPI/%): dichotomous index measured along the mucosal margin around implants; (2) Modified Bleeding on Probing (MBoP%): bleeding during probing around implants, dichotomous index [20]; (3) Peri-implant Probing Depth (PPD/mm): defined as the distance measured starting in the bottom of the peri-implant sulcus/pocket to the mucosal margin; (4) Peri-implant Margin Gingival (PMG/mm): measured from the implant platform to the peri-implant soft tissue margin; (5) Clinical Attachment Level (CAL/mm): calculated by adding PPD and PMG.
2.9. Peri-Implant Fluid Collection
Peri-implant crevicular fluid (PIF) was collected by the same examiner (BG) in each group using filter paper strips (PerioPaper; Oraflow, Hewlett, NY, USA) at baseline (immediately after prosthesis installation) and 12- and 24-months following implant insertion, as previously described [21,22]. Briefly, the site was dried, relative isolation was done with cotton rolls, and filter paper strips were placed into the peri-implant sulcus for 30 s (four sites in each implant). After, the fluid volume was measured in a calibrated device (Periotron 8000; Oraflow), and the strips were preserved in separate tubes containing phosphate-buffered saline (PBS)/Tween and the samples were stored at −80 °C.
2.10. Immunoenzymatic Evaluation by Multiplex Bead Immunoassay (Luminex)
Immuno-inflammatory markers concentration (interferon (IFN)-γ, interleukin-10, -1β, -17, -33, -21, -4, -23, -6, and tumor necrosis factor (TNF)-α in the crevicular fluid of implants were measured through commercial kits (H-Th17 HTH17MAG-14K, Millipore Corporation, Billerica, MA, USA) and the Luminex/MAGpix™ platform (Alameda, CA, USA).
2.11. Tomographic Evaluation
Before implant placement, dental cone beam computed tomography (CB) was done for surgical planning. All patients performed CBs at baseline and 12 and 24 months after prosthesis installation for bone level evaluation. All scans were acquired utilizing identical apparatus (KODAK 9000 Extraoral Imaging system, Carestream Health, Rochester, NY, USA).
The exposure settings selected were FOV of 240 × 190 mm, 74 kV, and 10 mA, with a 10.68-s acquisition time, allowing secure protocol with minimal radiation [23]. The software used was CS3D Imaging (Carestream Dental, Atlanta, GA, USA).
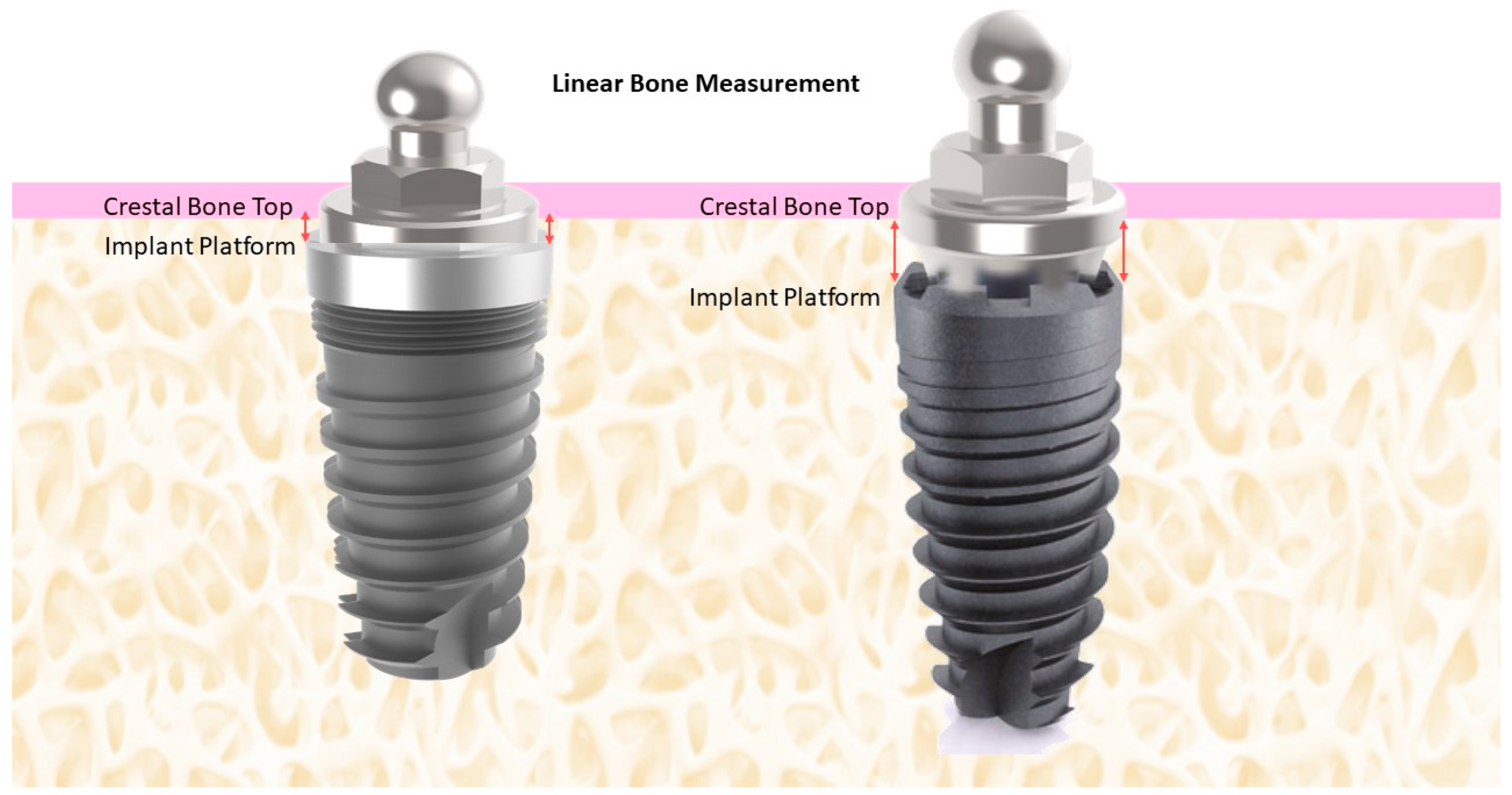
Peri-implant bone level (mm) corresponded to the linear measurement made from the implant platform to the crestal bone (mesial, distal, buccal, and lingually) in the sagittal and coronal slices, recorded every 2 mm (Figure 1). A single research investigator viewed the scans (PHFD) and made all tomographic measurements. Intra-examiner calibration was done using 15 images from patients who received implants for overdenture rehabilitation. The measure was performed twice in 24 h. The reproducibility was calculated as 90%.
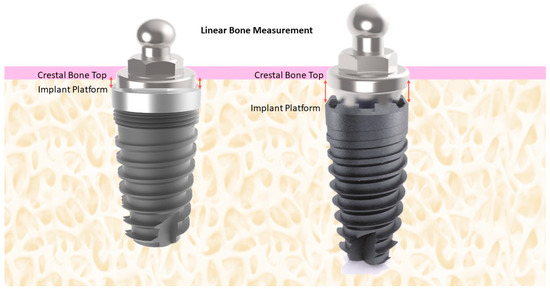
Figure 1.
Schematic illustration of the method of measurement of the crestal bone height.
2.12. Statistical Analysis
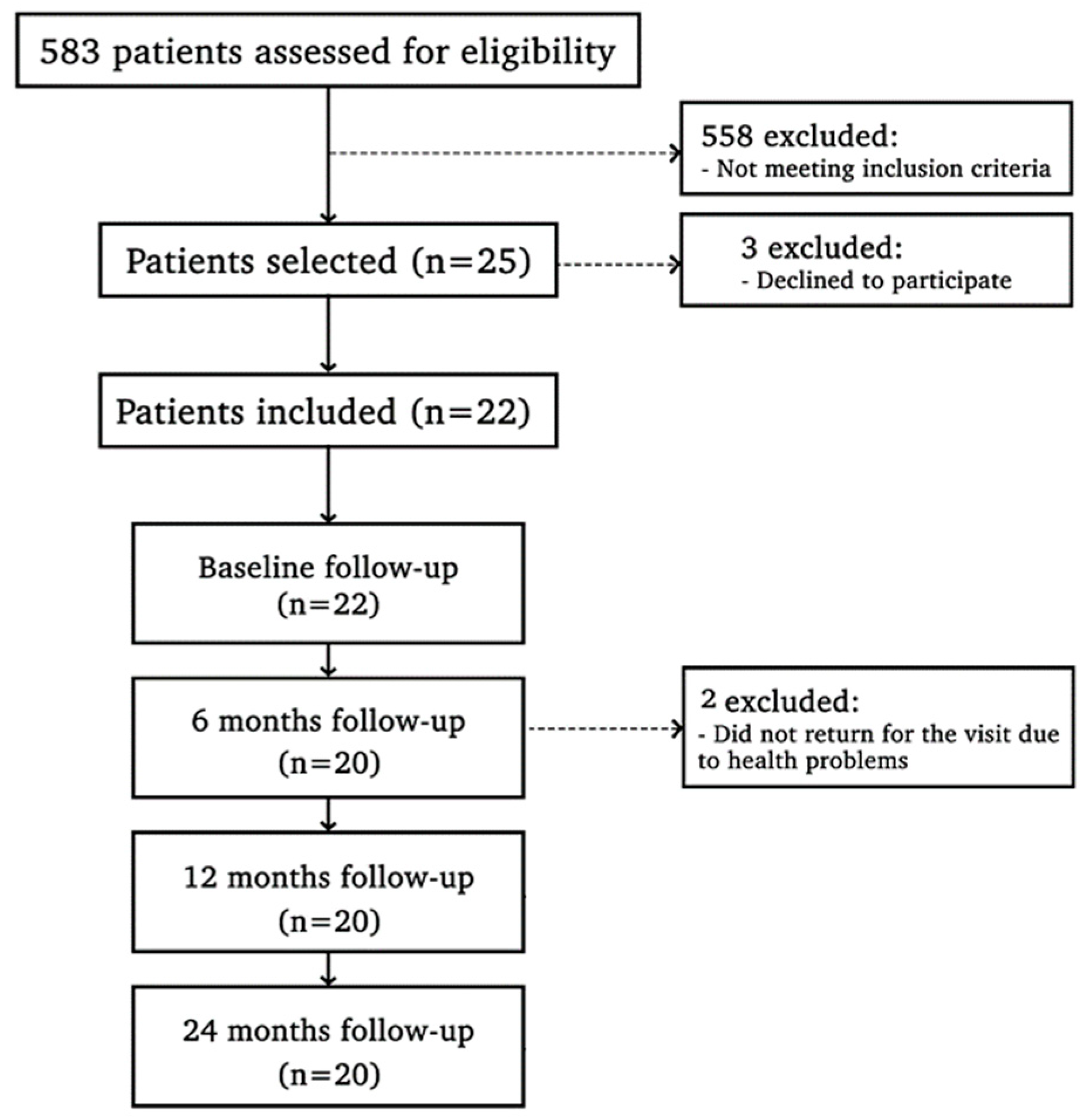
The primary outcome was set as crestal bone level. Additional outcomes included the clinical parameters and inflammatory marker levels. Initially, 22 individuals were part of the trial. One patient did not come for the 12-month visit, and two patients did not attend the 24-month visit; thus, intention-to-treat analyses were adopted on these individuals (Figure 2). The percentage of sites with modified MBoP and MPI and the mean of PPD, CAL, and PGM were calculated, separately, for the two groups. Data were inspected for normality using the Shapiro–Wilk test. Once the distribution was not normal, the Wilcoxon test was used to distinguish inter-group differences and the Friedman test for intra-group analysis in clinical variables (MBoP, MPI, PGM, PPD, and CAL) and tomographic assay. For immunoenzymatic markers, a generalized linear mixed model was applied for repeated measures over time, and for FPG and HbA1c levels, Repeated Measures ANOVA was applied. The biostatistician was blinded to the treatment allocation of the quadrants. Statistical tests were carried out using software programs (R version 4.0.5—The R Foundation for Statistical Computing; IBM SPSS Statistics V22 program), and α was set at 5%.
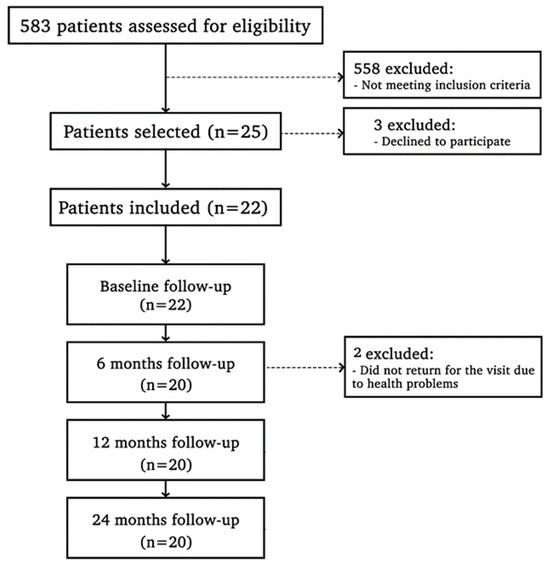
Figure 2.
Study flowchart.
3. Results
Patient enrollment started in October 2014 and finished by the end of September 2016. The clinical events and assessments were conducted between May 2016 and October 2018. Data entry and statistical analyses were completed in May 2019.
3.1. Clinical Outcomes
Table 1 represents the characteristics of the study patients. HbA1c and FPG levels did not differ significantly during the study (p > 0.05, Table 2).

Table 1.
Demographic characteristics of the study population (mean ± SD).

Table 2.
Study population FPG and HbA1c over time (mean ± SD).
There was no significant difference in modified plaque and bleeding on probing index between groups or time of evaluation (p > 0.05; Table 3).

Table 3.
Percentage (mean ± SD) of modified plaque and bleeding on probing index at experimental sites at baseline, 12 and 24 months.
The mean PPD and CAL were significantly higher in the control group than the test group at baseline and 12 months (p < 0.05; Table 4). No significant intra-group differences in PPD and CAL were observed at any point (p > 0.05; Table 3). The values of PMG did not present statistically significant differences in inter-group analysis throughout the study (p > 0.05; Table 4).

Table 4.
Peri-implant PPD, CAL, and PMG (mean ± SD) at experimental sites at baseline, 12 and 24 months.
3.2. Immunoinflammatory Marker Levels
The levels of immunoinflammatory markers are represented in Table 5. Immunoenzymatic assays demonstrated higher concentrations of IL-1β and IL-17f in test implants compared to control implants at 12 months. On the other hand, the test group presented lower concentrations of IL-10, and pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-6, IL-17, IL-21, IL-23, and IL-33 than the control group at 24 months (p < 0.05). Intra-group analysis showed an increase in levels of IL-4-, IL-10, and TNF-α in 12 months compared to baseline for both groups (p < 0.05). The test group showed reduced levels of IL-4, IL-10, IL-6, and TNF-α and an increase in IL-21 at 24 months compared to 12 months (p < 0.05). The control group presented lower levels of IL-4 and higher levels of IL-17, IL-1β, IL-33, IL-21, IFN-γ, IL-23, IL-6, and TNF-α at 24 months (p < 0.05).

Table 5.
Level of inflammatory markers for test and control groups over time (pg/mL).
3.3. Tomographic Analysis
Annual bone loss in the two study groups is presented in Table 6. Increased peri-implant bone loss was detected during the first 12 months of implant function compared to during the second year in both test and control groups (p < 0.05). The control group showed higher peri-implant bone loss in the first and second years than the test group (p < 0.05) (Figure 3).

Table 6.
Annual changes (mean ± SD) in the crestal bone level during the study in both groups (mm).
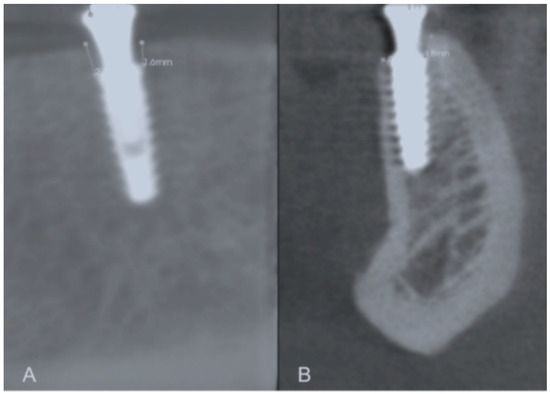
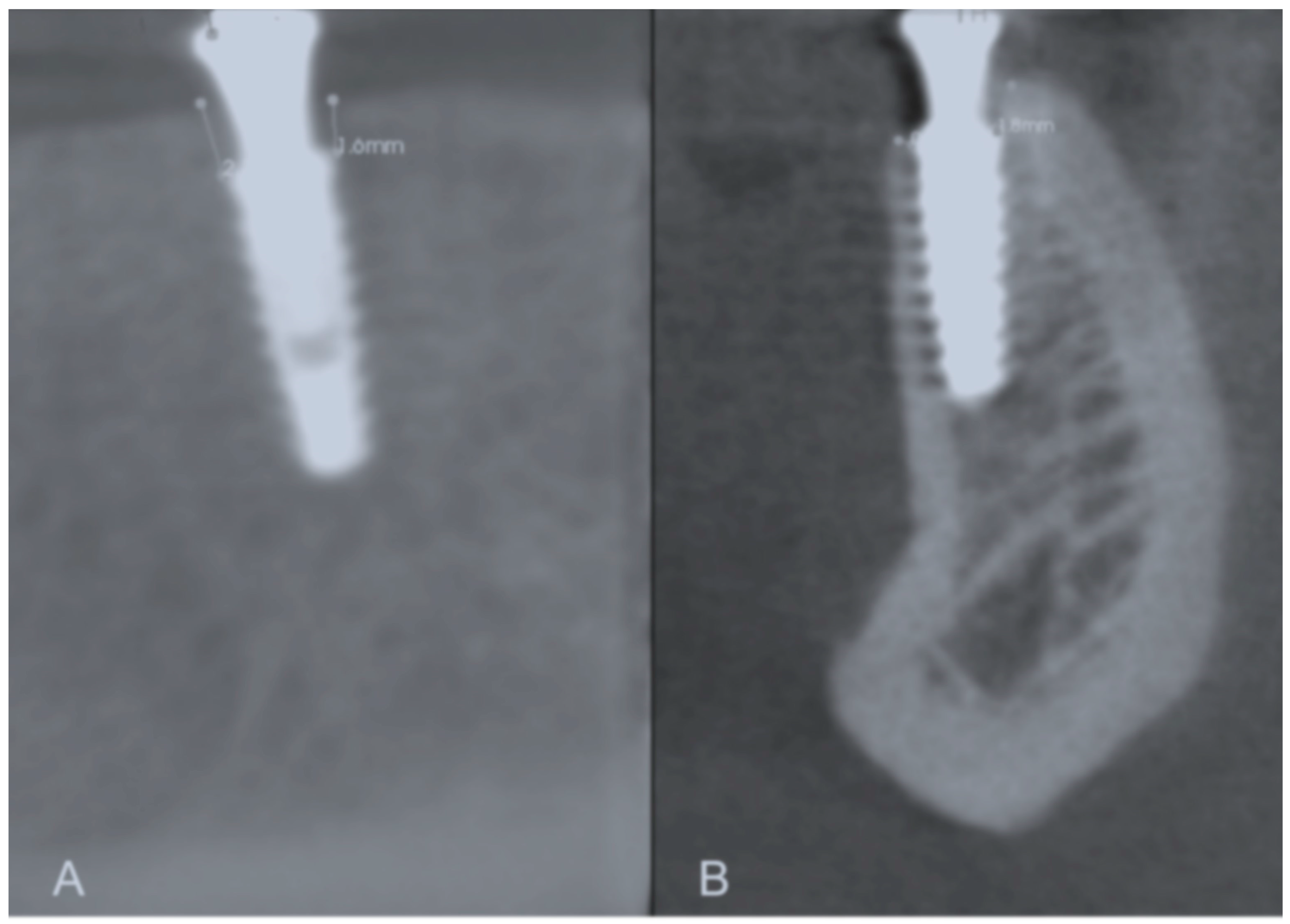
Figure 3.
Representative images of the buccal, mesial, lingual, and distal bone level measurements. (A) Mesial and distal bone level measurement; (B) Buccal and lingual bone level measurement.
4. Discussion
Overall, our results showed that morse taper implants with an abutment diameter smaller than the implant platform led to a better pattern of clinical and bone parameters, with lower peri-implant bone loss and lower PPD and CAL in T2DM rehabilitated with implant muco-retained overdentures, with positive modulation of inflammatory mediators compared to the control implants, which consisted of external hexagon implants with a standard connection.
In this trial, dental implants in the test group were based on the platform-switching concept. The idea behind “platform switching” is that the trans-mucosal abutment diameter is smaller than the implant shoulder diameter. Implants inserted with this idea have an implant–abutment junction nearer to the center of the implant. This concept led to minimal marginal peri-implant bone loss compared to implants with similar diameters of abutment and implant body, due to the positive effect of platform switching on marginal bone levels [9,24]. These findings agree with the tomographic outcomes of the present study that detected in the annual bone loss analysis that there was a lower amount of bone loss in the test group both in the first and second years when compared to the control group (p < 0.05). Although data concerning marginal bone loss in diabetic populations has been scarce until now, systematic reviews also support that platform switching has a positive influence on peri-implant marginal bone loss and appears beneficial in limiting bone resorption [10].
Different degrees of peri-implant crestal bone remodeling are influenced by the implant–abutment connection designs after functional loading. Pessoa et al. [15] demonstrated this in a split-mouth RCT that evaluated 12 edentulous patients with four customized implants in the mandibular interforaminal zone. The macroscopic design of the implants was the same and used external hexagon or morse-taper connections, and a standardized periapical X-ray evaluation found greater remodeling of the crestal bone with a higher loss around the external hexagon compared to the morse-taper. Considerable impact on peri-implant crestal bone level is related to the implant–abutment connection [25]. Additionally, in an animal study, Castro et al. [26] histologically and histomorphologically evaluated bone resorption around implants, and morse taper presented less bone loss compared to external hexagon. According to Melo et al. [27], periapical radiographs showed significantly greater bone loss in the external hexagon connection than morse taper implants in patients rehabilitated with immediately loaded mandibular overdentures during a 1-year follow-up, in agreement with the data observed in this study.
Some authors justify this remodeling according to the zone of stress. Lower concentration and better distribution of stress/strains in the peri-implant bone were observed in finite element analysis evaluating morse taper implant–abutment connections compared to external hexagonal connections [12,14,15,28]. These data agree with the outcomes presented in this study and with previous investigations that related decreased bone loss in implants with morse taper connections compared to other connection types [15,25,27]. The taper interface in the internal conical connection resists the loading and allows stable retention of position by frictional force [12,14,28]. Lateral forces are dissipated by the lateral wall of morse taper abutments, which also protects the abutment screw from excessive stress [12,14,28].
Remarkably, platform switching may also influence the location and the reestablishment of the biological width after implant placement, minimizing the marginal bone resorption [12]. This hypothesis is because the distance between the implant–abutment, micro gap contamination, and the crestal bone can be increased by a narrower abutment, controlling bone remodeling and contributing to the adequate formation of biological width [16,29]. These data are in line with the results of this trial that showed that a smaller abutment compared to those with a diameter equal to that of the implant can produce an area responsible for reducing the invasion of the biological width [30].
Concerning the molecular analysis performed in the current study, test implants presented reduced levels of pro-inflammatory mediators (IFN-γ, TNF-α, IL-6, IL-17, IL-21, IL-23, and IL-33) at 24 months compared to the control group (p < 0.05). These results are supported not only by the tomographic data of this RCT, which demonstrated greater alterations in the crestal bone in control implants, but are also consistent with our clinical outcomes that showed higher PPD in this experimental group until 12 months (p < 0.05).
Even though the clinical data obtained during this investigation suggest, in general, a low percentage of peri-implant inflammation sites in both implant groups, it could be speculated that the control group presents an at-risk-for-harm condition due to the pro-inflammatory pattern of local molecules observed, and this condition could predispose to peri-implantitis [31]. This disturbance in local levels of pro-inflammatory mediators in implants with an external hexagonal and standard connection seems to be exacerbated with time since, in this trial, control implants showed higher levels of pro-inflammatory molecules IFN-γ, TNF-α, IL-6, IL-17, IL-21, IL-23, and IL-33 at 24 months in comparison with baseline (p < 0.05). Furthermore, IL-6, IL-17, IL-21, and TNF-α were up-regulated at 24 months in control implants in comparison with 12 months (p < 0.05). Importantly, previous data confirm that the peri-implant fluid augmented the presence of IL-6 and IL-33 in intensifying the local inflammatory process, contributing to the risk of peri-implantitis [32,33]. Accordingly, a significantly positive association between IL-6 levels in the saliva and peri-implant disease was observed [34]. Another important pro-inflammatory marker that was up-regulated in control implants in this study was TNF-α, a well-established mediator related to peri-implantitis-related bone loss [35]. Remarkably, both TNF-α and IL-17 are related to osteoclast formation and bone resorption, including around dental implants [36]. The biochemical outcomes of peri-implant crevicular fluid may characterize biological processes in peri-implant tissues and, according to our results, it could be assumed that external hexagonal implants placed at crestal bone level with a standard platform could harmfully modulate the local pattern of inflammatory markers in the crevicular fluid of implants, even in clinical situations of health, encouraging peri-implant bone loss.
Given that a tendency to develop peri-implant diseases and complications is observed in diabetic patients [37,38], the benefits of the use of the platform-switching concept in this patient profile could be extrapolated to, or at least be useful in, the implant success over time in type 2 diabetic individuals. However, it is necessary to highlight that one limitation of the study could be the specificity of this trial population (Brazilian ethnicity), and other populations could respond in a different pattern.
While the clinical analyses in this investigation demonstrated augmented PPD and CAL in the control group compared to the test group at baseline and 12 months, peri-implant probing depth means were inferior to 3 mm, and parameters regarding plaque and bleeding on probing indexes also remained low throughout the time of follow-up, clinically representing healthy conditions. It is noteworthy that this study employed a rigorous schedule for supportive therapies, and evidence supports the relevance of supportive therapy for the prevention of pathological disturbance [39], reducing peri-implantitis risk in 26% at the patient level [40]. Thus, it can be assumed that the favorable clinical outcomes (compatible with healthy conditions) achieved in both the control and test implant placement strategies were related to the strict implementation of supportive therapy in the current study. However, patients do not always adhere to the proper maintenance therapy in clinical practice, and distinct results from those observed in the present trial might be observed if peri-implant maintenance was not performed with adequate care, especially in diabetic individuals, who are considered at risk for peri-implant biological complications. Given the better clinical outcomes achieved in the test implant approach in this trial, we hypothesize that long-term biological complications might be prevented using the test implants, principally in diabetic conditions.
5. Conclusions
In conclusion, our trial demonstrated reduced bone loss and improved clinical parameters, with lower PPD and CAL in the first years and positive modulation of inflammatory markers, with lower inflammatory pattern in the test implants in type 2 diabetic patients rehabilitated with mandibular implant-supported overdentures compared to control implants.
Author Contributions
B.G.—Worked in the experimental phase (clinical procedures and patient recruitment); M.G.C.—Analysis and interpretation of data for the work, laboratory analysis; F.V.R.—Drafting the work; F.R.C.—Substantial contributions to the conception or design of the work; P.H.F.D.—Tomographic evaluation; S.P.P.—Final approval of the version to be published, clinical procedures; M.Z.C.—Final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors agree to be personally accountable for the author’s contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Counsel of Technological and Scientific Development (CNPq), Brasília, DF, Brazil—Processes 421504/2018-8, and 301563/2017-9.
Institutional Review Board Statement
This trial was approved by the ethics committee of Paulista University (Protocol 077310/2014). Trial identification UTN code: U1111-1239-3638 (4 June 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos, V.R.; Lima, J.A.; Gonçalves, T.E.; Bastos, M.F.; Figueiredo, L.C.; Shibli, J.A.; Duarte, P.M. Receptor activator of nuclear factor-kappa B ligand/osteoprotegerin ratio in sites of chronic periodontitis of subjects with poorly and well-controlled type 2 diabetes. J. Periodontol. 2010, 81, 1455–1465. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; IDF: Brussels, Belgium, 2017. [Google Scholar]
- Botero, J.E.; Yepes, F.L.; Roldán, N.; Castrillón, C.A.; Hincapie, J.P.; Ochoa, S.P.; Ospina, C.A.; Becerra, M.A.; Jaramillo, A.; Contreras, A. Tooth and periodontal clinical attachment loss are associated with hyperglycemia in patients with diabetes. J. Periodontol. 2012, 83, 1245–1250. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, Y.G.; Jin, M.U.; Cho, J.H.; Lee, J.M. Relationship of tooth mortality and implant treatment in Type 2 diabetes mellitus patients in Korean adults. J. Adv. Prosthodont. 2013, 5, 51–57. [Google Scholar] [CrossRef]
- Kutkut, A.; Bertoli, E.; Frazer, R.; Pinto-Sinai, G.; Fuentealba, H.R.; Studts, J. A systematic review of studies comparing conventional complete denture and implant retained overdenture. J. Prosthodont. Res. 2018, 62, 1–9. [Google Scholar] [CrossRef]
- Aghaloo, T.; Pi-Anfruns, J.; Moshaverinia, A.; Sim, D.; Grogan, T.; Hadaya, D. The Effects of Systemic Diseases and Medications on Implant Osseointegration: A Systematic Review. Int. J. Oral Maxillofac. Implants 2019, 34, s35–s49. [Google Scholar] [CrossRef]
- de Souza, J.G.; Neto, A.R.; Filho, G.S.; Dalago, H.R.; de Souza, J.M., Jr.; Bianchini, M.A. Impact of local and systemic factors on additional peri-implant bone loss. Quintessence Int. 2013, 44, 415–424. [Google Scholar]
- Isidor, F. Histological evaluation of peri-implant bone at implants subjected to occlusal overload or plaque accumulation. Clin. Oral Implants Res. 1997, 8, 1–9. [Google Scholar] [CrossRef]
- Lazzara, R.J.; Porter, S.S. Platform switching: A new concept in implant dentistry for controlling postrestorative crestal bone levels. Int. J. Periodontics Restor. Dent. 2006, 26, 9–17. [Google Scholar]
- Al-Nsour, M.M.; Chan, H.L.; Wang, H.L. Effect of the platform-switching technique on the preservation of peri-implant marginal bone: A systematic review. Int. J. Oral Maxillofac. Implants 2012, 27, 138–145. [Google Scholar] [PubMed]
- Telleman, G.; Raghoebar, G.M.; Vissink, A.; Meijer, H.J. Impact of platform switching on inter-proximal bone levels around 8.5 mm implants in the posterior region; 5-year results from a randomized clinical trial. J. Clin. Periodontol. 2017, 44, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.S.; Muraru, L.; Júnior, E.M.; Vaz, L.G.; Sloten, J.V.; Duyck, J.; Jaecques, S.V. Influence of implant connection type on the biomechanical environment of immediately placed implants–CT-based nonlinear, three-dimensional finite element analysis. Clin. Implant Dent. Relat. Res. 2010, 12, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Perrotti, V.; Shibli, J.A.; Novaes, A.B.; Piattelli, A.; Iezzi, G. Equicrestal and subcrestal dental implants: A histologic and histomorphometric evaluation of nine retrieved human implants. J. Periodontol. 2011, 82, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.S.; Bezerra, F.J.; Sousa, R.M.; Vander, S.J.; Casati, M.Z.; Jaecques, S.V. Biomechanical evaluation of platform switching: Different mismatch sizes, connection types, and implant protocols. J. Periodontol. 2014, 85, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.S.; Sousa, R.M.; Pereira, L.M.; Neves, F.D.; Bezerra, F.J.; Jaecques, S.V.; Sloten, J.V.; Quirynen, M.; Teughels, W.; Spin-Neto, R. Bone Remodeling Around Implants with External Hexagon and Morse-Taper Connections: A Randomized, Controlled, Split-Mouth, Clinical Trial. Clin. Implant Dent. Relat. Res. 2017, 19, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.S.; Schoolfield, J.D.; Schenk, R.K.; Buser, D.; Cochran, D.L. Influence of the size of the microgap on crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged implants in the canine mandible. J. Periodontol. 2001, 72, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- De Melo, L.A.; de Farias, D.B.; de Medeiros, A.K.B.; Barbosa, G.A.S.; Dantas, E.M.; Carreiro, A.D.F.P. Comparative evaluation of peri-implant tissues in patients wearing mandibular overdenture with different implant platforms. J. Indian Soc. Periodontol. 2017, 21, 473–477. [Google Scholar] [PubMed]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: Systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef]
- Pozzi, A.; Agliardi, E.; Tallarico, M.; Barlattani, A. Clinical and radiological outcomes of two implants with different prosthetic interfaces and neck configurations: Randomized, controlled, split-mouth clinical trial. Clin. Implant Dent. Relat. Res. 2014, 16, 96–106. [Google Scholar] [CrossRef]
- Mombelli, A.; Lang, N.P. Clinical parameters for the evaluation of dental implants. Periodontol. 2000 1994, 4, 81–86. [Google Scholar] [CrossRef]
- Ghiraldini, B.; Conte, A.; Casarin, R.C.; Casati, M.Z.; Pimentel, S.P.; Cirano, F.R.; Ribeiro, F.V. Influence of Glycemic Control on Peri-Implant Bone Healing: 12-Month Outcomes of Local Release of Bone-Related Factors and Implant Stabilization in Type 2 Diabetics. Clin. Implant Dent. Relat. Res. 2016, 18, 801–809. [Google Scholar] [CrossRef]
- Verrastro Neto, A.; Andrade, R.; Corrêa, M.G.; Casarin RC, V.; Casati, M.Z.; Pimentel, S.P.; Cirano, F.R. The impact of different torques for the insertion of immediately loaded implants on the peri-implant levels of angiogenesis- and bone-related markers. Int. J. Oral Maxillofac. Surg. 2018, 47, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Feragalli, B.; Rampado, O.; Abate, C.; Macrì, M.; Festa, F.; Stromei, F.; Caputi, S.; Guglielmi, G. Cone beam computed tomography for dental and maxillofacial imaging: Technique improvement and low-dose protocols. Radiol. Med. 2017, 122, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.; Dhakne, V.M.; Jadhawar, S.; Kadam, I.; Mishra, K.; Patil, P. Bone Level Measurements Around Platform Switched and Platform Matched Implants: A Comparative Study. Niger. J. Surg. 2019, 25, 9–13. [Google Scholar] [CrossRef]
- Koo, K.T.; Lee, E.J.; Kim, J.Y.; Seol, Y.J.; Han, J.S.; Kim, T.I.; Lee, Y.M.; Ku, Y.; Wikesjö, U.M.; Rhyu, I.C. The effect of internal versus external abutment connection modes on crestal bone changes around dental implants: A radiographic analysis. J. Periodontol. 2012, 83, 1104–1109. [Google Scholar] [CrossRef]
- Castro, D.S.; Araujo, M.A.; Benfatti, C.A.; de Araujo, C.D.R.P.; Piattelli, A.; Perrotti, V.; Iezzi, G. Comparative histological and histomorphometrical evaluation of marginal bone resorption around external hexagon and Morse cone implants: An experimental study in dogs. Implant Dent. 2014, 23, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.A.; Souza, M.B.C.; Barbosa, G.A.S.; Carreiro, A.D.F.P. Peri-Implant Bone Loss of External Hexagon and Morse Taper in Patients Wearing Immediately Loaded Overdentures. Braz. Dent. J. 2017, 28, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.M.; Simamoto-Junior, P.C.; Fernandes-Neto, A.J.; Sloten, J.V.; Jaecques, S.V.; Pessoa, R.S. Influence of Connection Types and Implant Number on the Biomechanical Behavior of Mandibular Full-Arch Rehabilitation. Int. J. Oral Maxillofac. Implants 2016, 31, 750–760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Todescan, F.F.; Pustiglioni, F.E.; Imbronito, A.V.; Albrektsson, T.; Gioso, M. Influence of the microgap in the peri-implant hard and soft tissues: A histomorphometric study in dogs. Int. J. Oral Maxillofac. Implants 2002, 17, 467–472. [Google Scholar]
- Morris, H.F.; Ochi, S.; Crum, P.; Orenstein, I.H.; Winkler, S. AICRG, Part I: A 6-year multicentered, multidisciplinary clinical study of a new and innovative implant design. J. Oral Implantol. 2004, 30, 125–133. [Google Scholar] [CrossRef]
- Sanz, M.; Chapple, I.L. Working Group 4 of the VIII European Workshop on Periodontology. Clinical research on peri-implant diseases: Consensus report of Working Group 4. J. Clin. Periodontol. 2012, 39, 202–206. [Google Scholar] [CrossRef]
- Severino, V.O.; Beghini, M.; de Araújo, M.F.; de Melo, M.L.R.; Miguel, C.B.; Rodrigues, W.F.; de Lima Pereira, S.A. Expression of IL-6, IL-10, IL-17, and IL-33 in the peri-implant crevicular fluid of patients with peri-implant mucositis and peri-implantitis. Arch. Oral Biol. 2016, 72, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Ghassib, I.; Chen, Z.; Zhu, J.; Wang, H.L. Use of IL-1 β, IL-6, TNF-α, and MMP-8 biomarkers to distinguish peri-implant diseases: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2019, 21, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.; de Oliveira, D.W.; Costa, F.O. Could the biomarker levels in saliva help distinguish between healthy implants and implants with peri-implant disease? A systematic review. Arch. Oral Biol. 2018, 96, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Dutzan, N.; García-Sesnich, J.; Abusleme, L.; Dezerega, A.; Silva, N.; González, F.E.; Vernal, R.; Sorsa, T.; Gamonal, J. Host-pathogen interactions in progressive chronic periodontitis. J. Dent. Res. 2011, 90, 1164–1170. [Google Scholar] [CrossRef]
- Monje, A.; Caballé-Serrano, J.; Nart, J.; Peñarrocha, D.; Wang, H.L.; Rakic, M. Diagnostic accuracy of clinical parameters to monitor peri-implant conditions: A matched case-control study. J. Periodontol. 2018, 89, 407–417. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Cionca, N.; Mombelli, A. Systemic conditions and treatments as risks for implant therapy. Int. J. Oral Maxillofac. Implants 2009, 24, 12–27. [Google Scholar]
- Li, J.Y.; Yu, M.; Tyagi, A.M.; Vaccaro, C.; Hsu, E.; Adams, J.; Bellido, T.; Weitzmann, M.N.; Pacifici, R. IL-17 Receptor Signaling in Osteoblasts/Osteocytes Mediates PTH-Induced Bone Loss and Enhances Osteocytic RANKL Production. J. Bone Miner. Res. 2019, 34, 349–360. [Google Scholar] [CrossRef]
- Monje, A.; Aranda, L.; Diaz, K.T.; Alarcón, M.A.; Bagramian, R.A.; Wang, H.L.; Catena, A. Impact of Maintenance Therapy for the Prevention of Peri-implant Diseases: A Systematic Review and Meta-analysis. J. Dent. Res. 2016, 95, 372–379. [Google Scholar] [CrossRef]
- Costa, F.O.; Takenaka-Martinez, S.; Cota, L.O.; Ferreira, S.D.; Silva, G.L.; Costa, J.E. Peri-implant disease in subjects with and without preventive maintenance: A 5-year follow-up. J. Clin. Periodontol. 2012, 39, 173–181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).