Abstract
The aim of this study is to comparatively investigate changes in mechanical properties by adding five different types of boron derivatives to a microhybrid dental composite structure. In this study, which evaluated upper and lower surface microhardness (VHN), roughness (Ra), and color stability (∆E), a total of 126 discs were used (n = 7; per each subgroup). All boron derivatives were added to the dental composite structure in equal proportions in a dark room to create experimental composites (5% w/w). To enable comparison, a default composite without the addition of any boron derivative served as the negative control group. Before measurements, all samples were incubated at 37 °C for 24 h. For surface microhardness, roughness, and color stability, all experimental groups were statistically significant within themselves (p < 0.001). The group with borax pentahydrate exhibited the highest VHN value on the upper surface, while the lowest value was observed in the group with etifert. It was found that all experimental groups showed a decrease in lower surface microhardness compared to the control group (p < 0.001). Although the eticol-ceramic-added group had the lowest roughness values, this group also exhibited significantly higher ∆E values compared to the other groups. Surface roughness showed a negative correlation with ∆E and upper surface microhardness values for all experimental groups (respectively p = 0.038; r = −0.185/p = 0.006; r = −0.245). To sum up, the addition of boron derivatives to composites, except for etifert, increased upper surface microhardness values; however, except for eticol-ceramic, surface roughness values also increased. Nevertheless, the addition of boron derivatives, except for eticol-ceramic, ensures color stabilization.
1. Introduction
Boron is classified as a metalloid element in Group 13 (IIIA). Despite exhibiting chemical properties that bear a closer resemblance to carbon and silicon than to other elements in Group 13, it is noteworthy that boron, as elaborated upon below, manifests a more pronounced electron deficiency than both carbon and silicon [1]. It is considered a prebiotic chemical with many roles and is essential for plants [2,3]. The importance of boron in our world is strongly related to its effects on the prebiotic origins of genetic material [4]. The assumption that boron compounds have undertaken certain biological roles throughout history and played a prebiotic agent role in cellular-level biogenesis activities is expressed based on geological studies related to the origin of life. Its presence in the structure of chemical molecules essential for the evolution of life, coupled with its selective use as a substrate by host microorganisms, elevates boron material to a valuable point in the evolutionary process [2]. Throughout the evolution of life, boron has provided thermal and chemical stability [4]. Based on these, it can be said that boron is an essential mineral in the emergence and evolution of life and enters into both aspects of the definition of prebiotic as a micronutrient that has an important role in the nutrition of plants and humans [5].
Boron exists within the human body, with the greatest amount found in bones and teeth [6]. However, increasing the intake of boron changes the mineral composition of the teeth and shows beneficial effects on the alveolar bone in moderate amounts [7]. Boric acid can reduce alveolar bone loss [8]. Boron enhances the odontogenic and osteogenic differentiation processes in human dental germ stem cells, promoting the proliferation of human dental pulp stem cells. Moreover, it exerts a substantial influence on the growth and development of both bones and teeth [9]. AN0128 is a compound containing boron and possesses antibacterial and anti-inflammatory characteristics. A study conducted to test its potential effectiveness in the treatment of periodontal disease in rats shows that when experimental periodontitis is created by placing ligatures, histological and micro-CT evaluations significantly reduce the occurrence of inflammatory leakage and reduce bone density [10]. The addition of boron to silicate bioactive glass has recently emerged as an effective way to strengthen the mechanical properties thanks to the ability to form a boron network in the glass structure. Bioactive glass containing boron has shown improved biocompatibility and a certain antibacterial activity [9]. In studies with different materials, boron provides. For example, the addition of a small amount of boron significantly improves the casting structure and strength of titanium alloys [11].
Today, the most commonly utilized and preferred material for restorative treatments in dentistry is resin-based dental composite materials. Since the introduction of resin-based composites in dentistry, many strategies have been tested to obtain a new material with superior physical and chemical properties, such as mechanical and antibacterial properties [12]. Boron-doped bioactive glass nanoparticles (BG-NPS) are used on dental pulp stem cells and are also used in dental tissue engineering as a coating material for dental implants [13]. Methacrylate-based adhesive resin containing boron nitride nanotubes (BNNTs) can be successfully incorporated as a filler [14].
In addition to including some metal oxides such as titanium, silver, and zinc oxides to increase the antibacterial activity of the composite on secondary caries, which are the causes of failure in composite fillings [3], boron nitride nanolayers and their zinc oxide-modified derivatives have also achieved good mechanical properties [12]. In a study related to adhesives, it was also concluded that boron nitride nanotubes were successfully incorporated into a resin-containing adhesive, which is beneficial for their improved physicochemical properties and long-term bond stability without reducing the viability of fibroblast cell growth [15]. In the context of preceding studies, this investigation was orchestrated to examine the impact of incorporated boron material on dental composites, with a specific focus on elucidating which type of boron would prove more efficacious, particularly concerning various mechanical properties such as surface roughness, surface microhardness, and color stability. The hypothesis of the study is that in all experimental groups with added boron, surface roughness, upper surface microhardness, and color stability values will yield better results compared to the control group.
2. Material & Methods
2.1. The Calculation of Sample Size
The study was conducted at the Altınbaş University Faculty of Dentistry Hard Tissue Laboratory. Power analysis was performed using the statistical analysis program named G. Power 3.1.7 to determine the sample size in each subgroup. The power of the study, expressed as 1-ß (ß = Type II error probability), was considered. Taking into account the values from the study by [16], with α: 0.05 and an acceptable margin of error (d) of 3.2937 to achieve 95% power, it was calculated that each subgroup should include at least 4 composite discs. A decision was made to use a minimum of 7 composite discs for each methodology employed in the study.
2.2. The Selection of Composite & Boron Materials and the Design of the Study
A novel experimental composite was not created from scratch for this study. Experimental composites were obtained by incorporating five distinct boron derivatives into a pre-determined traditional microhybrid composite restorative material (Charisma Smart, Kulzer GmbH, Hanau, Germany) through laboratory procedures. The composite material without the addition of any boron derivative served as the control group. The added boron varieties were identified as boric acid, eticol-ceramic, borax pentahydrate, etifert, and zinc borate.
The employed boron derivatives were meticulously introduced into composite matrices at a concentration of 5% (w/w), giving rise to the development of five unique experimental composites. The procedural intricacies unfolded within the confines of an obscured environment, deliberately shrouded in a low-lit room during the boron integration phase, strategically implemented to avert the untimely polymerization of the experimental composites. The incorporation of components transpired under the guidance of a non-adherent titanium spatula within aseptic petri dishes. The amalgamating process persisted until the last vestiges of boron powder vanished, culminating only when the composite achieved a state of impeccable homogeneity [3,17]. The names and contents of all materials used in the study are provided in Table 1.

Table 1.
The contents of the boron derivatives included in the study.
2.3. Surface Microhardness
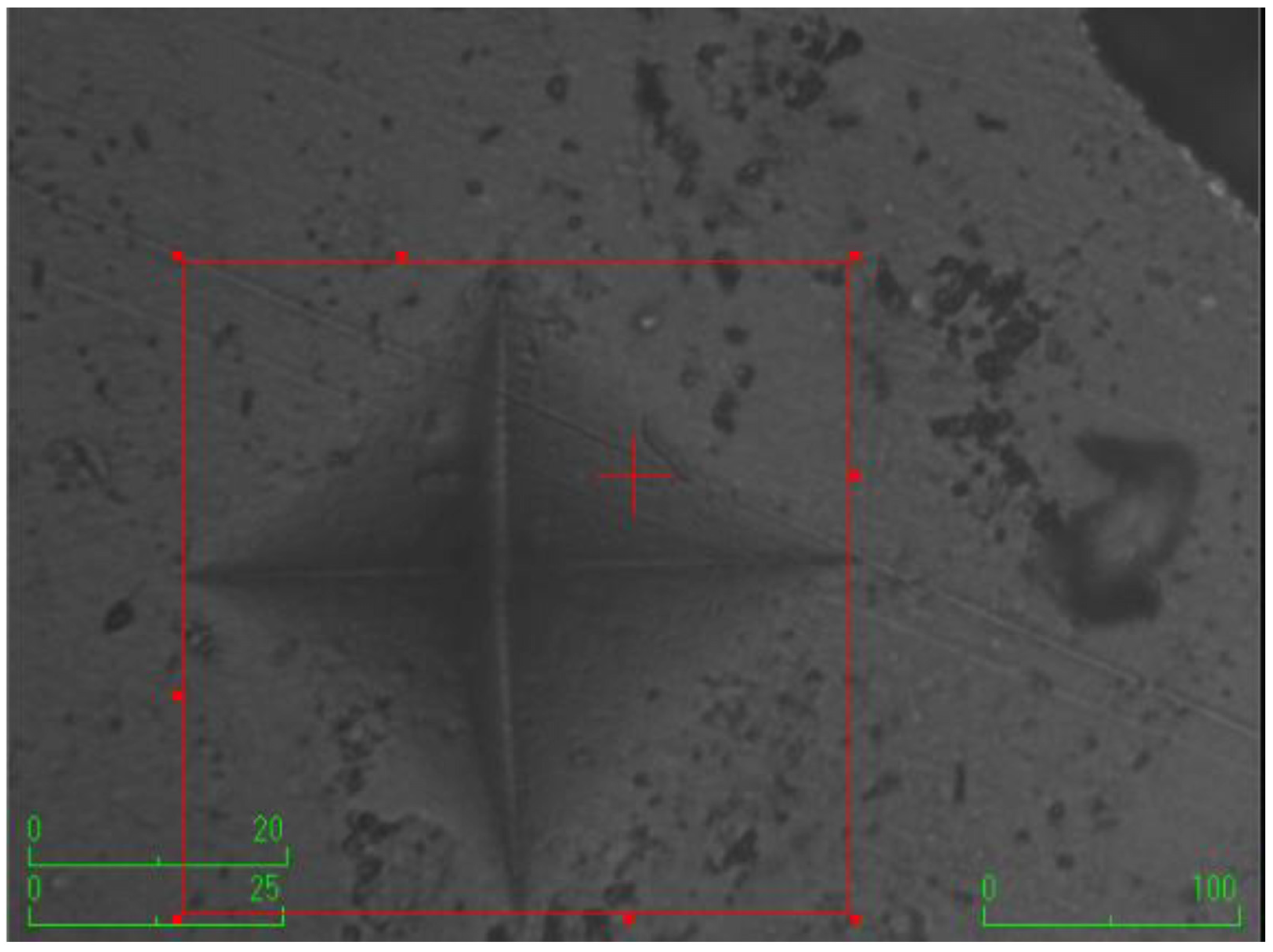
The samples were prepared using Teflon molds (2 mm width × 5 mm diameter) (n = 7). A mylar strip was initially placed on a microscope slide, followed by the arrangement of mylar strip and microscope slide successively onto the Teflon mold. The discs were obtained by carefully placing composites into the Teflon mold using a titanium spatula to ensure the absence of air bubbles. The prepared samples were incubated at 37 °C for 24 h. Before conducting measurements, the top surface of the composite discs underwent a precision finishing and polishing procedure utilizing a low-speed micromotor and a specialized finishing-polishing kit (Sof-Lex Disc 3M ESPE, St. Paul, MN, USA). This process aimed to establish a consistent measurement area and ensure standardization. Subsequently, a Vickers hardness testing machine (Shimadzu, HMV-G31D, Shimadzu Corporation, Kyoto, Japan) was employed for hardness measurement. The hardness measurements were conducted on both the upper and lower surfaces of the composite discs. The test load of the machine was set at approximately 1 N with a holding time of 15 s. The measurements were taken from 3 random regions with a distance of 150 µm between edges. The area of the resulting rhombus on the disc surface was calculated using the machine. The arithmetic average of three measurements was recorded as the Vickers hardness number (VHN) for both upper and lower surfaces. The surface microhardness was assessed using the equation: H = 1854.4 (Pd−2), where H denotes the Vickers hardness (kg/mm2), P represents the applied force (g), and d signifies the average length of the diagonals (µm).
2.4. Surface Roughness
The samples were prepared using Teflon molds (2 mm width × 6 mm diameter) (n = 7). The prepared samples were incubated at 37 °C for 24 h before experimental process. To ensure standardization in measurements, no finishing or polishing system was employed, and surface roughness was measured using a contact profilometer (Surtronic S128, Taylor Hobson, Leicester, UK). To guarantee uniformity, a sole researcher (M.K.U.) conducted both the preparation and measurement of all samples. Prior to assessing each composite group, the apparatus underwent calibration through its calibration mode. The measurements were taken from three distinct regions for every sample, and their averages were computed. Utilizing a portable contact profilometer, the roughness values for each sample were determined in terms of Ra. The profilometer device was operated with a stylus featuring a 0.25 mm cut-off, a 1.6 mm evaluation length, all within the range of up to 400 µm.
2.5. The Stabilization of Color
The samples, prepared using a Teflon mold with a thickness of 2 mm and a diameter of 6 mm, were incubated in the incubator at 37 °C for 24 h prior to the experimental stage (n = 7). Initially, the baseline CIE L*a*b* values were measured three times for discs obtained from the control group composite material without incorporated boron, and their arithmetic averages were calculated. Subsequently, the CIE L*a*b* values of discs obtained from experimental composites were measured in a similar manner and compared with the control group (VITA Easyshade V (VITA Zahnfabrik, Bad Säckingen, Germany)). The total color change (∆E*) was then calculated using the following formula:
To assess color stabilization, the L1* a1* b1* values obtained from the control group were noted. Subsequently, the values obtained in all experimental groups were recorded as L2* a2* b2*. Then, using the formulation, the ∆E values were calculated.
2.6. Statistical Analysis
The normality of continuous measurements in the study was assessed using the Shapiro–Wilk (n < 50) and Skewness-Kurtosis tests. As the measurements were normally distributed, parametric tests were applied. Descriptive statistics for continuous variables in the study, including mean, standard deviation, sample size (n), and percentage (%), were expressed. “One-Way Analysis of Variance (ANOVA)” was utilized for comparing continuous measurements among groups. Following variance analysis, the “Duncan test” was employed for post hoc analysis to determine differences between groups. Pearson correlation coefficients were calculated to assess relationships between measurements. A significance level of p < 0.05 was adopted for calculations, and the statistical package program SPSS (IBM SPSS for Windows, ver. 26) was used for analyses.
3. Results
Parametric tests were deemed appropriate for comparisons, as the Shapiro–Wilk values of measurements in each subgroup for every methodology indicated a normal distribution within the range of (±1.5) when n < 50.
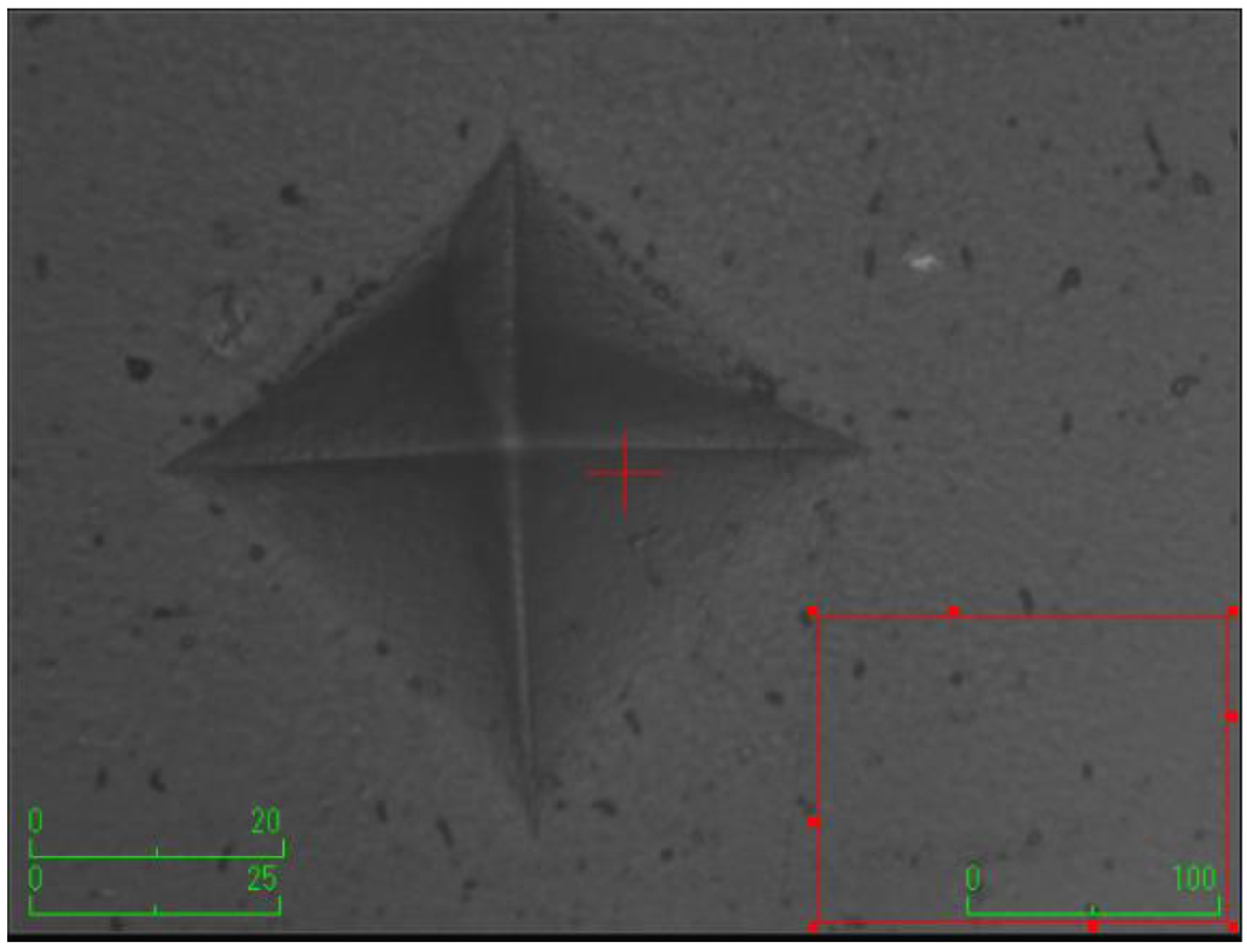
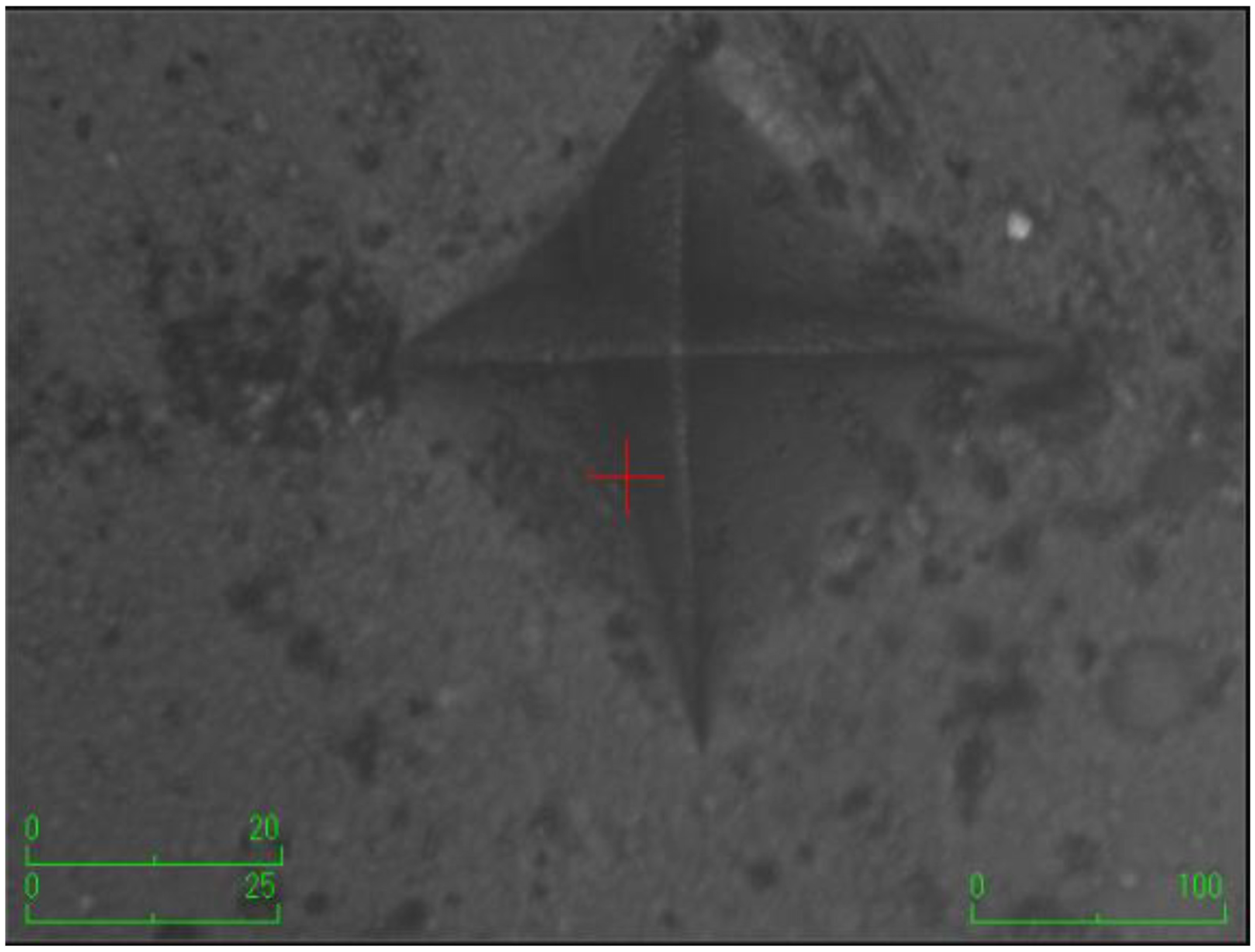
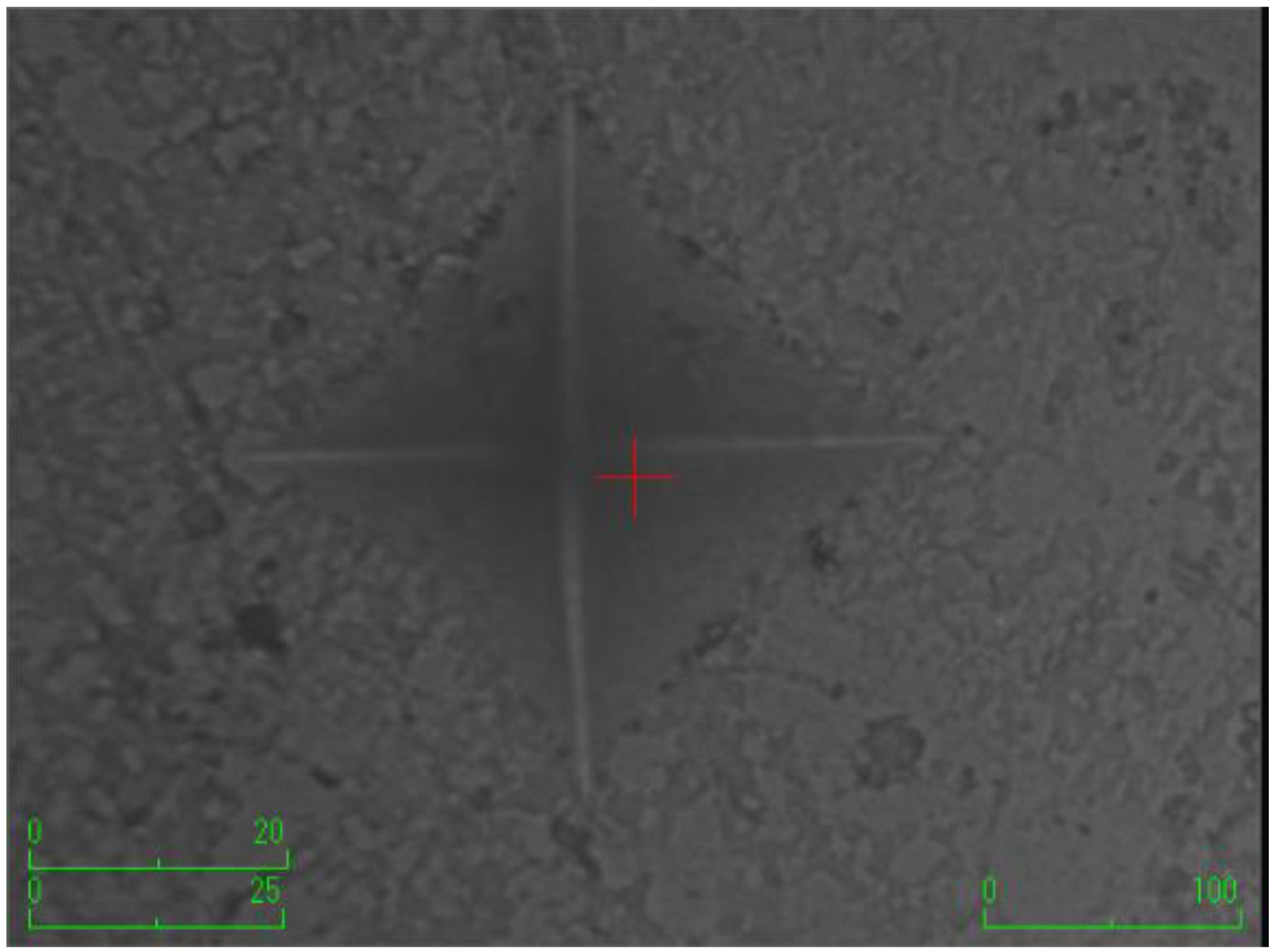
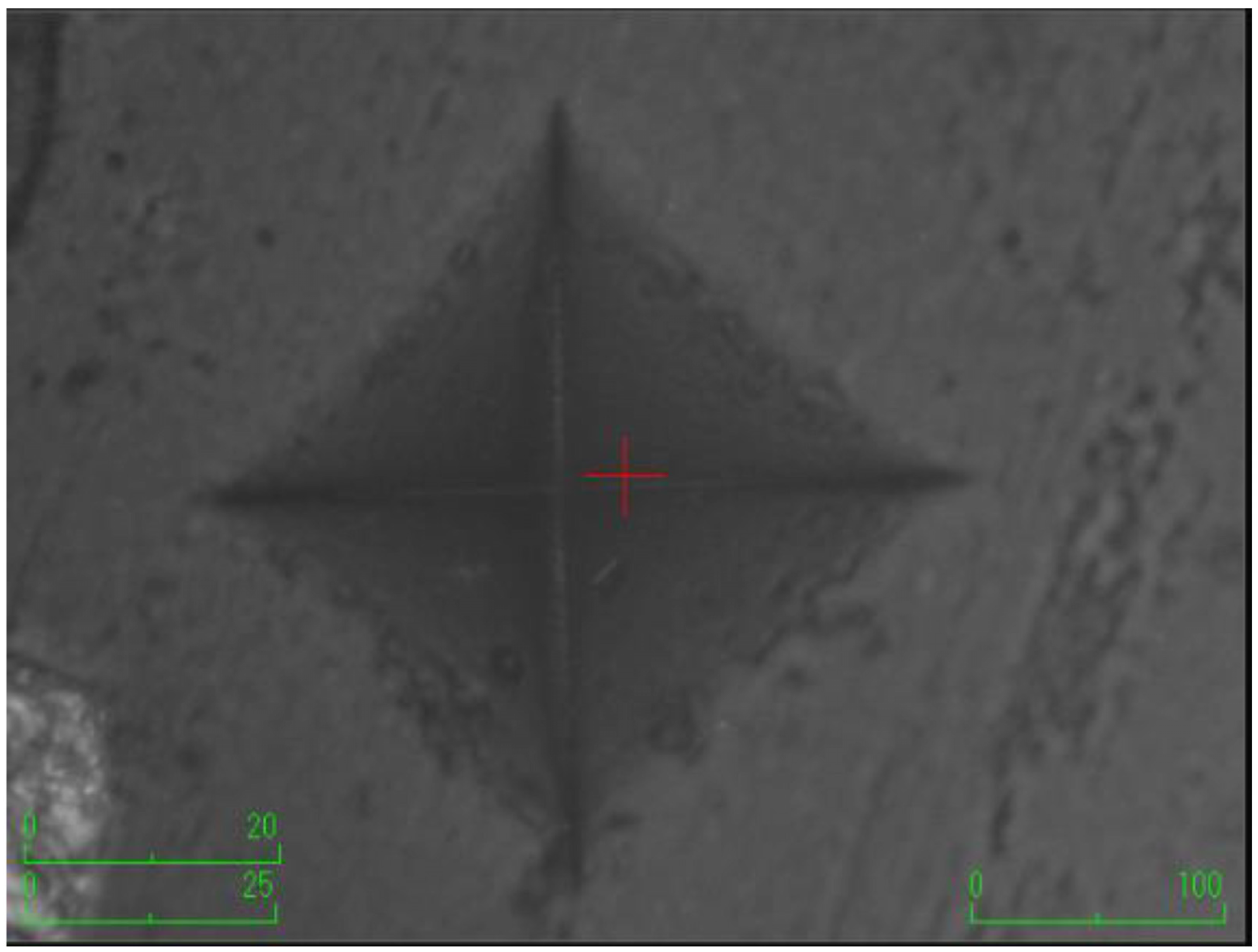
According to the surface microhardness values, there is a statistically significant difference among groups in terms of the upper and lower surfaces, and differences in upper–lower variations (p < 0.001). Compared to the control group, all experimental groups, excluding the etifert group (37.12 ± 4.20), demonstrated an increase in surface microhardness values. This increase was statistically significant only in the group with added zinc borate (p < 0.001). Looking at the lower surfaces, the values in the experimental groups were found to be lower than those in the control group, and this difference was statistically significant (p < 0.001). The lowest microhardness value on the lower surface was obtained in the eticol-ceramic added group (17.01 ± 5.75), while the highest value was observed in the group with added boric acid (36.95 ± 6.85). When considering the difference between the upper and lower surfaces, the smallest difference was observed in the control group. The highest difference was identified in the experimental group with eticol-ceramic (28.81 ± 6.96) (p < 0.001). Except for boric acid, all other groups showed a statistically significant difference compared to the control group (p < 0.001). All surface microhardness values are presented in Table 2. Furthermore, the figures of surface microhardness for all groups are presented in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6.

Table 2.
Comparison of the surface microhardness values among all groups.
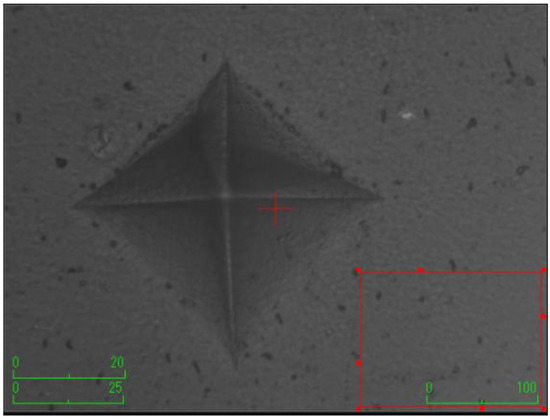
Figure 1.
Control (without the addition of any boron material).
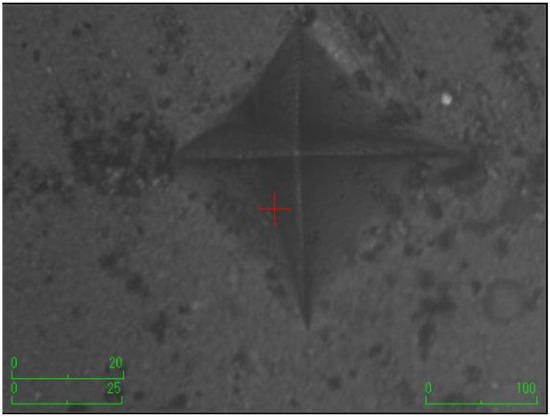
Figure 2.
The group with the addition of zinc borate.
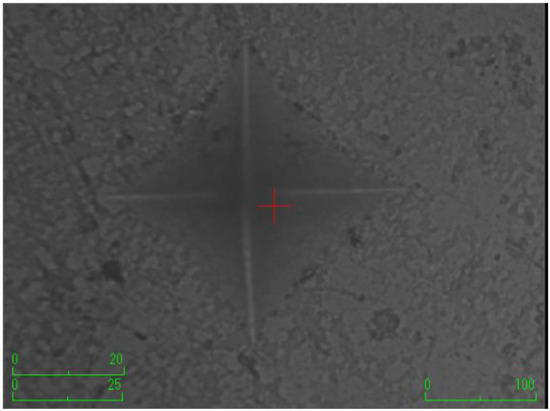
Figure 3.
The group with the addition of borax pentahydrate.
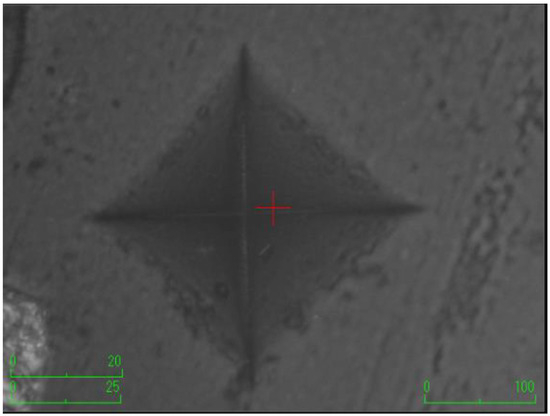
Figure 4.
The group with the addition of etifert.
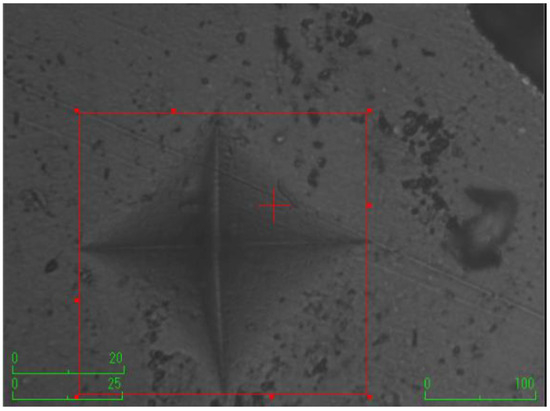
Figure 5.
The group with the addition of eticol-ceramic.
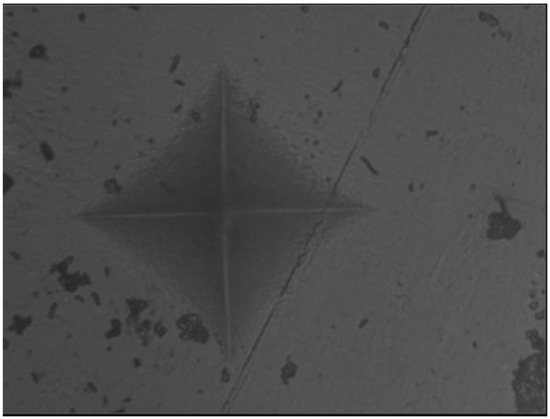
Figure 6.
The group with the addition of boric acid.
Statistically significant differences were observed among all groups based on roughness values (p < 0.001) (Table 3). Except for the eticol-ceramic group (0.53 ± 0.35), Ra values were found to be higher in the experimental groups with the added boron compared to the control group. Although the Ra value in the eticol-ceramic group was lower than that of the control group, no significant difference was observed between them (p > 0.05). The highest roughness value was encountered in the boric acid group (1.40 ± 1.25). All roughness values are presented in Table 3.

Table 3.
Comparison of the surface roughness values among all groups.
According to the ΔE values in the experimental groups, the most significant color change occurred in the experimental group with eticol-ceramic, and this difference was statistically significant compared to the other groups (18.58 ± 2.02). The ΔE values in the other four experimental groups did not show statistically significant differences (p > 0.05) (Table 4).

Table 4.
Comparison of the color stabilization values among all groups.
The results of the inter-measurement correlation analysis across all groups are presented in Table 5. Correlation coefficients indicating a significant relationship between measurements were identified. According to the obtained results, surface roughness values exhibited negative correlations with color stability and surface microhardness values for all groups, respectively (p = 0.038; r = −0.185/p = 0.006; r = −0.245). However, it was also observed that color stability showed a negative correlation with lower microhardness values (p < 0.001; r = −0.557).

Table 5.
Inter-measurement correlation (relationship) analysis results across all groups.
4. Discussion
The hypothesis of the study is partially rejected as statistically significant value losses or unfavorable results were obtained in some groups of boron derivatives for each method compared to the control group. Contemporary composites are comprised of an organic polymer matrix phase, an inorganic phase containing filler particles, and an intermediate phase [18]. The structure of the organic polymer matrix phase in resin composites includes dimethacrylate oligomers such as Bis-GMA or UDMA, along with initiator and accelerator systems for free radical polymerization, and stabilizer systems to enhance chemical and storage stability. Due to the high viscosity of UDMA and Bis-GMA, low molecular weight diacrylates such as TEGDMA are added to reduce viscosity [19]. Free radical polymerization is a key synthesis route for obtaining a wide variety of different polymers and material composites, and it involves the successive addition of free radicals to form a polymer chain. Additionally, living free radical polymerization and controlled free radical polymerization are techniques used to produce organic/inorganic hybrid composites with well-dispersed polymer solid composites [20]. In recent years, various therapeutic agents, strength-enhancing additives, antibacterial, and similar substances can be incorporated into the structure of dental composites [12,21,22]. Boron is an element of significant importance that can be examined as a material used in the dental field. Some studies suggest that boron may impart positive effects on structural strength, antibacterial activity, vital situation and progression. However, there is a limited number of studies on this subject, especially regarding biocompatibility [5]. Therefore, further research is needed to explore the potential use of boron in dental materials. In this study, five different boron materials, namely zinc borate, boric acid, borax pentahydrate, etifert, and eticol-ceramic, were incorporated into a microhybrid-based dental composite structure at 5% (w/w) using a precision balance. The experimental composites were produced by adding these materials to achieve the desired composition.
Boron elements, which play an active role in various systems such as neurological development, the endocrine system, the functioning of the body’s defense mechanisms, and bone structure, can induce osteogenic differentiation even at very low levels. Boron derivatives such as zinc borate and boric acid activate genes related to bone formation by significantly stimulating alkaline phosphatase activity and also contribute to calcium storage. Particularly, the significance of zinc borate as a crucial osteoinductive agent for osteoblastic differentiation has been substantiated through the utilization of human exfoliated deciduous teeth [23]. The incorporation of zinc borate into the structure of non-dental composites based on polycarbonate or polypropylene has been identified as a means to enhance the mechanical properties of the structure. It has been observed that the tribological and hardness performance also improves [24,25]. The impact of adding boric acid, colemanite, and borax to polymethyl methacrylate (PMMA)-based denture base material on flexural strength and surface hardness values was examined. As a result of this study, it was determined that 1% colemanite improves the mechanical properties of PMMA, and at the same time, boric acid was observed to enhance surface microhardness [26]. Additionally, various boron derivatives were utilized in various studies to investigate their cytotoxicity, anticancer, and antibacterial properties. It has been proven that borax pentahydrate disrupts the metabolism of cancer cells, reducing their proliferation and suggesting its potential as a novel antiangiogenic agent [27]. The addition of boron nitride nanosheets (BNNSs) to the structure of dental composites has been proposed to exhibit improved mechanical and antibacterial efficacy [12]. It has been demonstrated that hexagonal boron nitrate (h-BN) can inhibit bacterial growth in a cell culture medium [28], and the incorporation of sodium pentaborate pentahydrate into the structure of dental composites at specific ratios has been proven to significantly suppress bacterial growth according to direct contact test results [3]. Additionally, the addition of boron nitride nanotubes with fissure sealant has been found to reduce the surface energy of the composite structure and enhance the bioactivity of the fissure sealant [16]. When the literature is thoroughly examined, the presence of a limited number of studies is evident, and in existing studies, it is often observed that one or two types of boron materials are used and added to the structure in different percentages. The majority of these studies focus on antibacterial activity, whether or not there is therapeutic efficacy. At this crucial point, what becomes important is the size of the impact of the added boron material on the mechanical properties of the composite structure. One of the most crucial factors for a restoration to endure within the oral cavity for an extended period of time is its ability to withstand chewing forces. It is an undeniable fact that there are already numerous therapeutic agents available for use when performing a restoration in the oral cavity. In studies related to boron, it is essential to first examine to what extent the mechanical properties are affected if added to the composite structure. Then, work with numerous boron materials, and organize subsequent studies by selecting the most suitable boron materials. After obtaining the data, the antibacterial efficacy or cytotoxic effect of the relevant boron material should be evaluated. Based on this, this study was designed to work with numerous boron materials, with all materials added to the structure in equal proportions.
In this study, a microhybrid-type composite material was used as the base, and the same composite was used in all experimental groups. Currently, there is no in vitro evidence in the literature suggesting that nanofill or submicron resin composites exhibit better smoothness or gloss compared to traditional microhybrids [29]. Of course, it is an undeniable fact that nanofil or nanohybrid-based dental composites can demonstrate clinical performance, marginal adaptation, and mechanical properties similar to traditional composites. However, according to the conducted study, it has been revealed that nearly three-quarters of dentists preferentially choose a microhybrid composite material for direct restorations [30]. In this context, it was decided to incorporate a microhybrid-based composite in our study. Additionally, in such studies, boron derivatives can be introduced using different methods [3,9,17]. The sol-gel method facilitates the formation of nano-sized filler particles and provides a higher content of nanofillers compared to traditional microfilled composites [31]. At the same time, boron materials can be incorporated into the polymer chain by hydroborating organic double bonds through the hydroboration method. This method can reveal polymerization efficiency and yield controllable polymer structures [32]. Instead of these methods, the technique employed in the study by Demirci et al. [3] was applied due to financial reasons and laboratory facilities. This situation constitutes one of the limitations of the study.
Surface microhardness tests are a testing methodology that is widely utilized not only in industrial settings but also in the dental field, offering crucial insights into the durability, performance, and quality of materials. These tests ensure control over materials and manufactured components by measuring a material’s resistance to scratching. According to conducted research, various tests such as Brinell, Rockwell, Vickers, Knoop, Barcol, and Shore are commonly employed to determine the hardness of dental materials [33,34]. In the realm of dental science, the Knopp and Vickers examinations stand out prominently in the academic literature. The Vickers test, being a non-destructive methodology, finds widespread application across diverse materials. Furthermore, automated devices facilitate the precise measurement of the diagonal length of the square formed by the diamond indenter, allowing for the automatic computation of the parameters within a computerized environment [35]. Furthermore, the magnitude of the applied force and the duration of its application have been determined within the framework of ISO standards [36]. In line with the literature, surface hardness values were determined in this study by applying a force of 100 g for a holding time of 15 s. According to the obtained results, the groups containing etifert and boric acid exhibited inferior values compared to the control group. The decrease in etifert was statistically significant, but that of boric acid was not found to be significant. The addition of boron derivatives to the structure can be evaluated in terms of inorganic material incorporation, and in such a scenario, an increase in microhardness values compared to the control group can be anticipated. The observed decrease, especially due to the addition of boric acid, is a result contrary to the literature [26]. When evaluated from the perspective of the subsurface, the presence of moisture in the structure of eticol-ceramic may be a factor inhibiting polymerization conversion. It has been proven that dental polymer networks are sensitive to varying degrees of moisture and hydrolytic effects depending on their chemistry and structure [37]. The nature of the material to which the boron derivative is added (acrylic resin, composite, etc.) is a factor that will positively or negatively influence the emerging results. However, considering the composite’s inclusion of numerous materials, the presence of an organic matrix phase, and the conversion from monomer to polymer, boric acid and etifert may have hindered the polymerization mechanism, affecting the conversion values. It should not be overlooked that the physical properties of composite resins can be influenced by inter-particle voids [38]. Additionally, since the hardness of inorganic fillers directly affects the material’s hardness, obtaining different hardness values due to the different geometries and sizes of all boron derivatives is natural [39,40]. One of the limitations considered in this study was the absence of an examination of the FTIR conversion value, and to address this, the upper and lower microhardness values were separately analyzed, aiming to indirectly draw conclusions about the conversion. The subsurface microhardness values of all materials comprising the experimental group being lower than those of the control group support the notion of hindrance to the polymerization process. All microhardness values on the lower surface were found to be statistically significantly lower than the control group, raising questions that need to be addressed. The ratio of the composite resin’s lower surface values to upper surface values can be used as a boundary value for determining the degree of polymerization in surface hardness measurement, with a generally accepted threshold of 80% or higher [41]. However, it has been noted that this method can sometimes provide misleading results, suggesting an insufficient polymerization process based on the lower/upper surface ratio [42]. Therefore, in our study, statistical significance was determined based on mean differences rather than ratios.
The surface roughness of a restoration can lead to drawbacks in its aesthetic appearance, such as discoloration and microbial adhesion [40]. Factors influencing the surface roughness of restorative materials include the size, quantity, and hardness of the fillers, as well as the resin matrix structure. In this study, surface roughness was measured in two dimensions using a tool known as a contact profilometer, and the measurements were expressed in terms of Ra. While this method may yield lower values compared to three-dimensional and non-contact methods, it is often preferred due to its cost-effectiveness and accessibility [40,43]. While there is no universally accepted threshold value for the roughness of an ideal composite material, a limit of 0.2 Ra has been suggested to prevent microbial colonization on the dental composite surface [44]. In our study, it was observed that the group with the addition of eticol-ceramic exhibited surface roughness values lower than the control group, although not statistically significant. We attribute this result to the smaller particle size of eticol-ceramic compared to other types. In the group with the addition of etifert, which has larger particle size, higher values might be expected. However, in the etifert group, the upper surface hardness ratios were found to be at the lowest level. The addition of filler material with smaller size and rounded geometry allows for an increase in the degree of polymerization and improves polishability, resulting in smoother surfaces [45].
Color stability is a significant criterion in evaluating the clinical performance of restorative materials and can be attributed to both internal and external factors. Factors such as incomplete polymerization, the presence of surface roughness, water absorption, and the type and amount of filler can influence color stability. Spectrophotometers are the most commonly used devices for measuring color stability [46]. Such devices prevent the influence of external light conditions on measurements and allow photo-optical measurements to be converted into a numerical value in the CIE L*a*b* color space. As a consequence, it has been proven that, compared to traditional and subjective interpretations, objective and high-precision matching can be achieved in the majority of cases. For this purpose, we used an intraoral spectrophotometer device, VITA Easyshade V (VITA Zahnfabrik, Germany), in the present study [47]. According to the literature, color change values in the L*, a*, b* axes can be interpreted separately to assess color stability. However, in this study, the focus was solely on ΔE, and this aspect can be expressed as one of the limitations of the study. Although various threshold values have been reported in the literature, it has been suggested that the ΔE value should be between 1 and 3.3. Additionally, it has been reported that color changes can be easily distinguishable clinically when values exceed 3.3 [48,49]. According to the data obtained in our study, ΔE values for all groups except the eticol-ceramic group were found to be below the critical threshold of 3.3, indicating preserved color stability (visually observable). In the eticol-ceramic group, a dramatic color change was observed, easily noticeable to the naked eye (the discs were observed to darken towards black). Despite successful results in terms of smoothness and upper surface hardness values, the addition of eticol-ceramic significantly compromised the aesthetic properties. Despite being in a powder form and appearing white, we believe that the difference arises from factors in the structure of eticol-ceramic powder, such as a small amount of Fe2O3, Al2O3, SO4, and moisture. While the exact chemistry causing instability is not fully known, we assume that Fe2O3 can be tainted with Al2O3 and traces of other elements. Under normal conditions, Fe2O3 has a reddish-brown color, and sulfates can interact with metal ions, leading to color changes. Additionally, we posit that the moisture in the powder may have influenced polymerization transformation, potentially compromising color stability.
By incorporating the most suitable boron derivative into the dental composite structure in the most accurate proportions, superior mechanical properties can be achieved for restorative materials. This situation can be viewed positively from an industrial and commercial perspective. At the same time, it may pave the way for new projects and study designs. In this study, materials homogeneously incorporated into the structure by manual methods are planned to be introduced into the structure in a factory environment in collaboration with a local manufacturer, utilizing various methods. It might be appropriate to subject the dental composite materials obtained in this way to different mechanical tests. One significant limitation of this study is that all boron derivatives were added to the dental composite structure in similar proportions. Various negative results emerging in this study could be mitigated by adding the relevant boron derivatives in lower or higher proportions. Aging was not performed in this study. The long-term performance of experimental composites (solution and thermal aging) is a subject of interest. An indirect inference was made for the polymerization conversion value. To address this limitation, it is recommended to perform calculations using FT-IR or thermal differential scanning calorimetry. The structure of the dental composite with added boron, as well as its physical qualities such as internal atomic arrangement, particle sizes, and material characteristics, can be controlled through XRD or SEM-EDX analyses. Certainly, the boron derivatives that demonstrate successful results in these physical and mechanical tests should be investigated for their potential antibacterial effects or toxicity to surrounding cells.
5. Conclusions
Dental composites incorporated with zinc borate demonstrate superior upper microhardness values in comparison to other boron derivatives. Conversely, the introduction of etifert results in a substantial reduction in upper microhardness values. Among the experimental groups, boric acid exhibits the least decline in subsurface microhardness, implying that the addition of boric acid has the least pronounced impact on the degree of polymerization in dental composites. Despite attaining the most refined surfaces through the inclusion of eticol-ceramic, it significantly compromises the color stabilization of the dental composite. Successful color stabilization is achieved with the remaining boron derivatives, and their values fall within a clinically safe range. In summary, while the incorporation of boron derivatives into the composite structure positively affects certain mechanical properties, it may lead to the deterioration of some mechanical features.
Author Contributions
M.K.U. (Mehmet Kutluay Uçuk): Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing—Original Draft, Writing—Review and Editing, Supervision, Project administration, Funding acquisition; M.K.U. (Musa Kazim Ucuncu): Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing—Original Draft, Reviewing & Editing, Visualization, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- DeFrancesco, H.; Dudley, J.; Coca, A. Boron Chemistry: An Overview. In Boron Reagents in Synthesis; American Chemical Society: Washington, DC, USA, 2016; pp. 1–25. [Google Scholar]
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Ciocîlteu, M.V.; Bejenaru, C.; Radu, A.; Bejenaru, L.E.; Rău, G.; Mogoşanu, G.D.; Neamţu, J.; et al. New insights into boron essentiality in humans and animals. Int. J. Mol. Sci. 2022, 23, 9147. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Kaya, M.S.; Doğan, A.; Kalay, Ş.; Altın, N.Ö.; Yarat, A.; Akyüz, S.H.; Şahin, F. Antibacterial and cytotoxic properties of boron-containing dental composite. Turk. J. Bıol. 2015, 39, 417–426. [Google Scholar] [CrossRef]
- Scorei, R. Is Boron a Prebiotic Element? A Mini-Review of the Essentiality of Boron for the Appearance of Life on Earth. Orig. Life Evol. Biosph. 2012, 42, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Mitruţ, I.; Scorei, I.R.; Manolea, H.O.; Biţă, A.; Mogoantă, L.; Neamţu, J.; Bejenaru, L.E.; Ciocîlteu, M.V.; Bejenaru, C.; Rău, G.; et al. Boron-containing compounds in Dentistry: A narrative review. Rom. J. Morphol. Embryol. 2022, 63, 477. [Google Scholar] [CrossRef] [PubMed]
- Abdulai, D.; Cengiz, S. The effect of boron addition on the prosthetic dental materials: A review. Int. J. Appl. Dent. Sci. 2023, 9, 31–37. [Google Scholar] [CrossRef]
- Hakki, S.S.; Götz, W.; Dundar, N.; Kayis, S.A.; Malkoc, S.; Hamurcu, M.; Basoglu, A.; Nielsen, F.H. Borate and boric acid supplementation of drinking water alters teeth and bone mineral density and composition differently in rabbits fed a high protein and energy diet. J. Trace Elem. Med. Biol. 2021, 67, 126799. [Google Scholar] [CrossRef]
- Toker, H.; Ozdemir, H.; Yuce, H.B.; Goze, F. The effect of boron on alveolar bone loss in osteoporotic rats. J. Dent. Sci. 2016, 11, 331–337. [Google Scholar] [CrossRef]
- Sopcak, T.; Medvecky, L.; Jevinova, P.; Giretova, M.; Mahun, A.; Kobera, L.; Stulajterova, R.; Kromka, F.; Girman, V.; Balaz, M. Physico-chemical, mechanical and antibacterial properties of the boron modified biphasic larnite/bredigite cements for potential use in dentistry. Ceram. Int. 2023, 49, 6531–6544. [Google Scholar] [CrossRef]
- Luan, Q.; Desta, T.; Chehab, L.; Sanders, V.J.; Plattner, J.; Graves, D.T. Inhibition of Experimental Periodontitis by a Topical Boron-Based Antimicrobial. J. Dent. Res. 2008, 87, 148–152. [Google Scholar] [CrossRef]
- Zhu, J.; Kamiya, A.; Yamada, T.; Shi, W.; Naganuma, K. Influence of boron addition on microstructure and mechanical properties of dental cast titanium alloys. Mater. Sci. Eng. A 2003, 339, 53–62. [Google Scholar] [CrossRef]
- Alansy, A.S.; Saeed, T.A.; Al-Attab, R.; Guo, Y.; Yang, Y.; Liu, B.; Fan, Z. Boron nitride nanosheets modified with zinc oxide nanoparticles as novel fillers of dental resin composite. Dent. Mater. 2022, 38, e266–e274. [Google Scholar] [CrossRef] [PubMed]
- Rad, R.M.; Alshemary, A.Z.; Evis, Z.; Keskin, D.; Altunbaş, K.; Tezcaner, A. Structural and biological assessment of boron doped bioactive glass nanoparticles for dental tissue applications. Ceram. Int. 2018, 44, 9854–9864. [Google Scholar]
- Degrazia, F.W.; Leitune, V.C.B.; Samuel, S.M.W.; Collares, F.M. Boron nitride nanotubes as novel fillers for improving the properties of dental adhesives. J. Dent. 2017, 62, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Degrazia, F.W.; Leitune, V.C.B.; Visioli, F.; Samuel, S.M.W.; Collares, F.M. Long-term stability of dental adhesive incorporated by boron nitride nanotubes. Dent. Mater. 2018, 34, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Bohns, F.R.; Degrazia, F.W.; de Souza Balbinot, G.; Leitune, V.C.B.; Samuel, S.M.W.; García-Esparza, M.A.; Sauro, S.; Collares, F.M. Boron Nitride Nanotubes as Filler for Resin-Based Dental Sealants. Sci. Rep. 2019, 9, 7710. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Fidalgo-Pereira, R.; Evangelista Carpio, D.M.; Carvalho, Ó.; Catarino, S.; Torres, O.; MSouza, J.C. Relationship between the inorganic content and the polymerization of the organic matrix of resin composites for dentistry: A narrative review. Revsalus Rev. Científica Rede Académica Ciências Saúde Lusofonia 2022, 4, 136. [Google Scholar] [CrossRef]
- Kasseh, A.; Ait-Kadi, A.; Riedl, B.; Pierson, J.F. Organic/inorganic hybrid composites prepared by polymerization compounding and controlled free radical polymerization. Polymer 2003, 44, 1367–1375. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.T.; Xu, X. Antibacterial Dental Composites with Chlorhexidine and Mesoporous Silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Su, W.-T.; Chen, P.-H. Magnesium and zinc borate enhance osteoblastic differentiation of stem cells from human exfoliated deciduous teeth in vitro. J. Biomater. Appl. 2018, 32, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, S.A.A.; Rahimi, A.; Frounchi, M.; Radman, S. Investigation of flame retardancy and physical–mechanical properties of zinc borate and aluminum hydroxide propylene composites. Mater. Des. 2008, 29, 1051–1056. [Google Scholar] [CrossRef]
- Akinci, A. Study on the wear properties of zinc borate filled polycarbonate composites against 4140 steel under dry friction. Int. J. Polym. Anal. Charact. 2017, 22, 310–317. [Google Scholar] [CrossRef]
- Özdemir, A.K.; Özdemir Doğan, D.; Tuğut, F.; Demir, H.; Akın, H. Effects of boron on the mechanical properties of polymethylmethacrylate denture base material. Eur. Oral Res. 2021, 55, 45–53. [Google Scholar] [CrossRef]
- Yılmaz, H.; Ozbek, E.N.; Işel, E.; Butuner, B.D.; Anacak, G.Y.; Koparal, A.T.; Ulus, G. Evaluation of Borax Pentahydrate as a Novel Antiangiogenic Agent. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Kıvanç, M.; Barutca, B.; Koparal, A.T.; Göncü, Y.; Bostancı, S.H.; Ay, N. Effects of hexagonal boron nitride nanoparticles on antimicrobial and antibiofilm activities, cell viability. Mater. Sci. Eng. C 2018, 91, 115–124. [Google Scholar] [CrossRef]
- Kaizer, M.R.; de Oliveira-Ogliari, A.; Cenci, M.S.; Opdam, N.J.M.; Moraes, R.R. Do nanofill or submicron composites show improved smoothness and gloss? A systematic review of in vitro studies. Dent. Mater. 2014, 30, e41–e78. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Correa, M.B.; Opdam, N.; Demarco, F.F. Do Clinical Experience Time and Postgraduate Training Influence the Choice of Materials for Posterior Restorations? Results of a Survey with Brazilian General Dentists. Braz. Dent. J. 2013, 24, 642–646. [Google Scholar] [CrossRef]
- Miletic, V. Dental Composite Materials for Direct Restorations; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Oksal, B.S.; Gencer, A. Hydroboratıon Synthesıs, Characterızatıon and Bıologıcal Actıvıty of Novel Cyclıc Preceramıc Polymers: Transıtıon to SiBOC Ceramıcs. Ceram. Silikáty 2015, 59, 341–347. [Google Scholar]
- Dinçkal Yanıkoğlu, N.; Sakarya, R.E. Test methods used in the evaluation of the structure features of the restorative materials: A literature review. J. Mater. Res. Technol. 2020, 9, 9720–9734. [Google Scholar] [CrossRef]
- Nair, K.C.; Dathan, P.C.; Sreeba, S.B.; Soman, A.K. Hardness of Dental Materials Is an Essential Property That Determines the Life of Restorations—An Overview. ACTA Sci. Dent. Sci. 2022, 6, 129–134. [Google Scholar] [CrossRef]
- Filija, E.; Šalinović, I. Microhardness testing: Can we measure the repair of demineralized lesion? Sonda List Studenata Stomatol. Fak. Sveučilišta Zagreb. 2022, 21, 44–47. [Google Scholar]
- ISO 4049:2019; Dentistry—Polymer-Based Restorative Materials. ISO: Geneva, Switzerland, 2019.
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Manhart, J.; Kunzelmann, K.-H.; Chen, H.Y.; Hickel, R. Mechanical properties of new composite restorative materials. J. Biomed. Mater. Res. 2000, 53, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Topcu, F.T.; Erdemir, U.; Sahinkesen, G.; Yildiz, E.; Uslan, I.; Acikel, C. Evaluation of microhardness, surface roughness, and wear behavior of different types of resin composites polymerized with two different light sources. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Yildirim Ucuncu, M.; Ucuncu, M.K. Comparison of the Mechanical Properties of Various Microhybrid Dental Composites. Eurasian Dent. Res. 2023, 1, 58–64. [Google Scholar]
- Zhu, S.; Platt, J. Curing Efficiency of Three Different Curing Modes at Different Distances for Four Composites. Oper. Dent. 2011, 36, 362–371. [Google Scholar] [CrossRef]
- Leprince, J.; Devaux, J.; Mullier, T.; Vreven, J.; Leloup, G. Pulpal-Temperature Rise and Polymerization Efficiency of LED Curing Lights. Oper. Dent. 2010, 35, 220–230. [Google Scholar] [CrossRef]
- Ozan, G.; Sar Sancakli, H.; Yucel, T. Effect of black tea and matrix metalloproteinase inhibitors on eroded dentin in situ. Microsc. Res. Tech. 2020, 83, 834–842. [Google Scholar] [CrossRef]
- Bollen, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Jang, J.-H.; Park, S.-H.; Hwang, I.-N. Polymerization Shrinkage and Depth of Cure of Bulk-Fill Resin Composites and Highly Filled Flowable Resin. Oper. Dent. 2015, 40, 172–180. [Google Scholar] [CrossRef]
- Poggio, C.; Ceci, M.; Beltrami, R.; Mirando, M.; Wassim, J.; Colombo, M. Color stability of esthetic restorative materials: A spectrophotometric analysis. Acta Biomater. Odontol. Scand. 2016, 2, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, V.; Anilkumar, S.; Lylajam, S.; Rajesh, C.; Narayan, V. Comparison of accuracies of an intraoral spectrophotometer and conventional visual method for shade matching using two shade guide systems. J. Indian Prosthodont. Soc. 2016, 16, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Ceci, M.; Viola, M.; Rattalino, D.; Beltrami, R.; Colombo, M.; Poggio, C. Discoloration of different esthetic restorative materials: A spectrophotometric evaluation. Eur. J. Dent. 2017, 11, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.U.J.; Sim, C.P.C.; Loh, W.L.; Teo, J.H. Human-eye versus computerized color matching. Oper. Dent. 1999, 24, 358–363. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).