Do Vibrational Forces Induce an Anabolic Effect in the Alveolar Bone of Animal Models? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Methods for the Identification of Studies
2.2. Sources of Information
2.3. Eligibility Criteria
Inclusion Criteria
- -
- All published studies with animal models;
- -
- animal studies in which vibrations were applied;
- -
- articles that evaluated density or osteogenesis and their respective control group;
- -
- only articles published in English.
2.4. Exclusion Criteria
Inclusion Criteria
- -
- studies in which the frequency of vibrations is not mentioned;
- -
- models in which drugs or other treatments have been applied to the control group;
- -
- studies in which the results are unclear or incomplete.
2.5. Data Selection and Extraction Methods
2.5.1. Data Collection Process
2.5.2. Evaluation of Risk of Bias and Quality
2.6. Synthesis of Results
3. Results
3.1. Selection of Studies
3.2. General Characteristics of the Studies
3.3. Main Outcome Variables of the Study
3.3.1. Anabolic Effect Associated with Low Frequency Vibrations
3.3.2. Anabolic Effect Associated with High Frequency Vibrations
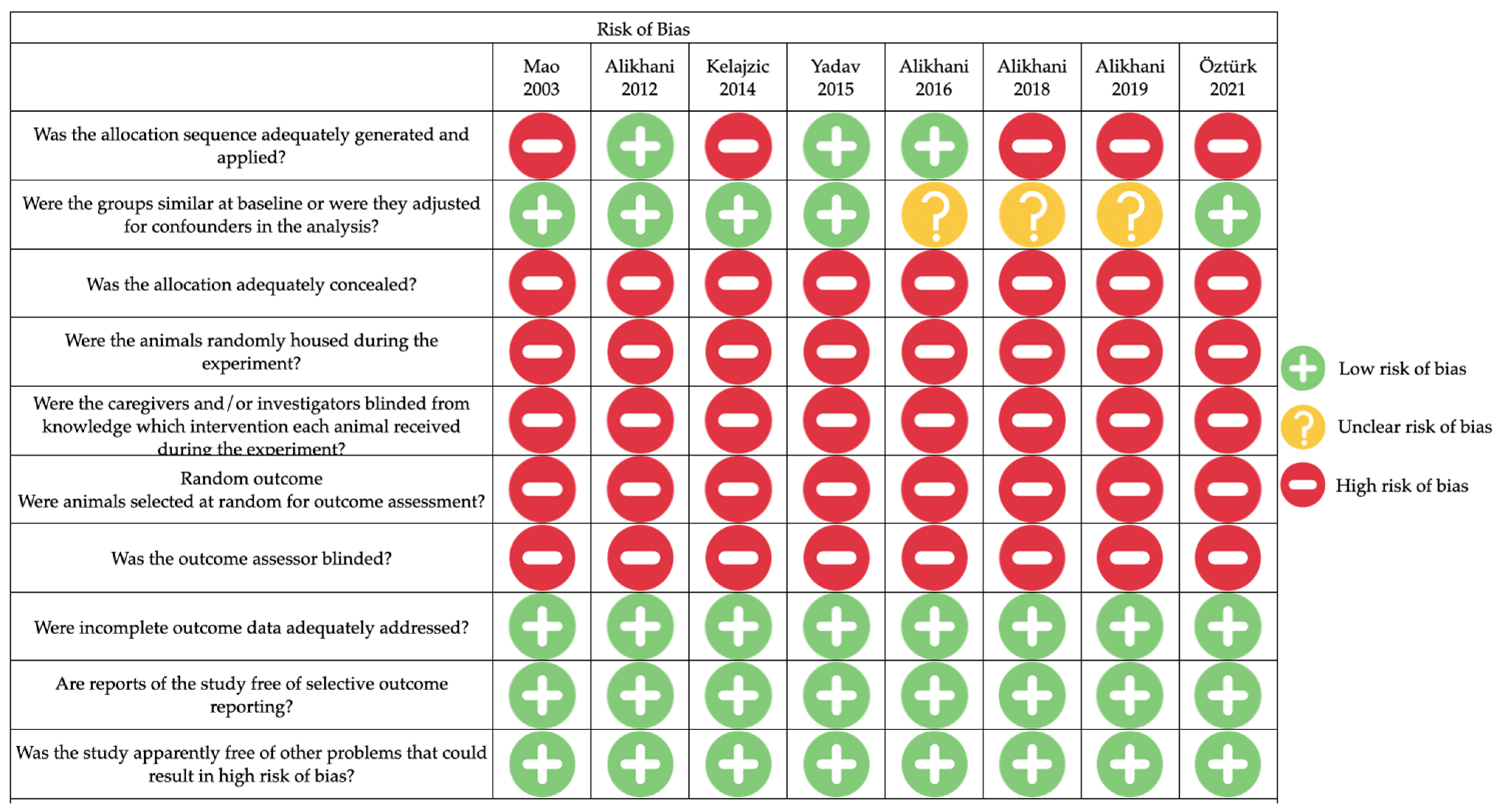
3.4. Risk of Bias Assessment
3.5. Evaluation of the Quality of the Included Studies
4. Discussion
Strengths and Limitations of This Systematic Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van der Zee, E.A.; Oroszi, T.; van Heuvelen, M.J.G.; Nyakas, C. Vibration detection: Its function and recent advances in medical applications. F1000Research 2020, 9. [Google Scholar] [CrossRef]
- Rubin, C.; Turner, A.S.; Bain, S.; Mallinckrodt, C.; McLeod, K. Low mechanical signals strengthen long bones. Nature 2001, 412, 603–604. [Google Scholar] [CrossRef]
- Alikhani, M.; Alansari, S.; Hamidaddin, M.A.; Sangsuwon, C.; Alyami, B.; Thirumoorthy, S.N.; Oliveira, S.M.; Nervina, J.M.; Teixeira, C.C. Vibration paradox in orthodontics: Anabolic and catabolic effects. PLoS ONE 2018, 13, e0196540. [Google Scholar] [CrossRef]
- ElDeeb, A.M.; Abdel-Aziem, A.A. Effect of Whole-Body Vibration Exercise on Power Profile and Bone Mineral Density in Postmenopausal Women with Osteoporosis: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2020, 43, 384–393. [Google Scholar] [CrossRef]
- DadeMatthews, O.O.; Agostinelli, P.J.; Neal, F.K.; Oladipupo, S.O.; Hirschhorn, R.M.; Wilson, A.E.; Sefton, J.M. Systematic review and meta-analyses on the effects of whole-body vibration on bone health. Complement. Ther. Med. 2022, 65, 102811. [Google Scholar] [CrossRef]
- Kalajzic, Z.; Peluso, E.B.; Utreja, A.; Dyment, N.; Nihara, J.; Xu, M.; Chen, J.; Uribe, F.; Wadhwa, S. Effect of cyclical forces on the periodontal ligament and alveolar bone remodeling during orthodontic tooth movement. Angle Orthod. 2014, 84, 297–303. [Google Scholar] [CrossRef]
- García Vega, M.F.; López Pérez-Franco, L.M.; Dib Kanán, A.; Román Méndez, C.D.; Soto Sainz, J.E.; Reyes Cervantes, E.; Cerda-Cristerna, B.I.; Salas Orozco, M.F.; Casillas Santana, M.A. Are mechanical vibrations an effective alternative to accelerate orthodontic tooth movement in humans? A systematic review. Appl. Sci. 2021, 11, 10699. [Google Scholar] [CrossRef]
- Keerthana, P.; Diddige, R.; Chitra, P. Performance comparison of vibration devices on orthodontic tooth movement—A systematic review and meta-analysis. J. Oral. Biol. Craniofacial Res. 2020, 10, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, T.; Gül Amuk, N. Three-dimensional imaging and molecular analysis of the effects of photobiomodulation and mechanical vibration on orthodontic retention treatment in rats: Effects of photobiomodulation and mechanical vibration on orthodontic retention treatment. J. Orofac. Orthop. 2022, 83 (Suppl. 1), 24–41. [Google Scholar] [CrossRef] [PubMed]
- Thammanichanon, P.; Kaewpitak, A.; Binlateh, T.; Leethanakul, C. Interval vibration reduces orthodontic pain via a mechanism involving down-regulation of TRPV1 and CGRP. In Vivo 2020, 34, 2389–2399. [Google Scholar] [CrossRef] [PubMed]
- Shipley, T.; Farouk, K.; El-Bialy, T. Effect of high-frequency vibration on orthodontic tooth movement and bone density. J. Orthod. Sci. 2019, 8, 15. [Google Scholar]
- Piancino, M.G.; Isola, G.; Cannavale, R.; Cutroneo, G.; Vermiglio, G.; Bracco, P.; Anastasi, G.P. From periodontal mechanoreceptors to chewing motor control: A systematic review. Arch. Oral. Biol. 2017, 78, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.; Alikhani, M.; Alansari, S.; Almansour, A.; Hamidaddin, M.A.; Khoo, E.; Lopez, J.A.; Nervina, J.M.; Nho, J.Y.; Oliveira, S.M.; et al. Therapeutic effect of localized vibration on alveolar bone of osteoporotic rats. PLoS ONE. 2019, 14, e0211004. [Google Scholar] [CrossRef] [PubMed]
- Pravitharangul, A.; Suttapreyasri, S.; Leethanakul, C. Mandible and iliac osteoblasts exhibit different Wnt signaling responses to LMHF vibration. J. Oral Biol. Craniofacial Res. 2019, 9, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhai, M.; Wang, P.; Zhang, X.; Cai, J.; Chen, X.; Shen, G.; Luo, E.; Jing, D. Low-level mechanical vibration enhances osteoblastogenesis via a canonical Wnt signaling-associated mechanism. Mol. Med. Rep. 2017, 16, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Steppe, L.; Liedert, A.; Ignatius, A.; Haffner-Luntzer, M. Influence of Low-Magnitude High-Frequency Vibration on Bone Cells and Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 595139. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Cariati, I.; Romagnoli, C.; D’Arcangelo, G.; Annino, G.; Tancredi, V. Whole Body Vibration: A Valid Alternative Strategy to Exercise? J. Funct. Morphol. Kinesiol. 2022, 7, 99. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; A Akl, E.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- El-Bialy, T. The Effect of High-Frequency Vibration on Tooth Movement and Alveolar Bone in Non-Growing Skeletal Class II High Angle Orthodontic Patients: Case Series. Dent. J. 2020, 8, 110. [Google Scholar] [CrossRef]
- Takano-Yamamoto, T.; Sasaki, K.; Fatemeh, G.; Fukunaga, T.; Seiryu, M.; Daimaruya, T.; Takeshita, N.; Kamioka, H.; Adachi, T.; Ida, H.; et al. Synergistic acceleration of experimental tooth movement by supplementary high-frequency vibration applied with a static force in rats. Sci. Rep. 2017, 7, 13969. [Google Scholar] [CrossRef]
- Sandercock, P.; Roberts, I. Systematic reviews of animal experiments. Lancet 2002, 360, 586. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Mao, J.J.; Wang, X.; Mooney, M.P.; Kopher, R.A.N.J. Strain Induced Osteogenesis of the Craniofacial Suture Upon Controlled Delivery of Low-Frequency Cyclic Forces. Front. Biosci. 2003, 8, a10–a17. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.; Khoo, E.; Alyami, B.; Raptis, M.; Salgueiro, J.; Oliveira, S.; Boskey, A.; Teixeira, C. Osteogenic effect of high-frequency acceleration on alveolar bone. J. Dent. Res. 2012, 91, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.; Lopez, J.A.; Alabdullah, H.; Vongthongleur, T.; Sangsuwon, C.; Alikhani, M.; Alansari, S.; Oliveira, S.M.; Nervina, J.M.; Teixeira, C.C. High-Frequency Acceleration: Therapeutic Tool to Preserve Bone following Tooth Extractions. J. Dent. Res. 2016, 95, 311–318. [Google Scholar] [CrossRef]
- Yadav, S.; Assefnia, A.; Gupta, H.; Vishwanath, M.; Kalajzic, Z.; Allareddy, V.; Nanda, R. The effect of low-frequency mechanical vibration on retention in an orthodontic relapse model. Eur. J. Orthod. 2015, 38, 44–50. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Fu, Z.; Huang, X.; Zhou, P.; Wu, B.; Cheng, L.; Wang, X.; Zhu, D. Protective effects of low-magnitude high-frequency vibration on high glucose-induced osteoblast dysfunction and bone loss in diabetic rats. J. Orthop. Surg. Res. 2021, 16, 650. [Google Scholar] [CrossRef]
- Kanzaki, H.; Nakamura, Y. Orthodontic tooth movement and bone density. J. Oral. Biosci. 2018, 60, 49–53. [Google Scholar] [CrossRef]
- Amuk, M.; Amuk, N.G.; Hacıoğlu, Z. Effects of orofacial applications of low-magnitude, high-frequency mechanical vibration on cranial sutures and calvarial bones—A micro-computed tomography study in rats. Am. J. Orthod. Dentofac. Orthop. 2022, 162, 459–468. [Google Scholar] [CrossRef]
- García-López, S.; Villanueva, R.E.; Massó-Rojas, F.; Páez-Arenas, A.; Meikle, M.C. Micro-vibrations at 30 Hz on bone cells cultivated in vitro produce soluble factors for osteoclast inhibition and osteoblast activity. Arch. Oral. Biol. 2020, 110, 104594. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Jitpukdeebodintra, S.; Leethanakul, C. Effects of low magnitude high frequency mechanical vibration combined with compressive force on human periodontal ligament cells in vitro. Eur. J. Orthod. 2018, 40, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration—What Do We Know So Far. Front. Cell Dev. Biol. 2019, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Kuroishi, K.N.; Gunjigake, K.K.; Ikeda, E.; Kawamoto, T. Expression of SOST/sclerostin in compressed periodontal ligament cells. J. Dent. Sci. 2016, 11, 272–278. [Google Scholar] [CrossRef]
- Shu, R.; Bai, D.; Sheu, T.; He, Y.; Yang, X.; Xue, C.; He, Y.; Zhao, M.; Han, X. Sclerostin promotes bone remodeling in the process of tooth movement. PLoS ONE. 2017, 12, e0167312. [Google Scholar] [CrossRef]
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics. 2015, 24, 407–419. [Google Scholar] [CrossRef]

| Author/Year/Country | Design of Study | Sample | Groups | Vibration Protocol | Measurement | Results | Conclusion |
|---|---|---|---|---|---|---|---|
| Mao (2003) USA [23] | In vivo | n = 21 M, Age: 6-weeks-old, White rabbits | EG1: n = 4, tensile cycled forces of 2 N with 0.1 Hz; EG2: n = 5, tensile cycled forces of 2 N with 0.2 Hz; EG3: n = 2 N of static forces over premaxilla suture; CG: n = 4, Not stimulations. | 0.2 Hz for 10 min/d over 12 days. | Craniofacial length. | Craniofacial length was significantly higher when cyclic forces at 0.2 Hz were applied. | Cyclic forces involve activation of stress-sensitive genes, leading to more craniofacial growth and osteogenesis. |
| Alikhani (2012) USA [24] | In vivo | n = 85 M, Age: 120 days, Sprague Dawley rats | EG: n = 43, different HFV (30–200 Hz); SC: n = 23, 4 µε of static load over the maxilla; CG: n = 19, not stimulations. | 30, 60, 100, and 200 Hz for 5 min/d over 28 days. | BV/TV; Osteogenic effect; Trabecular thickness; Trabecular space; Alveolar bone density; Growth factors; Transcription factors; Extracellular matrix; Mineralization. | The highest BV/TV was with 60 and 100 Hz rate. 60 Hz demonstrated a higher level compared with the static group in osteogenic effect, density, growth factors, transcription factors, extracellular matrix, mineralization, trabecular thickness, and the greatest decrease in trabecular space. | HFV vibration increase osteoblast activity, plays a key role during the mineralization process The higher response to vibration was in trabecular bone. |
| Kalajzic (2014) USA [6] | In vivo | n = 26 F, Age: 7 weeks old, Sprague Dawley rats | EG1: n = 4, received occlusal vibratory stimulus twice a week without orthodontic force; EG2: n = 9, received the orthodontic force only; EG3: n = 9, received the orthodontic force and additional vibratory stimulus twice a week CG: n = 4, not stimulations. | 30 Hz for 10 min/d, Twice per week with five Applications. | BV/TV; Alveolar bone density. | No significant difference between the control group and groups using vibrations in BV/TV and alveolar bone density. | LFV may cause different effects depending on force magnitude, frequency, or point of application. |
| Yadav (2015) USA [26] | In vivo | n = 30 M, Age: 12 weeks old, CD1 mice | EG1: n = 10, applied orthodontic force for 7 days, then, the orthodontic force was removed, and molar was allowed to relapse; EG2: n = 10, applied orthodontic force for 7 days, then exposed it to vibrational stimuli without orthodontic forces; CG: n = 10, applied orthodontic force for 7 days. | 30 Hz for 15 min/d over 7 days. | Bone volume; Alveolar bone density. | No significant differences in bone volume and density when comparing the CG and vibration group. Density was significantly more in the vibration group when compared to EG1. | LFV vibrations increase bone density despite inducing a process of bone resorption with orthodontic forces. |
| Alikhani (2016) USA [25] | In vivo | n = 85 M, Age: 4 months, Sprague Dawley rats | The maxillary right third molar was extracted in all rats; EG: received 120 Hz; SC: received 4 µε of static load; CG: Not stimulations. | 120 Hz for 5 min/d over 56 days. | BV/TV; Level of bone formation; Trabecular thickness; Trabecular number; Trabecular spacing; Tissue mineral density; Height; Osteogenic markers: ALP, Runx 2, OPN, OCN. | EG had significantly greater, BV/TV, level of bone formation, trabecular thickness, trabecular number, tissue mineral density and height. EG: obtained the least trabecular spacing. EG: exhibited significantly higher ALP, Runx 2, osteopontin and osteocalcin activity. | HFV promotes alveolar bone formation and decrease RANKL expression and osteoclast activation. |
| Alikhani (2018) USA [3] | In vivo | n = 206 M, Age: 120 days, Sprague Dawley rats | EG: received orthodontic force + HFV; OTM: received orthodontic force; CG: not received orthodontic force. | 120 Hz for 5 min/d over 14 days. | Alveolar bone density; Bone volume. | Alveolar bone density decreased more during tooth movement in experimental group. | HFV treatment during orthodontic tooth movement produces a catabolic effect HFV increased the number of osteoclasts. |
| Alikhani (2019) USA [13] | In vivo | n = 14 F, Age: 16 weeks old, Spices: Sprague Dawley rats | EG1: received bilateral ovariectomy; EG2: received ovariectomy and HFV; EG3: received ovariectomy and 10 με of static load; SG: received ovariectomy surgery without having the ovaries removed; CG: Not stimulations. | 120 Hz for 5 min/d over 28 and 56 days. | Alveolar bone density; BV/TV; BMD; Osteogenic markers: OPG, Runx2, Osterix, ALP, OCN, ColIα1, BMP2, Wnt. | HFV group reestablished the alveolar bone density the levels of the Sham group. BV/TV and BMD was significantly higher with HFV, compared to ovariectomy group. HFV restored osteogenic markers to levels similar to the Sham group. | HFV-induced bone remodeling depends on the inflammatory condition of the tissue. In absence of inflammatory condition, HFV is anabolic. The highest osteogenic effect of HFV was observed closest to the HFV application site. |
| Öztürk (2021) Turkey [9] | In vivo | n = 40, Age: not specified, Rats | CG: n = 6, not stimulations; CG2: n = 6, orthodontic tooth movement (10 days) + relapse (only relapse); PC1: n = 6, orthodontic tooth movement (10 days) + long-term retention (15 days); PC2: n = 6, orthodontic tooth movement (10 days) + short-term retention (7 days); EG1: n = 8, orthodontic tooth movement (10 days) + long-term retention (15 days) + 10–20–30 Hz cumulative increased frequencies mechanical vibration; EG2: n = 8, orthodontic tooth movement (10 days) + short-term retention (7 days) + 10–20–30 Hz cumulative increased frequencies mechanical vibration. | 10–20–30 Hz cumulative increased for 10 min/d first 3 days 10 Hz. Next 3 days 20 Hz Final 3 days 30 Hz. | Trabecular thickness; Trabecular number; OPG, RANKL, COX-2. | Trabecular thickness was lower in vibration Group than control groups. Trabecular number in vibration group was higher than CG and PC. No statistically significant intergroup difference in the OPG level. RANKL and COX-2 was significantly lower in EG. | HFV yielded a better bone structure by increasing trabecular number and levels of RANKL and COX-2. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas Aguilar, J.C.; García Vega, M.F.; Salas Orozco, M.F.; Aguilar Madrigal, R.M.; Reyes Cervantes, E.; Flores-Tochihuitl, J.; Soto Sainz, J.E.; Casillas Santana, M.A. Do Vibrational Forces Induce an Anabolic Effect in the Alveolar Bone of Animal Models? A Systematic Review. Appl. Sci. 2024, 14, 1118. https://doi.org/10.3390/app14031118
Villegas Aguilar JC, García Vega MF, Salas Orozco MF, Aguilar Madrigal RM, Reyes Cervantes E, Flores-Tochihuitl J, Soto Sainz JE, Casillas Santana MA. Do Vibrational Forces Induce an Anabolic Effect in the Alveolar Bone of Animal Models? A Systematic Review. Applied Sciences. 2024; 14(3):1118. https://doi.org/10.3390/app14031118
Chicago/Turabian StyleVillegas Aguilar, Julio César, María Fernanda García Vega, Marco Felipe Salas Orozco, Rosa Margarita Aguilar Madrigal, Eric Reyes Cervantes, Julia Flores-Tochihuitl, Jesús Eduardo Soto Sainz, and Miguel Angel Casillas Santana. 2024. "Do Vibrational Forces Induce an Anabolic Effect in the Alveolar Bone of Animal Models? A Systematic Review" Applied Sciences 14, no. 3: 1118. https://doi.org/10.3390/app14031118
APA StyleVillegas Aguilar, J. C., García Vega, M. F., Salas Orozco, M. F., Aguilar Madrigal, R. M., Reyes Cervantes, E., Flores-Tochihuitl, J., Soto Sainz, J. E., & Casillas Santana, M. A. (2024). Do Vibrational Forces Induce an Anabolic Effect in the Alveolar Bone of Animal Models? A Systematic Review. Applied Sciences, 14(3), 1118. https://doi.org/10.3390/app14031118








