1. Introduction
Parkinson’s disease (PD) is a highly prevalent, progressive neurological disease with complex motor and nonmotor symptoms which adversely affect mobility, functional skills, and quality of life [
1,
2]. There is a significantly greater risk of falls among individuals with PD compared to healthy older adults [
3,
4,
5]. Nearly 70% of individuals with PD experience at least one fall per year, and 39% report recurrent falls due to symptoms attributed to the disease and dynamic postural instability [
6,
7,
8]. Injurious falls are common and costly in PD as nearly 27% of individuals with PD will encounter a fall-related hip fracture within the first ten years of being diagnosed with the disease [
9,
10]. The motor symptoms associated with Parkinson’s disease include tremors, hypokinesia, bradykinesia, rigidity, postural instability, gait dysfunction, freezing of gait (FOG), and disuse-associated weakness and deconditioning [
11,
12,
13]. FOG can be characterized as a motor block or festinating steps during walking, where an individual’s center of gravity continues to accelerate forward as their feet appear stuck to the floor or are moving in fast, small, shuffling steps, leading to significantly increased fall risk [
4,
14]. Even individuals with very early stage PD may experience FOG (an estimated one in four persons), which can limit community mobility skills [
15,
16,
17]. For those in advanced disease stages, the incidence of FOG is estimated as high as 75%, and this gait deficit can markedly contribute to injurious falls [
15].
The underlying etiology of dynamic postural control impairments in individuals with PD is complex and not fully understood. Postural control requires multiple subsystems to interact simultaneously to maintain dynamic stability during both static and dynamic tasks as the individual interacts with a variety of environments. These subsystems include sensory organization, motor coordination, musculoskeletal properties, cognitive processes, and anticipatory and reactive postural control strategies [
18,
19,
20]. Sensory integration for balance control involves the weighting of somatosensory, visual, and vestibular information based on environmental and task demands. Research indicates that individuals with PD have impaired sensory organization, as evidenced by dynamic posturography testing, including ineffective use of somatosensory and vestibular cues and an overreliance on visual information [
21,
22,
23]. Reactive postural control, defined as the ability to bring in automatic postural responses following a perturbation or loss of balance, is a key contributor to increased fall risk in those with PD. Research demonstrates that individuals with PD have abnormal scaling and hypometric postural responses to perturbations both when standing and during walking tasks [
18,
24,
25,
26]. Anticipatory postural control strategies are employed preceding self-generated movements, such as in reaching, bending, and walking tasks to maintain dynamic equilibrium. These anticipatory postural adjustments are less effective in individuals with PD because they are smaller in magnitude and are prolonged compared to healthy adults [
19,
27,
28]. Anticipatory postural control and the ability to adapt to changes in postural demands in more complex mobility tasks such as walking and navigating obstacles deteriorate as the disease progresses, and this is a key intrinsic risk factor for falls in PD [
29].
One hallmark functional limitation in persons with PD is the progressive gait decline with disease progression. Even in the early stages of PD, biomechanical changes in gait are evident, such as slowed speed, reduced step length and arm swing, and impaired gait automaticity [
13,
30,
31,
32]. In a biomechanical analysis of gait kinematics in participants with PD as compared to healthy age-matched controls, Lewis et al. [
33] reported large variability among the PD cohort, but common characteristics seen were reduced peak joint angles, reduced or absent heel rocker at foot strike, reduced ankle power generation at push off, increased stance phase knee flexion, and reduced hip extension during midstance. These findings were variable based on disease presentation and severity and reflect the slowed, flatfoot contact and shuffling gait classically seen in clinical gait evaluations of individuals with PD [
32]. Dynamic postural control demands are high during walking as the center of mass (COM) travels outside of the base of support (BOS), i.e., the area within the perimeter created by every point of contact that the person makes with the supporting surface, during the gait cycle. The postural control must maintain a dynamic equilibrium while the BOS is moving. Impaired dynamic stability during gait tasks in persons with PD, particularly complex gait tasks such as changing directions and clearing obstacles, may underlie high fall risk as the individual attempts to adapt to environmental and task demands. Tripping and loss of balance during walking and obstacle crossing are commonly reported causes of falls in the PD population and often lead to injurious falls [
34].
Previous research on obstacle-crossing in healthy older adults revealed that older individuals employ a more conservative strategy for this gait task compared to young adults, evidenced by slowed gait crossing speed, shorter step length, reduced step width, and limited separation of the center of pressure (COP) from the COM to minimize postural instability [
35,
36,
37]. Older adults with postural instability demonstrate greater foot clearance over obstacles; have a greater risk of obstacle contact, especially with the trailing limb; and have increased frontal plane motion during obstacle crossing, all of which contribute to greater fall risk [
38,
39]. Postural instability and conservative gait adaptations during obstacle-crossing are more pronounced in individuals with PD compared to healthy older adults. Individuals with PD demonstrated slower gait velocity, shorter steps, increased step width, slowed obstacle-crossing speed, and difficulty lengthening their step over the obstacle compared to healthy controls, despite demonstrating similar foot clearance over the obstacle [
29,
40,
41,
42]. These gait alterations seen in PD are reflective of bradykinesia, a hallmark of this disease, and an attempt to maintain stability due to the postural threat of the obstacle-crossing task. Additionally, individuals with PD demonstrate increased step-to-step variability during the approach phase to an obstacle and plant their stance limb closer to the obstacle with significantly greater step length asymmetry than controls [
43,
44]. There was also greater variability in the toe clearance over the obstacle in the PD cohort compared to healthy controls [
45]. These findings in individuals with PD may reflect difficulty with gait control, rhythmicity, and adapting their gait during the obstacle-crossing task. The obstacle characteristics, e.g., height and depth, influence the necessary gait adaptations and postural stability demands. Higher obstacles pose a greater motor control and balance challenge for persons with PD as they approach the obstacle more slowly, with a wider BOS, and have a longer step duration over the obstacle; therefore, they spend an increased time in single-limb support with greater mediolateral instability compared to controls [
42,
46]. These gait adaptations can lead to increased trip risk or loss of balance in persons with PD.
The limited published research examining obstacle-crossing in persons with PD has focused primarily on gait spatiotemporal and kinematic measures. Stegemöller et al. [
29] examined dynamic postural stability during obstacle crossing by analyzing COM, COP, and the margin of stability (MOS), i.e., the linear distance between the COM and BOS. It is notable that during locomotion, the COP is often defined as the BOS. These researchers reported that individuals with PD had decrements in gait performance and employed gait adaptations to reduce the mechanical demands to enhance their postural stability during this task. They also reduced their range of COM displacement in the anteroposterior (A/P) direction, demonstrated a larger range of COM displacement in the mediolateral (M/L) direction, and generally showed a reduced mean distance between COM and COP compared to healthy controls. At all points in the gait cycle, individuals with PD demonstrated significantly increased MOS compared to controls [
29]. These findings reflect a conservative strategy to maintain dynamic balance during this challenging gait task. However, individuals with PD demonstrated reduced obstacle-crossing speed and increased single-limb stance time, which, combined with an increased M/L COM displacement, could predispose to increased fall risk, especially in the lateral direction. Liu et al. [
47] examined COM–COP inclination angles and the rate of change of inclination angles to reflect dynamic balance control during obstacle-crossing in persons with mild PD compared to age-matched controls. They found that the inclination angles and the rate of change of these angles in both the sagittal and frontal planes were greater in the individuals with PD compared to controls, and greater when the less-involved limb was leading compared to the involved limb (defined as the limb with initial motor symptoms), reflecting greater difficulty with balance control when the less involved limb was leading. Liu and colleagues interpreted their findings by noting that the greater the inclination angle during this gait task, the more effort was needed to control balance; therefore, even individuals with mild PD demonstrated impaired dynamic stability when crossing obstacles.
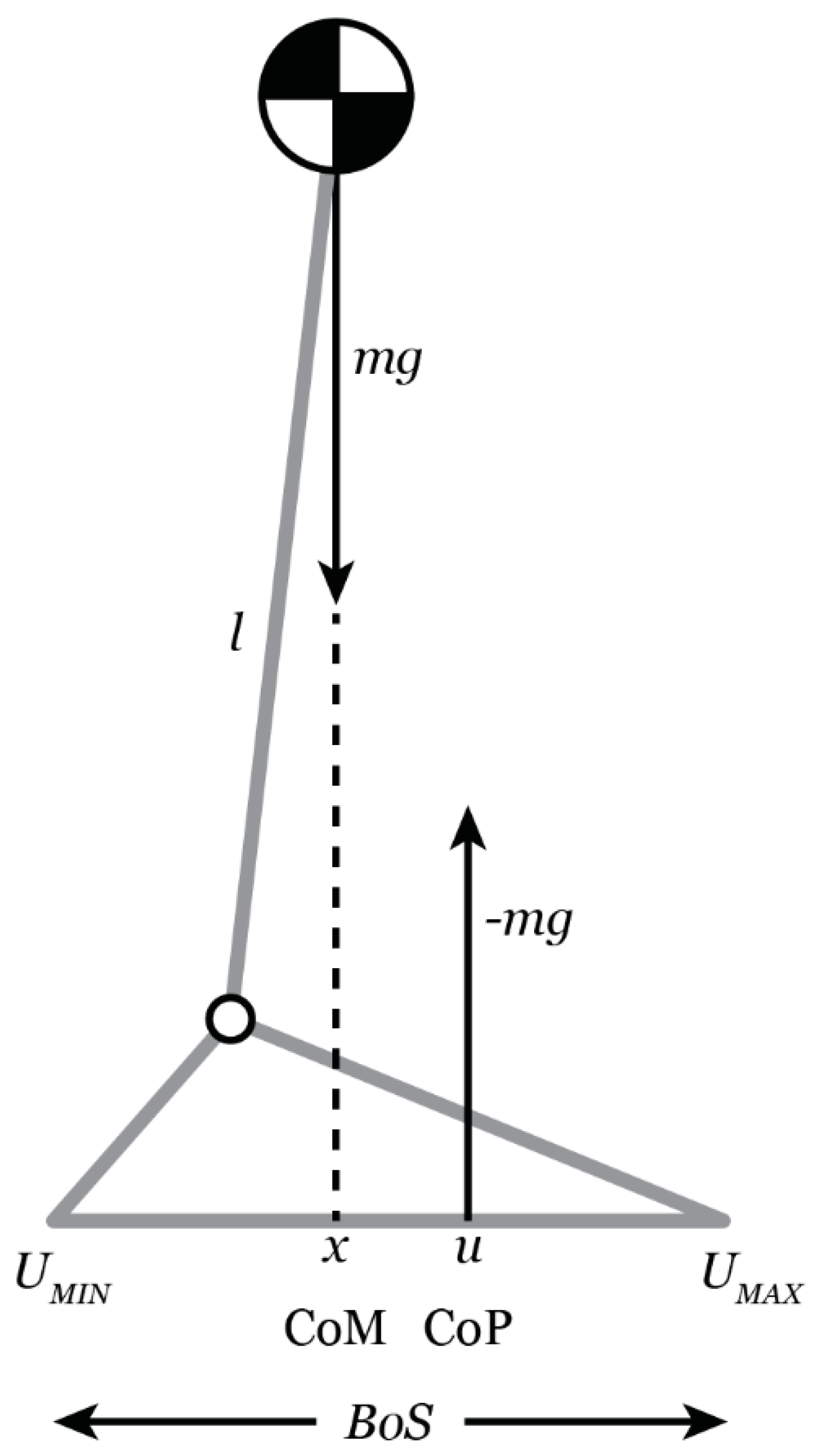
The theoretical model frequently utilized in clinical research to examine dynamic balance during gait, i.e., to examine the relationship between the COM and the COP, is the inverted pendulum model [
48,
49,
50,
51] (
Figure 1). The COM represents the line of gravitational pull on the body and the COP represents the position of the vertical ground reaction and is thought to represent the neurological response to changes in the position of the COM. Generally, when the medial horizontal distance from the COM to the COP is small, balance is expected to be maintained. If the COM is aligned with the COP but has a velocity directed outward, it may not be possible to maintain balance. On the other hand, balance may be maintained if the COM is beyond the BOS, but the COM velocity is directed inward [
48,
49,
50,
51,
52]. The magnitude of movement of the COM in the mediolateral (M/L) direction is an important predictor of falls and has been identified as a more sensitive measure of postural instability during gait than spatiotemporal gait parameters [
38]. Hof et al. [
48,
51] proposed a more nuanced variable to better understand the analysis of dynamic balance called the extrapolated center of mass (XCOM), which accounts for the position and velocity of the COM. Therefore, the trajectory of the COM is extrapolated in the direction of the COM velocity [
48,
51].
Individuals with PD are at an increased fall risk during complex gait tasks such as obstacle-crossing as they require adaptation of gait and challenge dynamic stability. Most gait research involving obstacle crossing in persons with PD has focused on spatiotemporal and kinematic variables but gives limited insight into postural control strategies applied during this challenging task. Examination of dynamic balance control during obstacle-crossing in individuals with PD is inadequately addressed in the literature. Therefore, the purpose of the present study was to examine if there were differences in dynamic balance, i.e., postural control, during self-paced walking and obstacle crossing between persons with mild-to-moderate PD and age-matched controls. We hypothesized that there would be differences in the margin of stability metrics (e.g., dynamic stability) between individuals with PD and healthy controls during the obstacle-crossing task. This research may provide insights into postural instability and factors contributing to fall risk during obstacle crossing in individuals with PD, which may guide clinicians in developing strategies to reduce falls with directed interventions.
2. Materials and Methods
2.1. Participants
The research was approved by the University Institutional Review Board (IRB reference#: 17-267-H). This project was part of a larger study assessing a variety of tasks during walking that challenged dynamic balance in people with PD and healthy controls. Data for these gait tasks were collected in a single session; therefore, the descriptions of the instrumentation and the procedures for data collection and reduction are similar to our previously published paper that reported on dynamic balance control during a turning task [
53]. The present paper will focus on the methods and results related to an obstacle-crossing task.
Participants consisted of a sample of convenience from the local tri-city area. Individuals with PD were recruited through retirement communities, PD support groups, PD-specific exercise classes, and the local Parkinson’s Association. An age- and gender-matched, but not paired, healthy control group (CON) was also recruited. Thirteen individuals with mild-to-moderate idiopathic PD and 11 CON individuals qualified and were enrolled in this study following written informed consent.
2.2. Participant Screening Tests
The inclusion and exclusion criteria for this study are outlined in
Table 1. Potential participants from each group were screened via phone interview to assess if they met the inclusion and exclusion criteria that could be determined with verbal reports. If individuals passed the phone screen interviewing process, they were subsequently evaluated in person in the biomechanics laboratory for the functional screening tests. The Berg Balance Scale (BBS) and Montreal Cognitive Assessment (MoCA) are assessment tools that are valid and reliable in persons with PD [
54]. The BBS is a standardized measure that assesses participants’ ability to maintain balance during sitting, transfers, and more challenging tasks such as alternating foot taps to stool and standing on one leg [
54]. Individuals were excluded from the study if they had a score of less than 36 on the BBS and/or a history of >2 falls per month, as this identified individuals as fallers or at high risk for falls [
54,
55]. The MoCA identifies mild cognitive impairments among the PD population and includes tests of the domains of executive and visuospatial function, memory, language, and attention [
56]. A score of less than 21 on the MoCA was an exclusion criterion for this study [
56]. Participants also completed a Freezing of Gait Questionnaire (FOG-Q) [
57]. There were no participants classified as having FOG based on their responses to question three. Additionally, no participants in either the PD or CON group reported a positive fall history in the past six months.
Three PD and two CON participants were omitted from data analysis due to an insufficient requisite number of gait cycles during obstacle crossing, e.g., large gaps in marker trajectories. One PD participant dropped out due to back pain on the test day (
Figure 2). Participant demographics are presented in
Table 2. Additionally, a descriptive summary of participants’ self-selected pace walking gait parameters is presented in
Appendix A. Scheduled testing appointments for this study aligned with PD participants’ “on” time in their medication schedule to reflect walking function in their usual medicated state.
2.3. Instrumentation
Kinematic and kinetic data were collected simultaneously. Three force plates (Advanced Mechanical Technology Inc., Watertown, MA, USA) were utilized to collect (1200 Hz) ground reaction force data. Sixteen Vicon motion capture cameras (eight T40S, two MXF40, six MX40) and Nexus v2.6.1 motion capture software (Vicon Motion System Ltd., Oxford Metrics, UK) were utilized to collect (120 Hz) the 3D marker trajectories. Vicon Nexus software was used to reconstruct marker trajectories, edit gaps, and filter raw trajectory data using a Woltring filter (MSE 15). A 6 Hz zero-lag 4th-order Butterworth filter was used to smooth raw ground reaction force data. Reflective markers placed on anatomical landmarks were consistent with the full-body Plug-in Gait model (PIG) [
53,
58].
2.4. Experimental Protocol
Physical examination included measures of height, weight, gross lower extremity range of motion, manual muscle testing of all major lower extremity joints, and screening integrity of nerve roots L4 and L5. Anthropometric measurements were taken for knee, ankle, and elbow width, wrist and hand thickness, leg length (distance from the anterior superior iliac spine (ASIS) to medial malleolus), pelvic width (distance between bilateral ASIS), pelvic height (perpendicular distance from a line connecting the right and left ASIS and the superior edge of the pubic tubercle), and pelvic depth (measurement from ASIS to posterior superior iliac spine). Pelvic measurements were utilized to estimate the location of each subject’s hip joint center [
59]. All participants wore tight-fitting shorts and their preferred athletic shoes. Physical examination measurements and marker placements were conducted by an examiner who had received three months of prior training (and who was supervised during data collection by the laboratory director). Forty spherical markers (14 mm diameter) were placed on anatomical landmarks according to a modified version of the full-body PIG model. Modifications of the PIG model included additional markers on the heads of the 5th metatarsals and placement of the thigh and tibial markers (as opposed to using thigh and tibial wands) on the mid-lateral thighs and tibiae. Refer to Alderink et al. [
53] for a detailed description of anatomical marker placement. Markers were placed by a single examiner to eliminate inter-examiner variability and checked between walking sessions for any displacement [
53].
Testing procedures included the following walking conditions: (1) self-selected pace; (2) walking with 90-degree turns at a normal pace; (3) termination of gait; and (4) walking with an obstacle-crossing task. Dynamic balance variables were analyzed for the 90-degree turn and during a gait cycle before the 90-degree turn in prior studies. This study focused on data analysis of dynamic balance during the obstacle-crossing task.
Following marker placement, a static calibration trial was performed in which participants were asked to stand still while assuming the anatomical position (arms slightly abducted and feet shoulder width apart) for approximately four seconds. The medial femoral condyle and medial malleolar markers were removed following the completion of the static calibration trial, before walking tasks.
Walking data were collected and processed for one gait trial at a comfortable speed to check for accurate thigh, femoral epicondyle, and tibial marker placement. Frontal and transverse hip and knee joint angle data were determined, graphed, and compared to the laboratory normal walking database (within one standard deviation) to ensure correct marker placement. If participants’ data fell within one standard deviation of the normal kinematic bands, the correct marker placement was verified and testing continued. To ensure the safety of the participants, a gait belt was available during the data collection process, but the use of the belt was up to the examiners’ discretion following the initial screening. Rest breaks were given as necessary. Marker placement was checked frequently in between walking trials and during rest breaks.
Participants performed practice walking trials along the 10-m pathway at a self-selected pace to find their normal gait pattern and feel comfortable with the walkway. Researchers identified the appropriate starting position for subjects to ensure accurate force plate contact, which was adjusted accordingly throughout trials. Trials were considered valid if the step taken before the obstacle cleanly contacted a force plate and the step after crossing the obstacle cleanly contacted the next force plate. Trials in which the subject’s foot was partially in contact with the force plates were repeated.
For obstacle-crossing trials, we used a method similar to Stegemöller et al. [
29]. The obstacle height was 10% of the participant’s height to within 1 cm. The obstacle was a 106 cm by 1 cm by 0.5 cm lightweight wooden dowel crossbar supported by two 56 cm vertical standards (
Figure 3). This method was chosen to minimize risk to the participant since the dowel would easily be dislodged forward with contact. The normalized obstacle height has been used previously in research [
29,
35,
36,
37,
38] and is similar to the height of a commonly encountered obstacle such as a curb or a step. Participants walked at a self-selected pace progressing along the negative
y-axis of the laboratory coordinate system (
Figure 3) and negotiated the obstacle for five trials with their left leg crossing first and then five trials with their right leg crossing first. Patients were encouraged to target the force plates as we believe that individuals in the community typically would watch their feet as they approach an obstacle. Standardized instructions were provided for crossing the obstacle as follows: “For this task, we will be examining how you step over an obstacle in your path. Please walk at a comfortable speed along the walkway, step over the obstacle in your path with your (left/right) foot and continue walking to the end of the walkway. You will complete five trials of this task on each leg. I will demonstrate the task for you”. One of the researchers subsequently provided a demonstration of this task. Additional instructions were provided at the researchers’ discretion. The obstacle-crossing task was completed once five valid trials were recorded with each limb.
2.5. Data Processing and Reduction
Marker and ground reaction force data were post-processed and trials were trimmed to a single obstacle-crossing gait cycle in Vicon Nexus 2.6.1 (see [
53] for more details). Six representative obstacle crossing trials (three right and three left) were selected and exported to Visual3D (C-Motion, Inc., Germantown, MD, USA) for the dynamic stability analysis. The primary variables of interest included a spatial inclination angle (COM–COP angle) and the M/L distances between COM and COP (COP–COM M/L), XCOM and COP (COP–XCOM M/L), and XCOM and the most lateral position of the BOS, i.e., U
MAX (U
MAX–XCOM M/L) (
Figure 4). These variables were measured to determine whether a difference existed between PD and CON participants during the stance phase at five specific time points: first double support (FDS1) and midstance (MST1) for the trailing limb (1st step); and first double support (FDS2), midstance (MST2), and second double support (SDS2) for the leading limb (2nd step) (
Figure 3).
Using a 15-segment model, total body COM was calculated as the weighted average of all segment COMs; segments were modeled as geometric shapes with masses were expressed as percentages of total body mass [
60,
61]. The velocity of the COM (
) was calculated with a first-order central difference formula [
60]. XCOM was then found using the following equation:
where
= angular eigenfrequency of the pendulum;
where
= 9.81 m/s
2 and
in m (distance between COM and ankle joint) [
48].
The toe and fifth metatarsal markers defined the anterior and lateral limits of the BOS (A/P and M/L U
MAX), respectively. The M/L direction was the focus of this study; therefore, the MOS during obstacle crossing was determined by the distance between the XCOM and M/L U
MAX.
The other dynamic stability variables, COP–COM and COP–XCOM, were also calculated in the M/L direction (i.e., along the
x-axis) of the lab coordinate system. The COM–COP inclination angle was calculated by finding the spatial angle between the vector from the COM to the vertical projection of the COM on the ground and the vector from the COM to the COP [
53].
The secondary variables of interest were the spatiotemporal gait parameters: stance time, swing time, and step length for both steps and stride length, stride width, and velocity for the gait cycle (averaged across the three right and three left gait cycles). For example, a left obstacle-crossing gait cycle consisted of a right leading limb and a left trailing limb (
Figure 3). In this case, the spatiotemporal gait parameters include (1) first stance time, i.e., the time spent from left (trailing limb) heel strike to left toe-off; (2) first swing time, i.e., the time spent from right (leading limb) toe-off to right heel strike; (3) first step length, i.e., the distance from the left heel to the right heel; (4) second stance time, i.e., the time spent from right heel strike to right toe-off; (5) second swing time, i.e., the time spent from left toe-off to left heel strike; and (6) second step length, i.e., the distance from the right heel to the left heel.
2.6. Data Analysis
Statistical analyses were performed using R Statistical Software (v4.2.2; R Core Team 2023) [
62] running in RStudio. [
63] Analysis models for spatiotemporal and dynamic balance variables (10 models) were mixed-model ANOVA models using lme from the nlme [
64] and emmeans packages [
65], with a significance level of α = 0.05. Units of analysis were not Subject but gait cycle sequences with Subject being a factor in the model. Each model had a random effect, limb within subject intercepts included. Limb was a fixed effect but grouping left and right limbs across subjects ignored individual left/right asymmetry, so it was nested within the randomly selected subjects. Variance was not expected to be constant across subjects but was assumed to be constant within subject, so constant variance within subjects was used for the variance structure. Normality was tested using Shapiro–Wilks tests. A few of these failed, but an examination of q–q plots for those variables indicated that the deviation from normality was mild and generally symmetric. ANOVA is known to be robust to normality, especially if the distribution is at least symmetric, so normality was accepted in these cases (see
Appendix B for details).
Three sets of fixed effects were used in the models: two for the spatiotemporal gait parameters and a third for the margin of stability, i.e., dynamic balance variables. The spatiotemporal data were split into two groups. One consisted of stride variables with data taken across the full obstacle-crossing stride. The stride variables included stride length, stride width, and gait velocity. The stride variables were modeled with a single fixed effect: Condition, i.e., CON and PD. Step variables had Step (1st and 2nd) added to the fixed effects along with the interaction between Step and Condition. The margin of stability fixed effects comprised Condition and Event (FDS1, MST1, FDS2, MST2, and SDS2), with their interaction. Event comprised five discrete stance phase points, requiring it to be modeled as a repeated measure.
Tables of p-values were generated for the Step and margin of stability models providing the overall ANOVA fixed effects significance. Since the stride variables had only a single two-level fixed factor, the F-test and post hoc t-test p-values were the same, so no ANOVA tables were generated for those variables. Almost all interactions were significant, so interaction analysis (when present in the model) was used for post hoc tables. Post hoc tables for the combined stride and step variables provide specifics for stride variables, the combination of the step variable for each of the first and second steps, and the margin of stability variables. Each line of the table includes group means and standard errors, differences in group means with p-values, and confidence intervals extracted from each analysis. Note that multiple comparison corrections were not needed for two-level factors.
4. Discussion
This study aimed to explore biomechanical variables reflective of dynamic postural control, specifically margin of stability metrics using 3D motion analysis, during walking and obstacle crossing in persons with Parkinson’s disease. Our study is also unique in that we examined gait and balance variables during the complete two-step sequence (leading limb and trailing limb step cycles) during this obstacle-crossing task. The primary purpose of this study was to determine if there were differences in dynamic balance control during self-paced walking and obstacle-crossing between persons with mild-to-moderate PD and age-matched controls. Our objective was to shed light on postural control strategies and compensations during walking with obstacle-crossing in the PD group as compared to healthy controls. Tripping or loss of balance during walking and obstacle-crossing is a common cause of injurious falls in persons with PD [
34]. Falls can have costly consequences in PD, leading to fear of movement, activity restrictions, and reduced independence. Injurious falls often result in hospitalization, admission to nursing homes, and increased mortality [
3,
6,
10]. Falls in persons with PD occur during complex gait tasks that require increased attention to the task and divided attention between gait adaptability and control of balance. Examination of dynamic balance control during obstacle-crossing in persons with PD has been inadequately studied in previous research, which has mainly focused on spatiotemporal gait parameters and primarily examined single leading-limb gait parameters. We sought to gain insights into how individuals with PD control dynamic balance during this challenging gait task, which may provide information pertaining to strategies to minimize falls in this population.
Qualitative analysis of the MOS metrics over the two-step sequence during obstacle-crossing revealed that the COM remained medial to the BOS throughout the gait task for both the PD and CON groups. This finding is consistent with the results reported by Galna et al. [
66] and may reflect a conservative strategy to minimize the risk of a lateral fall during this challenging gait task. Individuals with PD, however, demonstrated significantly greater mean values for COM–COP M/L at first double support for step one and COP–XCOM M/L at second double support for the second step as compared to controls. These time points in the two-step obstacle-crossing sequence represent critical transition points as the trailing limb moves over the obstacle and the individual continues forward gait progression. These findings may indicate a tendency for postural instability at these transition points during walking and obstacle-crossing in people with PD. Increased mediolateral excursion during these points in the step cycle may also reflect the possible use of a compensatory strategy to optimize the safe clearance of the trailing foot over the obstacle. Our findings agree with previous research [
29,
66]. Galna et al. reported that medio-lateral excursion was 21% greater in the PD group than in the control group during obstacle-crossing, and Stegemoller et al. [
29] reported that this M/L excursion was 36% greater in their PD cohort compared to controls. Consistent with our findings, Stegemoller et al. [
29], who also examined COM M/L across Events in the two-step sequence, reported significantly increased M/L COM excursion in the PD group at the double-limb support Event for both the leading and the trailing limb. It was interesting that we did not find significant differences in COM–COP M/L or COM–XCOM M/L between groups at the midstance (MST1, MST2) points in the two-step sequence during obstacle-crossing, which is the most unstable point in the gait cycles (steps one and two). This finding may indicate that individuals in our PD cohort who were in the early disease stage were successful at controlling balance during the single-limb stance demands of this obstacle-crossing task.
Increased medio-lateral excursion of the COM and XCOM is a clinically relevant finding as it may be indicative of increased fall risk in the PD group. Chou et al. reported that elderly fallers have significantly greater mediolateral COM motion when obstacle-crossing compared to healthy elderly controls [
38]. Lateral instability during this gait task is a significant concern as lateral falls result in a greater incidence of hip fractures in both persons with PD and elderly fallers [
10]. Several factors in PD may contribute to postural instability during the obstacle-crossing task; individuals with PD have bradykinetic (slowed) and hypokinetic (reduced amplitude) limb movements and anticipatory postural strategies [
12,
18,
27,
28], which may adversely affect dynamic postural control and gait adaptability for this task. Additionally, individuals with PD have impaired visual–spatial, limb proprioceptive, and sensorimotor integration for balance [
21,
22,
23,
67,
68]. The obstacle-crossing task requires spatial processing for successful task performance. Compensatory strategies to control balance may be employed to optimize foot clearance for both the leading and trailing limbs.
A strength of our study design was that we examined MOS metrics throughout the two-step obstacle-crossing sequence as dynamic balance control demands are high throughout this sequence, i.e., not just during the lead limb stepping over the obstacle. Trips and loss of balance can occur throughout the two-step cycle as the individual attempts to clear the obstacle with the first step, control single-limb stance during the first step, and then maintain balance while clearing the obstacle with the trailing limb (second step). We analyzed the interactive effects of Event (in this two-step sequence) and Condition (PD vs. CON) to shed light on the differences in dynamic postural control between the two groups across the obstacle-crossing task sequence. Our results revealed significant differences for the COP–COM and COP–XCOM variables for both Event and Event interaction with Condition (PD vs. CON), whereas significant differences for U
MAX–XCOM were only found for Event. These findings indicate that individuals with PD make different adjustments to control balance during this obstacle-crossing task than those of healthy controls. Previous research supports our finding that there are spatiotemporal gait changes seen in those with PD compared to healthy controls during the obstacle-crossing task, including slower gait velocity, shorter steps, increased step width, and slowed obstacle-crossing speed [
29,
40,
41,
42]. These conservative strategies may be an attempt to reduce postural instability and fall risk during this task. Our findings provide further support for there being differences in dynamic postural control strategies in those with mild PD and healthy controls. These differences are seen at critical Events in the two-step obstacle-crossing sequence. We found significant interaction effects between Step and Condition for swing time, stance time, and step length. Post hoc analysis revealed that there was a significantly shorter step length for the second step in PD compared to the CON group, which was consistent with the findings by Liu et al. [
47]. One possible explanation for this finding is that individuals with PD may shorten step length on their trailing leg to control balance (and reduce COM A/P excursion) in this recovery step. It is uncertain whether the difference of 0.08 m (3.15 inches) is clinically meaningful. This information may provide insights into strategy training to enhance postural stability during this challenging gait task in people with PD.
One other MOS metric that we examined was the COM–COP inclination angle, which is a spatial angle that accounts for both the A/P and M/L movement of COP relative to COM. We found a significant interaction effect for Event and Condition (PD vs. CON). Greater inclination angles in the frontal plane were previously reported as a good indicator by which to distinguish elderly fallers from non-fallers [
38]. In contrast to Liu et al.’s results [
47], we did not find greater inclination angles in the PD group compared to controls. Our post hoc analysis revealed a greater COM–COP inclination angle for the CON group at SDS2 (second double-limb support for step two). This finding could suggest that the healthy controls were more dynamically moving their center of mass as they transitioned to second double-limb support and gait continuation during the obstacle-crossing task. Our finding of no significant difference in inclination angle at other Events during obstacle-crossing may be due to the relatively mild disease stage for our PD cohort, or it could indicate a conservative strategy used by individuals with PD to limit the excursion of their COM. Differences in inclination angle findings between our study and the Liu paper may be due to methodological differences as we examined the inclination angle at different Events in the two-step sequence gait cycle.
Our findings support the contention that analyses of MOS metrics, specifically COM–COP M/L, COM–XCOM M/L, and U
MAX–XCOM M/L, are useful to assess dynamic stability during walking and obstacle-crossing and may provide insights into postural control strategies used by individuals with PD during this challenging gait task. This present work has laid a foundation from which to elucidate postural instability in persons with mild PD during this task. In addition to the small sample size, our study has several additional limitations that may have influenced our results. Subjects were instructed to target the first and second force plate with their steps during the obstacle-crossing task, which may have affected their gait strategy or step adjustments as they completed the task. Since most individuals, especially those with a fear of falling, use vision to safely clear an obstacle, we encouraged the use of vision throughout the task. Participants in our small PD cohort had mild disease severity, no freezing of gait, and no previous fall history (an exclusion criterion for the study was two or more falls in the past year). Therefore, our findings cannot be generalized to individuals with moderate-to-severe PD disease or frequent fallers who have more marked gait and balance deficits. Further research is warranted to examine dynamic balance control during obstacle-crossing in those in the middle-to-late disease stage and those with freezing of gait characteristics, as these individuals are at a much higher risk of falling during challenging gait tasks. Galna et al. found that individuals with PD with greater disease severity demonstrated greater postural instability during an obstacle-crossing task than those with mild PD [
66]. Understanding the mechanisms underlying this postural instability may help to guide treatment strategies for fall risk reduction in those with PD. Finally, only a few studies have utilized MOS variables to characterize dynamic balance control during obstacle-crossing in PD [
29,
47,
66]. Identifying which variables are the most meaningful and sensitive indicators of postural instability is needed in future studies. Terry et al. [
69] and Kazanski et al. [
70] have suggested that Hof et al.’s [
48,
50] original work on the margin of stability for static postures may need to be modified for use in gait, so additional work is needed in that area. Furthermore, Nguyen et al. [
71] recently reported that body size was correlated with the margin of stability in both mediolateral and anteroposterior directions. They proposed that expressing stability margins as an impulse should be considered in future work, which may be necessary for additional research involving individuals with PD.