Integrating Artificial Intelligence for Academic Advanced Therapy Medicinal Products: Challenges and Opportunities
Abstract
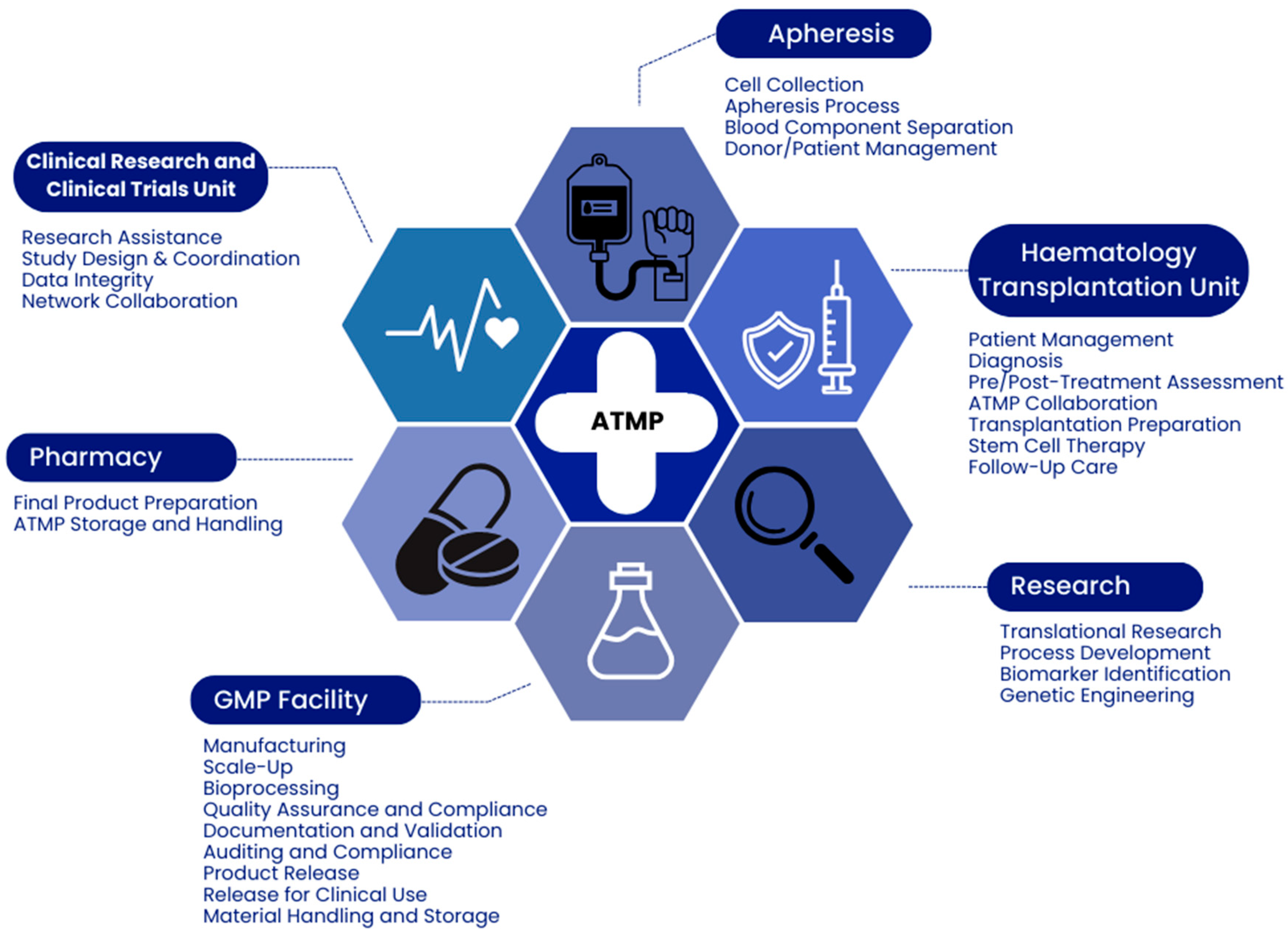
:1. Introduction
2. Advantages of On-Site Hospital Facilities
2.1. Implementing GMPs in Hospitals
2.2. Investment in Infrastructure and Personnel
3. Enhancing Hospital Facilities with New Technologies
4. Scaling Gene and Cell Therapy ATMPs from Research to the Clinic with AI
5. Analysis of the Role and Potential Impact of AI in Academic ATMP Manufacturing, with Additional Details on the Key Advantages AI Can Provide
5.1. Process Monitoring
5.2. Automation
5.3. Dynamic Control
5.4. Data Management
5.5. Systems Biology and AI
5.6. Advanced Control Systems Leveraging AI
6. Conclusions
Funding
Conflicts of Interest
References
- Johanna, I.; Daudeij, A.; Devina, F.; Nijenhuis, C.; Nuijen, B.; Romberg, B.; de Haar, C.; Haanen, J.; Dolstra, H.; Bremer, E.; et al. Basics of advanced therapy medicinal product development in academic pharma and the role of a GMP simulation unit. Immuno-Oncol. Technol. 2023, 20, 100411. [Google Scholar] [CrossRef]
- Elverum, K.; Whitman, M. Delivering cellular and gene therapies to patients: Solutions for realizing the potential of the next generation of medicine. Gene Ther. 2020, 27, 537–544. [Google Scholar] [CrossRef]
- Iancu, E.M.; Kandalaft, L.E. Challenges and advantages of cell therapy manufacturing under Good Manufacturing Practices within the hospital setting. Curr. Opin. Biotechnol. 2020, 65, 233–241. [Google Scholar] [CrossRef]
- Bersenev, A.; Fesnak, A. Place of Academic GMP Facilities in Modern Cell Therapy. Methods Mol. Biol. 2020, 2097, 329–339. [Google Scholar] [CrossRef]
- McGuirk, J.; Waller, E.K.; Qayed, M.; Abhyankar, S.; Ericson, S.; Holman, P.; Keir, C.; Myers, G.D. Building blocks for institutional preparation of CTL019 delivery. Cytotherapy 2017, 19, 1015–1024. [Google Scholar] [CrossRef]
- Amini, L.; Silbert, S.K.; Maude, S.L.; Nastoupil, L.J.; Ramos, C.A.; Brentjens, R.J.; Sauter, C.S.; Shah, N.N.; Abou-El-Enein, M. Preparing for CAR T cell therapy: Patient selection, bridging therapies and lymphodepletion. Nat. Rev. Clin. Oncol. 2022, 19, 342–355. [Google Scholar] [CrossRef]
- Abou-El-Enein, M.; Römhild, A.; Kaiser, D.; Beier, C.; Bauer, G.; Volk, H.D.; Reinke, P. Good Manufacturing Practices (GMP) manufacturing of advanced therapy medicinal products: A novel tailored model for optimizing performance and estimating costs. Cytotherapy 2013, 15, 362–383. [Google Scholar] [CrossRef]
- Viganò, M.; Giordano, R.; Lazzari, L. Challenges of running a GMP facility for regenerative medicine in a public hospital. Regen. Med. 2017, 12, 803–813. [Google Scholar] [CrossRef]
- Jackson, M.R. Accommodating clinical trials and other externally manufactured cellular therapy products: Challenges, lessons learned and creative solutions. Cytotherapy 2022, 24, 37–44. [Google Scholar] [CrossRef]
- Sutherland, V.; Buffo, M.J.; Whiteside, T.L. Impact of contracted manufacturing organization protocols on operations in an aca demically based Current Good Manufacturing Practice facility. Cytotherapy 2022, 24, 32–36. [Google Scholar] [CrossRef]
- Coppens, D.G.M.; Hoekman, J.; De Bruin, M.L.; Slaper-Cortenbach, I.C.M.; Leufkens, H.G.M.; Meij, P.; Gardarsdottir, H. Advanced therapy medicinal product manufacturing under the hospital exemption and other exemption pathways in seven European Union countries. Cytotherapy 2020, 22, 592–600. [Google Scholar] [CrossRef]
- Priesner, C.; Hildebrandt, M. Advanced Therapy Medicinal Products and the Changing Role of Academia. Transfus Med Hemother. 2022, 49, 158–162. [Google Scholar] [CrossRef]
- European Commission. Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products. Eur. Comm. J. 2017, 4, 1–32. [Google Scholar]
- Digiusto, D.L.; Melsop, K.; Srivastava, R.; Tran, C.A.T. Proceedings of the first academic symposium on developing, qualifying and operating a cell and gene therapy manufacturing facility. Cytotherapy 2018, 20, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, S.; Guchelaar, H.-J.; Zandvliet, M.L.; Meij, P. Clinical development of gene- and cell-based therapies: Overview of the European landscape. Mol. Ther. Methods Clin. Dev. 2016, 3, 16073. [Google Scholar] [CrossRef]
- Chauca Strand, G.; Bonander, C.; Jakobsson, N.; Johansson, N.; Svensson, M. Assessment of the clinical and cost-effectiveness evidence in the reimbursement decisions of new cancer drugs. ESMO Open 2022, 7, 100569. [Google Scholar] [CrossRef]
- Harrison, R.P.; Medcalf, N.; Rafiq, Q.A. Cell therapy-processing economics: Small-scale microfactories as a stepping stone toward large-scale macrofactories. Regen. Med. 2018, 13, 159–173. [Google Scholar] [CrossRef]
- Gladwell, D.; Ciani, O.; Parnaby, A.; Palmer, S. Surrogacy and the Valuation of ATMPs: Taking Our Place in the Evidence Generation/Assessment Continuum. Pharmacoeconomics. 2024, 42, 137–144. [Google Scholar] [CrossRef]
- Bäckel, N.; Hort, S.; Kis, T.; Nettleton, D.F.; Egan, J.R.; Jacobs, J.J.L.; Grunert, D.; Schmitt, R.H. Elaborating the potential of Artificial Intelligence in automated CAR-T cell manufacturing. Front. Mol. Med. 2023, 3, 1250508. [Google Scholar] [CrossRef]
- Hort, S.; Herbst, L.; Bäckel, N.; Erkens, F.; Niessing, B.; Frye, M.; König, N.; Papantoniou, I.; Hudecek, M.; Jacobs, J.J.L.; et al. Toward Rapid, Widely Available Autologous CAR-T Cell Therapy—Artificial Intelligence and Automation Enabling the Smart Manufacturing Hospital. Front. Med. 2022, 9, 913287. [Google Scholar] [CrossRef]
- Harrison, R.P.; Ruck, S.; Medcalf, N.; Rafiq, Q.A. Decentralized manufacturing of cell and gene therapies: Overcoming challenges and identifying opportunities. Cytotherapy 2017, 19, 1140–1151. [Google Scholar] [CrossRef]
- Gerzon, G.; Sheng, Y.; Kirkitadze, M. Process Analytical Technologies—Advances in bioprocess integration and future perspectives. J. Pharm. Biomed. Anal. 2022, 207, 114379. [Google Scholar] [CrossRef]
- Majors, B.S.; Betenbaugh, M.J.; Chiang, G.G. Links between metabolism and apoptosis in mammalian cells: Applications for anti-apoptosis engineering. Metab. Eng. 2007, 9, 317–326. [Google Scholar] [CrossRef]
- Ahmed, S.; Chauhan, V.M.; Ghaemmaghami, A.M.; Aylott, J.W. New generation of bioreactors that advance extracellular matrix modelling and tissue engineering. Biotechnol. Lett. 2019, 41, 1–25. [Google Scholar] [CrossRef]
- Harrison, R.P.; Chauhan, V.M. Enhancing cell and gene therapy manufacture through the application of advanced fluorescent optical sensors (Review). Biointerphases 2017, 13, 01A301. [Google Scholar] [CrossRef] [PubMed]
- Jaccard, N.; Griffin, L.D.; Keser, A.; Macown, R.J.; Super, A.; Veraitch, F.S.; Szita, N. Automated method for the rapid and precise estimation of adherent cell culture characteristics from phase contrast microscopy images. Biotechnol. Bioeng. 2014, 111, 504–517. [Google Scholar] [CrossRef]
- Tanemura, H.; Kitamura, R.; Yamada, Y.; Hoshino, M.; Kakihara, H.; Nonaka, K. Comprehensive modeling of cell culture profile using Raman spectroscopy and machine learning. Sci. Rep. 2023, 13, 21805. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Paes, B.C.M.F.; Fulber, J.P.C.; Tran, M.Y.; Farnós, O.; Kamen, A.A. Development of an Integrated Continuous Manufacturing Process for the rVSV-Vectored SARS-CoV-2 Candidate Vaccine. Vaccines 2023, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.; Cronin, J.; Haer, M.; Krouse, J.; Prosperi, W.; Drolet-Vives, K.; Lieve, M.; Soika, M.; Balmer, M.; Kirkitadze, M. In-line monitoring of surfactant clearance in viral vaccine downstream processing. Comput. Struct. Biotechnol. J. 2021, 19, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.; Cavaco, D.G.; Faria, T.Q.; Alves, P.M.; Carrondo, M.J.T.; Peixoto, C. Advances in Lentivirus Purification. Biotechnol. J. 2021, 16, 2000019. [Google Scholar] [CrossRef]
- Williams, T.; Kalinka, K.; Sanches, R.; Blanchard-Emmerson, G.; Watts, S.; Davies, L.; Knevelman, C.; McCloskey, L.; Jones, P.; Mitrophanous, K.; et al. Machine learning and metabolic modelling assisted implementation of a novel process analytical technology in cell and gene therapy manufacturing. Sci. Rep. 2023, 13, 834. [Google Scholar] [CrossRef]
- Odeh-Couvertier, V.Y.; Dwarshuis, N.J.; Colonna, M.B.; Levine, B.L.; Edison, A.S.; Kotanchek, T.; Roy, K.; Torres-Garcia, W. Predicting T-cell quality during manufacturing through an artificial intelligence-based integrative multiomics analytical platform. Bioeng. Transl. Med. 2022, 7, e10282. [Google Scholar] [CrossRef]
- Li, S.; An, J.; Li, Y.; Zhu, X.; Zhao, D.; Wang, L.; Sun, Y.; Yang, Y.; Bi, C.; Zhang, X.; et al. Automated high-throughput genome editing platform with an AI learning in situ prediction model. Nat. Commun. 2022, 13, 7386. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y. Enhancing single-cell biology through advanced AI-powered microfluidics. Biomicrofluidics 2023, 17, 51301. [Google Scholar] [CrossRef]
- Emerson, J.; Kara, B.; Glassey, J. Multivariate data analysis in cell gene therapy manufacturing. Biotechnol. Adv. 2020, 45, 107637. [Google Scholar] [CrossRef]
- Kern, S.; Wander, L.; Meyer, K.; Guhl, S.; Mukkula, A.R.G.; Holtkamp, M.; Salge, M.; Fleischer, C.; Weber, N.; King, R.; et al. Flexible automation with compact NMR spectroscopy for continuous production of pharmaceuticals. Anal. Bioanal. Chem. 2019, 411, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Feng Báez, J.P.; George De la Rosa, M.V.; Alvarado-Hernández, B.B.; Romañach, R.J.; Stelzer, T. Evaluation of a compact composite sensor array for concentration monitoring of solutions and suspensions via multivariate analysis. J. Pharm. Biomed. Anal. 2023, 233, 115451. [Google Scholar] [CrossRef]
- Rathore, A.S.; Mishra, S.; Nikita, S.; Priyanka, P. Bioprocess Control: Current Progress and Future Perspectives. Life 2021, 11, 557. [Google Scholar] [CrossRef]
- Jabarivelisdeh, B.; Carius, L.; Findeisen, R.; Waldherr, S. Adaptive predictive control of bioprocesses with constraint-based modeling and estimation. Comput. Chem. Eng. 2020, 135, 106744. [Google Scholar] [CrossRef]
- Sarker, I.H. AI-Based Modeling: Techniques, Applications and Research Issues Towards Automation, Intelligent and Smart Systems. SN Comput. Sci. 2022, 3, 158. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.; Gómez-Schiavon, M.; Ng, A.H.; El-Samad, H. Design and Analysis of a Proportional-Integral-Derivative Controller with Biological Molecules. Cell Syst. 2019, 9, 338–353.e10. [Google Scholar] [CrossRef]
- Mitra, S.; Murthy, G.S. Bioreactor control systems in the biopharmaceutical industry: A critical perspective. Syst. Microbiol. Biomanuf. 2022, 2, 91–112. [Google Scholar] [CrossRef]
- Mujawar, S.; Deshpande, A.; Gherkar, A.; Simon, S.E.; Prajapati, B. Introduction to Human-Machine Interface. In Human-Machine Interface; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 1–23. ISBN 9781394200344. [Google Scholar]
- Zheng, Y. Optimization of computer programming based on mathematical models of artificial intelligence algorithms. Comput. Electr. Eng. 2023, 110, 108834. [Google Scholar] [CrossRef]
- Heaton, H.; Fung, S.W. Explainable AI via learning to optimize. Sci. Rep. 2023, 13, 10103. [Google Scholar] [CrossRef]
- Cheng, Y.; Bi, X.; Xu, Y.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Artificial intelligence technologies in bioprocess: Opportunities and challenges. Bioresour. Technol. 2023, 369, 128451. [Google Scholar] [CrossRef] [PubMed]
- Arinez, J.F.; Chang, Q.; Gao, R.X.; Xu, C.; Zhang, J. Artificial Intelligence in Advanced Manufacturing: Current Status and Future Outlook. J. Manuf. Sci. Eng. 2020, 142, 110804. [Google Scholar] [CrossRef]
- Lam, C.; van Velthoven, M.H.; Meinert, E. Developing a Blockchain-Based Supply Chain System for Advanced Therapies: Protocol for a Feasibility Study. JMIR Res. Protoc. 2020, 9, e17005. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dwivedi, A.D.; Srivastava, G. Internet of Things Based Blockchain for Temperature Monitoring and Counterfeit Pharmaceutical Prevention. Sensors 2020, 20, 3951. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, J.; Naeni, L.M.; Fathollahi-Fard, A.M.; Fiore, U. Blockchain in supply chain management: A review, bibliometric, and network analysis. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef]
- EMA. Multi-annual AI workplan 2023–2028 HMA-EMA Big Data Steering Group. 2023, 1–13. Available online: https://www.ema.europa.eu/en/news/artificial-intelligence-workplan-guide-use-ai-medicines-regulation (accessed on 1 February 2024).
- EMA. Reflection paper on the use of artificial intelligence in lifecycle medicines. Eur. Med. Agency 2023, 31, 1–17. [Google Scholar]

| Advantages of AI in ATMP Production | Disadvantages of AI in ATMP Production |
|---|---|
| Enhanced Process Monitoring | Data Security Concerns |
| Real-time oversight and data analysis for maintaining consistency and quality. | Risks associated with patient data privacy and data integrity in AI systems. |
| Automation of Manufacturing Tasks | High Initial Investment |
| Increases efficiency, precision, and scalability, reducing the workload of biotechnologists. | Significant upfront costs for AI integration and infrastructure development. |
| Dynamic Control and Adaptability | Limited Data in Early Development |
| AI systems can adjust to the variability in patient cell samples, optimizing batch consistency. | AI models may lack accuracy in early stages due to insufficient data for machine learning. |
| Improved Data Management | Requirement for Specialized Personnel |
| Effective handling of large datasets, enhancing process comprehension and decision-making. | Need for staff with expertise in AI and bioprocessing, who can be scarce. |
| Systems Biology Integration | Regulatory Challenges |
| AI aids in understanding complex biological relationships, enhancing predictive medicine. | Ambiguity in regulatory compliance for emerging AI applications in healthcare. |
| Aspect of ATMP Production | With AI Integration | Without AI Integration |
|---|---|---|
| Process Monitoring | Enhanced real-time monitoring and analysis using AI algorithms. | Relies on manual and periodic offline sample analysis. |
| Manufacturing Efficiency | Higher efficiency and scalability due to automation and dynamic control. | Less efficient, often limited by manual operations and static processes. |
| Data Management | Advanced handling of large and complex datasets, facilitating better decision-making. | Traditional data management, potentially leading to slower and less informed decisions. |
| Quality and Consistency | Improved product quality and batch consistency through predictive models and real-time adjustments. | Potential variability and quality issues due to lack of real-time monitoring and control. |
| Investment and Costs | Higher initial investment but potential long-term cost savings through efficiency and reduced error rates. | Lower initial costs but potentially higher long-term operational expenses. |
| Personnel Training and Expertise | Requires staff trained in AI and data science. | Relies more on traditional bioprocessing skills. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Gallardo, C.; Bonora-Centelles, A. Integrating Artificial Intelligence for Academic Advanced Therapy Medicinal Products: Challenges and Opportunities. Appl. Sci. 2024, 14, 1303. https://doi.org/10.3390/app14031303
Aguilar-Gallardo C, Bonora-Centelles A. Integrating Artificial Intelligence for Academic Advanced Therapy Medicinal Products: Challenges and Opportunities. Applied Sciences. 2024; 14(3):1303. https://doi.org/10.3390/app14031303
Chicago/Turabian StyleAguilar-Gallardo, Cristobal, and Ana Bonora-Centelles. 2024. "Integrating Artificial Intelligence for Academic Advanced Therapy Medicinal Products: Challenges and Opportunities" Applied Sciences 14, no. 3: 1303. https://doi.org/10.3390/app14031303






