Synthesis of Porous Carbon Nanomaterials from Vietnamese Coal: Fabrication and Energy Storage Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of Porous Carbon Materials
2.3. The Morphology, Element Composition, and Structures of Materials
2.4. Fabrication of Electrodes from Porous Carbon Materials
3. Results and Discussion
3.1. Physical Properties and Surface Characteristics of CP and CDPC
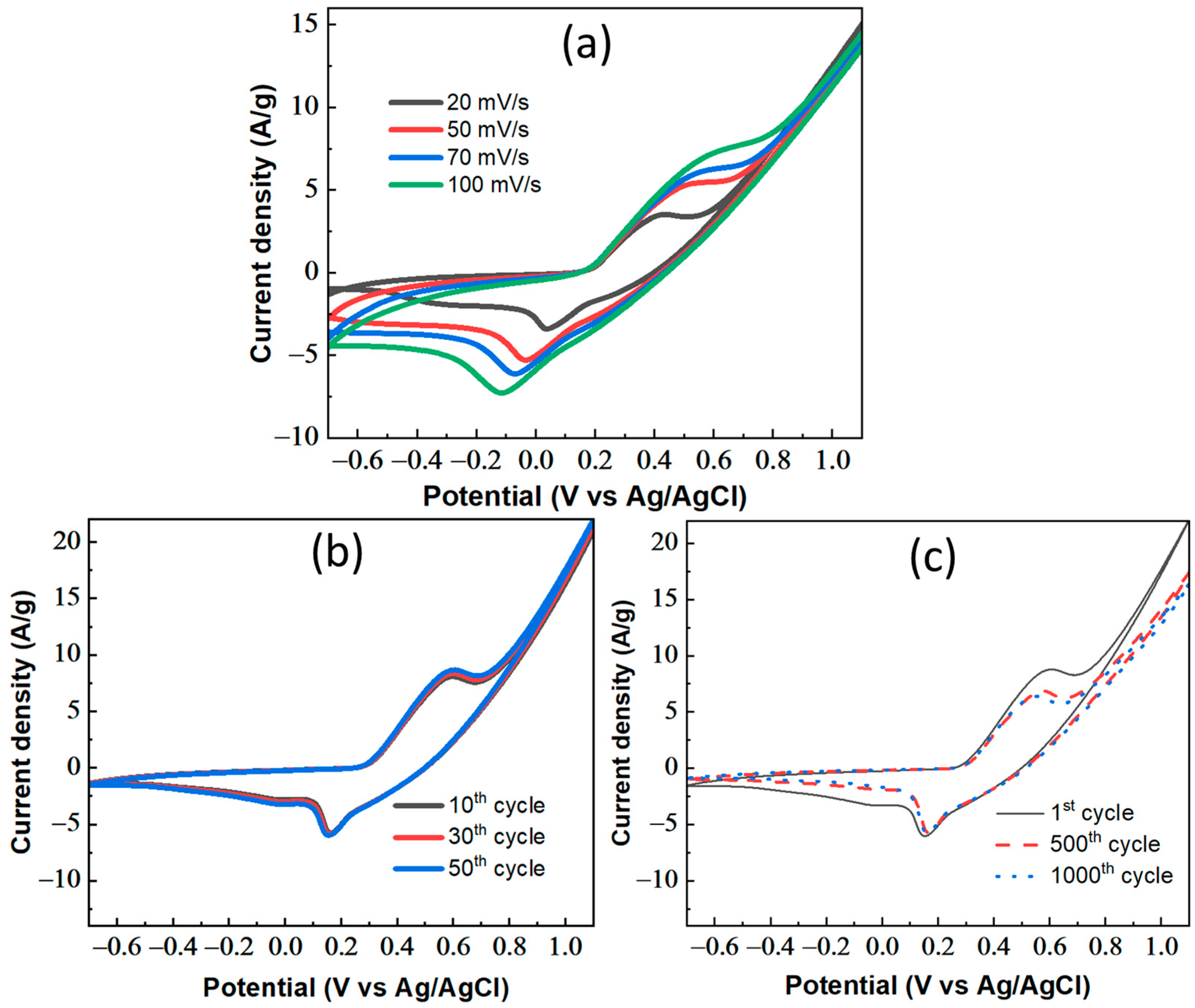
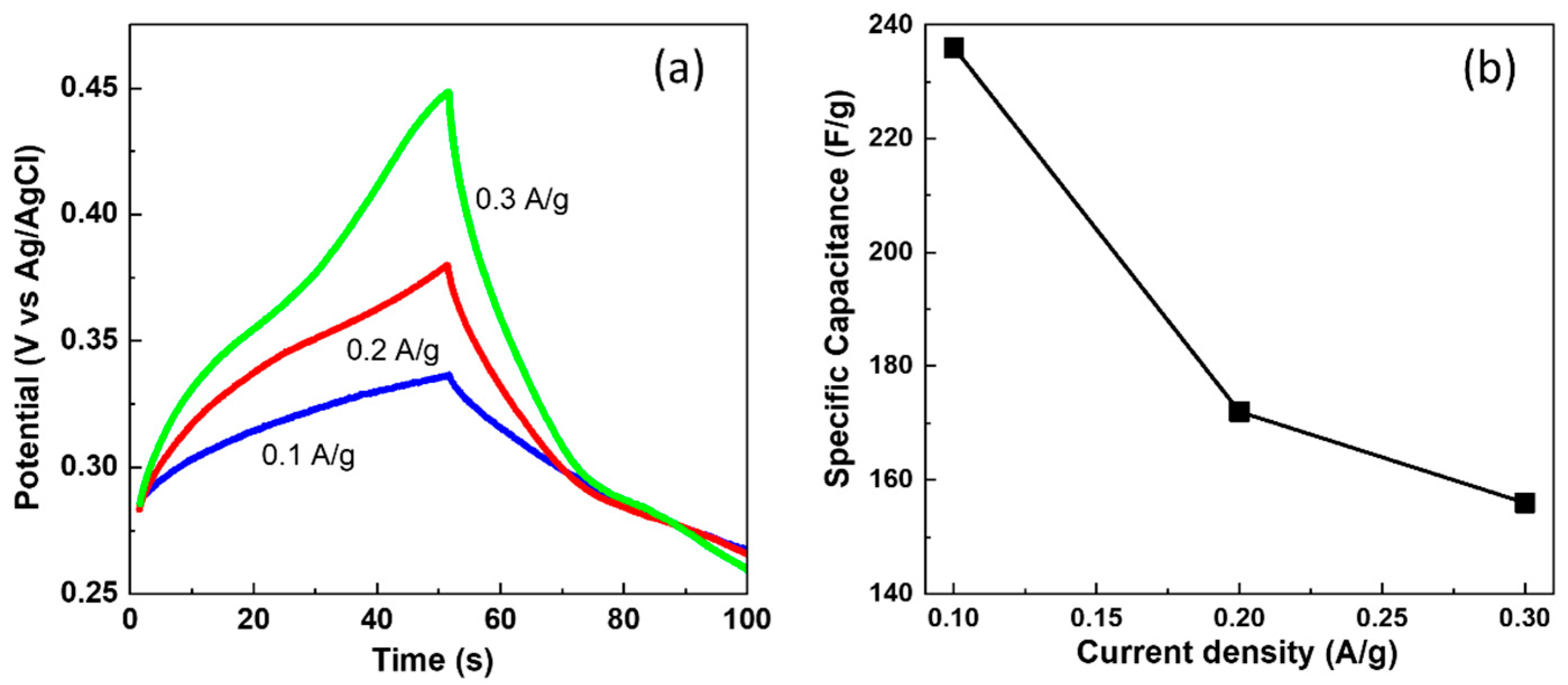
3.2. Electrochemical Measurement Results of CDPC Electrode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borchardt, L.; Zhu, Q.; Casco, M.E.; Berger, R.; Zhuang, X.; Kaskel, S.; Feng, X.; Xu, Q. Toward a molecular design of porous carbon materials. Mater. Today 2017, 20, 592–610. [Google Scholar] [CrossRef]
- Kang, B.T.; Ai, H.Q.; Lee, J.L. Single-atom vacancy induced changes in electronic and magnetic properties of graphyne. Carbon 2017, 116, 113–119. [Google Scholar] [CrossRef]
- Kumar, M.R.; Singh, S.; Fahmy, H.M.; Jaiswal, N.K.; Akin, S.; Shalan, A.E.; Mendez, S.L.; Salado, M. Next generation 2D materials for anodes in battery applications. J. Power Sources 2023, 5, 232256. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, H.; Kumar, G.; Sharma, S.; Aneja, R.; Sharma, A.K.; Kumar, R.; Kumar, P. Progress and challenges in electrochemical energy storage devices: Fabrication, electrode material, and economic aspects. Chem. Eng. J. 2023, 15, 143706. [Google Scholar] [CrossRef]
- Awasthi, K.; Srivastava, A.; Srivastava, O. Synthesis of carbon nanotubes. J. Nanosci. Nanotechnol. 2005, 5, 1616–1636. [Google Scholar] [CrossRef] [PubMed]
- Riya, T.; Balachandran, M. Luminescence and energy storage characteristics of coke-based graphite oxide. Mater. Chem. Phys. 2020, 257, 123854. [Google Scholar]
- Qin, Z.H. New advances in coal structure model. Int. J. Min. Sci. Technol. 2018, 28, 541–559. [Google Scholar] [CrossRef]
- Ma, X.M.; Dong, X.S.; Fan, Y.P. Prediction and characterization of the microcrystal structures of coal with molecular simulation. Energy Fuels 2018, 32, 3097–3107. [Google Scholar] [CrossRef]
- Mathews, J.P.; Chaffee, A.L. The molecular representations of coal—A review. Fuel 2012, 96, 1–14. [Google Scholar] [CrossRef]
- Vasireddy, S.; Morreale, B.; Cugini, A.; Song, C.; Spivey, J.J. Clean liquid fuels from direct coal liquefaction: Chemistry, catalysis, technological status and challenges. Energy Environ. Sci. 2011, 4, 311–345. [Google Scholar] [CrossRef]
- Haenel, M.W. Recent progress in coal structure research. Fuel 1992, 71, 1211–1223. [Google Scholar] [CrossRef]
- Thomas, R.; Manoj, B. Electrochemical Efficacies of Coal Derived Nanocarbons. Int. J. Coal Sci. Technol. 2021, 8, 459–472. [Google Scholar] [CrossRef]
- Xu, G.Z.; Yang, T.; Fang, Z.G.; Wang, Q.; Yang, C.; Zhao, X. Preparation and characterization of coal-based carbon foams by microwave heating process under ambient pressure. Diam. Relat. Mater. 2018, 86, 63–70. [Google Scholar] [CrossRef]
- Nilewski, L.; Mendoza, K.; Jalilov, A.S.; Berka, V.; Wu, G.; Sikkema, W.K.A.; Metzger, A.; Ye, R.; Zhang, R.; Luong, D.X.; et al. Highly oxidized graphene quantum dots from coal as efficient antioxidants. ACS Appl. Mater. Interfaces 2019, 11, 16815–16821. [Google Scholar] [CrossRef] [PubMed]
- Moothi, K.; Iyuke, S.E.; Meyyappan, M.; Falcon, R. Coal as a carbon source for carbon nanotube synthesis. Carbon 2012, 50, 2679–2690. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, Z.; Zhang, Y.; Meng, B.; Zhou, A.; Qiu, J. Graphene Sheets from Graphitized Anthracite Coal: Preparation, Decoration, and Application. Energy Fuels 2012, 26, 5186–5192. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent Developments in Nanostructured Anode Materials for Rechargeable Lithium-Ion Batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Tian, X.; Liu, Z. Coal-Based Semicoke-Derived Carbon Anode Materials with Tunable Microcrystalline Structure for Fast Lithium-Ion Storage. Nanomaterials 2022, 12, 4067. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, J.H.; Lee, Y.-S.; Bai, B.C.; Im, J.S. Effect of Crystallinity and Particle Size on Coke-Based Anode for Lithium Ion Batteries. Carbon Lett. 2021, 31, 911–920. [Google Scholar] [CrossRef]
- Fu, L.; Yang, L.; Shi, Y.; Wang, B.; Wu, Y. Synthesis of carbon coated nanoporous microcomposite and its rate capability for lithium ion battery. Microporous Mesoporous Mater. 2009, 117, 515–518. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef]
- Guo, Y.; Qi, J.; Jiang, Y.; Yang, S.; Wang, Z.; Xu, H. Performance of electrical double layer capacitors with porous carbons derived from rice husk. Mater. Chem. Phys. 2003, 80, 704–7030. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, J.; Zhang, H.; Yang, S.; Qi, J.; Wang, Z.; Xu, H. Use of rice husk-based porous carbon for adsorption of Rhodamine B from aqueous solutions. Dye. Pigment. 2005, 66, 123–128. [Google Scholar] [CrossRef]
- Vinayagam, M.; Babu, R.S.; Sivasamy, A.; de Barros, A.L.F. Biomass-derived porous activated carbon nanofibers from Sapindus trifoliatus nut shells for high-performance symmetric supercapacitor applications. Carbon Lett. 2021, 31, 1133–1143. [Google Scholar] [CrossRef]
- Vinayagam, M.; Babu, R.S.; Sivasamy, A.; de Barros, A.L.F. Biomass-derived porous activated carbon from Syzygium cumini fruit shells and Chrysopogon zizanioides roots for high-energy density symmetric supercapacitors. Biomass Bioenergy 2020, 143, 105838. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Jiang, D.; Huang, X. Mesoporous activated carbon decorated with MnO as anode materials for lithium ion batteries. J. Mater. Sci. 2015, 51, 3536–3544. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, S.; Zhang, D.; Xu, S. Synthesis and properties of magnetic Fe3O4-activated carbon nanocomposite particles for dye removal. Mater. Lett. 2008, 62, 645–647. [Google Scholar] [CrossRef]
- Hao, M.; Xiao, N.; Wang, Y.; Li, H.; Zhou, Y.; Liu, C.; Qiu, J. Pitch-derived N-doped porous carbon nanosheets with expanded interlayer distance as high-performance sodium-ion battery. Fuel Process. Technol. 2018, 177, 328–333. [Google Scholar] [CrossRef]
- Zhao, X.J.; Jia, W.; Wu, X.Y.; Lv, Y.; Qiu, J.; Guo, J.; Wang, X.; Jia, D.; Yan, J.; Wu, D. Ultrafine MoO3 anchored in coal-based carbon nanofibers as anode for advanced lithium-ion batteries. Carbon 2020, 156, 445–452. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Lin, C.; Yang, W.; Meng, Y.; Guo, Y.; Li, M.; Xiao, D. Hierarchically porous nitrogen-rich carbon derived from wheat straw as an ultra-high-rate anode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 9684–9690. [Google Scholar] [CrossRef]
- Linghu, R.; Liu, Y.; Zhang, Y.; Zhang, Y.; Gao, J.; Zhong, Y. Characterization of Delayed Coke and Fluid Coke Gasification Using Blast Furnace Slag as a Disposable Catalyst. Energy Fuels 2019, 33, 6734–6741. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.-J. The Chemical and Structural Analysis of Graphene Oxide with Different Degrees of Oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Le, T.H.; Ngo, V.H.; Nguyen, M.T.; Nguyen, V.C.; Vu, D.N.; Pham, T.D.; Tran, D.T. Enhanced Electrochemical Performance of Porous Carbon Derived from Cornstalks for Supercapacitor Applications. J. Electron. Mater. 2021, 50, 6854–6861. [Google Scholar] [CrossRef]
- Liu, T.; Wang, K.; Chen, Y.; Zhao, S.; Han, Y. Dominant Role of Wettability in Improving the Specific Capacitance. Green Energy Environ. 2019, 4, 171–179. [Google Scholar] [CrossRef]
- Xiao, H.; Yao, S.; Liu, H.; Qu, F.; Zhang, X.; Wu, X. NiO Nanosheet Assembles for Supercapacitor Electrode Materials. Prog. Nat. Sci. Mater. Int. 2016, 26, 271–275. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, L.; Sun, K.; Jiang, J.; Zhang, X. Preparation of Activated Carbon from Waste Camellia Oleifera Shell for Supercapacitor Application. J. Solid State Electrochem. 2012, 16, 2179–2186. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Manaf, S.A.B.A.; Kumar, A.; Chong, K.F.; Hegde, G. High Performance Supercapacitor Using Catalysis Free Porous Carbon Nanoparticles. J. Phys. D Appl. Phys. 2014, 47, 495307. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Tang, Y. A Novel Zinc-Ion Hybrid Supercapacitor for Long-Life and Low-Cost Energy Storage Applications. Energy Storage Mater. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Zhang, T.; Liu, Z. Nitrogen-Doped Graphene for Supercapacitor with Long-Term Electrochemical Stability. Energy 2014, 70, 612–617. [Google Scholar] [CrossRef]
- Gao, F.; Qu, J.Y.; Zhao, Z.B.; Quan, Z.; Beibei, L.; Jieshan, Q. A green strategy for the synthesis of graphene supported Mn3O4 nanocomposites from graphitized coal and their supercapacitor application. Carbon 2014, 80, 640–650. [Google Scholar] [CrossRef]
- Qiu, J.S.; Li, Y.F.; Wang, Y.P.; Wang, T.; Zhao, Z.; Zhou, Y.; Li, F.; Cheng, H. High-purity single-wall carbon nanotubes synthesized from coal by arc discharge. Carbon 2003, 41, 2159–2217. [Google Scholar] [CrossRef]


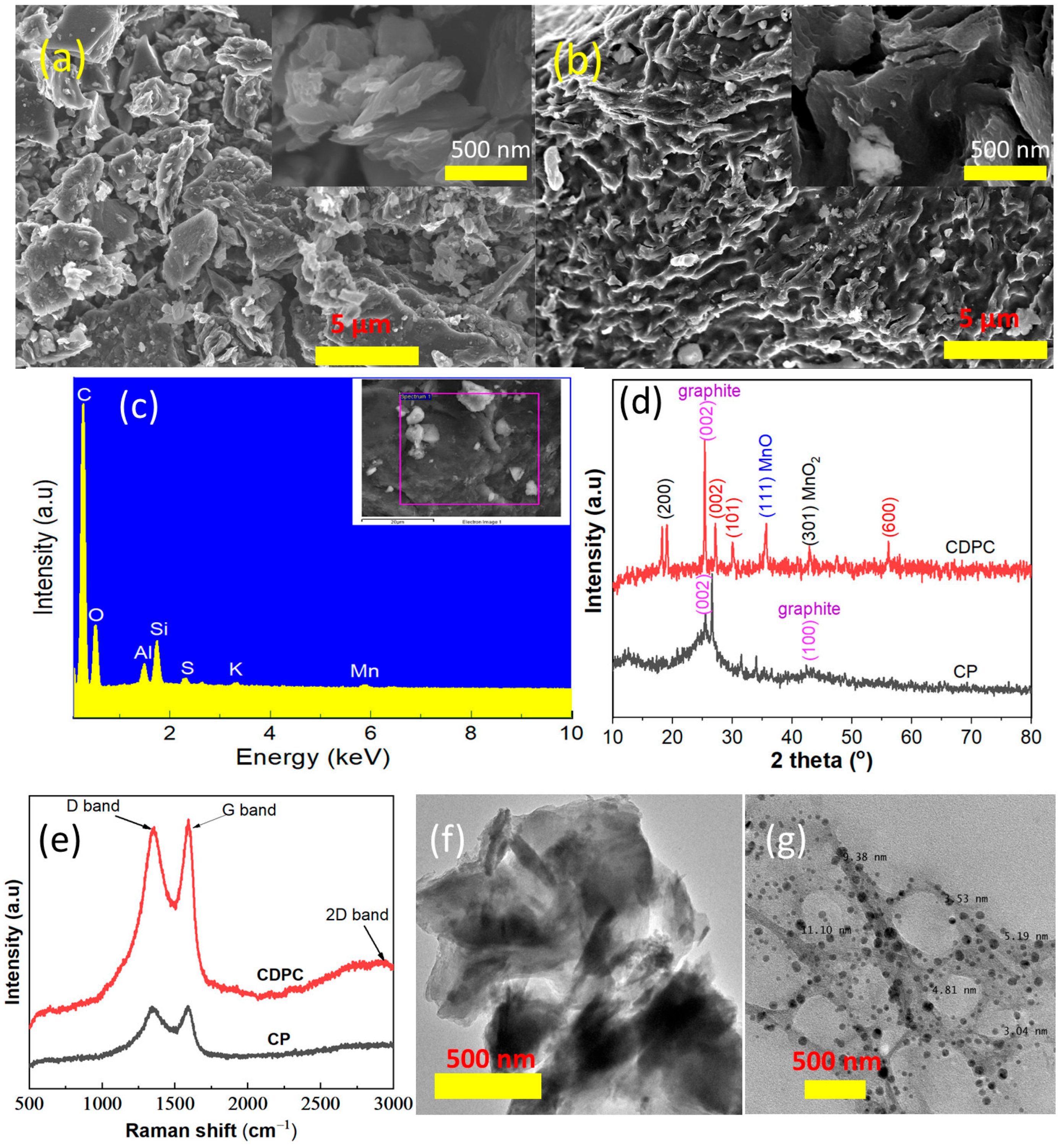

| Element | % Mass | % Atom |
|---|---|---|
| C | 80.88 | 85.53 |
| O | 17.18 | 13.64 |
| Al | 0.41 | 0.19 |
| Si | 0.87 | 0.39 |
| S | 0.51 | 0.20 |
| Mn | 0.15 | 003 |
| Total | 100.00 | 100.00 |
| Materials | Electrolyte | Charge/Discharge Current Density (A/g) | Specific Capacitance (F/g) | Reference |
|---|---|---|---|---|
| Nanoporous gold | [EMIM]BF4 | 0.14 | 79.1 | [34] |
| NiO nanosheet assembles | 3 M KOH | 0.5 | 81.67 | [35] |
| Active carbon (COS) | 1 M H2SO4 6 M KOH | 0.2 0.2 | 374 266 | [36] |
| Porous carbon nanoparticles (PCNs) | 6 M KOH | 0.06 | 309 | [37] |
| Zn-HSC | 1M Zn(CF3SO3)2 | 0.1 | 170 | [38] |
| Nitrogen-doped graphene | 6 M KOH | 0.5 5 | 197 151 | [39] |
| Graphene nanocapsules (from coal tar pitch using a nano-ZnO-template strategy coupled with in situ KOH activation) (GNCs) | 6 M KOH | 0.05 | 277 | [40] |
| Coal-derived porous carbon fibers (CPCFs) | 6 M KOH | 1 | 170 | [41] |
| CDPC | 6 M KOH | 0.1 0.3 | 236 156 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.H.; Nguyen, V.T.; Nguyen, T.N.; Ha, X.L.; Nguyen, Q.D.; Tran, T.K.N. Synthesis of Porous Carbon Nanomaterials from Vietnamese Coal: Fabrication and Energy Storage Investigations. Appl. Sci. 2024, 14, 965. https://doi.org/10.3390/app14030965
Do TH, Nguyen VT, Nguyen TN, Ha XL, Nguyen QD, Tran TKN. Synthesis of Porous Carbon Nanomaterials from Vietnamese Coal: Fabrication and Energy Storage Investigations. Applied Sciences. 2024; 14(3):965. https://doi.org/10.3390/app14030965
Chicago/Turabian StyleDo, Tra Huong, Van Tu Nguyen, Thi Nga Nguyen, Xuan Linh Ha, Quoc Dung Nguyen, and Thi Kim Ngan Tran. 2024. "Synthesis of Porous Carbon Nanomaterials from Vietnamese Coal: Fabrication and Energy Storage Investigations" Applied Sciences 14, no. 3: 965. https://doi.org/10.3390/app14030965






