Abstract
The high-intensity partial pressure of CO2 (pCO2) in karst underground rivers rapidly releases in open-flow sections. This is an important process affecting the global karst carbon cycle. This study focuses on the diurnal variation patterns and driving factors of CO2 exchange flux at the water–air interface in the open-flow sections of typical karst underground rivers in southwestern China. The inorganic carbon in water and water–air interface exchange fluxes are observed. Three representative survey stations, i.e., the outlet of the underground river (Q1), the river sections without submerged plants (H1), and the river sections with submerged plants (H2), are selected to study the CO2 exchange process and its influencing factors. The results show that the CO2 release flux at Q1 exhibits high pressure in the daytime and low pressure in the nighttime, while H1 and H2 exhibit the opposite pattern. The photosynthesis of submerged plants significantly inhibits the carbon release flux of the river, and in the river sections where submerged plants are distributed, their biological effects have inhibited approximately 0.131 Tg C/yr of carbon emissions. This study emphasizes the significant contribution of submerged plants in restraining the release of CO2, thereby promoting carbon sequestration and storage in karst water systems.
1. Introduction
Global carbon cycle research is crucial for understanding the fundamental mechanisms underpinning climate change. According to the current global carbon cycle model, there are carbon missing sinks ranging from 8 to 12 × 108 t C/a on land [1]. Therefore, reducing the uncertainty of carbon cycle is necessary to predict variations of atmospheric CO2 concentration accurately in the future. Karst carbon sinks play a significant role in identifying the missing carbon sinks worldwide [2]. Many studies on karst carbon sinks estimated that the karst carbon sink range from 2.2 to 6.08 × 108 t C/a worldwide [3,4]. With the changes in the global climate, the karst carbon sinks are anticipated to have greater significance in missing carbon calculation and global carbon cycle understanding [5]. Therefore, it is crucial to undertake an in-depth research on karst carbon sinks and estimate their flux and exchange trends precisely.
Karst carbon sinks have been recognized as a significant course for slowing climate change [6]. Karstification can increase the sink capacity of ecosystems and decrease the release capacity to the atmosphere [7,8]. However, the karst carbon cycle is complex, as it is sensitive to environmental factors. For instance, global warming may alter the hydrological cycle in karst regions, which inevitably affects the karst carbon cycle by influencing the carbonate rock weathering pace [9,10,11] and affecting the CO2 exchange at the water–air interface [12,13]. Under global climate change and ecological restoration, the karst carbon cycle is becoming more and more impacted by climate parameters such as precipitation, evapotranspiration, runoff, and temperature [14,15]. As reported by Zeng et al. [16], the karst carbon cycle is reacting to global climate change in a sensitive and quick manner.
Although inland water bodies only account for 1% of the Earth’s surface area, they play an important role in the carbon cycles of various ecosystems [17]. The water–rock interactions can change the chemical compositions and biological activities of water bodies. The chemical weathering and organic decomposition of underground rivers are two vital processes that contribute to the carbon formation [17,18,19]. The partial pressure of CO2 (pCO2) in underground river water is always more than 110 ppm [20]. The karst underground rivers in southwestern China alone total 1620.57 billion m3/a [21], which is equal to 16% of the Yangtze River’s annual runoff, and it is 2.8 times the Yellow River’s annual runoff. The underground river water in karst regions has a pCO2 above 680 ppm and peak reach up to 6166 ppm [22]. However, the degassed carbon flux of underground rivers has been found, in certain studies, to be equal to the net carbon accumulation on the continent (2.2 Pg C/yr) [23,24,25,26]. Research on the karst carbon cycle has focused on the open-flow sections of karst underground river water, where aquatic organisms absorb or release high-intensity pCO2 at a very rapid pace. Rapid CO2 release or biological absorption has a major influence on the carbon exchange process, particularly for karst groundwater habitats and ecosystems [2,5,27]. Thus, evaluating the resilience of karst carbon sinks requires investigating the CO2 exchange process at the water–air interfaces in the open-flow portions of karst underground rivers.
The current state of the karst carbon cycle calculation is hindered by numerous ambiguities regarding the migration and transformation processes of carbon in the open-flow sections of karst underground rivers. The research subject for this paper is a typical karst underground river basin in southwest China. The diurnal variation patterns and driving factors of CO2 respiration fluxes at the water–air interfaces in karst areas are evaluated by monitoring the carbon in water and water–air interface exchange fluxes at the outlets of underground rivers, river sections without submerged plants in underground rivers, and river sections with submerged plants in karst areas. Additionally, by using the end element method, it is possible to increase the accuracy of river CO2 flux calculations and strengthen the research on karst carbon sinks by examining the proportion of endogenous organic carbon in water bodies that contributes to organic carbon conversion, investigating the carbon in water conversion processes, and further elucidating the factors that control diurnal changes in carbon release in open-flow sections.
2. Materials and Methods
2.1. Study Area
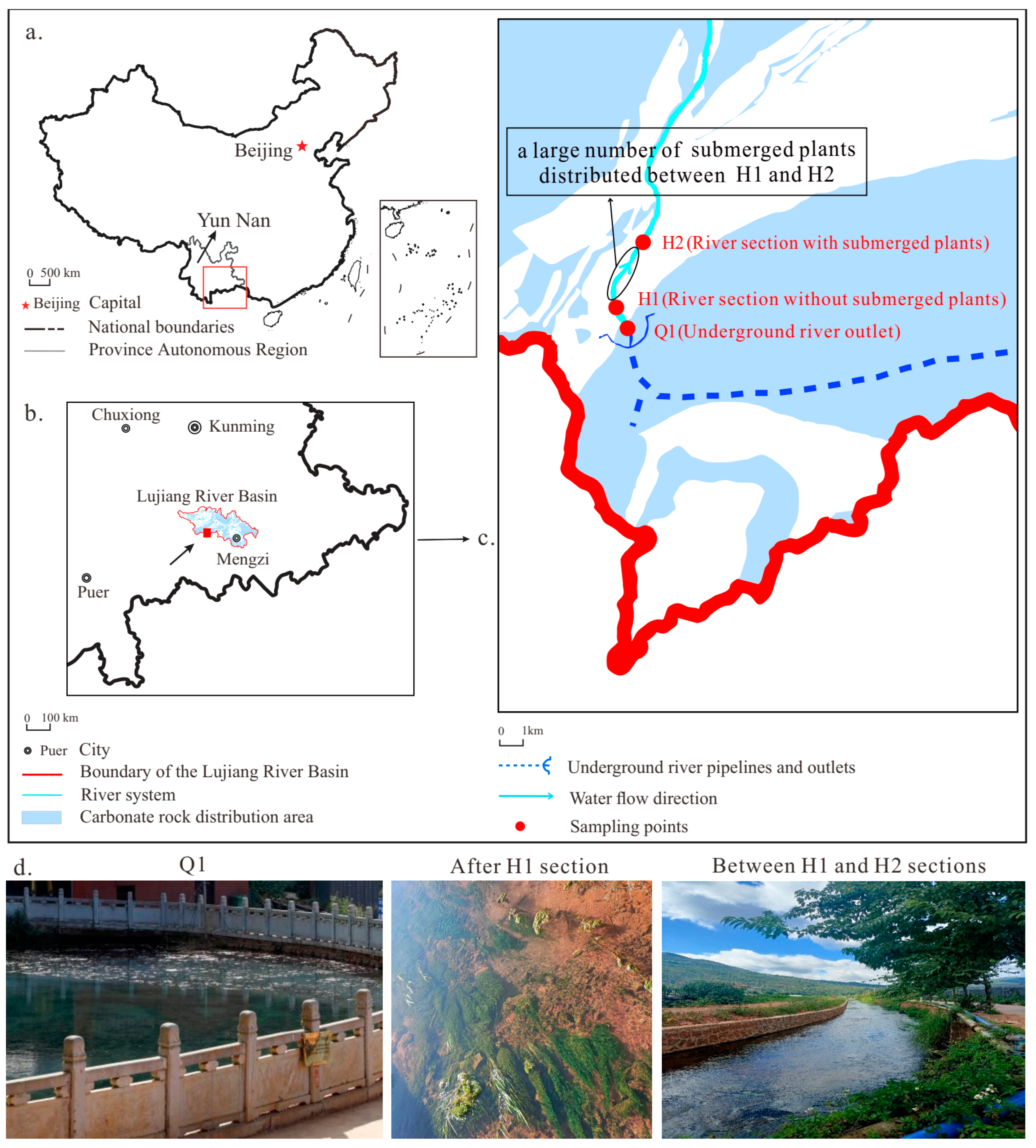
The Heilongtan Underground River is a typical karst underground river in Jianshui County, in the Yunnan–Guizhou Plateau southeast China (E102.84°~E102.85°, N23.47°~N23.49°) (Figure 1a). The Xiangchong River, a tributary of the Pear River of China (Figure 1b), originates from this location. The underground river empties into a 20 m tall sheer rock that emerges in the vicinity of Baixiang Mountain on Laogou Street. The annual rainfall in this basin region ranges from 800 to 900 mm. The underground river flows through the karst area and has an abundance of water resources. Water-bearing rocks mainly consist of limestone and dolomite. Underground rivers have a documented dry season flow of 425.06 L/s with a notable increase in flow during the rainy season. The study area receives around 2200 h of sunlight each year, providing sufficient energy for the growth of submerged plants. The dissolved water generally flows from east to west due to the blockage of crust rock in the shape of a bush on the old street, exiting into a fountain, and subsequently forming the surface of the river (Figure 1c). The dominant submerged plant species in this river, between H1 and H2, are Potamogeton crispus and Hydrilla verticillata. Both banks of the river are solid and stable, and the study region has consistent weather conditions. During the study period, the Trophic Level Index (TLI (Σ)) was monitored to be less than 30, which means a low level of eutrophication. The average water flow velocity was 0.15 m/s. Research has shown that the growth of algae is limited when the water flow rate exceeds 0.10 m/s, leading to a sharp decrease in cell abundance [28]. Therefore, primary photosynthesis is carried out by submerged plants from the H1 to H2 sections (Figure 1c,d).
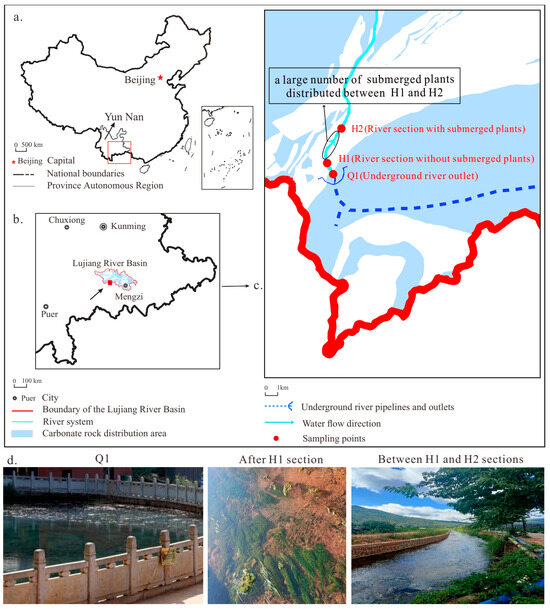
Figure 1.
Study area location and overview of the sampling points. (a) The relative position of the Lujiang River Basin, (b) the relative position of Heilongtan, (c) monitoring points, (d) specific situation of each monitoring point.
In this study area, the rainy season typically lasts from May to October, and the weather in 2023 is comparatively dry. The rainy season is expected to shift to mid-June. The average water depth during the observation period was 21 cm. The Xiangchong River is a natural waterway with fine sediment, and it serves as an irrigation source for around 20,000 acres in the region of Nanzhuang Town in Jianshui County. The Heilongtan Underground River is mainly used for irrigation and drinking water for people and livestock in the area. It is a significant water source, accounting for one-third of the primary water sources for Jianshui city.
2.2. Sample Collection and Processing
At the monitoring sites, there are three river sections: the outlet of the underground river (Q1), the sections without submerged plants (H1), and the sections with submerged plants (H2) (Figure 1d). Cloud coverage can have a significant impact on many environmental variables, including temperature and solar radiation [29]. Therefore, this study was conducted in favorable weather conditions, a gentle climate, consistent water flow, and pre-rainy season when water quality is stable. The study involved a three-day and two-night monitoring period from May 24 to 26, 2023, to observe the water’s physical and chemical indicators and continuously measure CO2 gas levels at the water–air interface. At each of the three sites (Q1, H1, and H2), a multiparameter meter (Ultrameter-II (6P), Myron L Company, Hilliard, OH, USA) was used to monitor the T (water temperature), EC (electrical conductivity), Ta (air temperature), and DO (dissolved oxygen) concentration of the river water at 4-h intervals. The specific EC, temperature, pH, and DO were 0.1 °C, 0.01 mg/L, and 1 μs/cm, respectively. River water samples were collected every four hours from these three sites, after which the samples were placed in a 2 L polyethylene bottle. An inductively coupled plasma emission spectrometer (Intrepid II XSP, Thermo Fisher Scientific, Waltham, MA, USA) was used in the laboratory to measure the cations (K+, Na+, Ca2+, and Mg2+) in the water samples after acidification with HNO3− to pH < 2. Additionally, ion chromatography (861 advanced compact IC Metrohm, Herisau, Switzerland) was employed to determine the anion content (F−, Cl−, NO3−, and SO42−). The sulfur content and total alkalinity were measured using the silicon molybdenum yellow colorimetric method [30]. The analysis was conducted at the Kunming General Survey of Natural Resources Center, China Geological Survey.
2.3. Monitoring of CO2 Exchange Flux at the Water-Air Interface
In this study, daily variations in greenhouse gas exchange at the water–air interface were measured using a static chamber method. Gas samples were collected every four hours using a flux box made of acrylic board, and a small fan was employed to ensure uniform air mixing inside the box [31]. To isolate the air inside the container from the external environment, the container was set on the water’s surface with a floating ring positioned above it [32]. Gas samples were extracted using a syringe and then injected into an aluminum foil storage bag. In the laboratory, gas chromatography was utilized to analyze the CO2 content with a TDX-01 column used to separate the CO2 before it was transformed by a methane converter and finally detected using an FID detector.
2.4. CO2 Exchange Flux Calculation
By the following formula [33], the CO2 exchange flux at the water–gas interface can be determined by monitoring the rate of change in the gas sample concentration:
where F is the gas exchange flux (mg·(m2·h)−1), F1 is the unit conversion factor of ppm and μg·m−3, F2 is the conversion coefficient between minutes and days, F3 is the unit conversion factor between μg and mg, V is the volume of air in the buoyancy chamber (m3), and A is the surface area of the floating tank above the water (m2). The linear slope (106·min−1) of the greenhouse gas concentration over time during the observation period is represented by the value of Δc/Δt. F > 0 denotes the release of greenhouse gases into the atmosphere from bodies of water, such as CO2, whereas F < 0 denotes absorption.
2.5. Data Analysis
The measured data in this investigation were processed and computed using Excel 2013. MAPGIS 67 software was used to construct a map of the study area. Phreeqc 3.7.3 software was used to calculate the pCO2 in river water and the SIc (saturation index of calcite) using a hydrochemical dataset, including pH; water temperature; and concentrations of K+, Na+, Ca2+, Mg2+, Cl−, SO42−, and HCO3−. The remaining data were drawn and analyzed by Origin 2022 and SPSS 25.
3. Results and Analysis
3.1. Characteristics of Hydrochemical Parameters
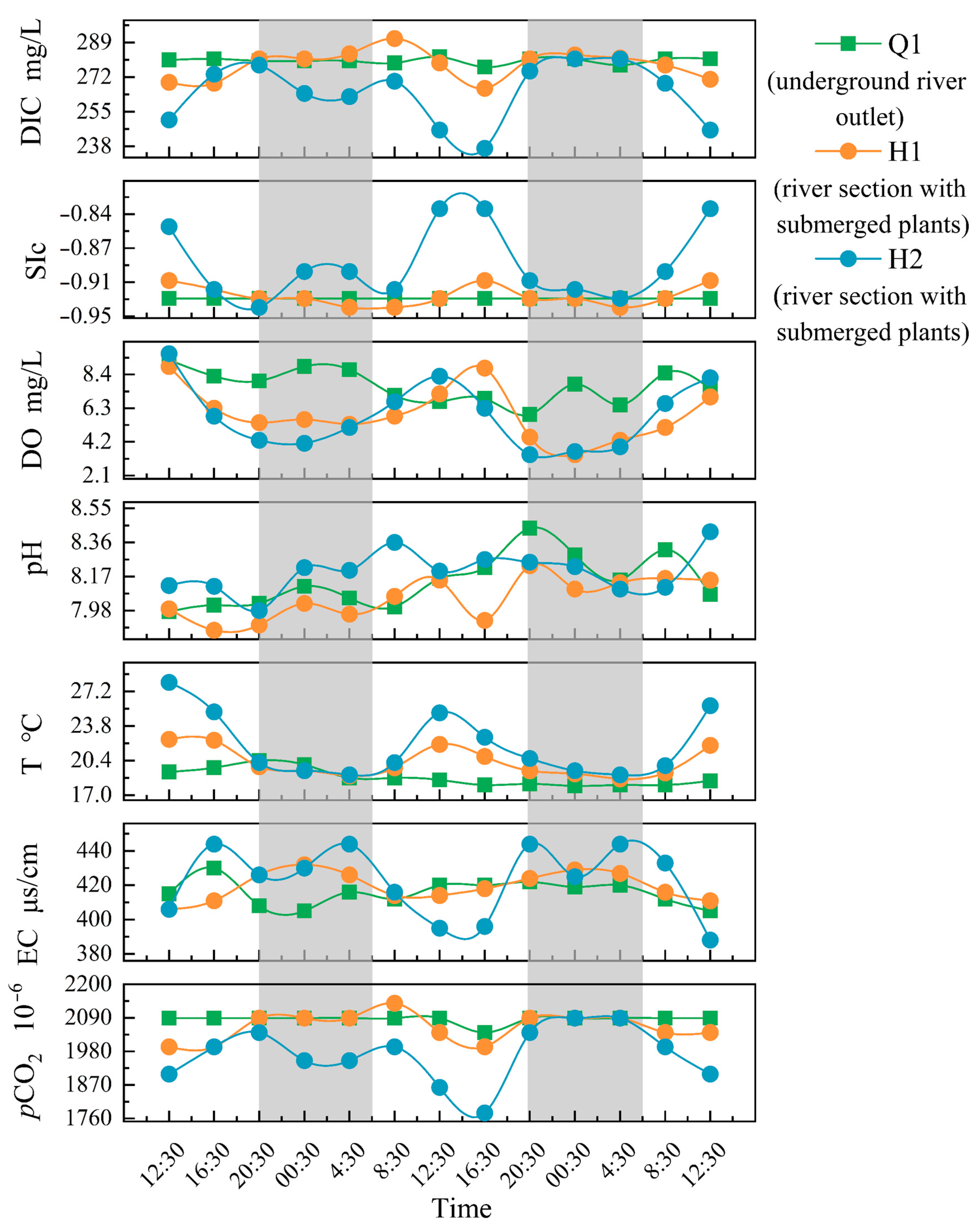
The hydrochemical parameters (DIC, SIc, DO, pH, T, EC, pCO2, and Ta) of the Q1, H1, and H2 varied throughout the monitoring period (Figure 2). Comparing H1 and H2 to Q1, it is clear that the former stations have larger daily variations. At Q1, there was no notable diurnal fluctuations, with only minor changes in DO and pH, and other metrics showing small variations. The diurnal variation pattern of DO at Q1 was low during the day and high at night, reaching its peak in the early morning and its lowest point in the late afternoon. In contrast to the variations in SIc, pH, DO, and water temperature, the diurnal variation patterns of DIC, EC, and pCO2 at H1 and H2 were low during the day and high at night with the highest and lowest values of DIC, SIc, DO, T, and pCO2 l occurring nearly simultaneously. At H1, the DIC, EC, and pCO2 decreased during the day, peaked in the afternoon, and increased in the evening. Before sunrise, the highest values of DIC and pCO2 were observed, and the maximum value of EC was observed prior to early morning. On the other hand, SIc, DO, and T increased during the day, peaked at midday, and decreased during the night, reaching their lowest values in the early morning.
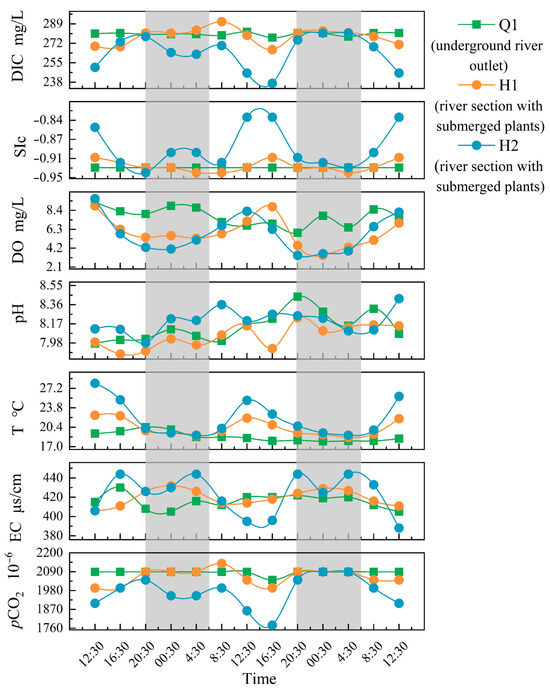
Figure 2.
The diurnal variation of parameters (note: The gray portion represents the period of night during the sampling period in the study area, while the white portion represents the period of daytime).
3.2. DIC Species
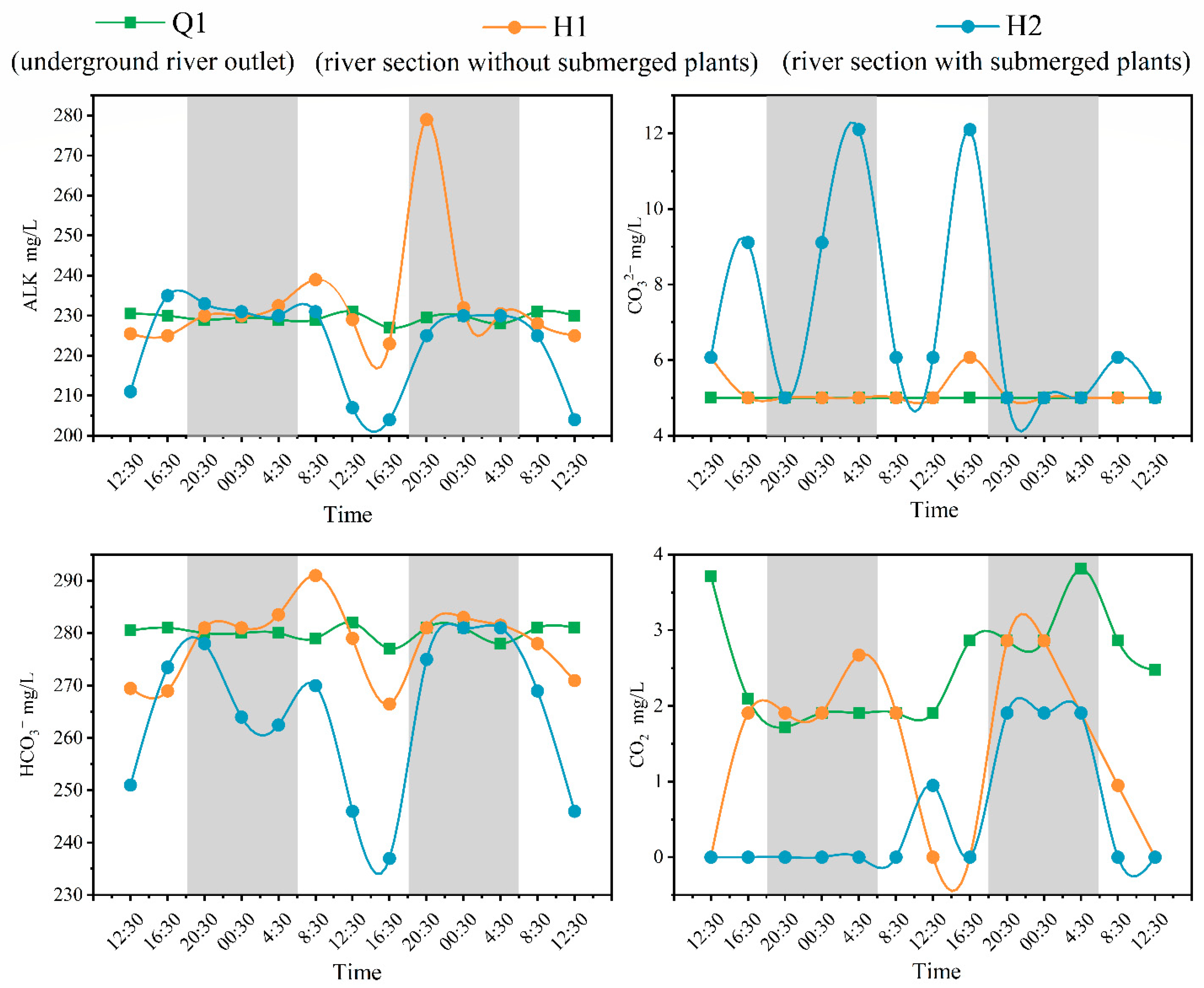
Dissolved inorganic carbon (DIC) is the most important component of carbon in water, mainly including HCO3−, CO32−, and dissolved CO2, in which HCO3− is the main component of DIC [34]. The average value of HCO3− at Q1 was 280, with a small change in amplitude, similar to the trend of DIC alkalinity (Figure 2 and Figure 3). The average values of HCO3− at H1 and H2 were 278 and 264, respectively, showing a trend of low at daytime and high at nighttime, similar to the trend of DIC alkalinity (Figure 2 and Figure 3). The concentrations of CO32− at Q1 were below 5; changes can be ignored below the method’s detection limit. Compared with the CO32− concentration change at H1, there was a significant change in H2, and the CO32− concentration at H1 only fluctuates slightly during the day. The average dissolved CO2 content at Q1 was 2.53, showing significant fluctuations compared to other DIC composition parameters in Q1. The concentration of CO32− at H2 was mostly less than 0.03 during most time periods, and only a few time periods showed fluctuations below the method’s detection limit.
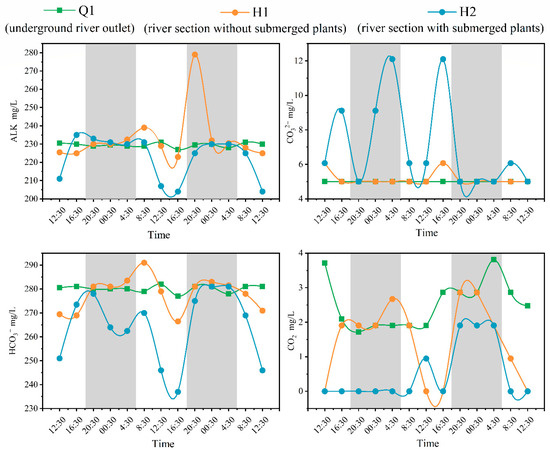
Figure 3.
Diurnal variation of DIC components in water bodies (note: The gray portion represents the period of night during the sampling period in the study area, while the white portion represents the period of daytime).
3.3. CO2 Fluxes
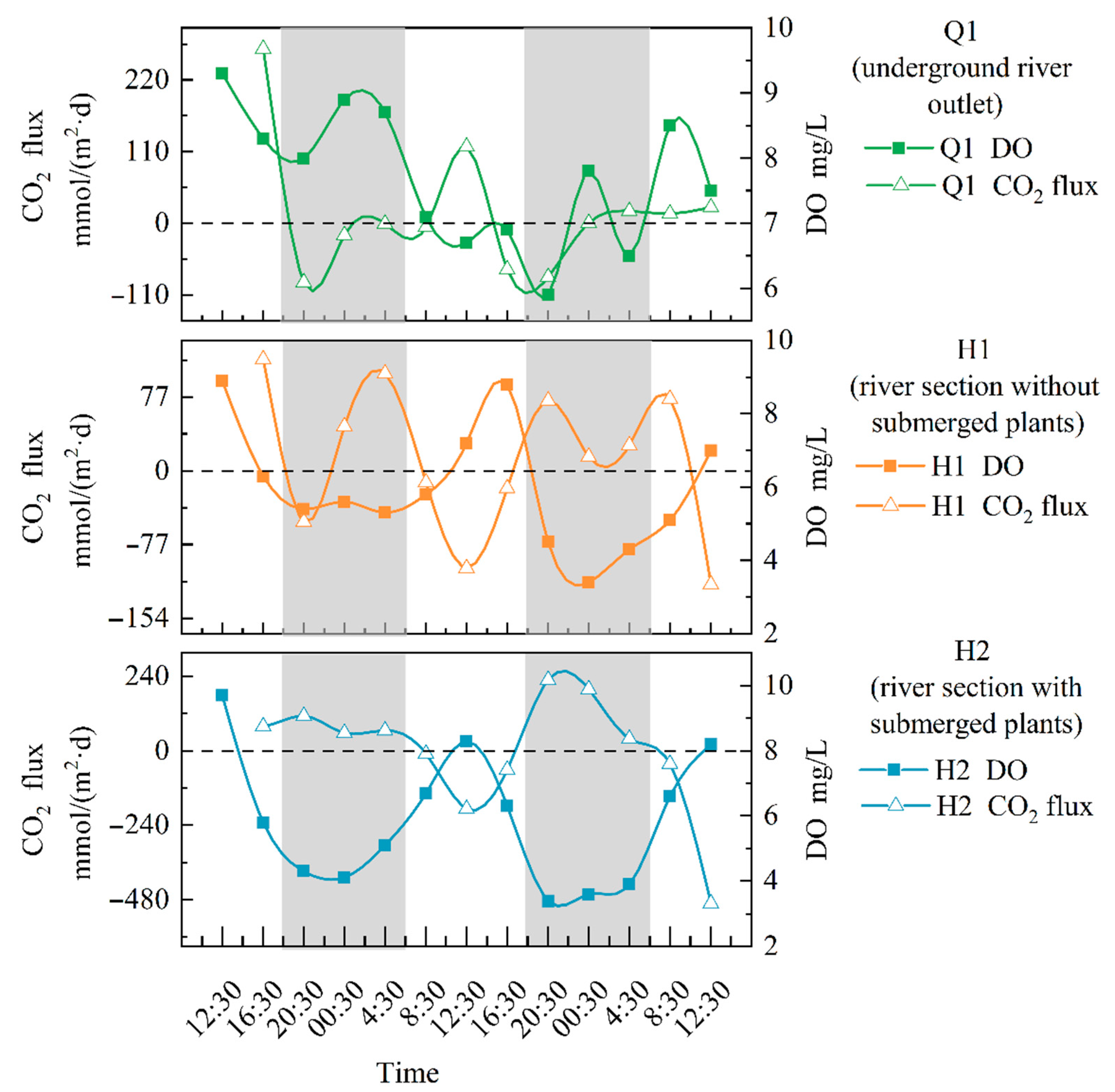
There was a significant difference (p < 0.01) between the average CO2 exchange flux and the DO concentration at H1 and H2, as shown in Figure 4. The CO2 exchange flux at H1 ranged from −118.49 to 116.53 mmol/(m2·d), with fluctuations that caused it to decrease during the day and increase at night. Throughout the entire sampling period, the average CO2 exchange flux at H1 was 12.62 mmol/(m2·d), indicating that this section may be a major gas source. The trend of the change in CO2 exchange flux at Q1 did not show significant fluctuations. The carbon flux calculated during most sampling time periods was positive, and the CO2 exchange flux at H2 ranged from −490.83 to 228.34 mmol/(m2·d). The maximum flux appeared in the late afternoon, while the minimum flux was observed in the morning, which is exactly the opposite of the DO. Throughout the sampling period, the average CO2 exchange flux at H2 was −0.68 mmol/(m2·d), indicating that the section may act as a sink for atmospheric CO2.
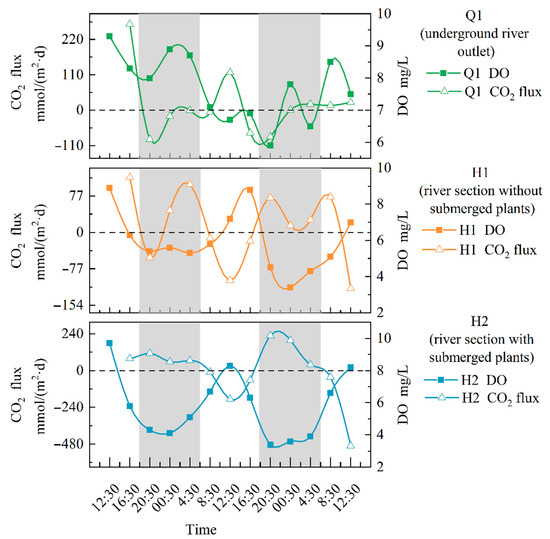
Figure 4.
Changes in CO2 flux at different points in the open-flow section (note: The gray portion represents the period of night during the sampling period in the study area, while the white portion represents the period of daytime).
4. Discussion
4.1. The Influence of Factors on CO2 Exchange Flux
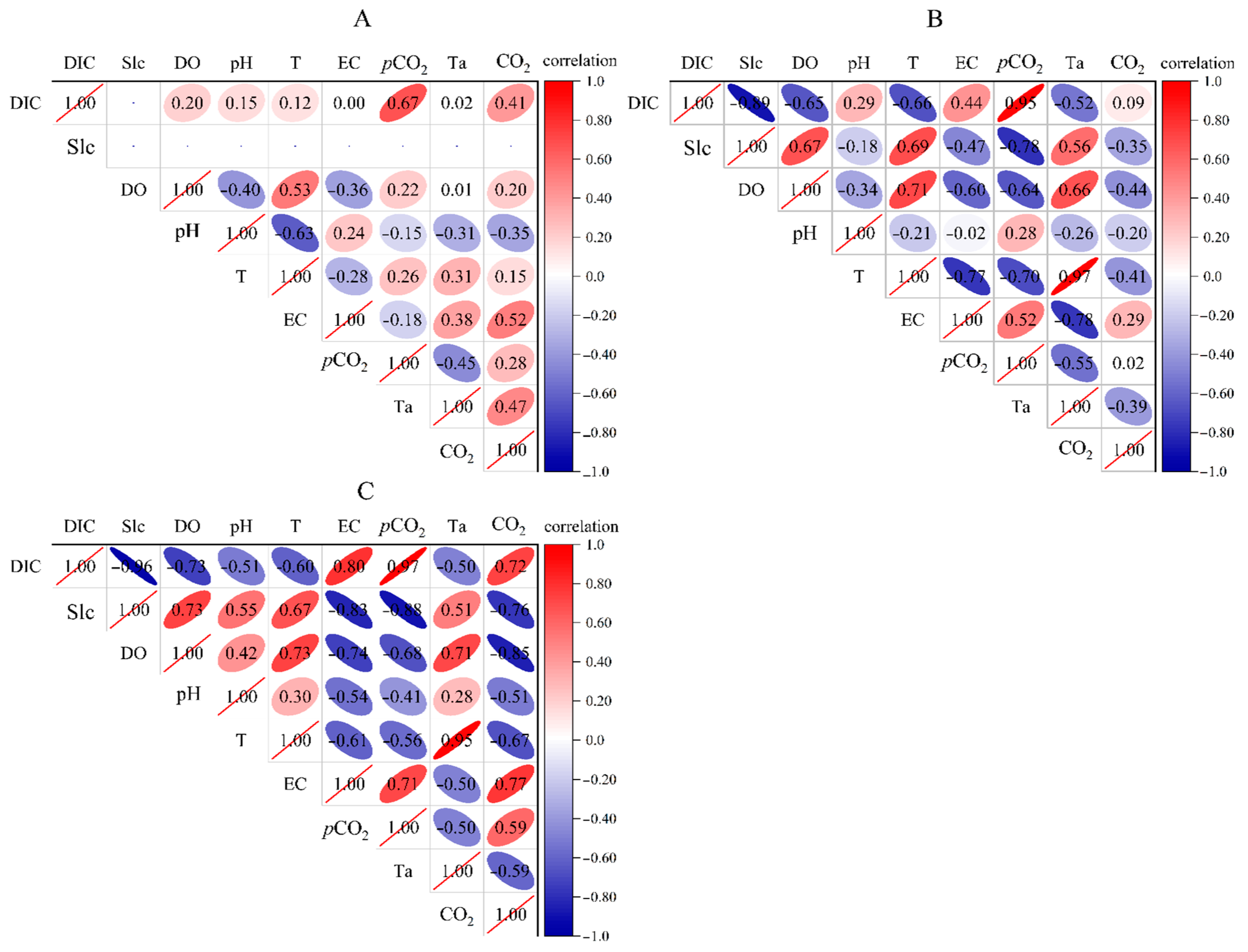
Temperature variations throughout the daytime have an impact on the CO2 flux [13,19]. At Q1, there is a weak correlation between most parameters with fewer submerged plants and low water flow. The correlation between CO2 flux and DIC (r = 0.41, p = 0.19), conductivity (r = 0.52, p = 0.08), and temperature (r = 0.47, p = 0.12) is significant and positively correlated. However, the correlation between CO2 flux and temperature is negative at H1 (r = −0.39, p = 0.21) and H2 (r = −0.59, p = 0.04). At Q1, the correlation between air temperature and water temperature is small, while at the river section, there is a strong correlation between air temperature and water temperature. Submerged plants thrive between H1 and H2. The correlation coefficient distribution map (Figure 5) shows that the correlation between various parameters and CO2 flux is more significant for H2 than for H1, indicating that river submerged plants are closely involved in the process of river carbon absorption and release as well as the alteration of water chemistry and physical indicators.
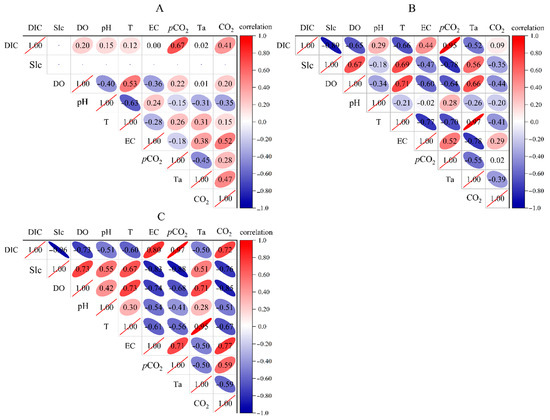
Figure 5.
Correlation of parameters at different points in the open-flow section (note: 1. (A–C) represent the distribution of correlation coefficients between various parameters at the underground river outlet (Q1), river section without submerged plants (H1), and river section with submerged plants (H2), respectively. 2. The shadow depth in the upper triangle area represents the correlation strength between different elements with red indicating a positive correlation between elements and blue indicating a negative correlation between elements. The darker the color is, the higher the saturation and the more significant the element correlation).
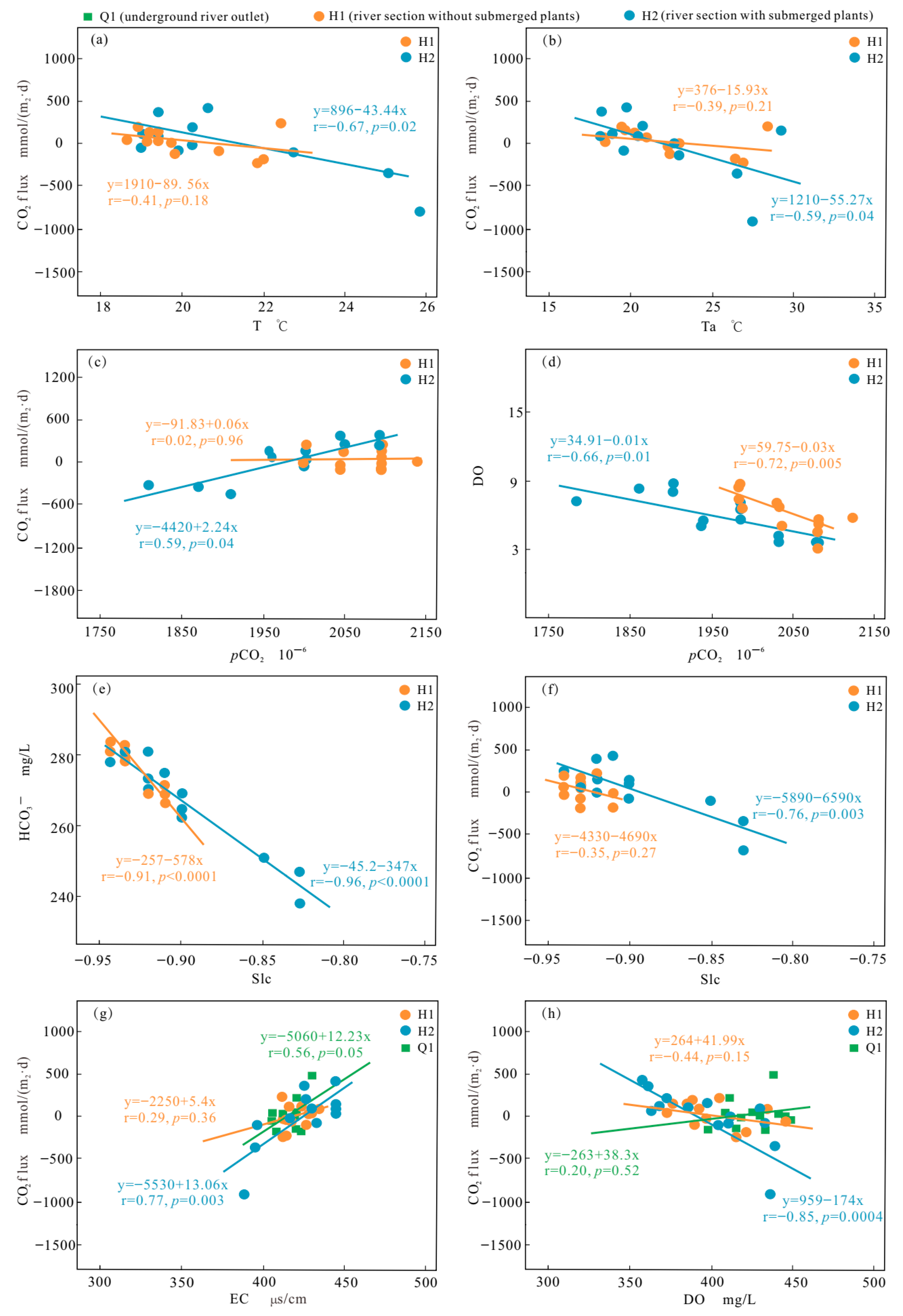
The solubility of CO2 in water influences the amount of CO2 released from streams and is negatively correlated to water temperature [33]. The H2 has a higher temperature than the H1, as shown in Figure 2. Theoretically, the CO2 flux will increase as CO2 solubility decreases. However, the water temperature is negatively correlated with the CO2 flux of the H1 (r = −0.41, p = 0.18) and H2 (r = −0.67, p = 0.02) (Figure 6a), which is consistent with the conclusion from the Lijiang River section [34]. In these two sections, water temperature shows a clear trend of higher temperature during the day and lower temperature at night (similar to the trend of temperature change) (Figure 6b), but the amplitude of change is not significant. Therefore, there are some other processes more influential than temperature in controlling CO2 release during river flow.
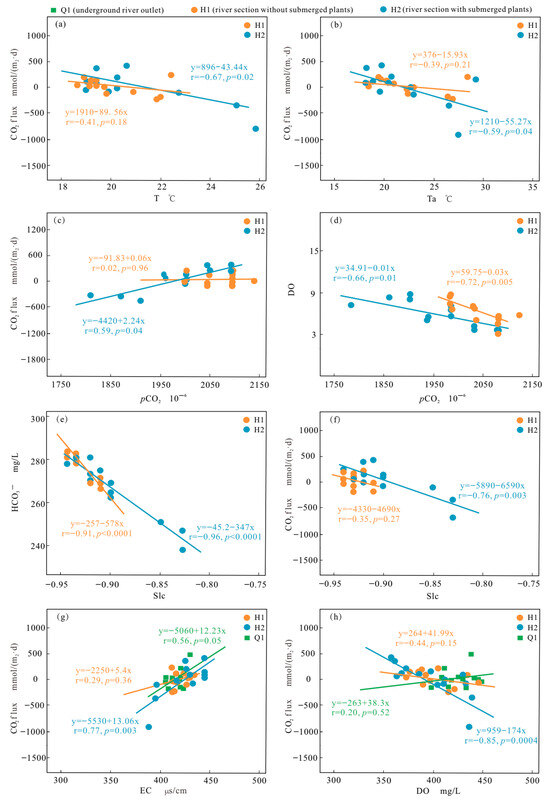
Figure 6.
Response relationship between CO2 flux and hydrochemical parameters (note: (a–h) are the results of the fitted line and correlation analysis).
Changes in DO and CO2 concentrations in river water are directly influenced by the metabolic activities of submerged plants and microbial communities (photosynthesis and respiration), and variations in pCO2 also affect pH levels [35,36]. Changes occurring during the day and night usually lead to periodic diurnal variations in CO2 degassing at the water–air interface [37]. Throughout this study, the relationship between pCO2 and CO2 flux was consistently positive. The study demonstrated an enhanced connection between pCO2 and CO2 flux at all points (Figure 6c), indicating that metabolic processes largely control CO2 release in the Heilongtan River. There was a significant negative correlation between the pCO2 concentration and DO concentration (Figure 6d), which reflects the photosynthesis and respiration of submerged plants. An increase in pCO2 raises the CO2 gradient between the air and water during respiration, leading to increased CO2 degassing [27]. Usually, CO2 in river water bodies is supersaturated, where the pCO2 in rivers is greater than that in the atmosphere [23]. During the day, photosynthesis may exceed respiration, and CO2 consumption may cause CO2 in the atmosphere to dissolve in river water. The diurnal variation in pCO2 at H2 reflects this difference.
The pCO2 concentration in rivers is mainly controlled by soil CO2 input, the respiration of aquatic microorganisms, and organic matter degradation, which increase pCO2, while the breakdown of carbonate rocks and submerged plant photosynthesis consume CO2, reducing pCO2 [38,39,40,41]. At Q1, the water had low fluidity, and the lowest pCO2 value occurred during the day due to the biological action of microorganisms on the water surface. At H2, the minimum pCO2 value during the day was lower than H1, indicating the biological role of submerged plants during water flow.
The influence of carbonate minerals on the concentration of dissolved CO2 can be controlled based on the water SIc value [27,42]. A positive SIc value indicates calcite precipitation, which facilitates CO2 degassing. Conversely, a negative SIc value indicates calcite dissolution, which restricts CO2 escape. During the entire sampling period, the SIc values at all points were <0, and the H1 (r = −0.91, p < 0.001) and H2 (r = −0.96, p < 0.001) and DIC (Figure 6e) showed a substantial negative association, indicating that the dissolution of calcite in the Heilongtan River transformed into HCO3− in the water, limiting the escape of CO2. However, the various indicator factors of calcite dissolution at Q1 are not correlated, indicating that calcite dissolution does not have a significant impact on the CO2 flux changes at the outlet point. There are also other factors that affect the source of HCO3−: for instance, pCO2 (r = 0.68, p = 0.01). Surprisingly, the SIc values of the H1 (r = −0.34, p = 0.27) and H2 (r = −0.76, p < 0.01) show a negative correlation with CO2 flux (Figure 6f), which contradicts our theoretical expectations. This indicates that there are other processes that have greater impacts than precipitation or the dissolution of carbonate rocks.
As the conductivity of the water body continuously changes, the CO2 flux at Q1, H1, and H2 shows different daily variation patterns. The conductivity is proportional to the total dissolved solids. The conductivity change in section H1 began to show the same change as that of Q1 on the second day, while the conductivity of Q1 was positively correlated with the change in CO2 flux (r = 0.56, p = 0.05) (Figure 6g). The changes in solutes affect the metabolic function of microorganisms in water, which may mainly determine the changes in CO2 flux at the H1 section during the flow process. However, during the process from H1 to H2, the dense distribution of submerged plants led to changes in CO2 flux at the H2 section. DO (p < 0.001) is the primary driver of changes in CO2 flux (Figure 6h), while EC (p = 0.003) has a significant positive effect (Figure 6g). CO2 flux exhibits a diurnal pattern and generally decreases with increasing DO concentration. Therefore, DO is an important variable that may regulate variations in CO2 flux.
4.2. Changes in the Sources and Processes of Carbon Components in Rivers
The composition of the basin affects the DIC content of river water, as carbonates and silicates dissolve at different rates [43,44]. In this study, HCO3− at Q1 mainly originates from carbonate rock weathering, as silicate rock weathering has a negligible impact [45]. DIC mainly includes HCO3−, CO32−, and dissolved CO2 [46], with HCO3− accounting for approximately 90% of the DIC composition in water. Carbonate rocks are the main source of river DIC in this study area.
The precipitation of carbonate minerals does not always result in the release of CO2 into the atmosphere, as DIC can be utilized by aquatic photoorganisms to form endogenous organic carbon sinks. Aquatic organisms can transform DIC, POC, DOC, and PIC into each other within the water body [5,26,47]. Photosynthesis increases DO and reduces DIC and HCO3−, while respiration releases CO2, increasing DIC and decreasing nighttime DO. There was a positive correlation between DO and HCO3− at H2 (r = 0.73, p < 0.01). Changes in DIC are related to pH (r = −0.67, p = 0.01) and are not significantly correlated with HCO3− or DO in water. Therefore, changes in DIC are not only related to the photosynthesis of submerged plants but also involve other biological processes.
The organic carbon C/N ratios of the Heilongtan Basin in this study ranged from 6.24 to 10.39 with an average of 8.01. The average organic carbon C/N ratio at the Q1 was 10.39, that at H1 was 7.40, and that at H2 was 6.24. The C/N ratio decreases in the clockwise direction, and the source of TOC is calculated based on the C/N ratio. Previous studies have shown that the C/N ratio of exogenous organic carbon is greater than 15 [48]. According to the typical product of photosynthesis formed by algae, C5.7H9.8O2.3N should have a C/N of 5.7; fungi (C10H17O6N) should have a C/N of 10; and bacteria (C5H7O2N) should have a C/N of 5. Therefore, the C/N ratio of endogenous organic matter should be between 5 and 10. With a C/N ratio of 5.7 as the endogenous endmember and 20.69 as the exogenous endmember [49], the proportion of endogenous organic carbon to total organic carbon can be calculated using a mixed endmember model. The equation is as follows:
where C/N is the C/N ratio of the sample; fau is the endogenous terminal element of the C/N ratio, taken as 5.7; fal is the external terminal element with a C/N ratio, taken as 20.69; and fB is the exogenous organic carbon fraction relative to the total organic carbon.
By combining the concentration values of TOC (POC + DOC) in the Heilongtan Basin, the concentration of endogenous carbon in total organic carbon (TOCau) can be calculated, and the equation is as follows:
In the formula, TOC represents the sample’s total organic carbon content; fB is the endogenous organic carbon fraction relative to the total organic carbon; and TOCau is the percentage of total organic carbon that is endogenous carbon.
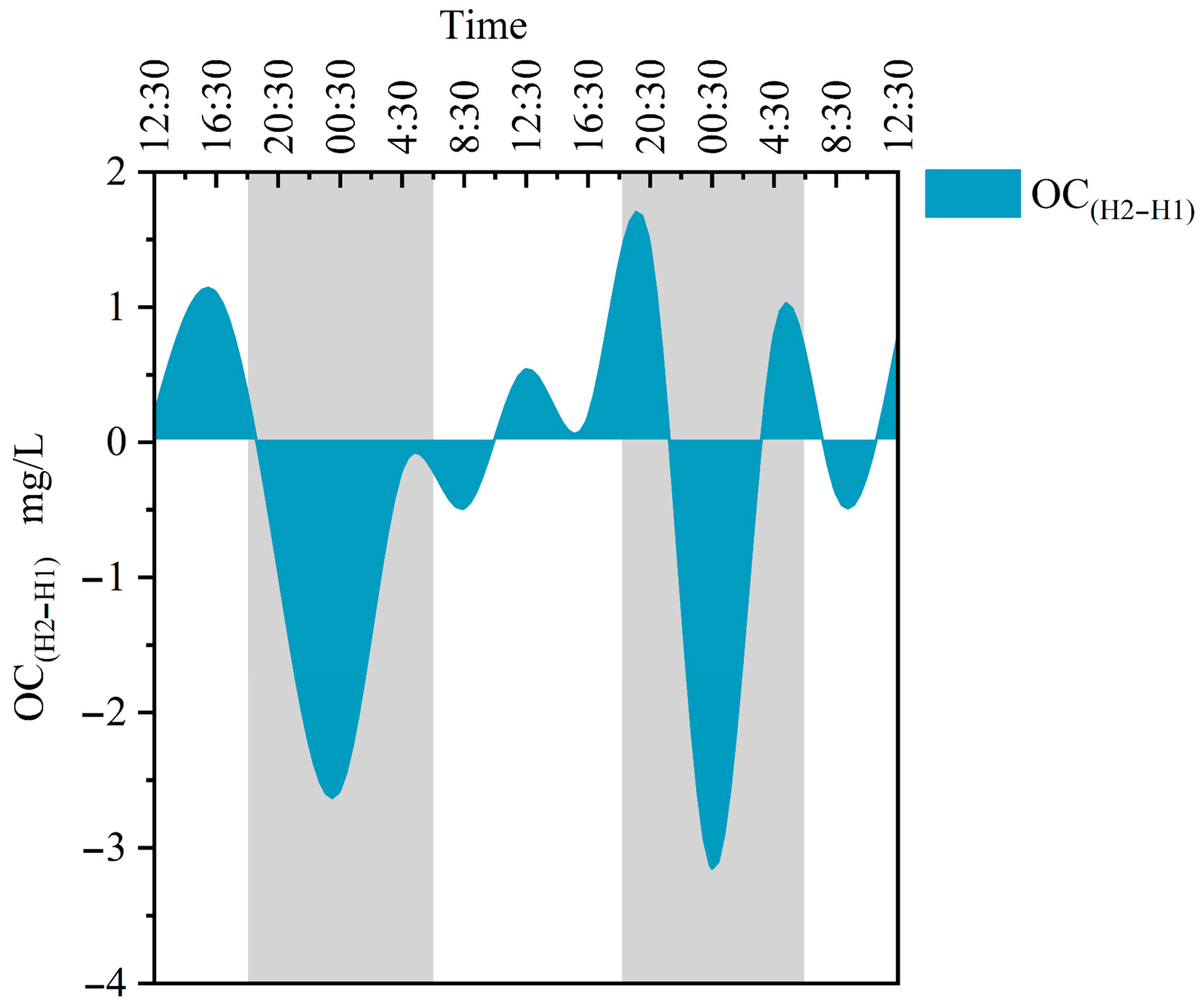
From Q1 to H1 and H2, the C/N ratio gradually drops as the water flows. The contribution ratios of endogenous organic carbon to TOC are 68.71%, 88.66%, and 96.40%, with an average of 84.59%. The contribution of endogenous organic carbon at Q1 is the least, and the water retention time at Q1 is long. Although there is an absence of submerged plants, the contribution of plankton cannot be ignored. Combining the previous CO2 flux analysis, it is clear that at Q1, the correlation between the photosynthesis of phytoplankton and CO2 carbon flux is low, and it may even promote carbon emissions. Moreover, it also indicates that apart from the organic carbon produced by the water’s biological carbon pump effect,, there is also a 31.29% contribution of exogenous organic carbon at Q1. This is mainly due to Q1 being situated at the outlet of groundwater, where tree leaves on the water surface wither and organic carbon leaches from the upstream soil. The contribution of endogenous organic carbon at H2 is 7.74% higher than H1, and it is close to 1. This indicates that the source of TOC at H2 is mainly organic carbon formed by the biological carbon pump effect, which is a net carbon sink. At the same time, the HCO3− content and DIC value in the water at H2 are lower than those shown for Q1 and H1, and there are a large number of dense submerged plants distributed between H1 and H2, proving that submerged plants utilize DIC in water and convert it into organic carbon (OC). However, it is worth noting that the difference between OC(H2–H1) is positive and negative, showing a diurnal trend of positive and negative (Figure 7), indicating that during the day, submerged plants use DIC as a carbon source for photosynthesis to synthesize OC, and this lifetime yield is greater than the OC consumed by respiration. At night, there is no photosynthesis, and non-autotrophic organisms consume OC, resulting in a decrease in water OC. This consumption rate depends on the type of microorganisms, temperature, and size of organic carbon molecules [50].
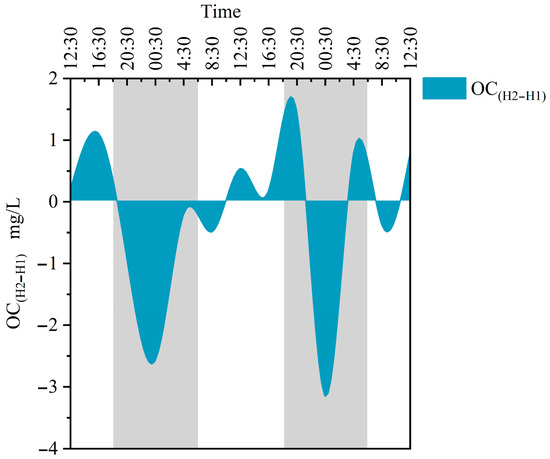
Figure 7.
Differences in organic carbon changes between H2 and H1. H1 represents the river sections without submerged plants, and H2 represents the river sections with submerged plants. The gray portion represents the period of night during the sampling period in the study area, while the white portion represents the daytime period.
The Beijiang River, Xijiang River and Dongjiang River are the three major tributaries of the Pearl River Basin, each with distinct sources of endogenous organic carbon. The organic carbon C/N ratios of Beijiang, Xijiang, and Dongjiang are 14.44, 15.22, and 9.99, respectively [46]. Calculations show that the contribution sources of endogenous organic carbon in the Beijiang and Xijiang Rivers account for 41.69% and 35.49%, respectively, while the Dongjiang River’s contribution is 71.38%. Compared with that in the Pearl River tributary, the contribution of endogenous organic carbon in the Heilongtan River water body was 84.59%, surpassing that of the Dongjiang River, which is largely due to the distribution of submerged plants. The hydraulic conditions of the Heilongtan River in this study area are similar to those of the Dongjiang River: clear water, a gentle riverbed slope, and slow water flow. In addition, a large number of submerged plants are distributed in the water body of the Heilongtan River, which increases the contribution of endogenous organic carbon to the water.
4.3. CO2 Exchange Flux Comparation of Study Area and the World Rivers
At Q1, H1, and H2, the average CO2 flux is 14.86 mmol/(m2·d), 12.62 mmol/(m2·d), and −0.68 mmol/(m2·d), respectively. The research results show that the Heilongtan Basin is an important net flux of CO2 from rivers to the atmosphere, similar to other major rivers in the world (Table 1). However, in the Heilongtan Basin, CO2 flux exhibits significant temporal and spatial changes, especially in terms of CO2 sinks appearing at H2. The average value from H1 to H2 in the Heilongtan Basin is 5.97 mmol/(m2·d), which is equivalent to the CO2 flux of Longchuan (6.87 mmol/(m2·d) [51]) but slightly higher than the observed values of other subtropical and tropical rivers [26,52,53,54]. It is worth noting that the CO2 flux in the Heilongtan Basin is even lower than in some temperate and northern rivers [55,56,57]. Research suggests that in Arctic tundra vegetation (moss), metabolic activity exceeds photosynthesis, leading to a significant release of CO2 [58]. However, this depends on the study area, temperature, and the amount of bicarbonate in the water. In this study area, the release of CO2 in the open-flow section of karst underground rivers has been reduced due to the biological effects of submerged plants. In inland waters, it has been found that the biological effects of phytoplankton can lead to the release of more CO2 from the water [59]. Phytoplankton are mostly found in lakes, reservoirs, or slow-moving water bodies, and the CO2 released from these water bodies is often much greater than that from rivers [32,60]. The CO2 flux at Q1 is similar to that in reservoirs and may be influenced by phytoplankton. Therefore, the different distribution and geological conditions of submerged plants in water bodies may have a more important impact on CO2 flux than the climate conditions in the Heilongtan Basin. This further emphasizes the important role of aquatic ecosystems dominated by submerged plants in suppressing CO2 emissions in karst areas.

Table 1.
Comparison of CO2 flux of Heilongtan under different climatic conditions.
The impact of CO2 balance systems and submerged plant photosynthesis on CO2 flux are the first two categories of influencing factors for research and analysis. The third category includes the effects of meteorological environmental factors and water-soluble organic and inorganic carbon on CO2 flux. DO is an important variable that may regulate variations in CO2 flux. By analyzing the CO2 flux of the widely distributed river section (from H1 to H2) of submerged plants in the Heilongtan Basin, it was found that the photosynthesis of submerged plants significantly inhibits the carbon flux at H2 with metabolism inhibiting carbon emissions of approximately 0.131 Tg C/yr from H1 to H2. At the same time, it indicates that high-intensity pCO2 is easily absorbed by submerged plants, indicating a process from release to absorption. Therefore, this study emphasizes the process change in CO2 flux in the open-flow section release to absorption under the metabolism of submerged plants, providing an effective way for carbon sequestration and sink enhancement in karst water bodies and helping to improve the accuracy of CO2 flux accounting in rivers.
4.4. The Limitations of Research
This study explores the diurnal variation pattern and driving factors of CO2 flux at the water–air interface in the open-flow section of a typical karst underground river basin in southwestern China. The study reveals the process of CO2 flux from release to absorption and evaluates the transformation process of carbon in the river. The study uses the end element mixing model to demonstrate the importance of submerged plants metabolism in the carbon cycle and provides an effective way for carbon sequestration and sink enhancement. However, the study has limitations, including its focus on only one karst underground river basin in southwestern China, the lack of consideration of other factors that may affect the carbon cycle in karst water bodies, and the impact of human activities such as land use change and pollution. Future research could conduct long-term observations of CO2 flux in different karst underground river basins and study the carbon sequestration effects of submerged plants under different land use types in the open-flow section.
5. Conclusions
1. The CO2 flux in the karst underground river open-flow section of the Heilongtan River shows a pattern of underground river outlet > river sections without submerged plants > river sections with submerged plants (<0). The CO2 flux at the underground river outlet is high during the day and low at night, while the CO2 flux in the river sections is low during the day and high at night.
2. The river sections with submerged plants exhibited a substantial correlation (p < 0.05) with the CO2 flux, although the underground river outlet and river sections without submerged plants showed weaker correlations (p > 0.05). DO (p < 0.001) is an important variable that may regulate variations in CO2 flux.
3. The end element mixed model calculation shows that at the underground river outlet, river sections without submerged plants, and river sections with submerged plants, the contribution ratio of endogenous organic carbon to TOC is 68.71%, 88.66%, and 96.40%, respectively. This is significantly higher than the ratios found in the Beijiang and Xijiang Rivers of the Pearl River, and it close to the ratio of the Dongjiang River. Combining the distribution of submerged plants with the hydrological circumstances of the Heilongtan and Dongjiang Rivers, it is evident that the metabolism of submerged plants plays a significant regulatory function in the variation of CO2 flux.
4. The photosynthesis of submerged plants significantly inhibits the carbon flux at the river sections with submerged plants, with metabolism inhibiting carbon emissions of approximately 0.131 Tg C/yr from the river sections without submerged plants to the river sections with submerged plants. In addition, it shows that submerged plants can absorb high-intensity pCO2, suggesting a mechanism that goes from release to absorption.
Author Contributions
Conceptualization, D.L. and L.Z.; data curation, D.L., X.P. and C.H.; validation, C.H. and L.Z.; formal analysis, D.L., C.L., H.L. and X.X.; investigation, D.L., L.Z. and G.C.; writing—original draft preparation, D.L.; writing—review and editing, L.Z. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by (Geological Survey Project of China Geological Survey Bureau) grant number (DD20230111, DD20230512).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The dates are not publicly available due to the privacy of the data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Davies, B.T.; Valdes, P.J.; Singarayer, J.S.; Wiltshire, A.J.; Jones, C.D. Quantifying the relative importance of land cover change from climate and land use in the representative concentration pathways. Glob. Biogeochem. Cycles 2019, 29, 842–853. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W. Significance of the carbon sink produced by H2O–carbonate–CO2–aquatic phototroph interaction on land. Sci. Bull. 2015, 60, 182–191. [Google Scholar] [CrossRef]
- Yuan, D.X. Sensitivity of karst process to environmental change along the Pep II transect. Quat. Int. 1997, 37, 105–113. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Wang, H.J. A potentially important CO2 sink generated by the global water cycle. Sci. Bull. 2007, 52, 2418–2422. [Google Scholar]
- Liu, Z.; Dreybrodt, W.; Wang, H. A new direction in effective accounting for the atmospheric CO2 budget: Considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms. Earth Sci. Rev. 2010, 99, 162–172. [Google Scholar] [CrossRef]
- Aufdenkampe, A.K.; Mayorga, E.; Raymond, P.A.; Melack, J.M.; Doney, S.C.; Alin, S.R. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front. Ecol. Environ. 2011, 9, 53–60. [Google Scholar] [CrossRef]
- Larson, C. Climate change. An unsung carbon sink. Science 2011, 334, 886–887. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Dreybrodt, W.; Liu, H. Atmospheric CO2 sink: Silicate weathering or carbonate weathering? Appl. Geochem. 2011, 26, 5292–5294. [Google Scholar] [CrossRef]
- Yu, S.; He, S.Y.; Sun, P.A.; Pu, J.B.; Huang, J.; Luo, H.X.; Li, Y.S.; Li, R.; Yuan, Y.Q. Impacts of anthropogenic activities on weathering and carbon fluxes: A case study in the Xijiang River basin, southwest China. Environ. Earth Sci. 2016, 75, 1–11. [Google Scholar] [CrossRef]
- Atekwana, E.A.; Fonyuy, E.W. Dissolved inorganic carbon concentrations and stable carbon isotope ratios in streams polluted by variable amounts of acid mine drainage. J. Hydrol. 2009, 372, 136–148. [Google Scholar] [CrossRef]
- Sharma, S.; Sack, A.; Adams, J.P.; Vesper, D.V.; Capo, R.C.; Hartsock, A.; Edenborn, H.M. Isotopic evidence of enhanced carbonate dissolution at a coal mine drainage site in Allegheny County, Pennsylvania, USA. Appl. Geochem. 2013, 29, 32–42. [Google Scholar] [CrossRef]
- Peter, H.; Singer, G.A.; Preiler, C.; Chifflard, P.; Steniczka, G.; Battin, T.J. Scales and drivers of temporal pCO2 dynamics in an Alpine stream. J. Geophys. Res. Biogeosci. 2014, 119, 1078–1091. [Google Scholar] [CrossRef]
- Wang, F.S.; Wang, Y.C.; Zhang, J.; Xu, H.; Wei, X.G. Human impact on the historical change of CO2 degassing flux in River Changjiang. Geochem. Trans. 2007, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Chen, J.; Yin, J.B.; Xu, C.Y.; Zhou, J.H. Responses of Precipitation and Runoff to Climate Warming and Implications for Future Drought Changes in China. Earth’s Future 2020, 8, 32. [Google Scholar] [CrossRef]
- Gong, S.H.; Wang, S.J.; Bai, X.Y.; Luo, G.J.; Zeng, C. Response of the weathering carbon sink in terrestrial rocks to climate variables and ecological restoration in China. Sci. Total Environ. 2020, 750, 141525. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.B.; Liu, Z.H.; Kaufmann, G. Sensitivity of the global carbonate weathering carbon-sink flux to climate and land-use changes. Nat. Commun. 2019, 10, 5749. [Google Scholar] [CrossRef] [PubMed]
- Battin, T.J.; Luyssaert, S.; Kaplan, L.A.; Aufdenkampe, A.K.; Richter, A.; Tranvik, L.J. The boundless carbon cycle. Nat. Geosci. 2009, 2, 598–600. [Google Scholar] [CrossRef]
- Hope, D.; Palmer, S.M.; Billett, M.F.; Dawson, J.J.C. Variations in dissolved CO2 and CH4 in a first-order stream and catchment: An investigation of soil–stream linkages. Hydrol. Process. 2004, 18, 3255–3275. [Google Scholar] [CrossRef]
- Lynch, J.K.; Beatty, C.M.; Seidel, M.P.; Jungst, L.J.; Degrandpre, M.D. Controls of riverine CO2 over an annual cycle determined using direct, high temporal resolution pCO2 measurements. J. Geophys. Res. 2010, 115, 3016. [Google Scholar] [CrossRef]
- Ni, M.F.; Li, S.Y. Partial Pressure of Carbon Dioxide and Water-air Exchange in a Typical Karst River. Quat. Sci. 2023, 43, 412–424. [Google Scholar]
- Cao, J.H.; Jiang, Z.C.; Yuan, D.X.; Xia, R.Y.; Zhang, C. The progress in the study of the karst dynamic system and global changes in the past 30 years. Geol. China 2017, 44, 874–900. (In Chinese) [Google Scholar]
- Zhang, T.; Li, J.H.; Pu, J.B.; Wu, F.H.; Li, L.; Yuan, D.X. Spatial Variations of CO2 Degassing Across Water-air Interface and Its Impact Factors in Summer in Guijiang River, China. Environ. Sci. 2017, 38, 11. (In Chinese) [Google Scholar]
- Regnier, P.; Friedlingstein, P.; Ciais, P.; Thullner, M. Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat. Geosci. 2013, 6, 597–607. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Erratum in Nature 2014, 503, 355–359. [Google Scholar] [CrossRef]
- Butman, D.; Raymond, P.A. Significant efflux of carbon dioxide from streams and rivers in the United States. Nat. Geosci. 2011, 4, 839–842. [Google Scholar] [CrossRef]
- Pu, J.B.; Li, J.H.; Khadka, M.B.; Martin, J.B.; Zhang, T.; Yu, S.; Yuan, D.X. In-stream metabolism and atmospheric carbon sequestration in a groundwater-fed karst stream. Sci. Total Environ. 2017, 579, 1343–1355. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, H.T.; Xiao, J.; Qiu, X.L.; Wang, B.L. Effects of river hydrology on phytoplankton dynamics in dammed rivers. Earth Environ. 2019, 47, 857–863. (In Chinese) [Google Scholar]
- Tan, S.C.; Shi, G.Y. Remote sensing for ocean primary productivity and its spatio-temporal variability in the China Seas. Acta Geogr. Sin. 2006, 61, 1189–1199. [Google Scholar]
- Wang, J.Y. Comparison Table of Water Environment Monitoring Parameters and Selection Standards. Water Conserv. Tech. Superv. 2004, 4, 53–63. (In Chinese) [Google Scholar]
- Zhang, T.; Li, J.H.; Pu, J.B.; Martin, J.B.; Khadka, M.B.; Wu, F.H.; Li, L.; Jiang, F.; Huang, S.Y.; Yuan, D.X. River sequesters atmospheric carbon and limits the CO2 degassing in karst area, southwest China. Sci. Total Environ. 2017, 609, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, E.; Lucotte, M.; Canuel, R.; Chamberland, A. Production of the greenhouse gases CH4 and CO2 by hydroelectric reservoirs of the boreal region. Glob. Biogeochem. Cycles 1995, 9, 529–540. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, S.B.; Wang, C.H.; Yang, Z.J.; Liu, D.F.; Guo, X.; Liu, L.; Lorke, A. Spatial and temporal variability of dissolved methane concentrations and diffusive emissions in the Three Gorges Reservoir. Water Res. 2021, 207, 117788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, J.H.; Pu, J.B.; Martin, J.B.; Yuan, D.X. Rainfall possibly disturbs the diurnal pattern of CO2 degassing in the Lijiang River, SW China. J. Hydrol. 2020, 590, 125540. [Google Scholar] [CrossRef]
- Odum, H.T. Primary Production in Flowing Waters. Limnol. Oceanogr. 1956, 1, 102–117. [Google Scholar] [CrossRef]
- Doctor, D.H.; Kendall, C.; Sebestyen, S.D.; Shanley, J.B.; Ohte, N.; Boyer, E.W. Carbon isotope fractionation of dissolved inorganic carbon (DIC) due to outgassing of carbon dioxide from a headwater stream. Hydrol. Process. 2008, 22, 2410–2423. [Google Scholar] [CrossRef]
- Wallin, M.; Audet, J.; Peacock, M.; Sahlee, E.; Winterdahl, M. Carbon dioxide dynamics in an agricultural headwater stream driven by hydrology and primary production. Biogeosciences 2020, 17, 2487–2498. [Google Scholar] [CrossRef]
- Abril, G.L.; Guérin, F.; Richard, S.; Delmas, R.; Corinne, G.L.; Gosse, P.; Tremblay, A. Carbon dioxide and methane emissions and the carbon budget of a 10-year old tropical reservoir (Petit Saut, French Guiana). Glob. Biogeochem. Cycles 2005, 19, 4. [Google Scholar] [CrossRef]
- Hotchkiss, E.R.; Hall, R.O.; Sponseller, R.A.; Butman, D.; Klaminder, J.; Laudon, H.; Rosvall, M.; Karlsson, J. Sources of and processes controlling CO2 emissions change with the size of streams and rivers. Nat. Geosci. 2015, 8, 696. [Google Scholar] [CrossRef]
- Yang, M.X.; Liu, Z.H.; Sun, H.L.; Yang, R.; Chen, B. Organic carbon source tracing and DIC fertilization effect in the Pearl River: Insights from lipid biomarker and geochemical analysis. Appl. Geochem. 2016, 73, 132–141. [Google Scholar] [CrossRef]
- Wang, W.F.; Li, S.L.; Zhong, J.; Wang, L.C.; Yang, H.; Xiao, H.Y.; Liu, C.Q. CO2 emissions from karst cascade hydropower reservoirs: Mechanisms and reservoir effect. Environ. Res. Lett. 2021, 16, 044013. [Google Scholar] [CrossRef]
- de Montety, V.; Martin, J.B.; Cohen, M.J.; Foster, C.; Kurz, M.J. Influence of diel biogeochemical cycles on carbonate equilibrium in a karst river. Chem. Geol. 2011, 283, 31–43. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley: Hoboken, NJ, USA, 1995; Volume 179, p. A277. [Google Scholar]
- Marx, A.; Hintze, S.; Sanda, M.; Jankovec, J.; Barth, J.A. Acid rain footprint three decades after peak deposition: Long-term recovery from pollutant sulphate in the Uhlirska catchment (Czech Republic). Sci. Total Environ. 2017, 598, 1037–1049. [Google Scholar] [CrossRef]
- Li, D.Y.; Zhang, L.K.; Li, C.F.; Wang, X.Y.; Wang, X.R.; Yang, Z.F.; Qian, L.T. Quantitative analysis of dissolved inorganic carbon sources in water bodies in the Lujiang River Basin. Carsologica Sin. 1–17. Available online: https://link.cnki.net/urlid/45.1157.P.20231016.1203.002 (accessed on 16 October 2023). (In Chinese).
- Zhang, L.K.; Qin, X.Q.; Yang, H.; Huang, Q.B.; Liu, P.Y. Characteristics of river carbon output flux and variation in the Pearl River Basin. Environ. Sci. 2013, 34, 10. (In Chinese) [Google Scholar] [CrossRef]
- Dagg, M.; Benner, R.; Lohrenz, S.; Lawrence, D. Transformation of dissolved and particulate materials on continental shelves influenced by large rivers: Plume processes. Cont. Shelf Res. 2004, 24, 833–858. [Google Scholar] [CrossRef]
- Kendall, C.; Silva, S.R.; Kelly, V.J. Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States. Hydrol. Process. 2010, 15, 7. [Google Scholar] [CrossRef]
- LaZerte, B.D. Stable carbon isotope ratios: Implications for the source of sediment carbon and for phytoplankton carbon assimilation in Lake Memphremagog, Quebec. Can. J. Fish. Aquat. Sci. 1983, 40, 1658–1666. [Google Scholar] [CrossRef]
- Hung, J.J.; Lin, L.L.; Liu, K.K. Dissolved and particulate organic carbon in the southern East China Sea. Cont. Shelf Res. 2000, 20, 4–5. [Google Scholar] [CrossRef]
- Li, Z.K.; Lan, J.; Wang, G.; Han, B.; Lu, Y.F.; He, Q.; Zhang, Z.H. Mutual inductance measurement of the superconducting coil for Joule Balance. IEEE Trans. Instrum. Meas. 2013, 62, 1531–1536. [Google Scholar] [CrossRef]
- Raymond, P.A.; Bauer, J.E.; Caraco, N.F.; Cole, J.J.; Longworth, B.; Petsch, S.T. Controls on the variability of organic matter and dissolved inorganic carbon ages in northeast US rivers. Mar. Chem. 2004, 92, 353–366. [Google Scholar] [CrossRef]
- Teodoru, C.R.; Giorgio, P.A.; Prairie, Y.T.; Camire, M. Patterns in pCO2 in boreal streams and rivers of northern Quebec, Canada. Glob. Biogeochem. Cycles 2009, 23, GB2012. [Google Scholar] [CrossRef]
- Varol, M.; Li, S.Y. Biotic and abiotic controls on CO2 partial pressure and CO2 emission in the Tigris River, Turkey. Chem. Geol. 2017, 449, 193. [Google Scholar] [CrossRef]
- Yao, G.R.; Gao, Q.Z.; Wang, Z.G.; Huang, X.K.; He, T.; Zhang, Y.L.; Jiao, S.L.; Ding, J. Dynamics of CO2 partial pressure and CO2 outgassing in the lower reaches of the Xijiang River, a subtropical monsoon river in China. Sci. Total Environ. 2007, 376, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Alin, S.R.; Rasera, M.L.; Salimon, C.I.; Richey, J.E.; Holtgrieve, G.W.; Krusche, A.V.; Snidvongs, A. Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets. J. Geophys. Res. Biogeosciences 2011, 116, g01009. [Google Scholar] [CrossRef]
- Li, S.Y.; Lu, X.X.; Bush, R.T. CO2 partial pressure and CO2 emission in the Lower Mekong River. J. Hydrol. 2013, 504, 40–56. [Google Scholar] [CrossRef]
- Rocher-Ros, G.; Harms, T.K.; Sponseller, R.A.; Visnen, M.; Mrth, C.; Giesler, R. Metabolism overrides photo-oxidation in CO2 dynamics of Arctic permafrost streams. Limnol. Oceanogr. 2020, 66, S169–S181. [Google Scholar] [CrossRef]
- Louis, V.S.; Kelly, C.A.; Duchemin, E.; Rudd, J.W.; Rosenberg, D.M. Reservoir surfaces as sources of greenhouse gases to the atmosphere: A global estimate. Bioscience 2000, 50, 766–775. [Google Scholar] [CrossRef]
- Ternon, J.F.; Oudot, C.; Dessier, A.; Diverres, D. A seasonal tropical sink for atmospheric CO2 in the Atlantic ocean: The role of the Amazon River discharge. Mar. Chem. 2000, 68, 183–201. [Google Scholar] [CrossRef]
- Yang, C.; Telmer, K.; Veizer, J. Chemical dynamics of the “St. Lawrence” riverine system: δDH2O, δ18OH2O, δ13CDIC, δ34S sulfate, and dissolved 87Sr/86Sr. Geochim. Et Cosmochim. Acta 1996, 60, 851–866. [Google Scholar] [CrossRef]
- Raymond, P.A.; Bauer, J.E.; Cole, J.J. Atmospheric CO2 evasion, dissolved inorganic carbon production, and net heterotrophy in the York River estuary. Limnol. Oceanogr. 2000, 45, 1707–1717. [Google Scholar] [CrossRef]
- Santos, M.A.; Rosa, L.P.; Sikar, B.; Sikar, E.; Santos, E.O. Gross greenhouse gas fluxes from hydro-power reservoir compared to thermo-power plants. Energy Policy 2006, 34, 481–488. [Google Scholar] [CrossRef]
- Liu, C.Q.; Wang, F.S.; Wang, Y.C.; Wang, B.L. Responses of Aquatic Environment to River Damming—From the Geochemical View. Resour. Environ. Yangtze Basin 2009, 18, 384–396. [Google Scholar]
- Yu, Y.X.; Liu, C.Q.; Wang, F.S.; Wang, B.L.; Liu, F. Spatiotemporal characteristics and diffusion flux of partial pressure of dissolved carbon dioxide (pCO2) in Hongjiadu reservoir. Chin. J. Ecol. 2008, 27, 1193–1199. [Google Scholar]
- Mei, H.Y.; Wang, F.S.; Yao, C.C.; Wang, B.L. Diffusion flux of partial pressure of dissolved carbon dioxide in Wan’an Reservoir in spring. Environ. Sci. 2011, 32, 58–63. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).