Unveiling the Essential Parameters Driving Mineral Reactions during CO2 Storage in Carbonate Aquifers through Proxy Models
Abstract
:Featured Application
Abstract
1. Introduction
- It adopts a comprehensive approach to characterize the mechanisms of CO2 trapping, encompassing solubility, residual (e.g., the trapped CO2 saturation due to relative permeability and capillarity hysteresis), and mineral trapping. A particular focus is on understanding the reactions involving carbonate minerals and the crucial parameters that govern these reactions. This study uses a stochastic approach to model the kinetic parameters, which is different from other studies conducted in the literature, which either model the geochemical reactions deterministically [11,25,26] or neglect them altogether [27,28].
2. Methodology
- Geomechanical aspects and caprock behavior are not modeled;
- There is no occurrence of natural fractures in the carbonate rock matrix. This concern was addressed by Machado et al. in [22,32], concluding that calcite dissolution is the primary reaction. However, the effects of calcite dissolution can be partially offset by the precipitation of halite and dawsonite, particularly in the areas surrounding the wellbore and within natural fractures;
- Pure CO2 stream is injected, e.g., without impurities and free water content;
- Dry-out effect due to water vaporization with CO2 injection is not modeled;
2.1. Modeling Geochemical Reactions
- Diffusion coefficient (D) for supercritical CO2 in brine equals 3.65 × 10−5 cm2/s, according to Ahmadi et al. [40], with an average value over the pressure range considered during the simulations.
- CO2 solubility in brine according to the method by Li and Nghiem [41] based on Henry’s law. This model is based on Henry’s constant calculation according to Equation (1) as a function of pressure and temperature. Still, the effect of salt on the gas solubility in the aqueous phase is modeled by the salting-out coefficient [42].
- Reactions with primary ((5)–(10)) and secondary minerals ((11) and (12)), using the Transition State Theory (TST)-derived rate laws [14]:
- o Keq is the chemical equilibrium constant;
- o A is the reactive surface area;
- o Ea is the activation energy;
- o k25 is the rate constant at T25 = 298.15 K (25 °C), and the rate constant (k) in different temperature conditions can be expressed as follows:
- Permeability alteration due to mineral precipitation or dissolution was computed by applying the Kozeny–Carman equation with an exponent value of 3, as Zeidouni et al. [51] recommended:
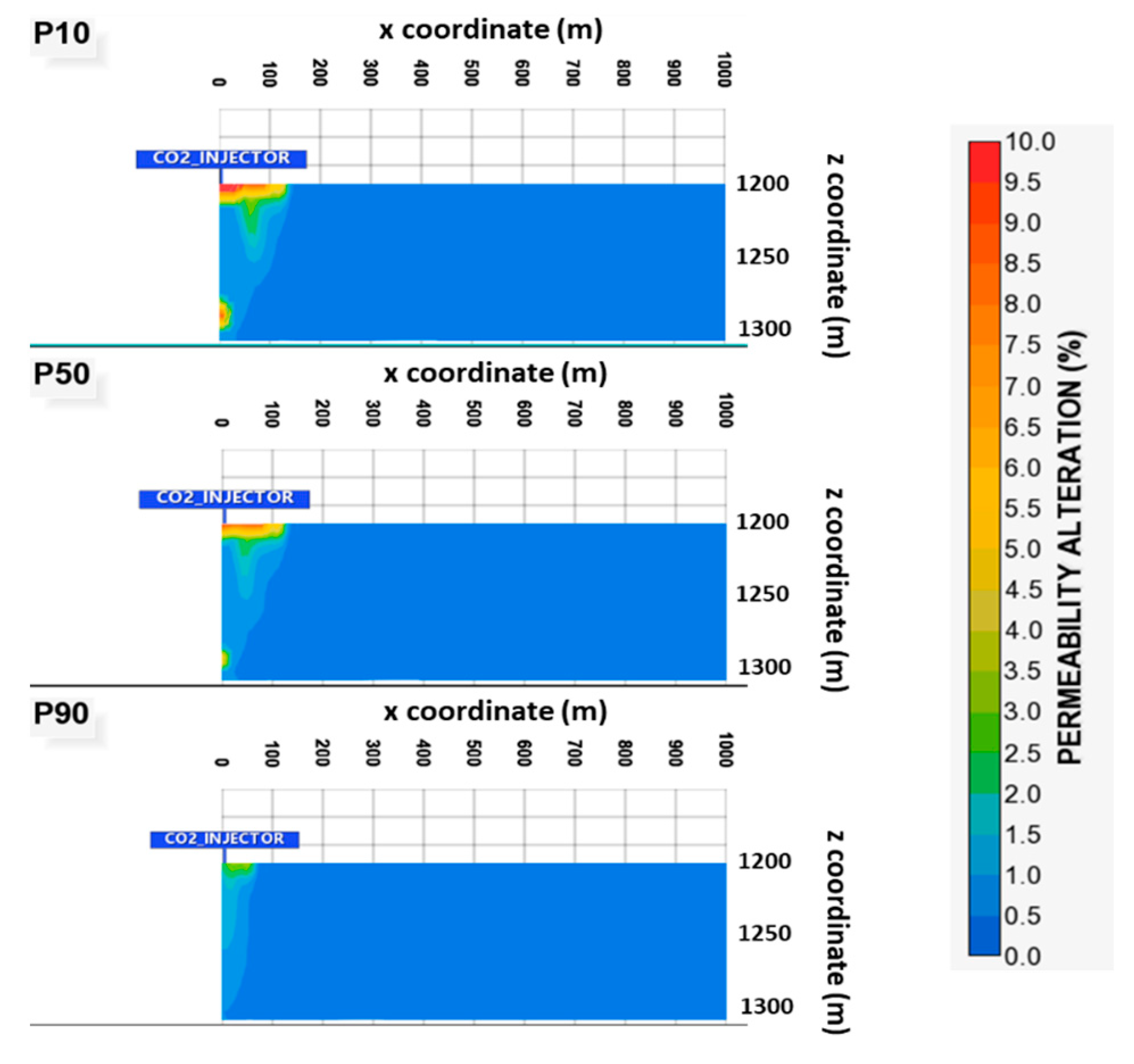
2.2. Geological Model
3. Results and Discussion
- II: the CO2 injectivity index after five years of injection, computed according to Equation (19):
- Mineralization_1000yr: Mineral dissolution or precipitation after 1000 years of CO2 redistribution;
- Calcite_1000yr: Calcite concentration change (in gmoles) after 1000 years of CO2 redistribution;
- Dolomite_1000yr: Dolomite concentration change (in gmoles) after 1000 years of CO2 redistribution;
- Quartz_1000yr: Quartz concentration change (in gmoles) after 1000 years of CO2 redistribution.
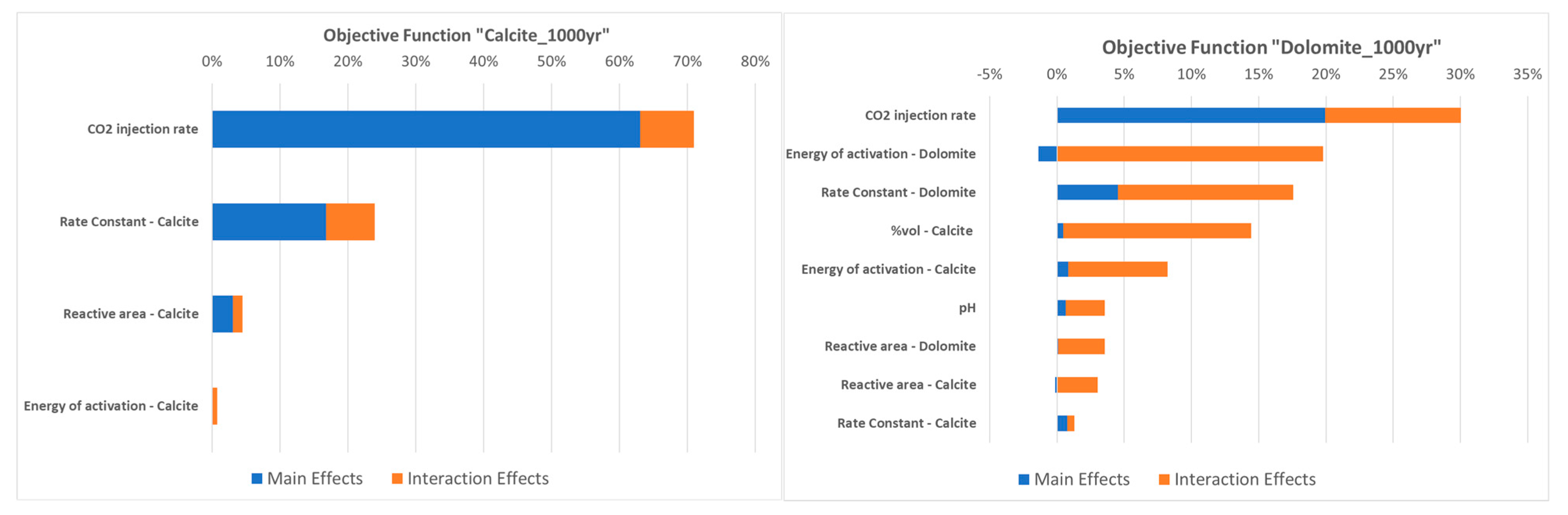
3.1. Parametric Analysis with Proxy Models for Calcite Dissolution
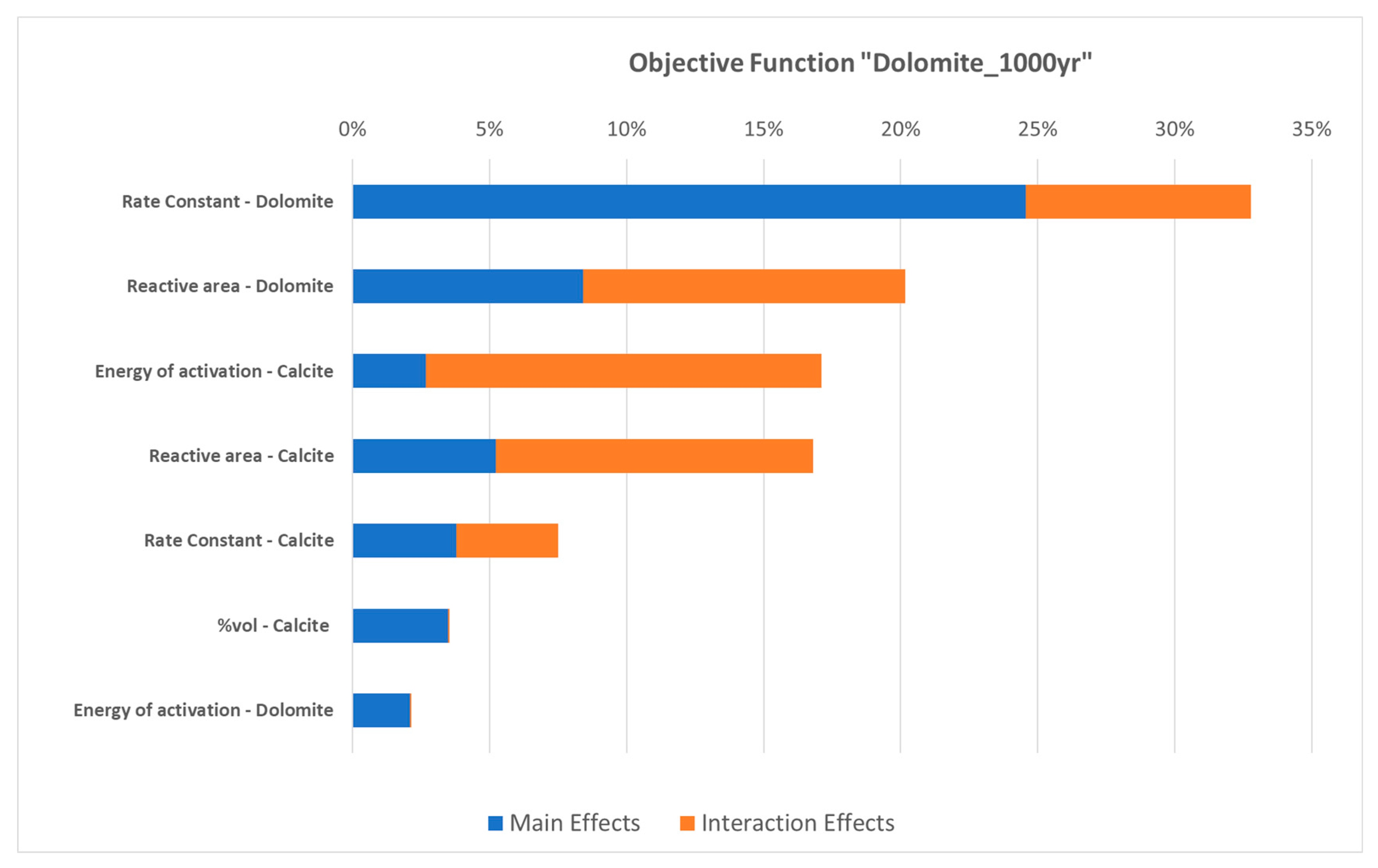
3.2. Parametric Analysis with Proxy Models for Dolomite Precipitation
3.3. Thorough Proxy Analysis
4. Conclusions
- The workflow employed in this study successfully mapped the uncertainty associated with the amount of CO2 mineralized in carbonate rocks. This is essential for defining mitigation measures to address the risk of leakage in storage targets containing reactive minerals within their matrix;
- Proxy models constructed using quadratic response surfaces effectively represented the objective functions used to evaluate CO2 injectivity and mineralization over extended periods of CO2 redistribution. These models performed well even with a relatively small number of runs, making them valuable decision-making tools;
- The development of proxy models allowed for an expanded parametric analysis and facilitated the examination of the relationships among kinetic parameters and other key properties, such as the CO2 injection rate, brine pH, and the initial percentage of calcite in the rock matrix. The findings highlight the significance of calcite dissolution as the primary mechanism of mineralization and the interplay between pH, available CO2, and dolomite and calcite kinetics for the formation of dolomite as a secondary mineral;
- The parametric assessment revealed that the rate constant emerges as the most significant kinetic parameter. This parameter holds direct control over the reaction rate, especially in the context of calcite dissolution. Therefore, the uncertainty on this parameter underscores the need for further research and data collection to improve our understanding and its characterization, as highlighted in our results;
- Both pH and the initial concentration of calcite exert significant control on dolomite precipitation, while the CO2 injection rate affects both calcite dissolution and dolomite generation. No changes in quartz content were observed in the conducted numerical experiments;
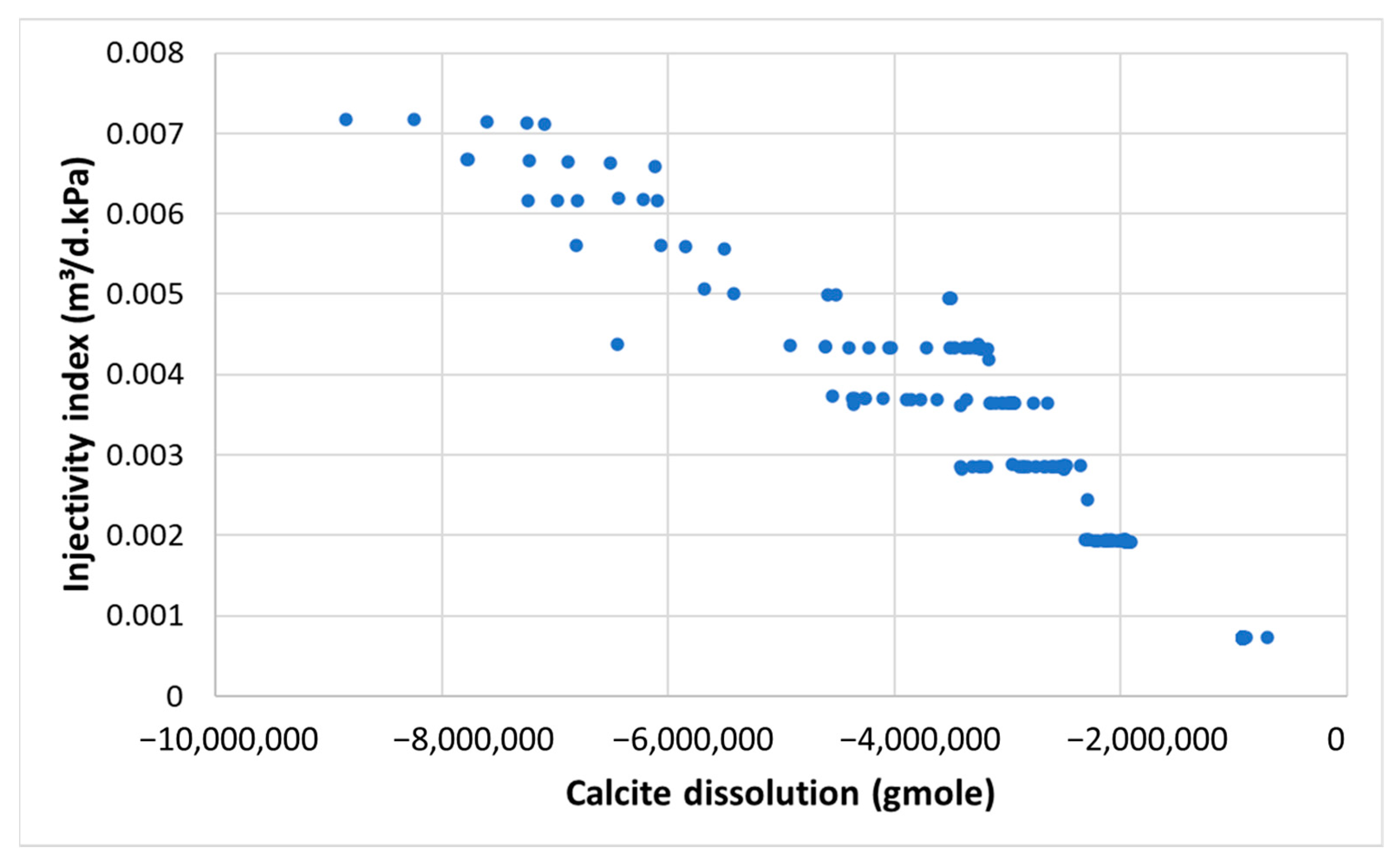
- The CO2 injectivity index has demonstrated its capability to reflect the uncertainty associated with carbonate mineral reactions. Therefore, tracking the injectivity index from field or lab tests provides a practical means of monitoring and quantifying the levels of uncertainty involved.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | reactive surface area, m2/m3; |
| ai | activity of component I, dimensionless; |
| Ea | activation energy, J/mol; |
| Henry’s constant at current pressure (p) and temperature (T), dimensionless; | |
| Henry’s constant at reference pressure (p*) and temperature (T), dimensionless; | |
| II | CO2 injectivity index, m3/d-kPa; |
| K | rock permeability, mD; |
| Keq | chemical equilibrium constant, dimensionless; |
| k | rate constant, mol/m2s; |
| nct | number of reactant components, dimensionless; |
| Pbh | bottom-hole pressure, kPa; |
| average aquifer pressure, kPa; | |
| Q | activity product, dimensionless; |
| bottom-hole CO2 volumetric injection rate, m3/d; | |
| r | reaction rate, mol/kg.s; |
| R | universal gas constant, 8.314 kPa·L/mol·K; |
| Sgt | residual gas saturation due to hysteresis, dimensionless; |
| Sg max | maximum gas saturation, dimensionless; |
| Sl | current fluid saturation, dimensionless; |
| T | temperature, K; |
| partial molar volume at infinite dilution, L/mol; | |
| %vol | volumetric percentage of calcite, %; |
| wi | activity power, dimensionless. |
| Greek symbols | |
| φ | rock porosity, dimensionless; |
| parameters from TST model, dimensionless. | |
| Subscripts | |
| l | fluid (w—water or CO2); |
| i | component. |
| Acronyms | |
| CCS | Carbon Capture and Storage; |
| IAP | ion activity product; |
| MCS | Monte Carlo simulation; |
| OF | objective function; |
| SR | simulation results; |
| TST | Transition State Theory. |
Appendix A
| Objective Function | Response Surface |
|---|---|
| “II” (m3/d.kPa) | II = 0.00256546 − 1.59671 × 10−6 × log10(Acalcite) + 5.30525 × 10−7 × log10(Adolomite) + 7.61884 × 10−11 × log10(Aquartz) − 6.03749 × 10−11 × Ea calcite − 1.09612 × 10−10 × Ea dolomite + 2.34945 × 10−11 × Ea quartz + 4.76277 × 10−7 × k25 calcite + 5.92785 × 10−7 × k25 dolomite + 1.99527 × 10−7 × k25 quartz − 1.01812 × 10−5 × %volcalcite + 1.05155 × 10−17 × log10(Acalcite) × log10(Adolomite) + 1.32612 × 10−11 × log10(Acalcite) × Ea dolomite − 3.73041 × 10−7 × log10(Acalcite) × k25 calcite − 2.09195 × 10−7 × log10(Adolomite) × log10(Adolomite) − 1.1835 × 10−11 × log10(Adolomite) × Ea dolomite − 7.6864 × 10−8 × log10(Adolomite) × k25 quartz − 4.15169 × 10−14 × log10(Aquartz) × log10(Aquartz) − 9.97032 × 10−15 × log10(Aquartz) × Ea calcite − 1.96966 × 10−11 × log10(Aquartz)× k25 calcite − 3.8904 × 10−11 × log10(Aquartz) × k25 dolomite +5.64032 × 10−10 × log10(Aquartz) × %volcalcite +8.70077 × 10−16 × Ea calcite × Ea dolomite +9.16404 × 10−16 × Ea calcite × Ea quartz − 1.31598 × 10−11 × Ea calcite × k25 calcite − 7.78977 × 10−12 × Ea calcite × k25 dolomite +6.09312 × 10−12 × Ea calcite × k25 quartz − 6.75202 × 10−12 × Ea dolomite × k25 quartz +3.8721 × 10−12 × Ea quartz × k25 calcite +5.05183 × 10−12 × Ea quartz × k25 quartz +6.21285 × 10−11 × Ea quartz × %volcalcite − 1.72748 × 10−7 × k25 calcite × k25 calcite − 1.33993 × 10−8 × k25 calcite × k25 dolomite +2.77643 × 10−8 × k25 calcite × k25 quartz +4.7949 × 10−7 × k25 calcite × %volcalcite +1.91192 × 10−8 × k25 dolomite × k25 dolomite − 2.7037 × 10−7 × k25 quartz × %volcalcite |
| “Mineralization_1000yr” (gmole) | Mineralization_1000yr = − 2.56092 × 106 − 78810.2 × log10(Acalcite) + 81857.1 × log10(Adolomite) + 5.47112 × log10(Aquartz) − 6.86334 × Ea calcite + 6.65629 × Ea dolomite–0.22563 × Ea quartz − 91559.1 × k25 calcite + 8129.79 × k25 dolomite + 6397.31 × k25 quartz + 81749.7 × %volcalcite − 19001.2 × log10(Acalcite) × log10(Acalcite) − 7830.34 × log10(Acalcite) × log10(Adolomite) − 0.804756 × log10(Acalcite) × Ea calcite − 0.836512 × log10(Acalcite) × Ea dolomite +0.224363 × log10(Acalcite) × Ea quartz − 32959.3 × log10(Acalcite) × k25 calcite − 4158.36 × log10(Acalcite) × k25 quartz − 29206.4 × log10(Acalcite) × %volcalcite + 1868.44 × log10(Adolomite) × k25 dolomite + 3510.25 × log10(Adolomite) × k25 quartz + 0.000293547 × log10(Aquartz) × Ea calcite − 0.000200297 × log10(Aquartz) × Ea dolomite + 0.730634 × log10(Aquartz) × k25 calcite + 1.11503 × log10(Aquartz) × k25 dolomite − 1.06921 × log10(Aquartz) × k25 quartz − 17.5626 × log10(Aquartz) × %volcalcite − 4.29981 × 10−5 × Ea calcite × Ea dolomite − 2.42536 × 10−5 × Ea calcite × Ea quartz − 0.65069 × Ea calcite × k25 calcite − 0.411025 × Ea calcite × k25 quartz + 2.95256 × Ea calcite × %volcalcite − 5.45726 × 10−5 × Ea dolomite × Ea dolomite− 0.109345 × Ea dolomite × k25 calcite − 0.162247 × Ea dolomite × k25 dolomite + 3.78857 × Ea dolomite × %volcalcite− 0.159753 × Ea quartz × k25 calcite − 1.20835 × Ea quartz × %volcalcite − 18328.2 × k25 calcite × k25 calcite +1183.54 × k25 calcite × k25 dolomite − 979.136 × k25 calcite × k25 quartz − 16958.1 × k25 calcite × %volcalcite − 12464.1 × k25 dolomite × %volcalcite + 16942.6 × k25 quartz × %volcalcite |
| “Calcite_1000yr” (gmole) | Calcite_1000yr = − 2.56106 × 106 − 78790.8 × log10(Acalcite) + 81840.5 × log10(Adolomite) + 5.46552 × log10(Aquartz) − 6.86332 × Ea calcite + 6.65732 × Ea dolomite − 0.225763 × Ea quartz − 91561.3 × k25 calcite + 8129.3 × k25 dolomite + 6395.27 × k25 quartz + 81740.7 × %volcalcite − 19002.2 × log10(Acalcite) × log10(Acalcite) − 7829.64 × log10(Acalcite) × log10(Adolomite) − 0.80482 × log10(Acalcite) × Ea calcite − 0.836622 × log10(Acalcite) × Ea dolomite +0.224371 × log10(Acalcite) × Ea quartz − 32959.4 × log10(Acalcite) × k25 calcite − 4157.52 × log10(Acalcite) × k25 quartz − 29200.9× log10(Acalcite) × %volcalcite + 1866.9 × log10(Adolomite) × k25 dolomite + 3510.1 × log10(Adolomite) × k25 quartz + 0.000293499 × log10(Aquartz) × Ea calcite − 0.000200249 × log10(Aquartz) × Ea dolomite + 0.730337 × log10(Aquartz) × k25 calcite + 1.11491 × log10(Aquartz) × k25 dolomite − 1.06935 × log10(Aquartz) × k25 quartz − 17.561 × log10(Aquartz) × %volcalcite − 4.29945 × 10−5 × Ea calcite × Ea dolomite − 2.4253 × 10−5 × Ea calcite × Ea quartz − 0.650722 × Ea calcite × k25 calcite − 0.411022 × Ea calcite × k25 quartz + 2.95273 × Ea calcite × %volcalcite − 5.45765 × 10−5 × Ea dolomite × Ea dolomite − 0.109326 × Ea dolomite × k25 calcite − 0.16221 × Ea dolomite × k25 dolomite + 3.78793 × Ea dolomite × %volcalcite − 0.159745 × Ea quartz × k25 calcite − 1.20803 × Ea quartz × %volcalcite − 18328.2 × k25 calcite × k25 calcite +1183.34 × k25 calcite × k25 dolomite − 979.243 × k25 calcite × k25 quartz − 16957.4 × k25 calcite × %volcalcite − 12461 × k25 dolomite × %volcalcite + 16942.1 × k25 quartz × %volcalcite |
| “Dolomite_1000yr” (gmole) | Dolomite_1000yr = 41.2594 − 9.40417 × log10(Acalcite) + 8.53233 × log10(Adolomite) + 0.0024599× log10(Aquartz) − 0.000209106 × Ea calcite +2.73214 × 10−5 × Ea dolomite + 0.000210498 × Ea quartz + 0.580279 × k25 calcite + 5.29976 × k25 dolomite − 2.81628 × k25 quartz + 10.4168 × %volcalcite +0.422753 × log10(Acalcite) × log10(Acalcite) − 0.000383179 × log10(Acalcite) × log10(Aquartz) +5.71373 × 10−5 × log10(Acalcite) × Ea calcite + 4.98471 × 10−5 × log10(Acalcite) × Ea dolomite + 0.111153 × log10(Acalcite) × k25 calcite − 0.453818 × log10(Acalcite) × k25 quartz − 2.45546 × log10(Acalcite) × %volcalcite + 0.903354 × log10(Adolomite) × log10(Adolomite) − 5.24884 × 10−5 × log10(Adolomite) × Ea dolomite − 3.46002 × 10−5 × log10(Adolomite) × Ea quartz + 0.18789 × log10(Adolomite) × k25 calcite + 0.816175 × log10(Adolomite) × k25 dolomite−2.43872 × log10(Adolomite) × %volcalcite +1.84261 × 10−8 × log10(Aquartz) × Ea calcite − 2.77418 × 10−8 × log10(Aquartz) × Ea dolomite +1.12908 × 10−8 × log10(Aquartz) × Ea quartz + 0.000120776 × log10(Aquartz) × k25 calcite + 6.67237 × 10−5 × log10(Aquartz) × k25 dolomite − 0.000946578 × log10(Aquartz) × %volcalcite +1.49461 × 10−5 × Ea calcite × k25 calcite − 2.05779 × 10−9 × Ea dolomite × Ea quartz − 1.21957 × 10−5 × Ea dolomite × k25 calcite − 1.9527 × 10−5 × Ea dolomite × k25 dolomite + 0.000290175 × Ea dolomite × %volcalcite − 4.67258 ×10−6 × Ea quartz × k25 calcite − 0.000187437 × Ea quartz × %volcalcite + 0.123451 × k25 calcite × k25 dolomite + 0.0592084 × k25 calcite × k25 quartz +0.319923× k25 dolomite × k25 dolomite +0.0799563× k25 dolomite × k25 quartz − 1.76213 × k25 dolomite × %volcalcite–0.204805 × k25 quartz × k25 quartz |
| “Quartz_1000yr” | It was not possible to calibrate a response surface due to the absence of variation in quartz content. |
References
- Dai, Z.; Xu, L.; Xiao, T.; McPherson, B.; Zhang, X.; Zheng, L.; Dong, S.; Yang, Z.; Soltanian, M.R.; Yang, C.; et al. Reactive Chemical Transport Simulations of Geologic Carbon Sequestration: Methods and Applications. Earth-Sci. Rev. 2020, 208, 103265. [Google Scholar] [CrossRef]
- Yamasaki, A. An Overview of CO2 Mitigation Options for Global Warming-Emphasizing CO2 Sequestration Options. J. Chem. Eng. Jpn. (JCEJ) 2003, 36, 361–375. [Google Scholar] [CrossRef]
- Han, W.S.; McPherson, B.J.; Lichtner, P.C.; Wang, F.P. Evaluation of Trapping Mechanisms in Geologic CO2 Sequestration: Case Study of SACROC Northern Platform, a 35-Year CO2 Injection Site. Am. J. Sci. 2010, 310, 282–324. [Google Scholar] [CrossRef]
- Jun, Y.-S.; Giammar, D.E.; Werth, C.J. Impacts of Geochemical Reactions on Geologic Carbon Sequestration. Environ. Sci. Technol. 2013, 47, 3–8. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Thorstenson, D.C.; Plummer, L.N. PHREEQE: A Computer Program for Geochemical Calculations; U.S. Geological Survey: Denver, CO, USA, 1980. [Google Scholar]
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. MINTEQA2/PRODEFA2: A Geochemical Assessment Model for Environmental Systems: Version 3.0 User’s Manual; U.S. Environmental Protection Agency National Exposure Research Laboratory Ecosystems Research Division: Athens, GA, USA, 1991. [Google Scholar]
- Wolery, T.J. EQ3/6, a Software Package for Geochemical Modeling of Aqueous Systems: Package Overview and Installation Guide (Version 7.0); UCRL-MA-110662-Pt.1; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1992; p. 138894. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. In U.S. Geological Survey Techniques and Methods, Book 6; U.S. Geological Survey: Denver, CO, USA, 2013; p. 497. [Google Scholar]
- Usdowski, E. Synthesis of Dolomite and Geochemical Implications. In Dolomites; Purser, B., Tucker, M., Zenger, D., Eds.; Wiley: Hoboken, NJ, USA, 1994; ISBN 978-0-632-03787-2. [Google Scholar]
- Hellevang, H.; Pham, V.T.H.; Aagaard, P. Kinetic Modelling of CO2–Water–Rock Interactions. Int. J. Greenh. Gas. Control 2013, 15, 3–15. [Google Scholar] [CrossRef]
- Ates, H.; Gupta, A.; Chandrasekar, V.; Vaidya, R.; AlMajid, M.; Yousif, Z. Ranking of Geologic and Dynamic Model Uncertainties for Carbon Dioxide Storage in Saline Aquifers. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 21 February 2023; SPE: Manama, Bahrain, 2023; p. D031S110R003. [Google Scholar]
- Dethlefsen, F.; Haase, C.; Ebert, M.; Dahmke, A. Uncertainties of Geochemical Modeling during CO2 Sequestration Applying Batch Equilibrium Calculations. Environ. Earth Sci. 2012, 65, 1105–1117. [Google Scholar] [CrossRef]
- Lake, L.W.; Bryant, S.L.; Araque-Martinez, A.N. Geochemistry and Fluid Flow, 1st ed.; Developments in Geochemistry; Elsevier Science Ltd.: Amsterdam, The Netherlands; New York, NY, USA, 2002; ISBN 978-0-444-50569-9. [Google Scholar]
- Lasaga, A.C. Chemical Kinetics of Water-Rock Interactions. J. Geophys. Res. 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Gunter, W.D.; Bachu, S.; Benson, S. The Role of Hydrogeological and Geochemical Trapping in Sedimentary Basins for Secure Geological Storage of Carbon Dioxide. Geol. Soc. 2004, 233, 129–145. [Google Scholar] [CrossRef]
- Xu, T.; Yue, G.; Wang, F.; Liu, N. Using Natural CO2 Reservoir to Constrain Geochemical Models for CO2 Geological Sequestration. Appl. Geochem. 2014, 43, 22–34. [Google Scholar] [CrossRef]
- Bolourinejad, P.; Shoeibi Omrani, P.; Herber, R. Effect of Reactive Surface Area of Minerals on Mineralization and Carbon Dioxide Trapping in a Depleted Gas Reservoir. Int. J. Greenh. Gas Control 2014, 21, 11–22. [Google Scholar] [CrossRef]
- Jaafar Azuddin, F.; Azahree, A.I. Reservoir Simulation Study for CO2 Sequestration in a Depleted Gas Carbonate Reservoir: Impact of Mineral Dissolution and Precipitation on Petrophysical Properties. SSRN J. 2019. [Google Scholar] [CrossRef]
- Azuddin, F.J.; Davis, I.; Singleton, M.; Geiger, S.; Mackay, E. Modeling Mineral Reaction at Close to Equilibrium Condition During CO2 Injection for Storage in Carbonate Reservoir. In Proceedings of the EAGE 2020 Annual Conference & Exhibition Online, Online, 8–11 December 2020; European Association of Geoscientists & Engineers: Bunnik, The Netherlands, 2020; pp. 1–5. [Google Scholar]
- Azuddin, F.J. Numerical Simulation and Experimental Investigation of Reactive Flow in a Carbonate Reservoir; Heriot-Watt University: Edinburgh, UK, 2022. [Google Scholar]
- Altar, D.E.; Kaya, E.; Zarrouk, S.J.; Passarella, M.; Mountain, B.W. Reactive Transport Modelling under Supercritical Conditions. Geothermics 2023, 111, 102725. [Google Scholar] [CrossRef]
- Machado, M.V.B.; Delshad, M.; Sepehrnoori, K. Modeling Self-Sealing Mechanisms in Fractured Carbonates Induced by CO2 Injection in Saline Aquifers. ACS Omega 2023, 8, 48925–48937. [Google Scholar] [CrossRef]
- Tadjer, A.; Bratvold, R.B. Managing Uncertainty in Geological CO2 Storage Using Bayesian Evidential Learning. Energies 2021, 14, 1557. [Google Scholar] [CrossRef]
- Mahjour, S.K.; Faroughi, S.A. Selecting Representative Geological Realizations to Model Subsurface CO2 Storage under Uncertainty. Int. J. Greenh. Gas Control 2023, 127, 103920. [Google Scholar] [CrossRef]
- Khanal, A.; Shahriar, M.F. Physics-Based Proxy Modeling of CO2 Sequestration in Deep Saline Aquifers. Energies 2022, 15, 4350. [Google Scholar] [CrossRef]
- Machado, M.V.B.; Delshad, M.; Sepehrnoori, K. The Interplay between Experimental Data and Uncertainty Analysis in Quantifying CO2 Trapping during Geological Carbon Storage. Clean. Energy Sustain. 2024, 2, 10001. [Google Scholar] [CrossRef]
- Raza, Y. Uncertainty Analysis of Capacity Estimates and Leakage Potential for Geologic Storage of Carbon Dioxide in Saline Aquifers. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2006. [Google Scholar]
- Likanapaisal, P.; Lun, L.; Krishnamurthy, P.; Kohli, K. Understanding Subsurface Uncertainty for Carbon Storage in Saline Aquifers: PVT, SCAL, and Grid-Size Sensitivity. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 17 October 2023; SPE: San Antonio, TX, USA, 2023; p. D021S020R002. [Google Scholar]
- Mckay, M.D.; Beckman, R.J.; Conover, W.J. A Comparison of Three Methods for Selecting Values of Input Variables in the Analysis of Output From a Computer Code. Technometrics 2000, 42, 55–61. [Google Scholar] [CrossRef]
- Ma, Y.Z. Quantitative Geosciences: Data Analytics, Geostatistics, Reservoir Characterization and Modeling; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-17859-8. [Google Scholar]
- Saltelli, A.; Ratto, M.; Andres, T.; Campolongo, F.; Cariboni, J.; Gatelli, D.; Saisana, M.; Tarantola, S. Global Sensitivity Analysis. The Primer, 1st ed.; Wiley: Hoboken, NJ, USA, 2007; ISBN 978-0-470-05997-5. [Google Scholar]
- Machado, M.V.B.; Delshad, M.; Sepehrnoori, K. A Computationally Efficient Approach to Model Reactive Transport During CO2 Storage in Naturally Fractured Saline Aquifers; SSRN: Rochester, NY, USA, 2023. [Google Scholar]
- Iglauer, S.; Al-Yaseri, A.Z.; Rezaee, R.; Lebedev, M. CO2 Wettability of Caprocks: Implications for Structural Storage Capacity and Containment Security. Geophys. Res. Lett. 2015, 42, 9279–9284. [Google Scholar] [CrossRef]
- Wang, J.; Samara, H.; Jaeger, P.; Ko, V.; Rodgers, D.; Ryan, D. Investigation for CO2 Adsorption and Wettability of Reservoir Rocks. Energy Fuels 2022, 36, 1626–1634. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Ye, M. An Uncertainty Analysis of Subsidy for Carbon Capture and Storage (CCS) Retrofitting Investment in China’s Coal Power Plants Using a Real-Options Approach. J. Clean. Prod. 2016, 137, 200–212. [Google Scholar] [CrossRef]
- Koelbl, B.S.; Van Den Broek, M.A.; Faaij, A.P.C.; Van Vuuren, D.P. Uncertainty in Carbon Capture and Storage (CCS) Deployment Projections: A Cross-Model Comparison Exercise. Clim. Chang. 2014, 123, 461–476. [Google Scholar] [CrossRef]
- Van Der Spek, M.; Fout, T.; Garcia, M.; Kuncheekanna, V.N.; Matuszewski, M.; McCoy, S.; Morgan, J.; Nazir, S.M.; Ramirez, A.; Roussanaly, S.; et al. Uncertainty Analysis in the Techno-Economic Assessment of CO2 Capture and Storage Technologies. Critical Review and Guidelines for Use. Int. J. Greenh. Gas Control 2020, 100, 103113. [Google Scholar] [CrossRef]
- Oda, J.; Akimoto, K. An Analysis of CCS Investment under Uncertainty. Energy Procedia 2011, 4, 1997–2004. [Google Scholar] [CrossRef]
- CMG. GEM Compositional & Unconventional Simulator, (Version 2023.30); Windows, CMG: Calgary, AB, Canada, 2023. [Google Scholar]
- Ahmadi, H.; Erfani, H.; Jamialahmadi, M.; Soulgani, B.S.; Dinarvand, N.; Sharafi, M.S. Corrigendum to “Experimental Study and Modelling on Diffusion Coefficient of CO2 in Water” Fluid Phase Equilibria 523 (2020) 112,584. Fluid. Phase Equilibria 2021, 529, 112869. [Google Scholar] [CrossRef]
- Li, Y.-K.; Nghiem, L.X. Phase Equilibria of Oil, Gas and Water/Brine Mixtures from a Cubic Equation of State and Henry’s Law. Can. J. Chem. Eng. 1986, 64, 486–496. [Google Scholar] [CrossRef]
- Bakker, R.J. Package FLUIDS 1. Computer Programs for Analysis of Fluid Inclusion Data and for Modelling Bulk Fluid Properties. Chem. Geol. 2003, 194, 3–23. [Google Scholar] [CrossRef]
- Bethke, C.M. Geochemical Reaction Modeling: Concepts and Applications; Oxford University Press: Oxford, UK, 1996; ISBN 978-0-19-509475-6. [Google Scholar]
- Pitzer, K.S. Thermodynamics of Electrolytes. I. Theoretical Basis and General Equations. J. Phys. Chem. 1973, 77, 268–277. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Kim, J.J. Thermodynamics of Electrolytes. IV. Activity and Osmotic Coefficients for Mixed Electrolytes. J. Am. Chem. Soc. 1974, 96, 5701–5707. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Gunter, W.D.; Aggarwal, P.K.; Perkins, E.H.; DeBraal, J.D. SOLMINEQ.88; a Computer Program for Geochemical Modeling of Water-Rock Interactions. Department of the Interior, US Geological Survey: Washington, DC, USA, 1988. [Google Scholar]
- Delany, J.M.; Lundeen, S.R. The LLNL Thermochemical Data Base—Revised Data and File Format for the EQ3/6 Package; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1991. [Google Scholar]
- Luo, S.; Xu, R.; Jiang, P. Effect of Reactive Surface Area of Minerals on Mineralization Trapping of CO2 in Saline Aquifers. Pet. Sci. 2012, 9, 400–407. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K. Analysis of Mineral Trapping for CO2 Disposal in Deep Aquifers; LBNL-6992; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2001; p. 789133. [Google Scholar]
- Cui, G.; Zhu, L.; Zhou, Q.; Ren, S.; Wang, J. Geochemical Reactions and Their Effect on CO2 Storage Efficiency during the Whole Process of CO2 EOR and Subsequent Storage. Int. J. Greenh. Gas Control 2021, 108, 103335. [Google Scholar] [CrossRef]
- Zeidouni, M.; Pooladi-Darvish, M.; Keith, D. Analytical Solution to Evaluate Salt Precipitation during CO2 Injection in Saline Aquifers. Int. J. Greenh. Gas Control 2009, 3, 600–611. [Google Scholar] [CrossRef]
- Machado, M.V.B.; Delshad, M.; Sepehrnoori, K. A Practical and Innovative Workflow to Support the Numerical Simulation of CO2 Storage in Large Field-Scale Models. SPE Reserv. Eval. Eng. J. 2023; in review. [Google Scholar]
- Xiao, Y.; Xu, T.; Pruess, K. The Effects of Gas-Fluid-Rock Interactions on CO2 Injection and Storage: Insights from Reactive Transport Modeling. Energy Procedia 2009, 1, 1783–1790. [Google Scholar] [CrossRef]
- Bennion, D.B.; Bachu, S. Drainage and Imbibition Relative Permeability Relationships for Supercritical CO2/Brine and H2S/Brine Systems in Intergranular Sandstone, Carbonate, Shale, and Anhydrite Rocks. SPE Reserv. Eval. Eng. 2008, 11, 487–496. [Google Scholar] [CrossRef]
- Land, C.S. Calculation of Imbibition Relative Permeability for Two- and Three-Phase Flow from Rock Properties. Soc. Pet. Eng. J. 1968, 8, 149–156. [Google Scholar] [CrossRef]
- Carlson, F.M. Simulation of Relative Permeability Hysteresis to the Nonwetting Phase; SPE: San Antonio, TX, USA, 1981; SPE-10157-MS. [Google Scholar]
- Jarrell, P.M. (Ed.) Practical Aspects of CO₂ Flooding; SPE Monograph Series; Henry L. Doherty Memorial Fund of AIME Society of Petroleum Engineers: Richardson, TX, USA, 2002; ISBN 978-1-55563-096-6. [Google Scholar]
- Burnside, N.M.; Naylor, M. Review and Implications of Relative Permeability of CO2/Brine Systems and Residual Trapping of CO2. Int. J. Greenh. Gas Control 2014, 23, 1–11. [Google Scholar] [CrossRef]
- Cremon, M.A.; Christie, M.A.; Gerritsen, M.G. Monte Carlo Simulation for Uncertainty Quantification in Reservoir Simulation: A Convergence Study. J. Pet. Sci. Eng. 2020, 190, 107094. [Google Scholar] [CrossRef]
- Liu, Q.; Maroto-Valer, M.M. Investigation of the pH Effect of a Typical Host Rock and Buffer Solution on CO2 Sequestration in Synthetic Brines. Fuel Process. Technol. 2010, 91, 1321–1329. [Google Scholar] [CrossRef]
- Machado, M.V.B.; Delshad, M.; Sepehrnoori, K. Injectivity Assessment for CCS Field-Scale Projects with Considerations of Salt Deposition, Mineral Dissolution, Fines Migration, Hydrate Formation, and Non-Darcy Flow. Fuel 2023, 353, 129148. [Google Scholar] [CrossRef]
- Machado, M.V.B.; Delshad, M.; Sepehrnoori, K. Potential Benefits of Horizontal Wells for CO2 Injection to Enhance Storage Security and Reduce Leakage Risks. Appl. Sci. 2023, 13, 12830. [Google Scholar] [CrossRef]
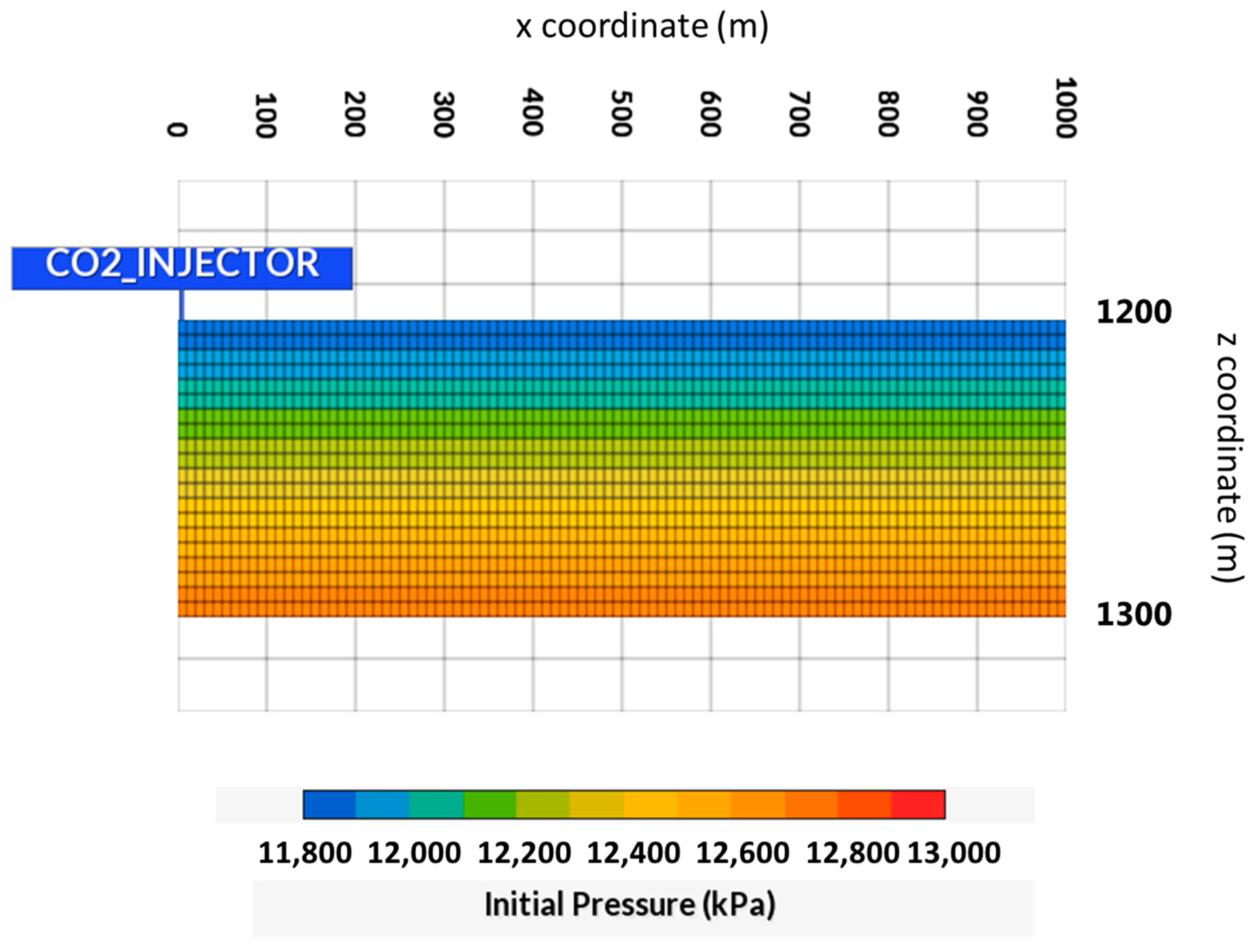

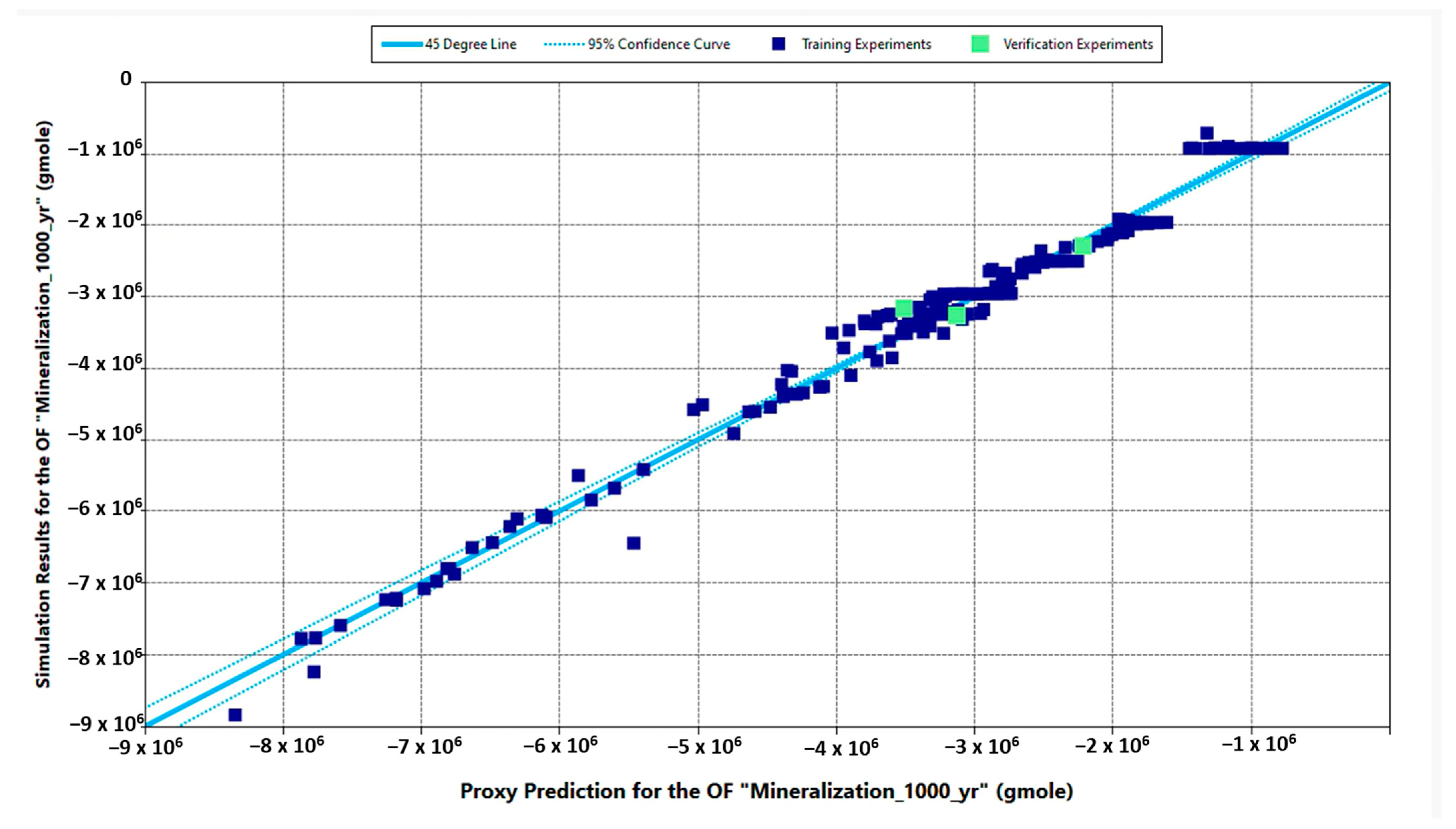
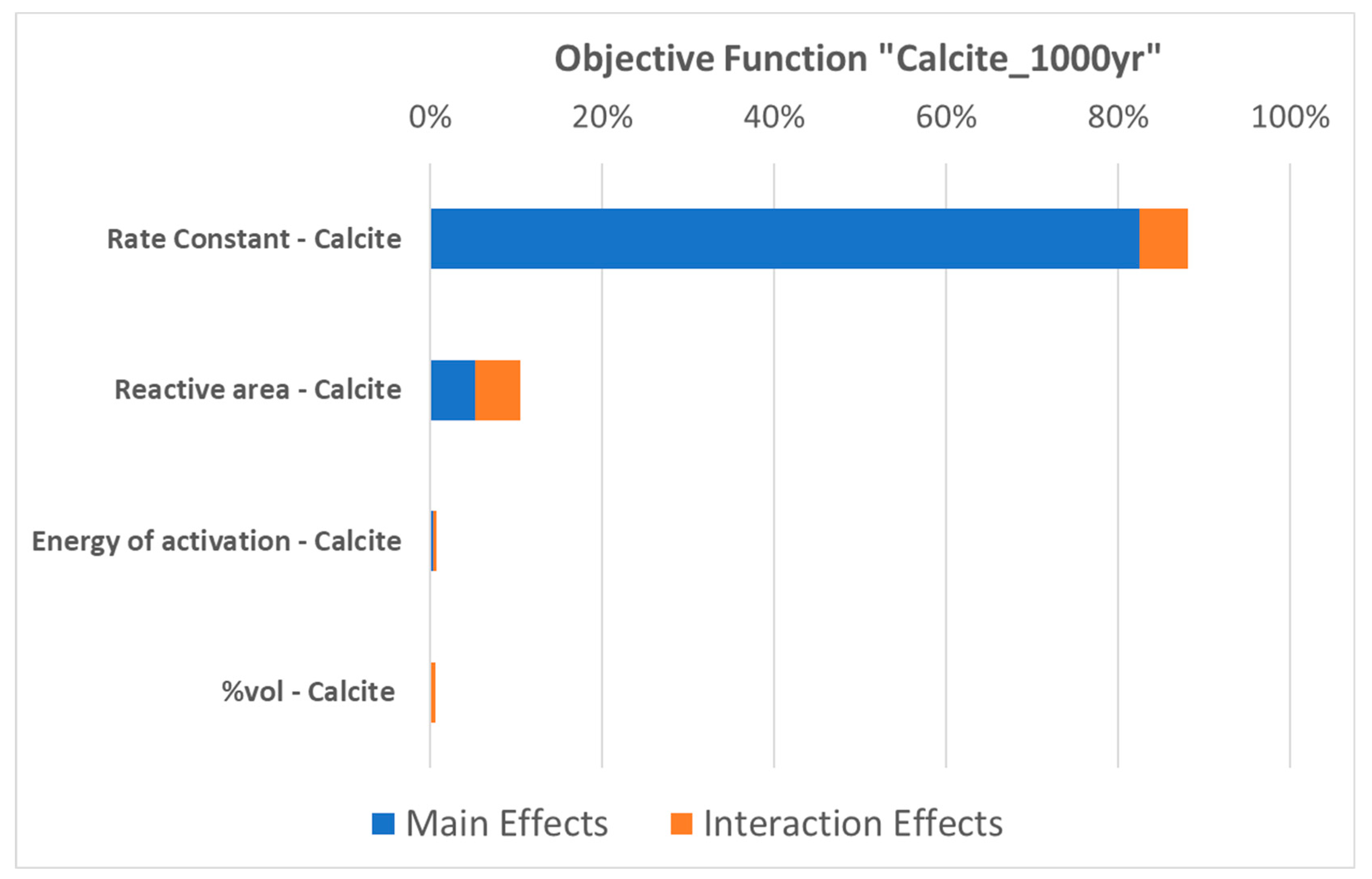
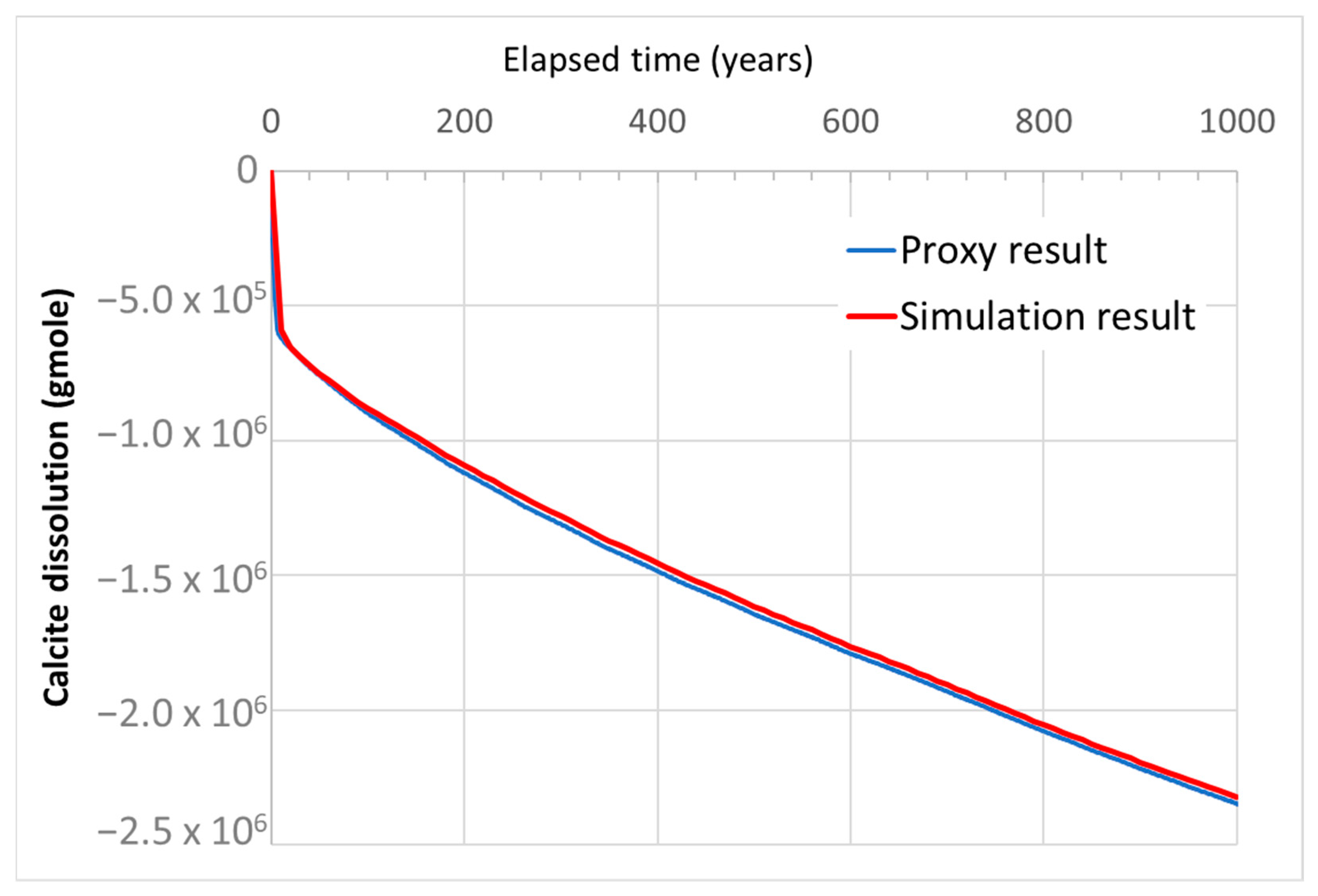

| Properties | Values |
|---|---|
| Initial pressure | 11,800 kPa @ 1200 m |
| Temperature | 80 °C |
| Salinity | 50,000 ppm (NaCl) |
| 5.66 × 105 |
| Ions | Concentration (mol/kgw) |
|---|---|
| H+ | 1.95618 × 10−6 |
| Ca2+ | 0.30308 |
| Mg2+ | 0.04185 |
| Na+ | 0.3234 |
| Cl− | 0.551 |
| HCO3− | 0.007482 |
| Mineral | Parameter | Value/Range | Source |
|---|---|---|---|
| Calcite | log10(Keq) | −8.38 | [5,8,46,47,48,49] |
| A | 88 to 23,000 m2/m3 | ||
| log10(k25) | −8.8 to −0.3 mol/m2s | ||
| Ea | 14,400 to 41,870 J/mol | ||
| Quartz | log10(Keq) | −4.38 | [5,8,46,47,49] |
| A | 2650 to 7128 m2/m3 | ||
| log10(k25) | −15 to −10 mol/m2s | ||
| Ea | 68,175 to 113,625 J/mol | ||
| Kaolinite | log10(Keq) | 9.80 | [5,8,46,47,50] |
| A | 1760 m2/m3 | ||
| log10(k25) | −13 mol/m2s | ||
| Ea | 62,760 J/mol | ||
| Albite | log10(Keq) | −19.7 | [5,8,16,46,47] |
| A | 2563.38 m2/m3 | ||
| log10(k25) | −10.16 mol/m2s | ||
| Ea | 65,000 J/mol | ||
| K-feldspar | log10(Keq) | −22.66 | [5,8,46,47,49] |
| A | 176 m2/m3 | ||
| log10(k25) | −12 mol/m2s | ||
| Ea | 67,830 J/mol | ||
| Illite | log10(Keq) | −43.28 | [5,8,16,46,47] |
| A | 41,888 m2/m3 | ||
| log10(k25) | −10.98 mol/m2s | ||
| Ea | 23,600 J/mol | ||
| Dolomite | log10(Keq) | −16.46 | [5,8,46,47,48,49] |
| A | 88 to 23,000 m2/m3 | ||
| log10(k25) | −9.22 to −3.19 mol/m2s | ||
| Ea | 36,100 to 62,760 J/mol | ||
| Siderite | log10(Keq) | −10.72 | [5,8,46,47] |
| A | 4046.67 m2/m3 | ||
| log10(k25) | −3.19 mol/m2s | ||
| Ea | 36,100 J/mol |
| Mineral Composition | Vol. % |
|---|---|
| Quartz | 1.0 |
| Kaolinite | 0.15 |
| Calcite | 68.68 |
| K-feldspar | 0.12 |
| Albite | 0.05 |
| Porosity | 30.0 |
| Property | Value |
|---|---|
| Initial pressure | 11,800 kPa @ 1200 m |
| Temperature | 80 °C |
| Horizontal permeability | 100 mD |
| Kv/Kh ratio | 0.1 |
| Porosity | 30% |
| Rock compressibility | 5.8 × 10−7 kPa−1 |
| Objective Function | Estimate | SR | MCS | Error SR and MCS (%) | Adjusted R2 (%) | Predicted R2 (%) |
|---|---|---|---|---|---|---|
| 600 Runs | 600 Runs | 600 Runs | 600 Runs | 600 Runs | ||
| “II” (m3/d.kPa) | P10 | 0.0025560 | 0.0025560 | 0.0 | 99.7 | 86.1 |
| P50 | 0.0025550 | 0.0025550 | 0.0 | |||
| P90 | 0.0025510 | 0.0025510 | 0.0 | |||
| “Mineralization_1000yr” (gmole) | P10 | −2.286 × 106 | −2.346 × 106 | 2.6 | 91.3 | 90.5 |
| P50 | −2.377 × 106 | −2.420 × 106 | 1.8 | |||
| P90 | −2.795 × 106 | −2.892 × 106 | 3.5 | |||
| “Calcite_1000yr” (gmole) | P10 | −2.286 × 106 | −2.346 × 106 | 2.6 | 91.3 | 90.5 |
| P50 | −2.377 × 106 | −2.420 × 106 | 1.8 | |||
| P90 | −2.796 × 106 | −2.892 × 106 | 3.4 | |||
| “Dolomite_1000yr” (gmole) | P10 | 57.70 | 55.2 | 4.3 | 82.0 | 80.0 |
| P50 | 53.45 | 53.0 | 0.8 | |||
| P90 | 51.71 | 50.4 | 2.5 | |||
| “Quartz_1000yr” (gmole) | P10 | It was not possible to calibrate a response surface because no changes were observed in relation to the initial content set for quartz throughout the simulation period. | ||||
| P50 | ||||||
| P90 | ||||||
| Property | Value | Unit |
|---|---|---|
| Rate Constant (k25)—Calcite (log10) | −8.44 | mol/m2s |
| Reactive area (A)—Calcite | 1202.26 | m2/m3 |
| Energy of activation (Ea)—Calcite | 28,047 | J/mol |
| Volumetric percentage (%vol)—Calcite | 34 | % |
| Numerical Experiment | P10/P90 Ratio for II |
|---|---|
| Section 3.1 and Section 3.2: Fixed CO2 injection rate of 0.5 metric tonne/day | 1.002 |
| Section 3.3: CO2 injection rate as a parameter, varying from 0.1 to 2.5 metric tonnes/day | 3.670 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, M.V.B.; Khanal, A.; Delshad, M. Unveiling the Essential Parameters Driving Mineral Reactions during CO2 Storage in Carbonate Aquifers through Proxy Models. Appl. Sci. 2024, 14, 1465. https://doi.org/10.3390/app14041465
Machado MVB, Khanal A, Delshad M. Unveiling the Essential Parameters Driving Mineral Reactions during CO2 Storage in Carbonate Aquifers through Proxy Models. Applied Sciences. 2024; 14(4):1465. https://doi.org/10.3390/app14041465
Chicago/Turabian StyleMachado, Marcos Vitor Barbosa, Aaditya Khanal, and Mojdeh Delshad. 2024. "Unveiling the Essential Parameters Driving Mineral Reactions during CO2 Storage in Carbonate Aquifers through Proxy Models" Applied Sciences 14, no. 4: 1465. https://doi.org/10.3390/app14041465





