Pre-Fermentative Cryogenic Treatments: The Effect on Aroma Compounds and Sensory Properties of Sauvignon Blanc and Chenin Blanc Wine—A Review
Abstract
:Highlights
- Pre-fermentative cryogenic treatments as a tool to increase the varietal thiol levels in Sauvignon blanc and Chenin blanc wines.
- Cryogenic pre treatment technologies as an innovative method for wine production.
Abstract
1. Introduction
2. Cryogenic Technologies
| Cryogenic Technology | Temperature (°C)/Time | Advantages | Disadvantages | Aroma Compounds Affected | Resultant Increase/ Decrease | References |
|---|---|---|---|---|---|---|
| Large capacity refrigerators | −8 to −20/9 h to 4 months | Cool large quantities of grapes/must/juice Conditions can be controlled | Increased costs and energy consumptionSlower rate of cooling Increased risk of undesirable flavour development | Bitter tannins | Decreases | [32,34,41,42,50] |
| Freeze-concentration | <0/10 min to 12 h | Wines produced from concentrated grape juice were superior in terms of chemical and sensory profiles Wineries with fermentation tanks with cooling systems have the potential to perform freeze-concentration without purchasing new equipment | High energy consumption, depending on the technology applied | Volatiles | Increase | [40,42,43,44,45,46,47,48,49] |
| Blast-freezing | −10 to −120/<25 min to 20 h | Allows for quick freezing of grape berries, <25 min | High energy consumption | Proanthocyanidins | Increase | [42,51,52] |
| Cryogenic agents, i.e., carbon dioxide (CO2) and nitrogen (N2) | −20/8 s to 24 h | Creates an inert atmosphere, protecting the grapes from oxidation Final wines were more aromatic than control wines | Increased cost | Terpenoids, hydroxylic compounds, fatty acids, anthocyanins | Increase | [32,36,42,53,54] |
3. Effect of Cryogenic Technologies on Physicochemical and Aroma Compounds of Grape Must and Wine
3.1. Physicochemical Parameters
3.2. Effect of Cryogenic Technologies on Grape Aroma Compounds
| Aroma Development Stage | Compound | Origin |
|---|---|---|
| Varietal | Precursors (free or bound) | Grape berry (skin and pulp) |
| Pre-fermentative | C6 compounds | Enzymatic/catalytic reactions due to processing (crushing of berries) |
| Fermentation | Ethyl esters, fusel alcohols, fatty acids, thiols | Microorganism metabolism (yeast and bacterial) |
| Post-fermentation | Oxidation of volatile aroma compounds; increase in fatty acids, esters, aldehydes, ketones, and polyphenols | Wine aging (bottle, barrel, storage, aging on lees) |
3.3. Effect of Cryogenic Technologies on Varietal Thiols
| Cultivar | Compound & Chemical Structure | Aroma Description | Aroma Perception in Wine (ng L–1) | Range in Wine (ng L–1) | Range in SA 1 Wine (ng L–1) |
|---|---|---|---|---|---|
| Sauvignon blanc | 4–methyl–4–sulfanylpentan–2–one (4MSP)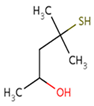 | Boxwood, blackcurrant | 0.8 | 0–88 | 0–21.9 |
| Chenin blanc | 0–23 | n.d. * | |||
| Sauvignon blanc | 3–sulfanylhexyl acetate (3SHA) 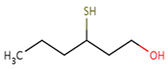 | Passionfruit, tropical, boxwood | 4 | 0–106 | 23–151 |
| Chenin blanc | 0–100 | 5–253 | |||
| Sauvignon blanc | 3–sulfanylhexan–1–ol (3SH) 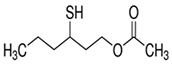 | Grapefruit, tropical, passionfruit | 60 | 350–5664 | 178–904 |
| Chenin blanc | 10–1368 | 99–1124 | |||
| Sauvignon blanc | benzyl mercaptan (BM)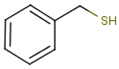 | Smoke, toasty, struck flint | 0.3 | 0.6–5.5 | n.d. * |
| Chenin Blanc | 30–40 | n.d. * | |||
| Sauvignon blanc | 2–furfurylthiol (FFT)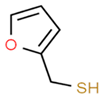 | Roasted Coffee | 0.4 | 1–36 | n.d. * |
| Chenin blanc | 14 | n.d. * |
3.4. Effect of Cryogenic Technologies on Methoxypyrazines
4. Effect of Cryogenic Technologies on Sensory Properties of Wine
5. Concluding Remarks and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neethling, E.; Petitjean, T.; Quénol, H.; Barbeau, G. Assessing local climate vulnerability and winegrowers’ adaptive processes in the context of climate change. Mitig. Adapt. Strat. Glob. Chang. 2017, 22, 777–803. [Google Scholar] [CrossRef]
- Kemp, B.; Botezatu, A.; Charnock, H.; Inglis, D.; Marchal, R.; Pickering, G.; Yang, F.; Willwerth, J. White winemaking in cold climates. In White Wine Technology; Academic Press: Cambridge, MA, USA, 2022; pp. 339–354, Chapter 26. [Google Scholar] [CrossRef]
- Reynolds, A.G. Viticultural and vineyard management practices and their effects on grape and wine quality. In Managing Wine Quality, 2nd ed.; Woodhead Publishing: Sawston, UK, 2022; Volume 1, pp. 443–539, Chapter 11. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2019, 99, 975–985. [Google Scholar] [CrossRef]
- Pinu, F.R.; Tumanov, S.; Grose, C.; Raw, V.; Albright, A.; Stuart, L.; Villas-Boas, S.G.; Martin, D.; Harker, R.; Greven, M. Juice Index: An integrated Sauvignon blanc grape and wine metabolomics database shows mainly seasonal differences. Metabolomics 2019, 15, 3. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Mattii, G.B. Effect of agronomic techniques on aroma composition of white grapevines: A Review. Agronomy 2021, 11, 2027. [Google Scholar] [CrossRef]
- Chen, K.; Li, J. A glance into the aroma of white wine. In White Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 313–326. [Google Scholar] [CrossRef]
- Robinson, J. Sauvignon Blanc Wine Grapes. 2021. Available online: https://www.jancisrobinson.com/learn/grape-varieties/white/sauvignon-blanc/ (accessed on 30 April 2021).
- Perestrelo, R.; Silva, C.; Pereira, J.; Câmara, J.S. Healthy effects of bioactive metabolites from Vitis vinifera L. grapes: A review. In Grapes: Production, Phenolic Composition and Potential Biomedical Effects; Câmara, J.S., Ed.; Nova Science Technology: Hauppauge, NY, USA, 2014; pp. 305–338. [Google Scholar]
- Prusova, B.; Humaj, J.; Sochor, J.; Baron, M. Formation, Losses, Preservation and Recovery of Aroma Compounds in the Winemaking Process. Fermentation 2022, 8, 93. [Google Scholar] [CrossRef]
- Parr, W.V.; Schlich, P.; Theobald, J.C.; Harsch, M.J. Association of selected viniviticultural factors with sensory and chemical characteristics of New Zealand Sauvignon blanc wines. Food Res. Int. 2013, 53, 464–475. [Google Scholar] [CrossRef]
- Hart, R.S.; Jolly, N.P.; Ndimba, B.K. Characterisation of hybrid yeasts for the production of varietal Sauvignon blanc wine-A review. J. Microbiol. Methods 2019, 165, 105699. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, C. Yeast and Its Ability to Release Thiols. 2021. Available online: https://www.wineland.co.za/yeast-and-its-ability-to-release-thiols/ (accessed on 5 March 2021).
- Průšová, B.; Sochor, J.; Baroň, M.; Kumšta, M. Effect of yeasts on the aroma profile of Sauvignon blanc varietal wine. Acta Univ. Agric. Silvic. Mendel. Brun. 2018, 66, 889–896. [Google Scholar] [CrossRef]
- Cerreti, M.; Esti, M.; Benucci, I.; Liburdi, K.; De Simone, C.; Ferranti, P. Evolution of S-cysteinylated and S-glutathionylated thiol precursors during grape ripening of Vitis vinifera L. cv. Grechetto, Malvasia del Lazio and Sauvignon blanc. Aust. J. Grape Wine Res. 2015, 21, 411–416. [Google Scholar] [CrossRef]
- Rice, S.; Tursumbayeva, M.; Clark, M.; Greenlee, D.; Dharmadhikari, M.; Fennell, A.; Koziel, J.A. Effects of harvest time on the aroma of white wines made from cold-hardy brianna and frontenac gris grapes using headspace solid-phase microextraction and gas chromatography-mass spectrometry-olfactometry. Foods 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine bunch rots: Impacts on wine composition, quality, and potential procedures for the removal of wine faults. J. Agric. Food Chem. 2016, 61, 5189–5206. [Google Scholar] [CrossRef] [PubMed]
- Dzedze, N.; van Breda, V.; Hart, R.S.; van Wyk, J. Wine chemical, sensory, aroma compound and protein analysis of wines produced from chemical and biological fungicide treated Chenin blanc grapes. Food Control 2019, 105, 265–276. [Google Scholar] [CrossRef]
- Hart, R.S.; Ndimba, B.K.; Jolly, N.P. Characterisation of thiol-releasing and lower volatile acidity-forming intra-genus hybrid yeast strains for Sauvignon blanc wine. S. Afr. J. Enol. Vitic. 2017, 38, 144–155. [Google Scholar] [CrossRef]
- Magyar, I.; Soos, J. Botrytized wines–current perspectives. Int. J. Wine Res. 2016, 8, 29–39. [Google Scholar] [CrossRef]
- Avizcuri-Inac, J.M.; González-Hernández, M.; Rosáenz-Oroz, D.; Martínez-Ruiz, R.; Vaquero-Fernández, L. Chemical and sensory characterisation of sweet wines obtained by different techniques. Cienc. Tec. Vitivinic. 2018, 33, 15–30. [Google Scholar] [CrossRef]
- Dankó, T.; Szelényi, M.; Janda, T.; Molnár, B.P.; Pogány, M. Distinct volatile signatures of bunch rot and noble rot. Physiol. Mol. Plant Pathol. 2021, 114, 101626. [Google Scholar] [CrossRef]
- Santos, H.; Augusto, C.; Reis, P.; Rego, C.; Figueiredo, A.C.; Fortes, A.M. Volatile metabolism of wine grape Trincadeira: Impact of infection with Botrytis cinerea. Plants 2022, 11, 141. [Google Scholar] [CrossRef]
- Cosme, F.; Gonçalves, B.; Inês, A.; Jordão, A.M.; Vilela, A. Grape and wine metabolites: Biotechnological approaches to improve wine quality. Grape Wine Biotechnol. 2016, 187–214. [Google Scholar] [CrossRef]
- Zhang, S.; Petersen, M.A.; Liu, J.; Toldam-Andersen, T.B. Influence of pre-fermentation treatments on wine volatile and sensory profile of the new disease tolerant cultivar Solaris. Molecules 2015, 20, 21609–21625. [Google Scholar] [CrossRef]
- Vilanova, M.; Escudero, A.; Graña, M.; Cacho, J. Volatile composition and sensory properties of Northwest Spain white wines. Food Res. Int. 2013, 54, 562–568. [Google Scholar] [CrossRef]
- Chen, L.; Capone, D.L.; Tondini, F.A.; Jeffery, D.W. Chiral polyfunctional thiols and their conjugated precursors upon winemaking with five Vitis vinifera Sauvignon blanc clones. J. Agric. Food Chem. 2018, 66, 4282–4674. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- Alti-Palacios, L.; Martínez, J.; Teixeira, J.A.; Câmara, J.S.; Perestrelo, R. Influence of cold pre-fermentation maceration on the volatilomic pattern and aroma of white wines. Foods 2023, 12, 1135. [Google Scholar] [CrossRef]
- Chen, L.; Capone, D.L.; Nicholson, E.L.; Jeffery, D.W. Investigation of intraregional variation, grape amino acids, and pre-fermentation freezing on varietal thiols and their precursors for Vitis vinifera Sauvignon blanc. Food Chem. 2019, 295, 637–645. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, A.; Durán-Guerrero, E.; Natera, R.; Palma, M.; Barroso, C.G. Influence of two different cryoextraction procedures on the quality of wine produced from muscat grapes. Foods 2020, 9, 1529. [Google Scholar] [CrossRef] [PubMed]
- Bestulić, E.; Rossi, S.; Plavša, T.; Horvat, I.; Lukić, I.; Bubola, M.; Peršurić, A.S.I.; Jeromel, A.; Radeka, S. Comparison of different maceration and non-maceration treatments for enhancement of phenolic composition, colour intensity, and taste attributes of Malvazija istarska (Vitis vinifera L.) white wines. J. Food Compos. Anal. 2022, 109, 104472. [Google Scholar] [CrossRef]
- Malićanin, M.; Danilović, B.; Stamenković Stojanović, S.; Cvetković, D.; Lazić, M.; Karabegović, I.; Savić, D. Pre-Fermentative Cold Maceration and Native Non-Saccharomyces Yeasts as a Tool to Enhance Aroma and Sensory Attributes of Chardonnay Wine. Horticulturae 2022, 8, 212. [Google Scholar] [CrossRef]
- Pedrosa-López, M.D.C.; Aragón-García, F.; Ruíz-Rodríguez, A.; Piñeiro, Z.; Durán-Guerrero, E.; Palma, M. Effects from the freezing of either whole or crushed grapes on the volatile compounds contents in Muscat wines. Foods 2022, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L.; du Toit, W. Cold maceration application in red wine production and its effects on phenolic compounds: A review. LWT Food Sci. Technol. 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Carillo, M.; Formato, A.; Fabiani, A.; Scaglione, G.; Pucillo, G.P. An inertizing and cooling process for grapes cryomaceration. Electron. J. Biotechnol. 2011, 14, 2–14. [Google Scholar] [CrossRef]
- Benucci, I.; Cerreti, M.; Liburdi, K.; Nardi, T.; Vagnoli, P.; Ortiz-Julien, A.; Esti, M. Pre-fermentative cold maceration in presence of non-Saccharomyces strains: Evolution of chromatic characteristics of Sangiovese red wine elaborated by sequential inoculation. Food Res. Int. 2018, 107, 257–266. [Google Scholar] [CrossRef] [PubMed]
- JagatiÄ, A.M.; Prusina, T.; IviÄ, S. Influence of cold maceration treatment on aromatic and sensory properties of Vugava wine (Vitis vinifera L.). J. Microbiol. Biotechnol. Food Sci. 2020, 10, 49–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Sheng, Q.; Chen, J.; Huang, W.; Zhan, J. Effect of suspension freeze-concentration technology on the quality of wine. S. Afr. J. Eno. Vitic. 2016, 37, 39–46. [Google Scholar] [CrossRef]
- Tomaz, I.; Šeparović, M.; Štambuk, P.; Preiner, D.; Maletić, E.; Karoglan Kontić, J. Effect of freezing and different thawing methods on the content of polyphenolic compounds of red grape skins. J. Food Process. Preserv. 2018, 42, e13550. [Google Scholar] [CrossRef]
- Tian, R.R.; Li, G.; Wan, S.B.; Pan, Q.H.; Zhan, J.C.; Li, J.M.; Zhang, Q.H.; Huang, W.D. Comparative study of 11 phenolic acids and five flavan-3-ols in cv. Vidal: Impact of natural icewine making versus concentration technology. Aust. J. Grape Wine Res. 2009, 15, 216–222. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Royo, J.B. Influence of the freezing method on the changes that occur in grape samples after frozen storage. J. Sci. Food Agric. 2013, 93, 3010–3015. [Google Scholar] [CrossRef] [PubMed]
- Petzold, G.; Niranjan, K.; Aguilera, J.M. Vacuum-assisted freeze concentration of sucrose solutions. J. Food Eng. 2013, 115, 357–361. [Google Scholar] [CrossRef]
- Petzold, G.; Moreno, J.; Lastra, P.; Rojas, K.; Orellana, P. Block freeze concentration assisted by centrifugation applied to blueberry and pineapple juices. Innov. Food Sci. Emerg. Technol. 2015, 30, 192–197. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Cheryan, M.; Sathe, S.K.; Salunkhe, D.K.; Luh, B.S. Freeze concentration of fruit juices. Critical Reviews in Food Sci. Nut. 1984, 20, 173–248. [Google Scholar] [CrossRef]
- Miyawaki, O.; Kato, S.; Watabe, K. Yield improvement in progressive freeze-concentration by partial melting of ice. J. Food Eng. 2012, 108, 377–382. [Google Scholar] [CrossRef]
- Ye, D.; Zhang, L.; Sun, S.; Chen, J.; Fang, T. Production of high-aroma instant tea powder using various innovative technologies. J. Food Proc. Eng. 2014, 37, 273–284. [Google Scholar] [CrossRef]
- Dempsey, P.; Bansal, P. The art of air blast freezing: Design and efficiency considerations. Appl. Therm. Eng. 2012, 41, 71–83. [Google Scholar] [CrossRef]
- Mencarelli, F.; Bellincontro, A. Recent advances in postharvest technology of the wine grape to improve the wine aroma. J. Sci. Food Agric. 2020, 100, 5046–5055. [Google Scholar] [CrossRef]
- Olarte Mantilla, S.M.; Collins, C.; Iland, P.G.; Kidman, C.M.; Jordans, C.; Bastian, S.E.P. Comparison of sensory attributes of fresh and frozen wine grape berries using Berry Sensory Assessment. Aust. J. Grape Wine Res. 2013, 19, 349–357. [Google Scholar] [CrossRef]
- Naviglio, D.; Formato, A.; Scaglione, G.; Montesano, D.; Pellegrino, A.; Villecco, F.; Gallo, M. Study of the grape cryo–maceration process at different temperatures. Foods 2018, 7, 107. [Google Scholar] [CrossRef]
- Naranjo, A.; Martínez-Lapuente, L.; Ayestarán, B.; Guadalupe, Z.; Pérez, I.; Canals, C.; Adell, E. Aromatic and sensory characterization of Maturana Blanca wines made with different technologies. Beverages 2021, 7, 10. [Google Scholar] [CrossRef]
- Jeffery, D.W. Spotlight on varietal thiols and precursors in grapes and wines. Aust. J. Chem. 2016, 69, 1323–1330. [Google Scholar] [CrossRef]
- Benkwitz, F.; Nicolau, L.; Lund, C.; Beresford, M.; Wohlers, M.; Kilmartin, P.A. Evaluation of key odorants in Sauvignon blanc wines using three different methodologies. J. Agric. Food Chem. 2012, 60, 6293–6302. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Coetzee, C.; du Toit, W.J. A comprehensive review on Sauvignon blanc aroma with a focus on certain positive volatile thiols. Food Res. Int. 2012, 45, 287–298. [Google Scholar] [CrossRef]
- October, F.M. Effect of Yeasts and Oenological Parameters on Acetaldehyde Production during Alcoholic Fermentation of South African Grape Musts. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2020. [Google Scholar]
- Pinu, F.R.; Edwards, P.J.; Jouanneau, S.; Kilmartin, P.A.; Gardner, R.C. Villas-Boas, S.G. Sauvignon blanc metabolomics: Grape juice metabolites affecting the development of varietal thiols and other aroma compounds in wines. Metabolomics 2014, 10, 556–573. [Google Scholar] [CrossRef]
- Moreno-Pérez, A.; Vila-López, R.; Fernández-Fernández, J.I.; Martínez-Cutillas, A.; Gil-Muñoz, R. Influence of cold pre-fermentation treatments on the major volatile compounds of three wine varieties. Food Chem. 2013, 139, 770–776. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Weightman, C.; Panzeri, V.; Nieuwoudt, H.H.; Du Toit, W.J. Effect of skin contact before and during alcoholic fermentation on the chemical and sensory profile of South African Chenin blanc white wines. S. Afr. J. Enol Vitic. 2015, 36, 366–377. [Google Scholar] [CrossRef]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Influence of harvesting technique and maceration process on aroma and phenolic attributes of Sauvignon blanc wine. Food Chem. 2015, 183, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, É.; Pedneault, K. Impact of frozen storage on the free volatile compound profile of grape berries. Am. J. Enol. Vitic. 2016, 67, 239–244. [Google Scholar] [CrossRef]
- Bonnaffoux, H.; Delpech, S.; Rémond, E.; Schneider, R.; Roland, A.; Cavelier, F. Revisiting the evaluation strategy of varietal thiol biogenesis. Food Chem. 2018, 268, 126–133. [Google Scholar] [CrossRef]
- Wilson, C.L. Chemical Evaluation and Sensory Relevance of Thiols in South African Chenin Blanc Wines. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2017. Available online: https://hdl.handle.net/10019.1/101250 (accessed on 15 March 2021).
- Coetzee, C.; Schulze, A.; Mokwena, L.; Du Toit, W.J.; Buica, A. Investigation of thiol levels in young commercial South African Sauvignon blanc and Chenin blanc wines using propiolate derivatization and GC-MS/MS. S. Afr. J. Enol. Vitic. 2018, 39, 180–184. [Google Scholar] [CrossRef]
- Wilson, C.; Brand, J.; du Toit, W.; Buica, A. Matrix effects influencing the perception of 3-mercaptohexan-1-ol (3MH) and 3-mercaptohexyl acetate (3MHA) in different Chenin blanc wines by Projective Mapping (PM) with Ultra Flash profiling (UFP) intensity ratings. Food Res. Int. 2019, 121, 633–640. [Google Scholar] [CrossRef]
- Hart, R.S.; Jolly, N.P.; Mohamed, G.; Booyse, M.; Ndimba, B.K. Characterisation of Saccharomyces cerevisiae hybrids selected for low volatile acidity formation and the production of aromatic Sauvignon blanc wine. Afr. J. Biotechnol. 2016, 15, 2068–2081. [Google Scholar] [CrossRef]
- Visan, L.; Tamba-Berehoiu, R.M.; Popa, C.N.; Danaila–Guidea, S.M.; Culea, R. Aromatic compounds in wines. Sci. Papers 2018, 18, 423–430. [Google Scholar]
- Dimopoulou, M.; Troianou, V.; Toumpeki, C.; Gosselin, Y.; Dorignac, É.; Kotseridis, Y. Effect of strains from different Saccharomyces species used in different inoculation schemes on chemical composition and sensory characteristics of Sauvignon blanc wine. OENO One 2020, 54, 745–759. [Google Scholar] [CrossRef]
- Capone, D.L.; Jeffery, D.W. Effects of transporting and processing Sauvignon blanc grapes on 3-mercaptohexan-1-ol precursor concentrations. J. Agric. Food Chem. 2011, 59, 4659–4667. [Google Scholar] [CrossRef]
- Capone, D.L.; Ristic, R.; Pardon, K.H.; Jeffery, D.W. Simple quantitative determination of potent thiols at ultratrace levels in wine by derivatization and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) analysis. Anal. Chem. 2015, 87, 1226–1231. [Google Scholar] [CrossRef]
- Casassa, L.F.; Sari, S.E. Sensory and chemical effects of two alternatives of pre-fermentative cold soak in Malbec wines during winemaking and bottle ageing. Int. J. Food Sci. Technol. 2015, 50, 1044–1055. [Google Scholar] [CrossRef]
- Suklje, K.; Gobler, N.; Coetzee, Z.; Lisjak, K.; Deloire, A. Methoxypyrazines and Greenness in Wines: Myth or Reality? A Few Perspectives. Wineland. 2013. Available online: https://www.wineland.co.za/methoxypyrazines-greenness-wines-myth-reality-perspectives/ (accessed on 2 December 2021).
- Sidhu, D.; Lund, J.; Kotseridis, Y.; Saucier, C. Methoxypyrazine analysis and influence of viticultural and enological procedures on their levels in grapes, musts, and wines. Crit. Rev. Food Sci. Nutr. 2015, 55, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Xie, S.; Guan, X.; Song, C.; Zhang, Z.; Meng, J. Methoxypyrazines biosynthesis and metabolism in grape: A review. Food Chem. 2018, 245, 1141–1147. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Alvarez, I.; Lizama, V.; Nieuwoudt, H.; Garcia, M.J.; Aleixandre, J.L.; Du Toit, W.J. Modelling phenolic and volatile composition to characterize the effects of pre-fermentative cold soaking in Tempranillo wines. LWT Food Sci. Technol. 2016, 66, 193–200. [Google Scholar] [CrossRef]
- Baron, M.; Prusova, B.; Tomaskova, L.; Kumsta, M.; Sochor, J. Terpene content of wine from the aromatic grape variety ‘Irsai Oliver’ (Vitis vinifera L.) depends on maceration time. Open Life Sci. 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 2. Chemical and sensory analysis. Am. J. Enol. Vitic. 2014, 65, 25–42. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Sykalia, D.; Mannu, A.; Badeka, A.V. Physico–chemical parameters complemented with aroma compounds fired up the varietal discrimination of wine using statistics. Eur. Food Res. Technol. 2020, 246, 2233–2248. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Longobardi, F.; Ventrella, A.; Agostiano, A.; Del Nobile, M.A. Effects of different vinification technologies on physical and chemical characteristics of Sauvignon blanc wines. Food Chem. 2012, 135, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
| Compound & Chemical Structure | Aroma Description | Aroma Perception in Water (ng L–1) | Aroma Perception in Wine (ng L–1) | Range in Wine (ng L–1) |
|---|---|---|---|---|
2–methoxy–3–isobutylpyrazine (ibMP)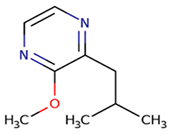 | vegetative, green pepper | 1–2 | 2–163 | 2–30 |
2–methoxy–3–isopropylpyrazine (ipMP) | earthy, mushroom, cooked, or canned asparagus, green beans | 1–2 | 2–16 | <10 |
2–methoxy–3–sec–butylpyrazine (sbMP)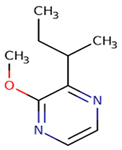 | green (peas, bell pepper, galbanum), ivy leaves, bell pepper | 1–2 | 2–16 | <10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Breda, V.M.; van Jaarsveld, F.P.; van Wyk, J. Pre-Fermentative Cryogenic Treatments: The Effect on Aroma Compounds and Sensory Properties of Sauvignon Blanc and Chenin Blanc Wine—A Review. Appl. Sci. 2024, 14, 1483. https://doi.org/10.3390/app14041483
van Breda VM, van Jaarsveld FP, van Wyk J. Pre-Fermentative Cryogenic Treatments: The Effect on Aroma Compounds and Sensory Properties of Sauvignon Blanc and Chenin Blanc Wine—A Review. Applied Sciences. 2024; 14(4):1483. https://doi.org/10.3390/app14041483
Chicago/Turabian Stylevan Breda, Valmary M., Francois P. van Jaarsveld, and Jessy van Wyk. 2024. "Pre-Fermentative Cryogenic Treatments: The Effect on Aroma Compounds and Sensory Properties of Sauvignon Blanc and Chenin Blanc Wine—A Review" Applied Sciences 14, no. 4: 1483. https://doi.org/10.3390/app14041483






