Pretreatments Applied to Wheat Straw to Obtain Bioethanol
Abstract
:1. Introduction
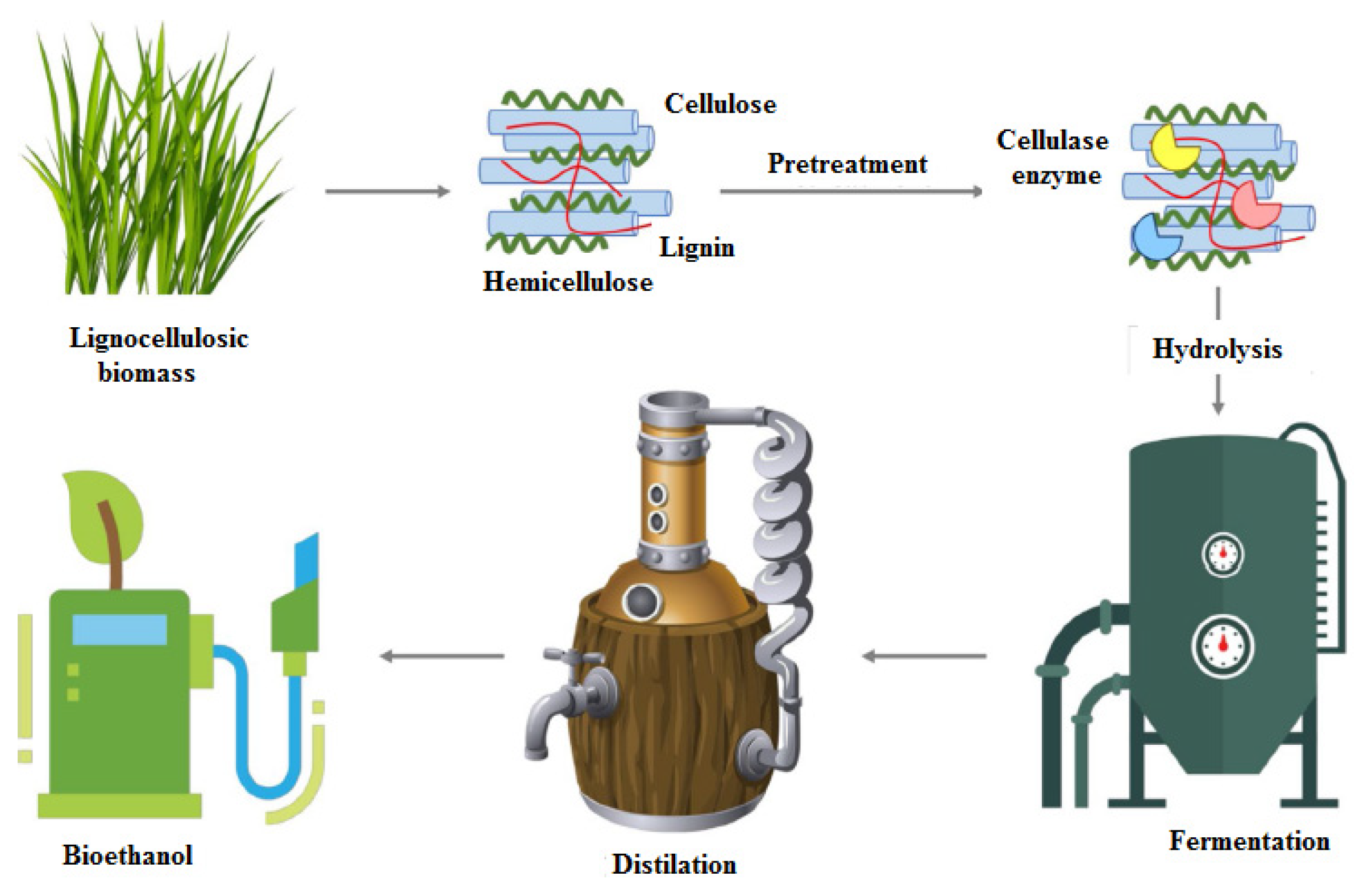
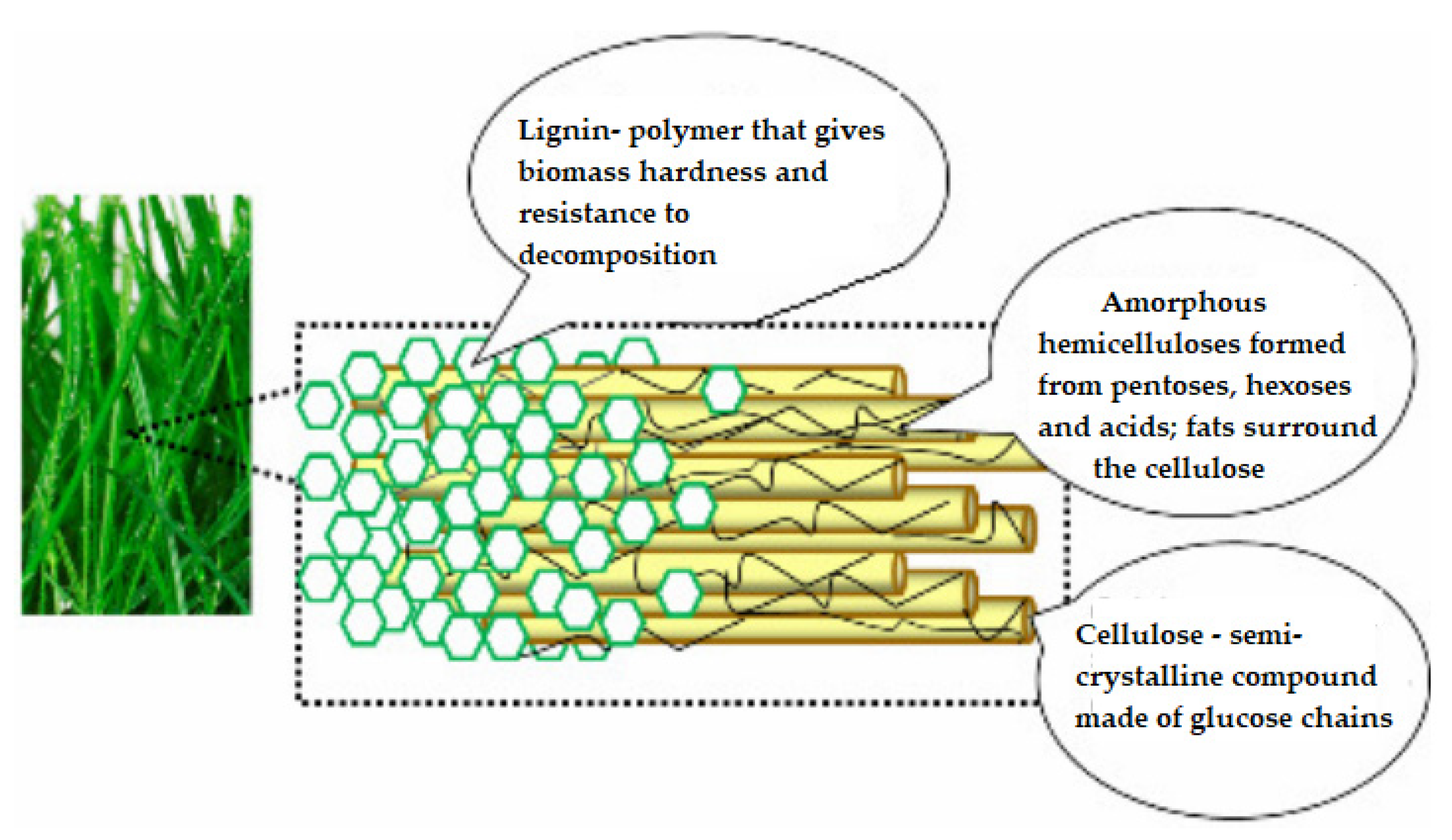
2. Structure of Lignocellulosic Biomass
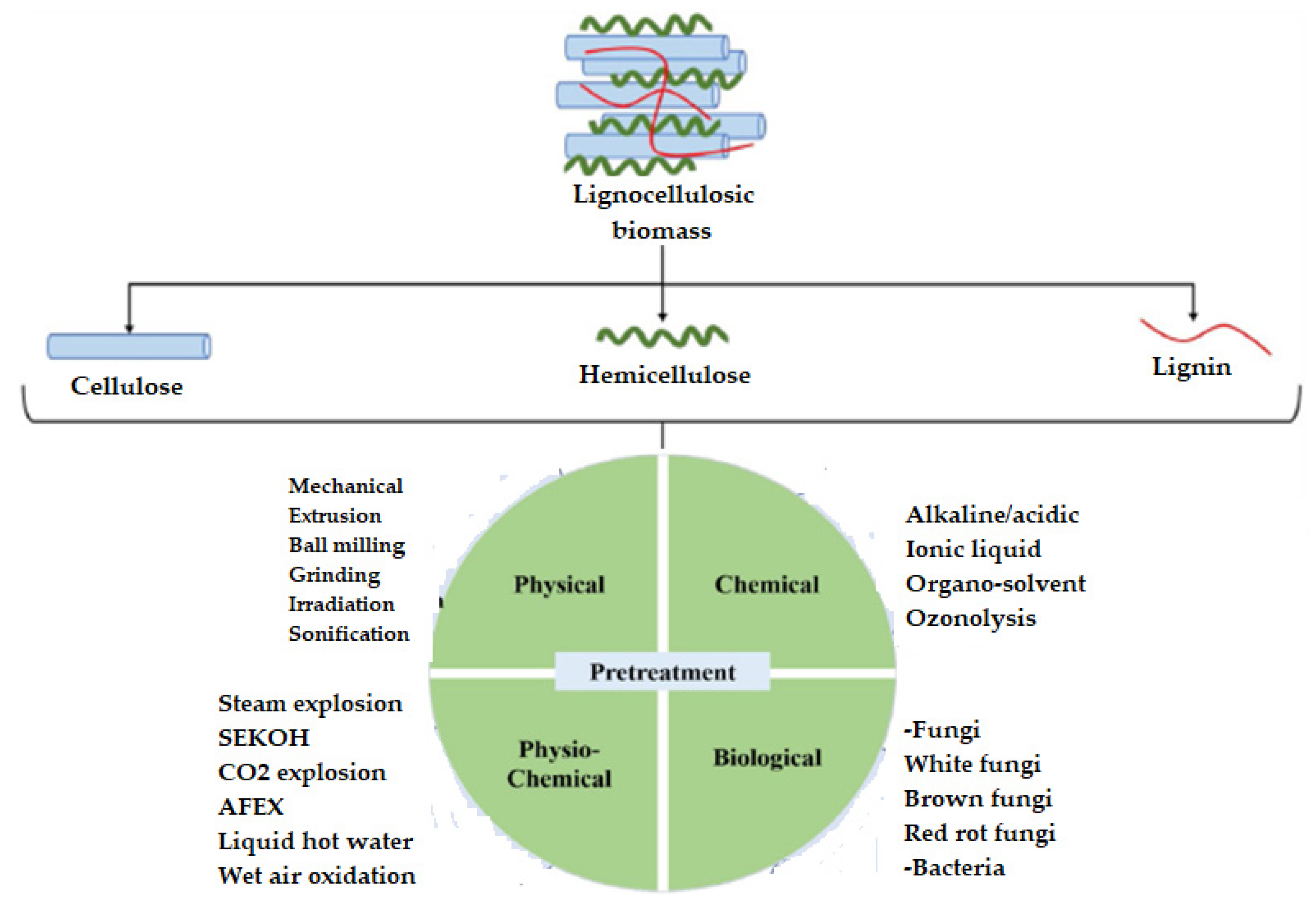
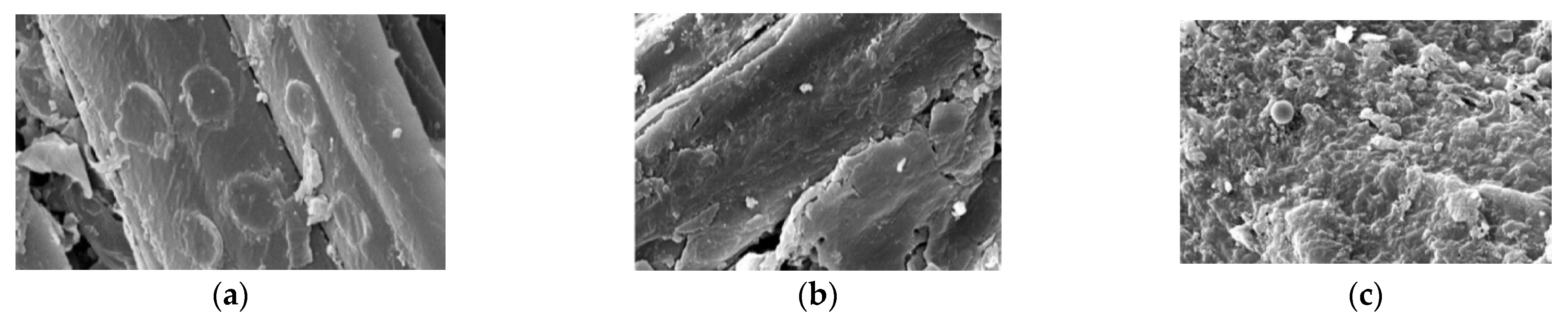
3. Pretreatments Applied to Wheat Straw
3.1. Physical Pretreatment
3.1.1. Mechanical Pretreatment
3.1.2. Irradiation of Wheat Straw
3.1.3. Pretreatment of Wheat Straw by Extrusion
3.2. Chemical Pretreatments Applied to Wheat Straw
3.2.1. Alkaline Pretreatment
3.2.2. Pretreatment with Organic Solvent
3.2.3. Pretreatment with Acid
3.2.4. Ionic Liquid Pretreatment
3.3. Physico-Chemical Pretreatment
3.3.1. Hydrothermal Pretreatment of Lignocellulosic Biomass
3.3.2. Pretreatment of Wheat Straw by Steam Explosion
3.4. Biological Pretreatment of Wheat Straw
4. Pretreatment Limitations
- -
- Pretreatment with an organic solvent has a low biomass recovery rate. The optimization of the pretreatment conditions depends on the pretreatment time and temperature, the type of catalysts and the solvent concentration [93];
- -
- During biological pretreatment, lignin derivatives can poison microorganisms.
- -
- -
- -
- In pretreatment with ionic liquid, there are no economic solutions for recycling the ionic liquid [40];
- -
- Chemical pretreatment with acid and alkaline implies a requirement of intensive energy, high costs, and harmful by-products [40].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EUR-Lex. Energii Regenerabile. Available online: https://eur-lex.europa.eu/RO/legal-content/summary/renewable-energy.html (accessed on 19 July 2023).
- Teme. Pactul Verde: Cheia unei Uniuni Neutre Climatic și Sustenabile. Available online: https://www.europarl.europa.eu/news/ro/headlines/society/20200618STO81513/pactul-verde-cheia-unei-uniuni-neutre-climatic-si-sustenabile?&at_campaign=20234-Green&at_medium=Google_Ads&at_platform=Search&at_creation=RSA&at_goal=TR_G&at_audience=pactul%20verde%20european&at_topic=Green_Deal&at_location=RO&gclid=Cj0KCQjw2eilBhCCARIsAG0Pf8uMUIGBaVGqGZ64gNTOhx2yrg8EsbP8OK2xgjMq2rrnkMC1M6ivG3waAtLHEALw_wcB (accessed on 19 July 2023).
- Fișe Descriptive despre Uniunea Europeană. Energia din Surse Regenerabile. Available online: https://www.europarl.europa.eu/factsheets/ro/sheet/70/energia-din-surse-regenerabile (accessed on 29 July 2023).
- Consiliul Uniunii Europene. Raport Al Comisiei Către Parlamentul European, Consiliu, Comitetul Economic Și Social European Și Comitetul Regiunilor. Available online: https://data.consilium.europa.eu/doc/document/ST-11866-2020-INIT/ro/pdf (accessed on 19 July 2023).
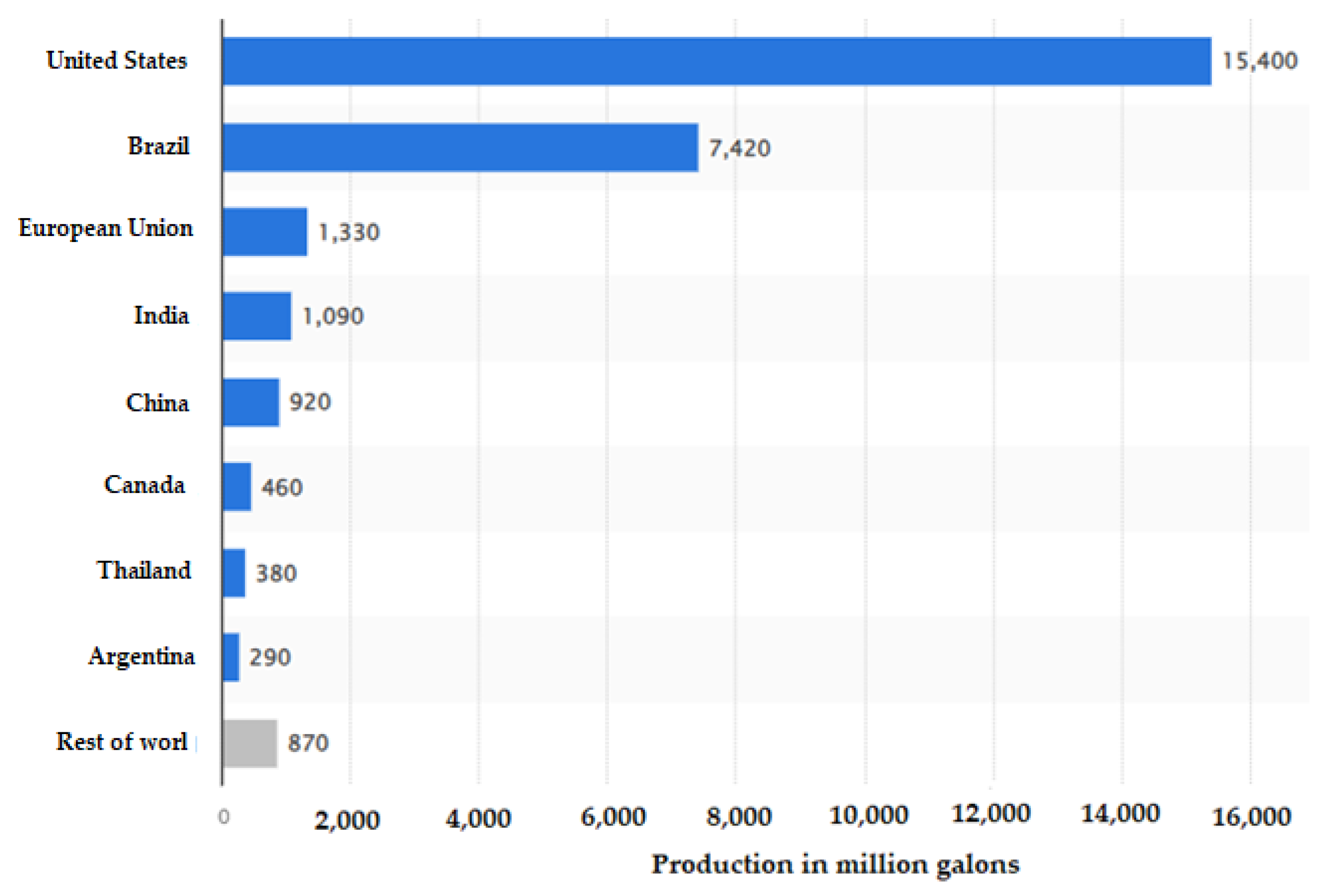
- Statista. Fuel Ethanol Production Worldwide in 2022, by Country. Available online: https://www.statista.com/statistics/281606/ethanol-production-in-selected-countries (accessed on 10 July 2023).
- Babu, S.; Rathore, S.S.; Singh, R.; Kumar, S.; Singh, V.K.; Yadav, S.K.; Yadav, V.; Raj, R.; Yadav, D.; Shekawat, K.; et al. Exploring agricultural waste biomass for energy, food and feed production and pollution mitigation: A review. Bioresour. Technol. 2022, 360, 127566. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Matarazzo, A.; Gorjian, S.; Adamczyk, J.; Failla, S.; Primerano, P.; Huisingh, D. Wheat-straw derived bioethanol production: A review of Life Cycle Assessments. Sci. Total Environ. 2021, 781, 146751. [Google Scholar] [CrossRef]
- Ibrahim, H.A.H. Pretreatment of straw for bioethanol production. Energy Procedia 2012, 14, 542–551. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Lamb, W.F.; Wiedmann, T.; Pongratz, J.; Andrew, R.; Crippa, M.; Olivier, J.G.J.; Wiedenhofer, D.; Mattioli, G.; Khourdajie, A.A.; House, J.; et al. A review of trends and drivers of greenhouse gas emissions by sector from 1990 to 2018. Environ. Res. Lett. 2021, 16, 7. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.; Serwa’nska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Efemwenkiekie, U.K.; Oyedepo, S.O.; Idiku, U.D.; Uguru-Okorie, D.C.; Kuhe, A. Comparative Analysis of a Four Stroke Spark Ignition Engine Performance Using Local Ethanol and Gasoline Blends. Procedia Manuf. 2019, 35, 1079–1086. [Google Scholar] [CrossRef]
- Gabisa, E.W.; Gheewala, S.H. Can Substitution of Imported Gasoline by Locally Produced Molasses Ethanol in Ethiopia Be Sustainable? An Eco-Efficiency Assessment. Renew. Sustain. Energy Rev. 2020, 123, 109770. [Google Scholar] [CrossRef]
- Iodice, P.; Senatore, A.; Langella, G.; Amoresano, A. Advantages of Ethanol–Gasoline Blends as Fuel Substitute for Last Generation Si Engines. Environ. Prog. Sustain. Energy 2017, 36, 1173–1179. [Google Scholar] [CrossRef]
- Anderson, S.T. The Demand for Ethanol as a Gasoline Substitute. J. Environ. Econ. Manag. 2012, 63, 151–168. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Current State-of-the-Art in Ethanol Production from Lignocellulosic Feedstocks. Microbiol. Res. 2020, 240, 126534. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, E.; Vandenberghe, L.P.S.; Soccol, C.R.; Sigoillot, J.-C.; Faulds, C. First Generation Bioethanol. In Green Fuels Technology: Biofuels; Green Energy and Technology; Soccol, C.R., Brar, S.K., Faulds, C., Ramos, L.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 175–212. ISBN 978-3-319-30205-8. [Google Scholar]
- Murawski de Mello, A.F.; Porto de Souza Vandenberghe, L.; Valladares-Diestra, K.K.; Amaro Bittencourt, G.; Martinez Burgos, W.J.; Soccol, C.R. Corn First-Generation Bioethanol Unities with Energy and Dried Grains with Solubles (DDGS) Production. In Liquid Biofuels: Bioethanol; Biofuel and Biorefinery Technologies; Soccol, C.R., Amarante Guimarães Pereira, G., Dussap, C.-G., Porto de Souza Vandenberghe, L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 109–132. ISBN 978-3-031-01241-9. [Google Scholar]
- Wati, L.; Kumari, S.; Kundu, B.S. Paddy straw as substrate for ethanol production. Ind. J. Microbiol. 2007, 47, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Vlasenko, E.Y.; Ding, H.; Labavitch, J.M.; Shoemaker, S.P. Enzymatic hydrolysis of pretreated rice straw. Bioresour. Technol. 1997, 59, 109–119. [Google Scholar] [CrossRef]
- Chen, Y.; Sharma-Shivappa, R.R.; Chen, C. Ensiling agricultural residues for bioethanol production. Appl. Biochem. Biotechnol. 2007, 143, 80–82. [Google Scholar] [CrossRef]
- Ogawa, M.; Yoshida, N. Nitrous oxide emission from the burning of agricultural residue. Atmos. Environ. 2005, 39, 3421–3429. [Google Scholar] [CrossRef]
- Sarkar, N.; Kumar Ghosh, S.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renewable Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- CarbonBrief. Q&A: European Commission calls for 90% cut in EU emissions by 2040. Available online: https://www.carbonbrief.org/qa-european-commission-calls-for-90-cut-in-eu-emissions-by-2040/ (accessed on 7 February 2024).
- Offshore Energy. Eight countries take steps to erase fossil fuels from EU’s power system as ‘ground-breaking’ new law comes to light. Available online: https://www.offshore-energy.biz/eight-countries-take-steps-to-erase-fossil-fuels-from-eus-power-system-as-ground-breaking-new-law-comes-to-light/ (accessed on 7 February 2024).
- Singh, R.; Srivastava, M.; Shukla, A. Environmental sustainability of bioethanol production from rice straw in India: A review. Renew. Sustain. Energy Rev. 2016, 54, 202–216. [Google Scholar] [CrossRef]
- Akhtar, N.; Goyal, D.; Goyal, A. Characterization of microwave-alkali-acid pre-treated rice straw for optimization of ethanol production via simultaneous saccharification and fermentation (SSF). Energy Convers. Manag. 2017, 141, 133–144. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Taherzadeh, M.; Karimi, K. Acid-based Hydrolysis Processes for Ethanol from Lignocellulosic Materials: A Review. BioResources 2007, 2, 472–499. [Google Scholar] [CrossRef]
- Tian, S.Q.; Zhao, R.Y.; Chen, Z.C. Review of the pretreatment and bioconversion of lignocellulosic biomass from wheat straw materials. Renew. Sustain. Energy Rev. 2018, 91, 483–484. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- García, J.C.; Díaz, M.J.; Garcia, M.T.; Feria, M.J.; Gómez, D.M.; López, F. Search for optimum conditions of wheat straw hemicelluloses cold alkaline extraction process. Biochem. Eng. J. 2013, 71, 127–133. [Google Scholar] [CrossRef]
- Bensah, E.C.; Mensah, M. Chemical pretreatment methods for the production of cellulosic ethanol: Technologies and innovations. Int. J. Chem. Eng. 2013, 21, 719607. [Google Scholar] [CrossRef]
- Adapa, P.; Tabil, L.; Schoenau, G. Grinding performance and physical properties of non-treated and steam exploded barley, canola, oat and wheat straw. Biomass Bioenergy 2010, 35, 549–561. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- García-Torreiro, M.; Lopez-Abelairas, M.; Lu-Chau, T.A.; Lema, J.M. Fungal pretreatment of agricultural residues for bioethanol production. Ind. Crop. Prod. 2016, 89, 486–492. [Google Scholar] [CrossRef]
- Dela Rosa, A.M.; Dela Mines, A.S.; Banzon, R.B.; Simbul-Nuguid, Z.F. Radiation pretreatment of cellulose for energy production. Radiat. Phys. Chem. 1983, 22, 861–867. [Google Scholar] [CrossRef]
- Huang, L.Z.; Ma, M.G.; Ji, X.X.; Choi, S.E.; Si, C. Recent Developments and Applications of Hemicellulose from Wheat Straw: A Review. Front. Bioeng. Biotechnol. 2021, 9, 690773. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Rehman, M.S.U.; Terán-Hilares, R.; Khalid, S.; Han, J.I. Optimization of twin gear-based pretreatment of rice straw for bioethanol production. Energy Convers. Manag. 2017, 141, 120–125. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Wang, S.; Wang, K.; Su, X. Comparison of irradiation with other pretreatments followed with simultaneous saccharification and fermentation on bioconversion of microcrystalline cellulose for bioethanol production. Bioresour Technol. 2015, 182, 289–295. [Google Scholar] [CrossRef]
- Harun, R.; Jason, W.S.Y.; Cherrington, T.; Danquah, M.K. Microalgal Biomass as Cellulosic Fermentation Feedstock for Bioethanol Production. Renew. Sust. Energy Rev. 2010, 85, 199–303. [Google Scholar] [CrossRef]
- Tian, D.; Shen, F.; Yang, G.; Deng, S.; Long, L.; He, J. Liquid hot water extraction followed by mechanical extrusion as a chemical-free pretreatment approach for cellulosic ethanol production from rigid hardwood. Fuel 2019, 252, 589–597. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, M. Extrusion as a pretreatment for lignocellulosic biomass: Fundamentals and applications. Renew. Energy 2017, 114, 1427–1441. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, D.; Bean, S.R.; Mo, X.; Sun, X.S.; Boyle, D. Ethanol production from supercritical fluid extrusion cooked sorghum. Ind. Crops Prod. 2006, 23, 304–310. [Google Scholar] [CrossRef]
- Karunanithy, C.; Muthukumarappan, K. Effect of extruder parameters and moisture content of switchgrass, prairie cord grass on sugar recovery from enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2010, 162, 1785–1803. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Negro, M.J.; Oliva, J.M.; Saez, F.; Ballesteros, M. Study of process configuration and catalyst concentration in integrated alkaline extrusion of barley straw for bioethanol production. Fuel 2014, 134, 448–454. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Y.; Hanna, M.A. Pretreatment of corn stover with twin-screw extrusion followed by enzymatic saccharification. Appl. Biochem. Biotechnol. 2012, 166, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Alavi, S.; Vadlani, P.; Amanor-Boadu, V. Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to fermentable sugars. Bioresour. Technol. 2011, 102, 7583–7590. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, Y.; Kapu, N.S.; Beatson, R. Evaluation of an organosolv-based biorefinery process to fractionate wheat straw into ethanol and co-products. Ind. Crop. Prod. 2018, 121, 294–302. [Google Scholar] [CrossRef]
- Sun, F.F.; Wang, L.; Hong, J.; Ren, J.; Du, F.; Hu, J.; Zhang, Z.; Zhou, B. The impact of glycerol organosolv pretreatment on the chemistry and enzymatic hydrolyzability of wheat straw. Bioresour. Technol. 2015, 187, 354–361. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, Y.; Li, G. Production of bioethanol and value added compounds from wheat straw through combined alkaline/alkaline-peroxide pretreatment. Bioresour. Technol. 2018, 259, 228–236. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic solvent effects in biomass conversion reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.T.; Huijgen, W.J.J. The promotional effect of water-soluble extractives on the enzymatic cellulose hydrolysis of pretreated wheat straw. Bioresour. Technol. 2017, 243, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Ali, M.F.; Abdeltawab, A.A.; Yakout, S.M.; Yu, G. Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for improving enzymatic hydrolysis. Bioresour. Technol. 2018, 263, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Tsapekos, P.; Kougias, P.G.; Egelund, H.; Larsen, U.; Pedersen, J.; Trénel, P.; Angelidaki, I. Mechanical pretreatment at harvesting increases the bioenergy output from marginal land grasses. Renew. Energy 2017, 111, 914–921. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatmentof lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Naresh Kumar, M.; Ravikumar, R.; Thenmozhi, S.; Kirupa Sankar, M. Development of natural cellulase inhibitor mediated intensified biological pretreatment technology using Pleurotus florida for maximum recovery of cellulose from paddy straw under solid state condition. Bioresour. Technol. 2017, 244, 353–361. [Google Scholar] [CrossRef]
- Sharma, H.K.; Xu, C.; Qin, W. Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: An overview. Waste Biomass Valoriz. 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Xie, C.; Gong, W.; Yang, Q.; Zhu, Z.; Yan, L.; Hu, Z.; Peng, Y. White-rot fungi pretreatment combined with alkaline/oxidative pretreatment to improve enzymatic saccharification of industrial hemp. Bioresour Technol. 2017, 243, 188–195. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Supriya, R.D.; Sindhu, R.; Binod, P.; Nair, R.B.; Pandey, A.; Gnansounou, E. Biological pretreatment of lignocellulosic biomass-current trends and future perspectives. In Second and Third Generation of Feedstocks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–212. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, R.; Abomohra, A.E.; Xie, S.; Yu, Z.; Guo, Q. A sustainable approach for efficient conversion of lignin into biodiesel accompanied by biological pretreatment of corn straw. Energy Convers. Manag. 2019, 199, 111928. [Google Scholar] [CrossRef]
- da Costa Lopes, A.M.; João, K.G.; Rubik, D.F.; Bogel-Łukasik, E.; Duarte, L.C.; Andreaus, J.; Bogel-Łukasik, R. Pre-treatment of lignocellulosic biomass using ionic liquids: Wheat straw fractionation. Bioresour. Technol. 2013, 142, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Sathitsuksanoh, N.; George, A.; Zhang, Y.; Percival, H. New lignocellulose pretreatments using cellulose solvents: A review. J. Chem. Technol. Biotechnol. 2013, 88, 169–180. [Google Scholar] [CrossRef]
- Maki-Arvela, P.; Anugwom, I.; Virtanen, P.; Sjoholm, R.; Mikkola, J.P. Dissolution of lignocellulosic materials and its constituents using ionic liquids—A review. Ind. Crop. Prod. 2010, 32, 175–201. [Google Scholar] [CrossRef]
- Tan, S.S.Y.; MacFarlane, D.R. Ionic liquids in biomass processing. Top Curr. Chem. 2009, 290, 311–339. [Google Scholar]
- Ziaei-Rad, Z.; Fooladi, J.; Pazouki, M.; Gummadi, S. Lignocellulosic biomass pre-treatment using low-cost ionic liquid for bioethanol production: An economically viable method for wheat straw fractionation. Biomass Bioenergy 2021, 151, 106140. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- Brandt, A.; Grasvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Goto, M. Ionic liquid pretreatment of lignocellulosic biomass for enhanced enzymatic delignification. In Application of Ionic Liquids in Biotechnology. Advances in Biochemical Engineering/Biotechnology; Springer: Cham, Switzerland, 2018; Volume 168. [Google Scholar]
- Elgharbawy, A.A.; Alam, M.Z.; Moniruzzaman, M.; Goto, M. Ionic liquid pretreatment as emerging approaches for enhanced enzymatic hydrolysis of lignocellulosic biomass. Biochem. Eng. J. 2016, 109, 252–267. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.J. Pretreatment of biomass using ionic liquids: Research updates. Renew. Energy 2017, 111, 77–84. [Google Scholar] [CrossRef]
- Asim, A.M.; Uroos, M.; Naz, S.; Sultan, M.; Griffin, G.; Muhammad, N.; Khan, A. Acidic ionic liquids: Promising and cost-effective solvents for processing of lignocellulosic biomass. J. Mol. Liq. 2019, 287, 110943. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martín, J.M.; Fierro, J.L. Fractionation of lignocellulosic biomass by selective precipitation from ionic liquid dissolution. Appl. Sci. 2019, 9, 1862. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Mathew, A.K.; Parameshwaran, B.; Sukumaran, R.K.; Pandey, A. An evaluation of dilute acid and ammonia fiber explosion pretreatment for cellulosic ethanol production. Bioresour. Technol. 2016, 199, 13–20. [Google Scholar] [CrossRef]
- Kossatz, H.L.; Rose, S.H.; Viljoen-Bloom, M.; van Zyl, W.H. Production of ethanol from steam exploded triticale straw in a simultaneous saccharification and fermentation process. Process Biochem. 2017, 53, 10–16. [Google Scholar] [CrossRef]
- Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I.; Chamorro, M.A.; Saez, F.; Ballesteros, M.; Moreno, A. A sequential steam explosion and reactive extrusion pretreatment for lignocellulosic biomass conversion within a fermentation-based biorefinery perspective. Fermentation 2017, 3, 15. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Zacharopoulou, M.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Topakas, E. Sequential high gravity ethanol fermentation and anaerobic digestion of steam explosion and organosolv pretreated corn stover. Bioresour. Technol. 2017, 244, 1129–1136. [Google Scholar] [CrossRef]
- Damay, J.; Boboescu, I.Z.; Duret, X.; Lalonde, O.; Lavoie, J.M. A novel hybrid first and second generation hemicellulosic bioethanol production process through steam treatment of dried sorghum biomass. Bioresour. Technol. 2018, 263, 103–111. [Google Scholar] [CrossRef]
- Monschein, M.; Nidetzky, B. Effect of pretreatment severity in continuous steam explosion on enzymatic conversion of wheat straw: Evidence from kinetic analysis of hydrolysis time courses. Bioresour. Technol. 2016, 200, 287–296. [Google Scholar] [CrossRef]
- Damay, J.; Duret, X.; Ghislain, T.; Lalonde, O.; Lavoie, J.M. Steam explosion of sweet sorghum stems: Optimisation of the production of sugars by response surface methodology combined with the severity factor. Ind. Crop. Prod. 2018, 111, 482–493. [Google Scholar] [CrossRef]
- Zhao, S.; Li, G.; Zheng, N.; Wang, J.; Yu, Z. Steam explosion enhances digestibility and fermentation of corn stover by facilitating ruminal microbial colonization. Bioresour. Technol. 2018, 253, 244–251. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, G.; Hegg, E.L. Enhancement of sugar recovery and ethanol production from wheat straw through alkaline pre-extraction followed by steam pretreatment. Bioresour. Technol. 2018, 266, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C.; Yoshida, T.; Cotta, M.A.; Sonomoto, K. Hydrothermal pretreatment and enzymatic saccharification of corn stover for efficient ethanol production. Ind. Crop. Prod. 2013, 44, 367–372. [Google Scholar] [CrossRef]
- Wagner, A.; Lackner, N.; Mutschlechner, M.; Prem, E.; Markt, R.; Illmer, P. Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Koupaie, E.; Dahadha, S.; Bazyar Lakeh, A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Vasco-Correa, J.; Shah, A. Techno-economic bottlenecks of the fungal pretreatment of lignocellulosic biomass. Fermentatio 2019, 5, 30. [Google Scholar] [CrossRef]
- Akyol, Ç.; Ince, O.; Bozan, M.; Ozbayram, E.G.; Ince, B. Biological pretreatment with Trametes versicolor to enhance methane production from lignocellulosic biomass: A metagenomic approach. Ind. Crop. Prod. 2019, 140, 111659. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Luo, X.; Li, Y.; Shah, A. Comparative study of changes in composition and structure during sequential fungal pretreatment of nonsterile lignocellulosic feedstocks. Ind. Crop. Prod. 2019, 133, 383–394. [Google Scholar] [CrossRef]
- Deswal, D.; Gupta, R.; Nandal, P.; Kuhad, R.C. Fungal pretreatment improves amenability of lignocellulosic material for its saccharification to sugars. Carbohydr. Polym. 2014, 99, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kautto, J.; Realff, M.J.; Ragauskas, A.J.; Kassi, T. Economic analysis of an organosolv process for bioethanol production. BioResources 2014, 9, 6041–6072. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv 2012, 30, 1447–1457. [Google Scholar] [CrossRef]
- Li, H.; Qu, Y.; Yang, Y.; Chang, S.; Xu, J. Microwave irradiation e a green and efficient way to pretreat biomass. Bioresour. Technol. 2016, 199, 34–41. [Google Scholar] [CrossRef]
- Kostas, E.T.; Beneroso, D.; Robinson, J.P. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass. Renew. Sustain. Energy Rev. 2017, 77, 12–27. [Google Scholar] [CrossRef]
- Chandel, A.K.; Chan, E.S.; Rudravaram, R.; Narasu, M.L.; Rao, L.V.; Ravindra, P. Economics and environmental impact of bioethanol production technologies: An appraisal. Biotechnol. Mol. Biol. Rev. 2007, 2, 14–32. [Google Scholar]
- Palonen, H. Role of Lignin in Enzymatic Hydrolysis of Lignocellulose; VTT Technical Research Centre of Finland: Espoo, Finland, 2004; Volume 11−22, pp. 26–32. [Google Scholar]
- Hamelinck, C.N.; van Hooijdonk, G.A.; Faaij, P.C. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]

| Methods | Chemical Reagent | Pretreatment Effect | R |
|---|---|---|---|
| Ionic liquid pretreatment | [BMiM] MeSO4-H2SO4 [emim] [CH3COO] | Cellulose digestibility 77%, Glucose yield 76% | [31] |
| Acid pretreatment | Oxalic acid dihydrate, dilute sulfuric acid 2% H2SO4 | Total reducing sugar 42%, glucose yield 90% | [31] |
| Alkaline pretreatment | Cold alkaline (aqueous ammonia 10% NaOH | Glucose yield 93.1%, Lignin removals 59.1%, Xylan digestiblity 88%, Glucose yield 73.8% | [33] |
| Organic solvent pretreatment | 5–10% Formic acid acid-free organosolv glycerol organosolv | Glucose yield 40% Theoretical ethanol production yield of 96% Lignin removals 65% | [34] |
| Raw Material | Alkaline Type | Pretreatment Condition | Reducing Sugars | Delignification Rate (%) | Fermentation Condition | Bioethanol Yield (g/L) | R |
|---|---|---|---|---|---|---|---|
| Wheat straw | Na2CO3 (11%) | 75 °C; 10–85 min | Xylose: 85.7% | 70.4 | 30 °C; 96 h | 65 | [51] |
| Wheat straw | NaOH/H2O2 | 50 °C, 3–15 h | 61.9 g/L | 60 | 37 °C; 96 h | 31.1 | [53] |
| Raw Material | Pretreatment Type | Pretreatment Condition | Reducing Sugars | Delignification Rate (%) | Fermentation Condition | Bioethanol Yield [g/L] | R |
|---|---|---|---|---|---|---|---|
| Wheat straw | Organosolv | 190 °C, 1 h | Glucose: 99 | 84.5 | 50 °C; 72 h. | NA | [51] |
| Wheat straw | Eutectic solvents | 70 °C, 9 h | Glucose: 79.7 | 71.4 | 50 °C; 72 h | 89.8% | [53] |
| Raw Material | Acid Type | Pretreatment Condition | Reducing Sugars | Delignification Rate (%) | Fermentation Condition | Bioethanol Yield [g/L] | R |
|---|---|---|---|---|---|---|---|
| Wheat straw | H2SO4 (2%) | 180 °C; 10 min | 43 g/L | NA | 30 °C; 72 h | 0.44 | [40] |
| Wheat straw | H2SO4 and Na2SO3 | 180 °C; 30 min | NA | 21 | 48 h | 17.25 g/g | [40] |
| Types | Temperature (T) and Pressure (P) | %Yield of Sugar | Advantage | Disadvantage |
|---|---|---|---|---|
| Wet air oxidation | T: 170–200 °C P: 10–20 MPa | 67% Cellulose content, 89% lignin removal, | Simple method suitable for lignin-enriched biomass residue | High cost to maintain it, cellulose is less affected |
| AFEX process | T: 60–100 °C P: 1.7–201 MPa 27% (w/w) ammonia concentration | 42% lignin reduction | Reduce crystallinity and amorphous structure of cellulose | High energy is required to maintain process temperature |
| Steam explosion | T: 180–280 °C P: 2.5–7 MPa | Glucose recovery 57–63% | Simple biomass pretreatment | -Generate toxically compounds—Disturb the sustainability of enzymes |
| CO2 explosion | T: 190 °C 20–60 bar CO2 pressure | Glucose yields 80.7% | Cheaper Higher yield Low-temperature requirement | Not relevant for biomass with less moisture amount |
| Liquid hot water | T: 160–240 °C P: >5 MPa | Glucose recovery 73.1% | Simple process Low capital Low maintenance costs Non-corrosion problem High sugar yields | Large amounts of energy are required due to high water consumption |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusănescu, C.O.; Ciobanu, M.; Rusănescu, M.; Dinculoiu, R.L. Pretreatments Applied to Wheat Straw to Obtain Bioethanol. Appl. Sci. 2024, 14, 1612. https://doi.org/10.3390/app14041612
Rusănescu CO, Ciobanu M, Rusănescu M, Dinculoiu RL. Pretreatments Applied to Wheat Straw to Obtain Bioethanol. Applied Sciences. 2024; 14(4):1612. https://doi.org/10.3390/app14041612
Chicago/Turabian StyleRusănescu, Carmen Otilia, Maria Ciobanu, Marin Rusănescu, and Raluca Lucia Dinculoiu. 2024. "Pretreatments Applied to Wheat Straw to Obtain Bioethanol" Applied Sciences 14, no. 4: 1612. https://doi.org/10.3390/app14041612






