Abstract
The use of cricket protein in emulsions is in line with the growing interest in sustainable food sources, as crickets require minimal resources and produce lower greenhouse gas emissions than traditional livestock. Research in this area suggests that incorporating cricket protein into emulsions not only improves their nutritional value but also contributes to the development of environmentally friendly and functional food products. This study proposes the use of cricket protein for the stabilization of emulsions formulated with avocado oil as a dispersed phase. This oil is rich in monounsaturated fats, particularly oleic acid, and a variety of bioactive compounds. In the first part of this study, we assessed the influence of the emulsifier concentration and found that 2 wt.% is the optimum because a depletion flocculation effect was produced. Subsequently, processing was optimized using ultrasonication so that the higher energy input produced emulsions with a droplet diameter of less than 700 nm. Finally, rhamsan gum was added to the formulation, producing emulgels with improved pseudoplastic behavior and physical stability. This study demonstrates that cricket protein in combination with rhamsan gum is capable of forming stable, low-droplet-size emulgels with potential applications in encapsulation systems.
1. Introduction
In recent years, the global demand for sustainable and nutritious food sources has driven innovative research on alternative protein sources. Among these, cricket protein has emerged as a promising candidate due to its nutritional profile and environmental sustainability [1]. The development of emulsions using cricket protein represents a new way of creating functional and sustainable food products.
Cricket flours, derived from edible crickets such as Gryllodes sigillatus and Acheta domesticus, have attracted attention due to their high protein content, essential amino acid profile and environmentally friendly production compared to traditional livestock farming [2]. Emulsions, colloidal systems composed of two immiscible phases stabilized by an emulsifier, play a crucial role in the formulation of various food products, including dressings, sauces and beverages [3]. Incorporating cricket protein into emulsions not only enhances their nutritional value but also contributes to sustainable development goals by reducing dependence on resource-intensive protein sources [4].
The success of emulsion development with cricket protein lies in understanding its unique physicochemical properties. Cricket protein contains a diverse range of proteins, lipids and chitin that impart distinctive functional properties to emulsions. Studies have shown that cricket protein has emulsifying properties due to the amphiphilic nature of its proteins, which can adsorb at the oil–water interface and stabilize the emulsion [5]. Furthermore, the presence of chitin, a polysaccharide in the cricket exoskeleton, may contribute to the mechanical stability of emulsions through interactions with proteins and lipids [6]. In addition to its functional properties, the nutritional benefits of cricket protein in emulsions are noteworthy. Cricket protein is a rich source of essential amino acids, vitamins and minerals, making it a valuable ingredient in emulsion-based food products designed to meet consumers’ nutritional needs. In addition, cricket protein has a lower environmental footprint compared to traditional protein sources, which is in line with growing consumer demand for sustainable and ethical food choices [7,8].
Avocado oil, derived from the fruit of the Persea americana tree, has gained considerable attention in recent years for its potential health benefits. This natural oil has been a staple in traditional diets, especially in regions where avocados are native, and its consumption is now gaining popularity worldwide. Scientific studies have examined the nutritional composition of avocado oil and found it to be high in antioxidants such as tocopherols, carotenoids and polyphenols. These compounds contribute to the oil’s potential anti-inflammatory and antioxidant properties, which are crucial for maintaining overall health and preventing chronic disease [9,10]. Avocado oil has also been linked to cardiovascular health benefits. Research suggests that the monounsaturated fats found in avocado oil may help improve lipid profiles by reducing LDL cholesterol levels while increasing HDL cholesterol, ultimately promoting a healthier cardiovascular system [11,12]. The versatility of avocado oil extends beyond its culinary uses; it is also gaining recognition for its use in skin care. Studies have demonstrated the oil’s potential to promote skin health due to its moisturizing properties and ability to enhance the absorption of essential nutrients [13,14]. In this context, exploring the applications of avocado oil and the scientific evidence surrounding its benefits is crucial for people looking to optimize their dietary and skincare routines. This scientific study proposes the use of avocado oil as a dispersed phase in emulsions and emulgels. Recent studies have already demonstrated the possibility of using avocado oil in such dispersed systems and with sustainable biological resources as emulsifying agents [15]. The main novelty of this study lies in the use of cricket protein as an interface stabilizer.
Rhamsan gum is a polysaccharide derived from the fermentation of Xanthomonas campestris, a bacterium commonly found in soil. This exopolysaccharide is known for its remarkable thickening and stabilizing properties, making it a key ingredient in various industries such as food, pharmaceuticals and cosmetics. With its unique molecular structure and versatile functionality, rhamsan gum has gained widespread recognition as a valuable additive in a variety of applications [16]. This biopolymer’s ability to form stable gels and increase viscosity in aqueous solutions has led to its widespread use in the food industry, where it contributes to the texture, mouthfeel and stability of a wide range of products [17]. Beyond the culinary sphere, rhamsan gum has also found utility in pharmaceutical formulations, providing controlled release and improved rheological properties. In addition, its role in personal care products underlines its importance in the cosmetics industry, where it acts as a reliable thickener and stabilizer in various formulations.
The development of emulsions using high-energy methods has revolutionized the field of colloidal science and industrial processing. Using techniques such as ultrasonication, high-pressure homogenization and microfluidization, researchers can efficiently produce stable emulsions with finely tuned droplet sizes and distributions [18]. Ultrasonic emulsification, which uses high-frequency sound waves to break immiscible liquids into tiny droplets, is a powerful technique for developing emulsions. In this process, ultrasonic energy is applied to a mixture of oil and water, resulting in the formation of stable emulsions with uniform droplet sizes. The cavitation phenomenon induced by the ultrasonic waves creates intense local heating and pressure fluctuations, facilitating the dispersion of the dispersed phase into the continuous phase. This method offers several advantages, including improved emulsion stability, reduced droplet size distribution and efficient encapsulation of active ingredients, making it a promising approach in various industries, such as food, pharmaceuticals and cosmetics [19].
As the food industry continues to explore alternative protein sources, the development of cricket-protein-based emulsions holds great promise. The main objective of this study was to develop emulsions and emulgels with cricket flour as an emulsifying agent in combination with rhamsan gum as a thickener. This study demonstrates the ability of cricket flour to stabilize avocado oil-in-water emulsions processed using ultrasonication. These systems could be used as matrices and encapsulation systems for bioactive principles in the food and cosmetic–dermatological industries.
2. Materials and Methods
2.1. Materials
The continuous phase was formulated using cricket flour (70 wt.% protein) as the emulsifier, purchased from Origen Farms (Albacete, Spain), and distilled water. The dispersed phase was formed with avocado oil, supplied by Bidah Chaumel (Murcia, Spain). Rhamsan gum, kindly supplied by CP Kelco (Atlanta, GA, USA), was used as a rheology modifier. All compounds were natural products. A certificate of analysis was provided.
2.2. Preparation of Emulgels
The compositions of the emulsions studied in the first part of this research (in the absence of a biopolymer as a rheology modifier) were formulated with 10 wt.% avocado oil and different concentrations of cricket flour in the range of 0.5–2.5 wt.%. A total of 250 g of sample was prepared for each of the batches. First, the continuous phase components, distilled water and cricket flour, were mixed in a magnetic stirrer for 10 min at 500 rpm. Subsequently, primary homogenization was carried out by adding the dispersed phase on top of the continuous phase in an Ultraturrax T25 rotor–stator system (Ika, Staufen, Germany) at 13,500 rpm for 120 s. In the second part of this research, once the concentration of cricket flour as the emulsifying agent was optimized, the system with 2 wt.% cricket flour was selected for secondary homogenization. This process was carried out with a high-energy Ultrasonic Processor CV334 (Sonics, Newtown, CT, USA) at three different amplitudes (25%, 50% and 75%). In all cases, the total homogenization time was equal to 10 min, with intervals of 10 s with energy input and 10 s without energy input. The temperature was set by means of an external bath at 25 °C.
In the second stage of this study, the emulsion with the optimized cricket flour concentration and ultrasonication amplitude regarding the secondary homogenization was used as a basis for the incorporation of the biopolymeric stabilizer (rhamsan gum). First, an aqueous dispersion of rhamsan gum was prepared with a total concentration of 1 wt.%. The appropriate amount of biopolymer was added over distilled water and stirred at 600 rpm for 1 h using an Ikavisc MR-D1 system (IKA, Staufen im Breisgau, Germany). This solution was then kept at 5 °C for 24 h to allow complete hydration of the polysaccharide. The final emulgels with different concentrations of rhamsan gum (0.1, 0.3 and 0.5 wt.%) were prepared by mixing the primary dispersion and the optimal emulsion using the Ikavisc MR-D1 at 400 rpm for 30 min.
2.3. Droplet Size Distributions
Droplet size distributions were determined via static light scattering using a Mastersizer 2000 (Malvern Instruments, Worcestershire, UK) using refractive indices of 1.335 and 1.472 for the continuous and dispersed phases, respectively. All emulsion samples were vortexed for 30 s and diluted to 10–15% opacity prior to scattering measurements. The droplet sizes were recorded as volume mean diameter (D4,3):
where di is the droplet diameter, ni is the number of droplets of diameter di and N is the total number of droplets. In addition, to assess the polydispersity of the target size distributions, the span parameter was used:
where d(v,0.9), d(v,0.5) and d(v,0.10) are the diameters at 90%, 50% and 10% cumulative volume.
2.4. Rheological Characterization
The rheological behavior of the emulsions and emulgels was analyzed using an AR2000 controlled stress rotational rheometer (TA Instruments, New Castle, DE, USA) with a 60 mm serrated plate–plate geometry and a 1 mm gap. All rheological tests were performed in triplicate at 25 °C. Multi-step flow curve tests were first performed to obtain the flow properties of the different systems. The results were recorded as shear rate and viscosity at each sampling point. Frequency sweep tests were performed in the limit of the linear viscoelastic region to determine the frequency dependence of the elastic (G′) and viscous (G″) moduli.
2.5. Physical Stability
For the study of the physical stability of the developed emulsions and emulgels, the samples were monitored using a Turbiscan Lab Expert (Formulaction, Toulouse, France). The samples were introduced into measuring cells and kept at room temperature. The Turbiscan uses light scattering technology to monitor changes in the physical properties of emulsions over time. By analyzing the intensity of the backscattered light, it provides valuable insight into the stability and structural evolution of emulsions. The Turbiscan stability index derived from the collected data provides a quantitative measure of emulsion stability, allowing researchers and formulators to assess the impact of different formulations, storage conditions and processing parameters on the long-term stability of emulsified systems. Higher TSI values indicate poorer physical stability and were calculated as follows:
where scanref and scani are the initial value of the backscattering and the value at a given time, respectively, and hj is the given height of the measuring cell.
2.6. Statistical Analysis
The results obtained via the laser diffraction measurements and rheological tests were analyzed using one-way analysis of variance (ANOVA) using Microsoft Excel 2016. Every sample was measured in triplicate for the laser diffraction technique but in duplicate for the rheological tests. All statistical calculations were carried out at a significance level of p < 0.05.
3. Results and Discussion
In this section, we present and discuss our results from the three different studies that were carried out: first, we studied the influence of the cricket flour on the emulsions; second, we evaluated the influence of the use of ultrasound equipment and different amplitudes; and finally, we assessed the influence of the addition of a physical stabilizer on the rheological properties and physical stability of the emulsions.
3.1. Influence of Cricket Flour Concentration
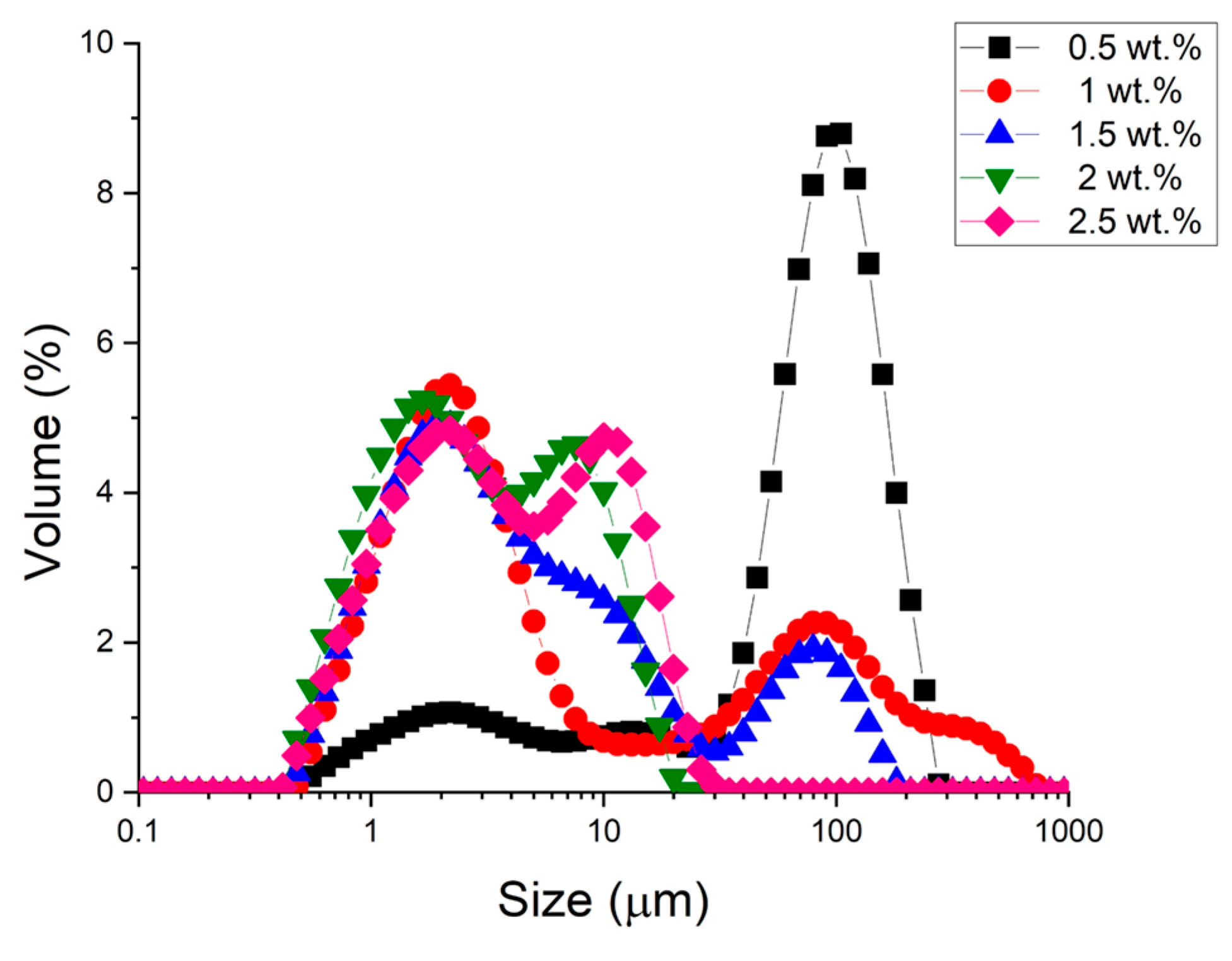
Figure 1 shows the droplet size distributions, where the volume percentage versus droplet diameter is plotted as a function of the emulsifier concentration.
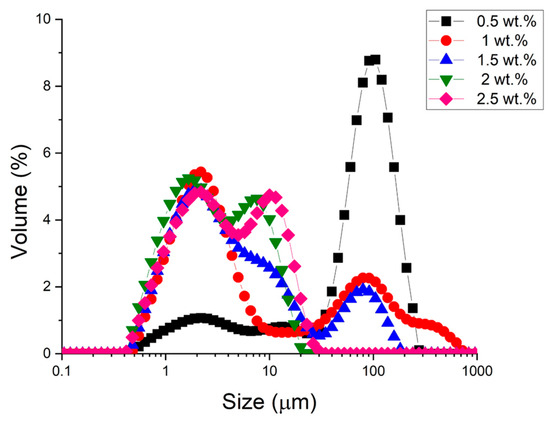
Figure 1.
Droplet size distribution of all studied emulsions as a function of cricket flour concentration used.
We observed that as the concentration of cricket flour increases, the droplet size distributions shift towards smaller sizes. At a concentration equal to or higher than 1 wt.%, the main peak is around 1–2 microns. Furthermore, it is noteworthy that at a concentration equal to or higher than 2 wt.%, the peak around one hundred microns disappears. These results highlight the need for a minimal amount of cricket flour, which acts as an emulsifier and a stabilizer at the oil–water interface, to obtain small droplet sizes. Finally, it should be noted that within the range of concentrations studied and with the processing used, all the distributions are multimodal.
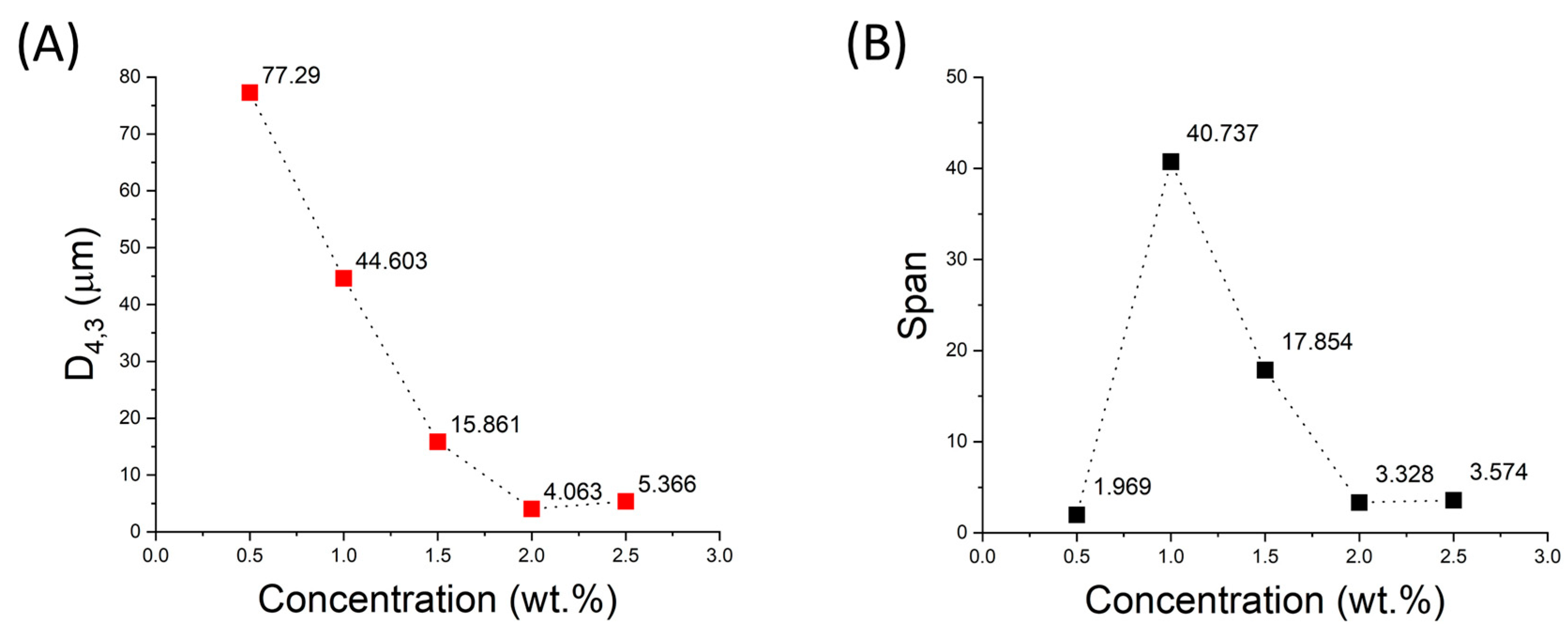
It should be noted that droplet size is a key factor influencing emulsion properties such as physical stability and rheological properties. Droplet size mainly depends on formulation and processing conditions. The smaller the droplet size, the less likely the emulsions are to be destabilized by creaming or coalescence [3]. Figure 2A shows the values of the volumetric mean diameter of the emulsions studied as a function of cricket flour concentration. A decrease in mean droplet size is clearly observed as the amount of emulsifier increases, as mentioned above. However, an optimum (minimum value) of this parameter is also shown for the 2 wt.% concentration. This could be explained by the fact that a depletion flocculation effect may be taking place at this concentration [20]. In the case of the span parameter, the lowest value is observed for the lowest emulsifier concentration. However, it should be noted that this is because the peak of the largest droplet volume is located at a large size, so that the lower polydispersity in this case is not an advantage. For the samples with the highest emulsifier concentration, the lowest value is again obtained for the 2 wt.% concentration, so this is the concentration that will be used in the following stages of this study.
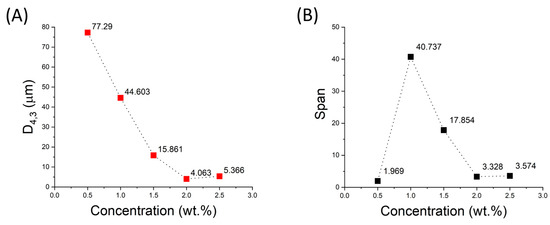
Figure 2.
Influence of cricket flour concentration on (A) volumetric mean diameter (D4,3) and (B) span parameter.
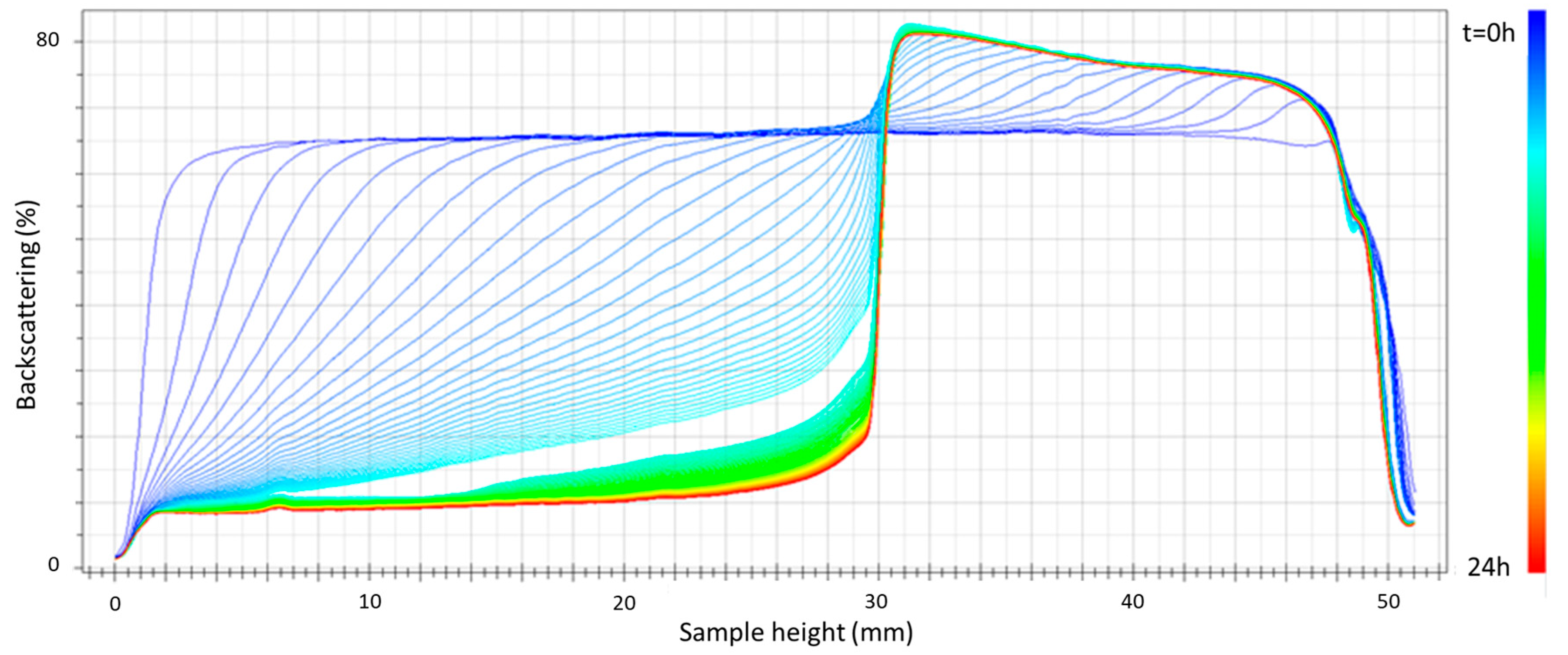
Figure 3 shows the plot of backscattering versus sample height after 24 h of aging for the emulsion containing 2 wt.% cricket flour as the emulsifier. Firstly, even in the first 24 h after preparation, there is a clear decrease in backscattering in the lower part of the emulsion measurement cell. As already mentioned, this fact is directly related to a mechanism of destabilization by creaming [21]. This mechanism is favored by large droplet sizes and the low viscosity of the samples. Taking into account the results already shown in which large droplet sizes were observed, the large degree of destabilization by creaming could be attributed to this last factor, which even led to the phase separation shown in Figure 3 that was observable with the naked eye approximately 5–6 h after the separation. All the emulsions studied showed phase separation by a creaming mechanism in less than 24 h.
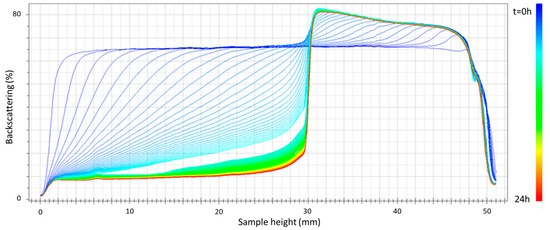
Figure 3.
Backscattering versus container height as a function of time for the emulsion formulated with 2 wt.% of cricket flour and developed using only primary homogenization.
3.2. Influence of Processing
In order to obtain emulsions with better properties, both in terms of droplet size and physical stability, a study of the influence of sample processing was carried out. The systems with a fixed concentration of 2 wt.% cricket flour and whose primary homogenization was carried out with the rotor–stator system were introduced into an ultrasonic system at three different amplitudes (25%, 50% and 75%).
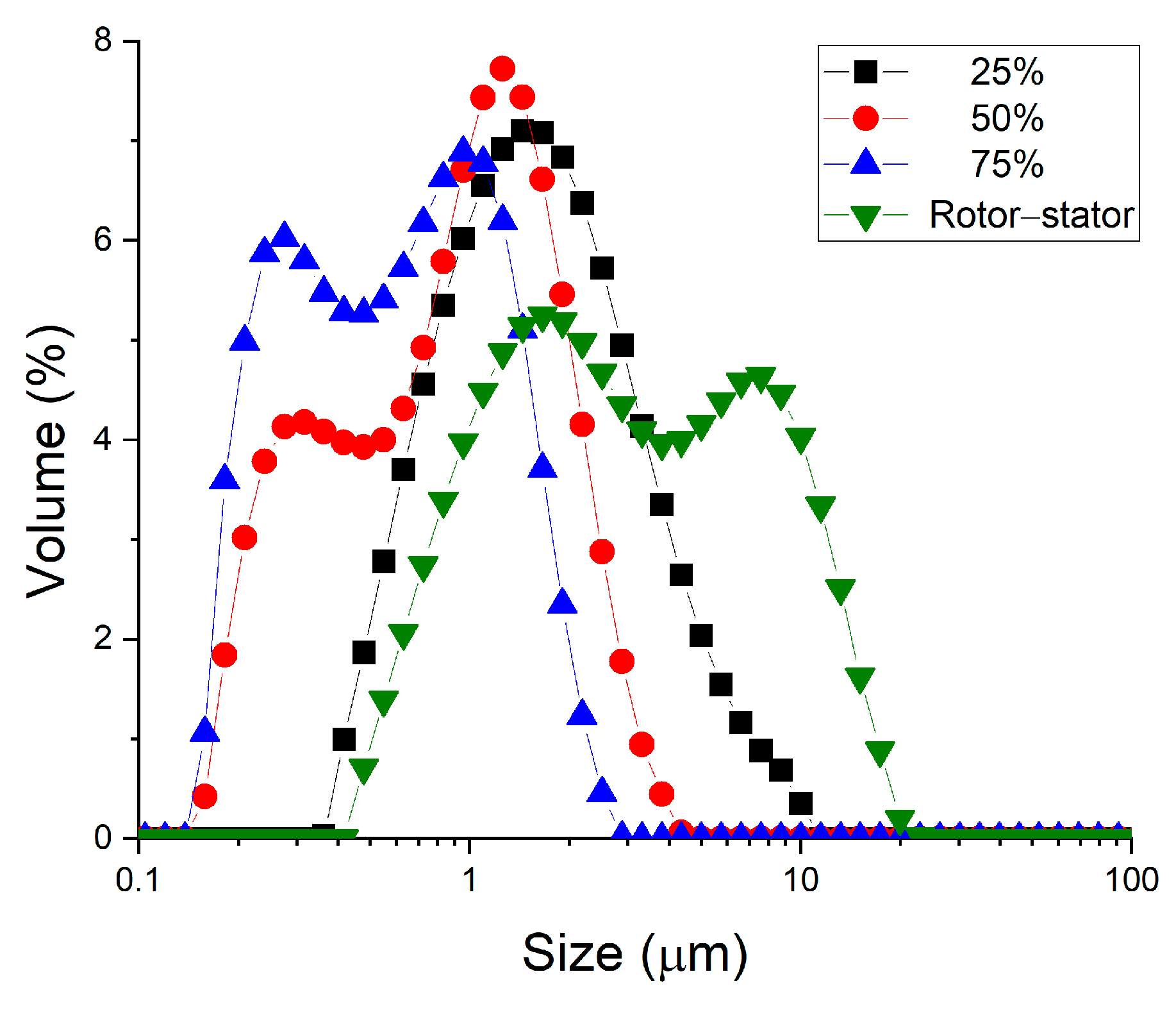
Figure 4 shows a clear improvement in results when secondary homogenization is carried out. As the amplitude (the applied energy) is increased, the droplet size distributions shifts towards smaller sizes. This shift in the droplet size distributions towards smaller sizes is reflected in significantly smaller values of the volumetric mean diameter as the energy input increases (see Table 1). In addition, the polydispersity of the samples is lower, although there is no clear trend. It is shown that no recoalescence phenomena are observed, so perhaps increasing the energy input (e.g., by increasing homogenization time) could lead to better results.
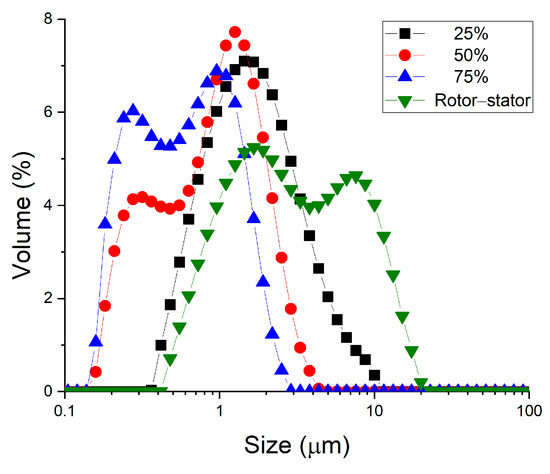
Figure 4.
Droplet size distribution of all studied emulsions containing 2 wt.% of cricket protein as a function of sample processing. The system indicated as rotor–stator involved only primary homogenization. The values of 25%, 50% and 75% correspond to the amplitude used in an ultrasonic system in secondary homogenization.

Table 1.
Volumetric mean diameter (D4,3) and span as a function of processing for the emulsions studied.
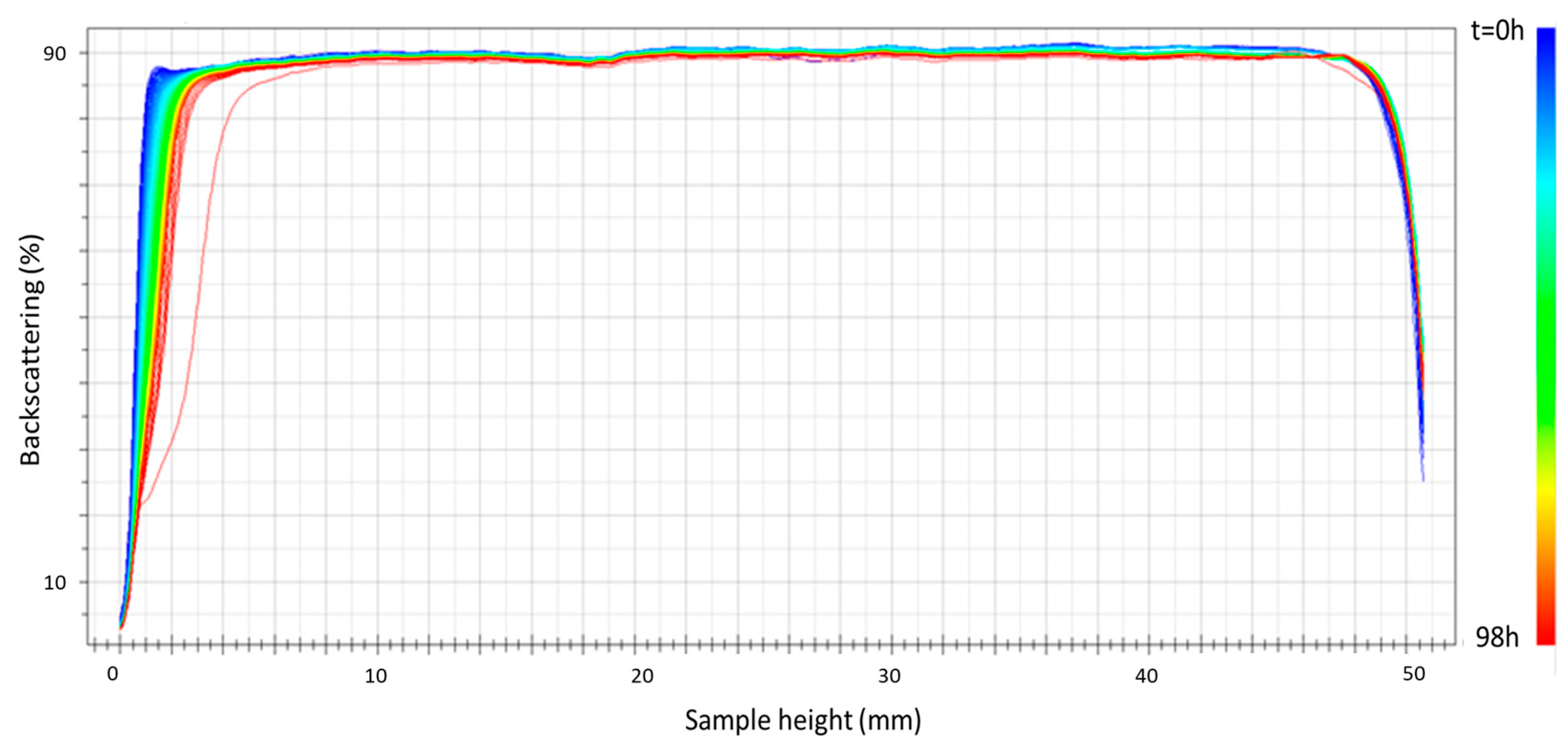
Figure 5 shows the results of the physical stability of the sample prepared using ultrasound at 75% amplitude as an example of the emulsions prepared with this emulsification method. A clear improvement in the stability results is shown for this emulsion for 98 h of aging time compared to the emulsion processed using only the rotor–stator device for 24 h (see Figure 3). This improvement must be attributed to the decrease in droplet diameters (see Table 1). It should be noted that in none of the samples was there any variation in backscattering in the intermediate zone of the vial, which could rule out changes in the droplet size or aggregation of the droplets. In any case, a physical stabilizer was incorporated into the formulation to inhibit this creaming destabilization mechanism.
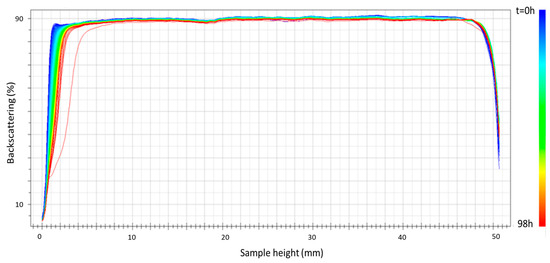
Figure 5.
Backscattering versus container height as a function of time for the emulsion formulated with 2 wt.% of cricket flour and prepared using ultrasound at 75% amplitude.
3.3. Incorporation of a Physical Stabilizer and Rheology Modifier
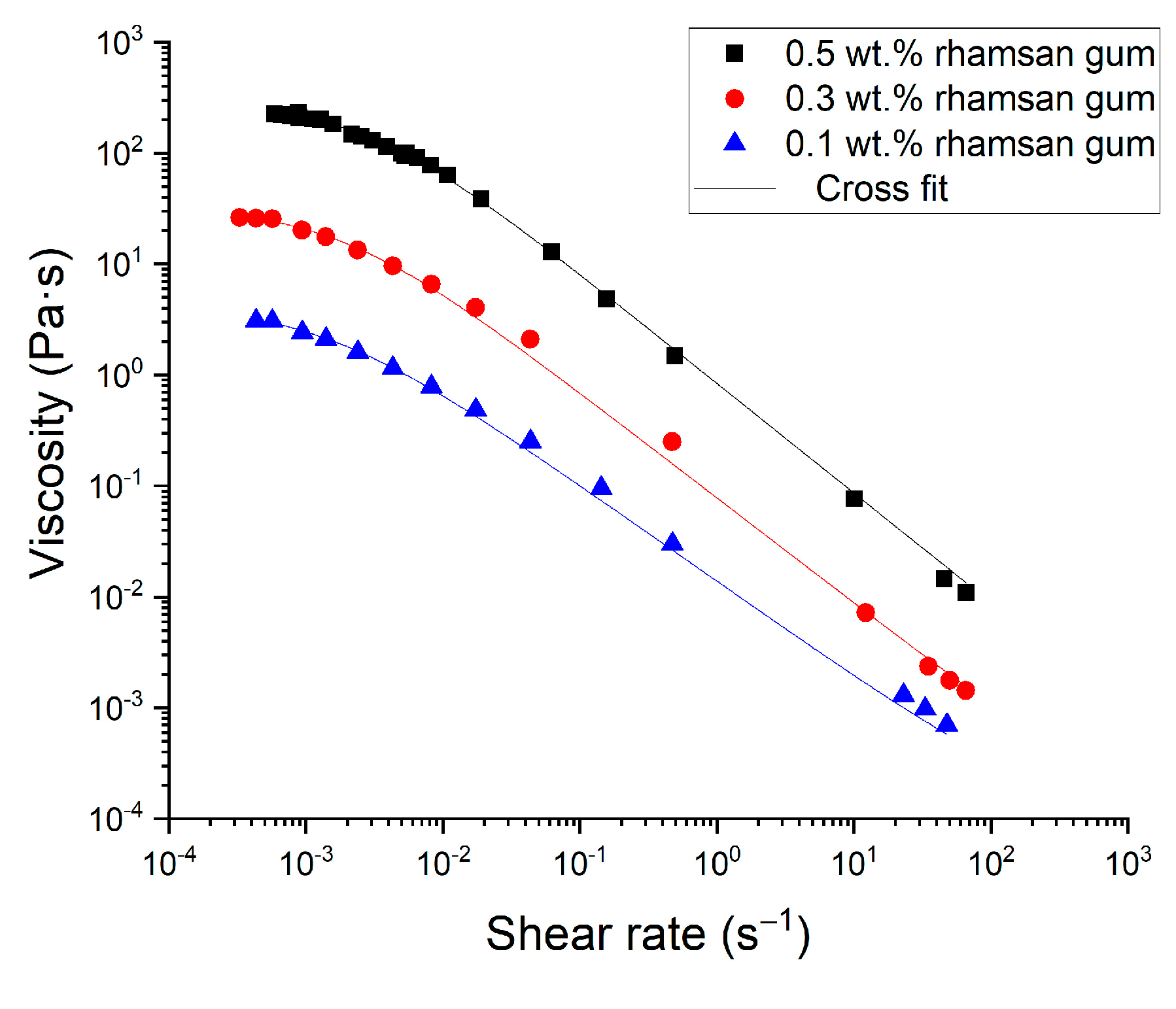
Figure 6 shows the flow behavior for the optimized emulsions containing 0.1, 0.3 and 0.5 wt.% rhamsan gum. All the samples studied exhibited shear thinning behavior, which was also fitted to a Cross model (R2 > 0.99):
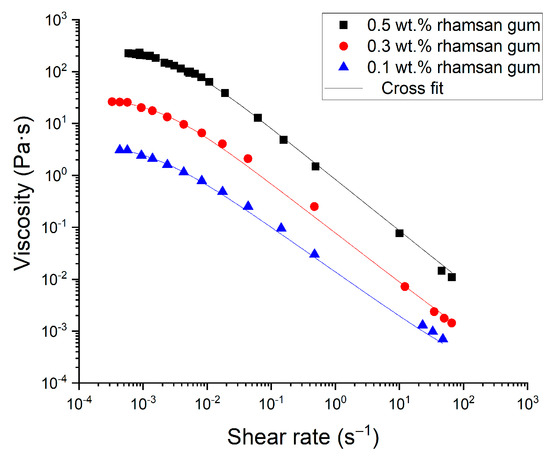
Figure 6.
Flow curves for all emulsions formulated with different rhamsan gum concentrations.
The fitting parameters are given in Table 2. The flow curve of the emulsion formulated without rhamsan gum, not shown in Figure 6, showed a Newtonian behavior with a constant viscosity of 2.74 mPa·s. In contrast, all the emulsions formulated with rhamsan gum show a very pseudoplastic behavior. The presence of the food grade biopolymer in the continuous phase induced a marked increase in viscosity and a pronounced decrease in the flow index. In addition, a higher flow resistance is shown, with higher k values as the concentration of the rubber increases. The results show that these emulsions behave similarly to other systems containing rhamsan gum as a stabilizer [22].

Table 2.
Cross model fitting parameters: ƞ0 is the viscosity at very low shear rates, ƞ∞ is the viscosity at very high shear rates and k is the inverse of the critical shear rate. The values were obtained using a nonlinear regression analysis.
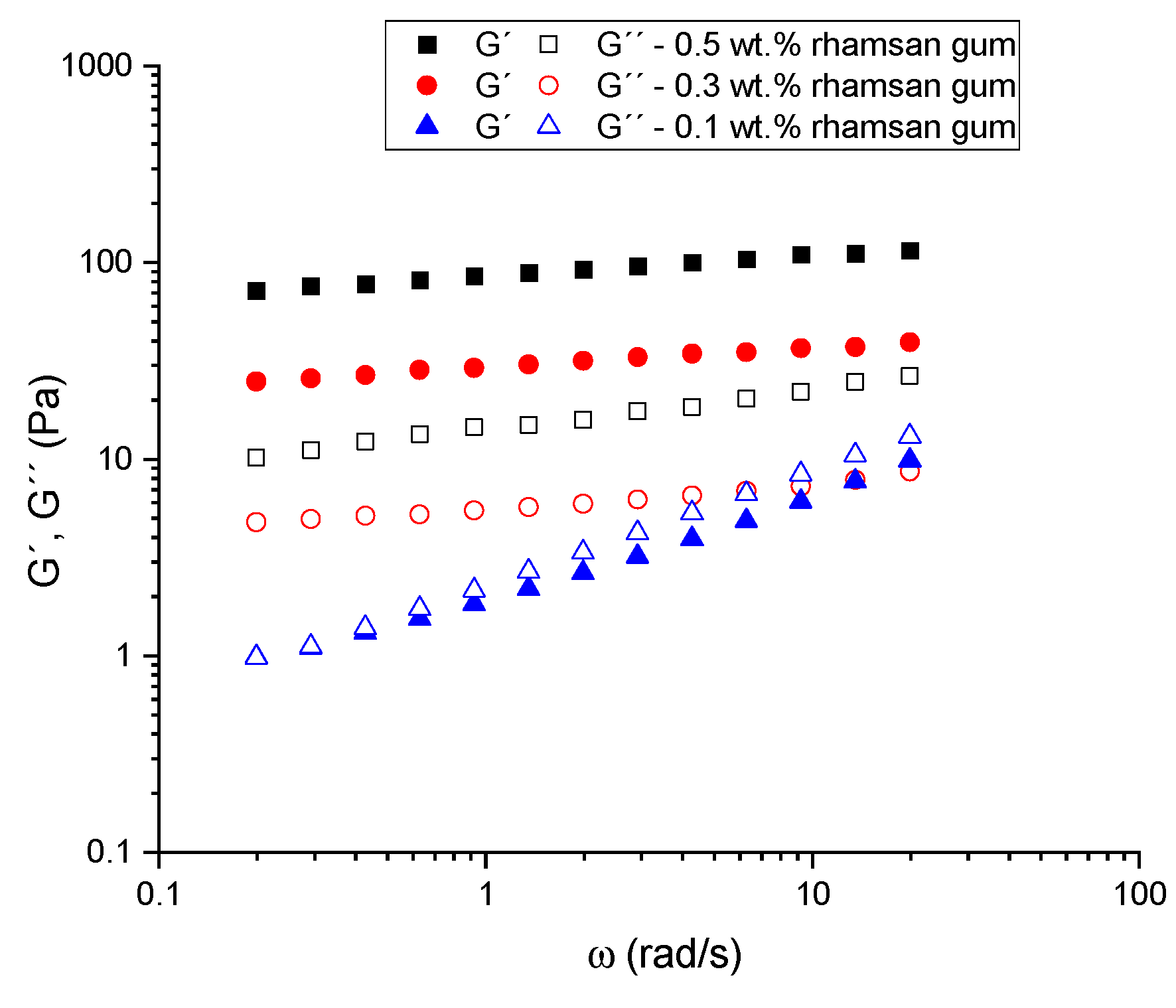
The frequency dependence of the linear viscoelastic functions (the storage modulus, G′, and the loss modulus, G″), which illustrates the influence of the biopolymer concentration, of the samples studied is shown in Figure 7. Samples formulated in the absence of rhamsan gum showed no linear viscoelastic range measurable by stress sweeps, so frequency sweeps were not carried out for these systems. Two types of behaviors are observed in the systems formulated with the biopolymer. For the 0.1 wt.% sample, a significant frequency dependence of the viscoelastic properties is observed, with G′ being higher than G″ over almost the whole range. There is therefore a low frequency crossover point where the viscous component dominates. All samples above 0.3 wt.% yield mechanical spectra of gel-like materials, with a higher storage modulus than loss modulus and little frequency dependence for either modulus. These results have previously been found in concentrated emulsions and seem to indicate the presence of three-dimensional networks similar to those of strong gels [23]. However, these emulgels formulated with rhamsan gum cannot be classified as strong gels, as they do not fulfill the criteria proposed by Ross-Murphy [24] for strong gels.
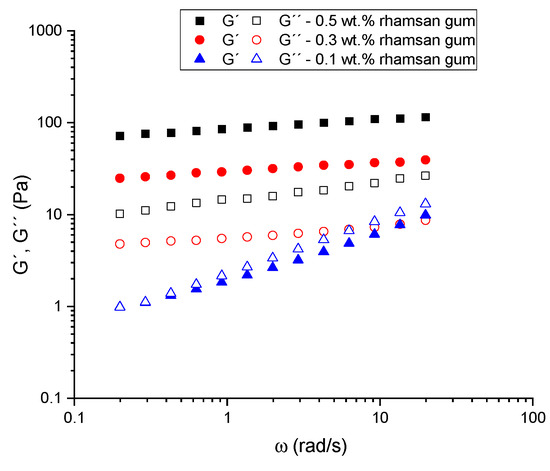
Figure 7.
Mechanical spectra for all emulsions formulated with different rhamsan gum concentrations.
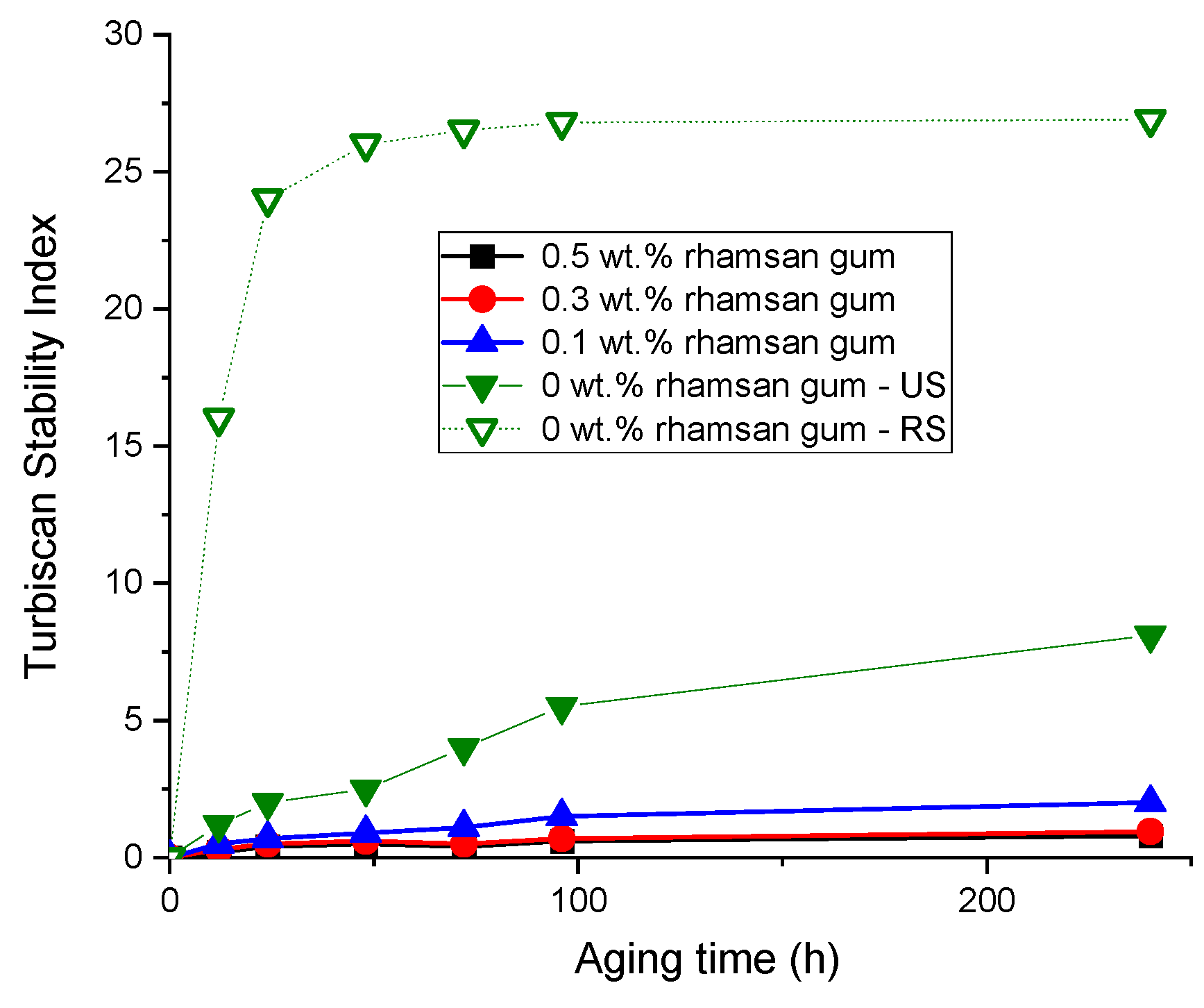
Finally, to check the effect of the addition of rhamsan gum on the physical stability of the emulsions, the results of the Turbiscan stability index (TSI) as a function of time are shown in Figure 8. Firstly, this parameter shows that the emulsions prepared with ultrasound (US) are more stable than those prepared only with a rotor–stator system (RS), confirming what has already been discussed in Figure 3 and Figure 5. On the other hand, the addition of rhamsan gum significantly improves the physical stability (lower TSI vales) of the systems, especially in emulsions with concentrations above 0.3 wt.%. This must be associated with the increase in viscosity discussed above as well as the viscoelastic properties.
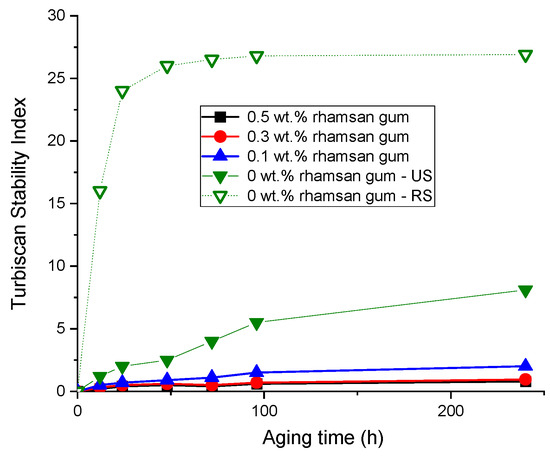
Figure 8.
Turbiscan stability index for the emulsions and emulgels as a function of rhamsan gum concentration and processing. Temperature = 20 °C.
Therefore, the addition of rhamsan gum as a rheology modifier not only imparts a gel-like property to the emulsions (i.e., they can be classified as emulgels) but also provides these systems with greater physical stability. In addition, between no addition of the biopolymer and obtaining the gel-like character, different behaviors are observed that allow us to obtain customized rheological properties (“tailored rheology”). The compatibility of cricket flour with this natural biopolymer is also demonstrated.
4. Conclusions
Our results showed that the optimal droplet sizes were obtained at a concentration equal to 2 wt.%. At higher concentrations, suboptimal results were observed, probably due to the phenomenon of depletion flocculation. Therefore, this study adds to the body of knowledge in the field of these dispersed systems, which are formulated with sustainable biological resources. The characterization and analysis carried out in this research not only expand our knowledge of the effect of cricket flour content on the performance of emulsions and emulgels of environmental friendly origin but also make us aware of the fact that a determination of the optimal amount of emulsifier is not an easy task from a technological point of view. Subsequently, the energy applied by ultrasonication was studied, and the smallest mean diameters were obtained at the largest amplitude (75%). Therefore, no recoalescence process due to excess mechanical energy input was observed during the secondary homogenization process. In addition, for each of the amplitudes studied, the results of this secondary homogenization were better than those obtained with a rotor–stator system on its own. Finally, rhamsan gum was added to the formulation in order to improve the physical stability of the emulsions, which were destabilized by a creaming mechanism. The addition of this food-grade biopolymer resulted in emulgels with improved physical stability. Thus, rheological characterization showed that they have pseudoplastic and gel-like characters, especially at a 0.3 wt.% concentration. The addition of the bio-based biopolymer allows us to obtain not only tailored rheological behaviors and properties but also enhanced physical stability. These systems developed with cricket flour, avocado oil and rhamsan gum could be used as matrices and encapsulation systems for bioactive principles in the food and cosmetic–dermatological industries.
Author Contributions
Conceptualization, I.G.-D. and A.R.-L.; methodology, J.S.; software, E.H.-F.; validation, L.A.T.-C. and J.S.; formal analysis, A.R.-L.; investigation, L.A.T.-C., I.G.-D., A.R.-L., E.H.-F. and J.S.; resources, L.A.T.-C.; data curation, E.H.-F.; writing—original draft preparation, L.A.T.-C.; writing—review and editing, L.A.T.-C. and J.S.; visualization, I.G.-D.; supervision, J.S.; project administration, J.S.; funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Programa Ramón y Cajal (Ministerio de Innovación y Ciencia, Gobierno de España).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional Value, Protein and Peptide Composition of Edible Cricket Powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O.; Borkovcova, M.; Bednarova, M. A Comprehensive Look at the Possibilities of Edible Insects as Food in Europe—A Review. Pol. J. Food Nutr. Sci. 2014, 64, 147–157. [Google Scholar] [CrossRef]
- Mcclements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Pilco-Romero, G.; Chisaguano-Tonato, A.M.; Herrera-Fontana, M.E.; Chimbo-Gándara, L.F.; Sharifi-Rad, M.; Giampieri, F.; Battino, M.; Vernaza, M.G.; Álvarez-Suárez, J.M. House Cricket (Acheta Domesticus): A Review Based on Its Nutritional Composition, Quality, and Potential Uses in the Food Industry. Trends Food Sci. Technol. 2023, 142, 104226. [Google Scholar] [CrossRef]
- Stone, A.K.; Tanaka, T.; Nickerson, M.T. Protein Quality and Physicochemical Properties of Commercial Cricket and Mealworm Powders. J. Food Sci. Technol. 2019, 56, 3355–3363. [Google Scholar] [CrossRef]
- Hirsch, A.; Cho, Y.-H.; Kim, Y.H.B.; Jones, O.G. Contributions of Protein and Milled Chitin Extracted from Domestic Cricket Powder to Emulsion Stabilization. Curr. Res. Food Sci. 2019, 1, 17–23. [Google Scholar] [CrossRef]
- Ordoñez-Araque, R.; Egas-Montenegro, E. Edible Insects: A Food Alternative for the Sustainable Development of the Planet. Int. J. Gastron. Food Sci. 2021, 23, 100304. [Google Scholar] [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the Development of Edible Insect-Based Foods in Europe. Foods 2021, 10, 766. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.; Roberts, T. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Avocado, Sunflower and Olive Oils as Replacers of Pork Back-Fat in Burger Patties: Effect on Lipid Composition, Oxidative Stability and Quality Traits. Meat Sci. 2012, 90, 106–115. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Razavi, B.M.; Hosseinzadeh, H. Effects of Avocado (Persea Americana) on Metabolic Syndrome: A Comprehensive Systematic Review: Avocado and Metabolic Syndrome. Phytother. Res. 2017, 31, 819–837. [Google Scholar] [CrossRef]
- Mahmassani, H.A.; Avendano, E.E.; Raman, G.; Johnson, E.J. Avocado Consumption and Risk Factors for Heart Disease: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 107, 523–536. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Flores, M.; Saravia, C.; Vergara, C.; Avila, F.; Valdés, H.; Ortiz-Viedma, J. Avocado Oil: Characteristics, Properties, and Applications. Molecules 2019, 24, 2172. [Google Scholar] [CrossRef]
- Tello, P.; Santos, J.; Calero, N.; Trujillo-Cayado, L.A. Formulation and Characterization of Sustainable Algal-Derived Nanoemulgels: A Green Approach to Minimize the Dependency on Synthetic Surfactants. Polymers 2024, 16, 194. [Google Scholar] [CrossRef]
- Podolsak, A.K.; Tiu, C.; Saeki, T.; Usui, H. Rheological Properties and Some Applications for Rhamsan and Xanthan Gum Solutions. Polym. Int. 1996, 40, 155–167. [Google Scholar] [CrossRef]
- Trujillo-Cayado, L.A.; Alfaro, M.C.; Raymundo, A.; Sousa, I.; Muñoz, J. Rheological Behavior of Aqueous Dispersions Containing Blends of Rhamsan and Welan Polysaccharides with an Eco-Friendly Surfactant. Colloids Surf. B Biointerfaces 2016, 145, 430–437. [Google Scholar] [CrossRef]
- Santana, R.C.; Perrechil, F.A.; Cunha, R.L. High- and Low-Energy Emulsifications for Food Applications: A Focus on Process Parameters. Food Eng. Rev. 2013, 5, 107–122. [Google Scholar] [CrossRef]
- Majid, I.; Nayik, G.A.; Nanda, V. Ultrasonication and Food Technology: A Review. Cogent Food Agric. 2015, 1, 1071022. [Google Scholar] [CrossRef]
- Jenkins, P.; Snowden, M. Depletion Flocculation in Colloidal Dispersions. Adv. Colloid Interface Sci. 1996, 68, 57–96. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayre, I.; Puech, K.; Snabre, P. Characterisation of Instability of Concentrated Dispersions by a New Optical Analyser: The TURBISCAN MA 1000. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 111–123. [Google Scholar] [CrossRef]
- Báez, L.A.; Santos, J.; Ramírez, P.; Trujillo-Cayado, L.A.; Muñoz, J. Development of Emulgels Formulated with Sweet Fennel Oil and Rhamsan Gum, a Biological Macromolecule Produced by Sphingomonas. Int. J. Biol. Macromol. 2019, 129, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.D. Viscoelastic Properties of Polymers; John Wiley & Sons: Hoboken, NJ, USA, 1980; ISBN 0-471-04894-1. [Google Scholar]
- Ross-Murphy, S.B. Structure–Property Relationships in Food Biopolymer Gels and Solutions. J. Rheol. 1995, 39, 1451–1463. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).