Enhanced Enzymatic Degradability of Livestock Blood Pretreated with Ultrasonic Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Livestock Blood Samples Used in the Study
2.1.1. Preparation of Livestock Blood Samples
2.1.2. Physical Properties of Livestock Blood Samples
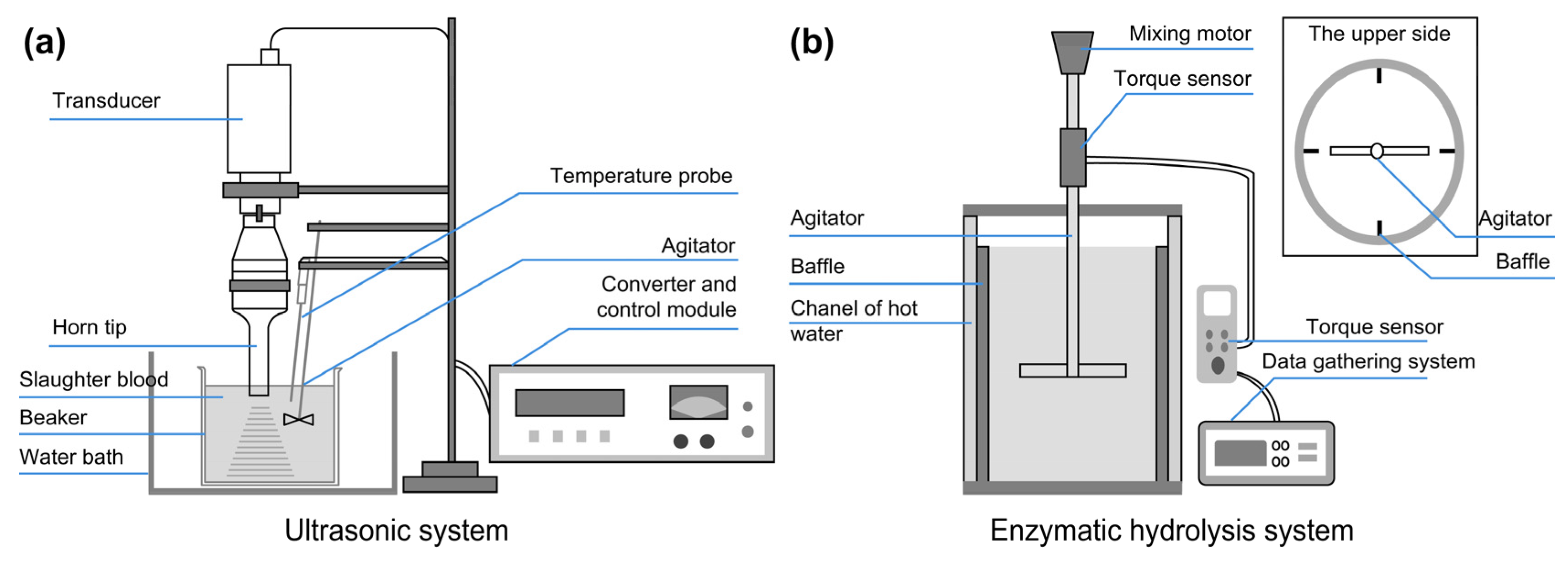
2.2. Method for Ultrasonic Treatment of Livestock Blood
2.3. Method for Enzymatic Hydrolysis of Ultrasonic-Pretreated Livestock Blood
2.4. Analysis of Physicochemical Properties of Samples
3. Results and Discussion
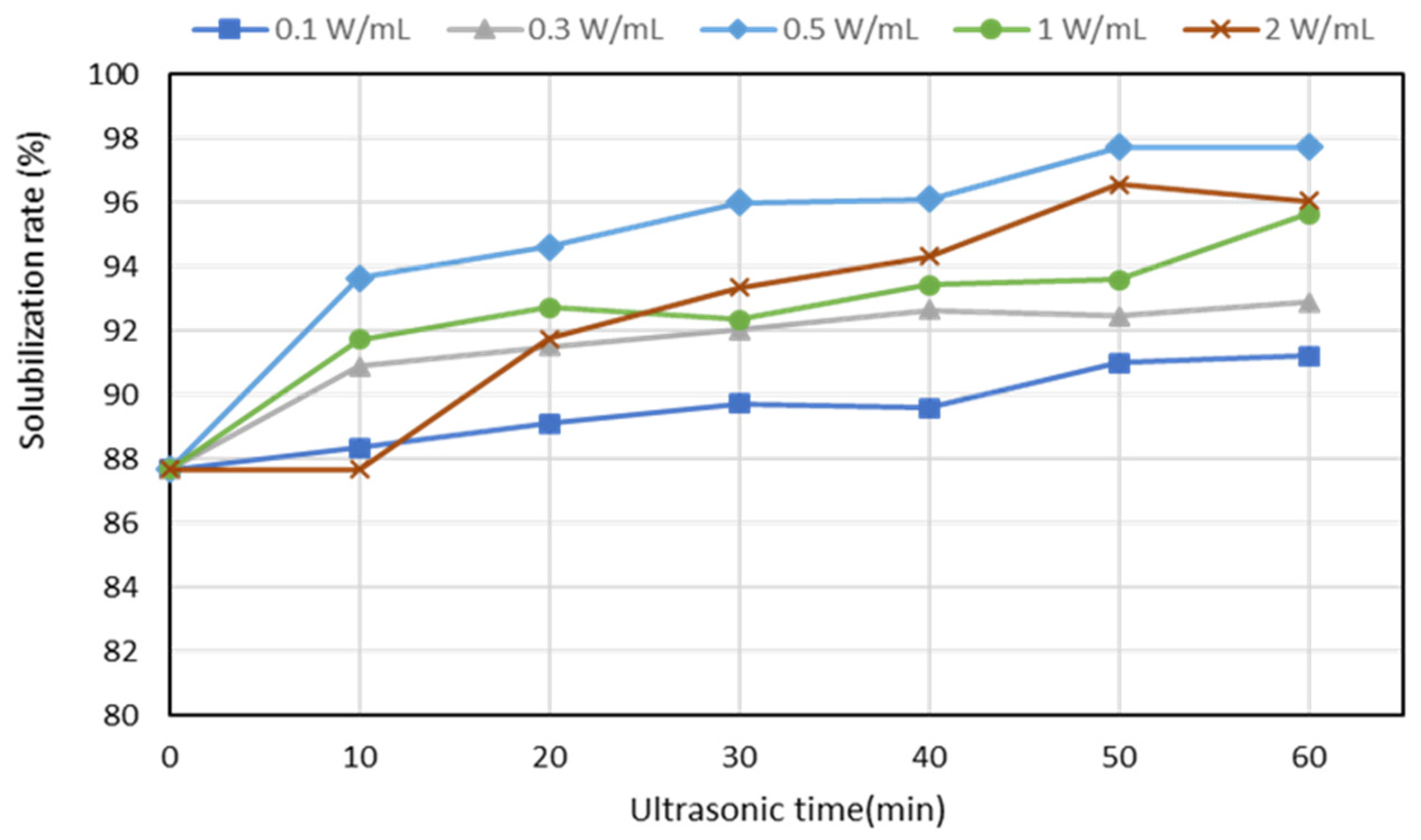
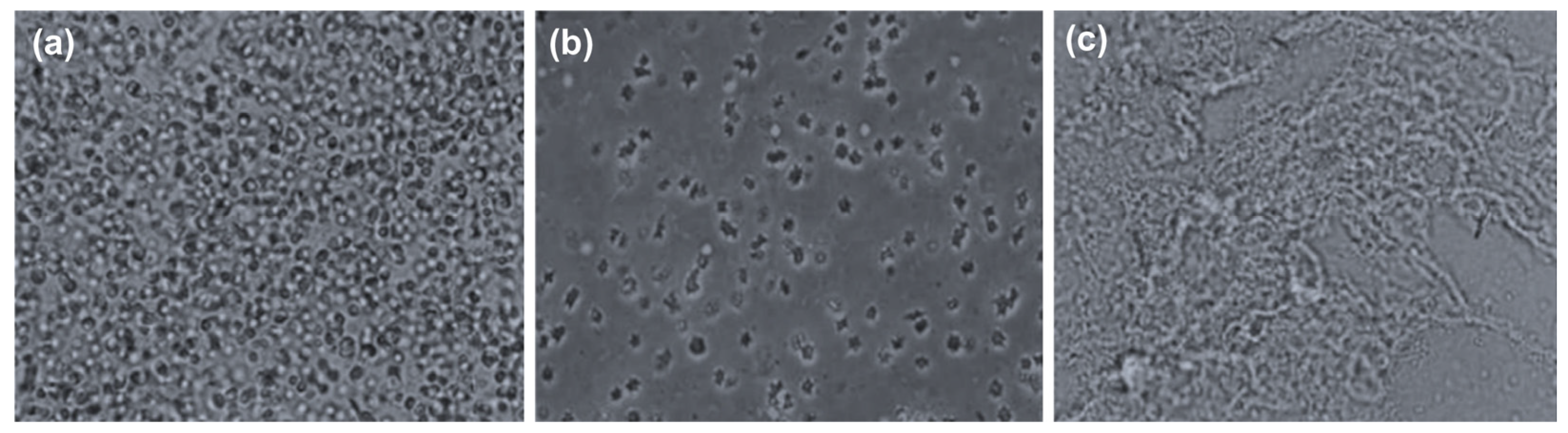
3.1. Protein Solubilization by Ultrasonic Pretreatment
3.2. Enzymatic Degradation of Untreated Livestock Blood
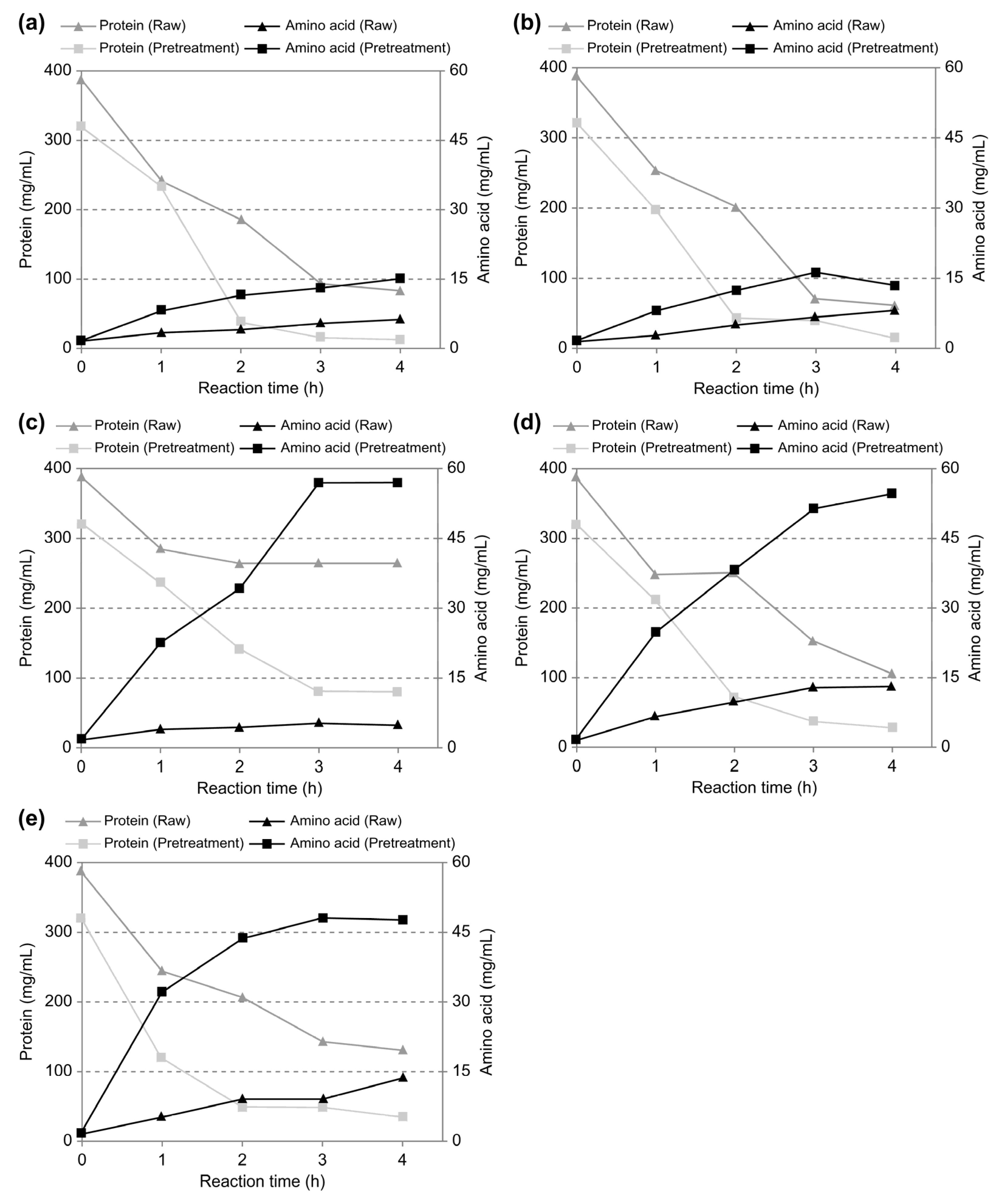
3.2.1. Effects on Protein Reduction and Amino Acid Conversion for Livestock Blood Depending on Enzyme Type
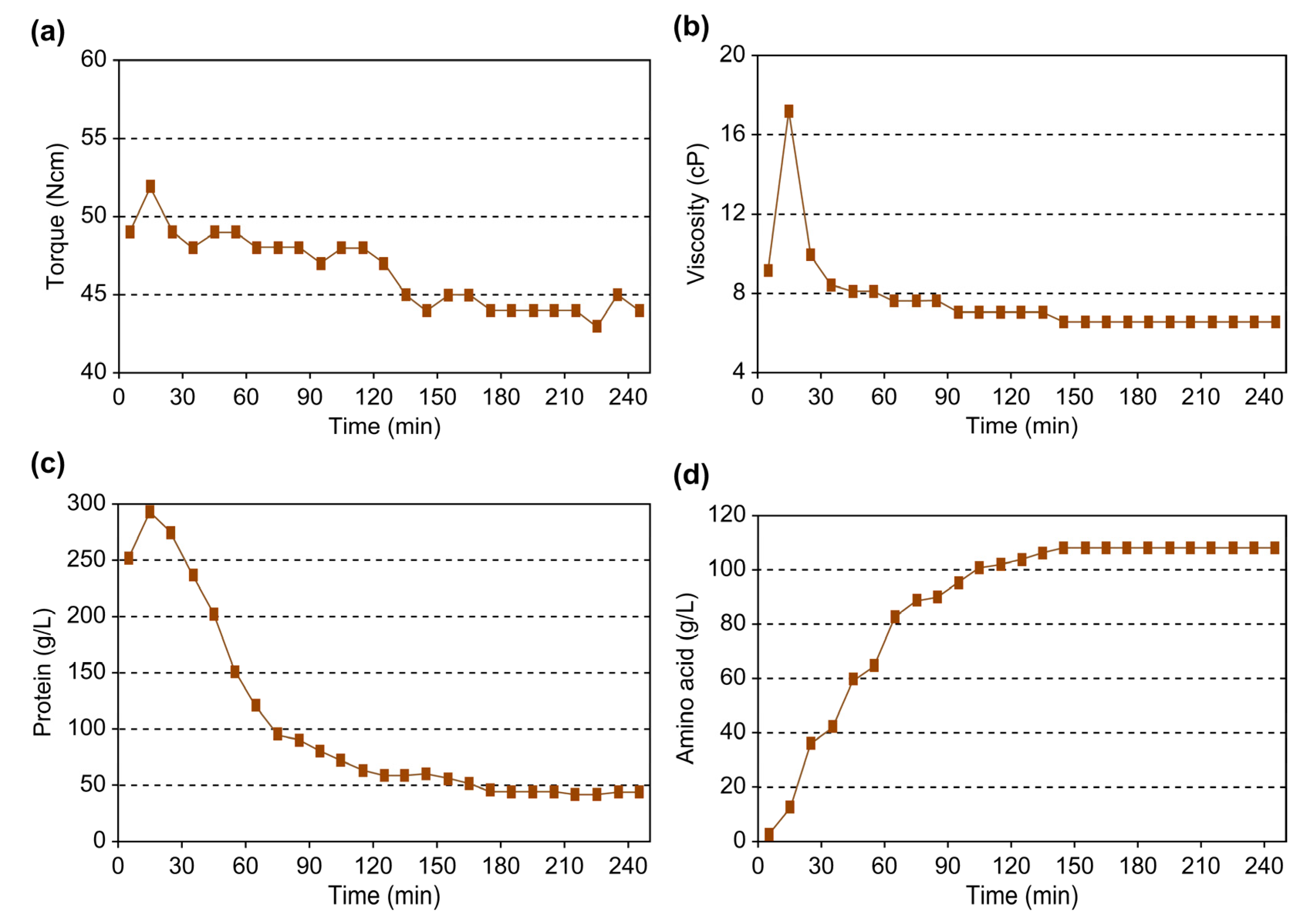
3.2.2. Analysis of the Torque of Reaction Stirrer According to Enzyme Reaction
4. Conclusions
- (1)
- Ultrasound pretreatment resulted in the improved enzymatic hydrolysis of the proteins in livestock blood, as evidenced by the high hemoglobin solubilization rate (97.6%) of the ultrasound-treated blood samples; it also resulted in particle size reduction and pathogen elimination.
- (2)
- Protein hydrolysis experiments using different types of enzymes showed that Flavourzyme®, an exo-type enzyme, exhibited the highest amino acid conversion efficiency. However, considering the relatively lower cost-effectiveness of exo-type enzymes, especially for large-scale processes, we determined the optimal condition to be the combination of the endo-type enzyme Savinase® and the exo-type enzyme Flavourzyme®. This combination yielded an amino acid conversion rate similar to that achieved with the single use of Flavourzyme®. Subsequent enzymatic hydrolysis experiments conducted on ultrasound-treated and untreated blood under the optimal conditions revealed that the amino acid concentration in ultrasound-treated blood reached 54.6 mg/mL, a nearly 4.2-fold increase compared to 13.1 mg/mL in untreated blood. This finding demonstrates that the ultrasonic treatment of livestock blood enhances proteolytic hydrolysis efficiency, particularly when using a combination of endo- and exo-type enzymes, resulting in a substantial increase in amino acid conversion.
- (3)
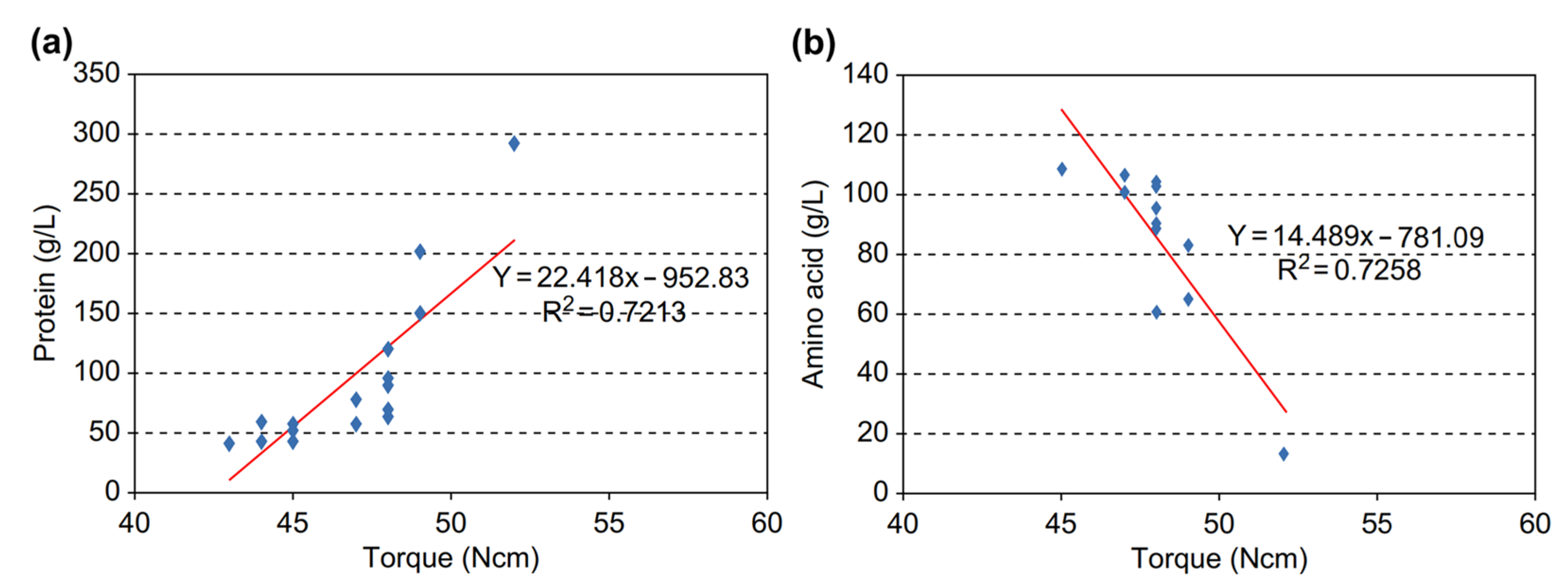
- As enzymatic hydrolysis progressed, the viscosity of livestock blood gradually decreased. Monitoring the agitator torque to gauge the reduction in applied physical force revealed a significant correlation between biological factors, such as protein and amino acid concentrations, and the mechanical factor, torque. Thus, it was confirmed that measuring torque during enzymatic reactions can be utilized to evaluate the extent of enzymatic reactions and thus serve as an indicator of reaction progress.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowak, B.; von Mueffling, T. Porcine blood cell concentrates for food products: Hygiene, composition, and preservation. J. Food Prot. 2006, 69, 2183–2192. [Google Scholar] [CrossRef]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.E.; García, C.A. Harnessing the potential of blood proteins as functional ingredients: A review of the state of the art in blood processing. Compr. Rev. Food Sci. 2017, 16, 330–344. [Google Scholar] [CrossRef]
- Lee, D.M.; Choi, M.S.; Woo, G.I.; Shin, Y.M.; Lee, K.H.; Chun, Y.P.; Chun, T.; Choi, I. Effect of gender-specific bovine serum supplemented medium on cell culture. J. Anim. Sci. Technol. 2009, 51, 413–420. [Google Scholar] [CrossRef]
- Hyun, C.K.; Shin, H.K. Production of angiotensin I converting enzyme inhibitory peptides from bovine blood plasma proteins. Korean J. Biotechnol. Bioeng. 1999, 14, 600–605. [Google Scholar]
- Aziz, A.; Basheer, F.; Sengar, A.; Irfanullah; Khan, S.U.; Farooqi, I.H. Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci. Total Environ. 2019, 686, 681–708. [Google Scholar] [CrossRef]
- Renata, T.D.; Miriam, C.C.; Valdemiro, C.S. Bovine blood components: Fractionation, composition, and nutritive value. J. Agric. Food. Chem. 1999, 47, 231–236. [Google Scholar]
- Bah, C.S.F.; Bekhit, A.E.-D.A.; Carne, A.; McConnell, M.A. Production of bioactive peptide hydrolysates from deer, sheep and pig plasma using plant and fungal protease preparations. Food Chem. 2015, 176, 54–63. [Google Scholar] [CrossRef]
- Moreto, M.; Miro, L.; Amat, C.; Polo, J.; Manichanh, C.; Perez-Bosque, A. Dietary supplementation with spray-dried porcine plasma has prebiotic effects on gut microbiota in mice. Sci. Rep. 2020, 10, 2926. [Google Scholar] [CrossRef]
- Nikhita, R.; Sachindra, N.M. Optimization of chemical and enzymatic hydrolysis for production of chicken blood protein hydrolysate rich in angiotensin-I converting enzyme inhibitory and antioxidant activity. Poultry Sci. 2021, 100, 101047. [Google Scholar] [CrossRef]
- Ann, S.W.; Kim, Y.C.; Hwang, I.S.; Cho, J.K.; Kim, M.S.; Lee, J.K.; Eum, W.Y. Effect of seafood amino acid fertilizer and Korean effective microorganisms on the fruit quality of fuji apple. J. Environ. Sci. 2010, 19, 1293–1299. [Google Scholar]
- Bah, C.S.F.; Carne, A.; McConnell, M.A.; Mros, S.; Bekhit, A.E.-D.A. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016, 202, 458–466. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Q.; Shan, A.; Zhang, H. Optimization of enzymatic hydrolysis of blood cells with a neutral protease. BioMed. Res. Int. 2013, 2013, 278927. [Google Scholar] [CrossRef]
- Fu, Y.; Bak, K.H.; Liu, J.; De Gobba, C.; Tøstesen, M.; Hansen, E.T.; Petersen, M.A.; Ruiz Carrascal, J.; Bredie, W.L.P.; Lametsch, R. Protein hydrolysates of porcine hemoglobin and blood: Peptide characteristics in relation to taste attributes and formation of volatile compounds. Food Res. Int. 2019, 121, 28–38. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, J.; Zhang, W.; Wæhrens, S.S.; Tøstesen, M.; Hansen, E.T.; Bredie, W.L.P.; Lametsch, R. Exopeptidase treatment combined with Maillard reaction modification of protein hydrolysates derived from porcine muscle and plasma: Structure-taste relationship. Food Chem. 2020, 306, 125613. [Google Scholar] [CrossRef]
- Fahlivi, M.; Campus, F.I.; Jonsson, A. Physicochemical Characteristics of Liquid Fertilizer from Fish Viscera. United Nations University Fisheries Training Programme, 2018, Iceland [Final Project]. Available online: http://www.unuftp.is/static/fellows/document/rizal15prf.pdf (accessed on 1 January 2023).
- Sun, T.; Xiao, W.; Jiang, C.; Wang, J.; Liu, Z. Producing amino acid fertilizer by hydrolysis of the fermented mash of food waste with the synergy of three proteases expressed by engineered Candida utilis. Bioresource Technol. Rep. 2019, 7, 100268. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, X.; Li, P.; Zhang, J.; Wang, S.; Ma, H. Effects of low intensity ultrasound on cellulase pretreatment. Bioresour. Technol. 2012, 117, 222–227. [Google Scholar] [CrossRef]
- de Carvalho Silvello, M.A.; Martínez, J.; Goldbeck, R. Low-frequency Ultrasound with Short Application Time Improves Cellulase Activity and Reducing Sugars Release. Appl. Biochem. Biotechnol. 2020, 191, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Kabawa, B.; Sampers, I.; Raes, K. Effect of ultrasonic treatment on enzymes: Decoupling the relation between the ultrasonic driven conformational change and enzyme activity. Ultrason. Sonochem. 2023, 101, 106720. [Google Scholar] [CrossRef] [PubMed]
- Koirala, S.; Prathumpai, W.; An, A.K. Effect of ultrasonication pretreatment followed by enzymatic hydrolysis of caprine milk proteins and on antioxidant and angiotensin converting enzyme (ACE) inhibitory activity of peptides thus produced. Int. Dairy J. 2021, 118, 105026. [Google Scholar] [CrossRef]
- Bougrier, C.; Carrere, H.; Delgenes, J.P. Solubilization of waste-activated sludge by ultrasonic treatment. Chem. Eng. J. 2005, 106, 163–169. [Google Scholar] [CrossRef]
- Timothy, J.M.; John, P.L. Applied Sonochemistry: Uses of Power Ultrasound in Chemistry and Processing; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Wang, F.; Lu, S.; Ji, M. Components of released liquid from ultrasonic waste activated sludge disintegration. Ultrason. Sonochem. 2006, 13, 334–338. [Google Scholar] [CrossRef]
- Lee, D.H.; Shin, H.J. Study on the process to decrease the molecular weight of β-(1,6)-branchedβ-(1-3)-D-Glucans. Korean J. Biotechnol. Bioeng. 2003, 18, 352–355. [Google Scholar]
- Portenlanger, G. Mechanical and radical effects of ultrasound: Ultrasound inwate water and sludge treatment. TU Hambg. Harbg. Rep. Sanit. Eng. 1999, 25, 139–151. [Google Scholar]
- Bah, C.S.F.; Bekhit, A.E.-D.A.; Carne, A.; McConnell, M.A. Slaughterhouse blood: An emerging source of bioactive compounds. Compr. Rev. Food Sci. Food Saf. 2013, 12, 314–331. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Krešić, G. Ultrasonic effect on physicochemical and functional properties of α-lactalbumin. LWT-Food Sci. Technol. 2010, 43, 254–262. [Google Scholar] [CrossRef]
- Santos, S.D.; Martins, V.G.; Salas-Mellado, M.; Prentice, C. Evaluation of functional properties in protein hydrolysates from bluewing searobin (Prionotus punctatus) obtained with different microbial enzymes. Food Bioprocess Technol. 2011, 4, 1399–1406. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association (APHA): Washington, DC, USA, 1995. [Google Scholar]
- Zwart, A.; Assendelft, O.W.; Bull, B.S.; England, J.M.; Lewis, S.M.; Zijlstra, W.G. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobincyanide standard (4th ed). J. Clin. Pathol. 1996, 49, 271–274. [Google Scholar] [CrossRef]
- Schönenbrücher, V.; Mallinson, E.T.; Bülte, M. A comparison of standard cultural methods for the detection of foodborne Salmonella species including three new chromogenic plating media. Int. J. Food Microbiol. 2008, 123, 61–66. [Google Scholar] [CrossRef]
- Tiehm, A.; Nickel, K.; Zellhorn, M.; Neis, U. Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Wat. Res. 2001, 35, 2003–2009. [Google Scholar] [CrossRef]
- Aliger, H. Ultrasonic Disruption. Am. Lab. 1975, 10, 75–85. [Google Scholar]
- Yarullina, L.G.; Akhatova, A.R.; Kasimova, R.I. Hydrolytic enzymes and their proteinaceous inhibitors in regulation of plant-pathogen interactions. Russ. J. Plant Physiol. 2016, 63, 193–203. [Google Scholar] [CrossRef]
- Broekman, S.; Pohlmann, O.; Beardwood, E.S.; Meulenaer, E.C. Ultrasonic treatment for microbiological control of water systems. Ultrason. Sonochem. 2010, 17, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Sheen, S.J.; Sheen, V.L. Characteristics of fraction-1-protein degradation by chemical and enzymic treatments. J. Agric. Food Chem. 1987, 35, 948–952. [Google Scholar] [CrossRef]
- Seeley, E.H.; Oppenheimer, S.R.; Mi, D.; Chaurand, P.; Caprioli, R.M. Enhancement of protein sensitivity for MALDI imaging mass spectrometry after chemical treatment of tissue sections. J. Am. Soc. Mass. Spectrom. 2008, 19, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Mótyán, J.A.; Tóth, F.; Tőzsér, J. Research applications of proteolytic enzymes in molecular Biology. Biomolecules 2013, 3, 923–942. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods 2015, 18, 1125–1137. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, J.; Zhang, Y.; Goh, K.L.; Wan, C.; Zheng, D.; Zheng, M. Preparation and investigation of novel endopeptidase-exopeptidase co-immobilized nanoflowers with improved cascade hydrolysis. Int. J. Biol. Macromol. 2023, 246, 125622. [Google Scholar] [CrossRef]
| Parameters | Concentration (wt.%) |
|---|---|
| Moisture content | 79.18 ± 0.14 |
| Inorganic content | 1.29 ± 0.14 |
| Organic content | 19.53 ± 0.15 |
| Protein | 18.26 ± 0.03 |
| Albumin | 2.03 ± 0.08 |
| Globulin | 2.05 ± 0.04 |
| Fibrinogen | 0.44 ± 0.46 |
| Hemoglobin | 14.08 ± 0.07 |
| Lipid | 0.17 ± 0.01 |
| Salt | 0.95 ± 0.01 |
| Enzyme | Characteristic | Source | Activity | |
|---|---|---|---|---|
| pH Range | Temperature Range (°C) | |||
| Savinase® | Endopeptidase | - | 8–12 (10) | 20–60 (55) |
| Alcalase® | Endopeptidase | Bacillus licheniformis | 6.5–8.5 | 45–65 (60) |
| Flavourzyme® | Exopeptidase | Aspergillus oryzae | 5–7 | 50 |
| Properties | Grinding Treated | Ultrasonic Treated | |
|---|---|---|---|
| Hemoglobin in whole blood (%) | 15.25 | ||
| Hemoglobin in plasma (%) | 5.59 | 14.63 | |
| Solubilization rate (%) | 36.32 | 95.91 | |
| pH | 7.48 | 7.14 | |
| Salinity (%) | 0.96 | 0.92 | |
| Particle size (um) | 131.16 | 44.32 | |
| 3-component | Moisture content (%) | 81.69 | 81.07 |
| Organic content (%) | 17.63 | 18.21 | |
| Inorganic content (%) | 0.69 | 0.72 | |
| Chemical component | Carbon (%) | 45.00 | 46.03 |
| Hydrogen (%) | 6.21 | 6.31 | |
| Oxygen (%) | 17.60 | 16.73 | |
| Nitrogen (%) | 12.65 | 13.11 | |
| Sulfur (%) | 0.14 | 0.15 | |
| Chlorine (%) | 1.07 | 1.09 | |
| Bacteria | General Bacteria (CFU/mL) | 1.5 × 105 | 1.3 × 102 |
| Total Coliforms (CFU/mL) | 2.0 × 103 | N.D. | |
| Salmonella (CFU/mL) | N.D. | N.D. | |
| Parameters | Case 1 c | Case 2 c | Case 3 c | Case 4 c | Case 5 c | |
|---|---|---|---|---|---|---|
| Protein (mg/mL) | Raw a | 83.3 | 61.3 | 263.0 | 104.9 | 130.4 |
| Ultrasonic-treated b | 12.5 | 13.8 | 78.4 | 27.8 | 33.8 | |
| Amino acid (mg/mL) | Raw a | 6.3 | 8.1 | 4.6 | 13.1 | 13.7 |
| Ultrasonic-treated b | 15.1 | 13.4 | 56.9 | 54.6 | 47.5 | |
| Protein Reduction Rate (%) d | Raw a | 78.5 | 84.2 | 32.1 | 72.9 | 66.3 |
| Ultrasonic-treated b | 96.1 | 95.7 | 75.6 | 91.3 | 89.5 | |
| Amino acid Conversion Rate (%) e | Raw a | 1.6 | 2.1 | 1.19 | 3.34 | 3.5 |
| Ultrasonic-treated b | 4.7 | 4.2 | 17.7 | 17.0 | 14.8 | |
| Properties | Enzymatic Degradation | ||
|---|---|---|---|
| Before | After | ||
| pH | 7.20 | 7.08 | |
| Salinity (%) | 0.91 | 0.91 | |
| Particle size (µm) | 48.24 | 8.84 | |
| 3-component | Moisture content (%) | 79.22 | 79.41 |
| Organic content (%) | 19.49 | 19.37 | |
| Inorganic content (%) | 1.29 | 1.22 | |
| Chemical component | Carbon (%) | 46.45 | 47.20 |
| Hydrogen (%) | 6.39 | 6.52 | |
| Oxygen (%) | 17.96 | 19.01 | |
| Nitrogen (%) | 12.89 | 13.41 | |
| Sulfur (%) | 0.12 | 0.11 | |
| Chlorine (%) | 1.11 | 1.20 | |
| Substrate concentration | Protein (mg/mL) | 319.2 | 27.8 |
| Amino acid (mg/mL) | 1.5 | 54.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, M.-J.; Jeon, Y.-W. Enhanced Enzymatic Degradability of Livestock Blood Pretreated with Ultrasonic Technique. Appl. Sci. 2024, 14, 1676. https://doi.org/10.3390/app14041676
Jeon M-J, Jeon Y-W. Enhanced Enzymatic Degradability of Livestock Blood Pretreated with Ultrasonic Technique. Applied Sciences. 2024; 14(4):1676. https://doi.org/10.3390/app14041676
Chicago/Turabian StyleJeon, Mi-Jin, and Yong-Woo Jeon. 2024. "Enhanced Enzymatic Degradability of Livestock Blood Pretreated with Ultrasonic Technique" Applied Sciences 14, no. 4: 1676. https://doi.org/10.3390/app14041676





