1. Introduction
Heavy rail steel is the main load-bearing component of railways. The general trend in the rapid development of railways has been toward high speed and heavy loads. Therefore, the quality requirements of heavy rail steel are becoming more and more strict, not only requiring high cleanliness but also needing to have high strength, high toughness and fatigue resistance. Inclusions in heavy rail steel are mainly divided into two categories: non-metallic inclusions and metal inclusions. Non-metallic inclusions include oxides, sulfides, silicates, etc. Metal inclusions are compounds formed by alloying elements in the melting process. The formation mechanism of inclusions in heavy rail steel is mainly affected by the composition of the raw materials, the reaction during melting and the physical and chemical changes during pouring and solidification. For example, elements such as oxygen, sulfur and phosphorus in raw materials easily react with iron in steel to produce their corresponding oxides, sulfides and phosphates. In addition, in the process of pouring and solidification, due to differences in cooling rates and crystallization rates, the formation of inclusions may also occur. Inclusions have a significant influence on the mechanical properties of heavy rail steel. On the one hand, inclusions are concentrated sources of stress, which reduce the fatigue resistance and toughness of the rail. On the other hand, inclusions also affect the hardness and strength of the rail, thus affecting its wear resistance and brittleness. Therefore, controlling the quantity and distribution of inclusions is very important in improving the performance of heavy rail steel.
In order to improve the cleanliness, strength, toughness and fatigue resistance of heavy rail steel, an aluminum deoxidation process is strictly implemented during the smelting process. However, non-metallic inclusions in steel are still the main factor affecting the cleanliness of the molten steel. Inclusions are also the key factors affecting rail performance [
1,
2,
3,
4,
5,
6,
7]. In the process of producing U75V heavy rail steel, several domestic steel mills have found that there are large particles of MgO–Al
2O
3 spinel inclusions in damaged rail samples of heavy rail steel [
8,
9,
10], which seriously affect the rail quality. MgO–Al
2O
3 spinel inclusions easily react with acids, alkalis and other substances, thus destroying the protective film on the steel surface and making it more susceptible to corrosion. This kind of inclusion produces stress concentration in steel, which leads to decreases in the fatigue strength of the steel and the easy formation of fatigue fractures. MgO–Al
2O
3 spinel inclusions form a brittle phase in steel, which reduces the plasticity and toughness of the steel and makes it prone to brittle fractures. Therefore, in order to reduce the harm of MgO-Al
2O
3 spinel inclusions, it is necessary to conduct in-depth research on its formation mechanism and take effective control measures to reduce its content and distribution.
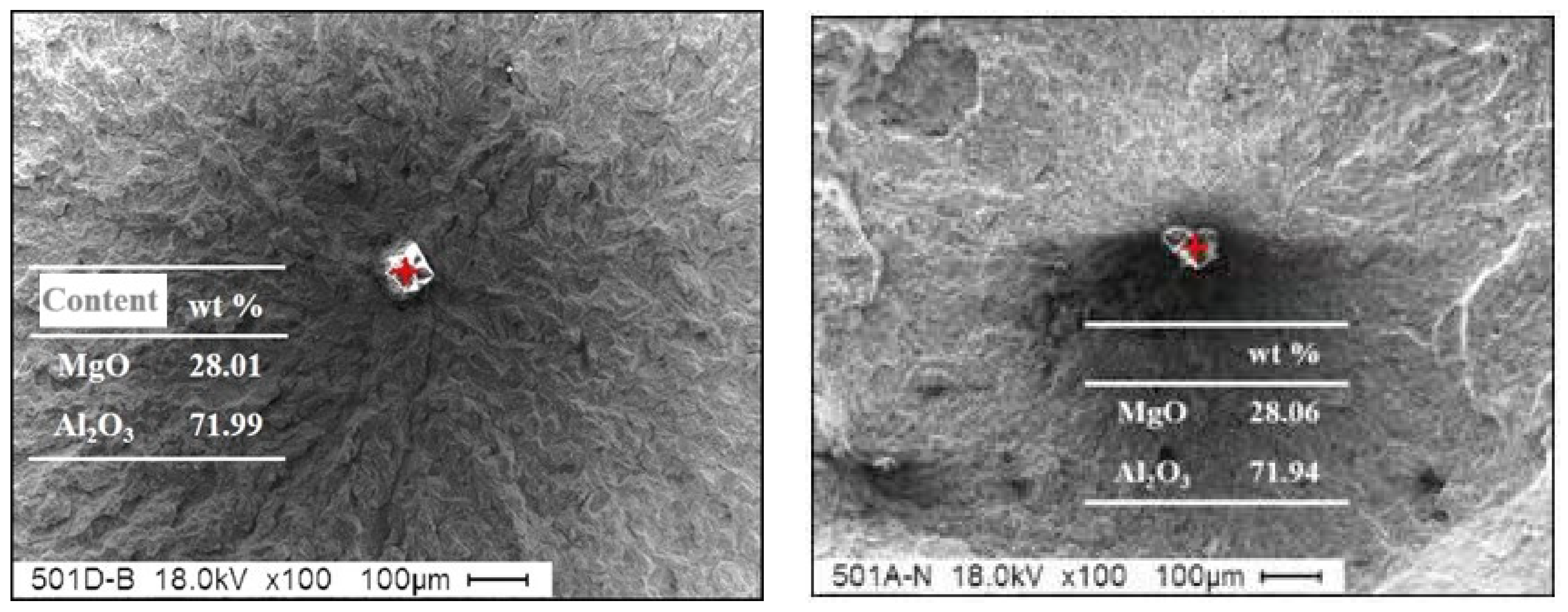
MgO–Al
2O
3 spinel inclusions have a stable, surface-centered cubic structure, with a melting point of 2135 °C and a high hardness. They do not easily deform during rolling, making them type-D point non-deformable inclusions. Most MgO–Al
2O
3 inclusions range in size from 2.0 μm to 6.0 μm, and exist in spherical, cubic and irregular shapes. As non-metallic inclusions in steel, MgO–Al
2O
3 spinel inclusions have a high melting point and poor deformation ability [
11], which seriously harm the plasticity, toughness and fatigue resistance of the steel matrix [
12]. As spinel inclusions do great harm to the quality of steel, many researchers have studied the formation mechanisms of such inclusions. Previous research results can be summarized into four models: (1) the carbon reduction model [
13,
14]; that is, C in the refractory first reduces MgO to generate CO and Mg vapor, which then diffuse into the molten steel and react with Al
2O
3 inclusions in the molten steel to generate spinel inclusions. (2) In the direct reaction model [
15,
16], the Al
2O
3 inclusions in the molten steel react directly when they contact the refractories involved in refining slag. (3) In the aluminum replacement model [
17], MgO enters the steel slag after the refractory is eroded, and the dissolved aluminum in the molten steel displaces the MgO in the steel slag and then forms spinel inclusions. (4) In the crystallography model [
18,
19], when the temperature of the molten steel decreases during continuous casting, the magnesium–aluminum spinel inclusion phase will crystallize inside CaO–SiO
2–Al
2O
3–MgO composite oxide inclusions with a low melting point. Such inclusions are more common in aluminum deoxidized steel [
20,
21]. The formation and control of spinel inclusions in aluminum deoxidized steel have been extensively reported. Harada A et al. [
22] and Park J et al. [
18,
23] pointed out that the formation of spinel inclusions was related to slag basicity and w
CaO/w
Al2O3. The formation of spinel inclusions could be inhibited by appropriately reducing the slag basicity and w
CaO/w
Al2O3. Bjorklund J et al. [
24] showed that a reduction in MgO content in the slag by a VD refining process could inhibit the formation of spinel inclusions. Chi Y et al. [
25] studied the influence of different refractories on steel cleanliness and pointed out that MgO and enamel refractories promote the formation of spinel inclusions in steel. In addition, Shin J et al. [
26] also studied the influence of the CaF
2 content in slag on the formation behavior of spinel inclusions, and pointed out that a w
CaF2 of less than 10% in the slag is conducive for spinel control.
In this paper, the formation, development and change of spinel inclusions in the actual production process of heavy rail steel are studied. In actual production, the formation of inclusions involves thermodynamics, dynamics, heat transfer and mass transfer. With the progress of metallurgical technologies and innovations in equipment, the formation conditions and process of inclusions are complicated and changeable. In this paper, the causes of spinel inclusions which affect the metallurgical quality of heavy rail steel are described from both thermodynamic and experimental aspects, and measures for avoiding spinel inclusions are proposed.