Adsorption Kinetics and Mechanism of Pb(II) and Cd(II) Adsorption in Water through Oxidized Multiwalled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.1.1. Materials
2.1.2. Instruments
2.2. Preparation of O-MWCNTS
2.3. Characterization of O-MWCNTS
2.4. Effect of pH
2.5. Effect of Temperature
2.6. Effect of Initial Concentrations
3. Results and Discussion
3.1. Characterization and Analysis of Results
3.1.1. TEM Analysis
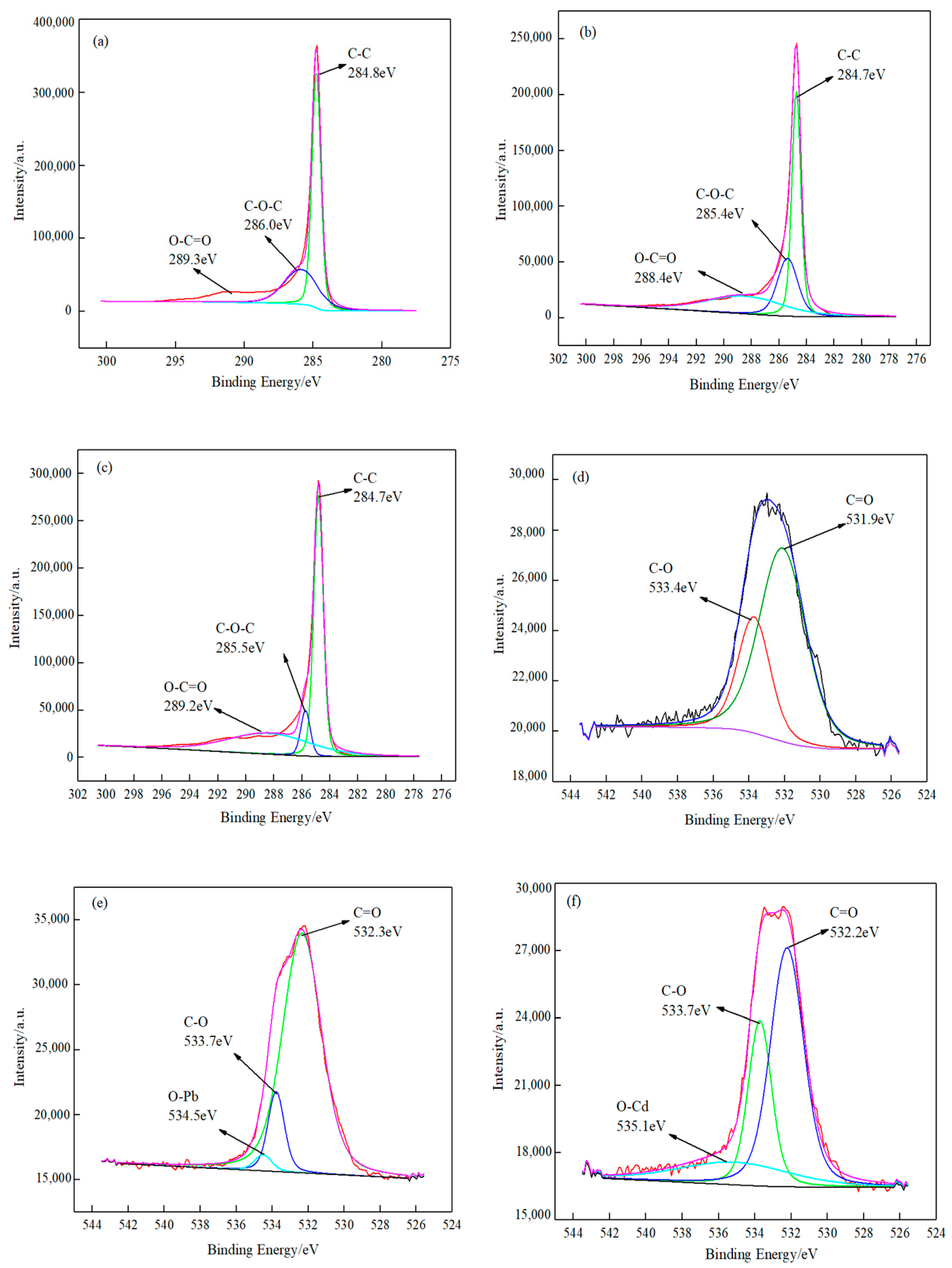
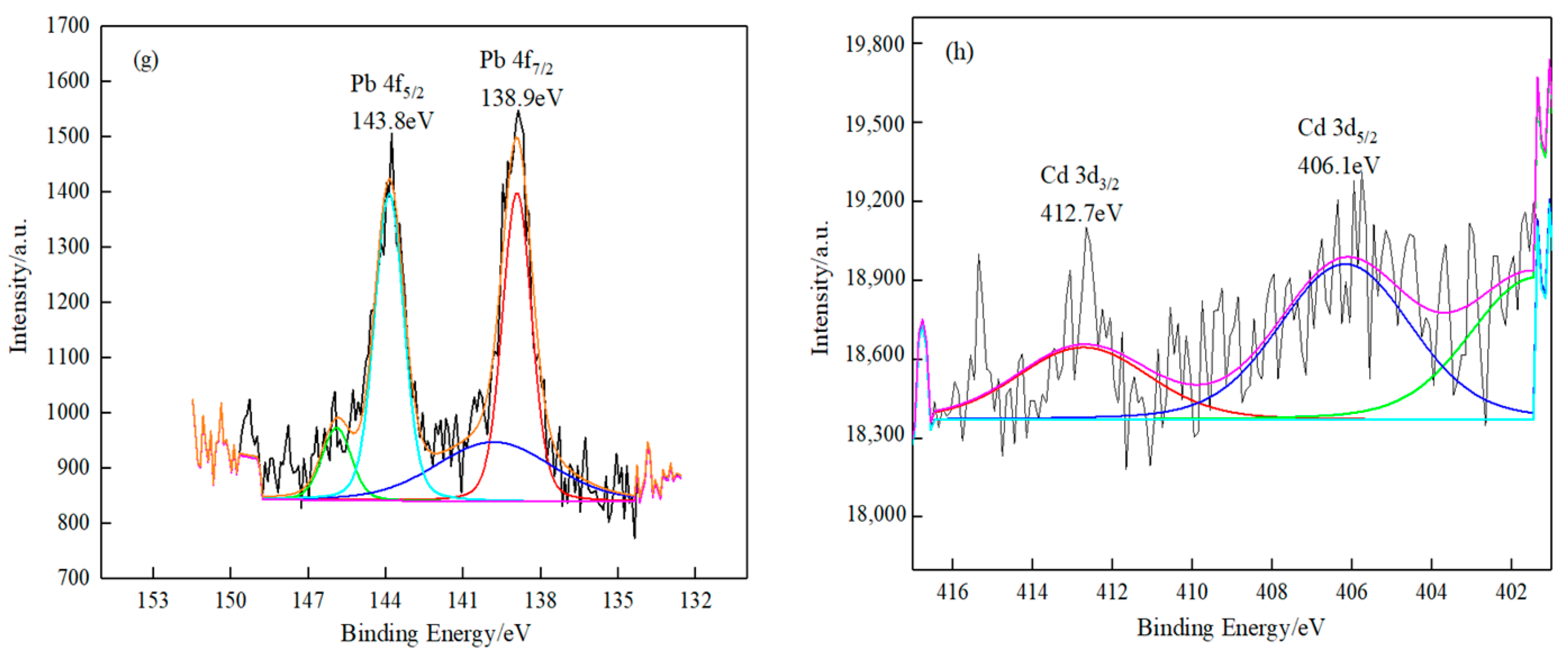
3.1.2. XPS Analysis
3.2. Adsorption Experiments of Pb(II)/Cd(II) with O-MWCNTS
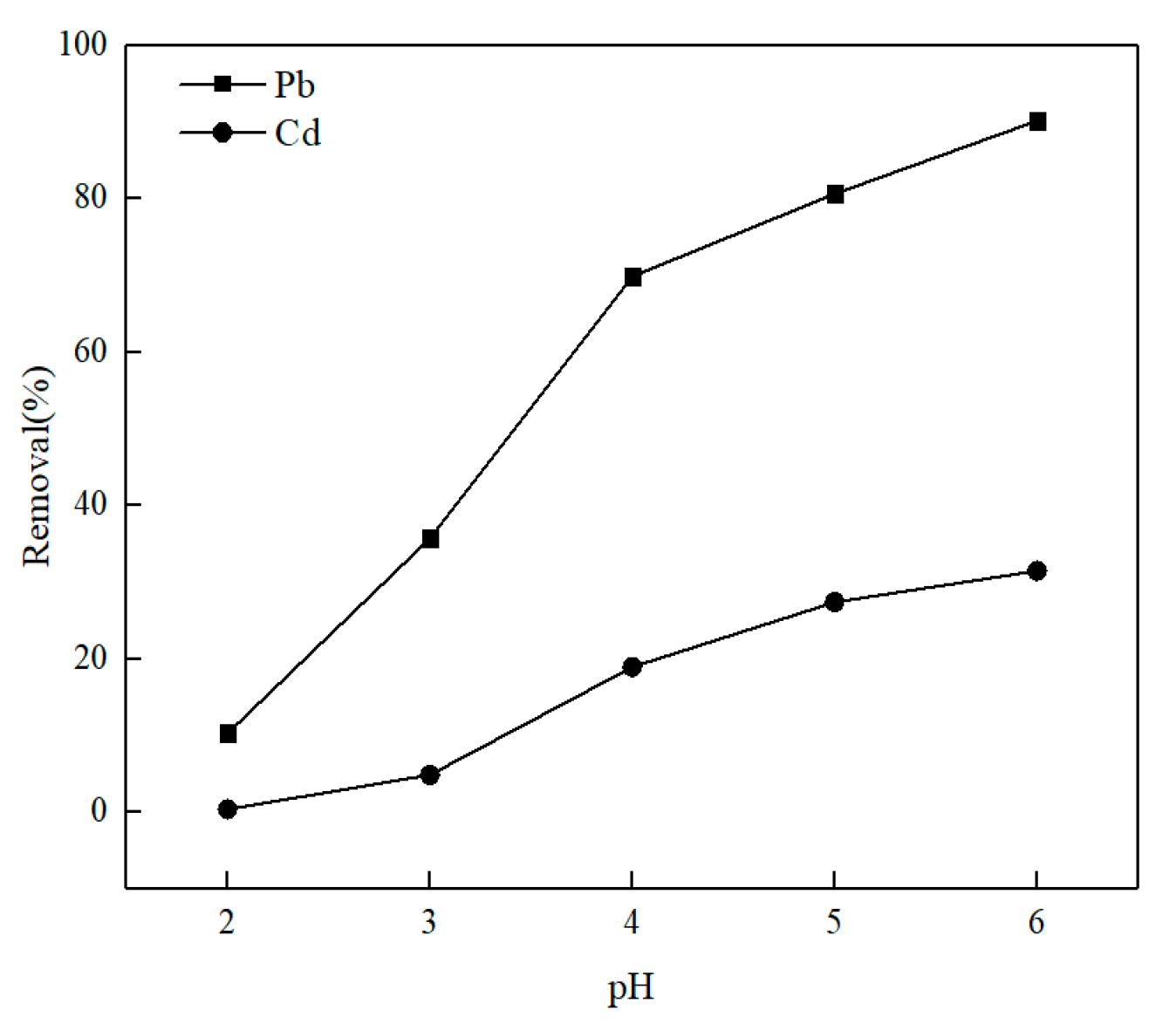
3.2.1. pH
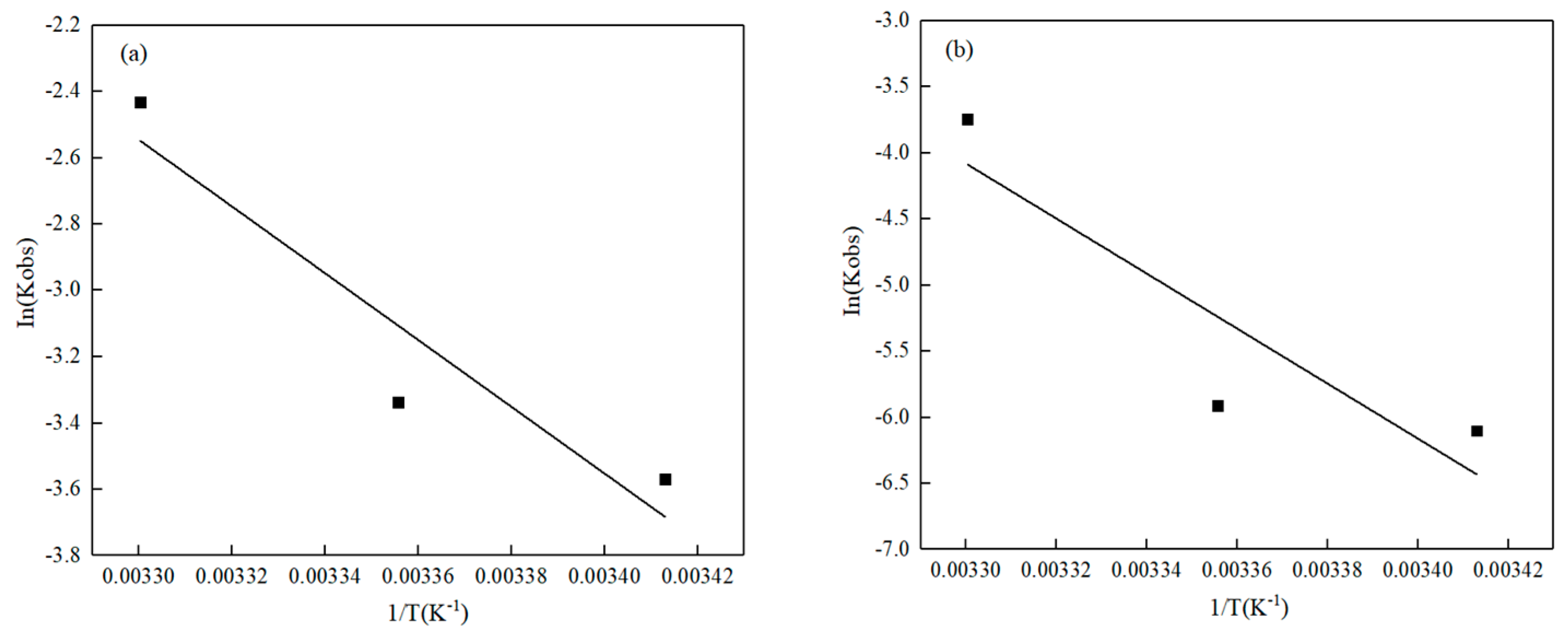
3.2.2. Temperature
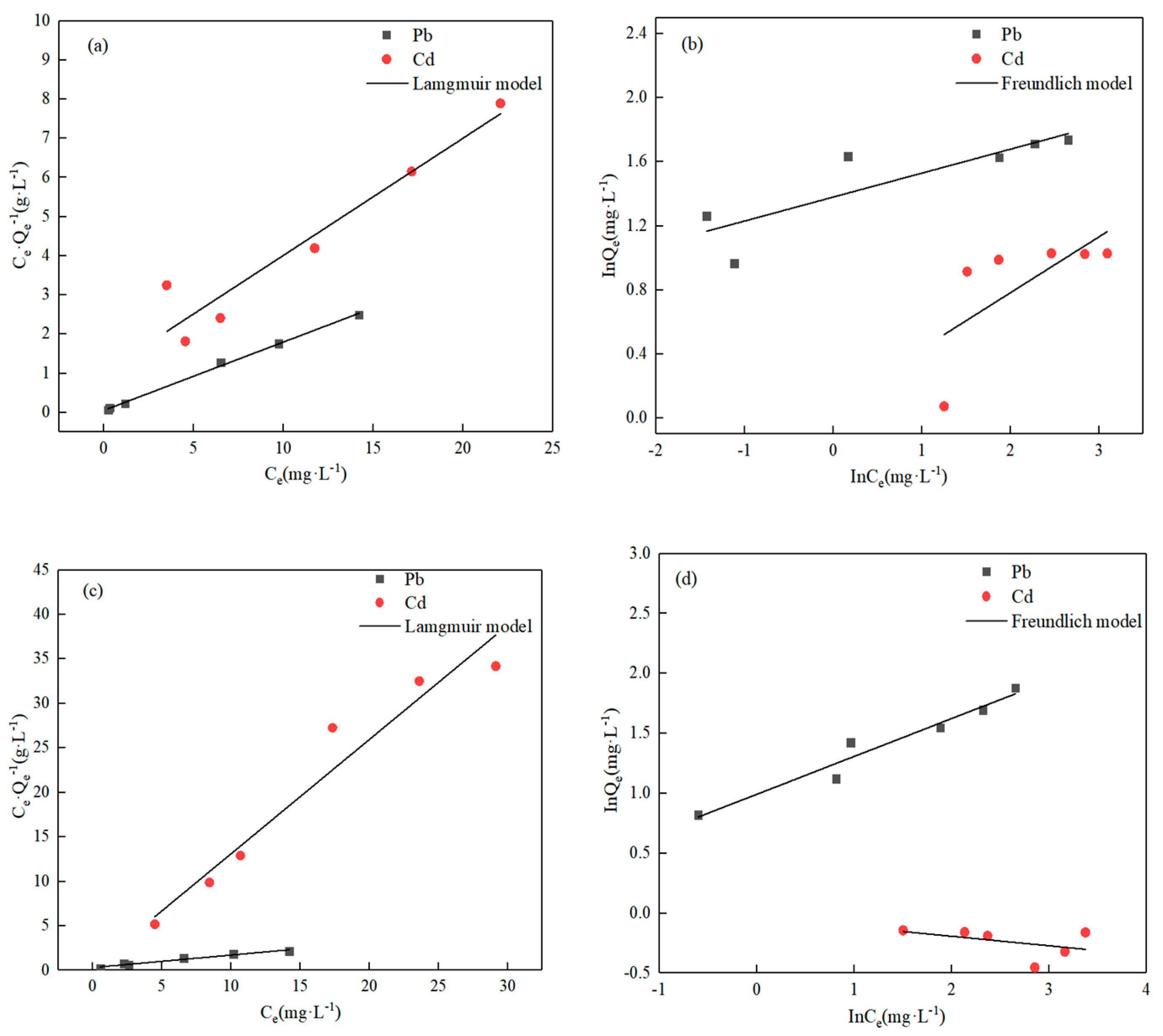
3.2.3. Initial Concentration
3.3. Adsorption Mechanism Analysis
4. Conclusions
- Batch kinetic experiments indicated that O-MWCNTS can effectively absorb metal ions Pb(II)/Cd(II) in water. The batch kinetic tests showed that a pH of 6 was most favorable for the absorption of Pb(II)/Cd(II). When the pH was increased to 6, the removal rate of Pb(II) and Cd(II) was increased to 90.15 and 31.47%, respectively. The removal rate of 76.34% decreased to 55.59% for Pb(II) and from 29.83% to 16.68% for Cd(II), when the starting concentration of Pb(II)/Cd(II) ranged from 5 to 15 mg·g−1. The removal rate in the competitive tests was about 60.46% with Pb(II) and 9.70% with Cd(II), and the tests proved that Pb(II) is more easily adsorbed on the surface of the O-MWCNTS. The Langmuir model was better at describing the absorptive data for both ions. And the Qm of Pb(II) was 5.73 mg·g−1, while that of Cd(II) was 3.34 mg·g−1 in the single-ion system; the Qm was 7.11 mg·g−1 with Pb(II) and 0.78 mg·g−1 with Cd(II) in the competitive system.
- Based on XPS analyses, it can be summarized that the absorbed lead/cadmium species on the surface of the O-MWCNTS was (-COO)2Pb and (-COO)Pb(-O)/(-COO)2Cd and (-COO)Cd(-O).
- Thermodynamic tests indicated that the activating energy was 83.68 kJ·mol−1 for Pb(II) and 172.88 kJ·mol−1 for Cd(II). The adsorption of Pb(II) on the surface of O-MWCNTS was more convenient than that of Cd(II).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Tawil, R.S.; El-Wakeel, S.T.; Abdel-Ghany, A.E.; Abuzeid, H.A.M.; Hashem, A.M. Silver/quartz nanocomposite as an adsorbent for removal of mercury (II) ions from aqueous solutions. Heliyon 2019, 5, e02415. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Kendüzler, E. Characterization and Adsorption/Sensing Applications of Novel Cadmium(II) Based Coordination Polymer. J. Environ. Chem. Eng. 2022, 10, 107989. [Google Scholar] [CrossRef]
- Nejad, M.S.; Sheibani, H. Super-Efficient Removal of Arsenic and Mercury Ions from Wastewater by Nanoporous Biochar-Supported Poly 2-Aminothiophenol. J. Environ. Chem. Eng. 2022, 10, 107363. [Google Scholar] [CrossRef]
- Zhao, R.L.; Chen, J.H.; Xie, X.X. Determination of heavy metals such as copper, lead, zinc, cadmium and nickel in industrial wastewater. Chin. Resour. Compr. Utili. 2022, 40, 25–27. [Google Scholar]
- Wang, H.; Wang, X.H.; Zhang, Y. Sulfate-reducing bacteria-based bioelectrochemical system for heavy metal wastewater treatment: Mechanisms, operating factors, and future challenges. J. Electroanal. Chem. 2023, 951, 117945. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017; p. 631. [Google Scholar]
- Pan, Z.; Liu, M.; Chen, Z. Removal of metalloids and heavy metals from acid mine drainage using biosynthesized Fe/Cu NPs. Biochem. Eng. J. 2024, 201, 109158. [Google Scholar] [CrossRef]
- Mustapha, S.; Tijani, J.O.; Ndamitso, M.M.; Abdulkareem, S.A.; Sumaila, A. The role of kaolin and kaolin/ZnO nanoadsorbents in adsorption studies for tannery wastewater treatment. Sci. Rep. 2022, 10, 13068. [Google Scholar] [CrossRef]
- Muzafar, S.; Tahir, H. Enhanced synthesis of silver nanoparticles by combination of plants extract and starch for the removal of cationic dye from simulated waste water using response surface methodology. J. Mol. Liq. 2018, 252, 368–382. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Tijani, J.O.; Ani, J.I.; Lisak, G. Taguchi optimization design of diameter-controlled synthesis of multi walled carbon nanotubes for the adsorption of Pb(II) and Ni(II) from chemical industry wastewater. Chemosphere 2020, 266, 128937. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Kovo, A.S.; Afolabi, E.A.; Tijani, J.O.; Bankole, M.T. Adsorption of Cr(VI), Ni(II), Fe(II) and Cd(II) ions by KIAgNPs decorated MWCNTs in a batch and fixed bed process. Sci. Rep. 2021, 11, 75. [Google Scholar] [CrossRef]
- Abdulkareem, A.S.; Hamzat, W.A.; Tijani, J.O.; Egbosiuba, T.C.; Mustapha, S.; Abubakre, O.K.; Babayemi, A.K. Isotherm, kinetics, thermodynamics and mechanism of metal ions adsorption from electroplating wastewater using treated and functionalized carbon nanotubes. J. Environ. Chem. Eng. 2023, 11, 109180. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Melgoza, L.L.; García-Depraect, O. Ongoing progress on novel nanocomposite membranes for the separation of heavy metals from contaminated water. Chemosphere 2021, 270, 129421. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R. A critical review on electrospun membranes containing 2D materials for seawater desalination. Desalination 2023, 555, 116528. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, O.A. Carbon derived nanomaterials for the sorption of heavy metals from aqueous solution: A review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100578. [Google Scholar] [CrossRef]
- Merillas, B.; Álvarez-Arenas, T.E.G.; Villafañe, F.; Rodríguez-Pérez, M.Á. Reaching a near zero radiative heat transfer by the inclusion of modified multiwalled-carbon nanotubes (MWCNTs) in polyurethane-polyisocyanurate aerogels. Mater. Today Chem. 2023, 34, 101789. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, H.; Yu, J.; Lin, S. Study on the competitive adsorption and correlational mechanism for heavy metal ions using the carboxylated magnetic iron oxide nanoparticles (MNPs-COOH) as efficient adsorbents. Appl. Surf. Sci. 2019, 473, 960–966. [Google Scholar] [CrossRef]
- Andalouci, A.; Roussigné, Y.; Gangloff, L.; Legagneux, P.; Farhat, S.; Chérif, S.M. 1D cobalt nanocrystals confined in vertically aligned carbon nanotubes: One-step synthesis and magnetic properties. J. Alloys Compd. 2023, 960, 170984. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, S.; Luan, Z. Adsorption of cadmium (II) from aqueous solution by surface oxidized carbon nanotubes. Carbon 2003, 41, 1057–1062. [Google Scholar] [CrossRef]
- Lin, S.H.; Wu, H.X.; Gao, L.P. Application status and prospect of modified carbon nanotubes and their composites in wastewater treatment. Chem. Ind. Eng. Prog. 2021, 40, 3466–3479. [Google Scholar]
- Li, Y.; Shang, Y.; He, J.; Li, M.; Yang, M. Low-loading oxidized multi-walled carbon nanotube grafted waterborne polyurethane composites with ultrahigh mechanical properties improvement. Diamond Relat. Mater. 2022, 130, 109427. [Google Scholar] [CrossRef]
- Guo, X.; Ge, H.; Sun, Z.; Zhao, Q.; Tian, Y.; Liu, D. Chemical coupling of manganese–cobalt oxide and oxidized multi-walled carbon nanotubes for enhanced lithium storage. J. Colloid Interface Sci. 2022, 618, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Wang, L.Z.; Li, X. Characterization and comparative analysis of nonmetallic inclusions in zirconium deoxidized steel. Spectrosc. Spectral Anal. 2023, 43, 2916–2921. [Google Scholar]
- Isaacs, M.A.; Drivas, C.; Lee, R.; Palgrave, R.; Parlett, C.M.; Morgan, D.J. XPS surface analysis of ceria-based materials: Experimental methods and considerations. Appl. Surf. Sci. Adv. 2023, 18, 100469. [Google Scholar] [CrossRef]
- Murakami, R.; Harada, Y.; Sonobayashi, Y.; Oji, H.; Makino, H.; Tanaka, H.; Yoshikawa, H. Correlation analysis with measurement conditions and peak structures in XPS spectral round-robin tests on MnO powder sample. J. Electron. Spectrosc. Relat. Phenom. 2023, 264, 147298. [Google Scholar] [CrossRef]
- Yan, M.H.; Zhang, Y.; Zhang, Z.F.; Li, P.; Hao, J.G.; Li, W.; Fu, P. Study of electrical conduction mechanisms of CaCu3Ti4O12–MgAl2O4 composite ceramics with NTC effect via in-situ XPS and complex impedance analysis. Solid State Sci. 2023, 146, 107372. [Google Scholar] [CrossRef]
- Wan, J.M.; Zhang, F.W.; Han, Z.T.; Song, P.P.; Bai, Y. Efficient adsorption of multiple heavy metal ions by alkali fusion combined with hydrothermal modification of biomass power plant ash. Environ. Eng. 2022, 40, 108–117. [Google Scholar]
- Zhang, M.Q.; Lou, X.F.; Chen, L.B. Study on isothermal adsorption and adsorption kinetics of propachlor in organobentonite. J. Guangxi Univ. Nat. Sci. Ed. 2022, 47, 756–762. [Google Scholar]
- Egbosiuba, T.C.; Egwunyenga, M.C.; Tijani, J.O.; Mustapha, S.; Abdulkareem, A.S.; Kovo, A.S.; Lisak, G. Activated multi-walled carbon nanotubes decorated with zero valent nickel nanoparticles for arsenic, cadmium and lead adsorption from wastewater in a batch and continuous flow modes. J. Hazard. Mater. 2022, 423, 126993. [Google Scholar] [CrossRef]
- Onu, D.C.; Babayemi, A.K.; Egbosiuba, T.C.; Okafor, B.O.; Ani, I.J.; Mustapha, S.; Abdulkareem, A.S. Isotherm, kinetics, thermodynamics, recyclability and mechanism of ultrasonic assisted adsorption of methylene blue and lead (II) ions using green synthesized nickel oxide nanoparticles. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100818. [Google Scholar]
- Wang, J.; Zheng, D.; Xie, G.N. Research progress on enhancement of directional alignment of carbon nanotubes. J. Xi’an Jiaotong Univ. 2022, 56, 35–46. [Google Scholar]
- Zhao, D.M.; Li, Z.W.; Liu, L.D. Progress in preparation and application of graphene/carbon nanotube composites. Acta Chim. Sin. 2014, 72, 185–200. [Google Scholar] [CrossRef]
- Shi, Y.L.; Yu, C.J.; Lin, Q. Performance and mechanism of adsorption of CdII) by biological carbon in coir. Sci. Tech. Eng. 2022, 22, 13187–13193. [Google Scholar]
- Oli, M.; Maleti, S.; Isakovski, M.K. Removing low levels of Cd(II) and Pb(II) by adsorption on two types of oxidized multiwalled carbon nanotubes. J. Environ. Chem. Eng. 2021, 9, 105402. [Google Scholar]
- Jiang, B.H.; Li, L.; Zhao, Y. Effect of pH Value on adsorption of Pb2+ in water by natural magnetite and its adsorption mechanism. J. Funct. Mater. 2013, 44, 3392–3396. [Google Scholar]
- Chen, Y.; Tang, J.; Wang, S.; Zhang, L.; Sun, W. Bimetallic coordination polymer for highly selective removal of Pb(II): Activation energy, isosteric heat of adsorption and adsorption mechanism. Chem. Eng. J. 2021, 425, 131474. [Google Scholar] [CrossRef]
- Rehman, B.S.U.; Shah, S.A. Adsorption of Cr(VI) by NaOH-modified microporous activated carbons derived from the wastes of Amaranthus retroflexus, Magnolia soulangeana, and Tanacetum Vulgar L.: Mechanism, isotherms, and kinetic studies. Environ. Sci. Pollut. Res. 2023, 30, 35808–35837. [Google Scholar]
- Zhao, M.; Huang, Z.; Wang, S.; Zhang, L. Ultrahigh efficient and selective adsorption of Au(III) from water by novel chitosan-coated MoS2 biosorbents: Performance and mechanisms. Chem. Eng. J. 2020, 401, 126006. [Google Scholar] [CrossRef]
- Mohammadreza, B.; Hossein, A.; Mohammad, E.; Ali, E.; Alireza, B. Comprehensive batch and continuous methyl orange removal studies using surfactant modified chitosan-clinoptilolite composite. Sep. Purif. Technol. 2021, 267, 118601. [Google Scholar]
- Qiu, F.G.; Wang, X.Q.; Tong, S.Y. Study on competitive adsorption of heavy metals by sludge from water feeders. Ind. Water Treat. 2022, 42, 72–78. [Google Scholar]
- Luengo, C.V.; Lopez, N.A.; Ramos, C.P.; Avena, M.J. Highly efficient arsenic adsorption onto Mg/Al/Fe layered double hydroxides: Kinetics, isotherm, XPS and Mössbauer spectroscopies. J. Water Process Eng. 2023, 56, 104542. [Google Scholar] [CrossRef]
- Huangfu, C.; Yu, S.; Tong, B.; Yang, A.; Lyu, J.; Guo, X. Efficient lithium extraction from aqueous solutions by MIL-100 (Fe): A study on adsorption kinetics, thermodynamics and mechanism. Sep. Purif. Technol. 2023, 322, 124365. [Google Scholar] [CrossRef]
- Li, K.; Chen, M.; Chen, L.; Zhao, S.; Pan, W.; Li, P.; Han, Y. Adsorption of tetracycline from aqueous solution by ZIF-8: Isotherms, kinetics and thermodynamics. Environ. Res. 2024, 241, 117588. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, H.Q.; Lu, M.M.; Yu, H. Preparation of nitrogen-doped activated carbon and kinetic study of its adsorption of gold-thiosulfate ligand ions. Hydrometall. China 2023, 42, 400–407. [Google Scholar]
- Yang, L.M.; Liu, Y.; Chen, X.S.; Zhang, J.H. Adsorption thermodynamics and kinetics of sisomicin extracted from two types of macroporous weakly acidic cation resins, DK-13 and DK-3. Chin. J. Antibiot. 2023, 48, 179–185. [Google Scholar]
- Zeng, H.; Su, Y.M.; Wang, X.Y. Adsorption behavior of L-cysteine-modified straw fiber on sulfamethoxazole in water: Qualitative analysis by XPS and quantitative calculation by DFT. Acta Sci. Circumstantiae 2023, 43, 86–95. [Google Scholar]
- Chubar, N. XPS determined mechanism of selenite (HSeO3−) sorption in absence/presence of sulfate (SO42−) on Mg-Al-CO3 Layered double hydroxides (LDHs): Solid phase speciation focus. J. Environ. Chem. Eng. 2023, 11, 109669. [Google Scholar] [CrossRef]
- Zhan, F.Q.; Li, Y.L.; Gai, A.J. Preparation and characterization of active boron nitride and its adsorption properties for heavy metal ions. Res. Environ. Sci. 2019, 36, 1317–1328. [Google Scholar]
- Abdelmoula, M.; Ruby, C.; Mallet, M.; Ghanbaja, J.; Coustel, R.; Scudiero, L.; Wang, W.J. Identification of a Fe(OH)2-like phase in the core–shell structure of nano-zero-valent Fe and its evolution when interacting with Pd2+ aq ions by Mössbauer spectroscopy, XPS, and TEM. J. Phys. Chem. Solids 2023, 172, 111066. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar]
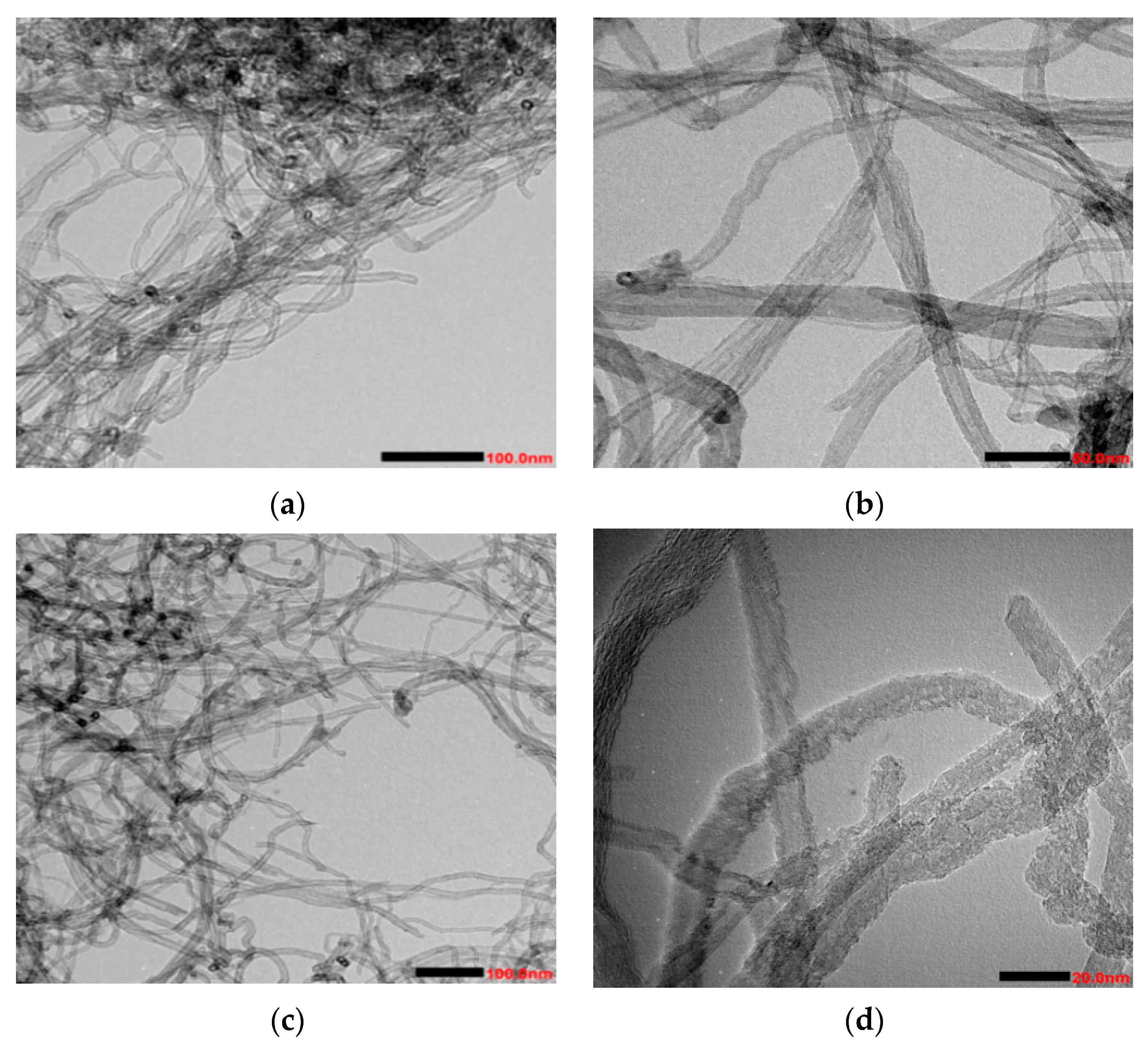
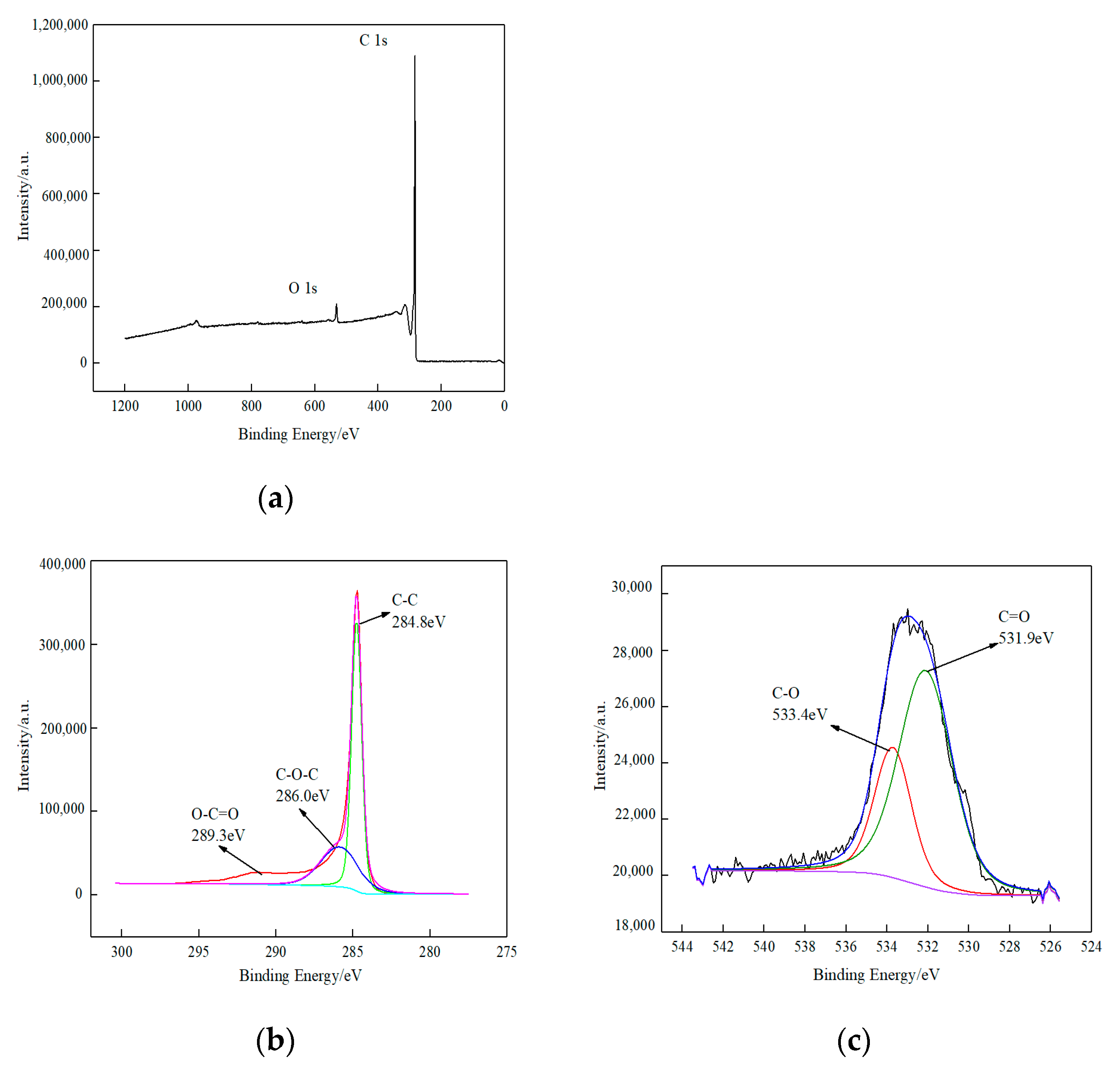

| Instrument Name | Model Number | Factory Owners | Type of Analysis |
|---|---|---|---|
| Electronic Analytical Balance | CPA225D | Sartorius, Göttingen, Germany | Weigh |
| Magnetic heating stirrer | MYP84-1 | Changzhou Ronghua Instrument Manufacturing Co., Changzhou, China | Stirring |
| pH meter | PHS-3C | Mettler Toledo Instruments Ltd., Shanghai, China | pH |
| Inductively Coupled Plasma Emission Spectrometer (ICPES) | Optima 7300 | Platinum Elmer USA Inc., Waltham, MA, USA | Determination of adsorption concentration |
| Dual Function Thermostat and Oscillator | SC240C | Jintan Jierier Electric Appliance Co., Jintan, China | Temperature control and shaking |
| Transmission electron microscope | Hatachi | Hitachi, Shizuoka, Japan | Characterize |
| X-ray photoelectron spectrometer | Nexsa | Thermo Fisher Scientific, Waltham, MA, USA | Determination of elemental binding states |
| Adsorption Conditions | Heavy Metal Ions | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| Qm (mg·g−1) | KL (L·mg−1) | R2 | nF | KF | R2 | ||
| Single | Pb(II) | 5.73 | 3.12 | 0.99 | 6.67 | 3.97 | 0.73 |
| Cd(II) | 3.34 | 0.29 | 0.91 | 2.86 | 1.09 | 0.47 | |
| Competitiveness | Pb(II) | 7.11 | 0.44 | 0.96 | 3.13 | 2.69 | 0.95 |
| Cd(II) | 0.78 | 4.84 | 0.94 | −12.5 | 0.97 | 0.2 | |
| Adsorption Conditions | Metal Type | Quasi-Primary Kinetic Fitting | Quasi-Secondary Kinetic Fitting | ||||
|---|---|---|---|---|---|---|---|
| k1 | Qe1 | R2 | k2 | Qe2 | R2 | ||
| Single | Pb | 0.1 | 6.89 | 0.99 | 0.01 | 7.41 | 0.97 |
| Cd | 0.08 | 2.46 | 0.95 | 0.05 | 2.65 | 0.96 | |
| Competitiveness | Pb | 0.11 | 5.97 | 0.98 | 0.02 | 6.33 | 0.98 |
| Cd | 0.01 | 1.07 | 0.83 | 0.45 | 1.12 | 0.88 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Cui, Y.; Du, W.; Cui, W.; Huo, L.; Liu, H. Adsorption Kinetics and Mechanism of Pb(II) and Cd(II) Adsorption in Water through Oxidized Multiwalled Carbon Nanotubes. Appl. Sci. 2024, 14, 1745. https://doi.org/10.3390/app14051745
Li X, Cui Y, Du W, Cui W, Huo L, Liu H. Adsorption Kinetics and Mechanism of Pb(II) and Cd(II) Adsorption in Water through Oxidized Multiwalled Carbon Nanotubes. Applied Sciences. 2024; 14(5):1745. https://doi.org/10.3390/app14051745
Chicago/Turabian StyleLi, Xin, Yating Cui, Wanting Du, Weiheng Cui, Lijuan Huo, and Hongfang Liu. 2024. "Adsorption Kinetics and Mechanism of Pb(II) and Cd(II) Adsorption in Water through Oxidized Multiwalled Carbon Nanotubes" Applied Sciences 14, no. 5: 1745. https://doi.org/10.3390/app14051745
APA StyleLi, X., Cui, Y., Du, W., Cui, W., Huo, L., & Liu, H. (2024). Adsorption Kinetics and Mechanism of Pb(II) and Cd(II) Adsorption in Water through Oxidized Multiwalled Carbon Nanotubes. Applied Sciences, 14(5), 1745. https://doi.org/10.3390/app14051745




