Abstract
Soil heavy metal pollution poses a formidable challenge for environmental protection professionals. This study delves into the impact of zinc and lead pollution on the particle size distribution, liquid-plastic limit, permeability, shear strength, and other pertinent physical and engineering properties of clay. The alterations in the microstructure of soil contaminated by heavy metals were scrutinized using a scanning electron microscope. The findings reveal that as the concentration of heavy metals in contaminated soil rises, there is a concurrent decrease in the liquid limit, plasticity index, and silt content. This, in turn, leads to the deterioration of the original fluidity and plasticity of the soil, accompanied by a reduction in fine particles. Resistivity tests indicate that an escalation in water content results in a decrease in resistivity, an increase in porosity leads to an increase in resistivity, and an elevation in the concentration of heavy metals precipitates a sharp decline in resistivity due to the heightened conductivity of heavy metal ions. Heavy metal pollution induces structural changes in the soil, particularly in pore size, thereby influencing the permeability coefficient.
1. Introduction
Over the last two decades, the swift advancement of urbanization in China has propelled the rapid growth of the regional economy while concurrently giving rise to severe urban environmental pollution. As a typical inorganic pollutant, heavy metal pollution has a varied geographical distribution in China due to factors such as the level of economic development, industrialization, and distribution of mineral resources. As shown in Figure 1, compared with the eastern and western regions, the central region of China has a larger number of metal mines and coal mines, and the level of industrialization is more developed, so soil heavy metal pollution is more prominent and the rate of soil heavy metal pollution accounts for about 34.23% of the total pollution rate [1].
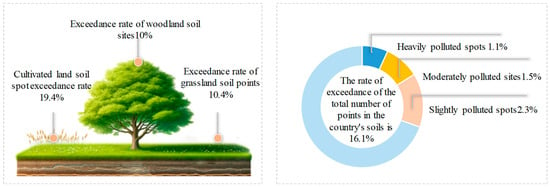
Figure 1.
Distribution of heavy metal content in the soil of major cities in China [1].
In 2014, the Ministry of Environmental Protection and the Ministry of Land and Resources released the National Soil Pollution Survey Communique that explicitly stated that the Yangtze River Delta, Pearl River Delta, and Northeast China’s mature industrial bases had, due to inadequate control measures and unregulated discharges, substantially surpassed the permissible levels for inorganic pollutants, notably heavy metals [2]. Currently, heavy metals have emerged as the primary contaminants influencing soil environmental quality in China. Residual heavy metals generated from industrial and domestic activities integrate with the soil matrix through processes such as self-diffusion, precipitation, and adsorption, culminating in soil corrosion, structural degradation, and alterations in physical and mechanical properties. These transformations exert an adverse influence on the quality of regional engineering construction [3].
To enhance the guidance of engineering practices, it is imperative to investigate the characteristics of soil contaminated with heavy metals. The exploration of contaminated soil commenced with the inaugural International Contaminated Soil Conference held in the Netherlands in 1985. Subsequently, numerous institutions embarked on research endeavors concerning contaminated soil. In contrast, the investigation of contaminated soil in China commenced at a later date, only gaining momentum in the 1980s when Tongji University initiated comprehensive research on the migration patterns of pollutants in soil [4].
At present, there are many branches of research on contaminated soil, and scholars at home and abroad have drawn a large number of conclusions. Zulfahmi et al. [5] found that the introduction of tin tailings results in a decrease in the liquid limit of the soil matrix, with the plasticity index exhibiting an incremental trend corresponding to the quantity of tin tailings incorporated. Concurrently, tin tailings exert an influence on the permeability and undrained strength characteristics of the soil mass. Liu et al. [6] found that the introduction of heavy metal ions led to a reduction in the clay particle content within contaminated loess.
Souli et al. [7] found that the introduction of Zn2+ into soil resulted in an increase in both soil particle size and the pore size between soil particles. Julien et al. [8] studied the permeability of expanded soil after Cu2+ immersion and found that the permeability coefficient of contaminated soil showed a trend of first decreasing and then increasing with the increase in Cu2+ ion concentration. Zheng et al. [9] found that the increase in heavy metal ion concentration increased the permeability coefficient of contaminated soil while reducing its unconfined compressive strength. Pan et al. [10] showed that the increase in heavy metal pollution concentration in test soil led to the decrease in unconfined compressive strength, the decrease in clay content, and the increase in soluble salt content. Lv et al. [11] found that the compressibility of heavy metal-contaminated expansive soil increased with the increase in heavy metal ion concentration through consolidation testing under cyclic loading. Chen et al. [12] found that the compressive strength of soil decreased with the increase in heavy metal pollutant content. Li et al. [13] conducted resistivity tests on cadmium-contaminated soil solidified by rice husk ash and cement, and found that the resistivity of contaminated soil samples fluctuated with the increase in heavy metal ion content before decreasing.
Generally, limited research has been conducted on the geotechnical properties, microstructural alterations, and mechanical characteristics of soil contaminated with heavy metal ions. In this study, laboratory geotechnical tests were conducted on contaminated soil samples containing varying concentrations of heavy metal ions, focusing on the examination of undrained shear strength, resistivity, particle size, and microstructure of the contaminated soil.
2. Materials and Methods
2.1. Materials
The test soil used in this study was the national standard kaolin. Prior to the preparation of the remolded soil sample, the soil underwent a drying process and screening to eliminate impurities, followed by a comprehensive basic soil test. Table 1 presents the fundamental parameters of the sample.

Table 1.
Physical and mechanical properties of sandstone.
2.2. Preparation of Heavy Metal-Contaminated Soil
China is a large mineral-rich country with lead and zinc being the dominant minerals found in resource-rich lead and zinc mines that are widely distributed across the country. However, while the rapid development of the lead and zinc mining industry has made outstanding contributions to the country’s economic development, it has also caused serious environmental pollution issues due to a lack of technology and poor management during the mining process, with heavy metal pollution in particular becoming increasingly serious. Therefore, zinc and lead were selected as the heavy metal pollution sources for this paper.
The specific characteristics of heavy metal ions are shown in Table 2.

Table 2.
Common indicators of pollutants.
The soil was flushed several times using deionized water to remove excess heavy metal ions. The conductivity value of the rinse solution was tested and when the conductivity was less than 600 µs/cm, the soil was considered to be completely cleaned of excess metal ions. The clean pure soil samples were crushed, sieved, and baked in an oven at 105–110 °C until they reached a constant weight, and were then removed from the oven and cooled to room temperature in a desiccator. The prepared pollution solution with constant concentration and water content was mixed into the prepared pure clay powder and stirred thoroughly. The prepared heavy metal-contaminated soil samples were sealed in self-sealing bags and kept in a standard room at constant temperature (room temperature 25 ± 0.5 °C, relative humidity >90%) for subsequent tests.
2.3. Test Equipment and Scheme
According to the provisions of the “Standard for Geotechnical Test Methods” (GB/T 50123-2019) [14], liquid-plastic limit testing, direct shear testing, plastic index testing, and fractional testing were carried out to analyze the physical and mechanical properties of the prepared heavy metal-contaminated soil.
Microstructure is intrinsic to the composition of macroscopic soils and is also the main factor affecting the engineering properties of soils. In order to determine the microstructural characteristics of the studied heavy metal-contaminated soils, SEM tests were carried out. In this paper, SEM tests were performed according to the following requirements of the experimental specifications: collect soil specimens before and after contamination; use tweezers to adjust the specimen to keep the fresh side up; use double-sided tape to fix the specimen onto the carrier table; try to avoid the impact of disturbances such as touching or vibration; gently move the carrier table to the vacuum chamber in preparation for spraying the specimen with gold; start the vacuum pumps to exclude the air in the vacuum chamber and to maintain the indoor vacuum; spray the surface of the specimen with gold to ensure the surface of the specimen produces good electrical conductivity, to prevent electrical charge build-up at the surface and the phenomenon of electrical discharges, and to increase the quality and clarity of the image.
Indoor test methods for soil resistivity can be classified into two-electrode and four-electrode methods depending on the number of electrodes. The principle of the two-electrode test method is to measure the voltage difference and current value at both ends of the soil sample to calculate the resistivity value per unit size of the soil sample. Although this method is simple in structure and convenient during testing, the electrodes at the two ends of the soil sample causes the electrode polarization effect, which reduces the stability of the test data and leads to failure of the test to meet the requirements of high accuracy. The principle of the four-electrode test method is to set the electrodes at both ends of the soil sample as transmitter electrodes for measuring the current value inside the soil sample; at the same time, a section inside the soil sample is selected at random and a measuring electrode is applied to measure the voltage difference of the section, which is then used to calculate the resistivity value per unit size of the section of the soil sample. The four-electrode test method can effectively eliminate the influence of the polarization effect of the electrodes at both ends of the soil sample on the conductive properties of the soil sample, thus improving the accuracy and stability of the resistivity measurement value of the soil sample. This method is more suitable for conductivity measurements that require high accuracy.
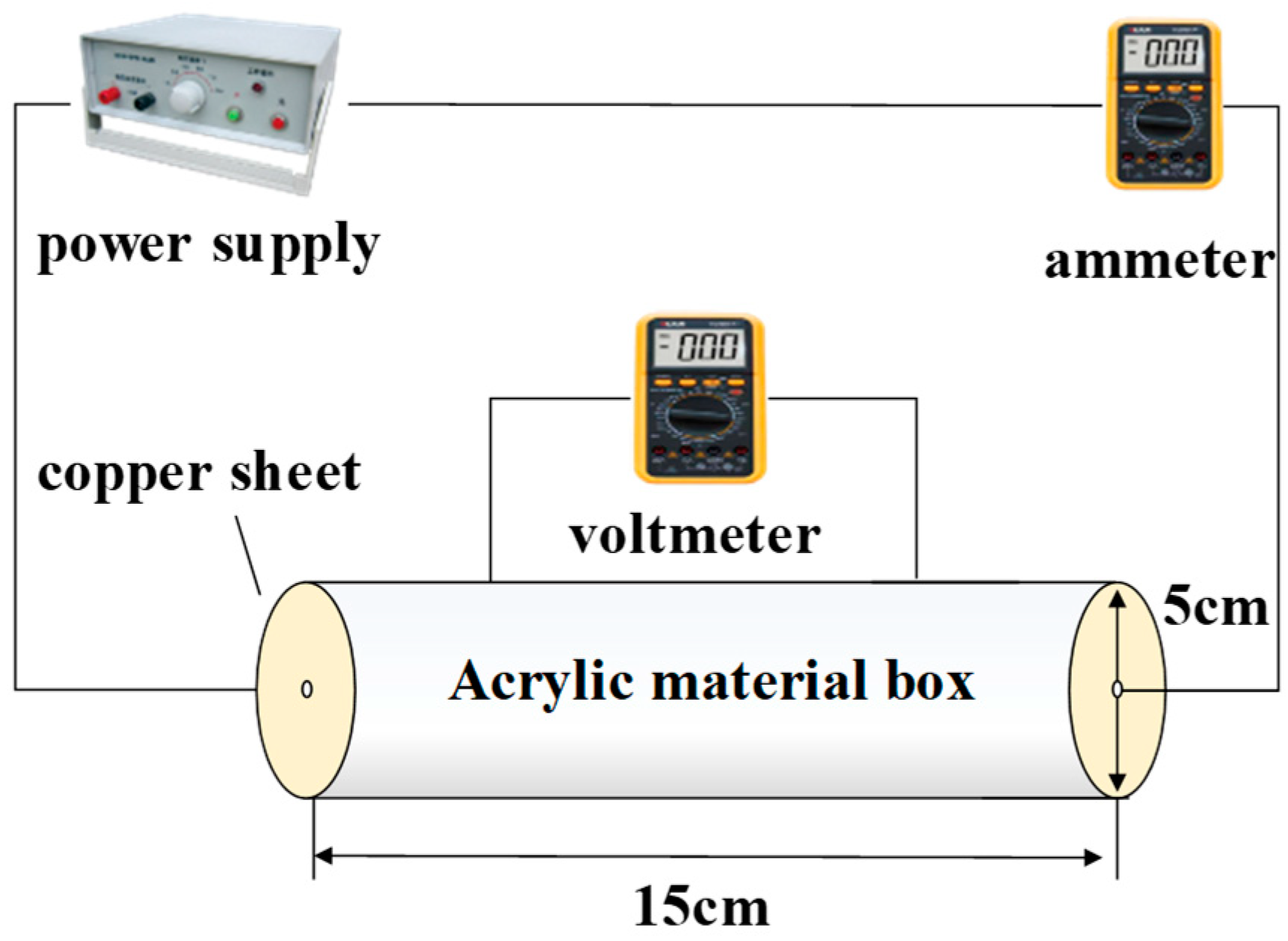
In this paper, an improved circular four-electrode resistivity measuring device is used to analyze the resistivity change of the heavy metal-contaminated soil. The schematic diagram of this test device is shown in Figure 2.
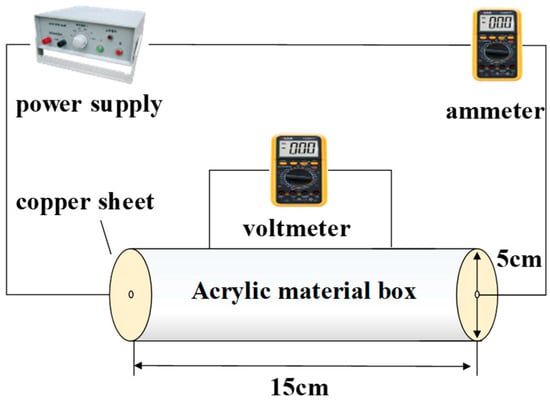
Figure 2.
Electrical resistivity test device circuit diagram.
The main body of the test device is a hollow cylinder made out of acrylic material, with a diameter of 5 cm and a length of 15 cm. On the side wall of the cylinder, there are two holes, 5 cm apart, for voltage measurement at both ends of the soil sample. There are 5 cm diameter conductive copper sheets on both sides to measure the current passing through the soil sample. The device used in this paper has relatively stable and accurate current and voltage readings, and it is easy to convert these data into the true resistivity of the soil samples. The cylindrical acrylic outer wall allows for the observation of the effect of the soil sample during compaction.
The specific steps for resistivity measurements are as follows:
Firstly, place a pre-prepared sample of concentration-contaminated soil into the test device and compact it with hydrostatic pressure. Next, connect the test device to the power supply and an ammeter/voltmeter, ensuring that the current and voltage are stable and constant at a frequency of 50 Hz. During this process, the corresponding resistivity values are measured. The test scheme is shown in Table 3.

Table 3.
Heavy metal-contaminated soil resistivity test scheme.
Based on the proportion of heavy metal ion mass to the total mass of dry soil, the content of heavy metal pollutants designed in this paper was 0.5 mg/kg, 5 mg/kg, 50 mg/kg, 500 mg/kg, and 5000 mg/kg. Meanwhile, in order to study the influence of state parameters such as water content and porosity on the characteristics of heavy metal-contaminated soil, the water content of the prepared soil samples was 20%, 25%, 30%, 35%, and 40%. The porosity was 0.4, 0.425, 0.45, 0.475, and 0.50.
The shear rate was set to 0.8 mm/min. The vertical stress of the test was 100 kPa, 200 kPa, 300 kPa, and 400 kPa. During data processing, the shear strength was the peak value of shear stress within 6 mm of the displacement on the shear stress and shear displacement curve. When there was no peak value, the shear stress corresponding to the shear displacement of 4 mm was taken as the shear strength. The test flow is shown in Figure 3.
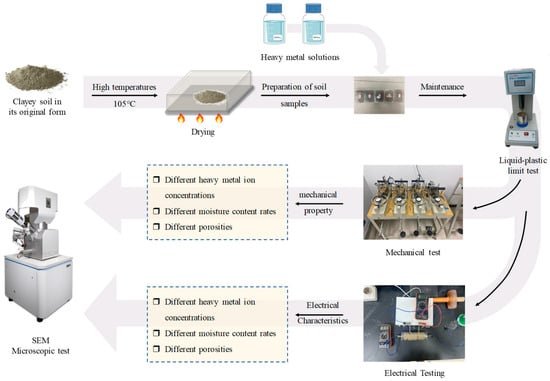
Figure 3.
Test flow chart.
3. Results
3.1. Physical Test Analysis
3.1.1. Study on Particle Gradation of Heavy Metal-Contaminated Soil
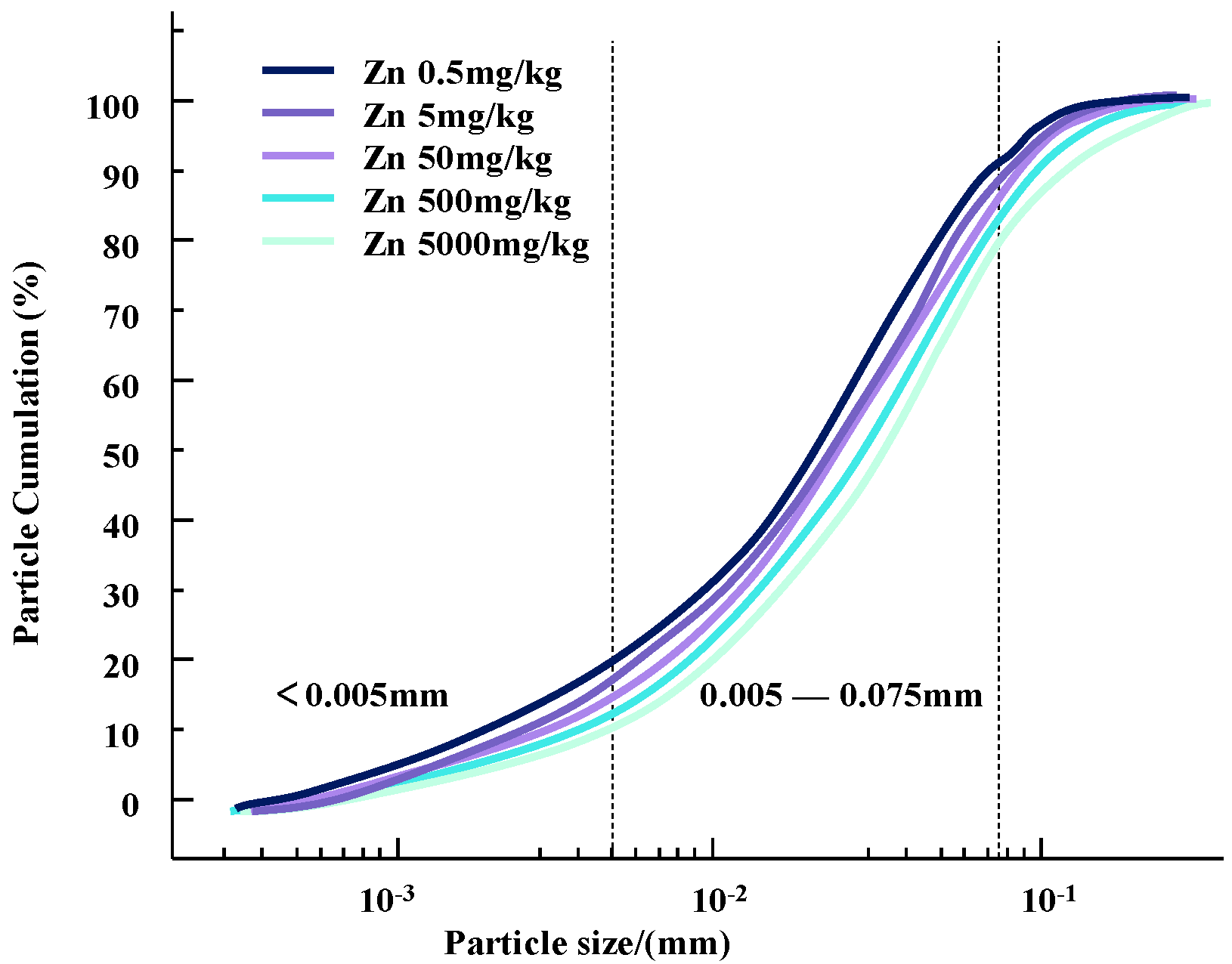
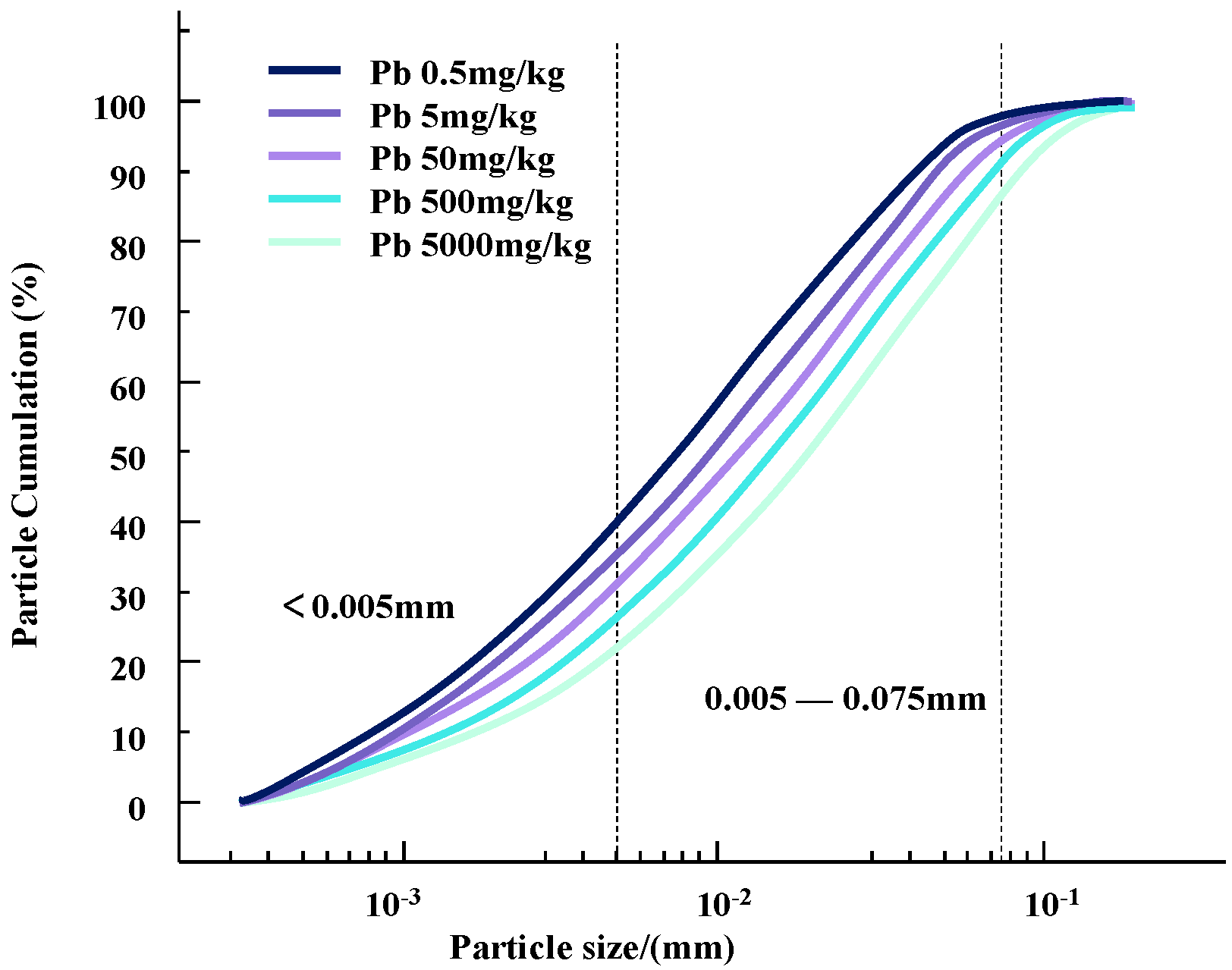
The particle size composition test is an important research tool and method to study the relative content of various particle size groups in soil and to classify soil. The particle size and its distribution in soil samples with different concentrations of Zn and Pb contaminants were determined using a laser particle sizer. Changes in particle content and particle size distribution curves for different concentrations of heavy metal pollutants are shown in Figure 4 and Figure 5.
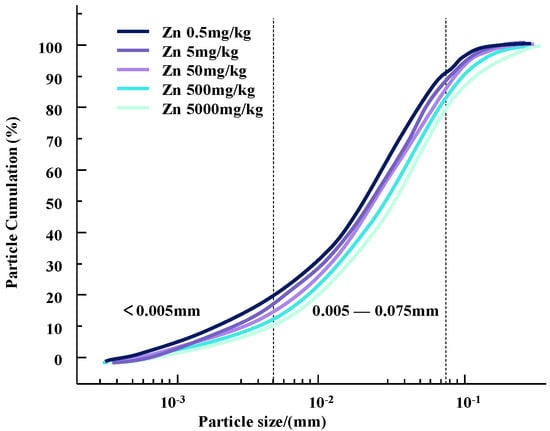
Figure 4.
Gradation curves of zinc-contaminated soil with different concentrations.
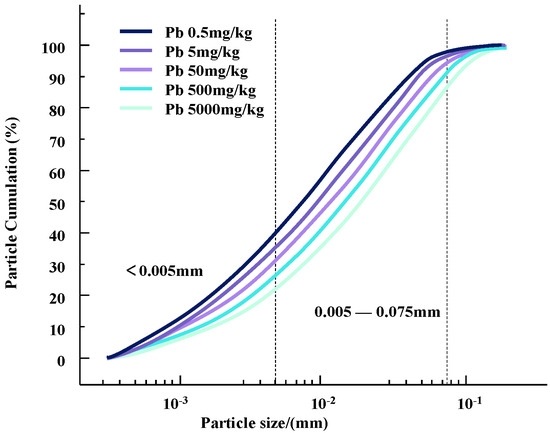
Figure 5.
Gradation curves of lead-contaminated soil with different concentrations.
It can be seen that as the concentration of heavy metal pollutants increases, the particle size of the soil changes and the gradation curve shifts downward. Since the selected heavy metal ions are all +2 valent ions, the changes in silt content and clay content of the two heavy metal-contaminated clayey soils have a similar trend with the concentration of heavy metal contaminants.
This change may be due to the infiltration of heavy metal ions, resulting in a change in the contact between soil particles [13]. During this process, some colloids bind heavy metal contaminants in the soil, causing the surface of the clay particles to appear double-charged. High-priced heavy metal ions may replace the original low-priced positive charges on the surface of soil particles to form new pollution bonds. This will reduce the thickness of the double electric layer on the surface of the soil particles, strengthen the bonding force between the particles, and form a polymer, which causes the agglomeration of contaminated soil particles and gradually increases the particle size of the soil particles [15]. Therefore, compared with natural soil, the content of clay particles is increased and the content of silt particles is decreased [16].
3.1.2. Study on Liquid Limit and Plastic Limit of Heavy Metal-Contaminated Soil
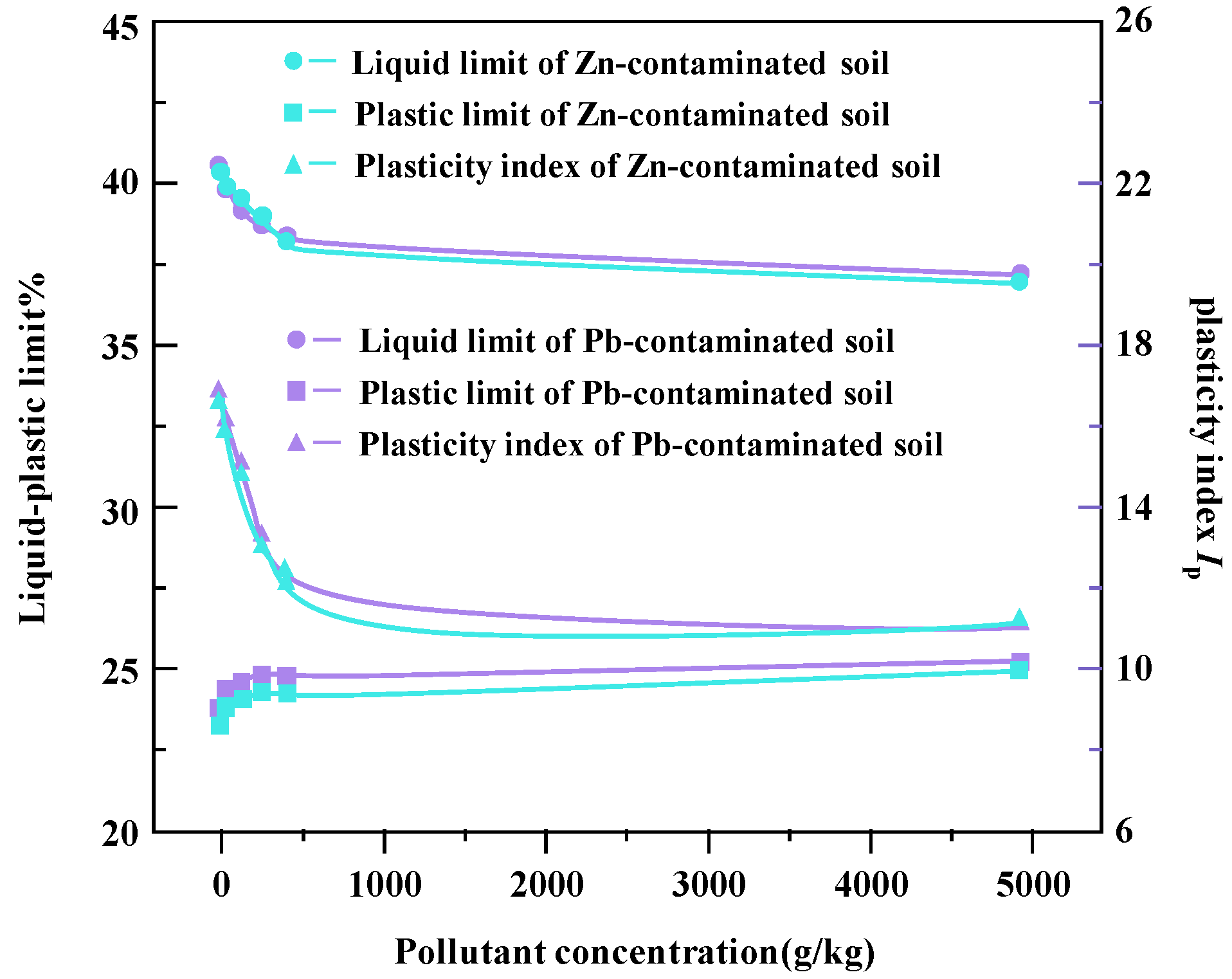
In Figure 6, the change curves of liquid limit, plastic limit, and plastic index of Zn- and Pb-contaminated soils with different concentrations are shown. The test results show that heavy metal ions have significant effects on the liquid limit and plastic index of the sample soil. With the increase in heavy metal ion concentration, the liquid limit and plasticity index showed a decreasing trend, which indicates that heavy metal pollution would cause the weakening of soil fluidity and plasticity.
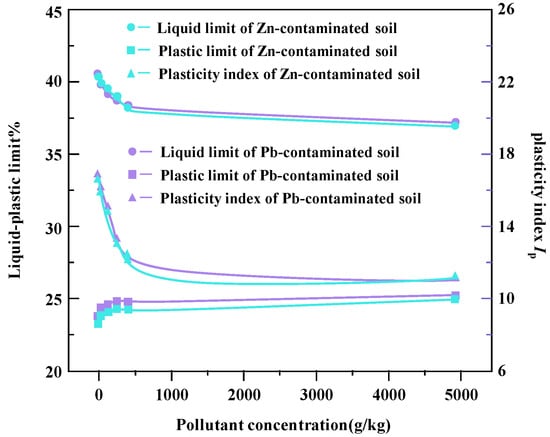
Figure 6.
Change curves for plastic limit and plastic index of Zn- and Pb-contaminated soil liquids.
The reason for this change may be that with the increase in heavy metal ion concentration, the surface double layer of soil particles becomes thinner [17]. This results in a polymerization effect between particles, resulting in a decrease in the surface area and the water retention capacity between particles, which, in turn, leads to a decrease in both the liquid limit water content and the plasticity index [18]. Metal ions further enhance this effect by reducing the repulsive forces between particles and allowing particles to move more freely at lower water content. This process leads to the decrease in both the liquid limit and the plastic index of heavy metal-contaminated soil [19].
3.2. Direct Shear Test Analysis
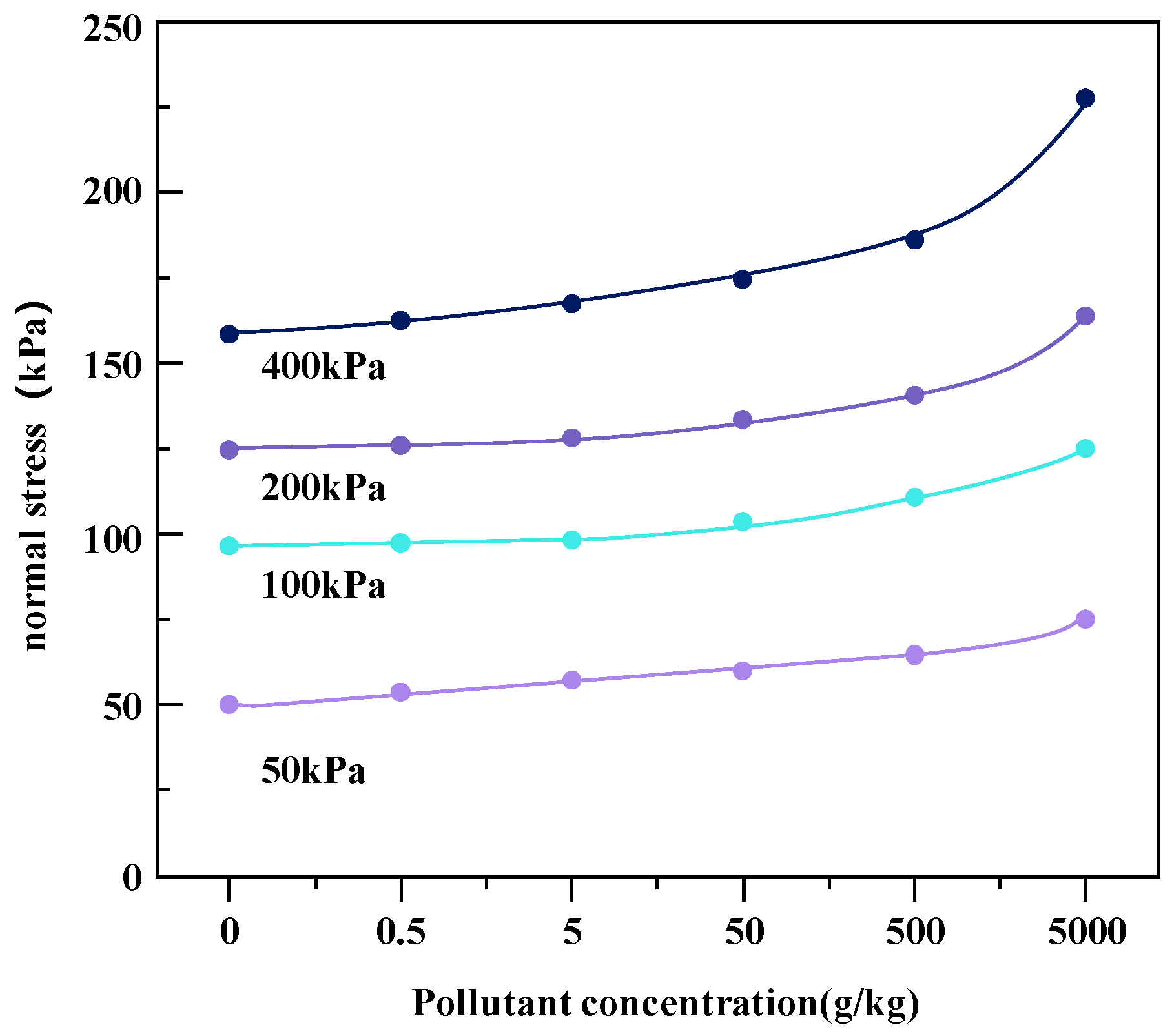
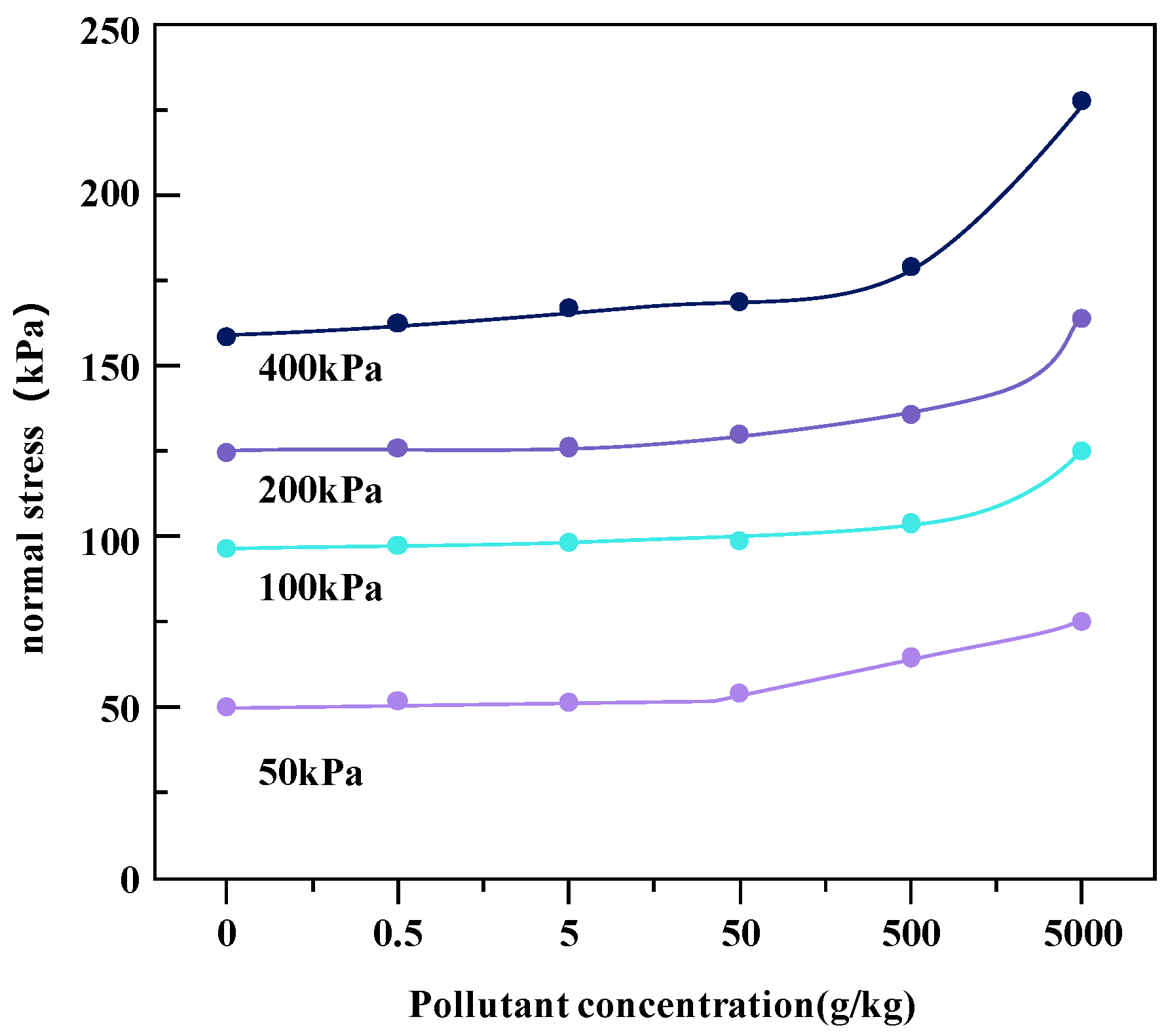
The effects of heavy metal concentrations on the shear strength of soil under different coaxial loads (400, 200, 100, and 50 kPa) of metal-contaminated soil samples are shown in Figure 7 and Figure 8.
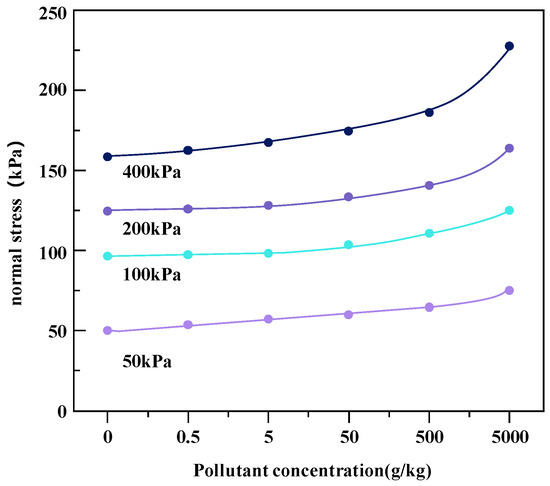
Figure 7.
Change in shear strength of Zn heavy metal-contaminated soil under different coaxial loads.
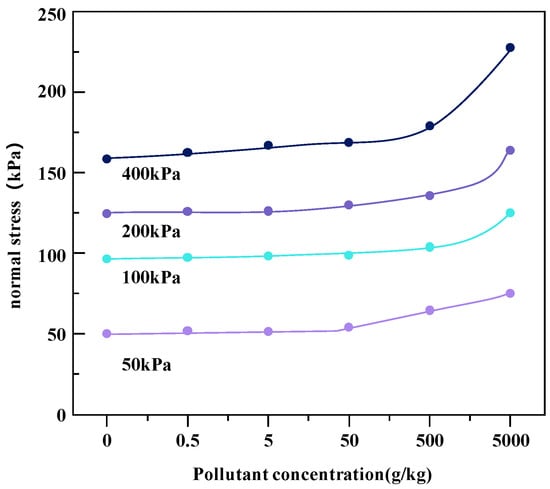
Figure 8.
Change in shear strength of Pb heavy metal-contaminated soil under different coaxial loads.
Heavy metal pollutants change the interactions between soil particles, resulting in changes in the strength properties of soil. Under the four axial loads, with the increase in heavy metal pollution concentration, the shear strength of the two kinds of heavy metal-contaminated soils increased. The reason for this phenomenon may be that with the increase in the concentration of heavy metal ions, the ionization balance between soil particles and water molecules is destroyed. The double electric layer plays a key role in the cohesion of soil, and heavy metal ions may reduce the thickness of the double electric layer on the surface of clay particles [20]. Heavy metal pollution may also damage the cohesive structure of the clay as the hydration film shrinks and cracks due to the presence of heavy metals. In addition, with the thickening of the water film on the surface of the soil particles, the attraction between the particles is greatly enhanced, resulting in particle aggregation, which changes the connection characteristics between the soil particles, resulting in a reduction in the contact area, an increase in friction resistance, and an increase in shear strength. Therefore, the shrinkage of the hydration film leads to the aggregation and cementation of soil particles, which helps to improve the shear strength, that is, the lower repulsive force leads to greater shear strength [21].
3.3. Resistivity Test Analysis
The change trend of resistivity after pollution was studied by controlling water content, porosity, and heavy metal pollution concentration.
3.3.1. Influence of Moisture Content and Pollutant Concentration on Resistivity
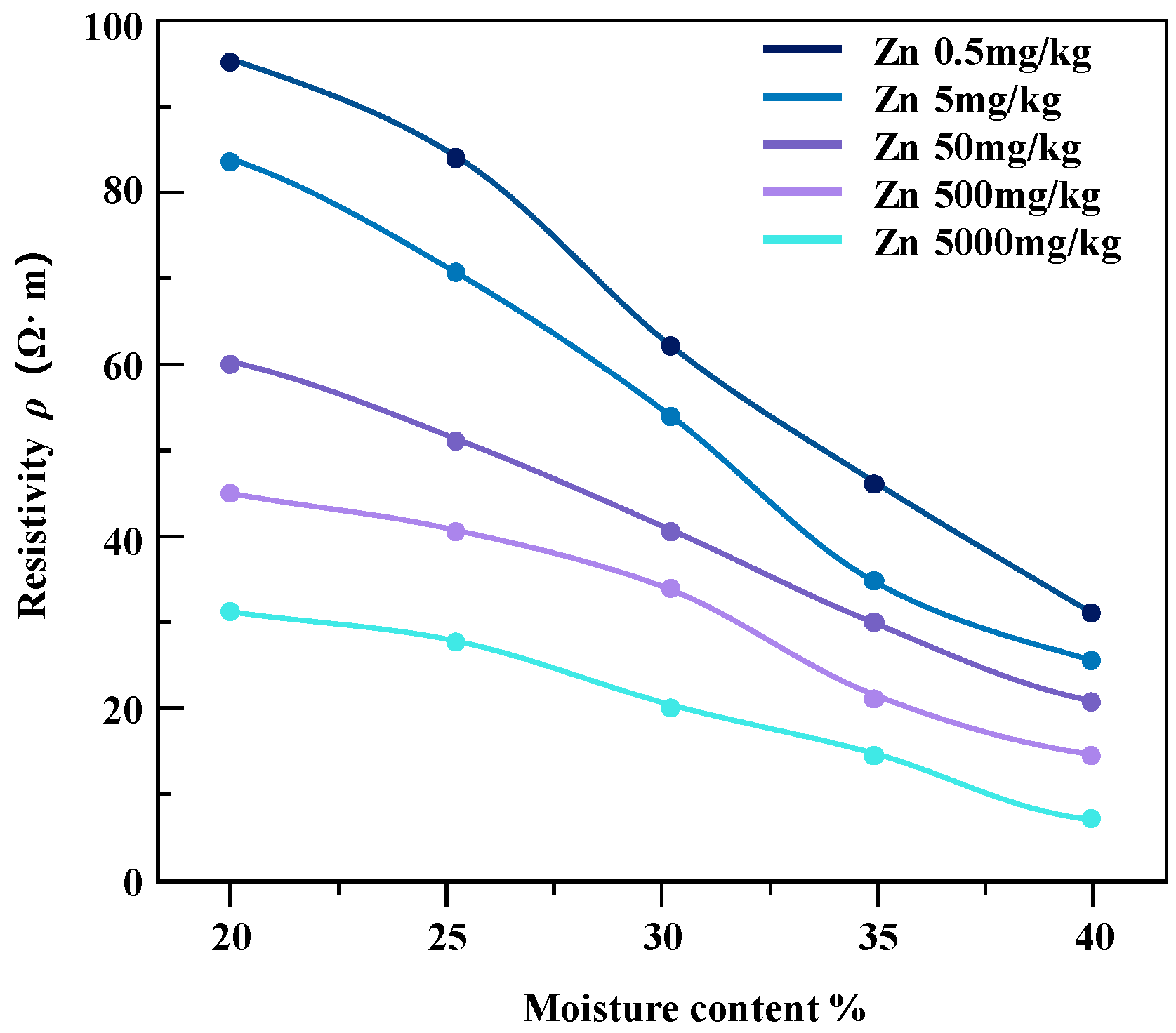
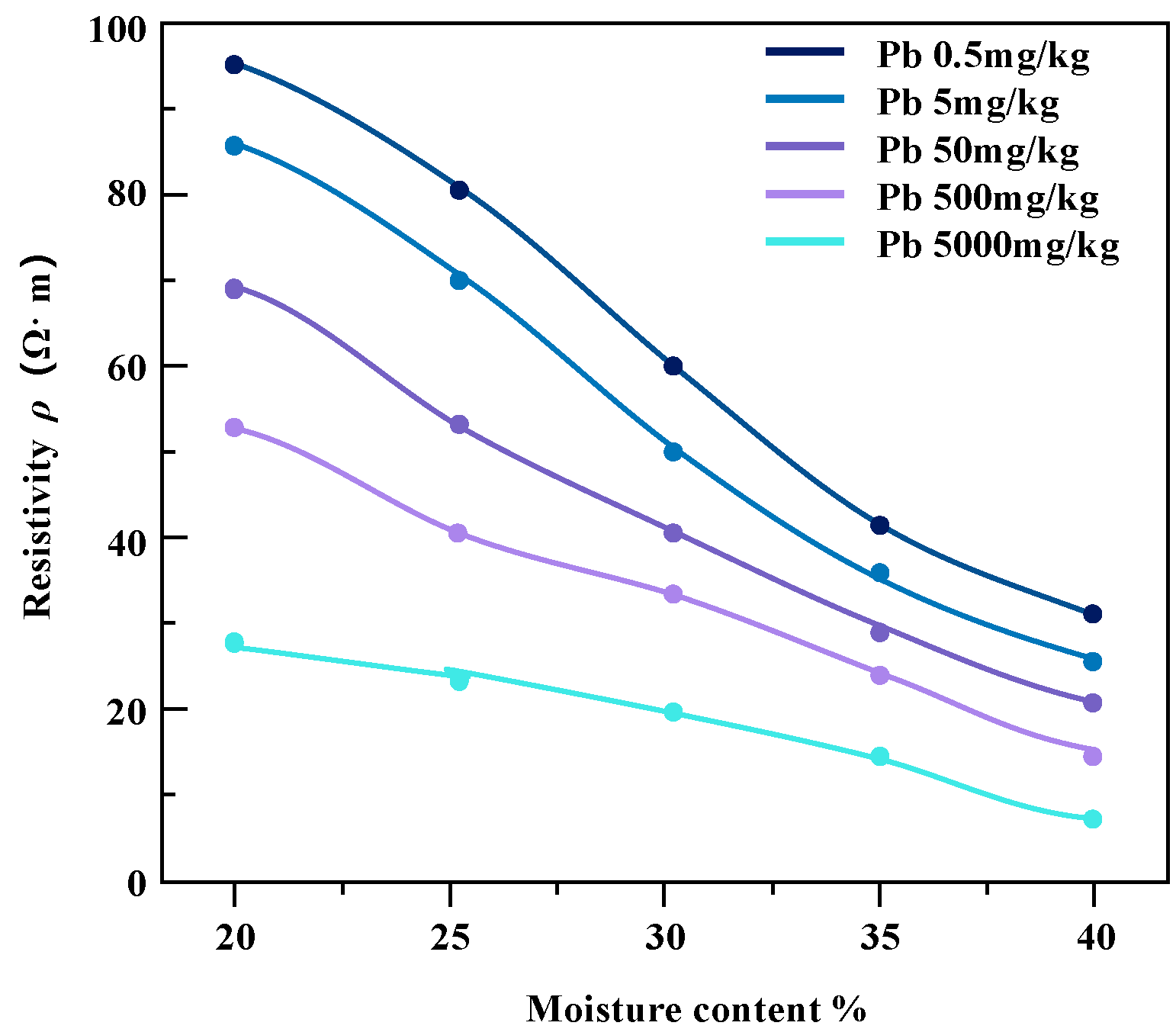
The pollution concentration and water content of contaminated soil samples were controlled and variation relationship diagrams of resistivity of soil samples under different conditions were obtained (Figure 9 and Figure 10).
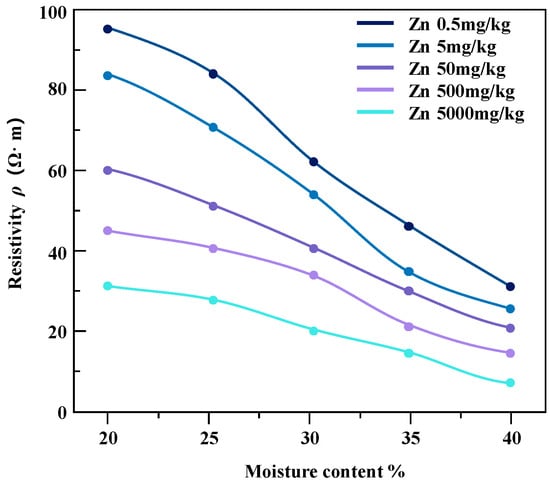
Figure 9.
The resistivity of zinc-contaminated soil varies with water content.
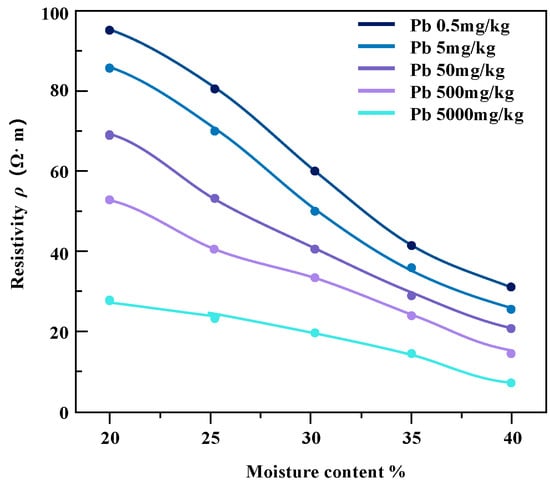
Figure 10.
The resistivity of lead-contaminated soil varies with water content.
For the studied heavy metal-contaminated soil samples, the invasion of heavy metal ions enhances the pore fluid conductivity, which further affects the overall soil conductivity. From the results in the above figures, it can be seen that the resistivity of soil samples contaminated with the same concentration of heavy metals shows a decreasing trend with the increase in water content, and the magnitude of the change is higher in the low-contamination concentration soil samples than in the high-contamination concentration soil samples. Zinc-contaminated soil samples and lead-contaminated soil samples show a similar trend.
In the case of the same water content, the resistivity of the soil sample decreases with the increase in pollutant concentration and the change range decreases gradually. The reason for this phenomenon may be that heavy metal pollution penetrates pore water in soil samples. In the saturated state, the conductivity channel mainly conducts through the pore water path, which maintains the relative stability of the resistivity of the contaminated soil sample to a certain extent [22].
3.3.2. The Effect of Porosity on Resistivity
The pollution concentration and porosity of contaminated soil samples were controlled, and the relation diagrams of resistivity change of soil samples under different conditions were obtained (Figure 11 and Figure 12).
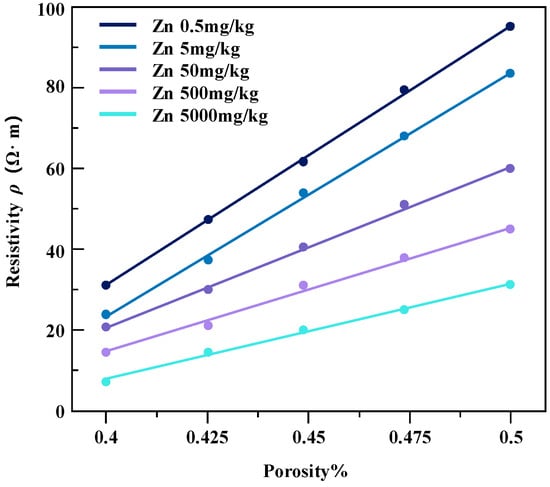
Figure 11.
The resistivity of zinc-contaminated soil varies with the porosity.
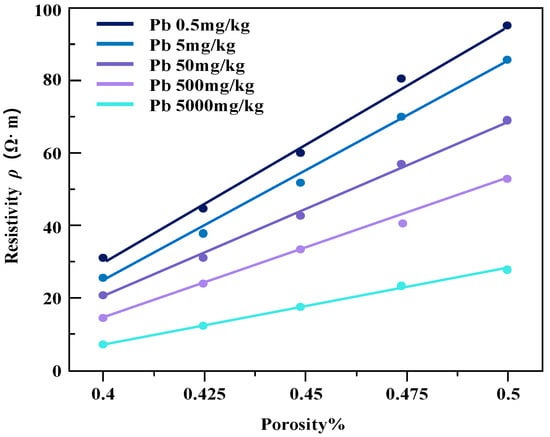
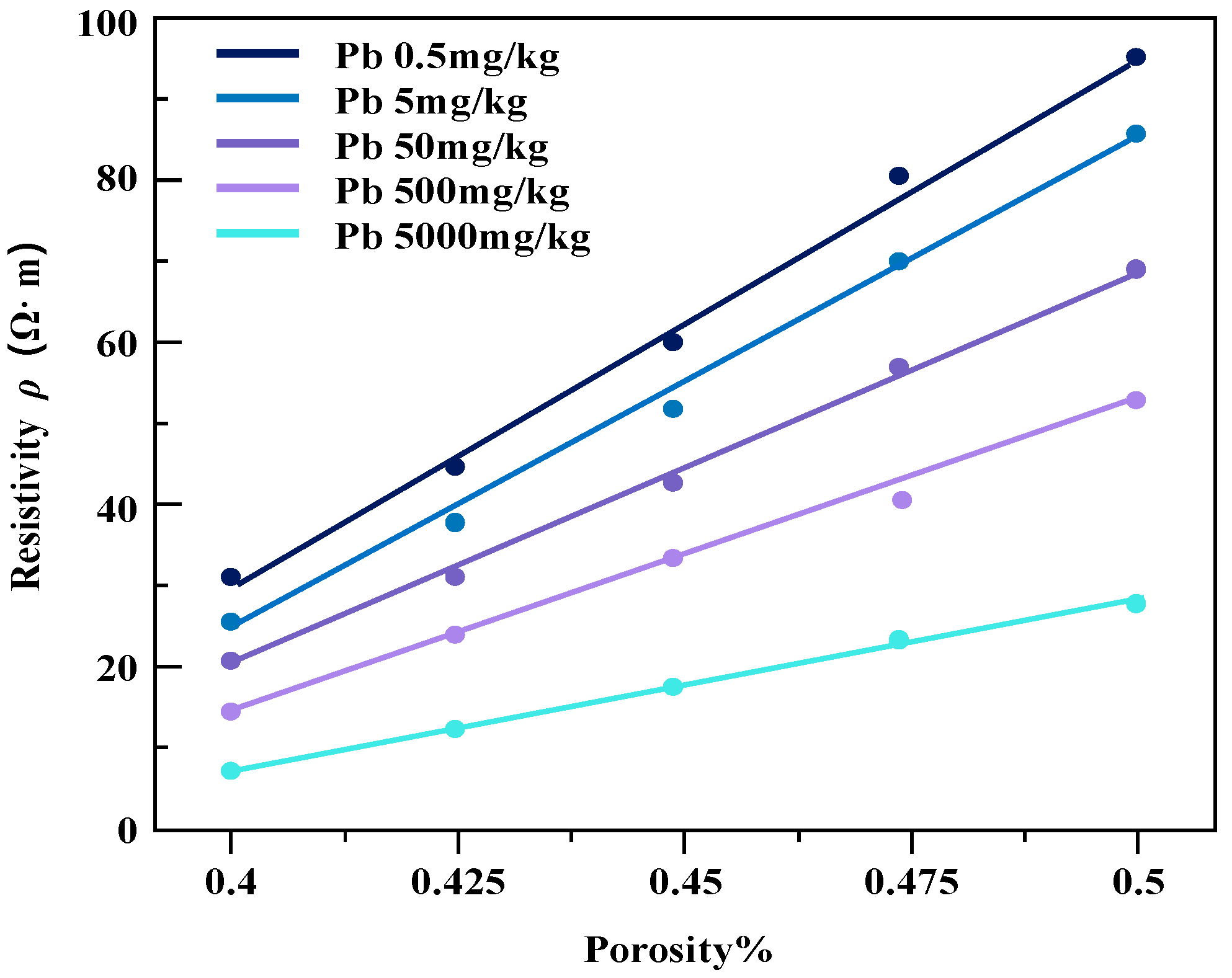
Figure 12.
The resistivity of lead-contaminated soil varies with the porosity.
Under the same water content conditions, the change in specimen resistivity shows a positive correlation with the increase in the porosity of the soil sample, resulting in an increase in resistivity. Under the same porosity conditions, the resistivity of the soil samples decreases continuously with increasing pollutant concentration. It is also noted that the magnitude of change in resistivity of the two contaminated clayey soils does not vary much with the increase in soil porosity.
The reason for this phenomenon may be that in certain water content conditions, with the increase in soil porosity, pore water cannot fill the original pore space, causing the soil to produce excess pore space, so that the overall conductivity path of the soil body is reduced and the soil resistivity drop is elevated. With the further increase in porosity of the soil body and the pore water gradually discontinuing, the pore water resistivity on the overall resistivity of the degree of influence becomes larger, so that the soil resistivity tends to infinity. The resistivity of the two kinds of heavy metal-contaminated powdery clay with porosity change trend is basically the same. The reason for this is that the three heavy metal ions used (Zn, Pb, Cd) are all +2 valence metal ions with similar ionic activities [23].
3.4. Experimental Analysis of Microscopic Characteristics
Through complex physical and chemical processes, soil has formed a multiphase system composed of solid phase, liquid phase, and gas phase, forming a loose particle accumulation. These factors jointly restrict the engineering properties of soil [24]. When heavy metal ions pollute soil, they interact with soil, resulting in changes in the composition and microstructure of soil. These changes will affect the engineering properties of soil. Therefore, microscopic analysis becomes the basis for understanding this process, especially when studying the microstructure of contaminated soil, which helps to deeply explore the key content of the corrosion mechanism of contaminated soil [25].
In this paper, a scanning electron microscope was used to obtain high-resolution stereoscopic imaging of the soil in order to observe and analyze the changes in the microscopic properties of the test soil samples with different heavy metal contamination concentrations. When analyzing the soil samples before and after heavy metal contamination, it was found that, as shown in Figure 13, when the test soil was not contaminated by heavy metals, its soil particles were compactly arranged with more small pores, and when the heavy metal concentration of the soil samples was increased with the increase in heavy metal ion concentrations, the soil body formed a dense and stable structure with more large pores and rounded particles, and gradually transformed from edge-to-edge and side-to-surface contact to face-to-surface contact in the soil body The pore space between particles gradually transformed from dense and fine voids to a larger pore structure.

Figure 13.
Experimental results of microscopic characteristics of Zn- and Pb-contaminated soils with different concentrations.
The reason for this phenomenon may be that the invasion of heavy metal ions reduces the specific surface area of the soil, increases the particle diameter, and makes the agglomeration of clay minerals more obvious [26]. With the increase in heavy metal ion concentration, the small pores in the soil sample become smaller and the large pores increase, and the contact between soil particles appears in various forms [27].
4. Conclusions
We carried out physical mechanics and microscopic experimental tests on heavy metal-contaminated soil, explored the microstructural change mechanism of heavy metal-contaminated soil, and studied the change rule of engineering characteristics related to heavy metal-contaminated soil with different pollution types and different pollution concentrations after the invasion of heavy metal ions. The relevant results are as follows:
- With the increase in heavy metal concentration in contaminated soil, the properties of several soil samples changed. The increase in heavy metal ion concentration leads to the decrease in liquid limit, plastic index, and clay content. This indicates that, to a certain extent, the soil has lost some of its original physical properties, such as fluidity and plasticity, and the content of fine particles in the soil is also reduced.
- According to the results of the resistivity test, it can be observed that the resistivity of heavy metal-contaminated soil shows a complex relationship with water content, porosity, and heavy metal concentration. With the increase in soil moisture content, the resistivity tends to decrease, because the addition of water changes the conductivity characteristics of the soil and makes the resistivity gradually stable. As the porosity increases, the resistivity tends to rise, because more pore space leads to a lengthening of the conductive path, thereby increasing the resistance. With the increase in heavy metal ion concentration, the resistivity drops sharply because heavy metal ions display electrical conductivity in soil.
- Before and after soil is polluted by heavy metals, its pores, particles, and colloidal morphology will change. The fundamental reason for the change of permeability coefficient before and after pollution is that metal ions change the internal structure of soil, especially the size of pores.
After the invasion of heavy metal ions into soil, a complex process of physical, chemical, and biological reactions occurs. The results of this study can provide a theoretical basis for basic research on heavy metal-contaminated soil. Research focusing on the engineering properties of heavy metal-contaminated soil is still in the preliminary stages. A stable and mature theory on the soil joint action of the engineering properties of the soil, from a single-heavy-metal ion pollution to a variety of heavy metal ions, is yet to be formed and need to be further studied.
Author Contributions
Data curation, G.Z., T.L., H.L. and X.Y.; Formal analysis, G.Z., Z.W. and D.Y.; Funding acquisition, T.L. and Z.W.; Investigation, G.Z. and Z.W.; Methodology, X.H.; Project administration, T.L. and X.H.; Resources, T.L.; Supervision, X.H.; Validation, D.Y.; Visualization, T.L., H.L. and X.Y.; Writing—original draft, G.Z.; Writing—review and editing, T.L. and X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China: U2006213; National Natural Science Foundation of China: 42277139.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the confidentiality of the subject research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peng, C.; He, Y.L.; Guo, C.H.; Xiao, X.Y.; Zhang, Y. Characteristics and Risk Assessment of Heavy Metals in Urban Soils of Major Cities in China. Environ. Sci. 2022, 43, 1–10. [Google Scholar]
- Cheng, N.C.; Zheng, Y.J.; He, X.F.; Li, X.F.; Zhang, X.X. Analysis of the Report on the national general survey of soil contamination. J. Agro-Environ. Sci. 2017, 36, 1689–1692. [Google Scholar]
- Wang, J.; Mou, C.; Zhao, H.R.; Ding, J.W. Experimental study on quantitative relationship of heavy-meta-contaminated soils. J. Civ. Environ. Eng. 2020, 42, 30–36. [Google Scholar]
- Coby, W.; Xiang, D.L. Analysis of heavy metal contaminated soils. Pract. Period. Hazard. Toxic Radio-Act. Waste Manag. 2003, 7, 1–27. [Google Scholar]
- Zulfahmi, A.R.; ZuhairiW, Y.; Ralhan, M.T.; Sahibin, A.R.; Razi, I.; Tukimat, L.; Syakireen, Z.; Noorulakma, A. Influence of amang (tin tailing) on geotechnical properties of clay soil. Sains Malays. 2012, 41, 303–312. [Google Scholar]
- Liu, G.; Yang, X.J.; Fan, H.H.; Shi, X.; Li, P. Effects of heavy metal ions on physical-chemical and engineering properties of loess. J. Northwest A F Univ. (Nat. Sci. Ed.) 2017, 45, 213–218. [Google Scholar]
- Souli, H.; Fleureau, M.; Ayadi, T.M.; Besnard, M. Physicochemical analysis of permeability changes in the presence of zinc. Geoderma 2008, 145, 1–7. [Google Scholar] [CrossRef]
- Jullien, A.; Proust, C.; Forestier, L.L.; Baillif, P. Hydro-chemio-mechanical coupling effects on permeability and swelling behaviour of a Ca smectite soaked by Cu solutions. Appl. Clay Sci. 2002, 21, 143–153. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Zhang, Y.; Wan, D.; Han, S.; Duan, M. Experimental Study on Physical and Mechanical Properties of Expansive Soil Polluted by Heavy Metals. IOP Conf. Ser. Earth Environ. Sci. 2019, 218, 012022. [Google Scholar] [CrossRef]
- Pan, C. Experimental Study on Evaluation of Heavy Metal Contaminated Soil by Thermal Conductivity Method; Southeast University: Nanjing, China, 2019. [Google Scholar]
- Lv, W.H. Experimental Study on the Physical Mechanical Properties of Heavy Metal Contaminated Expansive Soil in Nan Yang; Zhong Yuan University of Technology: Zhengzhou, China, 2018. [Google Scholar]
- Cheng, R.G.; Ma, F.R.; Pang, Y.B. Experimental analysis of the strength properties of the heavy metal contaminated soil. J. Civ. Archit. Environ. Eng. 2014, 36, 94–98. [Google Scholar]
- Li, L.H.; Yue, Y.W.; Xiao, H.L.; Li, W.T.; Han, Q.P. Performance and influence mechanism of Cd-contaminated soil solidified by rice husk ash-cement. Chin. J. Geotech. Eng. 2023, 45, 252–261. [Google Scholar]
- GB/T 50123-1999; National Standard of the People’s Republic of China. Standard for Geotechnical Test Methods. Ministry of Water Resources of the People’s Republic of China: Bejing, China, 1999.
- Xia, L. Engineering Properties of Heavy Metal Contaminated Soil; Hefei University of Technology: Hefei, China, 2014. [Google Scholar]
- Morvan, M.; Espinat, D.; Lambard, J.; Zemb, T. Ultrasmall-and small-angle X-ray scattering of smectite clay suspensions. Colloids Surf. A Physicochem. Eng. Asp. 1994, 82, 193–203. [Google Scholar] [CrossRef]
- Li, J.S.; Xue, Q.; Wang, P.; Li, Z.Z. Effect of lead (II) on the mechanical behavior and microstructure development of a Chinese clay. Appl. Clay Sci. 2015, 105, 192–199. [Google Scholar] [CrossRef]
- Shariatmadari, N.; Salami, M.; Karimpour, F.M. Effect of inorganic salt solutions on some geotechnical properties of soil-bentonite mixtures as barriers. Int. J. Civ. Eng. 2011, 9, 103–110. [Google Scholar]
- Sunil, B.; Shrihari, S.; Nayak, S. Shear strength characteristics and chemical characteristics of leachate-contaminated lateritic soil. Eng. Geol. 2009, 106, 20–25. [Google Scholar] [CrossRef]
- Wen, B.P.; He, L. Influence of lixiviation by irrigation water on residual shear strength of weathered red mudstone in Northwest China: Implication for its role in landslides’ reactivation. Eng. Geol. 2012, 151, 56–63. [Google Scholar] [CrossRef]
- Tiwari, B.; Tuladhar, G.R.; Marui, H. Variation in residual shear strength of the soil with the salinity of pore fluid. J. Geotech. Geoenviron. Eng. 2005, 131, 1445–1456. [Google Scholar] [CrossRef]
- Jiang, J.F.; Yu, C.; Liao, R.P.; Zhu, C.P. Tests on electrical resistivity characteristics of Wenzhou soft clay with Cu2+. J. Civ. Archit. Environ. Eng. 2018, 40, 85–90. [Google Scholar]
- Sun, Y.K.; Liu, Y.Q.; Neng, C.X.; Dong, L. Development of Resistivity Method in the Investigation of Contaminated Soils. Environ. Sci. Technol. 2011, 34 (Suppl. S2), 165–171. [Google Scholar]
- Zhang, Z.H.; Li, H.Y.; Shi, Y.M. Experimental study on permeability properties and micro-structure of clay contaminated by Cu2+. China Civ. Eng. J. 2014, 47, 122–129. [Google Scholar]
- Liu, Z.B.; Fang, W.; Cheng, Z.L.; Yu, C. Experimental study of influence of zinc ions on one-dimensional compressibility of bentonite. Rock Soil Mech. 2013, 34, 2211–2217. [Google Scholar]
- Liu, G.; Zhang, C.C. Experimental study on the microscopic properties of cadmium-contaminated kaolin clay. Sci. Technol. Eng. 2016, 16, 254–258. [Google Scholar]
- He, Y.Y.; Chen, J.H. Effect cadmium contamination on the mechanical properties and mechanism analysis of the soil. Sci. Technol. Eng. 2019, 19, 276–280. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).