Microplastics in Mediterranean Mussel Mytilus galloprovincialis: Comparison between Cultured and WildType Mussels from the Northern Adriatic
Abstract
1. Introduction
2. Materials and Methods
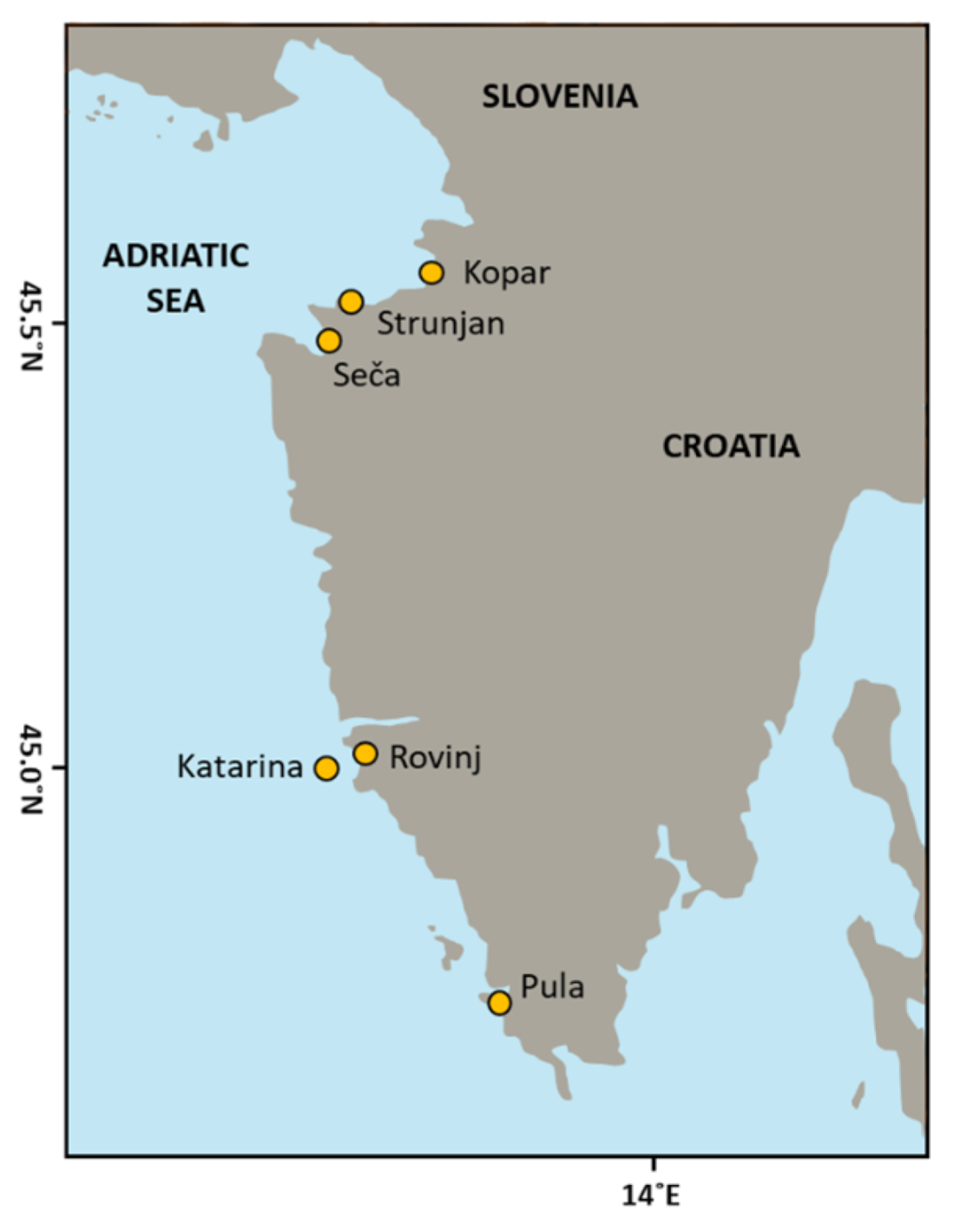
2.1. Sample Collection
2.2. Tissue Preparation
2.3. Observation and Validation of Microplastic
2.4. Data Analysis
3. Results and Discussion
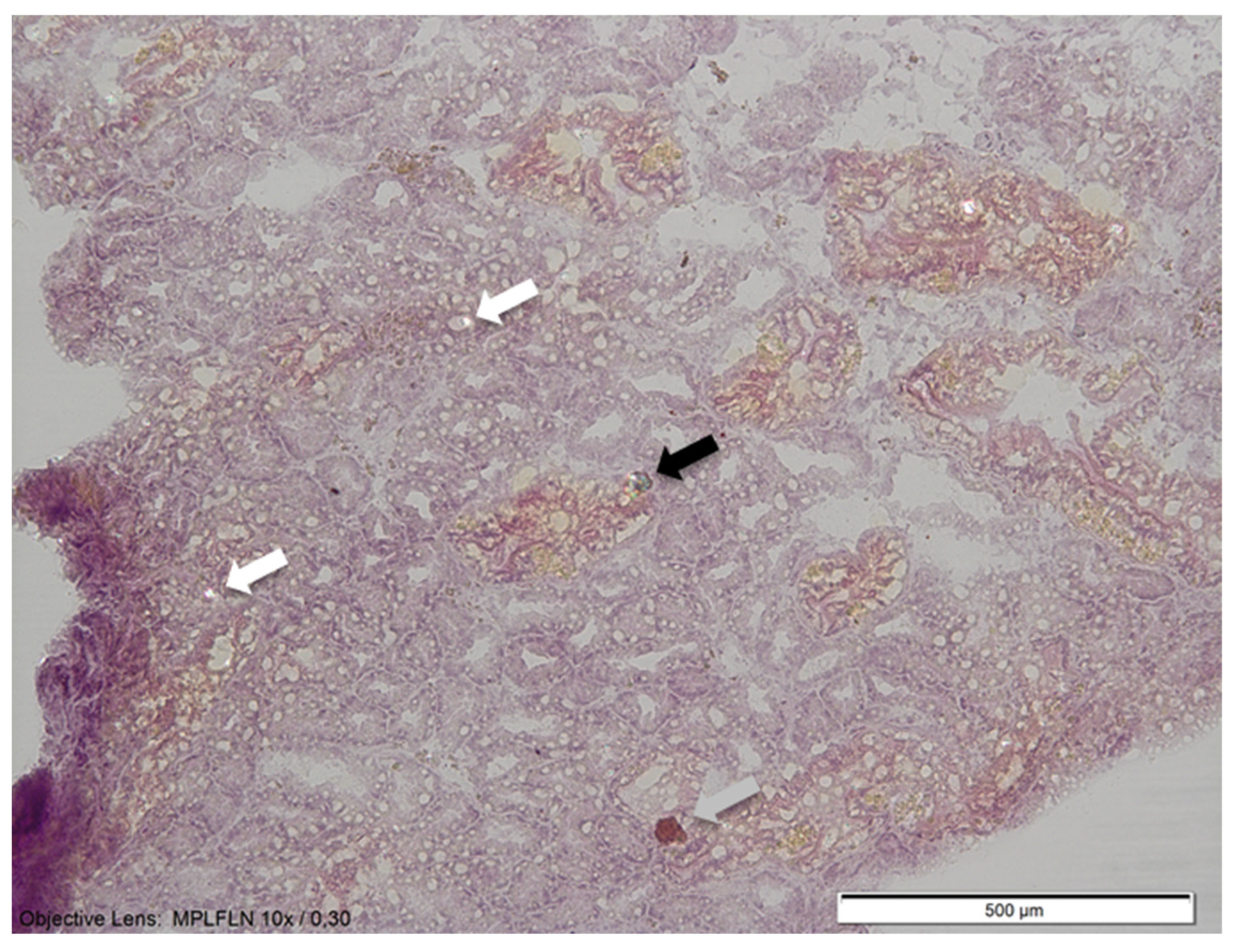
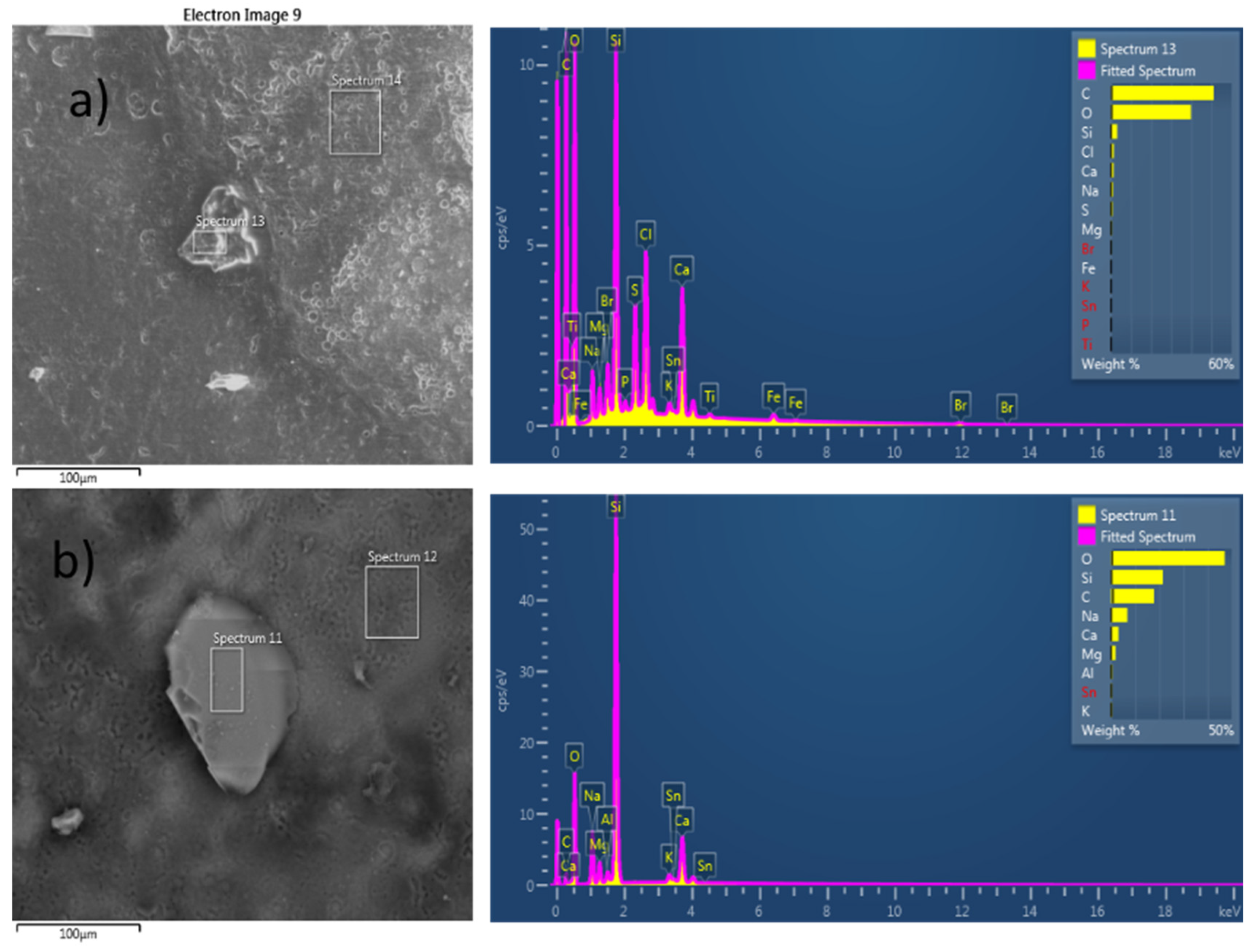
3.1. Identification of Microplastics Found in Mussel Digestive Glands Using Scanning-Electron Microscopy
3.2. Abundance of Microplastics Found in Mussel Digestive Glands
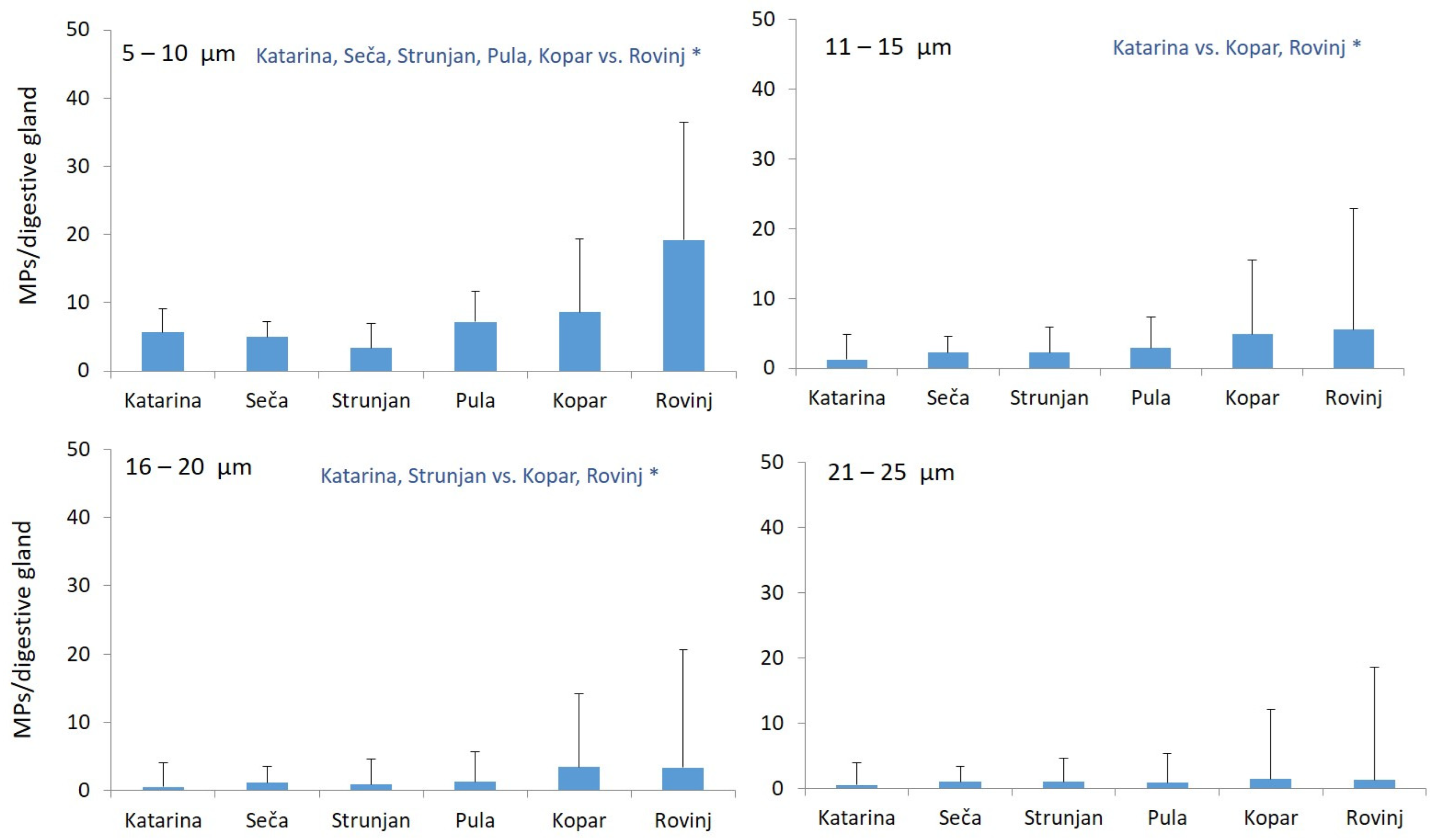
3.3. Size Distribution of Microplastics Found in Mussel Digestive Glands
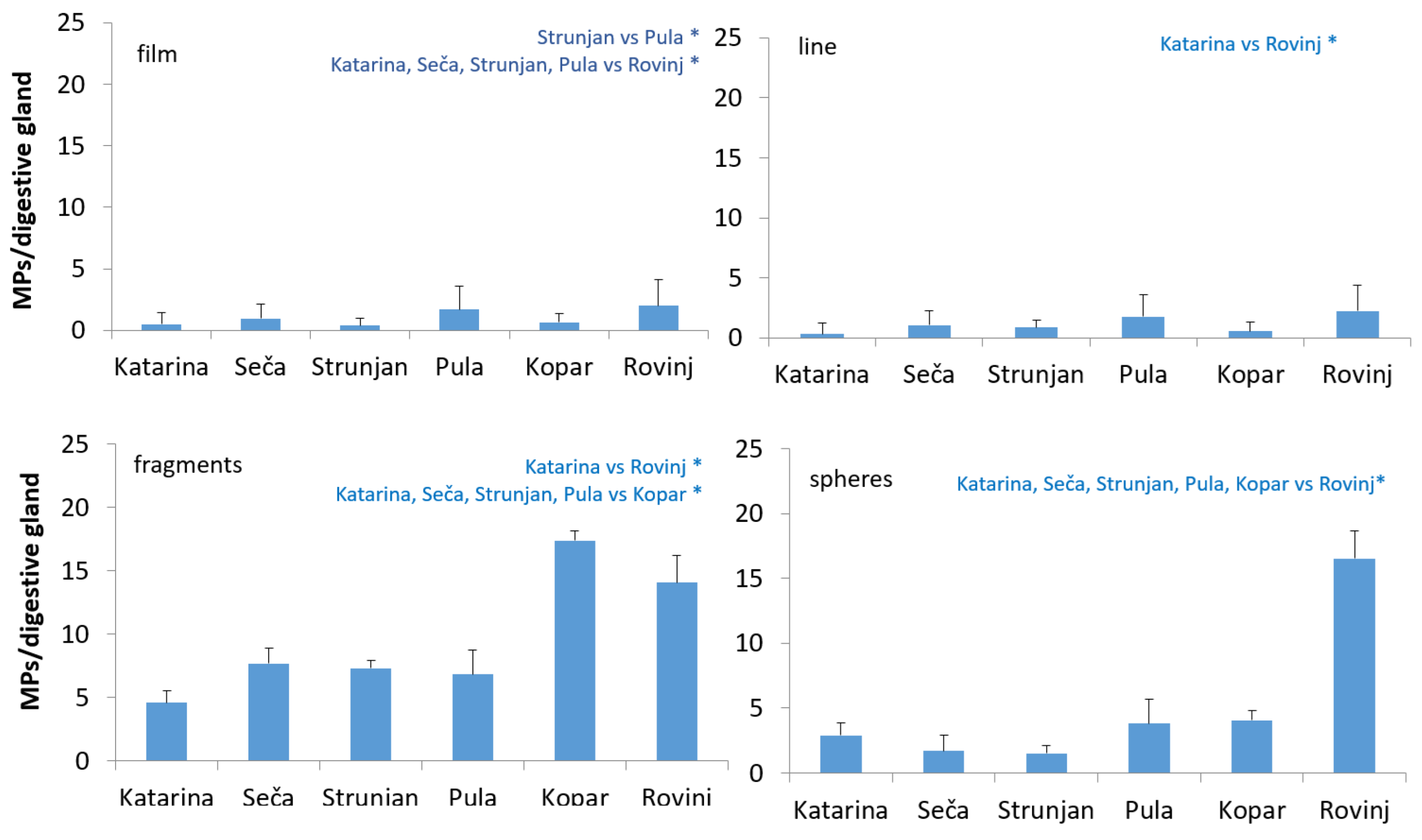
3.4. Different Shapes of Microplastics Found in Mussel Digestive Glands
3.5. Color of Microplastics Found in Mussel Digestive Glands
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Arthur, C., Baker, J.E., Bamford, H.A., Eds.; Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris. In Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, University of Washington Tacoma, Tacoma, WA, USA, 9–11 September 2008. [Google Scholar]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Gutow, L.; Klages, M. (Eds.) Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; p. 447. [Google Scholar] [CrossRef]
- La Daana, K.K.; Asmath, H.; Gobin, J.F. The status of marine debris/litter and plastic pollution in the Caribbean Large Marine Ecosystem (CLME): 1980–2020. Environ. Pollut. 2022, 300, 118919. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Cole, M.; Liddle, C.; Consolandi, G.; Drago, C.; Hird, C.; Lindeque, P.K.; Galloway, T.S. Microplastics, microfibres and nanoplastics cause variable sub-lethal responses in mussels (Mytilus spp.). Mar. Pollut. Bull. 2020, 160, 111552. [Google Scholar] [CrossRef]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef]
- Raju, P.; Santhanam, P.; Perumal, P. Impacts of microplastics on marine organisms: Present perspectives and the way forward. Egypt. J. Aquat. Res. 2022, 48, 205–209. [Google Scholar] [CrossRef]
- Zarfl, C.; Fleet, D.; Fries, E.; Galgani, F.; Gerdts, G.; Hanke, G.; Matthies, M. Microplastics in oceans. Mar. Pollut. Bull. 2011, 62, 1589–1591. [Google Scholar] [CrossRef]
- Vasilakopoulos, P.; Palialexis, A.; Boschetti, S.T.; Cardoso, A.C.; Druon, J.-N.; Konrad, C.; Kotta, M.; Magliozzi, C.; Palma, M.; Piroddi, C.; et al. Marine Strategy Framework Directive, Thresholds for MSFD Criteria: State of Play and Next Steps; EUR 31131 EN; Publications Office of the European Union: Luxembourg, 2022; ISBN 978-92-76-53689-5. JRC128344. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; Kershaw, P.J., Ed.; Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: Paris, France, 2015; 96p. [Google Scholar]
- Jiang, X.; Conner, N.; Lu, K.; Tunnell, J.W.; Liu, Z. Occurrence, distribution, and associated pollutants of plastic pellets (nurdles) in coastal areas of South Texas. Sci. Total Environ. 2022, 842, 156826. [Google Scholar] [CrossRef]
- Rubin, A.E.; Gnaim, R.; Levi, S.; Zucker, I. Risk assessment framework for microplastic in marine environments. Sci. Total Environ. 2023, 901, 166459. [Google Scholar] [CrossRef] [PubMed]
- Pinlova, B.; Nowack, B. From cracks to secondary microplastics-surface characterization of polyethylene terephthalate (PET) during weathering. Chemosphere 2024, 352, 141305. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; Volume 10, p. 43. [Google Scholar] [CrossRef]
- Efimova, I.; Bagaeva, M.; Bagaev, A.; Kileso, A.; Chubarenko, I.P. Secondary microplastics generation in the sea swash zone with coarse bottom sediments: Laboratory experiments. Front. Mar. Sci. 2018, 5, 313. [Google Scholar] [CrossRef]
- Huber, M.; Archodoulaki, V.M.; Pomakhina, E.; Pukánszky, B.; Zinöcker, E.; Gahleitner, M. Environmental degradation and formation of secondary microplastics from packaging material: A polypropylene film case study. Polym. Degrad. Stab. 2022, 195, 109794. [Google Scholar] [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Rotchell, J.M.; Shen, X.; Li, Q.; Zhu, J. Where are we? Towards an understanding of the selective accumulation of microplastics in mussels. Environ. Pollut. 2021, 286, 117543. [Google Scholar] [CrossRef]
- Gajšt, T.; Bizjak, T.; Palatinus, A.; Liubartseva, S.; Kržan, A. Sea surface microplastics in Slovenian part of the Northern Adriatic, Mar. Pollut. Bull. 2016, 113, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Munari, C.; Scoponi, M.; Mistri, M. Plastic debris in the Mediterranean Sea: Types, occurrence and distribution along Adriatic shorelines. Waste. Manag. 2017, 67, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Mistri, M.; Scoponi, M.; Sfriso, A.A.; Munari, C.; Curiotto, M.; Sfriso, A.; Orlando-Bonaca, M.; Lipej, L. Microplastic Contamination in Protected Areas of the Gulf of Venice. Water Air Soil Pollut. 2021, 113, 379. Available online: https://link.springer.com/article/10.1007/s11270-021-05323-9 (accessed on 1 January 2024). [CrossRef]
- Korez, Š.; Gutow, L.; Saborowski, R. Microplastics at the strandlines of Slovenian beaches. Mar. Pollut. Bull. 2019, 145, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Bošković, N.; Joksimović, D.; Peković, M.; Perošević-Bajčeta, A.; Bajt, O. Microplastics in Surface Sediments along the Montenegrin Coast, Adriatic Sea: Types, Occurrence, and Distribution. J. Mar. Sci. Eng. 2021, 9, 841. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Pellini, G.; Gomiero, A.; Fortibuoni, T.; Ferrà, C.; Grati, F.; Tassetti, A.N.; Polidori, P.; Fabi, G.; Scarcella, G. Characterization of microplastic litter in the gastrointestinal tract of Solea solea from the Adriatic Sea. Environ. Pollut. 2018, 234, 943–952. [Google Scholar] [CrossRef]
- Bošković, N.; Joksimović, D.; Bajt, O. Microplastics in fish and sediments from the Montenegrin coast (Adriatic Sea): Similarities in accumulation. Sci. Total Environ. 2022, 850, 158074. [Google Scholar] [CrossRef]
- Martinelli, M.; Gomiero, A.; Guicciardi, S.; Frapiccini, E.; Strafella, P.; Angelini, S.; Domenichetti, F.; Belardinelli, A.; Colella, S. Preliminary results on the occurrence and anatomical distribution of microplastics in wild populations of Nephrops norvegicus from the Adriatic Sea. Environ. Pollut. 2021, 278, 116872. [Google Scholar] [CrossRef]
- Kanduč, T.; Šlejkovec, Z.; Falnoga, I.; Mori, N.; Budič, B.; Kovačić, I.; Pavičić-Hamer, D.; Hamer, B. Environmental status of the NE Adriatic Sea, Istria, Croatia: Insights from mussel Mytilus galloprovincialis condition indices, stable isotopes and metal (loid)s. Mar. Pollut. Bull. 2018, 126, 525–534. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Hamer, B.; Korlević, M.; Durmiši, E.; Nerlović, V.; Bierne, N. Nuclear marker Me 15–16 analyses of Mytilus galloprovincialis populations along the eastern Adriatic coast. Cah. Biol. Mar. 2012, 53, 35. [Google Scholar]
- Woods, M.N.; Stack, M.E.; Fields, D.M.; Shaw, S.D.; Matrai, P.A. Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Mar. Pollut. Bull. 2018, 137, 638–645. [Google Scholar] [CrossRef]
- Gonçalves, C.; Martins, M.; Sobral, P.; Costa, P.M.; Costa, M.H. An assessment of the ability to ingest and excrete microplastics by filter-feeders: A case study with the Mediterranean mussel. Environ. Pollut. 2019, 245, 600–606. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.I.; Cavallo, R.A. Mytilus galloprovincialis filter feeding on the bacterial community in a Mediterranean coastal area (Northern Ionian Sea, Italy). Water Res. 2005, 39, 469–477. [Google Scholar] [CrossRef]
- Mubiana, V.K.; Blust, R. Effects of temperature on scope for growth and accumulation of Cd, Co, Cu and Pb by the marine bivalve Mytilus edulis. Mar. Environ. Res. 2007, 63, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Signa, G.; Di Leonardo, R.; Vaccaro, A.; Tramati, C.D.; Mazzola, A.; Vizzini, S. Lipid and fatty acid biomarkers as proxies for environmental contamination in caged mussels Mytilus galloprovincialis. Ecol. Indic. 2015, 57, 384–394. [Google Scholar] [CrossRef]
- Coppola, F.; Almeida, Â.; Henriques, B.; Soares, A.M.; Figueira, E.; Pereira, E.; Freitas, R. Biochemical responses and accumulation patterns of Mytilus galloprovincialis exposed to thermal stress and Arsenic contamination. Ecotoxicol. Environ. Saf. 2018, 147, 954–962. [Google Scholar] [CrossRef]
- Musella, M.; Wathsala, R.; Tavella, T.; Rampelli, S.; Barone, M.; Palladino, G.; Biagi, E.; Brigidi, P.; Turroni, S.; Franzellitti, S.; et al. Tissue-scale microbiota of the Mediterranean mussel (Mytilus galloprovincialis) and its relationship with the environment. Sci. Total Environ. 2020, 717, 137209. [Google Scholar] [CrossRef]
- Pizzurro, F.; Recchi, S.; Nerone, E.; Salini, R.; Barile, N.B. Accumulation Evaluation of Potential Microplastic Particles in Mytilus galloprovincialis from the Goro Sacca (Adriatic Sea, Italy). Microplastics 2022, 1, 303–318. [Google Scholar] [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Pavičić Hamer, D.; Kovačić, I.; Koščica, L.; Hamer, B. Physiological indices of maricultured mussel Mytilus galloprovincialis Lamarck, 1819 in Istria, Croatia: Seasonal and transplantation effect. J. World Aquac. Soc. 2016, 47, 768–778. [Google Scholar] [CrossRef]
- Santana, M.F.M.; Ascer, L.G.; Custódio, M.R.; Moreira, F.T.; Turra, A. Microplastic contamination in natural mussel beds from a Brazilian urbanized coastal region: Rapid evaluation through bioassessment. Mar. Pollut. Bull. 2016, 106, 183–189. [Google Scholar] [CrossRef]
- Bråte, I.L.N.; Hurley, R.; Iversen, K.; Beyer, J.; Thomas, K.V.; Steindal, C.C.; Green, N.W.; Olsen, M.; Lusher, A. Mytilus spp. as sentinels for monitoring microplastic pollution in Norwegian coastal waters: A qualitative and quantitative study. Environ. Pollut. 2018, 243, 383–393. [Google Scholar] [CrossRef]
- Khoironi, A.; Anggoro, S.; Sudarno. The existence of microplastic in Asian green mussels. In IOP Conference Series: Earth and Environmental Science, Proceedings of the International Conference on Green Agro-industry and Bioeconomy (ICGAB 2017), Batu, Indonesia, 24–25 October 2017; IOP Publishing: Bristol, UK, 2018; Volume 131, p. 012050. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Hossain, M.S.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Li, J.; Ju, P.; Li, F. Microplastics in four bivalve species and basis for using bivalves as bioindicators of microplastic pollution. Sci. Total Environ. 2021, 782, 146830. [Google Scholar] [CrossRef]
- Masiá, P.; Ardura, A.; Garcia-Vazquez, E. Microplastics in seafood: Relative input of Mytilus galloprovincialis and table salt in mussel dishes. Food Res. Int. 2022, 153, 110973. [Google Scholar] [CrossRef]
- Joyce, P.W.; Falkenberg, L.J. Microplastic abundances in co-occurring marine mussels: Species and spatial differences. Reg. Stud. Mar. Sci. 2023, 57, 102730. [Google Scholar] [CrossRef]
- Patterson, J.; Jeyasanta, K.I.; Laju, R.L.; Edward, J.P. Microplastic contamination in Indian edible mussels (Perna perna and Perna viridis) and their environs. Mar. Pollut. Bull. 2021, 171, 112678. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Pinheiro, M.; Lahbib, Y.; Neuparth, T.; Santos, M.M.; Trigui El Menif, N. Effects of environmentally relevant levels of polyethylene microplastic on Mytilus galloprovincialis (Mollusca: Bivalvia): Filtration rate and oxidative stress. Environ. Sci. Pollut. Res. 2021, 28, 26643–26652. [Google Scholar] [CrossRef]
- Calmão, M.; Blasco, N.; Benito, A.; Thoppil, R.; Torre-Fernandez, I.; Castro, K.; Izagirre, U.; Garcia-Velasco, N.; Soto, M. Time-course distribution of fluorescent microplastics in target tissues of mussels and polychaetes. Chemosphere 2023, 311, 137087. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.M.; Benedetti, M.; d’Errico, G.; Regoli, F.; Bebianno, M.J. Polystyrene nanoplastics in the marine mussel Mytilus galloprovincialis. Environ. Pollut. 2023, 333, 122104. [Google Scholar] [CrossRef] [PubMed]
- Kolandhasamy, P.; Su, L.; Li, J.; Qu, X.; Jabeen, K.; Shi, H. Adherence of microplastics to soft tissue of mussels: A novel way to uptake microplastics beyond ingestion. Sci. Total Environ. 2018, 610, 635–640. [Google Scholar] [CrossRef]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef] [PubMed]
- Détrée, C.; Gallardo-Escárate, C. Polyethylene microbeads induce transcriptional responses with tissue-dependent patterns in the mussel Mytilus galloprovincialis. J. Molluscan Stud. 2017, 83, 220–225. [Google Scholar] [CrossRef]
- Masiá, P.; Ardura, A.; García-Vázquez, E. Virgin polystyrene microparticles exposure leads to changes in gills DNA and physical condition in the Mediterranean mussel Mytilus galloprovincialis. Animals 2021, 11, 2317. [Google Scholar] [CrossRef]
- Faggio, C.; Tsarpali, V.; Dailianis, S. Mussel digestive gland as a model tissue for assessing xenobiotics: An overview. Sci. Total Environ. 2018, 636, 220–229. [Google Scholar] [CrossRef]
- Kinjo, A.; Mizukawa, K.; Takada, H.; Inoue, K. Size-dependent elimination of ingested microplastics in the Mediterranean mussel Mytilus galloprovincialis. Mar. Pollut. Bull. 2019, 149, 110512. [Google Scholar] [CrossRef]
- Rivera-Hernández, J.R.; Fernández, B.; Santos-Echeandia, J.; Garrido, S.; Morante, M.; Santos, P.; Albentosa, M. Biodynamics of mercury in mussel tissues as a function of exposure pathway: Natural vs microplastic routes. Sci. Total Environ. 2019, 674, 412–423. [Google Scholar] [CrossRef]
- Pedersen, A.F.; Gopalakrishnan, K.; Boegehold, A.G.; Peraino, N.J.; Westrick, J.A.; Kashian, D.R. Microplastic ingestion by quagga mussels, Dreissena bugensis, and its effects on physiological processes. Environ. Pollut. 2020, 260, 113964. [Google Scholar] [CrossRef]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef]
- Détrée, C.; Gallardo-Escárate, C. Single and repetitive microplastics exposures induce immune system modulation and homeostasis alteration in the edible mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 2018, 83, 52–60. [Google Scholar] [CrossRef]
- Alnajar, N.; Jha, A.N.; Turner, A. Impacts of microplastic fibres on the marine mussel, Mytilus galloprovinciallis. Chemosphere 2021, 262, 128290. [Google Scholar] [CrossRef] [PubMed]
- Vasanthi, R.L.; Arulvasu, C.; Kumar, P.; Srinivasan, P. Ingestion of microplastics and its potential for causing structural alterations and oxidative stress in Indian green mussel Perna viridis—A multiple biomarker approach. Chemosphere 2021, 283, 130979. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A.; Besseling, E.; Foekema, E.M.; Kamermans, P.; Koelmans, A.A. Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.). Environ. Toxicol. Chem. 2012, 31, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Birnstiel, S.; Soares-Gomes, A.; da Gama, B.A. Depuration reduces microplastic content in wild and farmed mussels. Mar. Pollut. Bull. 2019, 140, 241–247. [Google Scholar] [CrossRef]
- Ward, J.E.; Rosa, M.; Shumway, S.E. Capture, ingestion, and egestion of microplastics by suspension-feeding bivalves: A 40-year history. Anthr. Coasts 2019, 2, 39–49. [Google Scholar] [CrossRef]
- Ward, J.E.; Zhao, S.; Holohan, B.A.; Mladinich, K.M.; Griffin, T.W.; Wozniak, J.; Shumway, S.E. Selective ingestion and egestion of plastic particles by the blue mussel (Mytilus edulis) and eastern oyster (Crassostrea virginica): Implications for using bivalves as bioindicators of microplastic pollution. Environ. Sci. Technol. 2019, 53, 8776–8784. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo [a] pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef]
- Zhao, S.; Ward, J.E.; Danley, M.; Mincer, T.J. Field-based evidence for microplastic in marine aggregates and mussels: Implications for trophic transfer. Environ. Sci. Technol. 2018, 52, 11038–11048. [Google Scholar] [CrossRef]
- Qu, X.; Su, L.; Li, H.; Liang, M.; Shi, H. Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci. Total Environ. 2018, 621, 679–686. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef]
- Verdú, I.; González-Pleiter, M.; Leganés, F.; Rosal, R.; Fernandez-Pinas, F. Microplastics can act as vector of the biocide triclosan exerting damage to freshwater microalgae. Chemosphere 2021, 266, 129193. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Xia, S.; Zhao, J. Cu (II) adsorption on Poly (Lactic Acid) Microplastics: Significance of microbial colonization and degradation. Chem. Eng. J. 2022, 429, 132306. [Google Scholar] [CrossRef]
- Vandermeersch, G.; Van Cauwenberghe, L.; Janssen, C.R.; Marques, A.; Granby, K.; Fait, G.; Kotterman, M.J.J.; Diogène, J.; Bekaert, K.; Robbens, J.; et al. A critical view on microplastic quantification in aquatic organisms. Environ. Res. 2015, 143, 46–55. [Google Scholar] [CrossRef]
- Fernández, B.; Albentosa, M. Insights into the uptake, elimination and accumulation of microplastics in mussel. Environ. Pollut. 2019, 249, 321–329. [Google Scholar] [CrossRef]
- Cutroneo, L.; Reboa, A.; Besio, G.; Borgogno, F.; Canesi, L.; Canuto, S.; Dara, M.; Enrile, F.; Forioso, I.; Greco, G.; et al. Microplastics in seawater: Sampling strategies, laboratory methodologies, and identification techniques applied to port environment. Environ. Sci. Pollut. Res. 2020, 27, 8938–8952. [Google Scholar] [CrossRef]
- Pavičić-Hamer, D.; Kovačić., I.; Sović, T.; Marelja, M.; Lyons, D.M. Exposure to Polymethylmethacrylate Microplastics Induces a Particle Size-Dependent Immune Response in Mediterranean Mussel Mytilus galloprovincialis. Fishes 2022, 7, 307. [Google Scholar] [CrossRef]
- Gedik, K.; Eryaşar, A.R. Microplastic pollution profile of Mediterranean mussels (Mytilus galloprovincialis) collected along the Turkish coasts. Chemosphere 2020, 260, 127570. [Google Scholar] [CrossRef]
- Wesch, C.; Bredimus, K.; Paulus, M.; Klein, R. Towards the suitable monitoring of ingestion of microplastics by marine biota: A review. Environ. Pollut. 2016, 218, 1200–1208. [Google Scholar] [CrossRef]
- Blair, R.M.; Waldron, S.; Phoenix, V.R.; Gauchotte-Lindsay, C. Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland, UK. Environ. Sci. Pollut. Res. 2019, 26, 12491–12504. [Google Scholar] [CrossRef]
- Stollberg, N.; Kröger, S.D.; Reininghaus, M.; Forberger, J.; Witt, G.; Brenner, M. Uptake and absorption of fluoranthene from spiked microplastics into the digestive gland tissues of blue mussels, Mytilus edulis L. Chemosphere 2021, 279, 130480. [Google Scholar] [CrossRef] [PubMed]
- CDI–Marine Data Access. Available online: http://harmonia.maris2.nl/search (accessed on 23 October 2013).
- Ding, L.; fan Mao, R.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Dahl, M.; Bergman, S.; Björk, M.; Diaz-Almela, E.; Granberg, M.; Gullström, M.; Leiva-Dueñas, C.; Magnusson, K.; Marco-Méndez, C.; Piñeiro-Juncal, N.; et al. A temporal record of microplastic pollution in Mediterranean seagrass soils. Environ. Pollut. 2021, 273, 116451. [Google Scholar] [CrossRef]
- Liubartseva, S.; Coppini, G.; Lecci, R.; Creti, S. Regional approach to modeling the transport of floating plastic debris in the Adriatic Sea. Mar. Pollut. Bull. 2016, 103, 115–127. [Google Scholar] [CrossRef]
- Atwood, E.C.; Falcieri, F.M.; Piehl, S.; Bochow, M.; Matthies, M.; Franke, J.; Carniel, S.; Sclavo, M.; Laforsch, C.; Siegert, F. Coastal accumulation of microplastic particles emitted from the Po River, Northern Italy: Comparing remote sensing and hydrodynamic modelling with in situ sample collections. Mar. Pollut. Bull. 2019, 138, 561–574. [Google Scholar] [CrossRef]
- Foo, Y.H.; Ratnam, S.; Lim, E.V.; Abdullah, M.; Molenaar, V.J.; Hwai, A.T.S.; Zhang, S.; Li, H.; Zanuri, N.B.M. Microplastic ingestion by commercial marine fish from the seawater of Northwest Peninsular Malaysia. PeerJ 2022, 10, e13181. [Google Scholar] [CrossRef]
- Miserli, K.; Lykos, C.; Kalampounias, A.G.; Konstantinou, I. Screening of Microplastics in Aquaculture Systems (Fish, Mussel, and Water Samples) by FTIR, Scanning Electron Microscopy–Energy Dispersive Spectroscopy and Micro-Raman Spectroscopies. Appl. Sci. 2023, 13, 9705. [Google Scholar] [CrossRef]
- Bihari, N.; Mičić, M.; Fafanđel, M.; Jakšić, Ž.; Batel, R. Testing quality of sea water from the Adriatic coast of Croatia with toxicity, genotoxicity and DNA integrity tests. Acta Adriat. 2004, 45, 75–81. [Google Scholar]
- Jakšić, Ž.; Batel, R.; Bihari, N.; Mičić, M.; Zahn, R.K. Adriatic coast as a microcosm for global genotoxic marine contamination––A long-term field study. Mar. Pollut. Bull. 2005, 50, 1314–1327. [Google Scholar] [CrossRef]
- Leung, M.M.L.; Ho, Y.W.; Maboloc, E.A.; Lee, C.H.; Wang, Y.; Hu, M.; Cheung, S.G.; Fang, J.K.H. Determination of microplastics in the edible green-lipped mussel Perna viridis using an automated mapping technique of Raman microspectroscopy. J. Hazard. Mater. 2021, 420, 126541. [Google Scholar] [CrossRef]
- Webb, S.; Ruffell, H.; Marsden, I.; Pantos, O.; Gaw, S. Microplastics in the New Zealand green lipped mussel Perna canaliculus. Mar. Pollut. Bull. 2019, 149, 110641. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef]
- Berglund, E.; Fogelberg, V.; Nilsson, P.A.; Hollander, J. Microplastics in a freshwater mussel (Anodonta anatina) in Northern Europe. Sci. Total Environ. 2019, 697, 134192. [Google Scholar] [CrossRef]
- Mathalon, A.; Hill, P. Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef]
- Renzi, M.; Guerranti, C.; Blašković, A. Microplastic contents from maricultured and natural mussels. Mar. Pollut. Bull. 2018, 131, 248–251. [Google Scholar] [CrossRef]
- Gomiero, A.; Strafella, P.; Øysæd, K.B.; Fabi, G. First occurrence and composition assessment of microplastics in native mussels collected from coastal and offshore areas of the northern and central Adriatic Sea. Environ. Sci. Pollut. Res. 2019, 26, 24407–24416. [Google Scholar] [CrossRef]
- Vianello, A.; Da Ros, L.; Boldrin, A.; Marceta, T.; Moschino, V. First evaluation of floating microplastics in the Northwestern Adriatic Sea. Environ. Sci. Pollut. Res. 2018, 25, 28546–28561. [Google Scholar] [CrossRef]
- Catarino, A.I.; Thompson, R.; Sanderson, W.; Henry, T.B. Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 2017, 36, 947–951. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Çevik, C.; Ataş, N.T. Stuffed with microplastics: Microplastic occurrence in traditional stuffed mussels sold in the Turkish market. Food Biosci. 2020, 37, 100715. [Google Scholar] [CrossRef]



| Source of Variation | SS a | Df b | MS c | F d |
|---|---|---|---|---|
| Total number | 8570.87 | 5 | 1714.17 | 7.76 * |
| Shape | ||||
| Film | 44.37 | 5 | 8.87 | 4.69 * |
| Line | 54.97 | 5 | 10.99 | 3.14 * |
| Fragment | 2441.80 | 5 | 488.36 | 6.82 * |
| Sphere | 3265.07 | 5 | 653.01 | 5.94 * |
| Size (µm) | ||||
| 5–10 | 3524.74 | 5 | 650.95 | 8.21 * |
| 11–15 | 280.66 | 5 | 56.13 | 3.81 ** |
| 16–20 | 163.36 | 5 | 32.67 | 4.65 * |
| 21–25 | 10.96 | 5 | 2.19 | 0.90 |
| >25 | 112.87 | 5 | 22.57 | 2.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačić, I.; Štefanko, K.; Špada, V.; Pustijanac, E.; Buršić, M.; Burić, P. Microplastics in Mediterranean Mussel Mytilus galloprovincialis: Comparison between Cultured and WildType Mussels from the Northern Adriatic. Appl. Sci. 2024, 14, 2056. https://doi.org/10.3390/app14052056
Kovačić I, Štefanko K, Špada V, Pustijanac E, Buršić M, Burić P. Microplastics in Mediterranean Mussel Mytilus galloprovincialis: Comparison between Cultured and WildType Mussels from the Northern Adriatic. Applied Sciences. 2024; 14(5):2056. https://doi.org/10.3390/app14052056
Chicago/Turabian StyleKovačić, Ines, Karla Štefanko, Vedrana Špada, Emina Pustijanac, Moira Buršić, and Petra Burić. 2024. "Microplastics in Mediterranean Mussel Mytilus galloprovincialis: Comparison between Cultured and WildType Mussels from the Northern Adriatic" Applied Sciences 14, no. 5: 2056. https://doi.org/10.3390/app14052056
APA StyleKovačić, I., Štefanko, K., Špada, V., Pustijanac, E., Buršić, M., & Burić, P. (2024). Microplastics in Mediterranean Mussel Mytilus galloprovincialis: Comparison between Cultured and WildType Mussels from the Northern Adriatic. Applied Sciences, 14(5), 2056. https://doi.org/10.3390/app14052056








