A Review of the Impacts of Implant Stiffness on Fracture Healing
Abstract
:Featured Application
Abstract
1. Introduction
2. Importance of Interfragmentary Movement in Fracture Healing
3. Clinical Problems in Bridge Plating for Fracture Treatment
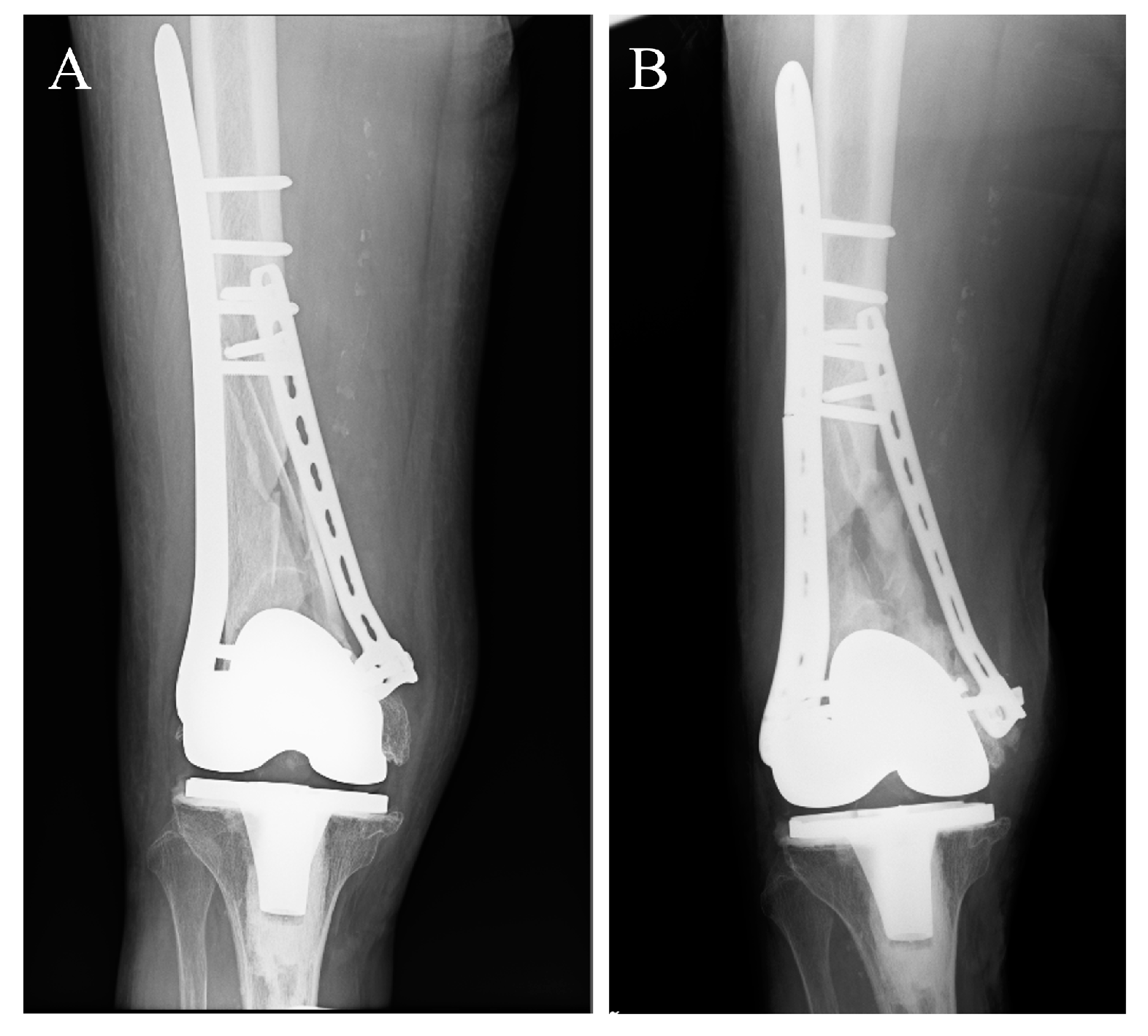
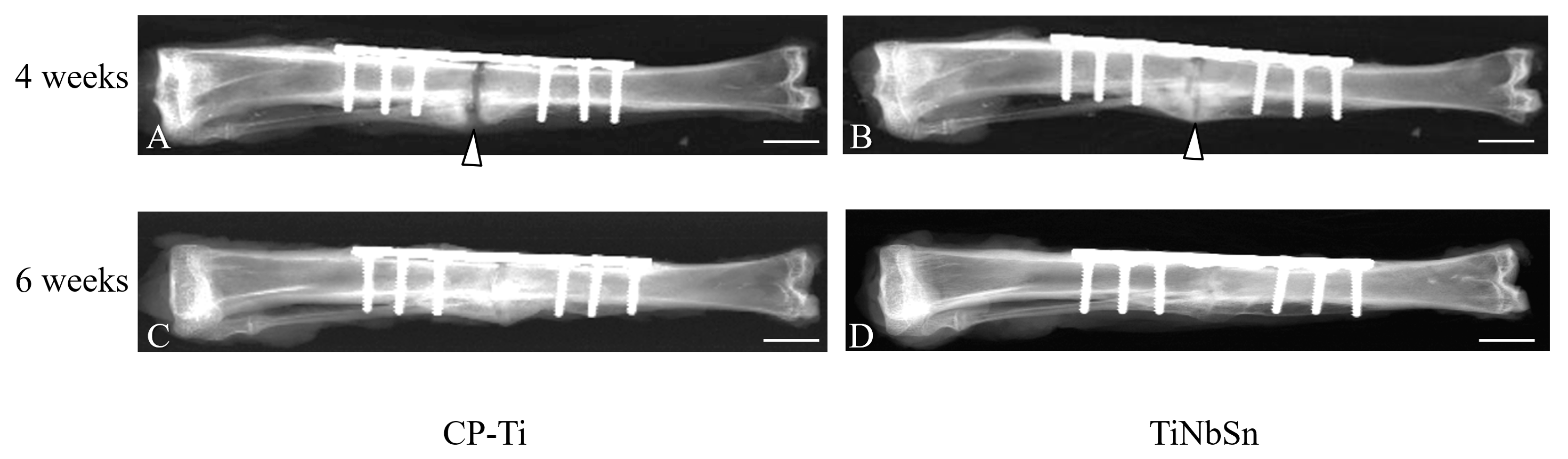
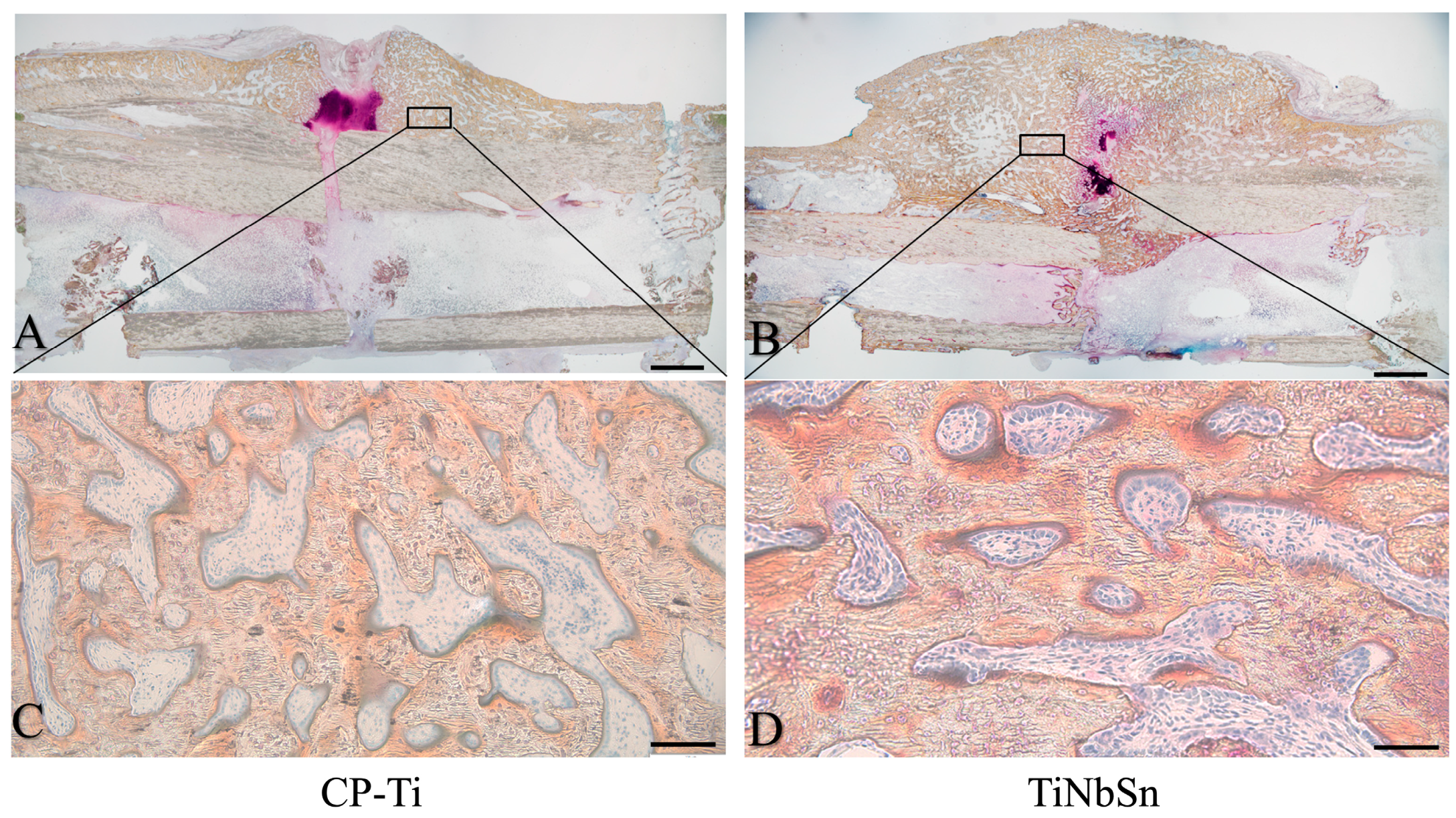
4. Clinical Applications of Low Young’s Modulus Metallic Materials to Accelerate Fracture Healing
5. Issues in the Clinical Setting
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claes, L.E.; Heigele, C.A.; Neidlinger-Wilke, C.; Kaspar, D.; Seidl, W.; Margevicius, K.J.; Augat, P. Effects of mechanical factors on the fracture healing process. Clin. Orthop. Relat. Res. 1998, 355, S132–S147. [Google Scholar] [CrossRef]
- Foster, A.L.; Moriarty, T.F.; Zalavras, C.; Morgenstern, M.; Jaiprakash, A.; Crawford, R.; Burch, M.A.; Boot, W.; Tetsworth, K.; Miclau, T.; et al. The influence of biomechanical stability on bone healing and fracture-related infection: The legacy of Stephan Perren. Injury 2021, 52, 43–52. [Google Scholar] [CrossRef]
- Glatt, V.; Evans, C.H.; Tetsworth, K. A Concert between Biology and Biomechanics: The Influence of the Mechanical Environment on Bone Healing. Front. Physiol. 2016, 7, 678. [Google Scholar] [CrossRef]
- Mori, Y.; Adams, D.; Hagiwara, Y.; Yoshida, R.; Kamimura, M.; Itoi, E.; Rowe, D.W. Identification of a progenitor cell population destined to form fracture fibrocartilage callus in Dickkopf-related protein 3-green fluorescent protein reporter mice. J. Bone Miner. Metab. 2016, 34, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, M.; Mori, Y.; Sugahara-Tobinai, A.; Takai, T.; Itoi, E. Impaired Fracture Healing Caused by Deficiency of the Immunoreceptor Adaptor Protein DAP12. PLoS ONE 2015, 10, e0128210. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Wong, M. The role of mechanical loading histories in the development of diarthrodial joints. J. Orthop. Res. 1988, 6, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Wong, M. Mechanical stresses and endochondral ossification in the chondroepiphysis. J. Orthop. Res. 1988, 6, 148–154. [Google Scholar] [CrossRef]
- Grundnes, O.; Reikeras, O. Effects of instability on bone healing. Femoral osteotomies studied in rats. Acta Orthop. Scand. 1993, 64, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Augat, P.; Simon, U.; Liedert, A.; Claes, L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos. Int. 2005, 16, S36–S43. [Google Scholar] [CrossRef] [PubMed]
- Ganse, B.; Orth, M.; Roland, M.; Diebels, S.; Motzki, P.; Seelecke, S.; Kirsch, S.M.; Welsch, F.; Andres, A.; Wickert, K.; et al. Concepts and clinical aspects of active implants for the treatment of bone fractures. Acta Biomater. 2022, 146, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Mori, Y.; Kamimura, M.; Koguchi, M.; Kurishima, H.; Koyama, T.; Mori, N.; Masahashi, N.; Hanada, S.; Itoi, E.; et al. beta-type TiNbSn Alloy Plates With Low Young Modulus Accelerates Osteosynthesis in Rabbit Tibiae. Clin. Orthop. Relat. Res. 2022, 480, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.C.; Judkins, L.M.; Manogharan, G.; Mehta, S.; Hast, M.W. Current concepts in fracture healing: Temporal dynamization and applications for additive manufacturing. OTA Int. 2022, 5, e164. [Google Scholar] [CrossRef]
- Sudkamp, N.; Bayer, J.; Hepp, P.; Voigt, C.; Oestern, H.; Kaab, M.; Luo, C.; Plecko, M.; Wendt, K.; Kostler, W.; et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J. Bone Jt. Surg. Am. 2009, 91, 1320–1328. [Google Scholar] [CrossRef]
- Beltran, M.J.; Collinge, C.A.; Gardner, M.J. Stress Modulation of Fracture Fixation Implants. J. Am. Acad. Orthop. Surg. 2016, 24, 711–719. [Google Scholar] [CrossRef]
- Bottlang, M.; Lesser, M.; Koerber, J.; Doornink, J.; von Rechenberg, B.; Augat, P.; Fitzpatrick, D.C.; Madey, S.M.; Marsh, J.L. Far cortical locking can improve healing of fractures stabilized with locking plates. J. Bone Jt. Surg. Am. 2010, 92, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Bottlang, M.; Tsai, S.; Bliven, E.K.; von Rechenberg, B.; Klein, K.; Augat, P.; Henschel, J.; Fitzpatrick, D.C.; Madey, S.M. Dynamic Stabilization with Active Locking Plates Delivers Faster, Stronger, and More Symmetric Fracture-Healing. J. Bone Jt. Surg. Am. 2016, 98, 466–474. [Google Scholar] [CrossRef]
- Henderson, C.E.; Kuhl, L.L.; Fitzpatrick, D.C.; Marsh, J.L. Locking plates for distal femur fractures: Is there a problem with fracture healing? J. Orthop. Trauma 2011, 25 (Suppl. S1), S8–S14. [Google Scholar] [CrossRef]
- Lujan, T.J.; Henderson, C.E.; Madey, S.M.; Fitzpatrick, D.C.; Marsh, J.L.; Bottlang, M. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J. Orthop. Trauma 2010, 24, 156–162. [Google Scholar] [CrossRef]
- Carter, D.R.; Blenman, P.R.; Beaupre, G.S. Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J. Orthop. Res. 1988, 6, 736–748. [Google Scholar] [CrossRef]
- Claes, L.; Augat, P.; Suger, G.; Wilke, H.J. Influence of size and stability of the osteotomy gap on the success of fracture healing. J. Orthop. Res. 1997, 15, 577–584. [Google Scholar] [CrossRef]
- Chao, E.Y.; Aro, H.T.; Lewallen, D.G.; Kelly, P.J. The effect of rigidity on fracture healing in external fixation. Clin. Orthop. Relat. Res. 1989, 241, 24–35. [Google Scholar] [CrossRef]
- Miramini, S.; Zhang, L.; Richardson, M.; Mendis, P.; Oloyede, A.; Ebeling, P. The relationship between interfragmentary movement and cell differentiation in early fracture healing under locking plate fixation. Australas. Phys. Eng. Sci. Med. 2016, 39, 123–133. [Google Scholar] [CrossRef]
- Augat, P.; Margevicius, K.; Simon, J.; Wolf, S.; Suger, G.; Claes, L. Local tissue properties in bone healing: Influence of size and stability of the osteotomy gap. J. Orthop. Res. 1998, 16, 475–481. [Google Scholar] [CrossRef]
- Augat, P.; Burger, J.; Schorlemmer, S.; Henke, T.; Peraus, M.; Claes, L. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J. Orthop. Res. 2003, 21, 1011–1017. [Google Scholar] [CrossRef]
- Einhorn, T.A. The science of fracture healing. J. Orthop. Trauma 2005, 19, S4–S6. [Google Scholar] [CrossRef]
- Augat, P.; Merk, J.; Ignatius, A.; Margevicius, K.; Bauer, G.; Rosenbaum, D.; Claes, L. Early, full weightbearing with flexible fixation delays fracture healing. Clin. Orthop. Relat. Res. 1996, 328, 194–202. [Google Scholar] [CrossRef]
- Schell, H.; Thompson, M.S.; Bail, H.J.; Hoffmann, J.E.; Schill, A.; Duda, G.N.; Lienau, J. Mechanical induction of critically delayed bone healing in sheep: Radiological and biomechanical results. J. Biomech. 2008, 41, 3066–3072. [Google Scholar] [CrossRef]
- Klein, P.; Schell, H.; Streitparth, F.; Heller, M.; Kassi, J.P.; Kandziora, F.; Bragulla, H.; Haas, N.P.; Duda, G.N. The initial phase of fracture healing is specifically sensitive to mechanical conditions. J. Orthop. Res. 2003, 21, 662–669. [Google Scholar] [CrossRef]
- Epari, D.R.; Schell, H.; Bail, H.J.; Duda, G.N. Instability prolongs the chondral phase during bone healing in sheep. Bone 2006, 38, 864–870. [Google Scholar] [CrossRef]
- Rechenmacher, A.J.; Helmkamp, J.; Brown, M.; Paul, A.V.; Campbell, S.T.; Pean, C.A.; DeBaun, M.R. Interfragmentary strain measurement post-fixation to guide intraoperative decision making: A narrative review. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 3299–3305. [Google Scholar] [CrossRef]
- Tsai, S.; Fitzpatrick, D.C.; Madey, S.M.; Bottlang, M. Dynamic locking plates provide symmetric axial dynamization to stimulate fracture healing. J. Orthop. Res. 2015, 33, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wu, J.; Deng, G.; Bi, C.; Wang, J.; Wang, Q. Axial Micromotion Locking Plate Construct Can Promote Faster and Stronger Bone Healing in an Ovine Osteotomy Model. Front. Bioeng. Biotechnol. 2020, 8, 593448. [Google Scholar] [CrossRef]
- Madey, S.M.; Tsai, S.; Fitzpatrick, D.C.; Earley, K.; Lutsch, M.; Bottlang, M. Dynamic Fixation of Humeral Shaft Fractures Using Active Locking Plates: A Prospective Observational Study. Iowa Orthop. J. 2017, 37, 1–10. [Google Scholar]
- Ebraheim, N.A.; Martin, A.; Sochacki, K.R.; Liu, J. Nonunion of distal femoral fractures: A systematic review. Orthop. Surg. 2013, 5, 46–50. [Google Scholar] [CrossRef]
- Ricci, W.M.; Streubel, P.N.; Morshed, S.; Collinge, C.A.; Nork, S.E.; Gardner, M.J. Risk Factors for Failure of Locked Plate Fixation of Distal Femur Fractures: An Analysis of 335 Cases. J. Orthop. Trauma 2014, 28, 83–89. [Google Scholar] [CrossRef]
- Rodriguez, E.K.; Boulton, C.; Weaver, M.J.; Herder, L.M.; Morgan, J.H.; Chacko, A.T.; Appleton, P.T.; Zurakowski, D.; Vrahas, M.S. Predictive factors of distal femoral fracture nonunion after lateral locked plating: A retrospective multicenter case-control study of 283 fractures. Injury 2014, 45, 554–559. [Google Scholar] [CrossRef]
- Rodriguez, E.K.; Zurakowski, D.; Herder, L.; Hall, A.; Walley, K.C.; Weaver, M.J.; Appleton, P.T.; Vrahas, M. Mechanical Construct Characteristics Predisposing to Non-union After Locked Lateral Plating of Distal Femur Fractures. J. Orthop. Trauma 2016, 30, 403–408. [Google Scholar] [CrossRef]
- Hoffmann, M.F.; Jones, C.B.; Sietsema, D.L.; Tornetta, P., 3rd; Koenig, S.J. Clinical outcomes of locked plating of distal femoral fractures in a retrospective cohort. J. Orthop. Surg. Res. 2013, 8, 43. [Google Scholar] [CrossRef]
- Henderson, C.E.; Lujan, T.J.; Kuhl, L.L.; Bottlang, M.; Fitzpatrick, D.C.; Marsh, J.L. 2010 mid-America Orthopaedic Association Physician in Training Award: Healing complications are common after locked plating for distal femur fractures. Clin. Orthop. Relat. Res. 2011, 469, 1757–1765. [Google Scholar] [CrossRef]
- Harvin, W.H.; Oladeji, L.O.; Della Rocca, G.J.; Murtha, Y.M.; Volgas, D.A.; Stannard, J.P.; Crist, B.D. Working length and proximal screw constructs in plate osteosynthesis of distal femur fractures. Injury 2017, 48, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Weight, M.; Collinge, C. Early results of the less invasive stabilization system for mechanically unstable fractures of the distal femur (AO/OTA types A2, A3, C2, and C3). J. Orthop. Trauma 2004, 18, 503–508. [Google Scholar] [CrossRef]
- Southeast Fracture, C. LCP Versus LISS in the Treatment of Open and Closed Distal Femur Fractures: Does it Make a Difference? J. Orthop. Trauma 2016, 30, e212–e216. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Huang, L.; Huang, X. The impact of plate length, fibula integrity and plate placement on tibial shaft fixation stability: A finite element study. J. Orthop. Surg. Res. 2019, 14, 52. [Google Scholar] [CrossRef]
- Moloney, G.B.; Pan, T.; Van Eck, C.F.; Patel, D.; Tarkin, I. Geriatric distal femur fracture: Are we underestimating the rate of local and systemic complications? Injury 2016, 47, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Li, S.; Cui, Y.; Tao, A.; Wang, C.; Li, H.; Zhang, L.; Yu, H.; Jiang, J.; Li, C. Comparison of the osteoblastic activity of low elastic modulus Ti-24Nb-4Zr-8Sn alloy and pure titanium modified by physical and chemical methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 111018. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, H.H.; Morgan, E.F.; Niebur, G.L.; Morris, G.E.; Wong, E.K.; Keaveny, T.M. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 2004, 37, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.H.; Bobyn, J.D.; Tanzer, M. New femoral designs: Do they influence stress shielding? Clin. Orthop. Relat. Res. 2006, 453, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Guo, Z.; Fu, J.; Li, J.; Yuan, C.F.; Shi, L.; Li, S.J. The effects of nail rigidity on fracture healing in rats with osteoporosis. Acta Orthop. 2009, 80, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Watanabe, S.; Hanada, S. Beta TiNbSn alloys with low young’s modulus and high strength. Mater. Trans. 2005, 46, 1070–1078. [Google Scholar] [CrossRef]
- Masahashi, N.; Mori, Y.; Tanaka, H.; Kogure, A.; Inoue, H.; Ohmura, K.; Kodama, Y.; Nishijima, M.; Itoi, E.; Hanada, S. Bioactive TiNbSn alloy prepared by anodization in sulfuric acid electrolytes. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 753–763. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Wang, B.L.; Wang, J.G.; Li, C.; Zhao, L.C. Corrosion behaviour of Ti-Nb-Sn shape memory alloys in different simulated body solutions. Mat. Sci. Eng. A Struct. 2006, 438, 891–895. [Google Scholar] [CrossRef]
- Rosalbino, F.; Maccio, D.; Scavino, G.; Saccone, A. In vitro corrosion behaviour of Ti-Nb-Sn shape memory alloys in Ringer’s physiological solution. J. Mater. Sci. Mater. Med. 2012, 23, 865–871. [Google Scholar] [CrossRef]
- Fujisawa, H.; Mori, Y.; Kogure, A.; Tanaka, H.; Kamimura, M.; Masahashi, N.; Hanada, S.; Itoi, E. Effects of intramedullary nails composed of a new beta-type Ti-Nb-Sn alloy with low Young’s modulus on fracture healing in mouse tibiae. J. Biomed. Mater. Res. B 2018, 106, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Kogure, A.; Mori, Y.; Tanaka, H.; Kamimura, M.; Masahashi, N.; Hanada, S.; Itoi, E. Effects of elastic intramedullary nails composed of low Young’s modulus Ti-Nb-Sn alloy on healing of tibial osteotomies in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Fujisawa, H.; Kamimura, M.; Kogure, A.; Tanaka, H.; Mori, N.; Masahashi, N.; Aizawa, T. Acceleration of Fracture Healing in Mouse Tibiae Using Intramedullary Nails Composed of β-Type TiNbSn Alloy with Low Young’s Modulus. Tohoku J. Exp. Med. 2021, 255, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, M.; Mori, Y.; Kamimura, M.; Ito, K.; Tanaka, H.; Kurishima, H.; Koyama, T.; Mori, N.; Masahashi, N.; Aizawa, T. Low Young’s Modulus TiNbSn Alloy Locking Plates Accelerate Osteosynthesis in Rabbit Tibiae. Tohoku J. Exp. Med. 2023, 261, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Henschel, J.; Tsai, S.; Fitzpatrick, D.C.; Marsh, J.L.; Madey, S.M.; Bottlang, M. Comparison of 4 Methods for Dynamization of Locking Plates: Differences in the Amount and Type of Fracture Motion. J. Orthop. Trauma 2017, 31, 531–537. [Google Scholar] [CrossRef]
- Sumitomo, N.; Noritake, K.; Hattori, T.; Morikawa, K.; Niwa, S.; Sato, K.; Niinomi, M. Experiment study on fracture fixation with low rigidity titanium alloy: Plate fixation of tibia fracture model in rabbit. J. Mater. Sci. Mater. Med. 2008, 19, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Chiba, D.; Yamada, N.; Mori, Y.; Oyama, M.; Ohtsu, S.; Kuwahara, Y.; Baba, K.; Tanaka, H.; Aizawa, T.; Hanada, S.; et al. Mid-term results of a new femoral prosthesis using Ti-Nb-Sn alloy with low Young’s modulus. BMC Musculoskelet. Disord. 2021, 22, 987. [Google Scholar] [CrossRef]
- Baba, K.; Mori, Y.; Chiba, D.; Kuwahara, Y.; Kurishima, H.; Tanaka, H.; Kogure, A.; Kamimura, M.; Yamada, N.; Ohtsu, S.; et al. TiNbSn stems with gradient changes of Young’s modulus and stiffness reduce stress shielding compared to the standard fit-and-fill stems. Eur. J. Med. Res. 2023, 28, 214. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Yu, Z.; Ren, L.; Zhao, X.; Wang, J. Study on Osseointegration Capability of beta-Type Ti-Nb-Zr-Ta-Si Alloy for Orthopedic Implants. Materials 2024, 17, 472. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Das, S.; Suwas, S.; Chatterjee, K. Engineering the next-generation tin containing beta titanium alloys with high strength and low modulus for orthopedic applications. J. Mech. Behav. Biomed. Mater. 2018, 78, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, L.; Li, C.; Li, S.; Hou, W.; Hao, Y.; Lu, Y.; Li, L. Synergistic Amelioration of Osseointegration and Osteoimmunomodulation with a Microarc Oxidation-Treated Three-Dimensionally Printed Ti-24Nb-4Zr-8Sn Scaffold via Surface Activity and Low Elastic Modulus. ACS Appl. Mater. Interfaces 2024, 16, 3171–3186. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, K.; Li, X.; Zhang, X.; Gong, X.; Zhu, Y.; Ren, Z.; Zhang, B.; Cheng, J. Biocompatibility and osseointegration properties of a novel high strength and low modulus beta- Ti10Mo6Zr4Sn3Nb alloy. Front. Bioeng. Biotechnol. 2023, 11, 1127929. [Google Scholar] [CrossRef]
- Acharya, S.; Bahl, S.; Dabas, S.S.; Hassan, S.; Gopal, V.; Panicker, A.G.; Manivasagam, G.; Suwas, S.; Chatterjee, K. Role of aging induced alpha precipitation on the mechanical and tribocorrosive performance of a beta Ti-Nb-Ta-O orthopedic alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109755. [Google Scholar] [CrossRef]
- Liu, H.; Niinomi, M.; Nakai, M.; Cho, K. beta-Type titanium alloys for spinal fixation surgery with high Young’s modulus variability and good mechanical properties. Acta Biomater. 2015, 24, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Niinomi, M.; Nakai, M.; Hieda, J.; Ishimoto, T.; Nakano, T. Optimization of Cr content of metastable beta-type Ti-Cr alloys with changeable Young’s modulus for spinal fixation applications. Acta Biomater. 2012, 8, 2392–2400. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, Y.; Kamimura, M.; Ito, K.; Koguchi, M.; Tanaka, H.; Kurishima, H.; Koyama, T.; Mori, N.; Masahashi, N.; Aizawa, T. A Review of the Impacts of Implant Stiffness on Fracture Healing. Appl. Sci. 2024, 14, 2259. https://doi.org/10.3390/app14062259
Mori Y, Kamimura M, Ito K, Koguchi M, Tanaka H, Kurishima H, Koyama T, Mori N, Masahashi N, Aizawa T. A Review of the Impacts of Implant Stiffness on Fracture Healing. Applied Sciences. 2024; 14(6):2259. https://doi.org/10.3390/app14062259
Chicago/Turabian StyleMori, Yu, Masayuki Kamimura, Kentaro Ito, Masashi Koguchi, Hidetatsu Tanaka, Hiroaki Kurishima, Tomoki Koyama, Naoko Mori, Naoya Masahashi, and Toshimi Aizawa. 2024. "A Review of the Impacts of Implant Stiffness on Fracture Healing" Applied Sciences 14, no. 6: 2259. https://doi.org/10.3390/app14062259
APA StyleMori, Y., Kamimura, M., Ito, K., Koguchi, M., Tanaka, H., Kurishima, H., Koyama, T., Mori, N., Masahashi, N., & Aizawa, T. (2024). A Review of the Impacts of Implant Stiffness on Fracture Healing. Applied Sciences, 14(6), 2259. https://doi.org/10.3390/app14062259







