DC Voltage Induces Quadratic Optical Nonlinearity in Ion-Exchanged Glasses at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
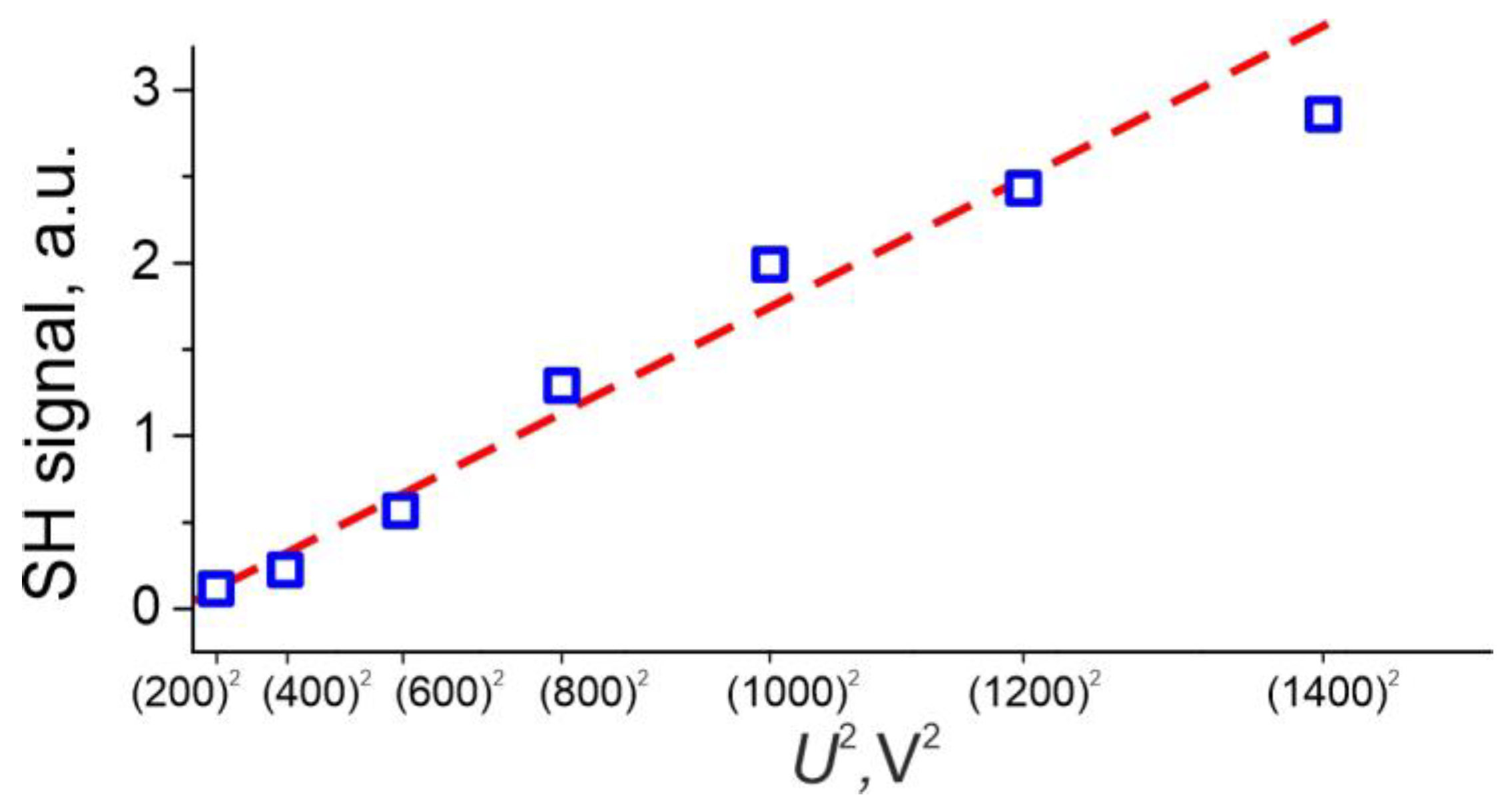
3.1. The EFISH Phenomenon in IE Glasses
3.2. The EFISH vs. the IE Duration
3.3. The Dynamics of the Phenomenon
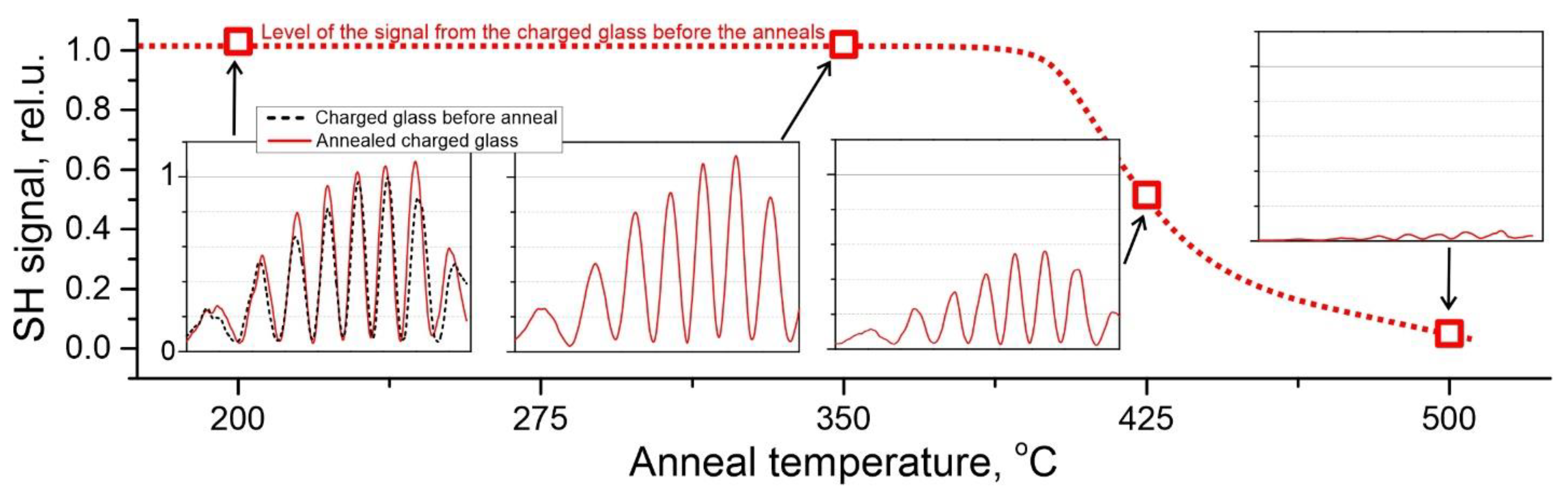
3.4. The Effect of Annealing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moore, D.T. Gradient-Index Optics: A Review. Appl. Opt. 1980, 19, 1035. [Google Scholar] [CrossRef] [PubMed]
- Yakhkind, A.K. Optical Graded-Index Elements Made from Glass. J. Opt. Technol. 2003, 70, 877. [Google Scholar] [CrossRef]
- Broquin, J.-E.; Honkanen, S. Integrated Photonics on Glass: A Review of the Ion-Exchange Technology Achievements. Appl. Sci. 2021, 11, 4472. [Google Scholar] [CrossRef]
- Berneschi, S.; Righini, G.C.; Pelli, S. Towards a Glass New World: The Role of Ion-Exchange in Modern Technology. Appl. Sci. 2021, 11, 4610. [Google Scholar] [CrossRef]
- Dussauze, M.; Cardinal, T. Nonlinear Optical Properties of Glass. In Springer Handbook of Glass; Musgraves, J.D., Hu, J., Calvez, L., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2019; pp. 193–225. [Google Scholar]
- Gy, R. Ion Exchange for Glass Strengthening. Mater. Sci. Eng. B 2008, 149, 159–165. [Google Scholar] [CrossRef]
- Li, X.; Jiang, L.; Wang, Y.; Mohagheghian, I.; Dear, J.P.; Li, L.; Yan, Y. Correlation between K+-Na+ Diffusion Coefficient and Flexural Strength of Chemically Tempered Aluminosilicate Glass. J. Non-Cryst. Solids 2017, 471, 72–81. [Google Scholar] [CrossRef]
- Tervonen, A.; West, B.R.; Honkanen, S. Ion-Exchanged Glass Waveguide Technology: A Review. Opt. Eng. 2011, 50, 071107. [Google Scholar] [CrossRef]
- da Silva, B.J.P.; de Melo, R.P.; Falcão-Filho, E.L.; de Araújo, C.B. Potassium Source for Ion-Exchange Glass Waveguide Fabrication. Appl. Opt. 1997, 36, 5949. [Google Scholar] [CrossRef]
- Jiao, Q.; Wang, X.; Qiu, J.; Zhou, D. Effect of Silver Ions and Clusters on the Luminescence Properties of Eu-Doped Borate Glasses. Mater. Res. Bull. 2015, 72, 264–268. [Google Scholar] [CrossRef]
- Li, J.; Wei, R.; Liu, X.; Guo, H. Enhanced Luminescence via Energy Transfer from Ag+ to RE Ions (Dy3+, Sm3+, Tb3+) in Glasses. Opt. Express 2012, 20, 10122. [Google Scholar] [CrossRef]
- Karvonen, L.; Rönn, J.; Kujala, S.; Chen, Y.; Säynätjoki, A.; Tervonen, A.; Svirko, Y.; Honkanen, S. High Non-Resonant Third-Order Optical Nonlinearity of Ag–Glass Nanocomposite Fabricated by Two-Step Ion Exchange. Opt. Mater. 2013, 36, 328–332. [Google Scholar] [CrossRef]
- Xiang, W.; Gao, H.; Ma, L.; Ma, X.; Huang, Y.; Pei, L.; Liang, X. Valence State Control and Third-Order Nonlinear Optical Properties of Copper Embedded in Sodium Borosilicate Glass. ACS Appl. Mater. Interfaces 2015, 7, 10162–10168. [Google Scholar] [CrossRef] [PubMed]
- Berneschi, S.; D’Andrea, C.; Baldini, F.; Banchelli, M.; de Angelis, M.; Pelli, S.; Pini, R.; Pugliese, D.; Boetti, N.G.; Janner, D.; et al. Ion-Exchanged Glass Microrods as Hybrid SERS/Fluorescence Substrates for Molecular Beacon-Based DNA Detection. Anal. Bioanal. Chem. 2021, 413, 6171–6182. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Karvonen, L.; Säynätjoki, A.; Ye, C.; Tervonen, A.; Honkanen, S. Ag Nanoparticles Embedded in Glass by Two-Step Ion Exchange and Their SERS Application. Opt. Mater. Express 2011, 1, 164. [Google Scholar] [CrossRef]
- Dussauze, M.; Rodriguez, V.; Lipovskii, A.; Petrov, M.; Smith, C.; Richardson, K.; Cardinal, T.; Fargin, E.; Kamitsos, E.I. How Does Thermal Poling Affect the Structure of Soda-Lime Glass? J. Phys. Chem. C 2010, 114, 12754–12759. [Google Scholar] [CrossRef]
- Reshetov, I.; Scherbak, S.; Kan, G.; Kaasik, V.; Pleshakov, O.; Melehin, V.; Lipovskii, A. Controlling the Sign and Magnitude of the Nonlinear Susceptibility of Poled Glasses at Room Temperature. J. Mater. Sci. 2023, 58, 11859–11871. [Google Scholar] [CrossRef]
- Reshetov, I.; Scherbak, S.; Tagantsev, D.; Zhurikhina, V.; Lipovskii, A. Giant Enhancement of Optical Second Harmonic in Poled Glasses by Cold Repoling. J. Phys. Chem. Lett. 2022, 13, 5932–5937. [Google Scholar] [CrossRef]
- Wagner, K.W. Erklärung Der Dielektrischen Nachwirkungsvorgänge Auf Grund Maxwellscher Vorstellungen. Arch. Für Elektrotechnik 1914, 2, 371–387. [Google Scholar] [CrossRef]
- Iwamoto, M. Maxwell–Wagner Effect. In Encyclopedia of Nanotechnology; Springer Netherlands: Dordrecht, The Netherlands, 2015; pp. 1–13. [Google Scholar]
- Li, C.; Chen, G.; Qiu, X.; Lou, Q.; Gao, X. A Direct Proof for Maxwell–Wagner Effect of Heterogeneous Interface. AIP Adv. 2021, 11, 065227. [Google Scholar] [CrossRef]
- Karam, L.; Adamietz, F.; Michau, D.; Murugan, G.S.; Cardinal, T.; Fargin, E.; Rodriguez, V.; Richardson, K.A.; Dussauze, M. Second-Order Optical Response in Electrically Polarized Sodo-Niobate Amorphous Thin Films: Particularity of Multilayer Systems. Adv. Photonics Res. 2021, 2, 2000171. [Google Scholar] [CrossRef]
- Samet, M.; Kallel, A.; Serghei, A. Maxwell-Wagner-Sillars Interfacial Polarization in Dielectric Spectra of Composite Materials: Scaling Laws and Applications. J. Compos. Mater. 2022, 56, 3197–3217. [Google Scholar] [CrossRef]
- Vu, T.T.N.; Teyssedre, G.; Roy, S.L.; Laurent, C. Maxwell–Wagner Effect in Multi-Layered Dielectrics: Interfacial Charge Measurement and Modelling. Technologies 2017, 5, 27. [Google Scholar] [CrossRef]
- Liu, J.; Duan, C.G.; Mei, W.N.; Smith, R.W.; Hardy, J.R. Dielectric Properties and Maxwell-Wagner Relaxation of Compounds ACu3Ti4O12 (A=Ca, Bi 2/3,Y 2/3,La 2/3). J. Appl. Phys. 2005, 98, 093703. [Google Scholar] [CrossRef]
- High Quality Microscope Slides. Available online: https://www.agarscientific.com/high-quality-microscope-slides (accessed on 29 February 2024).
- Natrup, F.V.; Bracht, H.; Murugavel, S.; Roling, B. Cation Diffusion and Ionic Conductivity in Soda-Lime Silicate Glasses. Phys. Chem. Chem. Phys. 2005, 7, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Oven, R. Analytical Model of Electric Field Assisted Ion Diffusion into Glass Containing Two Indigenous Mobile Species, with Application to Poling. J. Non-Cryst. Solids 2021, 553, 120476. [Google Scholar] [CrossRef]
- Doremus, R.H. Exchange and Diffusion of Ions in Glass. J. Phys. Chem. 1964, 68, 2212–2218. [Google Scholar] [CrossRef]
- Bos, M.; Boukamp, B.A.; Vrielink, J.A.M. Determination of Diffusion Profiles of Silver Ions in Soda-Lime–Silica Glass by X-Ray Fluorescence Spectrometry. Anal. Chim. Acta 2002, 459, 305–311. [Google Scholar] [CrossRef]
- Jerphagnon, J.; Kurtz, S.K. Maker Fringes: A Detailed Comparison of Theory and Experiment for Isotropic and Uniaxial Crystals. J. Appl. Phys. 1970, 41, 1667–1681. [Google Scholar] [CrossRef]
- Scherbak, S.A.; Reshetov, I.V.; Zhurikhina, V.V.; Lipovskii, A.A. Second Harmonic Generation By Surface of Poled Glasses: Modeling and Measurement of Maker Fringes. St. Petersbg. State Polytech. Univ. J. Phys. Math. 2021, 14, 95–113. [Google Scholar] [CrossRef]
- Quiquempois, Y.; Martinelli, G.; Duthérage, P.; Bernage, P.; Niay, P.; Douay, M. Localisation of the Induced Second-Order Non-Linearity within Infrasil and Suprasil Thermally Poled Glasses. Opt. Commun. 2000, 176, 479–487. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Wang, F.X.; Canfield, B.K.; Cattaneo, S.; Kauranen, M. Multipolar Tensor Analysis of Second-Order Nonlinear Optical Response of Surface and Bulk of Glass. Opt. Express 2007, 15, 8695. [Google Scholar] [CrossRef] [PubMed]
- Reshetov, I.; Kaasik, V.; Kan, G.; Shestakov, S.; Scherbak, S.; Zhurikhina, V.; Lipovskii, A. SHG in Micron-Scale Layers of Glasses: Electron Beam Irradiation vs. Thermal Poling. Photonics 2022, 9, 733. [Google Scholar] [CrossRef]
- An, H.; Fleming, S. Second-Order Optical Nonlinearity in Thermally Poled Borosilicate Glass. Appl. Phys. Lett. 2006, 89, 181111. [Google Scholar] [CrossRef]
- Bengtsson, F.; Pehlivan, I.B.; Österlund, L.; Karlsson, S. Alkali Ion Diffusion and Structure of Chemically Strengthened TiO2 Doped Soda-Lime Silicate Glass. J. Non-Cryst. Solids 2022, 586, 121564. [Google Scholar] [CrossRef]
- Macrelli, G.; Varshneya, A.K.; Mauro, J.C. Simulation of Glass Network Evolution during Chemical Strengthening: Resolution of the Subsurface Compression Maximum Anomaly. J. Non-Cryst. Solids 2019, 522, 119457. [Google Scholar] [CrossRef]
- Ingram, M.D.; Wu, M.-H.; Coats, A.; Kamitsos, E.I.; Varsamis, C.-P.E.; Garcia, N.; Sola, M. Evidence from Infrared Spectroscopy of Structural Relaxation during Field Assisted and Chemically Driven Ion Exchange in Soda-Lime-Silica Glasses. Phys. Chem. Glas. 2005, 46, 84–89. [Google Scholar]




| Oxide | SiO2 | Na2O | CaO | MgO | K2O | Al2O3 | SO3 | Fe2O3 |
|---|---|---|---|---|---|---|---|---|
| Wt.% | 72.20 | 14.30 | 6.40 | 4.30 | 1.20 | 1.20 | 0.30 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherbak, S.; Kan, G.; Tagantsev, D.; Lipovskii, A. DC Voltage Induces Quadratic Optical Nonlinearity in Ion-Exchanged Glasses at Room Temperature. Appl. Sci. 2024, 14, 2305. https://doi.org/10.3390/app14062305
Scherbak S, Kan G, Tagantsev D, Lipovskii A. DC Voltage Induces Quadratic Optical Nonlinearity in Ion-Exchanged Glasses at Room Temperature. Applied Sciences. 2024; 14(6):2305. https://doi.org/10.3390/app14062305
Chicago/Turabian StyleScherbak, Sergey, Gennadiy Kan, Dmitry Tagantsev, and Andrey Lipovskii. 2024. "DC Voltage Induces Quadratic Optical Nonlinearity in Ion-Exchanged Glasses at Room Temperature" Applied Sciences 14, no. 6: 2305. https://doi.org/10.3390/app14062305
APA StyleScherbak, S., Kan, G., Tagantsev, D., & Lipovskii, A. (2024). DC Voltage Induces Quadratic Optical Nonlinearity in Ion-Exchanged Glasses at Room Temperature. Applied Sciences, 14(6), 2305. https://doi.org/10.3390/app14062305






