Nutritional and Physico-Chemical Characteristics of Innovative Bars Enriched with Aronia melanocarpa By-Product Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bar Formulation
2.3. Rheological Analyses
2.4. In Vitro Digestion
2.5. Antioxidant Activity
2.6. HPLC–DAD–ESI–MS Identification of the Phenolic Composition
2.6.1. Extraction of Phenolic Compounds
2.6.2. High-Performance Liquid Chromatography (HPLC DAD-ESI+) Conditions
2.7. Nutritional Properties
2.8. Influence of Storage Period on Microbiological Analysis
2.8.1. Total Viable Cell Count
2.8.2. Total Fungi-Yeast and Moulds
2.9. Statistical Analysest
3. Results and Discussions
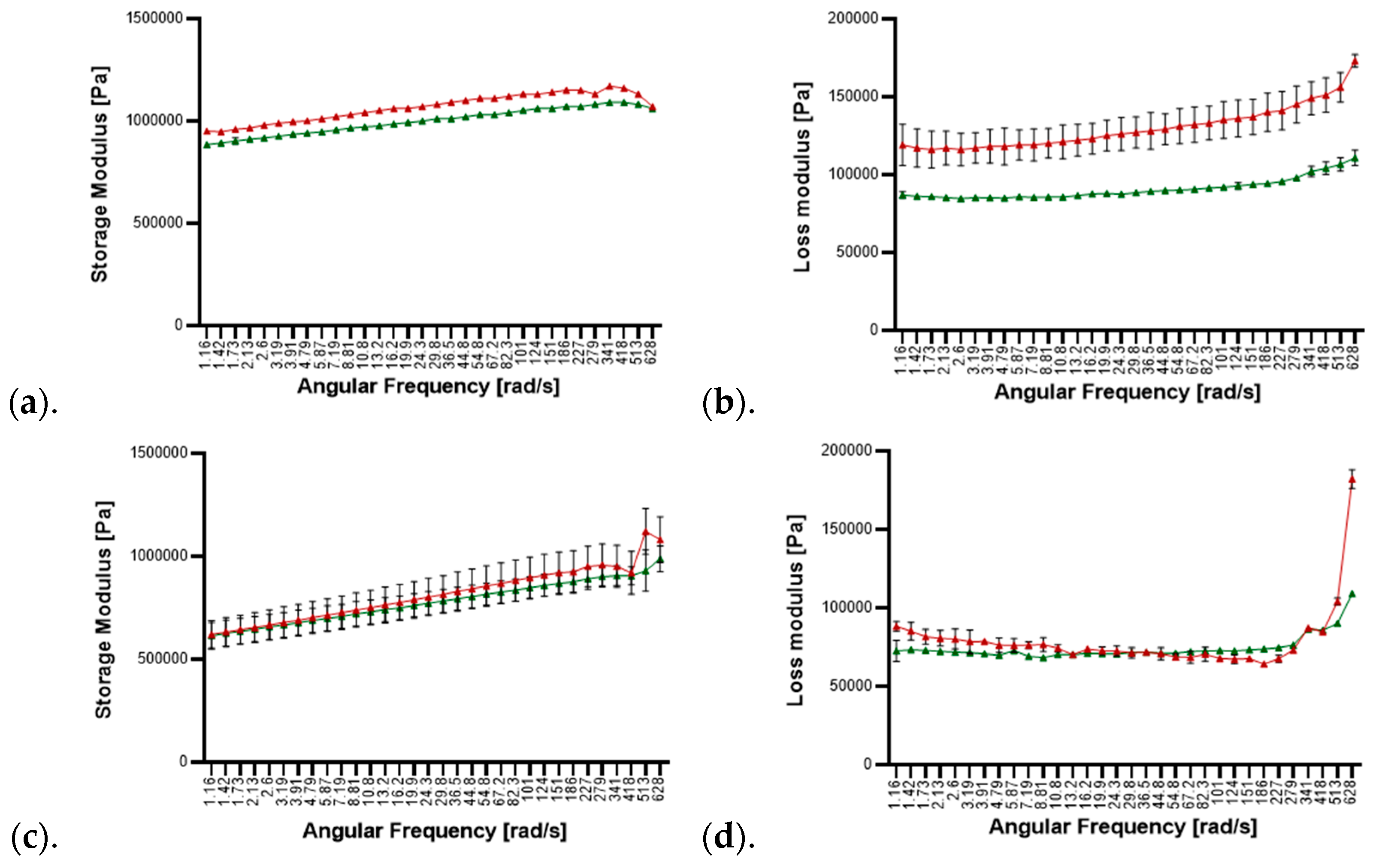
3.1. Rheological Analyses
3.2. Antioxidant Activity and Bioactive Compounds
3.3. In Vitro Digestion
3.4. Nutritional Properties
3.5. Influence of Time on Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horszwald, A.; Julien, H.; Andlauer, W. Characterisation of Aronia powders obtained by different drying processes. Food Chem. 2013, 141, 2858–2863. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, C.; Li, G.; Chrubasik, S. The clinical effectiveness of chokeberry: A systematic review. Phytother. Res. PTR 2010, 24, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Janković, B.; Marinović-Cincović, M.; Janković, M. Isoconversional kinetic study and accurate determination of lifetime properties for thermal and thermo-oxidative degradation processes of Aronia melanocarpa. Innov. Food Sci. Emerg. Technol. 2016, 33, 542–553. [Google Scholar] [CrossRef]
- Caruso, M.C.; Galgano, F.; Tolve, R.; Pecora, M.; Tedesco, I.; Favati, F.; Condelli, N. Nutraceutical properties of wild berry fruits from Southern Italy. J. Berry Res. 2016, 6, 321–332. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Soto, M.J.; Larrosa, M.; Garcia-Cantalejo, J.M.; Espin, J.C.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J. Nutr. Biochem. 2007, 18, 259–271. [Google Scholar] [CrossRef]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef]
- Research, Z.M. Aronia Berries Market: Global Industry Analysis, Size, Share, Growth, Trends, and Forecasts 2023–2030. Available online: https://www.zionmarketresearch.com/report/aronia-berries-market (accessed on 22 February 2024).
- Fava, F.; Totaro, G.; Diels, L.; Reis, M.; Duarte, J.; Carioca, O.B.; Poggi-Varaldo, H.M.; Ferreira, B.S. Biowaste biorefinery in Europe: Opportunities and research & development needs. N. Biotechnol. 2015, 32, 100–108. [Google Scholar] [CrossRef]
- Cvetanovic, A.; Zengin, G.; Zekovic, Z.; Svarc-Gajic, J.; Razic, S.; Damjanovic, A.; Maskovic, P.; Mitic, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef]
- Grunovaitė, L.; Pukalskienė, M.; Pukalskas, A.; Venskutonis, P.R. Fractionation of black chokeberry pomace into functional ingredients using high pressure extraction methods and evaluation of their antioxidant capacity and chemical composition. J. Funct. Foods 2016, 24, 85–96. [Google Scholar] [CrossRef]
- Rodrigues, S.; de Brito, E.S.; de Oliveira Silva, E. Elderberry—Sambucus nigra L. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 181–185. [Google Scholar] [CrossRef]
- Kaloudi, T.; Tsimogiannis, D.; Oreopoulou, V. Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components. Molecules 2022, 27, 4375. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, K.; Piskin, E.; Xiao, J.; Tomas, M.; Capanoglu, E. Fruit Juice Industry Wastes as a Source of Bioactives. J. Agric. Food Chem. 2022, 70, 6805–6832. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Loonis, M.; Delosiere, M.; Buffiere, C.; Hafnaoui, N.; Sante-Lhoutellier, V.; Remond, D. The matrix of fruit & vegetables modulates the gastrointestinal bioaccessibility of polyphenols and their impact on dietary protein digestibility. Food Chem. 2018, 240, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Teleky, B.E.; Martau, G.A.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Biofunctional soy-based sourdough for improved rheological properties during storage. Sci. Rep. 2022, 12, 17535. [Google Scholar] [CrossRef] [PubMed]
- Sparkman, K.; Joyner, H.S. Impact of formulation on high-protein bar rheological and wear behaviors. J. Texture Stud. 2019, 50, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Ștefănescu, B.E.; Socaci, S.A.; Fărcaș, A.C.; Nemeș, S.A.; Teleky, B.E.; Martău, G.A.; Călinoiu, L.F.; Mitrea, L.; Ranga, F.; Grigoroaea, D.; et al. Characterization of the Chemical Composition and Biological Activities of Bog Bilberry (Vaccinium uliginosum L.) Leaf Extracts Obtained via Various Extraction Techniques. Foods 2024, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yuan, Z.; Rong, L.; Zhang, Y.; Xiong, G.; Liu, Y.; Li, C. An Optimized Method for Extraction and Characterization of Phenolic Compounds in Dendranthema indicum var. aromaticum Flower. Sci. Rep. 2019, 9, 7745. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C.; Calinoiu, L.F.; Dulf, F.V.; Stefanescu, B.E.; Crisan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef]
- Palma-Duran, S.A.; Caire-Juvera, G.; Campa-Siqueiros, M.M.; Chávez-Suárez, K.M.; Robles-Burgueño, M.D.R.; Gutiérrez-Coronado, M.L.; Bermúdez-Almada, M.D.C.; Saucedo-Tamayo, M.D.S.; Grajeda-Cota, P.; Valenzuela-Quintanar, A.I. A Comprehensive HPLC-DAD-ESI-MS Validated Method for the Quantification of 16 Phytoestrogens in Food, Serum and Urine. Appl. Sci. 2020, 10, 8147. [Google Scholar] [CrossRef]
- Approved Methods Committee American Association of Cereal Chemists. Approved Methods of the American Association of Cereal Chemists; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Lazar, C.; Rotar, A.M.; Apostu, S.; Buzgau, G. Comparative Studies Concerning Microbial Quality of Ecological and Conventional Milk. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2010, 67, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Haș, I.M.; Vodnar, D.-C.; Bungau, A.F.; Tarce, A.G.; Tit, D.M.; Teleky, B.-E. Enhanced Elderberry Snack Bars: A Sensory, Nutritional, and Rheological Evaluation. Foods 2023, 12, 3544. [Google Scholar] [CrossRef] [PubMed]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsä-Kortelainen, S.; Sozer, N. Applicability of protein and fiber-rich food materials in extrusion-based 3D printing. J. Food Eng. 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Lazăr, M.-A.; Catană, M.; Catană, L.; Burnete, A.-G.; Teodorescu, R.I.; Asănică, A.C.; Belc, N. Valorisation of Aronia melanocarpa pomace for development of functional ingredients with high nutritional value and antioxidant capacity. Sci. Pap. B Hortic 2020, 64, 403–410. [Google Scholar]
- Blicharz-Kania, A.; Vasiukov, K.; Sagan, A.; Andrejko, D.; Fifowska, W.; Domin, M. Nutritional Value, Physical Properties, and Sensory Quality of Sugar-Free Cereal Bars Fortified with Grape and Apple Pomace. Appl. Sci. 2023, 13, 10531. [Google Scholar] [CrossRef]
- Munhoz, C.L.; Guimarães, R.D.C.A.; Nozaki, V.T.; Sanjinez-Argandoña, E.J.; Hiane, P.A.; Macedo, M.L.R. Preparation of a cereal bar containing bocaiuva: Physical, nutritional, microbiological and sensory evaluation. Acta Sci. Technol. 2014, 36, 553. [Google Scholar] [CrossRef]
- Banach, J.C.; Clark, S.; Metzger, L.E.; Lamsal, B.P. Textural performance of crosslinked or reduced-calcium milk protein ingredients in model high-protein nutrition bars. J. Dairy Sci. 2016, 99, 6061–6070. [Google Scholar] [CrossRef]
- Maleki, G.; Shadordizadeh, T.; Mozafari, M.R.; Attar, F.R.; Hesarinejad, M.A. Physicochemical and nutritional characteristics of nutrition bar fortified with cowpea protein. J. Food Meas. Charact. 2022, 17, 2010–2015. [Google Scholar] [CrossRef]
- Lima, J.R.; Garruti, D.S.; Bruno, L.M. Physicochemical, microbiological and sensory characteristics of cashew nut butter made from different kernel grades-quality. LWT—Food Sci. Technol. 2012, 45, 180–185. [Google Scholar] [CrossRef]
- Alfheeaid, H.A.; Barakat, H.; Althwab, S.A.; Musa, K.H.; Malkova, D. Nutritional and Physicochemical Characteristics of Innovative High Energy and Protein Fruit- and Date-Based Bars. Foods 2023, 12, 2777. [Google Scholar] [CrossRef]
- Szydłowska, A.; Zielińska, D.; Łepecka, A.; Trząskowska, M.; Neffe-Skocińska, K.; Kołożyn-Krajewska, D. Development of Functional High-Protein Organic Bars with the Addition of Whey Protein Concentrate and Bioactive Ingredients. Agriculture 2020, 10, 390. [Google Scholar] [CrossRef]
- Ciont, C.; Difonzo, G.; Pasqualone, A.; Chis, M.S.; Ranga, F.; Szabo, K.; Simon, E.; Naghiu, A.; Barbu-Tudoran, L.; Caponio, F.; et al. Phenolic profile of micro- and nano-encapsulated olive leaf extract in biscuits during in vitro gastrointestinal digestion. Food Chem. 2023, 428, 136778. [Google Scholar] [CrossRef] [PubMed]
- Munshi, R.; Kochhar, A.; Kaur, A. Nutrient selection and optimization to formulate a nutrient bar stable on storage and specific to women at risk of osteoporosis. J. Food Sci. Technol. 2020, 57, 3099–3107. [Google Scholar] [CrossRef] [PubMed]
- Veggi, N.; Voltarelli, F.A.; Pereira, J.M.N.; Silva, W.C.; Navalta, J.W.; Cavenaghi, D.F.L.D.C.; Barros, W.M.D. Quality of high-protein diet bar plus chia (Salvia hispanica L.) grain evaluated sensorially by untrained tasters. Food Sci. Technol. 2018, 38, 306–312. [Google Scholar] [CrossRef]


| Ingredients | Control Bar | Bar with By-Product |
|---|---|---|
| Cashew butter | 38.22% | 38.22% |
| Honey bees | 35.72% | 35.72% |
| Oat flakes | 21.06% | 18.06% |
| Coconut flakes | 5% | 5% |
| Aronia by-product | - | 3% |
| Freeze-dried aronia | 10,621.67 ± 0.28 μM Trolox/100 g dry weight |
| Fresh aronia | 4140.1 ± 0.18 μM Trolox/100 g fresh weight |
| Peak | Rt (min) | UV λmax (nm) | [M + H]+ (m/z) | Compounds | Fresh Aronia B | Freeze-Dried Aronia B |
|---|---|---|---|---|---|---|
| 1 | 3.04 | 270 | 139 | Hydroxybenzoic acid | 9198.83 ± 0.28 | 22,880.942 ± 1.20 *** |
| 2 | 10.32 | 530, 280 | 611 | Cyanidin diglucoside Delphinidin glucoside | 3751.54 ± 0.35 | 9411.872 ± 0.27 *** |
| 3 | 11.29 | 530, 280 | 465 | Cyanidin glucoside | 1203.94 ± 0.21 | 3010.610 ± 0.29 *** |
| 4 | 13.05 | 529, 280 | 449 | Cyanidin | 124.38 ± 0.09 | 308.464 ± 0.10 *** |
| 5 | 13.35 | 322 | 287 | Caffeic acid | 200.33 ± 0.11 | 764.026 ± 0.11 *** |
| 6 | 14.48 | 356, 256 | 181 | Kaempferol diglucoside | 266.31 ± 0.10 | 978.302 ± 0.11 *** |
| 7 | 14.88 | 360, 255 | 611 | Quercetin-acetyl rhamnoside | 331.80 ± 0.20 | 1068.519 ± 0.31 *** |
| 8 | 15.61 | 360, 255 | 491 | Quercetin-rutinoside (Rutin) | 383.25 ± 0.33 | 1265.660 ± 0.41 *** |
| 9 | 16.17 | 360, 255 | 611 | Quercetin glucoside | 581.29 ± 0.52 | 1700.039 ± 0.56 *** |
| 10 | 18.75 | 356, 256 | 465 | Kaempferol glucoside | 272.54 ± 0.12 | 1269.002 ± 0.18 *** |
| 11 | 21.37 | 360, 255 | 449 | Quercetin | 232.00 ± 0.18 | 974.960 ± 0.28 *** |
| 12 | 23.27 | 356, 256 | 303 | Kaempferol | 246.03 ± 0.07 | 741.064 ± 0.29 *** |
| Total Phenolics | 16,792.24 ± 3.78 | 44,373.46 ± 3.21 | ||||
| Peak | Rt (min) | Compound | Control Bar + Aronia before Digestion | Control Bar after Digestion | Control Bar + Aronia Powder after Digestion | Aronia Powder after Digestion |
|---|---|---|---|---|---|---|
| 1 | 3.04 | Hydroxybenzoic acid | 223.58 ± 0.38 | 335.99 ± 0.78 N.S. | 3592.40 ± 1.21 ** | 11,381.54 ± 4.27 * |
| 2 | 3.63 | Dihydroxybenzoic acid | n.d. | 259.86 ± 0.52 N.S. | 2828.27 ± 1.08 ** | 7125.05 ± 3.08 * |
| 3 | 9.65 | Protocatechuic acid | 315.68 ± 0.41 | 482.72 ± 0.69 N.S. | 3505.16 ± 1.57 ** | 11,548.09 ± 3.49 * |
| 4 | 10.32 | Cyanidin diglucoside Delphinidin glucoside | 253.42 ± 0.30 | n.d. | 74.62 ± 0.18 ** | 5197.59 ± 1.36 * |
| 5 | 11.29 | Cyanidin-glucoside | 101.53 ± 0.44 | n.d. | 71.99 ± 0.24 ** | 2696.02 ± 1.07 * |
| 6 | 13.05 | Cyanidin | 29.49 ± 0.20 | n.d. | n.d. | 124.19 ± 0.19 * |
| 7 | 13.35 | Caffeic acid | 40.69 ± 0.17 | 37.03 ± 0.20 N.S. | 88.39 ± 0.32 ** | 1395.14 ± 1.01 * |
| 8 | 14.48 | Kaempferol diglucoside | 30.96 ± 0.15 | n.d. | 40.49 ± 0.21 ** | 543.21 ± 0.78 * |
| 9 | 14.88 | Quercetin-acetyl-rhamnoside | 37.31 ± 0.21 | n.d. | 38.01 ± 0.17 ** | 681.32 ± 0.86 * |
| 10 | 15.61 | Quercetin rutinoside (Rutin) | 30.96 ± 0.18 | n.d. | 32.07 ± 0.14 ** | 624.89 ± 0.73 * |
| 11 | 16.17 | Quercetin glucoside | 34.84 ± 0.14 | n.d. | 65.73 ± 0.29 ** | 540.24 ± 0.66 * |
| 12 | 18.75 | Kaempferol glucoside | 42.25 ± 0.22 | n.d. | n.d. | 168.98 ± 0.31 * |
| 13 | 21.37 | Quercetin | 32.37 ± 0.28 | n.d. | n.d. | 112.54 ± 0.09 * |
| 14 | 23.27 | Kaempferol | 34.14 ± 0.11 | n.d. | n.d. | 102.15 ± 0.11 * |
| Total Phenolics | 1207.22 ± 0.56 | 1115.61 ± 0.91 | 10,337.12 ± 1.49 | 42,240.94 ± 4.47 | ||
| Total Anthocyanins | n.d. | 384.44 ± 0.49 | 146.61 ± 0.28 | 8017.80 ± 1.91 | ||
| Control Bar | Bar with Aronia | |
|---|---|---|
| Dry matter (%) | 90.52 ± 1.22 | 90.43 ± 1.35 N.S. |
| Humidity (%) | 9.47 ± 0.55 | 9.56 ± 0.43 N.S. |
| Ash (%) | 1.82 ± 0.01 | 1.87 ± 0.10 N.S. |
| Protein (%) | 12.20 ± 0.28 | 12.28 ± 0.22 N.S. |
| Fat (%) | 20.51 ± 0.61 | 20.46 ± 0.35 N.S. |
| Carbohydrates (%) | 45.41 ± 0.26 | 45.47 ± 0.33 N.S. |
| Fiber (%) | 10.58 ± 0.47 | 10.35 ± 0.38 N.S. |
| Kcal | 429.87 ± 1.23 | 430.01 ± 1.56 N.S. |
| Kj | 1798.59 ± 2.96 | 1799.16 ± 3.09 N.S. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bratosin, B.C.; Martău, G.-A.; Ciont, C.; Ranga, F.; Simon, E.; Szabo, K.; Darjan, S.; Teleky, B.-E.; Vodnar, D.C. Nutritional and Physico-Chemical Characteristics of Innovative Bars Enriched with Aronia melanocarpa By-Product Powder. Appl. Sci. 2024, 14, 2338. https://doi.org/10.3390/app14062338
Bratosin BC, Martău G-A, Ciont C, Ranga F, Simon E, Szabo K, Darjan S, Teleky B-E, Vodnar DC. Nutritional and Physico-Chemical Characteristics of Innovative Bars Enriched with Aronia melanocarpa By-Product Powder. Applied Sciences. 2024; 14(6):2338. https://doi.org/10.3390/app14062338
Chicago/Turabian StyleBratosin, Bogdan Constantin, Gheorghe-Adrian Martău, Călina Ciont, Floricuța Ranga, Elemér Simon, Katalin Szabo, Sorina Darjan, Bernadette-Emőke Teleky, and Dan Cristian Vodnar. 2024. "Nutritional and Physico-Chemical Characteristics of Innovative Bars Enriched with Aronia melanocarpa By-Product Powder" Applied Sciences 14, no. 6: 2338. https://doi.org/10.3390/app14062338
APA StyleBratosin, B. C., Martău, G.-A., Ciont, C., Ranga, F., Simon, E., Szabo, K., Darjan, S., Teleky, B.-E., & Vodnar, D. C. (2024). Nutritional and Physico-Chemical Characteristics of Innovative Bars Enriched with Aronia melanocarpa By-Product Powder. Applied Sciences, 14(6), 2338. https://doi.org/10.3390/app14062338










