Effect of MXene Nanosheet Sticking on Supercapacitor Device Performance
Abstract
:Featured Application
Abstract
1. Introduction
1.1. Background
1.2. Objectives of the Study
2. MXenes as Supercapacitor Electrodes
2.1. Overview of MXenes
2.2. Advantages of MXenes in Supercapacitors
2.2.1. MXenes with Various Materials: A Journey to Enhanced Supercapacitors
2.2.2. MXene–Carbon Allotropes: Pioneering Energy Storage Innovation
2.2.3. MXene–Polymer Composites: A Synergistic Approach to Enhanced Supercapacitors
3. Factors Influencing Sticking in MXene Electrodes
3.1. Morphology and Size of MXene Flakes
3.1.1. Size of MXene Flakes
3.1.2. Flake Thickness
3.1.3. Flake Surface Chemistry
3.1.4. Surface Area/Volume Ratio
3.2. Surface Chemistry of MXene Flakes
3.2.1. Surface Chemistry
3.2.2. Hydrophobicity
3.3. Electrode Processing Techniques
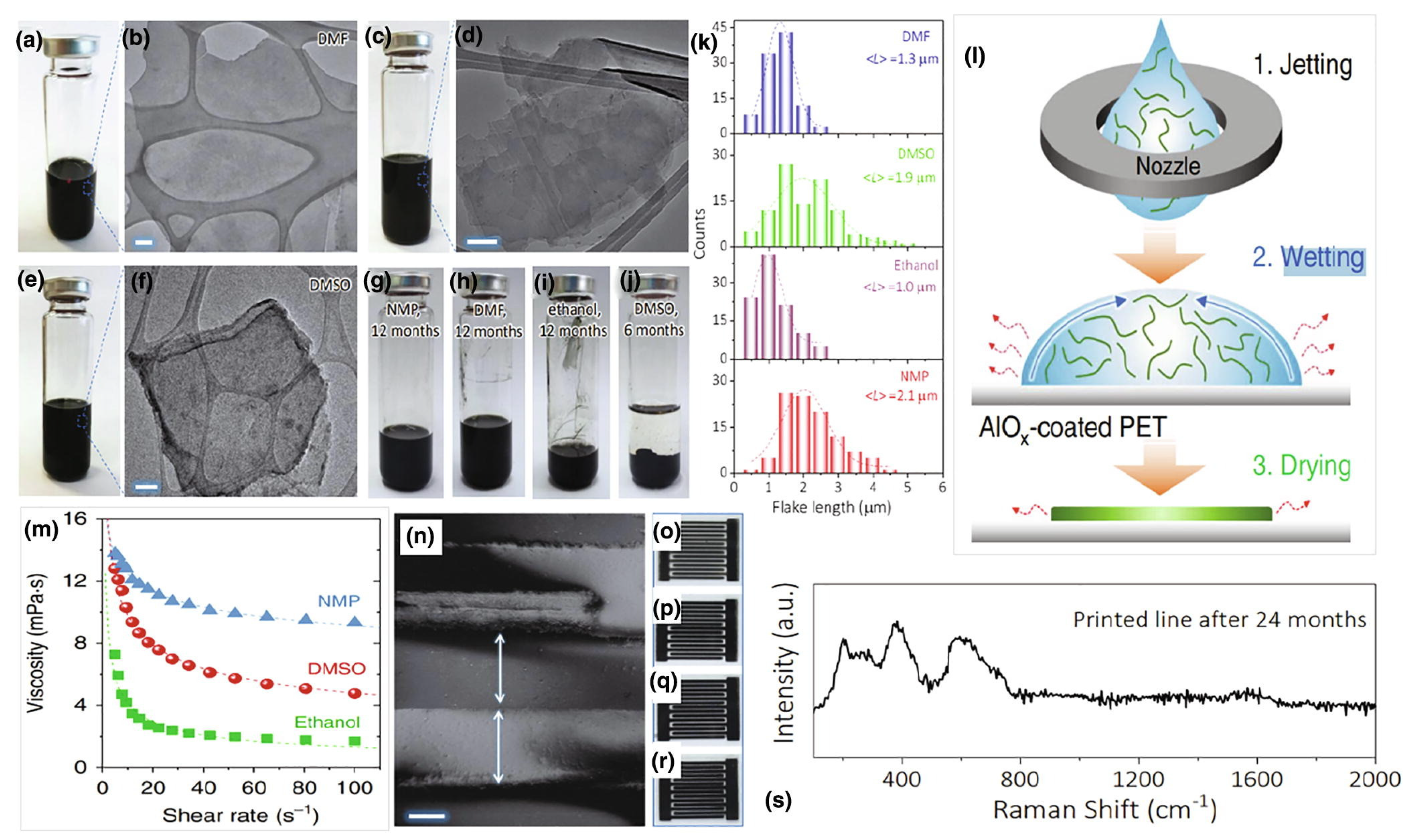
3.3.1. Dispersion Method
3.3.2. Solvent Selection
3.3.3. Additive Incorporation
3.3.4. Deposition Techniques
3.3.5. Drying Techniques
3.4. Environmental Factors
3.4.1. Temperature
3.4.2. Humidity
3.4.3. Other Environmental Factors
4. Approaches for Preventing Sticking of MXenes
4.1. Surface Functionalization and Passivation
4.2. Integration with Polymer Matrices or Carbon Nanomaterials
4.2.1. Polymer Matrices
4.2.2. Carbon Nanomaterials
4.2.3. Hybrid Composites
4.3. Electrode Processing Optimization
5. Conclusions
5.1. Emerging Strategies for Sticking Prevention
5.2. Challenges and Opportunities
5.3. Prospect of MXene-based Supercapacitors
5.4. Recommendations for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, R.; Shan, G.; Hu, M.; Huang, W. Two-dimensional transition metal carbides and/or nitrides (MXenes) and their applications in sensors. Mater. Today Phys. 2021, 21, 100527. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Thakur, N.; Kumar, P.; Sati, D.C.; Neffati, R.; Sharma, P. Recent advances in two-dimensional MXenes for power and smart energy systems. J. Energy Storage 2022, 50, 104604. [Google Scholar] [CrossRef]
- Du, W.; Miao, L.; Song, Z.; Zheng, X.; Lv, Y.; Zhu, D.; Gan, L.; Liu, M. Kinetics-driven design of 3D VN/MXene composite structure for superior zinc storage and charge transfer. J. Power Sources 2022, 536, 231512. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Han, Z.; Bo, Z.; Yan, J.; Cen, K.; Ostrikov, K.K. MXene-Based Electrodes for Supercapacitor Energy Storage. Energy Fuels 2022, 36, 2390–2406. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. MXene-based wearable supercapacitors and their transformative impact on healthcare. Mater. Adv. 2023, 4, 4317–4332. [Google Scholar] [CrossRef]
- Bilibana, M.P. Electrochemical properties of MXenes and applications. Adv. Sens. Energy Mater. 2023, 2, 100080. [Google Scholar] [CrossRef]
- Vattikuti, P.S.V.; Shim, J.; Rosaiah, P.; Mauger, A.; Julien, C.M. Recent Advances and Strategies in MXene-Based Electrodes for Supercapacitors: Applications, Challenges and Future Prospects. Nanomaterials 2024, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Nahirniak, S.; Ray, A.; Saruhan, B. Challenges and Future Prospects of the MXene-Based Materials for Energy Storage Applications. Batteries 2023, 9, 126. [Google Scholar] [CrossRef]
- Hu, C.; Qin, Y.; Song, Z.; Liu, P.; Miao, L.; Duan, H.; Lv, Y.; Xie, L.; Liu, M.; Gan, L. π-Conjugated molecule mediated self-doped hierarchical porous carbons via self-stacking interaction for high-energy and ultra-stable zinc-ion hybrid capacitors. J. Colloid Interface Sci. 2024, 658, 856–864. [Google Scholar] [CrossRef]
- Ren, X.; Liao, G.; Li, Z.; Qiao, H.; Zhang, Y.; Yu, X.; Wang, B.; Tan, H.; Shi, L.; Qi, X.; et al. Two-dimensional MOF and COF nanosheets for next-generation optoelectronic applications. Coord. Chem. Rev. 2021, 435, 213781. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, Z.T.; Sang, D.K.; Han, X.G.; Zhang, H.; Niu, C.M. Current progress in black phosphorus materials and their applications in electrochemical energy storage. Nanoscale 2017, 9, 13384–13403. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, B.; Ge, Y.; Zhu, Y.; Nie, G.; Song, Y.; Lim, C.-K.; Zhang, H.; Prasad, P.N. Chemistry, functionalization, and applications of recent monoelemental two-dimensional materials and their heterostructures. Chem. Rev. 2021, 122, 1127–1207. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, I.-D. Recent developments in 2D nanomaterials for chemiresistive-type gas sensors. Electron. Mater. Lett. 2018, 14, 221–260. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Energy Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Dhanjai; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. TrAC Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, L.; Sun, L.; Qian, Y.; Wang, J.; Li, M. (Cr2/3Ti1/3)3AlC2 and (Cr5/8Ti3/8)4AlC3: New MAX-phase Compounds in Ti–Cr–Al–C System. J. Am. Ceram. Soc. 2014, 97, 67–69. [Google Scholar] [CrossRef]
- Champagne, A.; Charlier, J.-C. Physical properties of 2D MXenes: From a theoretical perspective. J. Phys. Mater. 2020, 3, 032006. [Google Scholar] [CrossRef]
- Wu, Z.; Shang, T.; Deng, Y.; Tao, Y.; Yang, Q. The Assembly of MXenes from 2D to 3D. Adv. Sci. 2020, 7, 1903077. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jiao, T.; Li, M.; Li, Y.; Ma, L.; Mo, F.; Liang, G.; Wang, D.; Wang, Z.; Ruan, Z.; et al. In situ formation of NaTi2(PO4)3 cubes on Ti3C2 MXene for dual-mode sodium storage. J. Mater. Chem. A 2018, 6, 18525–18532. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.-S.; Zheng, S.; Wang, X.; Qin, J.; Wang, S.; Shi, X.; Bao, X. Ti3C2 MXene-derived sodium/potassium titanate nanoribbons for high-performance sodium/potassium ion batteries with enhanced capacities. ACS Nano 2017, 11, 4792–4800. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Shen, J.; Hu, Q.; Nie, W.; Wang, Z.; Zou, L.; Li, C. Vapor phase polymerized conducting polymer/MXene textiles for wearable electronics. Nanoscale 2021, 13, 1832–1841. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, H.; Hu, T.; Fan, B.; Wang, X.; Li, Z. Emerging 2D MXenes for supercapacitors: Status, challenges and prospects. Chem. Soc. Rev. 2020, 49, 6666–6693. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, M.; Huang, Y.; Pei, Z.; Li, H.; Wang, Z.; Xue, Q.; Zhi, C. Multifunctional energy storage and conversion devices. Adv. Mater. 2016, 28, 8344–8364. [Google Scholar] [CrossRef]
- Kar, T.; Godavarthi, S.; Pasha, S.K.; Deshmukh, K.; Martínez-Gómez, L.; Kesarla, M.K. Layered materials and their heterojunctions for supercapacitor applications: A review. Crit. Rev. Solid State Mater. Sci. 2021, 47, 357–388. [Google Scholar] [CrossRef]
- Zhao, M.; Ren, C.E.; Ling, Z.; Lukatskaya, M.R.; Zhang, C.; Van Aken, K.L.; Barsoum, M.W.; Gogotsi, Y. Flexible MXene/carbon nanotube composite paper with high volumetric capacitance. Adv. Mater. 2015, 27, 339–345. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, D.Y.; Kim, H.; Hyeon, J.S.; de Andrade, M.J.; Baughman, R.H.; Kim, S.J. Highly loaded MXene/carbon nanotube yarn electrodes for improved asymmetric supercapacitor performance. MRS Commun. 2019, 9, 114–121. [Google Scholar] [CrossRef]
- Wang, R.; Luo, S.; Xiao, C.; Chen, Z.; Li, H.; Asif, M.; Chan, V.; Liao, K.; Sun, Y. MXene-carbon nanotubes layer-by-layer assembly based on-chip micro-supercapacitor with improved capacitive performance. Electrochim. Acta 2021, 386, 138420. [Google Scholar] [CrossRef]
- Liang, W.; Zhitomirsky, I. MXene–carbon nanotube composite electrodes for high active mass asymmetric supercapacitors. J. Mater. Chem. A 2021, 9, 10335–10344. [Google Scholar] [CrossRef]
- Li, Y.; Pan, C.; Kamdem, P.; Jin, X.-J. Binder-free two-dimensional mxene/acid activated carbon for high-performance super-capacitors and methylene blue adsorption. Energy Fuels 2020, 34, 10120–10130. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Huang, H.; Wang, Z.; Xu, Z.; Zhao, H.; Wang, Y.; Chen, N.; Yang, W. High-voltage asymmetric MXene-based on-chip micro-supercapacitors. Nano Energy 2020, 74, 104928. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Kong, D.; Wang, X.; Li, B.; Cai, T.; Li, X.; Xu, J.; Li, Y.; Yan, Y.; et al. Stimulation of surface terminating group by carbon quantum dots for improving pseudocapacitance of Ti3C2Tx MXene based electrode. Carbon 2021, 180, 118–126. [Google Scholar] [CrossRef]
- Li, L.; Fu, J.; Cho, Y.-R.; Yun, J.M.; Jung, Y.S.; Kwon, S.H.; Kim, K.H. Hierarchically layered nanocomposite electrodes formed by spray-injected MXene nanosheets for ultrahigh-performance flexible supercapacitors. Appl. Surf. Sci. 2021, 549, 149226. [Google Scholar] [CrossRef]
- Li, X.; Ma, Y.; Yue, Y.; Li, G.; Zhang, C.; Cao, M.; Xiong, Y.; Zou, J.; Zhou, Y.; Gao, Y. A flexible Zn-ion hybrid micro-supercapacitor based on MXene anode and V2O5 cathode with high capacitance. Chem. Eng. J. 2022, 428, 130965. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Yan, J.; Ma, Y.; Zhang, C.; Li, X.; Liu, W.; Yao, X.; Yao, S.; Luo, S. Polypyrrole–MXene coated textile-based flexible energy storage device. RSC Adv. 2018, 8, 39742–39748. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Zhang, W.; Zhang, P.; He, W.; Chen, J.; Sun, Z. A multidimensional nanostructural design towards electrochemically stable and mechanically strong hydrogel electrodes. Nanoscale 2020, 12, 6637–6643. [Google Scholar] [CrossRef]
- Fan, Q.; Zhao, R.; Yi, M.; Qi, P.; Chai, C.; Ying, H.; Hao, J. Ti3C2-MXene composite films functionalized with polypyrrole and ionic liquid-based microemulsion particles for supercapacitor applications. Chem. Eng. J. 2020, 428, 131107. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Moncada, J.; Chen, H.; Kayali, E.; Orangi, J.; Carrero, C.A.; Beidaghi, M. Thick and freestanding MXene/PANI pseudocapacitive electrodes with ultrahigh specific capacitance. J. Mater. Chem. A 2018, 6, 22123–22133. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Liu, D.; Li, X.; Xiao, H.; Ma, Y.; Xu, M.; Yuan, G.; Chen, G. Opening MXene ion transport channels by intercalating PANI nanoparticles from the self-assembly approach for high volumetric and areal energy density supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 30633–30642. [Google Scholar] [CrossRef]
- Yuen, A.C.Y.; Chen, T.B.Y.; Lin, B.; Yang, W.; Kabir, I.I.; Cordeiro, I.M.D.C.; Whitten, A.E.; Mata, J.; Yu, B.; Lu, H.-D.; et al. Study of structure morphology and layer thickness of Ti3C2 MXene with Small-Angle Neutron Scattering (SANS). Compos. Part C Open Access 2021, 5, 100155. [Google Scholar] [CrossRef]
- Ye, F.; Xu, B.; Chen, R.; Li, R.; Chang, G. The Influence of Nano- and Micron-size of MXene Flakes on the Electrochemical Performance. Electron. Mater. Lett. 2023, 19, 527–533. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, R.; Cheng, W.; Ji, P.; Sheng, J.; Liao, Q.; Lai, H.; Fu, X.; Zhang, C.; Li, H. Synthesis of Large-Area MXenes with High Yields through Power-Focused Delamination Utilizing Vortex Kinetic Energy. Adv. Sci. 2022, 9, e2202748. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, J.; Gong, C.; Guan, Z.; Yang, D.; Shen, Z.; Yao, W.; Wu, H. Achieving high yield of Ti3C2Tx MXene few-layer flakes with enhanced pseudocapacior performance by decreasing precursor size. Chin. Chem. Lett. 2020, 31, 1039–1043. [Google Scholar] [CrossRef]
- Zeraati, A.S.; Mirkhani, S.A.; Sun, P.; Naguib, M.; Braun, P.V.; Sundararaj, U. Improved synthesis of Ti3C2Tx MXenes resulting in exceptional electrical conductivity, high synthesis yield, and enhanced capacitance. Nanoscale 2021, 13, 3572–3580. [Google Scholar] [CrossRef] [PubMed]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at every step, from their precursors to single flakes and assembled films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, L.; He, X.; Fang, L.; Wang, H.; Zhang, L.; Yu, L.; Zhu, G. Nitrogen-Doped Porous MXene (Ti3C2) for Flexible Supercapacitors with Enhanced Storage Performance. Molecules 2022, 27, 4890. [Google Scholar] [CrossRef]
- Liu, L.Y.; Orbay, M.; Luo, S.; Duluard, S.; Shao, H.; Harmel, J.; Rozier, P.; Taberna, P.L.; Simon, P. Exfoliation and delamination of Ti3C2Tx MXene prepared via molten salt etching route. ACS Nano 2022, 16, 111–118. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, N.; Seo, Y. MXenes: Versatile 2D materials with tailored surface chemistry and diverse applications. J. Energy Chem. 2024, 90, 253–293. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, X.; Li, X.L.; Bai, Y.; Xiao, H.H.; Liu, Y.; Liu, R.; Yuan, G.H. Engineering 3D Ion Transport Channels for Flexible MXene Films with Superior Capacitive Performance. Adv. Funct. Mater. 2019, 29, 1900326. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.-Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.-L.; Barsoum, M.W.; Simon, P.; et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2017, 2, 17105. [Google Scholar] [CrossRef]
- Ji, J.; Zhao, L.; Shen, Y.; Liu, S.; Zhang, Y. Covalent stabilization and functionalization of MXene via silylation reactions with improved surface properties. FlatChem 2019, 17, 100128. [Google Scholar] [CrossRef]
- Mozafari, M.; Soroush, M. Surface functionalization of MXenes. Mater. Adv. 2021, 2, 7277–7307. [Google Scholar] [CrossRef]
- Patra, S.; Kiran, N.U.; Mane, P.; Chakraborty, B.; Besra, L.; Chatterjee, S.; Chatterjee, S. Hydrophobic MXene with enhanced electrical conductivity. Surf. Interfaces 2023, 39, 102969. [Google Scholar] [CrossRef]
- Maleski, K.; Mochalin, V.N.; Gogotsi, Y. Dispersions of Two-Dimensional Titanium Carbide MXene in Organic Solvents. Chem. Mater. 2017, 29, 1632–1640. [Google Scholar] [CrossRef]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.-H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Sun, D.; Zhang, Y.; Liu, B.; Hu, Q.; Zhou, A. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Mater. Sci. Eng. B 2015, 191, 33–40. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Li, Z.; Chen, H. Dynamic simulation and experimental verification of foam transport in porous media based on level set method. Energy Sci. Eng. 2019, 7, 1795–1807. [Google Scholar] [CrossRef]
- Biswas, S.; Alegaonkar, P.S. MXene: Evolutions in Chemical Synthesis and Recent Advances in Applications. Surfaces 2022, 5, 1–34. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Min, B.K.; Yi, Y.; Kim, S.J.; Choi, C.-G. MXene(Ti3C2TX)/graphene/PDMS composites for multifunctional broadband electromagnetic interference shielding skins. Chem. Eng. J. 2020, 393, 124608. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, H.; Li, W.; Liu, G.; Zhang, Y.; Wang, Y. Sandwich-like Co3O4/MXene composites as high capacity electrodes for lithium-ion batteries. New J. Chem. 2020, 44, 5913–5920. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Wang, J.; Yang, Y.; Cao, Y.; Wang, W. New application of MXene in polymer composites toward remarkable anti-dripping performance for flame retardancy. Compos. Part A Appl. Sci. Manuf. 2019, 127, 105649. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, H.-B.; Liu, J.; Xie, X.; Yang, R.; Li, Y.; Hong, S.; Yu, Z.-Z. Highly Conductive Transition Metal Car-bide/Carbonitride (MXene)@polystyrene Nanocomposites Fabricated by Electrostatic Assembly for Highly Efficient Electro-magnetic Interference Shielding. Adv. Funct. Mater. 2017, 27, 1702807. [Google Scholar] [CrossRef]
- Redondo, E.; Pumera, M. MXene-functionalised 3D-printed electrodes for electrochemical capacitors. Electrochem. Commun. 2021, 124, 106920. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Dong, S.; Yang, J.; Liu, Y. Template-Free Synthesized 3D Macroporous MXene with Superior Performance for Supercapacitors. Appl. Mater. Today 2019, 16, 315–321. [Google Scholar] [CrossRef]
- Tseluikin, V.; Dzhumieva, A.; Tribis, A.; Tikhonov, D.; Tsyganov, A.; Gorshkov, N.; Lopukhova, M. Study of Electrodeposition and Properties of Composite Nickel Coatings Modified with Ti3C2TX MXene. Coatings 2023, 13, 1042. [Google Scholar] [CrossRef]
- Hu, G.; Yang, L.; Yang, Z.; Wang, Y.; Jin, X.; Dai, J.; Wu, Q.; Liu, S.; Zhu, X.; Wang, X.; et al. A general ink formulation of 2D crystals for wafer-scale inkjet printing. Sci. Adv. 2020, 6, eaba5029. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Jiang, X.; Zhang, H.; Qiu, J.; Zhang, C. Perspectives on solution processing of two-dimensional MXenes. Mater. Today 2021, 48, 214–240. [Google Scholar] [CrossRef]
- Iqbal, A.; Hong, J.; Ko, T.Y.; Koo, C.M. Improving oxidation stability of 2D MXenes: Synthesis, storage media, and conditions. Nano Converg. 2021, 8, 9. [Google Scholar] [CrossRef]
- Liu, L.; Ying, G.; Hu, C.; Zhang, K.; Ma, F.; Su, L.; Zhang, C.; Wang, C. Functionalization with MXene (Ti3C2) Enhances the Wettability and Shear Strength of Carbon Fiber-Epoxy Composites. ACS Appl. Nano Mater. 2019, 2, 5553–5562. [Google Scholar] [CrossRef]
- Ding, R.; Sun, Y.; Lee, J.; Nam, J.-D.; Suhr, J. Enhancing Interfacial Properties of Carbon Fiber Reinforced Epoxy Composites by Grafting MXene Sheets (Ti2C). Compos. Part B Eng. 2021, 207, 108580. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, G.; Hou, K.; Wang, H.; Wang, N.; Yang, S.; Wang, J. Environmentally-Adaptive Epoxy Lubricating Coating Using Self-Assembled PMXene@polytetrafluoroethylene Core-Shell Hybrid as Novel Additive. Carbon 2021, 184, 12–23. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Cao, M.; Shen, X.; Yang, Y.; Yi, J.; Guan, J.; Shen, J.; Xi, M.; Zhang, Y.; et al. Tribological and Thermo-Mechanical Properties of TiO2 Nanodot-Decorated Ti3C2/Epoxy Nanocomposites. Materials 2021, 14, 2509. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Jiang, Q.; Zhang, X.; Alshareef, H.N. Large Dielectric Constant Enhancement in MXene Percolative Polymer Composites. ACS Nano 2018, 12, 3369–3377. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Zhang, J.; Zhao, X.; Kong, N.; Tao, J.; Zhou, J. Understanding the Effect of Pore Size on Electrochemical Capacitive Performance of MXene Foams. Nano Micro Small 2022, 18, e2202203. [Google Scholar] [CrossRef] [PubMed]
- Ankitha, M.; Shabana, N.; Arjun, A.M.; Muhsin, P.; Rasheed, P.A. Ultrasensitive electrochemical detection of dopamine from human serum samples by Nb2CTx-MoS2 hetero structures. Microchem. J. 2023, 187, 108424. [Google Scholar] [CrossRef]
- Hemanth, N.R.; Kim, T.; Kim, B.; Jadhav, A.H. Kwangyeol Lee and Nitin K. Chaudhari, Transition metal dichalco-genide-decorated MXenes: Promising hybrid electrodes for energy storage and conversion applications. Mater. Chem. Front. 2021, 5, 3298–3321. [Google Scholar] [CrossRef]
- Natu, V.; Sokol, M.; Verger, L.; Barsoum, M.W. Effect of edge charges on stability and aggregation of Ti3C2Tz MXene colloidal suspensions. J. Phys. Chem. C 2018, 122, 27745–27753. [Google Scholar] [CrossRef]
- Greaves, M.; Mende, M.; Wang, J.; Yang, W.; Barg, S. Investigating the rheology of 2D titanium carbide (MXene) dispersions for colloidal processing: Progress and challenges. J. Mater. Res. 2021, 36, 4578–4600. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Heier, J.; Zhang, C. Printing and coating MXenes for electrochemical energy storage devices. J. Phys. Energy 2020, 2, 31004. [Google Scholar] [CrossRef]
- Kosnan, M.A.; Azam, M.A.; Safie, N.E.; Munawar, R.F.; Takasaki, A. Recent Progress of Electrode Architecture for MXene/MoS2 Supercapacitor: Preparation Methods and Characterizations. Micromachines 2022, 13, 1837. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Li, H.; Lin, S.; Ding, W.; Zhu, X.; Sheng, Z.; Wang, H.; Zhu, X.; Sun, Y. 2D/2D 1T-MoS2/Ti3C2 MXene Hetero-structure with Excellent Supercapacitor Performance. Adv. Funct. Mater. 2020, 30, 0190302. [Google Scholar] [CrossRef]
- Zhang, H.; He, H.; Huang, Y.; Pu, S.; Xie, Y.; Huang, J.; Jiang, X.; Wang, Y.; Wang, S.; Peng, H.; et al. Simultaneously addressing self-stacking and oxidative degradation issues of Ti3C2Tx MXene through biothermochemistry induced 3D cross-linking. Appl. Surf. Sci. 2023, 639, 158183. [Google Scholar] [CrossRef]
- Peng, M.; Wang, L.; Li, L.; Tang, X.; Huang, B.; Hu, T.; Yuan, K.; Chen, Y. Manipulating the Interlayer Spacing of 3D MXenes with Improved Stability and Zinc-Ion Storage Capability. Adv. Funct. Mater. 2022, 32, 2109524. [Google Scholar] [CrossRef]
- Su, T.; Ma, X.; Tong, J.; Ji, H.; Qin, Z.; Wu, Z. Surface engineering of MXenes for energy and environmental applications. J. Mater. Chem. A 2022, 10, 10265–10296. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, L.; Xiang, S.; Yu, S.; Johnson, H.M.; Wang, S.; Yin, J.; Zhao, J.; Luo, Y.; Chu, P.K. Unleashing the Potential of MXene-Based Flexible Materials for High-Performance Energy Storage Devices. Adv. Sci. 2024, 11, e2304874. [Google Scholar] [CrossRef] [PubMed]
- Ahaliabadeh, Z.; Kong, X.; Fedorovskaya, E.; Kallio, T. Extensive comparison of doping and coating strategies for Ni-rich positive electrode materials. J. Power Sources 2022, 540, 231633. [Google Scholar] [CrossRef]
- Parveena, S.; Cochran, E.W.; Zulfiqar, S.; Amin, M.A.; Warsi, M.F.; Chaudhary, K. Iron/vanadium co-doped tungsten oxide nanostructures anchored on graphitic carbon nitride sheets (FeV-WO3@g-C3N4) as a cost-effective novel electrode material for advanced supercapacitor applications. RSC Adv. 2023, 13, 26822–26838. [Google Scholar] [CrossRef]
- Qiu, J.; Duan, Y.; Li, S.; Zhao, H.; Ma, W.; Shi, W.; Lei, Y. Insights into Nano- and Micro-Structured Scaffolds for Advanced Electrochemical Energy Storage. Nano-Micro Lett. 2024, 16, 130. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, B.; Kaur, D. Achieving enhanced pseudocapacitance in MoS2 nanowires rationally sputtered over NiMnIn shape memory alloy for flexible Na-ion supercapacitor. J. Energy Storage 2023, 71, 231633. [Google Scholar] [CrossRef]
- Aldhaher, A.; Rabiee, N.; Iravani, S. Exploring the synergistic potential of MXene-MOF hybrid composites: A perspective on synth esis, properties, and applications. Hybrid Adv. 2024, 5, 100131. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Yadav, R.M. Graphene-metal oxide hybrid materials with 2D and 3D morphologies for advanced supercapacitor electrodes: Status, challenges and prospects. Mater. Today Nano 2023, 24, 100399. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, Y.; Huang, H.; Jiang, Q. Chemically functionalized two-dimensional titanium carbide MXene by in situ grafting-intercalating with diazonium ions to enhance supercapacitive performance. J. Phys. Chem. Solids 2018, 115, 172–179. [Google Scholar] [CrossRef]
- Khalafallah, D.; Li, X.; Zhi, M.; Hong, Z. Nanostructuring Nickel–Zinc–Boron/Graphitic Carbon Nitride as the Positive Electrode and BiVO4-Immobilized Nitrogen-Doped Defective Carbon as the Negative Electrode for Asymmetric Capacitors. ACS Appl. Nano Mater. 2021, 4, 14258–14273. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Y.; Cheng, L.; Guo, S.; Liu, T.; Hu, Z.; Yu, H.; Chen, D.; Li, Y.; Yuan, H. Structure design and assembly mode of carbon nanotube-based flexible electrode materials and flexible supercapacitors. J. Energy Storage 2023, 73, 109179. [Google Scholar] [CrossRef]
- Venkateshalu, S.; Tomboc, G.R.M.; Nagalingam, S.P.; Kim, J.; Sawaira, T.; Sehar, K.; Pollet, B.G.; Kim, J.Y.; Grace, A.N.; Lee, K. Synergistic MXene/LDH heterostructures with extensive interfacing as emerging energy conversion and storage materials. J. Mater. Chem. A 2023, 11, 14469–14488. [Google Scholar] [CrossRef]
- Wang, G.; Liang, R.; Liu, L.; Zhong, B. Improving the specific capacitance of carbon nanotubes-based supercapacitors by combining introducing functional groups on carbon nanotubes with using redox-active electrolyte. Electrochim. Acta 2014, 115, 183–188. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, J.; Sanchez, J.S.; Xia, Z.; Xiao, L.; Chen, R.; Palermo, V. Surface chemistry and structure manipulation of graphene-related materials to address the challenges of electrochemical energy storage. Chem. Commun. 2023, 59, 2571–2583. [Google Scholar] [CrossRef]
- You, L.; Liu, B.; Hua, H.; Jiang, H.; Yin, C.; Wen, F. Energy Storage Performance of Polymer-Based Dielectric Composites with Two-Dimensional Fillers. Nanomaterials 2023, 13, 2842. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Y.; Fu, Z.; Su, B.-L. Hierarchically structured porous materials: Synthesis strategies and applications in energy storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [Google Scholar] [CrossRef]
- Kim, J.-M.; Zhang, X.; Zhang, J.-G.; Manthiram, A.; Meng, Y.S.; Xu, W. A review on the stability and surface modification of layered transition-metal oxide cathodes. Mater. Today 2021, 46, 155–182. [Google Scholar] [CrossRef]
- Ge, K.; Shao, H.; Taberna, P.-L.; Simon, P. Understanding Ion Charging Dynamics in Nanoporous Carbons for Electrochemical Double Layer Capacitor Applications. ACS Energy Lett. 2023, 8, 2738–2745. [Google Scholar] [CrossRef]
- Fajardo, G.J.P.; Belekoukia, M.; Bolloju, S.; Fiamegkou, E.; Menon, A.S.; Ruff, Z.; Shen, Z.; Shah, N.; Björklund, E.; Zuba, M.J.; et al. Understanding improved capacity retention at 4.3 V in modified single crystal Ni-rich NMC//graphite pouch cells at elevated temperature. RSC Appl. Interfaces 2024, 1, 133–146. [Google Scholar] [CrossRef]
- Lovett, A.J.; Daramalla, V.; Nayak, D.; Sayed, F.; Mahadevegowda, A.; Ducati, C.; Spencer, B.; Dutton, S.; Grey, C.P.; Driscoll, J.L. Vertically Aligned Nanocomposite Thin Films Incorporating 3D-Architectures for Micro-Battery Applications. ECS Meet. Abstr. 2023, 244, 3079. [Google Scholar] [CrossRef]
- Liu, L.; Raymundo-Piñero, E.; Sunny, S.; Taberna, P.L.; Simon, P. Role of Surface Terminations for Charge Storage of Ti3C2T x MXene Electrodes in Aqueous Acidic Electrolyte. Angew. Chem. Int. Ed. 2024, e202319238. [Google Scholar] [CrossRef]
- Misra, S.; A Hachtel, J.; Boebinger, M.G.; Muraleedharan, M.G.; Konečná, A.; Mathis, T.S.; Kent, P.R.C.; Naguib, M.; Gogotsi, Y.; Unocic, R.R. Understanding the Surface Chemistry Dependent Plasmon Response in Ti3C2Tx MXenes using Monochromated STEM-EELS. Microsc. Microanal. 2022, 28 (Suppl. S1), 2460–2461. [Google Scholar] [CrossRef]
- Hassan, O.; Franklin, G.F.; Simon, P.; Dubois, L.; Taberna, P.-L.; Duclairoir, F. Pillared Graphene for Supercapacitor Applications. ECS Meet. Abstr. 2023, 244, 994. [Google Scholar] [CrossRef]


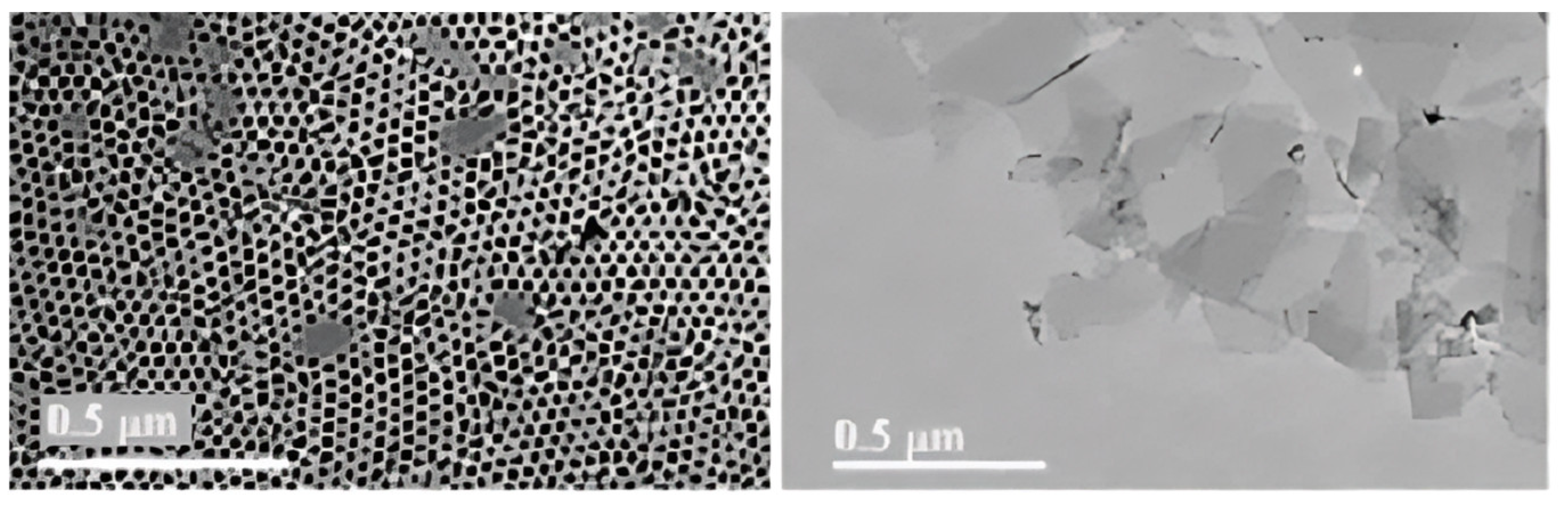
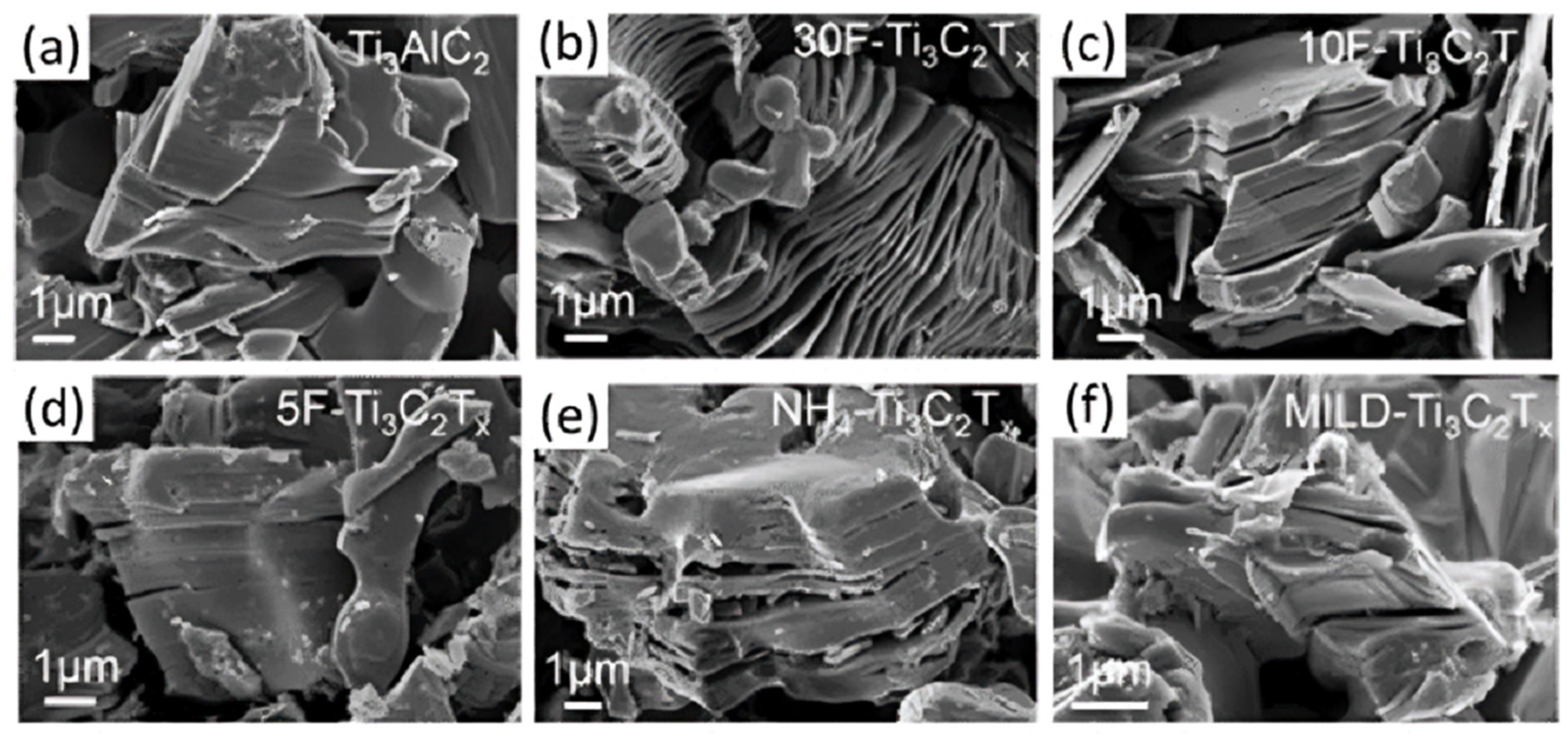
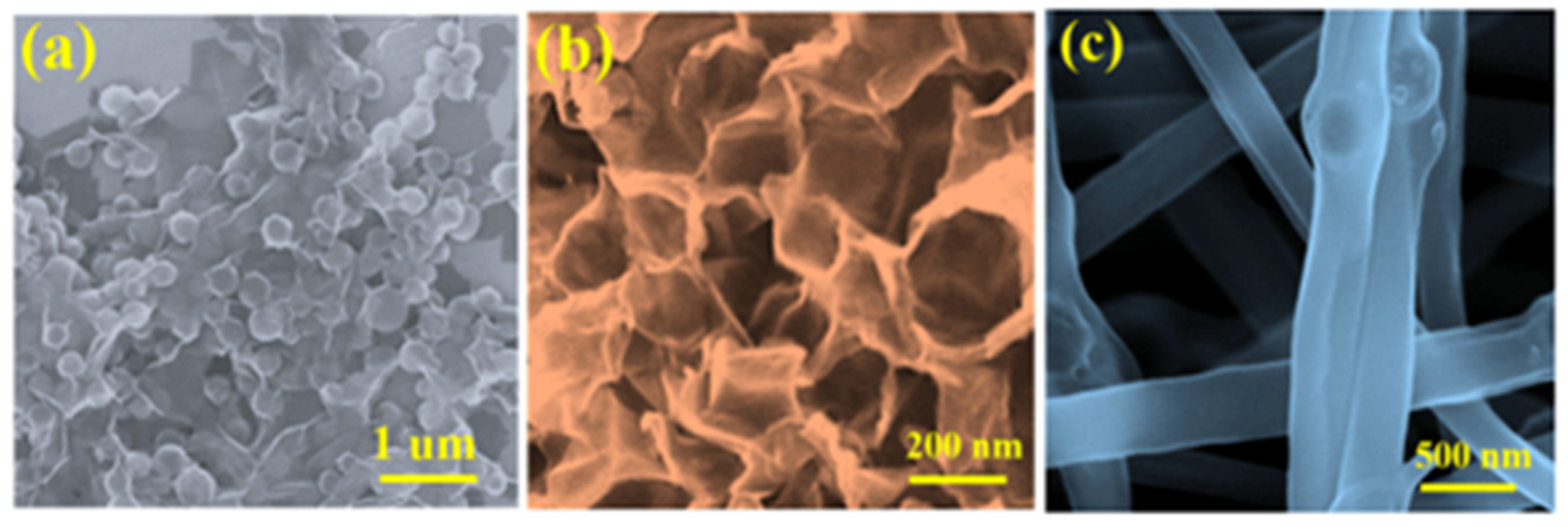

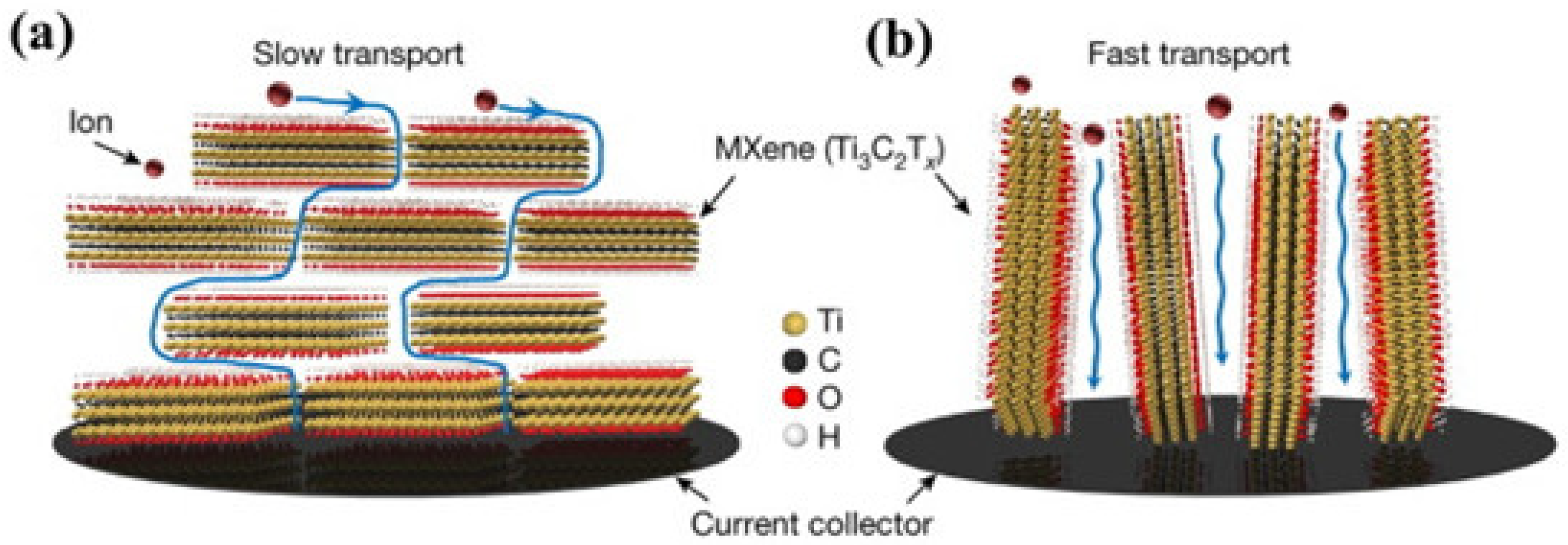
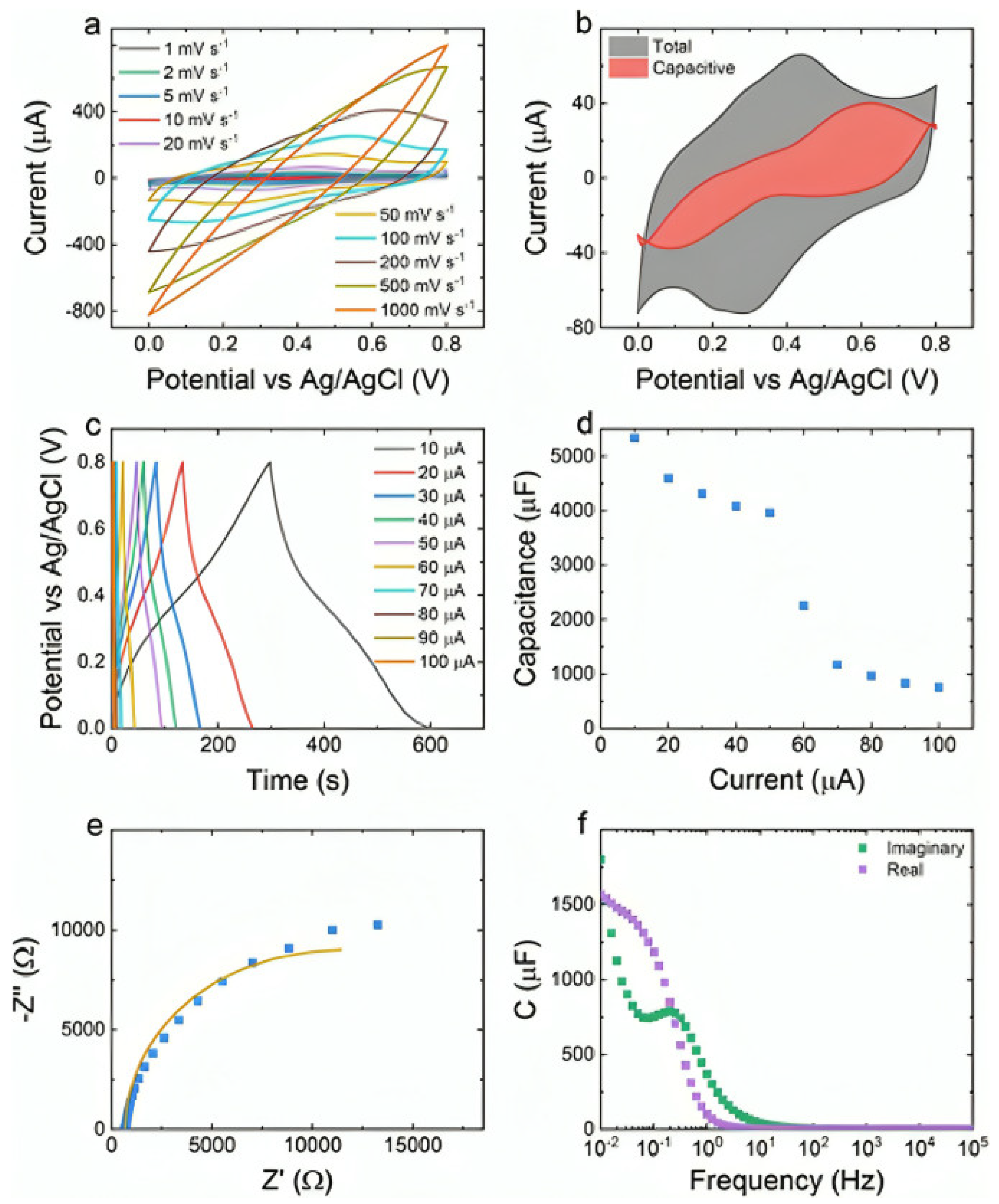


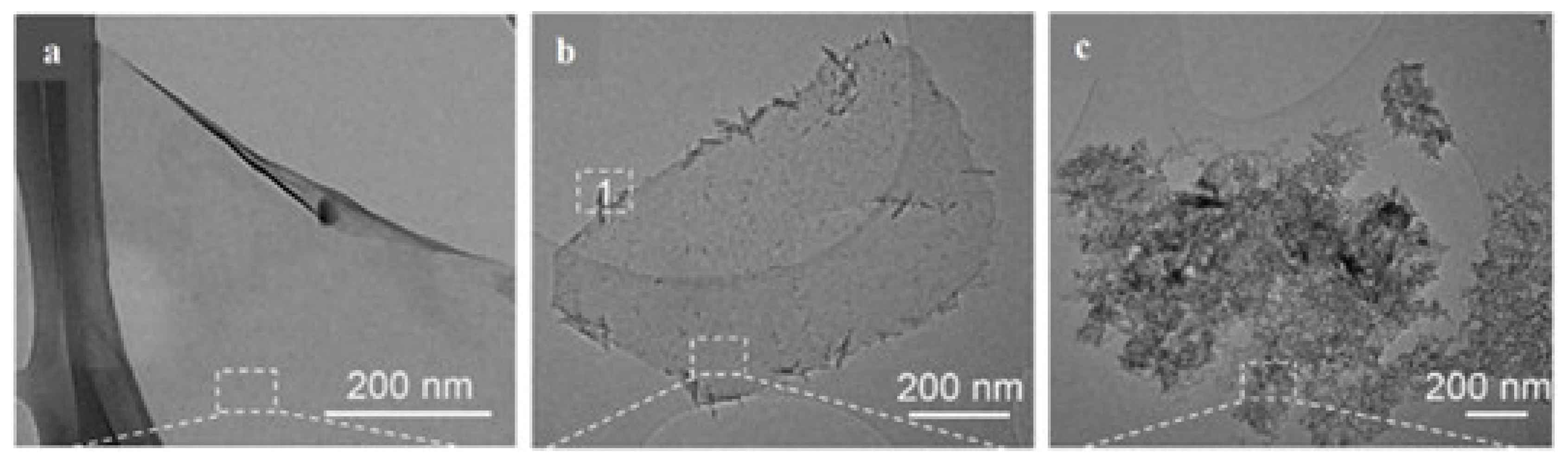
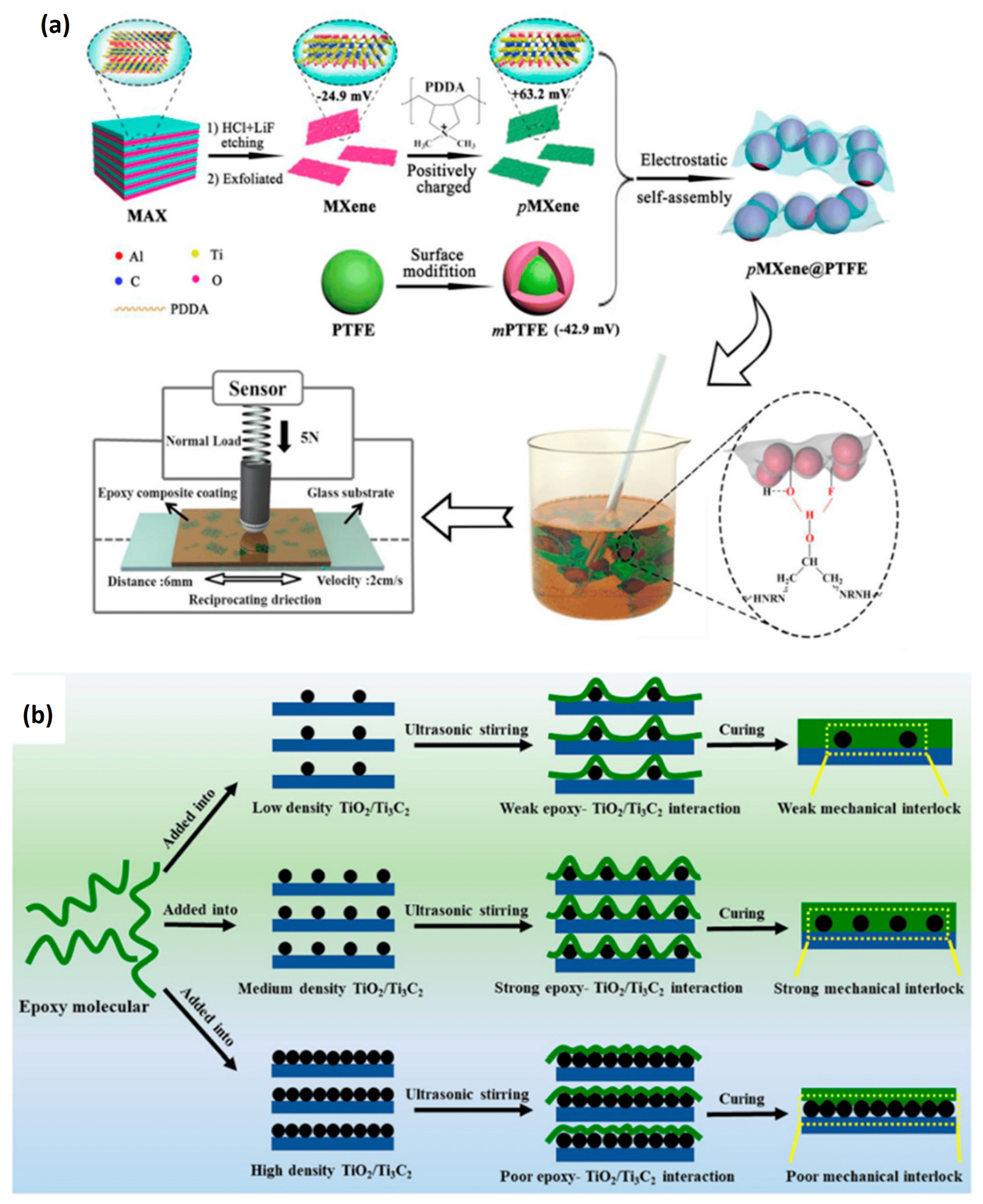

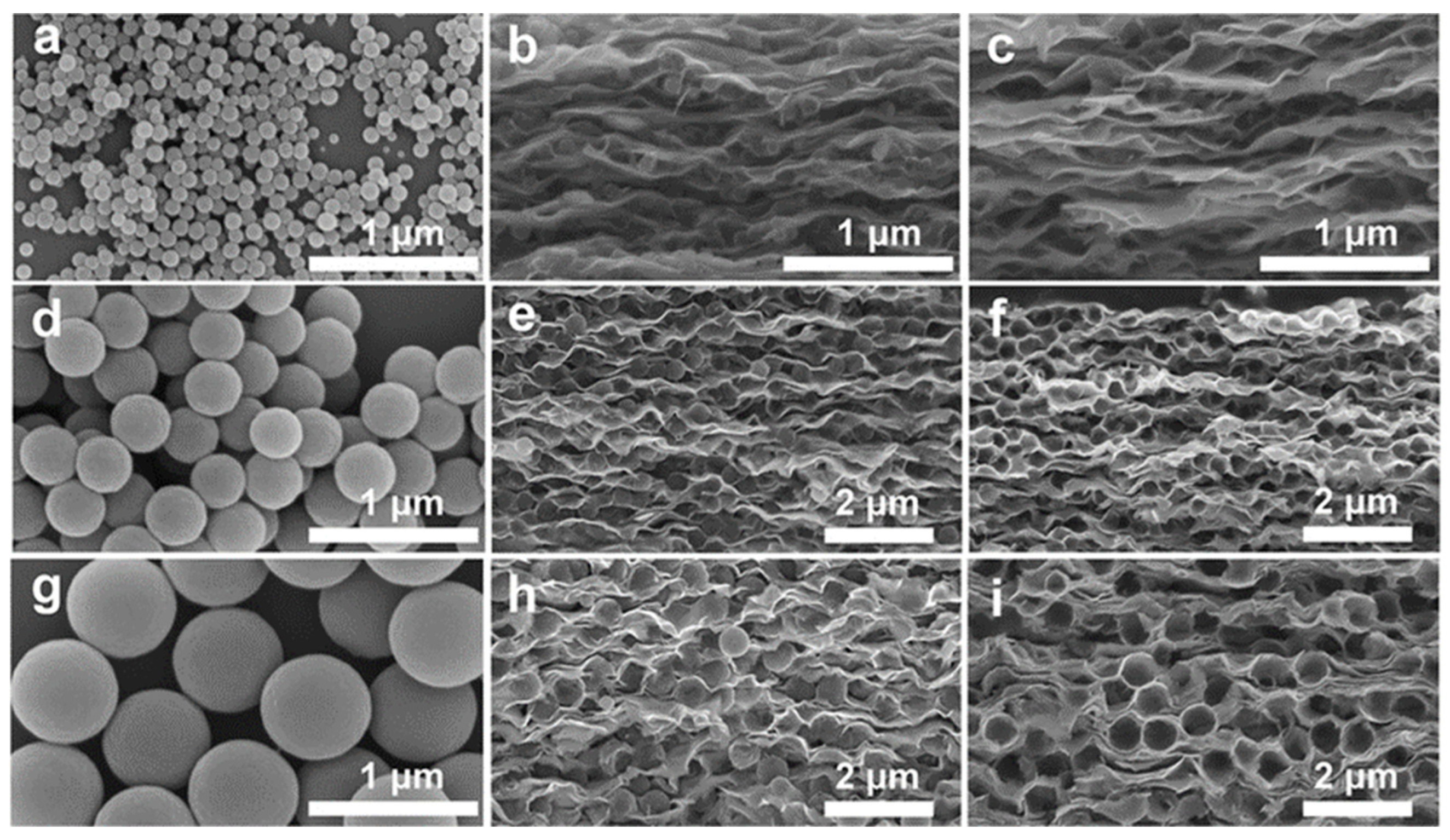
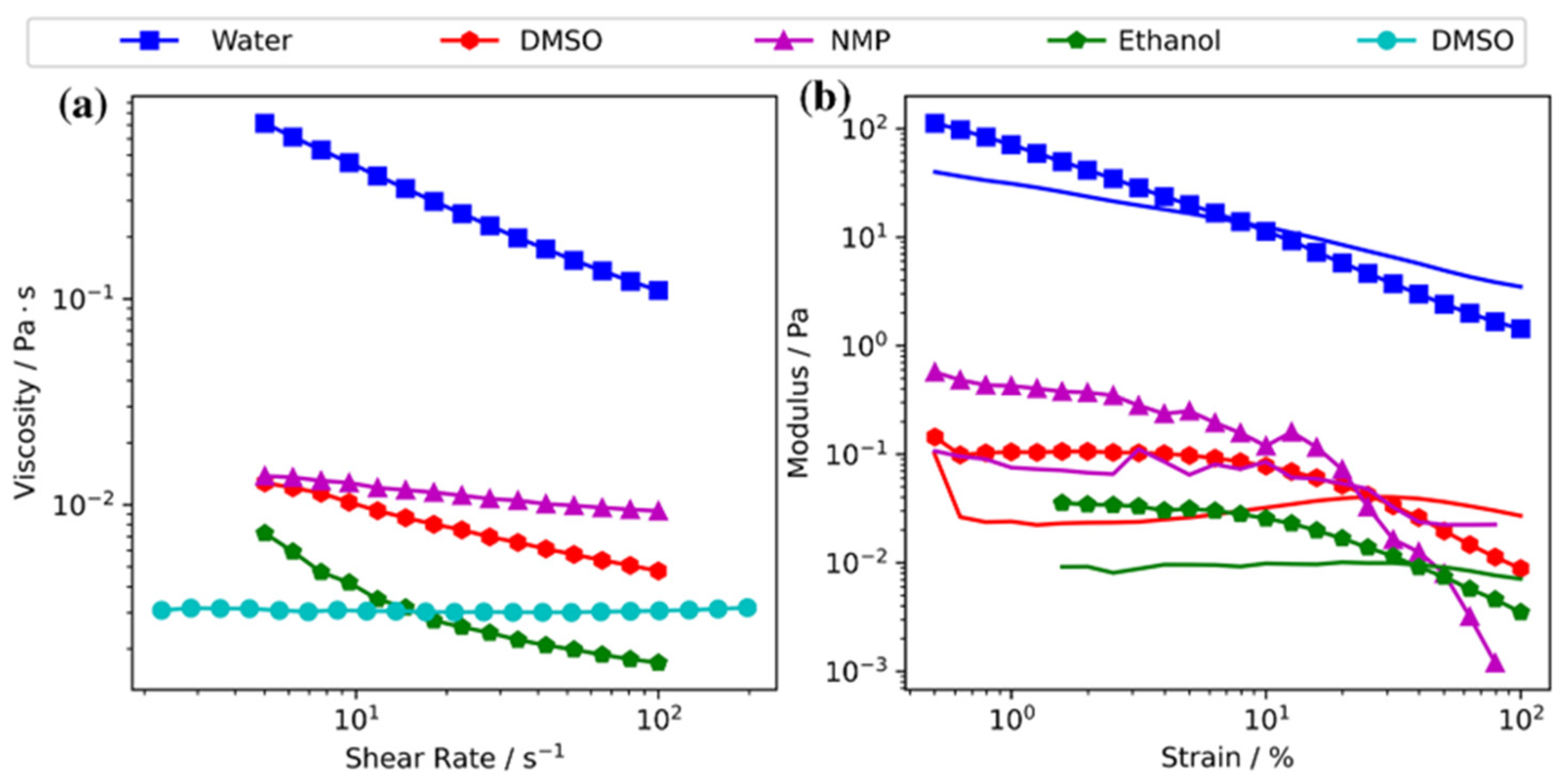
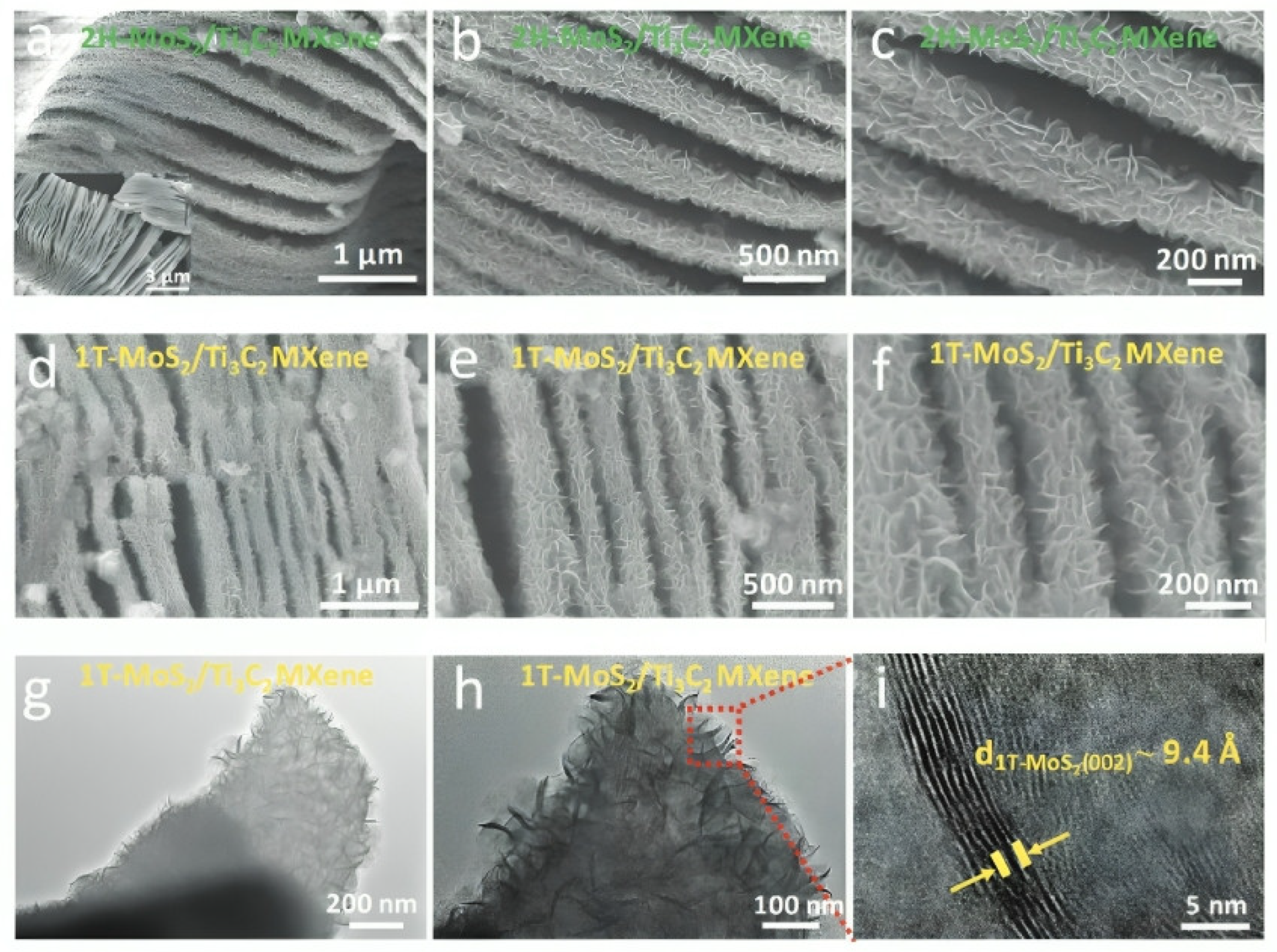
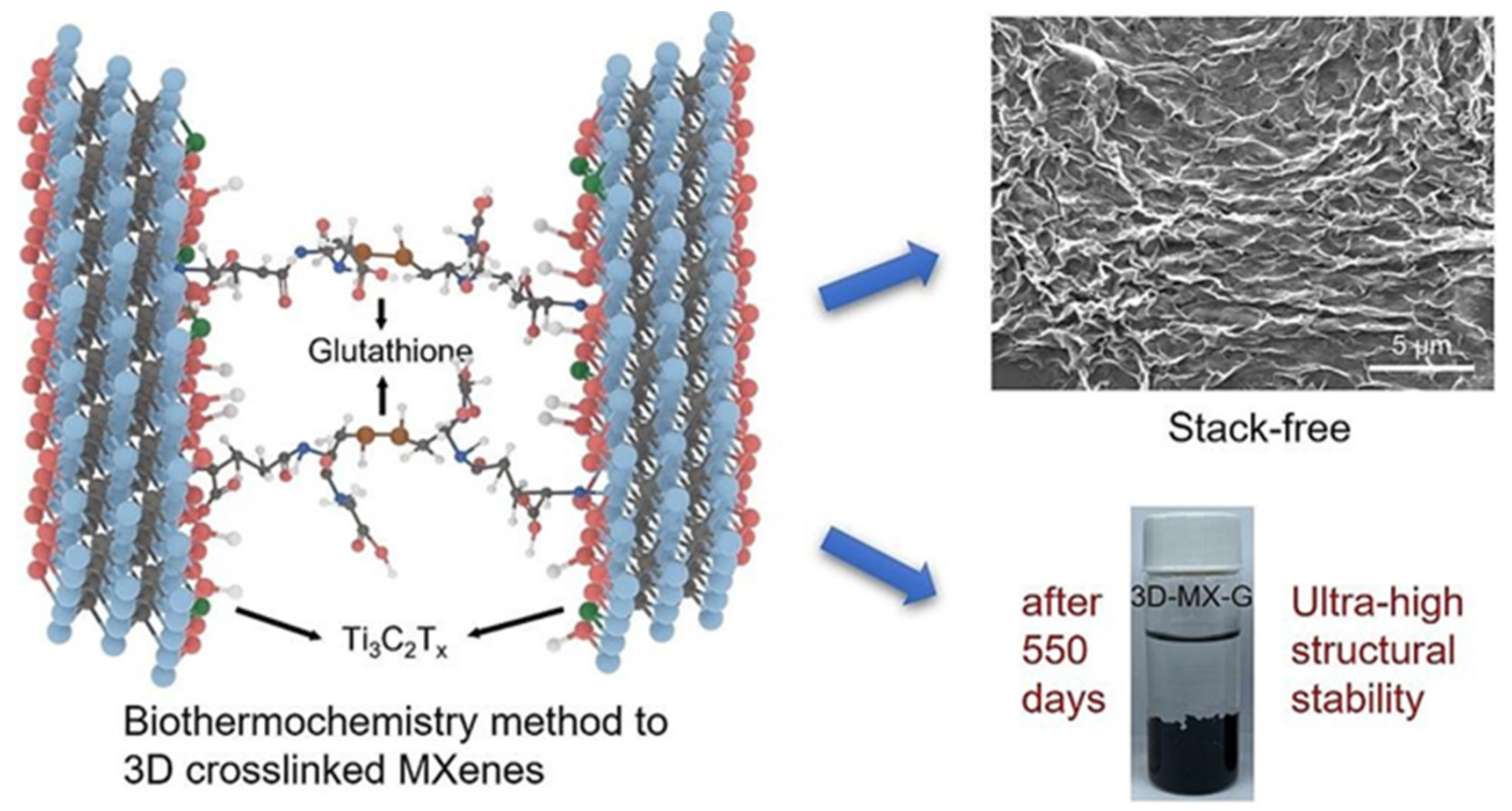
| Strategy | Performance Metrics | Merits | Demerits | Refs. |
|---|---|---|---|---|
| MXene coating | High specific capacitance | Improved electrical conductivity; enhanced ion accessibility | Potential issues with scalability; stability concerns | [87,88] |
| Doping | Fast charge/discharge rates | Increased specific capacitance; improved cycling stability | Dependent on choice of dopant; cost implications | [89,90] |
| Nanostructuring | High energy density | Enhanced surface area; improved electrolyte penetration | Complexity in fabrication; potential loss of mechanical strength | [91,92] |
| Hybrid materials | Long-term cycling stability | Combined benefits of different materials; complementary properties | Synthesis challenges; compatibility issues | [93,94] |
| Material | Stackability Characteristics | Factors Influencing Nanosheet Cohesion | Impact on Supercapacitor Performance | Refs. |
|---|---|---|---|---|
| MXene coating | Potential issues with stackability due to strong inter-particle bonds | Coating stability, uniformity, and adhesion to substrates | Influence on capacitance, electrical properties, and overall stability | [95] |
| Doping | Reliability of nanosheet stacking dependent on dopant choice | Integration of dopants, impact on nanosheet interactions | Effects on capacitance, cycling stability, and cost implications | [96] |
| Nanostructuring | Challenges in stacking during fabrication; potential impact on strength | Complexity of fabrication process, nanosheet alignment | Relationship to energy density, surface area enhancement, and electrolyte penetration | [97] |
| Hybrid materials | Stackability influenced by material compatibility and synthesis issues | Compatibility between materials, stacking interactions | Impact on long-term stability, leveraging diverse material benefits | [98] |
| MXene Strategy for Enhancing Storage Device Performance | Performance Metrics | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Surface functionalization | Enhanced specific capacitance | Improved wettability; increased ion accessibility | Potential process complexity; stability concerns | [99,100] |
| Nanocomposites | Higher energy density | Synergistic effects with additives; enhanced structural stability | Challenges in achieving homogeneous dispersion; synthesis complexity | [101] |
| Templated growth | Improved rate capability | Controlled morphology; facilitates ion transport pathways | Limited scalability; template removal process | [102] |
| Surface modification | Enhanced cycling stability | Improved conductivity; resistance to electrolyte-induced degradation | Reversibility issues; potential impact on mechanical properties | [103] |
| Sticking Prevention Approach | Effects on Supercapacitor Metrics | Merits | Demerits | Refs. |
|---|---|---|---|---|
| Surface coating | Improved specific capacitance | Enhanced stability; prevention of restacking | Potential impact on electrical conductivity; additional processing steps | [104] |
| Functional spacer molecules | Enhanced energy density | Effective prevention of restacking; promotes ion diffusion | Complexity in molecule design; cost implications | [105,106] |
| Intercalation | Increased rate capability | Facilitates ion transport; enhances cycling stability | Dependency on guest species; potential structural distortions | [107] |
| Surface roughening | Improved electrolyte accessibility | Enhanced active sites; prevents restacking issues | Need for precise control in surface modification; potential structural changes | [108] |
| Nanostructure design | Increased surface area; improved electrical conductivity | Enhanced ion accessibility; structural stability | Fabrication complexity; potential performance variability | [109] |
| Sticking Prevention Approach | Synthesis Complexity | Environmental Impact | Long-Term Stability | Cost Analysis |
|---|---|---|---|---|
| Surface coating | Moderate | Low impact | Good stability observed | Medium |
| Functional spacer molecules | Moderate | Low impact | Stable cycling behavior | High |
| Intercalation | High | Moderate impact | Enhanced life cycle | High |
| Surface roughening | High | Moderate impact | Impact on long-term performance | Medium |
| Nanostructure design | High | High impact | Improved durability | High |
| MXene Composite | Application/Enhancement | Key Findings |
|---|---|---|
| MXene–Carbon Nanotube (CNT) and MXene–Reduced Graphene Oxide (rGO) | Improved volumetric capacitance | Sandwich-like structure improved volumetric capacitance up to 350 F cm−3 at 5 A g−1; MXene–rGO composite demonstrated volumetric capacitance of 435 F cm3 with cycling stability [27]. |
| Asymmetric yarn supercapacitor | Achieved high energy density | Utilized MXene–CNT anode, MnO2/CNT cathode, and achieved an energy density of 100 mWh cm2 [28]. |
| Ti3C2Tx MXene–CNT composite | High areal capacitance | Fabricated through photolithography and vacuum filtration, achieving areal capacitance of 61.38 mF cm−2 at 0.5 mA cm−2 [29]. |
| Ti3C2Tx MXene–Multiwalled CNT (MCNT) composite | Demonstrated high areal capacitance | Asymmetric supercapacitors exhibited areal capacitance of 0.94 F cm−2 with Na2SO4 electrolyte [30]. |
| MXene–carbon allotropes (AAC) | Preventing aggregation, enhanced electrochemical performance | Asymmetric supercapacitor with MXene–AAC (2:1) achieved specific capacitance of 177 F g−1 at 0.5 A g−1 with 97.4% retention over 10,000 cycles [31]; micro supercapacitor with MXene as cathode and activated carbon as anode showed areal capacitance of 7.8 mF cm−2, energy density of 3.5 mWh cm−3, and power density of 100 mW cm−3 [32]. |
| MXene–carbon quantum dots (CQD) | Superior capacitance | Ti3C2Tx MXene–CQD composite electrode demonstrated capacitance of 441.3 F g−1 at 1 A g−1 with 100% retention after 10,000 cycles [33]. |
| Ti3C2Tx/NiCO2S4 @ CC composite (TNSC) | Enhanced gravimetric capacitance, cyclic stability | Gravimetric capacitance of 2326 F g−1 at 1 A g−1 with cyclic stability of 93.8% at 10 A g−1, resulting in quasi-solid-state flexible supercapacitor [34]. |
| Zinc ion hybrid micro supercapacitors | Utilizing hydrogel electrolytes for improved performance | Constructed with V2O5 cathode and Ti3C2Tx MXene anode in PAM hydrogel electrolyte, offering 129 mF cm−2 areal capacitance and 48.9 mWh cm−2 energy density with 77% capacitance retention over 10,000 cycles [35]. |
| MXene–Polymer Composites | Application/Enhancement | Key Findings |
| MXene–polypyrrole (PPy) composite | Enhanced specific capacitance | Electrodeposited composite (PPy) on MXene textile electrodes resulted in specific capacitance of 343.20 F g−1 compared to pristine MXene electrodes [37]. |
| MXene–PPy composite | Improved tensile strength, specific capacitance | Specific capacitance of 614 F g−1 at 1 A g−1, retaining 100% capacitance over 10,000 cycles [38]. |
| PPy–MXene–IL–mic composite | Enhanced energy density, capacitance retention | Energy density of 31.2 Wh kg−1, capacitance retention of 91% over 2000 cycles and 91% coulombic efficiency across temperature range of 4 °C to 50 °C [39]. |
| MXene–Polyaniline (PANI) composite | Volumetric and gravimetric capacitance improvement | Synthesized composite exhibited volumetric capacitance of 1682 F cm−3 and gravimetric capacitance of 503 F g−1, with specific capacitance reaching 336 F g−1 with 98.3% capacitance retention over 10,000 cycles [40]. |
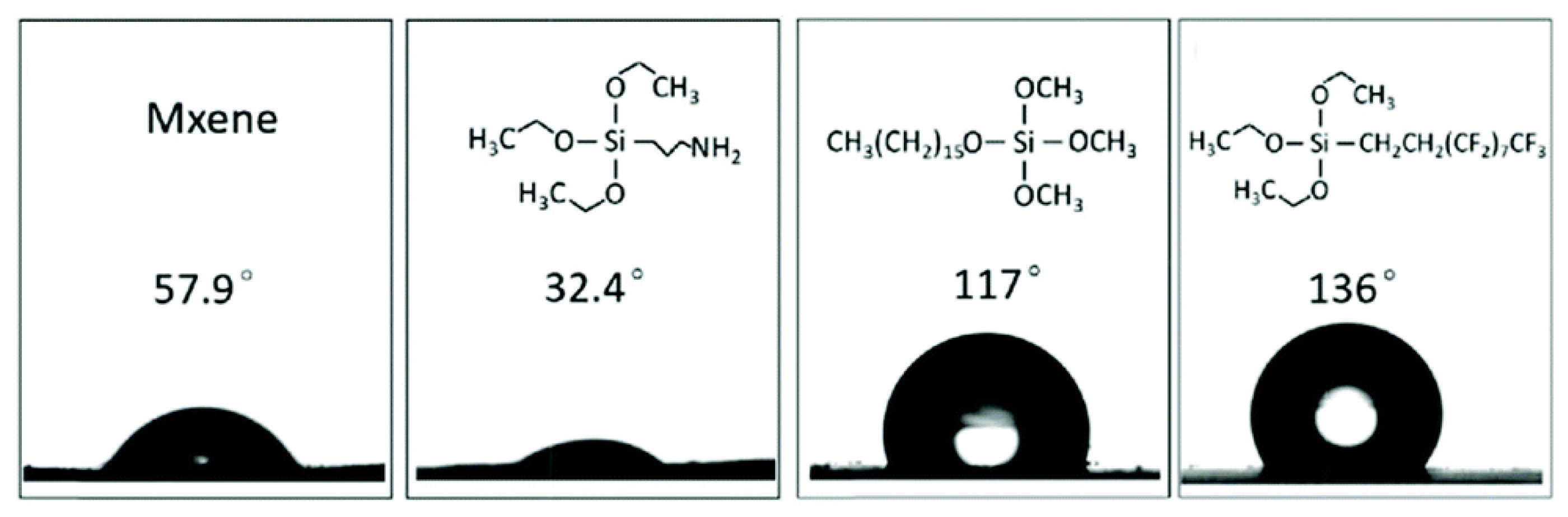
| Functionalization with silylation reagents | Tailoring surface wetting properties | Functionalization with silylation agents can modify MXene flake hydrophobicity, hindering water contact and promoting hydrophobic characteristics [54]. |
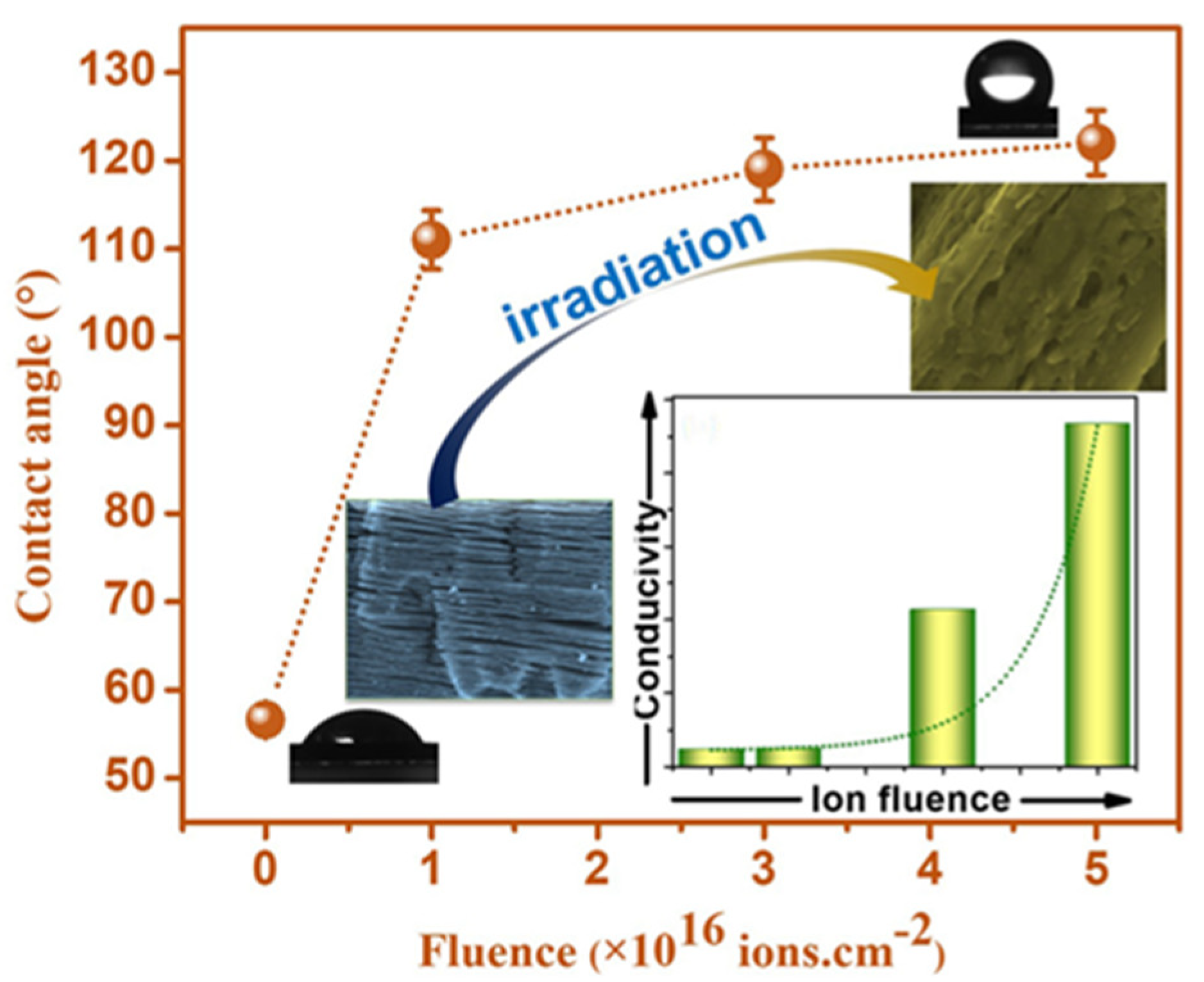
| Ion irradiation for hydrophobic transformation | Altering surface chemistry to hydrophobic nature | N+ ion irradiation method selectively eliminates surface-terminating groups, leading to a hydrophobic nature in MXenes, enhancing electrical conductivity [58]. |
| Additive manufacturing for electrode fabrication | Precise control over electrode architecture | Electrochemically activating 3D-printed nanocarbon electrodes and functionalizing with Ti3C2 demonstrated enhanced electrochemical capacitance in Ti3C2@3DnCEs [68]. |
| Surface functionalization and passivation | Enhancement of interfacial shear strength in carbon fibers; Improved mechanical engagement in epoxy resin matrix | Functionalization of acid-treated carbon fiber using Ti3C2TX led to a 186% increase in interfacial shear strength. Modified carbon fibers enhanced mechanical engagement effect during debonding, showing improved tensile strength compared to non-coated fibers [74]. |
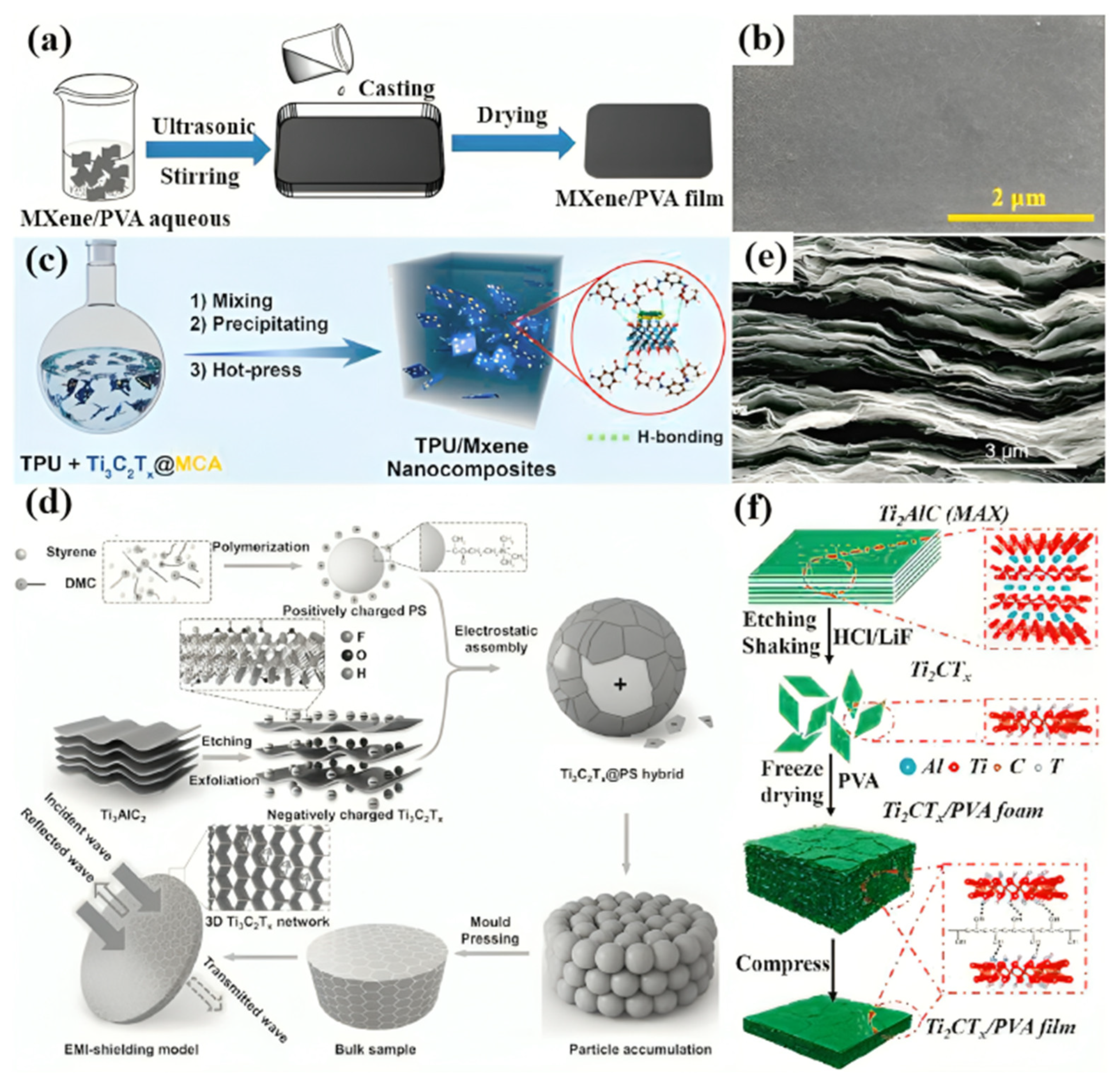
| Integration with polymer matrices | Prevention of sticking, enhancement of dispersion stability, improved electrode performance | Integration of MXenes within P(VDF-TrFE-CFE) polymer matrices prevented sticking, provided dispersion stability, and improved electrode performance. MXene flakes were evenly dispersed throughout the polymer matrix without forming aggregates, showing improved dielectric permittivity [80]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrova, M.; Kurtev, N.; Pandiev, I. Effect of MXene Nanosheet Sticking on Supercapacitor Device Performance. Appl. Sci. 2024, 14, 2452. https://doi.org/10.3390/app14062452
Aleksandrova M, Kurtev N, Pandiev I. Effect of MXene Nanosheet Sticking on Supercapacitor Device Performance. Applied Sciences. 2024; 14(6):2452. https://doi.org/10.3390/app14062452
Chicago/Turabian StyleAleksandrova, Mariya, Nikolay Kurtev, and Ivailo Pandiev. 2024. "Effect of MXene Nanosheet Sticking on Supercapacitor Device Performance" Applied Sciences 14, no. 6: 2452. https://doi.org/10.3390/app14062452
APA StyleAleksandrova, M., Kurtev, N., & Pandiev, I. (2024). Effect of MXene Nanosheet Sticking on Supercapacitor Device Performance. Applied Sciences, 14(6), 2452. https://doi.org/10.3390/app14062452








