Effect of the Silanization of Aerosil OX50 in the Properties of Light-Cured Dental Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silanization of Aerosil OX50
2.2. Preparation of Dental Composite Formulations
2.3. Characterization of the Filler and Experimental Composites
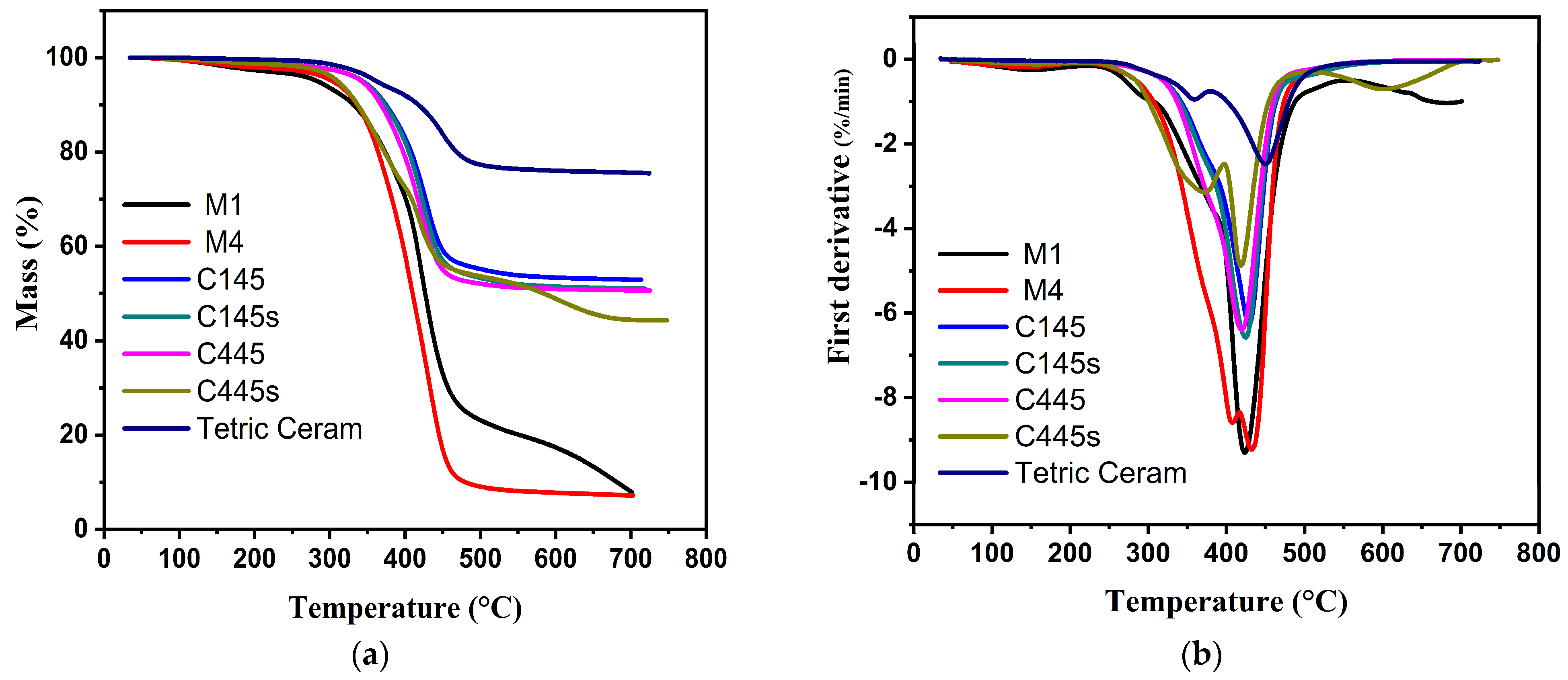
3. Results
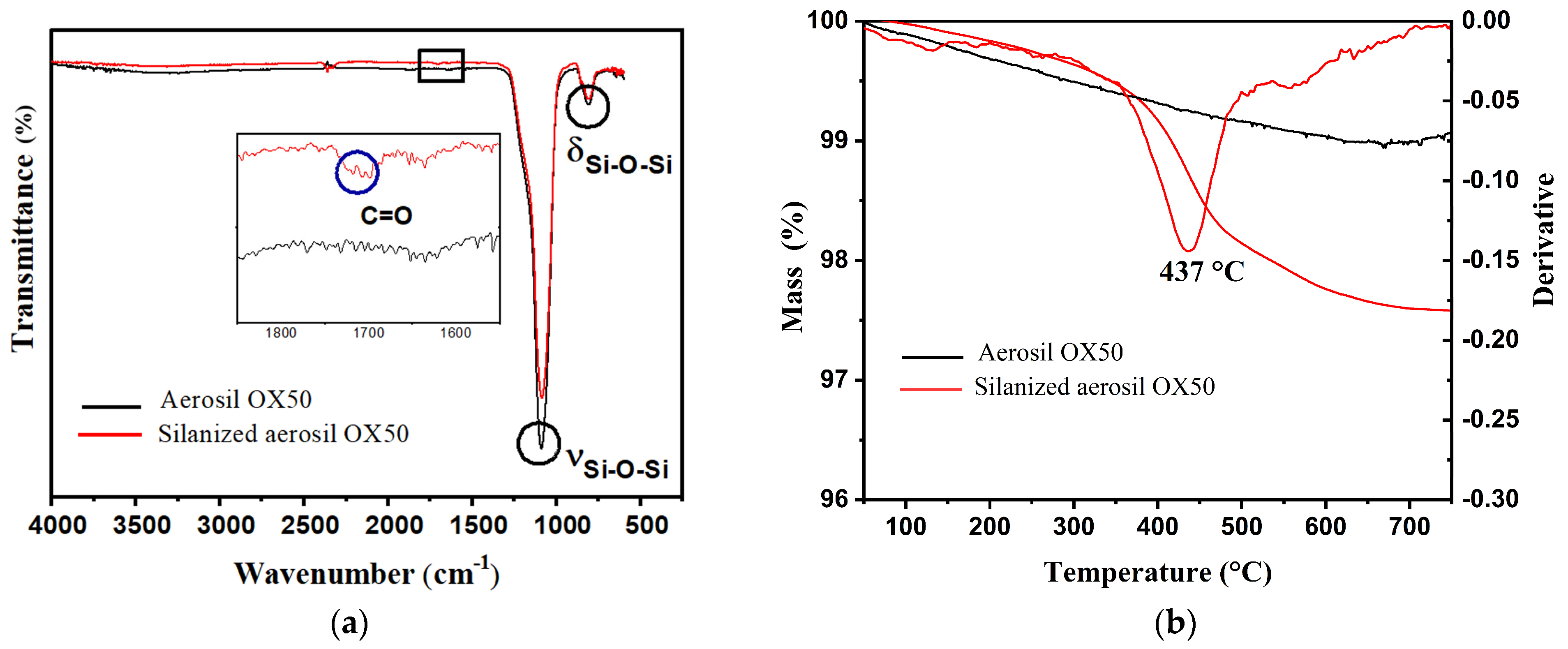
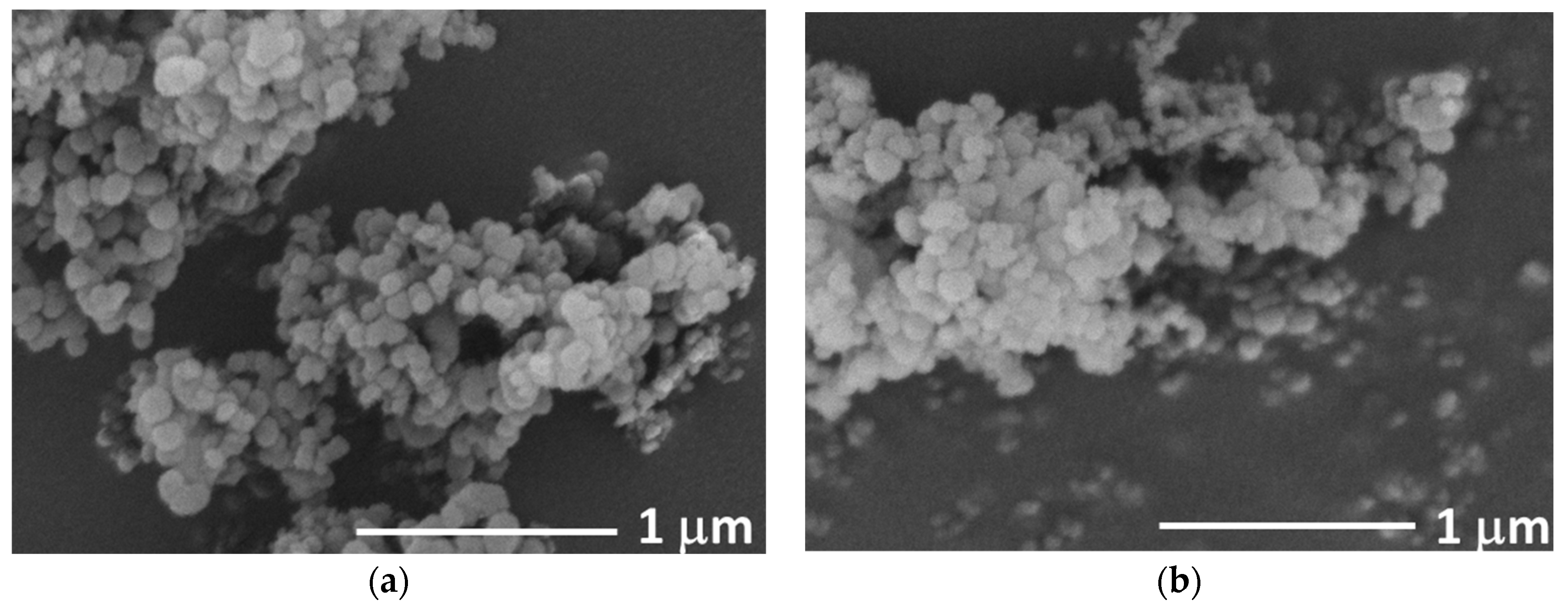
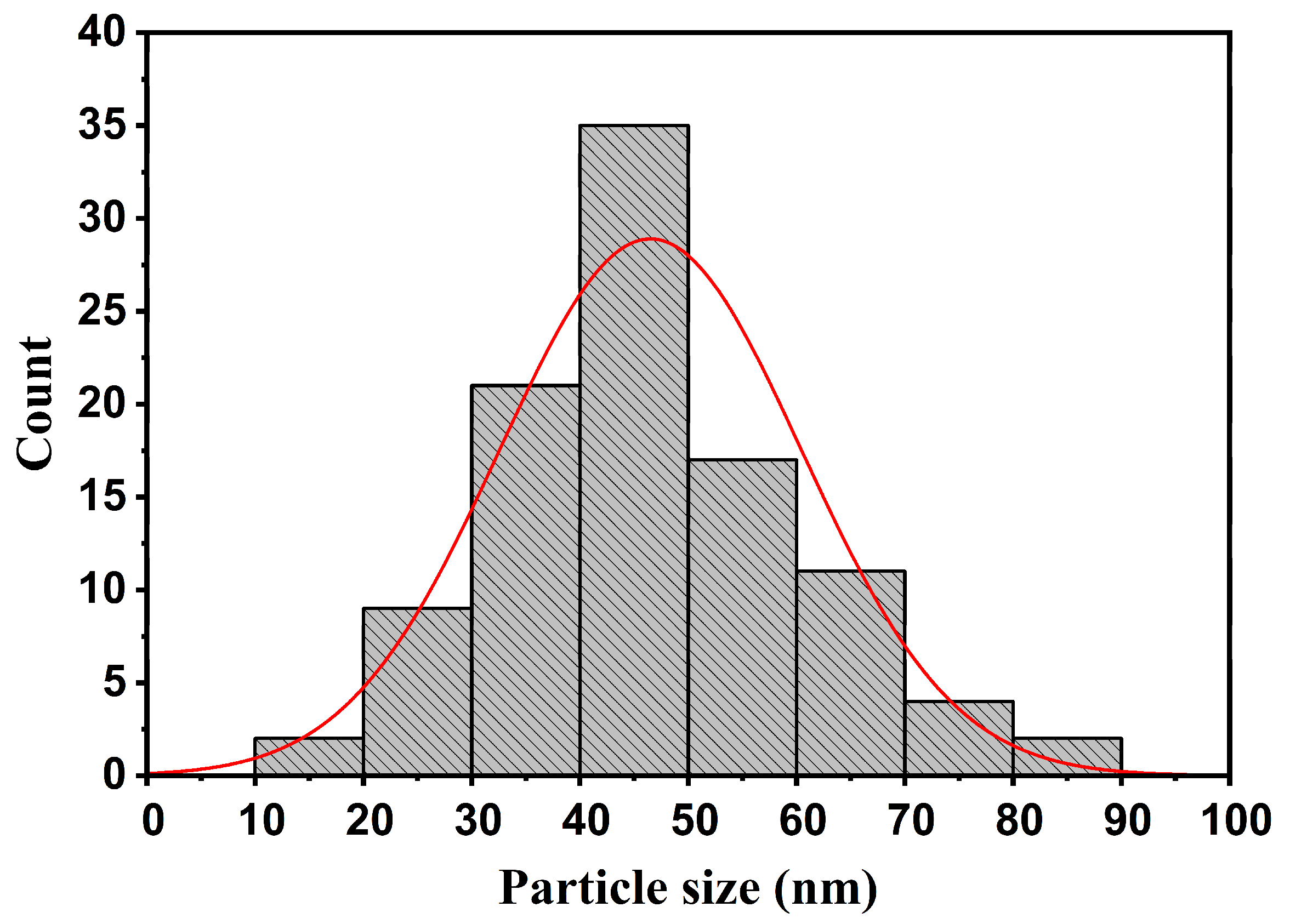
3.1. Characterization of the Filler
3.2. Physical Appearance of the Experimental Composites
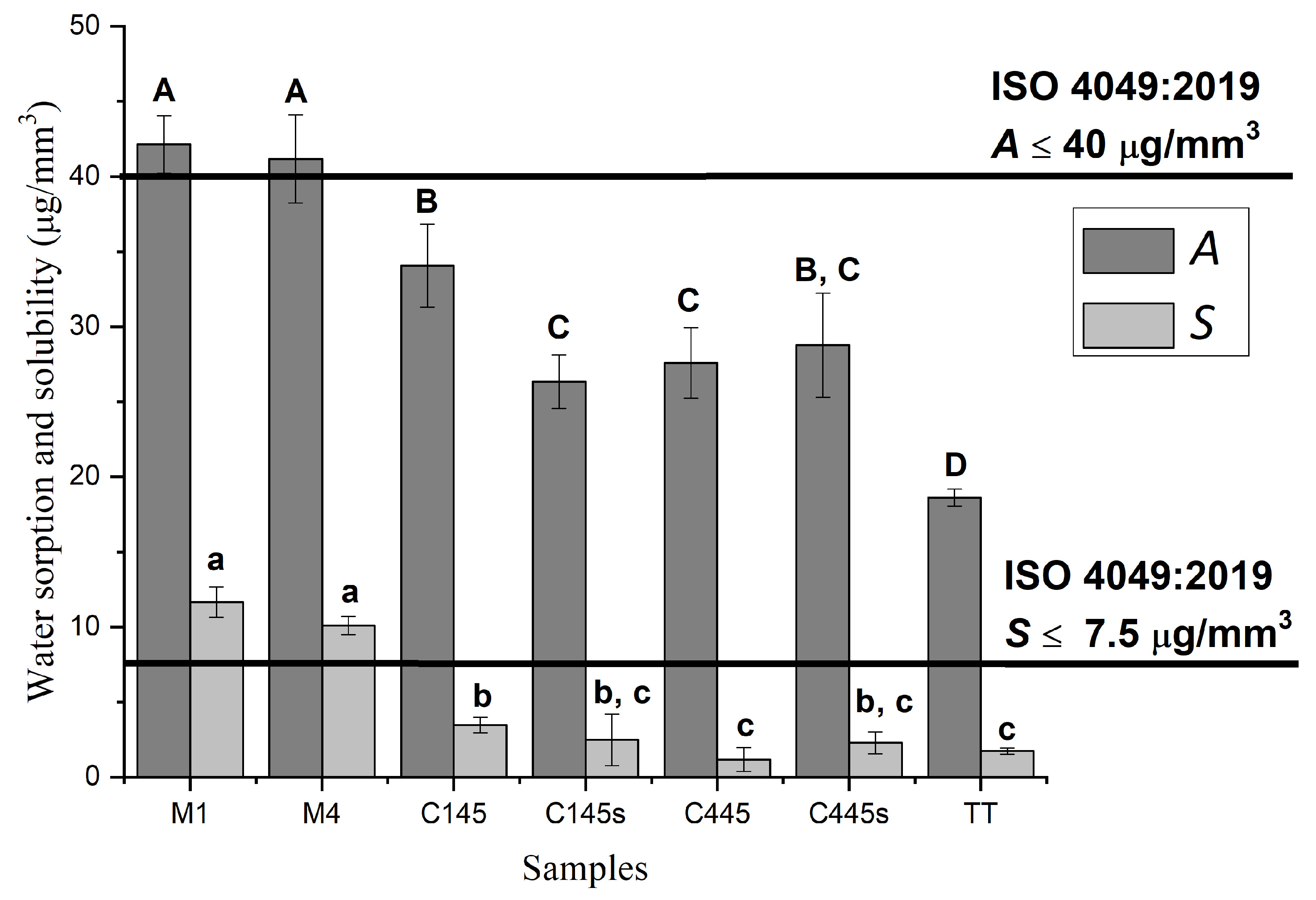
3.3. Characterization of the Experimental Composites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte de Oliveira, F.J.; Ferreira da Silva Filho, P.S.; Fernandes Costa, M.J.; Rabelo Caldas, M.R.G.; Dutra Borges, B.C.; Gadelha de Araújo, D.F. A comprehensive review of the antibacterial activity of dimethylaminohexadecyl methacrylate (DMAHDM) and its influence on mechanical properties of resin-based dental materials. Jpn. Dent. Sci. Rev. 2021, 57, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Convenio de Minamata Sobre el Mercurio—Texto y Anexos; Programa de Naciones Unidas para el Medio Ambiente: Nairobi, Kenya, 2019; pp. 3–15.
- Gomez, G.J. Perspectivas del Uso de la Amalgama Dental bajo el Convenio de Minamata: Tendencias Nacionales e Internacionales. CES Odontol. 2020, 33, 53–63. [Google Scholar] [CrossRef]
- Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; González-López, J.A.; Trejo-Carbajal, N.; Meléndez-Rodríguez, M.; Herrera-González, A.M. Preparation and evaluation of a BisGMA-free dental composite resin based on a novel trimethacrylate monomer. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Martim, G.C.; Kupfer, V.L.; Moisés, M.P.; dos Santos, A.; Buzzetti, P.H.M.; Rinaldi, A.W.; Rubira, A.F.; Girotto, E.M. Physical-chemical properties of dental composites and adhesives containing silane-modified SBA-15. J. Mech. Behav. Biomed. Mater. 2018, 80, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A review of dental composites: Challenges, chemistry aspects, filler influences, and future insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P. Physicochemical and biological assessment of silver nanoparticles immobilized Halloysite nanotubes-based resin composite for dental applications. Heliyon 2020, 6, e03601. [Google Scholar] [CrossRef]
- Moragues, A.; Ruiz Soto, A.; De Miguel, M.; Reyes, E.; Cristina, A. Nano-scale aluminium interaction in synthetic hydrated calcium silicate gel studied by 29Si MAS-NMR. Bol. Soc. Esp. Ceram. Vidr. 2022, 62, 388–401. [Google Scholar] [CrossRef]
- Nader, R.; Hamieh, T.; Villieras, F.; Angelina.Razafitianamaharav; Toufaily, J.; McHeik, A.S.; Thomas, F. Study of Gas Adsorption on Biphasic Nanostructured Surfaces. Phys. Procedia 2014, 55, 373–382. [Google Scholar] [CrossRef]
- ISO 4049 E; Dentistry—Polymer-Based Restorative Materials. ISO: Geneva, Switzerland, 2019.
- Haji, Z.; Ghafoor, R. Effect of Curing Modes on Depth-of-Cure in Bulk-Fill Composite. Int. Dent. J. 2021, 71, S40. [Google Scholar] [CrossRef]
- Alkhouri, N.; Xia, W.; Ashley, P.; Young, A. The effect of varying monocalcium phosphate and polylysine levels on dental composite properties. J. Mech. Behav. Biomed. Mater. 2023, 145, 106039. [Google Scholar] [CrossRef]
- Thadathil Varghese, J.; Cho, K.; Raju; Farrar, P.; Prentice, L.; Prusty, B.G. Influence of silane coupling agent on the mechanical performance of flowable fibre-reinforced dental composites. Dent. Mater. 2022, 38, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, L.M.; Ferraz, L.G.; Antunes, K.B.; Garcia, I.M.; Schneider, L.F.J.; Collares, F.M. Silane content influences physicochemical properties in nanostructured model composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2021, 37, e85–e93. [Google Scholar] [CrossRef] [PubMed]
- Karabela, M.M.; Sideridou, I.D. Synthesis and study of properties of dental resin composites with different nanosilica particles size. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2011, 27, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Cisneros-Pineda, O.G.; Herrera Kao, W.; Loría-Bastarrachea, M.I.; Veranes-Pantoja, Y.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Towards optimization of the silanization process of hydroxyapatite for its use in bone cement formulations. Mater. Sci. Eng. C 2014, 40, 157–163. [Google Scholar] [CrossRef]
- Vidal, M.L.; Rego, G.F.; Viana, G.M.; Cabral, L.M.; Souza, J.P.B.; Silikas, N.; Schneider, L.F.; Cavalcante, L.M. Physical and chemical properties of model composites containing quaternary ammonium methacrylates. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Goulart, M.; Fugolin, A.P.; Lewis, S.H.; Rodrigues, J.A.; Erhardt, M.C.; Pfeifer, C.S. Thiourethane filler functionalization for dental resin composites: Concentration-dependent effects on toughening, stress reduction and depth of cure. Mater. Sci. Eng. C 2021, 118, 111535. [Google Scholar] [CrossRef]
- Abid Althaqafi, K.; Alshabib, A.; Satterthwaite, J.; Silikas, N. Properties of A Model Self-Healing Microcapsule-Based Dental Composite Reinforced with Silica Nanoparticles. J. Funct. Biomater. 2022, 13, 19. [Google Scholar] [CrossRef]
- Alayola, J.J. Efecto de la Incorporación de Nanoarcillas Sobre las Propiedades de Materiales Compuestos Dentales. Master’s Thesis, Centro de Investigaciones Científicas de Yucatán, A.C., Mérida, Mexico, 2017. [Google Scholar]
- Zha, C.; Wang, W.; Lu, Y.; Zhang, L. Constructing covalent interface in rubber/clay nanocomposite by combining structural modification and interlamellar silylation of montmorillonite. ACS Appl. Mater. Interfaces 2014, 6, 18769–18779. [Google Scholar] [CrossRef]
- Zubrzycki, J.; Klepka, T.; Marchewka, M.; Zubrzycki, R. Tests of Dental Properties of Composite Materials Containing Nanohybrid Filler. Materials 2023, 16, 348. [Google Scholar] [CrossRef]
- Althaqafi, K.A.; Satterthwaite, J.; Silikas, N. A review and current state of autonomic self-healing microcapsules-based dental resin composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, 329–342. [Google Scholar] [CrossRef]
- Susila Anand, V.; Balasubramanian, V. Effect of resin chemistry on depth of cure and cytotoxicity of dental resin composites. Mater. Sci. Eng. B 2014, 181, 33–38. [Google Scholar] [CrossRef]
- Cherchali, F.Z.; Attik, N.; Mouzali, M.; Tommasino, J.B.; Abouelleil, H.; Decoret, D.; Seux, D.; Grosgogeat, B. Structural stability of DHMAI antibacterial dental composite following in vitro biological aging. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Azlisham, N.A.F.; Johari, Y.; Mohamad, D.; Yhaya, M.F.; Mahmood, Z. Degree of conversion and physicomechanical properties of newly developed flowable composite derived from rice husk using urethane dimethacrylate monomer. J. Eng. Med. 2023, 237, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Encalada-Alayola, J.J.; Veranes-Pantoja, Y.; Uribe-Calderón, J.A.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Effect of Type and Concentration of Nanoclay on the Mechanical and Physicochemical Properties of Bis-GMA/TTEGDMA Dental Resins. Polymers 2020, 12, 601. [Google Scholar] [CrossRef] [PubMed]
- Kamalak, H.; Aksu Canbay, C.; Yigit, O.; Altin, S. Physico-mechanical and thermal characteristics of commercially available and newly developed dental flowable composites. J. Mol. Struct. 2017, 1156, 314–319. [Google Scholar] [CrossRef]
- Al Ayyan, W.; Al Halabi, M.; Hussein, I.; Khamis, A.H.; Kowash, M. A systematic review and meta-analysis of primary teeth caries studies in Gulf Cooperation Council States. Saudi Dent. J. 2018, 30, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tarano-Artigas, O.; Cauich-Rodriguez, J.V.; Cervantes-Uc, J.M.; Veleva-Muleshkova, L.; Veranes-Pantoja, Y. Evaluación termo-mecánica de composites dentales fotopolimerizables. Rev. Cuba. Química 2022, 34, 194–210. [Google Scholar]


| Composites | Organic Matrix | Inorganic Filler |
|---|---|---|
| C145 | Bis-GMA/TEEGDMA/MPS (M1) | Aerosil OX50 (45%) |
| C445 | Bis-GMA/TEEGDMA (M4) | |
| C145s | Bis-GMA/TEEGDMA/MPS (M1) | Aerosil OX50 silanized (45%) |
| C445s | Bis-GMA/TEEGDMA (M4) | |
| TT | Bis-GMA/TEGDMA | Mixtures of oxides and copolymers (82%) |
| Samples | DC (%) | Depth of Cure (mm) |
|---|---|---|
| Property Value | 55–80 | ≥2 |
| M1 | 86.3 (0.1) a | 2.95 (0.01) a |
| M4 | 85 (2) a,b | 2.94 (0.01) a,b |
| C145 | 71 (5) c | 2.93 (0.02) b |
| C145s | 79 (2) d | 2.93 (0.01) b |
| C445 | 80 (3) b,d | 2.93 (0.01) a,b |
| C445s | 76 (6) c,d | 2.93 (0.02) a,b |
| TT | 63 (4) e | 2.67 (0.09) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Rodríguez, S.d.l.C.; Tarano-Artigas, O.; Herrera-Kao, W.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M.; Rosa-Sainz, A.; La Serna, A.A.; Veranes-Pantoja, Y. Effect of the Silanization of Aerosil OX50 in the Properties of Light-Cured Dental Composites. Appl. Sci. 2024, 14, 2453. https://doi.org/10.3390/app14062453
Díaz-Rodríguez SdlC, Tarano-Artigas O, Herrera-Kao W, Cauich-Rodríguez JV, Cervantes-Uc JM, Rosa-Sainz A, La Serna AA, Veranes-Pantoja Y. Effect of the Silanization of Aerosil OX50 in the Properties of Light-Cured Dental Composites. Applied Sciences. 2024; 14(6):2453. https://doi.org/10.3390/app14062453
Chicago/Turabian StyleDíaz-Rodríguez, Selena de la Caridad, Oridayma Tarano-Artigas, Wilberth Herrera-Kao, Juan Valerio Cauich-Rodríguez, José Manuel Cervantes-Uc, Ana Rosa-Sainz, Amisel Almirall La Serna, and Yaymarilis Veranes-Pantoja. 2024. "Effect of the Silanization of Aerosil OX50 in the Properties of Light-Cured Dental Composites" Applied Sciences 14, no. 6: 2453. https://doi.org/10.3390/app14062453
APA StyleDíaz-Rodríguez, S. d. l. C., Tarano-Artigas, O., Herrera-Kao, W., Cauich-Rodríguez, J. V., Cervantes-Uc, J. M., Rosa-Sainz, A., La Serna, A. A., & Veranes-Pantoja, Y. (2024). Effect of the Silanization of Aerosil OX50 in the Properties of Light-Cured Dental Composites. Applied Sciences, 14(6), 2453. https://doi.org/10.3390/app14062453







