High-Sensitivity Polarization Sensitive Optical Coherence Tomography Based on Numerical Correction for Perfect Circularly Polarized Light
Abstract
:1. Introduction
2. Experimental Setup
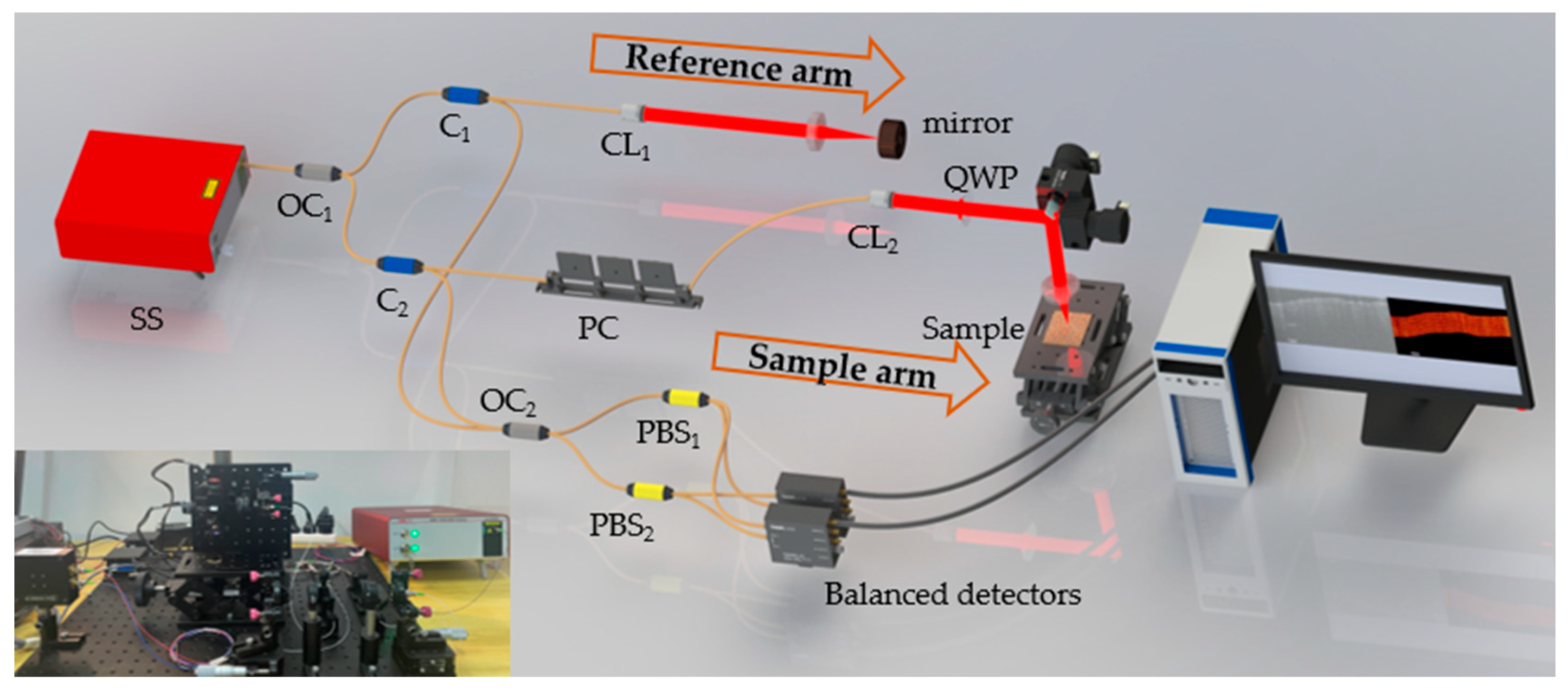
2.1. System Setup of Fiber-Based Polarization Sensitive Optical Coherence Tomography
2.2. Principle of Numerical Correction Method for Perfect Circularly Polarized Light
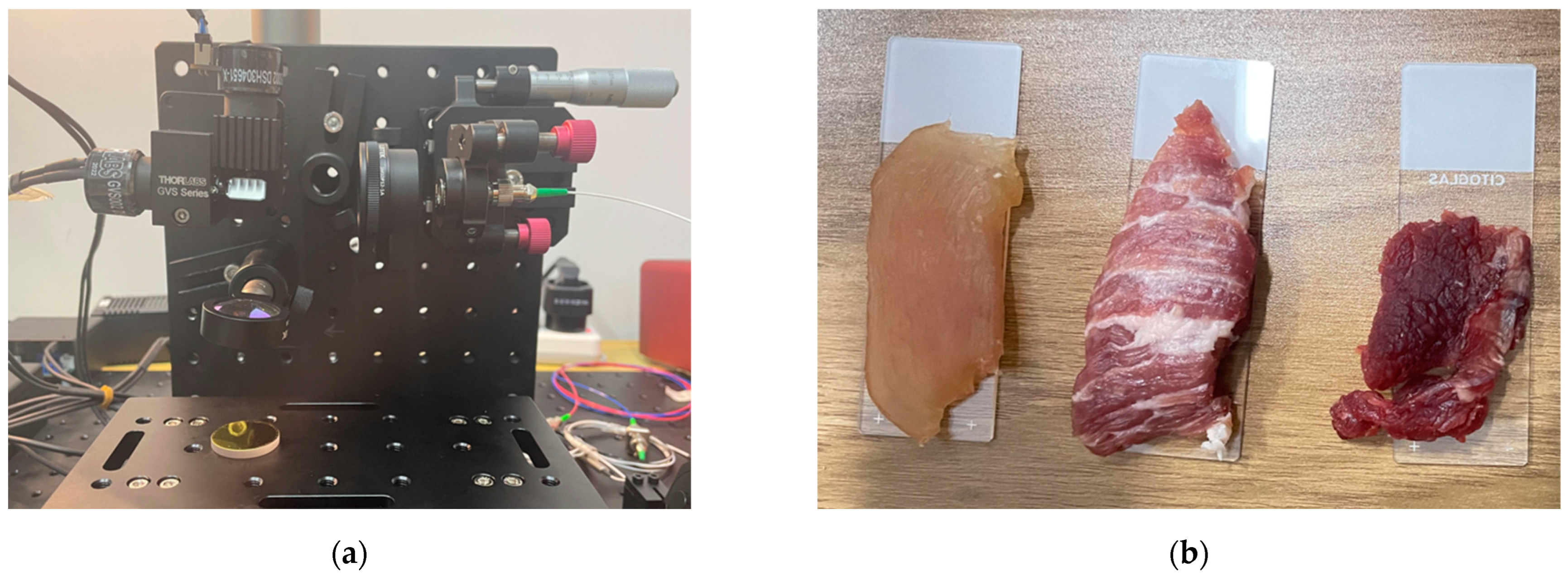
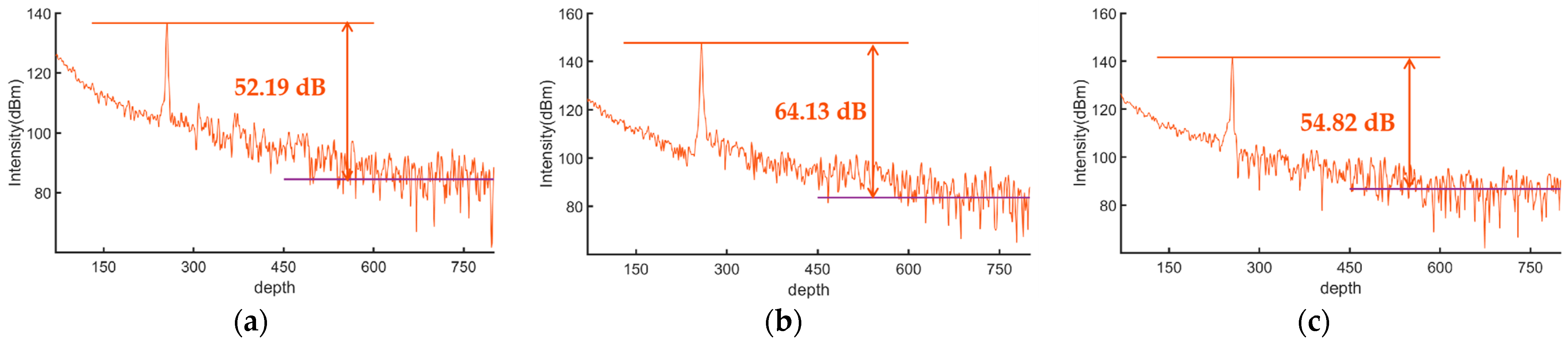
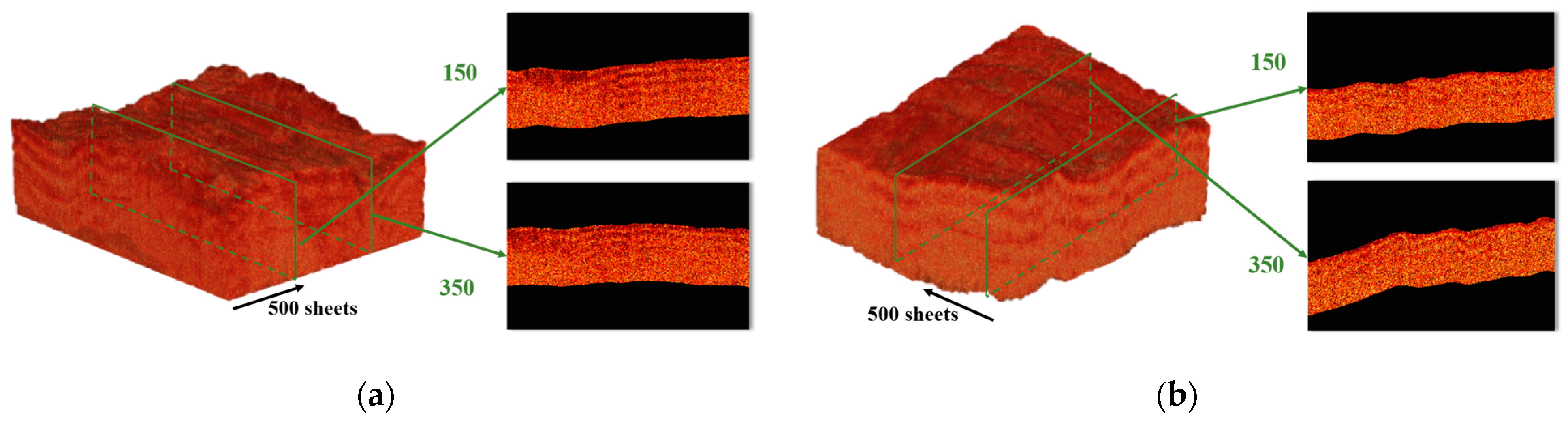
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, F.; Hu, L.; Lang, Z.; Liu, Q.; Cai, F.; Fu, L. A Stare-Down Video-Rate High-Throughput Hyperspectral Imaging System and Its Applications in Biological Sample Sensing. IEEE Sens. J. 2023, 23, 23629–23637. [Google Scholar] [CrossRef]
- Tearney, G.J.; Brezinski, M.E.; Bouma, B.E.; Boppart, S.A.; Pitris, C.; Southern, J.F.; Fujimoto, J.G. In Vivo Endoscopic Optical Biopsy with Optical Coherence Tomography. Science 1997, 276, 2037–2039. [Google Scholar] [CrossRef] [PubMed]
- Leggett, C.L.; Gorospe, E.C.; Chan, D.K.; Muppa, P.; Owens, V.; Smyrk, T.C.; Anderson, M.; Lutzke, L.S.; Tearney, G.; Wang, K.K. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett’s esophagus. Gastrointest. Endosc. 2016, 83, 880–888.e882. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.J.; Martin, H.C.; Cass, D.T. Laser Doppler imaging prediction of burn wound outcome in children. Burns 2002, 28, 11–17. [Google Scholar] [CrossRef]
- Jeng, J.C.; Bridgeman, A.; Shivnan, L.; Thornton, P.M.; Alam, H.; Clarke, T.J.; Jablonski, K.A.; Jordan, M.H. Laser Doppler imaging determines need for excision and grafting in advance of clinical judgment: A prospective blinded trial. Burns 2003, 29, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Laíns, I.; Wang, J.C.; Cui, Y.; Katz, R.; Vingopoulos, F.; Staurenghi, G.; Vavvas, D.G.; Miller, J.W.; Miller, J.B. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog. Retin. Eye Res. 2021, 84, 100951. [Google Scholar] [CrossRef]
- Larin, K.V.; Sampson, D.D. Optical coherence elastography—OCT at work in tissue biomechanics. Biomed. Opt. Express 2017, 8, 1172–1202. [Google Scholar] [CrossRef]
- Tang, P.; Wang, R.K. Polarization sensitive optical coherence tomography for imaging microvascular information within living tissue without polarization-induced artifacts. Biomed. Opt. Express 2020, 11, 6379–6388. [Google Scholar] [CrossRef]
- Eugui, P.; Harper, D.J.; Lichtenegger, A.; Augustin, M.; Merkle, C.W.; Woehrer, A.; Hitzenberger, C.K.; Baumann, B. Polarization-sensitive imaging with simultaneous bright- and dark-field optical coherence tomography. Opt. Lett. 2019, 44, 4040–4043. [Google Scholar] [CrossRef]
- Yao, G.; Duan, D. High-resolution 3D tractography of fibrous tissue based on polarization-sensitive optical coherence tomography. Exp. Biol. Med. 2020, 245, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Mariottoni, E.B.; Jammal, A.A.; Urata, C.N.; Berchuck, S.I.; Thompson, A.C.; Estrela, T.; Medeiros, F.A. Quantification of Retinal Nerve Fibre Layer Thickness on Optical Coherence Tomography with a Deep Learning Segmentation-Free Approach. Sci. Rep. 2020, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Fialová, S.; Augustin, M.; Fischak, C.; Schmetterer, L.; Handschuh, S.; Glösmann, M.; Pircher, M.; Hitzenberger, C.K.; Baumann, B. Posterior rat eye during acute intraocular pressure elevation studied using polarization sensitive optical coherence tomography. Biomed. Opt. Express 2017, 8, 298–314. [Google Scholar] [CrossRef]
- Hee, M.R.; Huang, D.; Swanson, E.A.; Fujimoto, J.G. Polarization-sensitive low-coherence reflectometer for birefringence characterization and ranging. J. Opt. Soc. Am. B 1992, 9, 903–908. [Google Scholar] [CrossRef]
- Wang, H.; Akkin, T.; Magnain, C.; Wang, R.; Dubb, J.; Kostis, W.J.; Yaseen, M.A.; Cramer, A.; Sakadžić, S.; Boas, D. Polarization sensitive optical coherence microscopy for brain imaging. Opt. Lett. 2016, 41, 2213–2216. [Google Scholar] [CrossRef]
- Lin, H.; Kao, M.C.; Lai, C.M.; Huang, J.C.; Kuo, W.C. All fiber optics circular-state swept source polarization-sensitive optical coherence tomography. J. Biomed. Opt. 2014, 19, 21110. [Google Scholar] [CrossRef]
- Al-Qaisi, M.K.; Akkin, T. Polarization-sensitive optical coherence tomography based on polarization-maintaining fibers and frequency multiplexing. Opt. Express 2008, 16, 13032–13041. [Google Scholar] [CrossRef]
- Trasischker, W.; Zotter, S.; Torzicky, T.; Baumann, B.; Haindl, R.; Pircher, M.; Hitzenberger, C.K. Single input state polarization sensitive swept source optical coherence tomography based on an all single mode fiber interferometer. Biomed. Opt. Express 2014, 5, 2798–2809. [Google Scholar] [CrossRef]
- Tang, P.; Kirby, M.A.; Le, N.; Li, Y.; Zeinstra, N.; Lu, G.N.; Murry, C.E.; Zheng, Y.; Wang, R.K.J.L. Polarization sensitive optical coherence tomography with single input for imaging depth-resolved collagen organizations. Sci. Appl. 2021, 10, 237. [Google Scholar] [CrossRef]
- Kiseleva, E.; Kirillin, M.; Feldchtein, F.; Vitkin, A.; Sergeeva, E.; Zagaynova, E.; Streltzova, O.; Shakhov, B.; Gubarkova, E.; Gladkova, N. Differential diagnosis of human bladder mucosa pathologies in vivo with cross-polarization optical coherence tomography. Biomed. Opt. Express 2015, 6, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Gubarkova, E.V.; Kirillin, M.Y.; Dudenkova, V.V.; Timashev, P.S.; Kotova, S.L.; Kiseleva, E.B.; Timofeeva, L.B.; Belkova, G.V.; Solovieva, A.B.; Moiseev, A.A.; et al. Quantitative evaluation of atherosclerotic plaques using cross-polarization optical coherence tomography, nonlinear, and atomic force microscopy. J. Biomed. Opt. 2016, 21, 126010. [Google Scholar] [CrossRef]
- Li, E.; Makita, S.; Hong, Y.-J.; Kasaragod, D.; Yasuno, Y. Three-dimensional multi-contrast imaging of in vivo human skin by Jones matrix optical coherence tomography. Biomed. Opt. Express 2017, 8, 1290–1305. [Google Scholar] [CrossRef]
- Xiong, Q.; Wang, N.; Liu, X.; Chen, S.; Braganza, C.S.; Bouma, B.E.; Liu, L.; Villiger, M. Constrained polarization evolution simplifies depth-resolved retardation measurements with polarization-sensitive optical coherence tomography. Biomed. Opt. Express 2019, 10, 5207–5222. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.C.; Szabari, M.V.; Lagares, D.; Mccrossan, A.F.; Hariri, L.P.; Tager, A.M.; Suter, M.J. Assessing the progression of systemic sclerosis by monitoring the tissue optic axis using PS-OCT. Sci. Rep. 2020, 10, 2561. [Google Scholar] [CrossRef]
- Braaf, B.; Vermeer, K.A.; de Groot, M.; Vienola, K.V.; de Boer, J.F. Fiber-based polarization-sensitive OCT of the human retina with correction of system polarization distortions. Biomed. Opt. Express 2014, 5, 2736–2758. [Google Scholar] [CrossRef] [PubMed]
- Lippok, N.; Villiger, M.; Jun, C.; Bouma, B. Single input state, single-mode fiber-based polarization-sensitive optical frequency domain imaging by eigenpolarization referencing. Opt. Lett. 2015, 40, 2025–2028. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, L.; Ke, M.; Schmetterer, L.; Barathi, V.A. Using image data to numerically correct the jitter in polarization depth encoding PS-OCT. Opt. Lett. 2021, 46, 1692–1695. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Saxer, C.; Xiang, S.; de Boer, J.F.; Nelson, J.S. Phase-resolved optical coherence tomography and optical Doppler tomography for imaging blood flow in human skin with fast scanning speed and high velocity sensitivity. Opt. Lett. 2000, 25, 114–116. [Google Scholar] [CrossRef]
- Nettelblad, H.; Thuomas, K.Å.; Sjöberg, F. Magnetic resonance imaging: A new diagnostic aid in the care of high-voltage electrical burns. Burns 1996, 22, 117–119. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Hu, L.; Cao, J. High-Sensitivity Polarization Sensitive Optical Coherence Tomography Based on Numerical Correction for Perfect Circularly Polarized Light. Appl. Sci. 2024, 14, 2525. https://doi.org/10.3390/app14062525
Li S, Hu L, Cao J. High-Sensitivity Polarization Sensitive Optical Coherence Tomography Based on Numerical Correction for Perfect Circularly Polarized Light. Applied Sciences. 2024; 14(6):2525. https://doi.org/10.3390/app14062525
Chicago/Turabian StyleLi, Sifan, Lantian Hu, and Jing Cao. 2024. "High-Sensitivity Polarization Sensitive Optical Coherence Tomography Based on Numerical Correction for Perfect Circularly Polarized Light" Applied Sciences 14, no. 6: 2525. https://doi.org/10.3390/app14062525




