Deletion of Transient Receptor Channel Vanilloid 4 Aggravates CaCl2-Induced Abdominal Aortic Aneurysm and Vascular Calcification: A Histological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CaCl2-Induced Abdominal Aortic Injury
2.3. Aorta Collection and Morphometry
2.4. Histological Analysis
2.5. Statistical Analysis
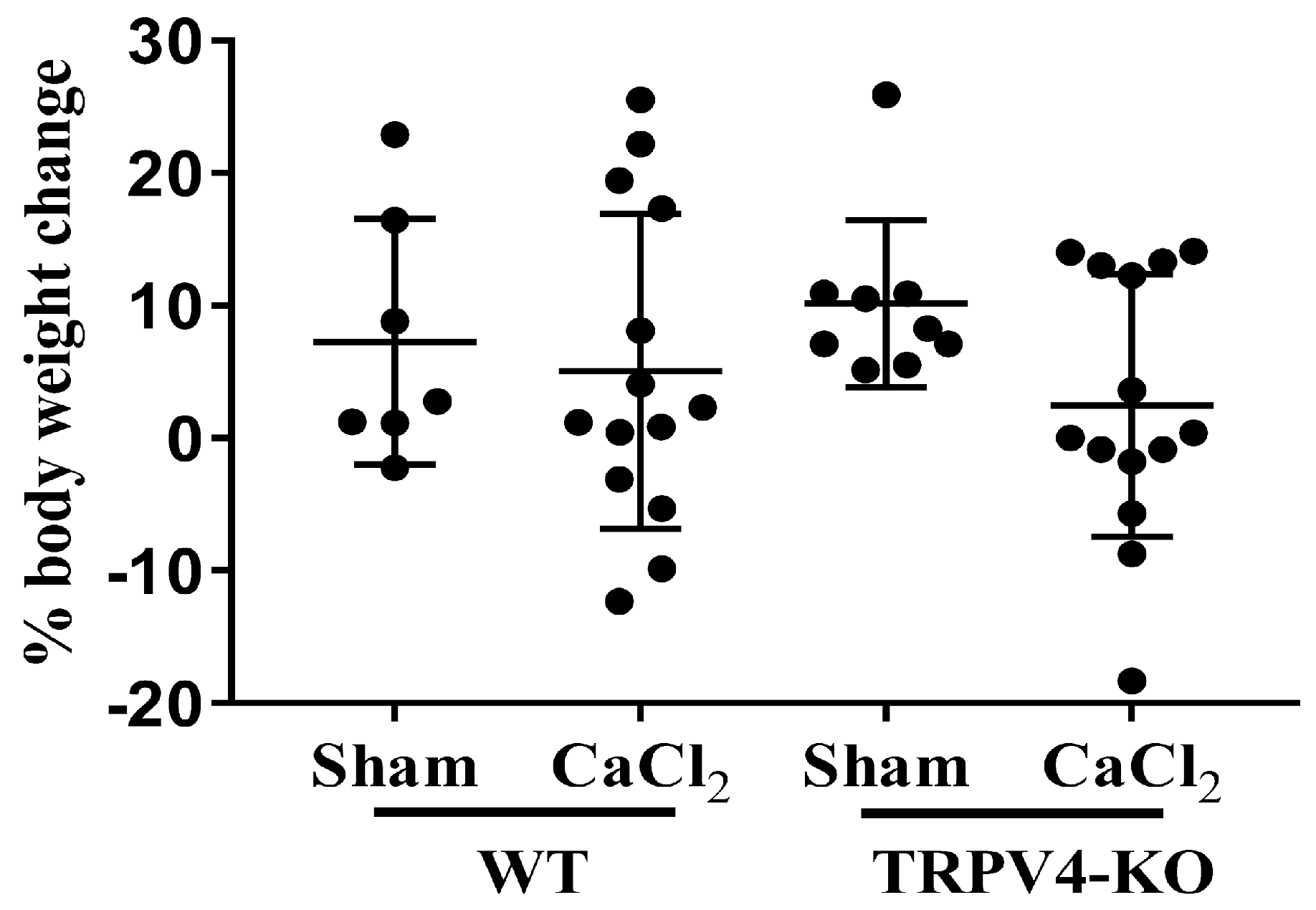
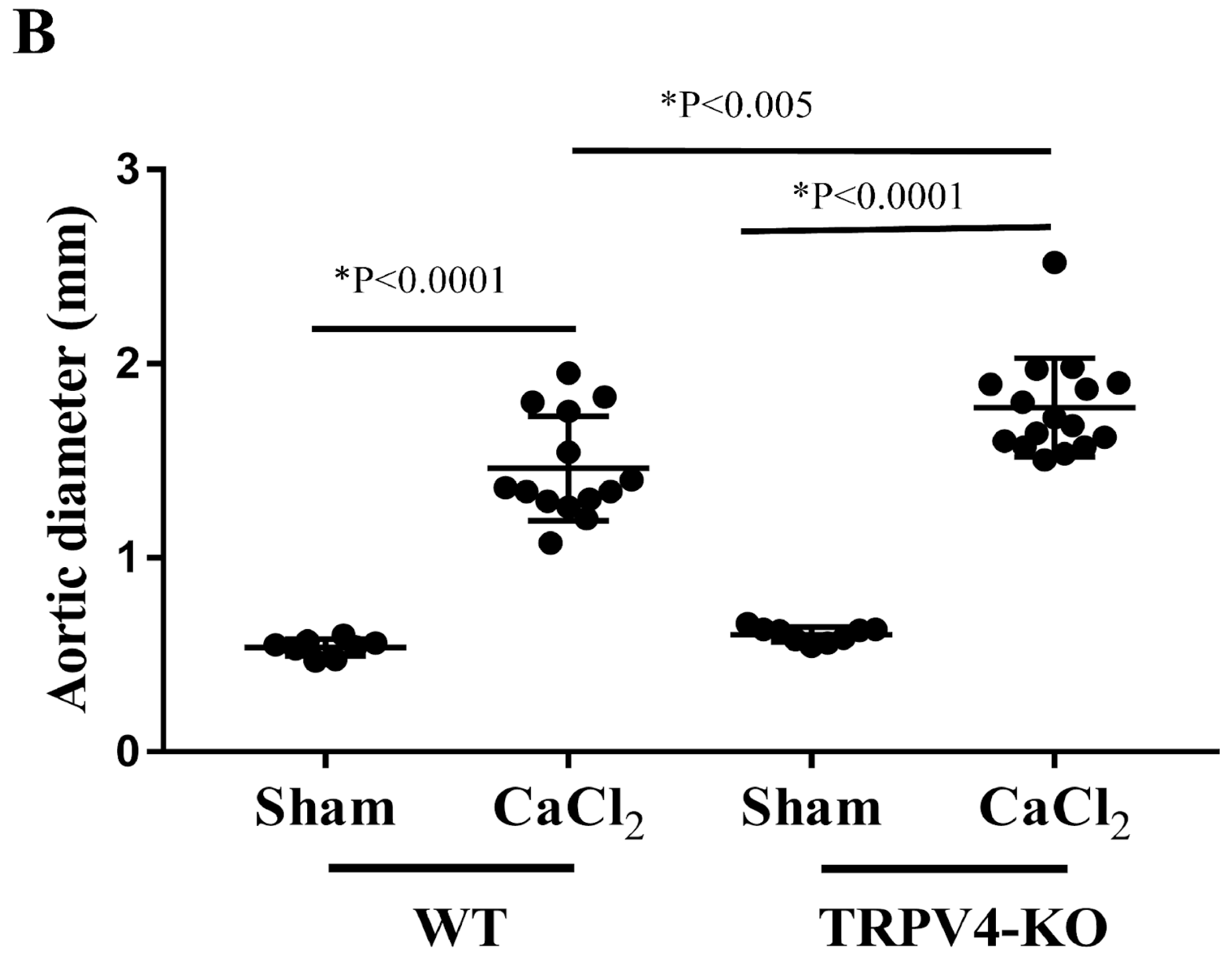
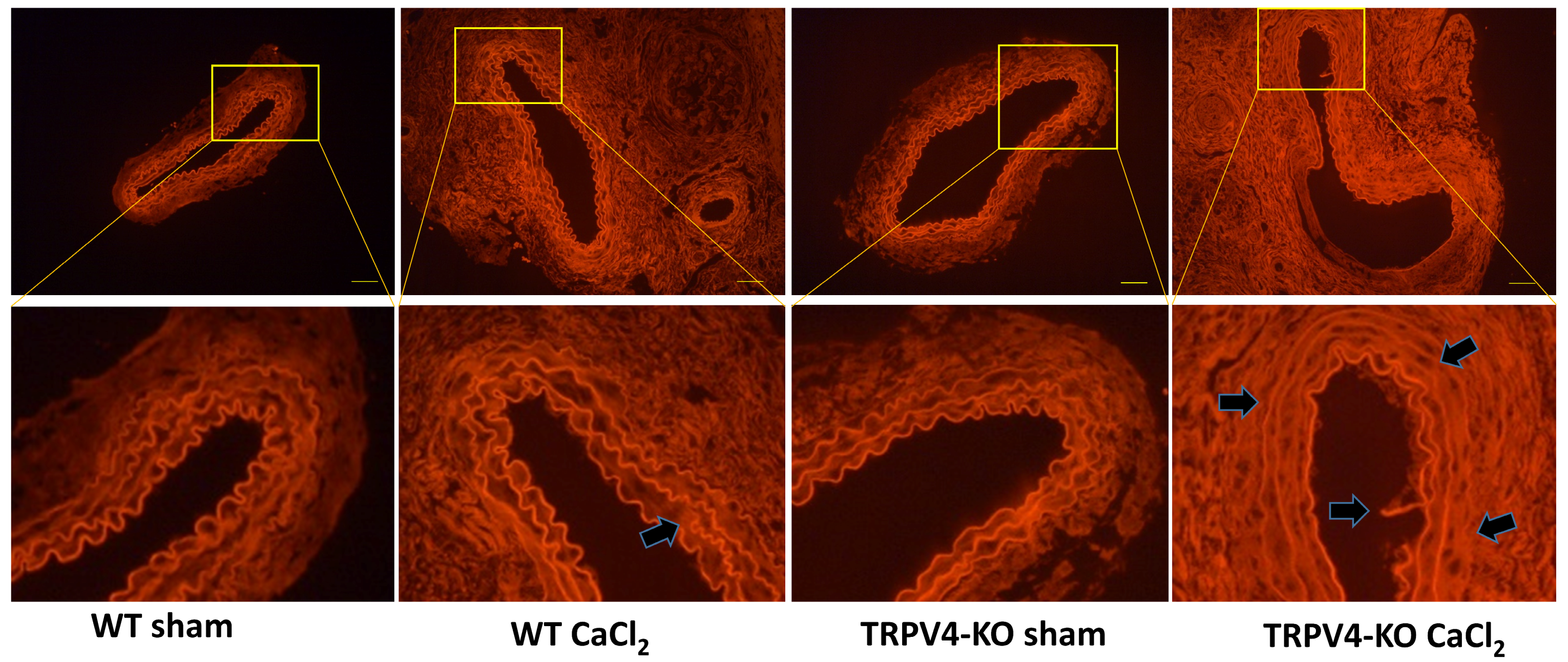
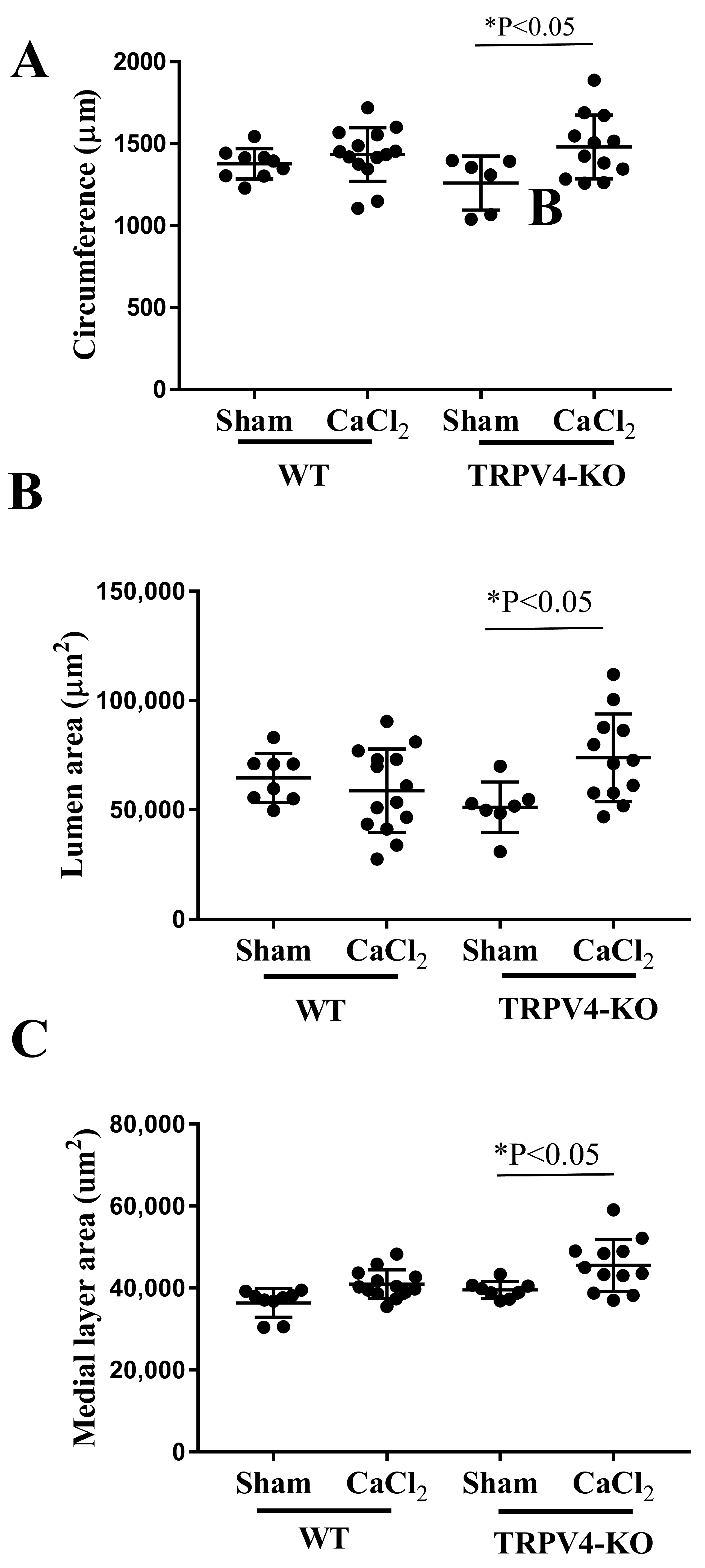
3. Results
3.1. Deletion of TRPV4 Exacerbates Aortic Diameters in CaCl2-Induced Aortic Injury
3.2. Deletion of TRPV4 Aggravates Tunica Media Damage in CaCl2-Induced Aortic Injury
3.3. Deletion of TRPV4 Increases Tunica Media Area and Lumen Circumference in CaCl2-Induced Aortic Injury
3.4. Deletion of TRPV4 Enhances Aortic Calcification in CaCl2-Induced Aortic Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, C.; Imaizumi, T.; Kawai, H.; Suda, K.; Honma, Y.; Ichihashi, M.; Ema, M.; Mizutani, K.I. Aging of the Vascular System and Neural Diseases. Front. Aging Neurosci. 2020, 12, 557384. [Google Scholar] [CrossRef] [PubMed]
- Tedjawirja, V.N.; Nieuwdorp, M.; Yeung, K.K.; Balm, R.; de Waard, V. A Novel Hypothesis: A Role for Follicle Stimulating Hormone in Abdominal Aortic Aneurysm Development in Postmenopausal Women. Front. Endocrinol. 2021, 12, 726107. [Google Scholar] [CrossRef]
- Kent, K.C.; Zwolak, R.M.; Egorova, N.N.; Riles, T.S.; Manganaro, A.; Moskowitz, A.J.; Gelijns, A.C.; Greco, G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J. Vasc. Surg. 2010, 52, 539–548. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Limet, R.; Defawe, O.D. Abdominal aortic aneurysm. Lancet 2005, 365, 1577–1589. [Google Scholar] [CrossRef]
- Chowdhury, M.M.; Zieliński, L.P.; Sun, J.J.; Lambracos, S.; Boyle, J.R.; Harrison, S.C.; Rudd, J.H.F.; Coughlin, P.A. Editor’s Choice—Calcification of Thoracic and Abdominal Aneurysms is Associated with Mortality and Morbidity. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2018, 55, 101–108. [Google Scholar] [CrossRef]
- Yuan, C.; Ni, L.; Zhang, C.; Hu, X.; Wu, X. Vascular calcification: New insights into endothelial cells. Microvasc. Res. 2021, 134, 104105. [Google Scholar] [CrossRef] [PubMed]
- Rantasalo, V.; Gunn, J.; Kiviniemi, T.; Hirvonen, J.; Saarenpää, I.; Kivelev, J.; Rahi, M.; Lassila, E.; Rinne, J.; Laukka, D. Intracranial aneurysm is predicted by abdominal aortic calcification index: A retrospective case-control study. Atherosclerosis 2021, 334, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; Moe, S.M. Vascular calcification: Pathophysiology and risk factors. Curr. Hypertens. Rep. 2012, 14, 228–237. [Google Scholar] [CrossRef]
- Disthabanchong, S. Vascular calcification in chronic kidney disease: Pathogenesis and clinical implication. World J. Nephrol. 2012, 1, 43–53. [Google Scholar] [CrossRef]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular calcification: An update on mechanisms and challenges in treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef]
- Yao, H.; Sun, Z.; Zang, G.; Zhang, L.; Hou, L.; Shao, C.; Wang, Z. Epidemiological Research Advances in Vascular Calcification in Diabetes. J. Diabetes Res. 2021, 2021, 4461311. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Z.; Cai, Z.; Sun, Y.; Li, L.; Yao, F.; Yang, L.; Zhou, Y.; Zhu, H.; Fu, Y.; et al. Runx2 (Runt-Related Transcription Factor 2)-Mediated Microcalcification Is a Novel Pathological Characteristic and Potential Mediator of Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1352–1369. [Google Scholar] [CrossRef]
- Neels, J.G.; Leftheriotis, G.; Chinetti, G. Atherosclerosis Calcification: Focus on Lipoproteins. Metabolites 2023, 13, 457. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.K.; Jeon, J.H. Vascular Calcification-New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef] [PubMed]
- Nemcsik, J.; Kiss, I.; Tislér, A. Arterial stiffness, vascular calcification and bone metabolism in chronic kidney disease. World J. Nephrol. 2012, 1, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Al-Huseini, I.; Ashida, N.; Kimura, T. Deletion of IκB-Kinase β in Smooth Muscle Cells Induces Vascular Calcification through β-Catenin-Runt-Related Transcription Factor 2 Signaling. J. Am. Heart Assoc. 2018, 7, e007405. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.; Bundy, K.; Roach, C.; Douglas, H.; Ventura, V.; Segars, M.F.; Schwartz, O.; Simpson, C.L. Mechanisms of the Osteogenic Switch of Smooth Muscle Cells in Vascular Calcification: WNT Signaling, BMPs, Mechanotransduction, and EndMT. Bioengineering 2020, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.D.; Eijken, M.; van de Peppel, J.; van Leeuwen, J.P. Calcifying vascular smooth muscle cells and osteoblasts: Independent cell types exhibiting extracellular matrix and biomineralization-related mimicries. BMC Genom. 2014, 15, 965. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc. Med. 2015, 25, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Bleichert, S.; Ibrahim, N.; Wortmann, M.; Eckstein, H.H.; Brostjan, C.; Wagenhäuser, M.U.; Goergen, C.J.; Maegdefessel, L. Translating mouse models of abdominal aortic aneurysm to the translational needs of vascular surgery. JVS Vasc. Sci. 2021, 2, 219–234. [Google Scholar] [CrossRef]
- Bumdelger, B.; Otani, M.; Karasaki, K.; Sakai, C.; Ishida, M.; Kokubo, H.; Yoshizumi, M. Disruption of Osteoprotegerin has complex effects on medial destruction and adventitial fibrosis during mouse abdominal aortic aneurysm formation. PLoS ONE 2020, 15, e0235553. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kuninaka, Y.; Nosaka, M.; Kimura, A.; Taruya, A.; Furuta, M.; Mukaida, N.; Kondo, T. Prevention of CaCl2-induced aortic inflammation and subsequent aneurysm formation by the CCL3–CCR5 axis. Nat. Commun. 2020, 11, 5994. [Google Scholar] [CrossRef] [PubMed]
- Son, B.K.; Kojima, T.; Ogawa, S.; Akishita, M. Testosterone inhibits aneurysm formation and vascular inflammation in male mice. J. Endocrinol. 2019, 241, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Baylie, R.L.; Brayden, J.E. TRPV channels and vascular function. Acta Physiol. 2011, 203, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Watanabe, H.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T.; Nilius, B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003, 424, 434–438. [Google Scholar] [CrossRef]
- Köhler, R.; Heyken, W.T.; Heinau, P.; Schubert, R.; Si, H.; Kacik, M.; Busch, C.; Grgic, I.; Maier, T.; Hoyer, J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1495–1502. [Google Scholar] [CrossRef]
- Earley, S.; Heppner, T.J.; Nelson, M.T.; Brayden, J.E. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ. Res. 2005, 97, 1270–1279. [Google Scholar] [CrossRef]
- Masuyama, R.; Vriens, J.; Voets, T.; Karashima, Y.; Owsianik, G.; Vennekens, R.; Lieben, L.; Torrekens, S.; Moermans, K.; Vanden Bosch, A.; et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008, 8, 257–265. [Google Scholar] [CrossRef]
- Wang, Y.; Krishna, S.; Golledge, J. The calcium chloride-induced rodent model of abdominal aortic aneurysm. Atherosclerosis 2013, 226, 29–39. [Google Scholar] [CrossRef]
- de Carvalho, H.F.; Taboga, S.R. Fluorescence and confocal laser scanning microscopy imaging of elastic fibers in hematoxylin-eosin stained sections. Histochem. Cell Biol. 1996, 106, 587–592. [Google Scholar] [CrossRef]
- Masuyama, R. Activation of TRPV4 promotes osteoclasts differentiation. Arthritis Res. Ther. 2012, 14 (Suppl. S1), P42. [Google Scholar] [CrossRef]
- Hu, K.; Sun, H.; Gui, B.; Sui, C. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2017, 91, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Stowers, R.; Chaudhuri, O. Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nat. Commun. 2019, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, Z.; Li, Y.; Chen, C.; Yang, H.; Lin, Q.; Hu, M.; Qin, X. The Cell Origin and Role of Osteoclastogenesis and Osteoblastogenesis in Vascular Calcification. Front. Cardiovasc. Med. 2021, 8, 639740. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Fu, H.; Liu, X.; Duan, K.; Lu, X.; Lu, M.; Sun, T.; Guo, T.; Weng, J. Cation Channel Transient Receptor Potential Vanilloid 4 Mediates Topography-Induced Osteoblastic Differentiation of Bone Marrow Stem Cells. ACS Biomater. Sci. Eng. 2019, 5, 6520–6529. [Google Scholar] [CrossRef]
- Goswami, R.; Merth, M.; Sharma, S.; Alharbi, M.O.; Aranda-Espinoza, H.; Zhu, X.; Rahaman, S.O. TRPV4 calcium-permeable channel is a novel regulator of oxidized LDL-induced macrophage foam cell formation. Free Radic. Biol. Med. 2017, 110, 142–150. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Leddy, H.A.; Benefield, H.C.; Liedtke, W.B.; Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. USA 2014, 111, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Hartmannsgruber, V.; Heyken, W.T.; Kacik, M.; Kaistha, A.; Grgic, I.; Harteneck, C.; Liedtke, W.; Hoyer, J.; Köhler, R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE 2007, 2, e827. [Google Scholar] [CrossRef] [PubMed]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Sánchez, A.C.; Koltsova, E.K. Immune and inflammatory mechanisms of abdominal aortic aneurysm. Front. Immunol. 2022, 13, 989933. [Google Scholar] [CrossRef]
- He, B.; Zhan, Y.; Cai, C.; Yu, D.; Wei, Q.; Quan, L.; Huang, D.; Liu, Y.; Li, Z.; Liu, L.; et al. Common molecular mechanism and immune infiltration patterns of thoracic and abdominal aortic aneurysms. Front. Immunol. 2022, 13, 1030976. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, M.A.; Madhur, M.S.; Merryman, W.D. Adaptive immune cells in calcific aortic valve disease. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H141–H155. [Google Scholar] [CrossRef]
- New, S.E.; Aikawa, E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ. Res. 2011, 108, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Immune mechanisms in medium and large-vessel vasculitis. Nat. Rev. Rheumatol. 2013, 9, 731–740. [Google Scholar] [CrossRef]
- Shannon, A.H.; Elder, C.T.; Lu, G.; Su, G.; Mast, A.; Salmon, M.D.; Montgomery, W.G.; Spinosa, M.D.; Upchurch, G.R., Jr.; Sharma, A.K. Pharmacologic inhibition of transient receptor channel vanilloid 4 attenuates abdominal aortic aneurysm formation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 9787–9801. [Google Scholar] [CrossRef]
- Dutta, B.; Arya, R.K.; Goswami, R.; Alharbi, M.O.; Sharma, S.; Rahaman, S.O. Role of macrophage TRPV4 in inflammation. Lab. Investig. A J. Techol. Methods Pathol. 2020, 100, 178–185. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Huseini, I.; Al-Ismaili, M.; Boudaka, A.; Sirasanagandla, S.R. Deletion of Transient Receptor Channel Vanilloid 4 Aggravates CaCl2-Induced Abdominal Aortic Aneurysm and Vascular Calcification: A Histological Study. Appl. Sci. 2024, 14, 2566. https://doi.org/10.3390/app14062566
Al-Huseini I, Al-Ismaili M, Boudaka A, Sirasanagandla SR. Deletion of Transient Receptor Channel Vanilloid 4 Aggravates CaCl2-Induced Abdominal Aortic Aneurysm and Vascular Calcification: A Histological Study. Applied Sciences. 2024; 14(6):2566. https://doi.org/10.3390/app14062566
Chicago/Turabian StyleAl-Huseini, Isehaq, Maryam Al-Ismaili, Ammar Boudaka, and Srinivasa Rao Sirasanagandla. 2024. "Deletion of Transient Receptor Channel Vanilloid 4 Aggravates CaCl2-Induced Abdominal Aortic Aneurysm and Vascular Calcification: A Histological Study" Applied Sciences 14, no. 6: 2566. https://doi.org/10.3390/app14062566





