The Effect of Lysozyme on the Aggregation and Charging of Oxidized Carbon Nanohorn (CNHox) in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Electrophoretic Mobility
2.2.2. Dynamic Light Scattering
3. Results and Discussion
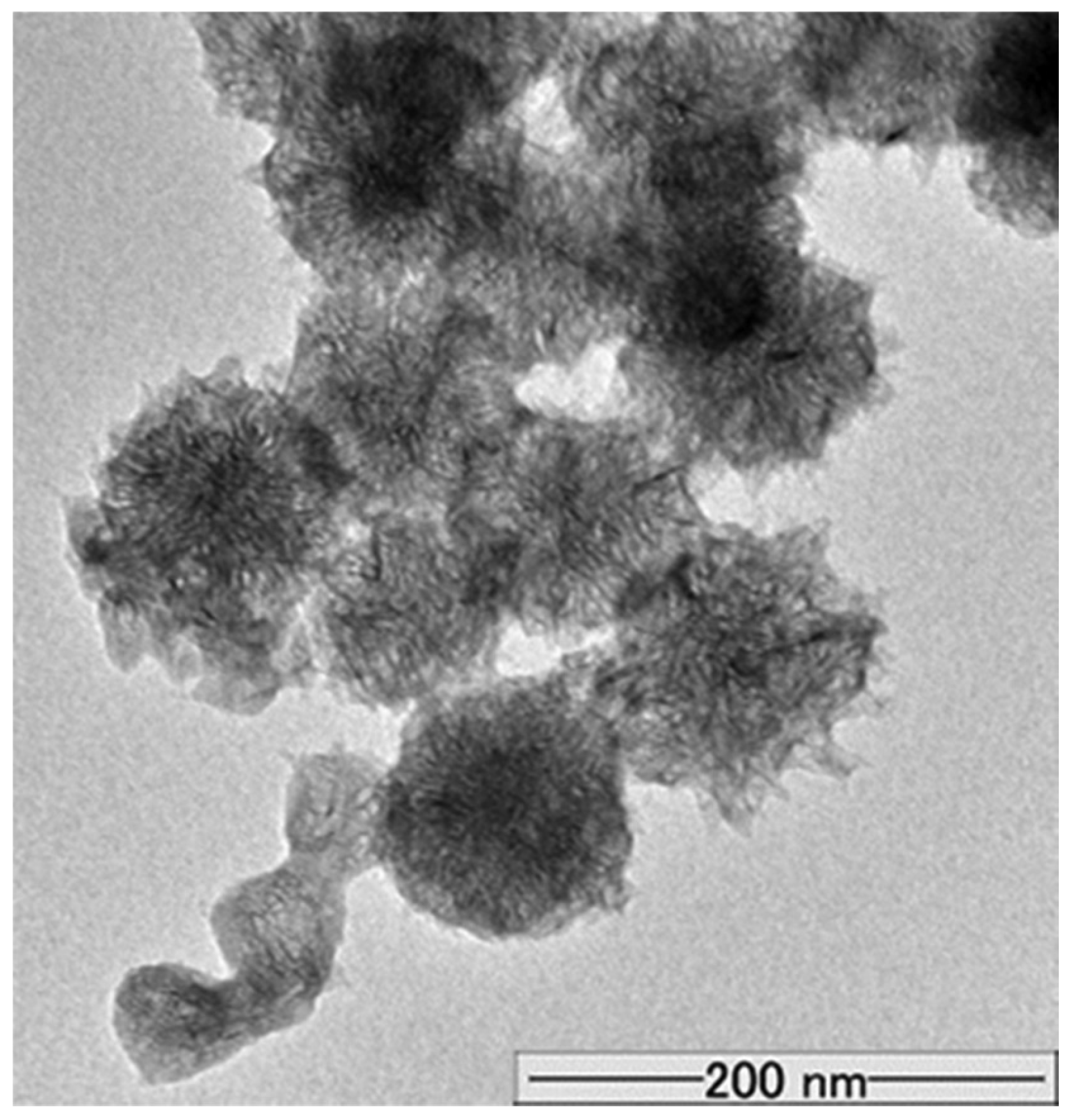
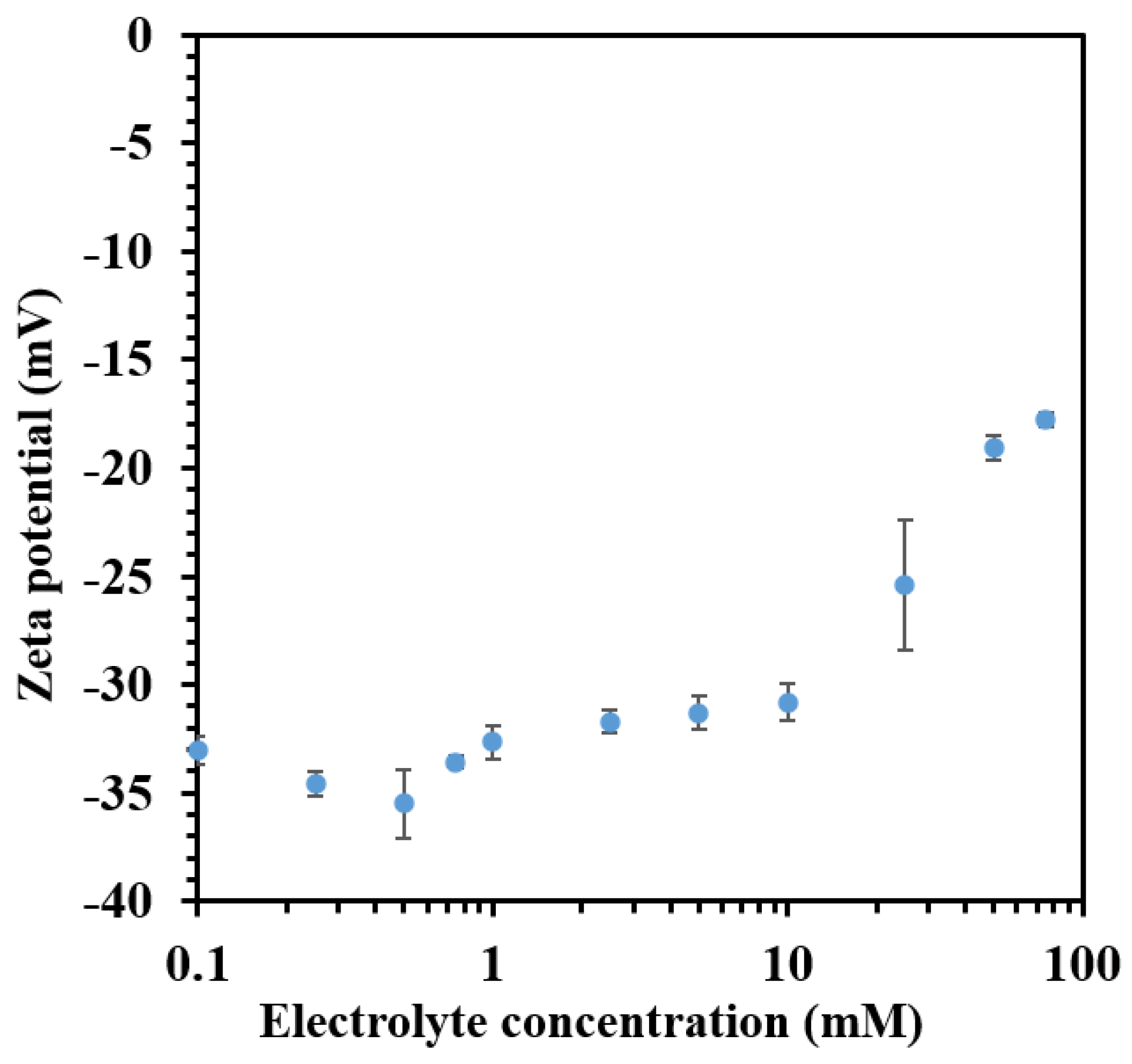
3.1. Charging Behavior of Bare CNHox
3.2. Aggregation Behavior of Bare CNHox
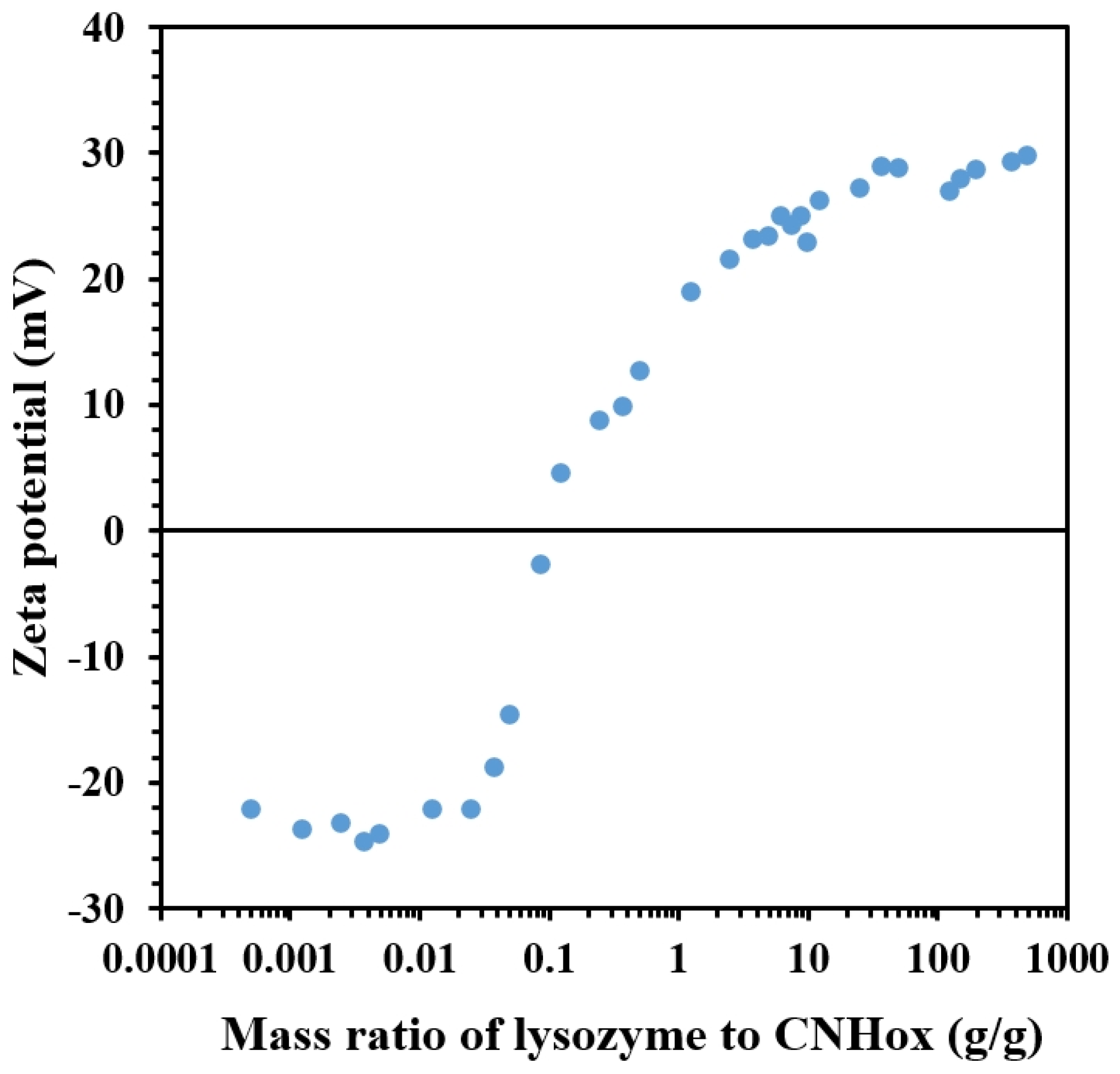
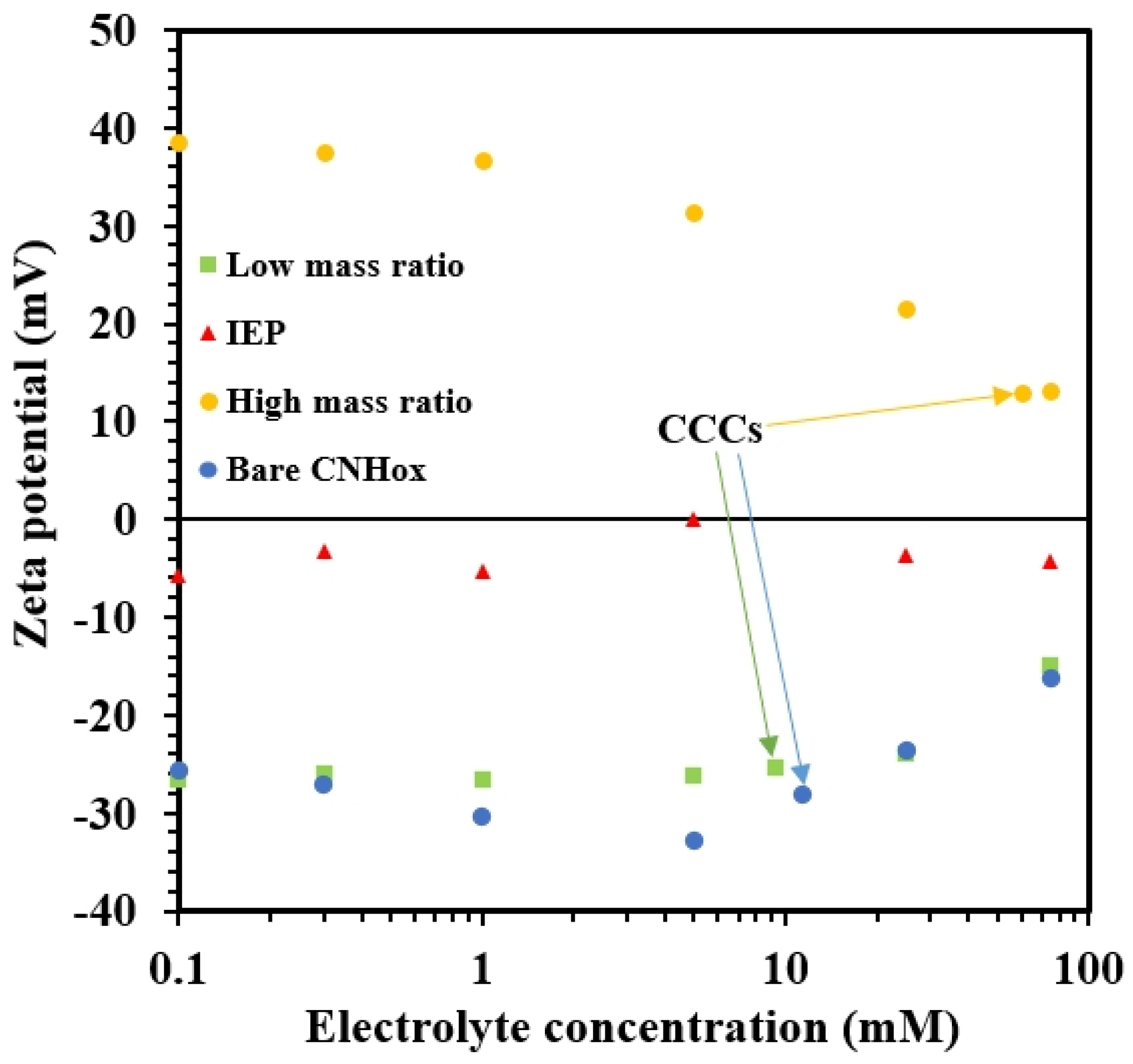
3.3. Effect of LSZ on the Charging Behavior of CNHox
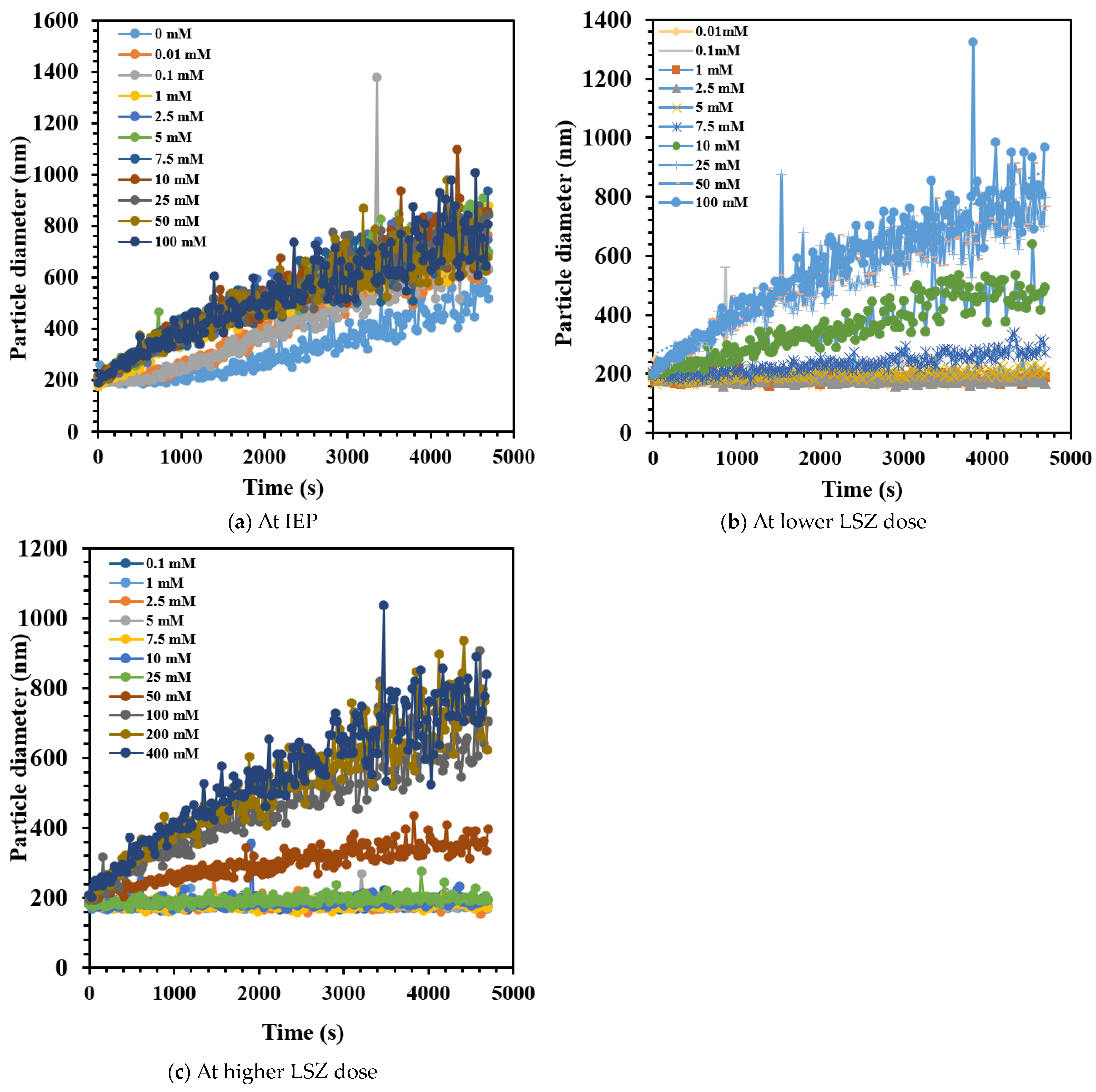
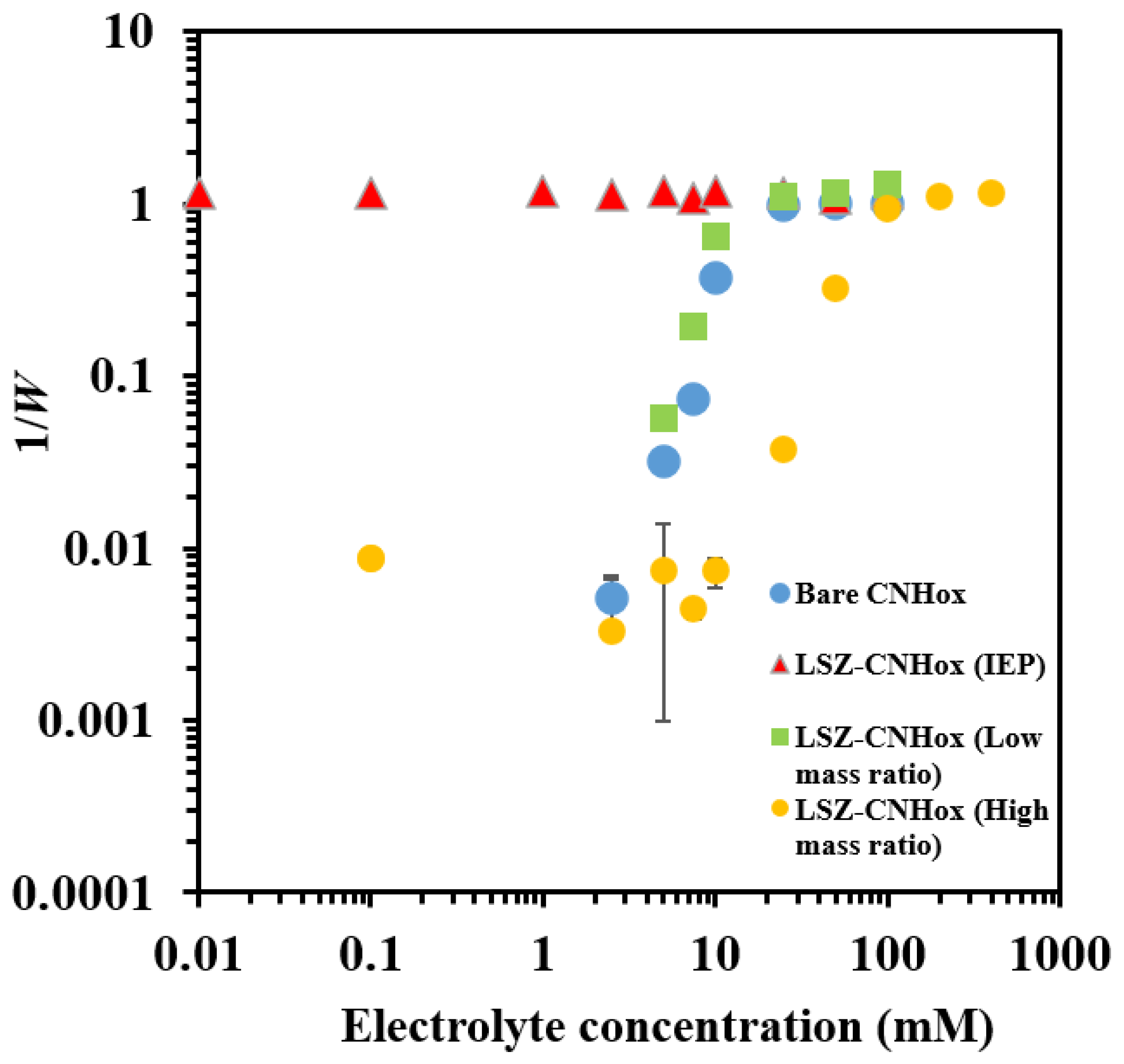
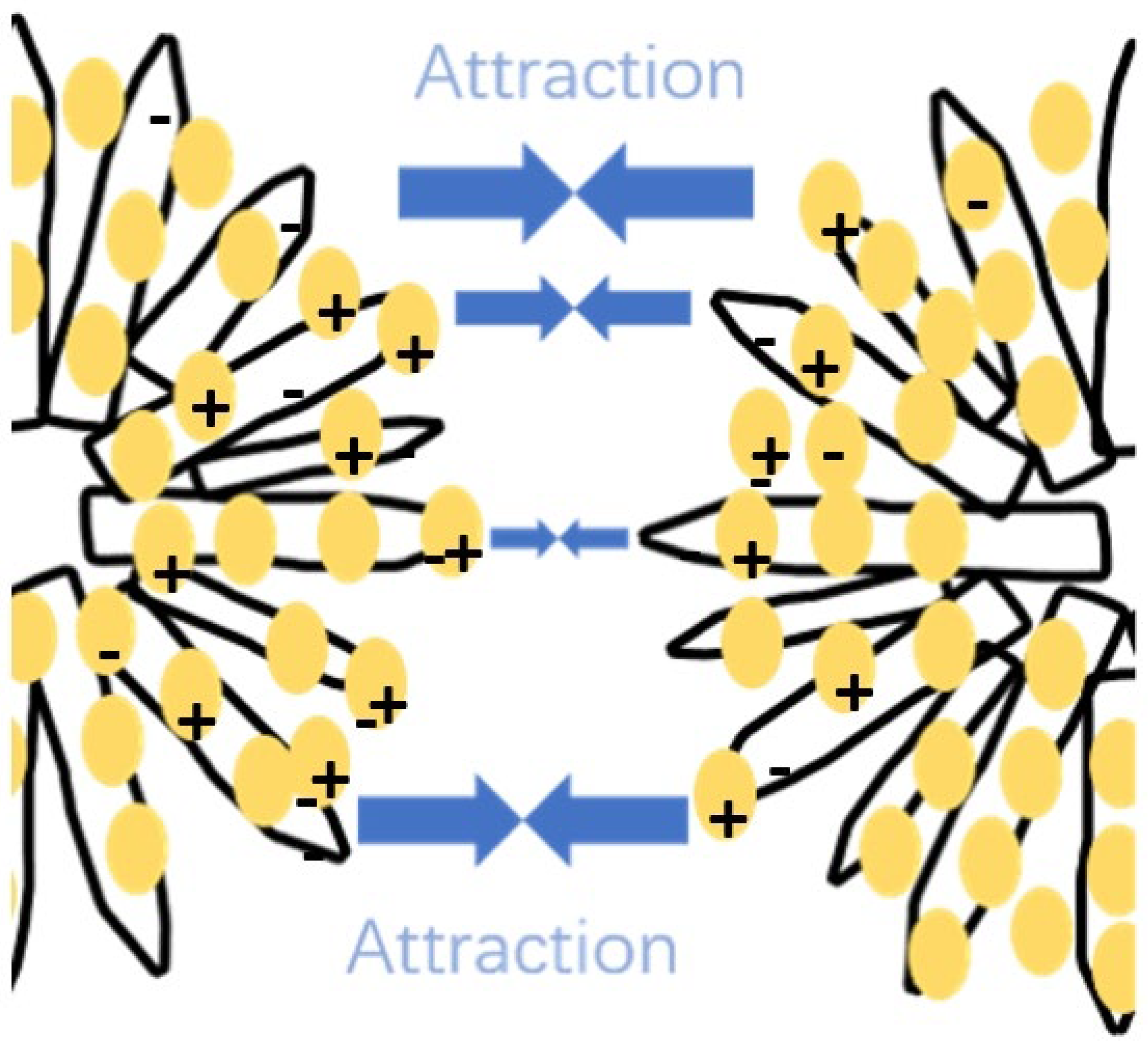
3.4. Effect of LSZ on the Aggregation Behavior of CNHox
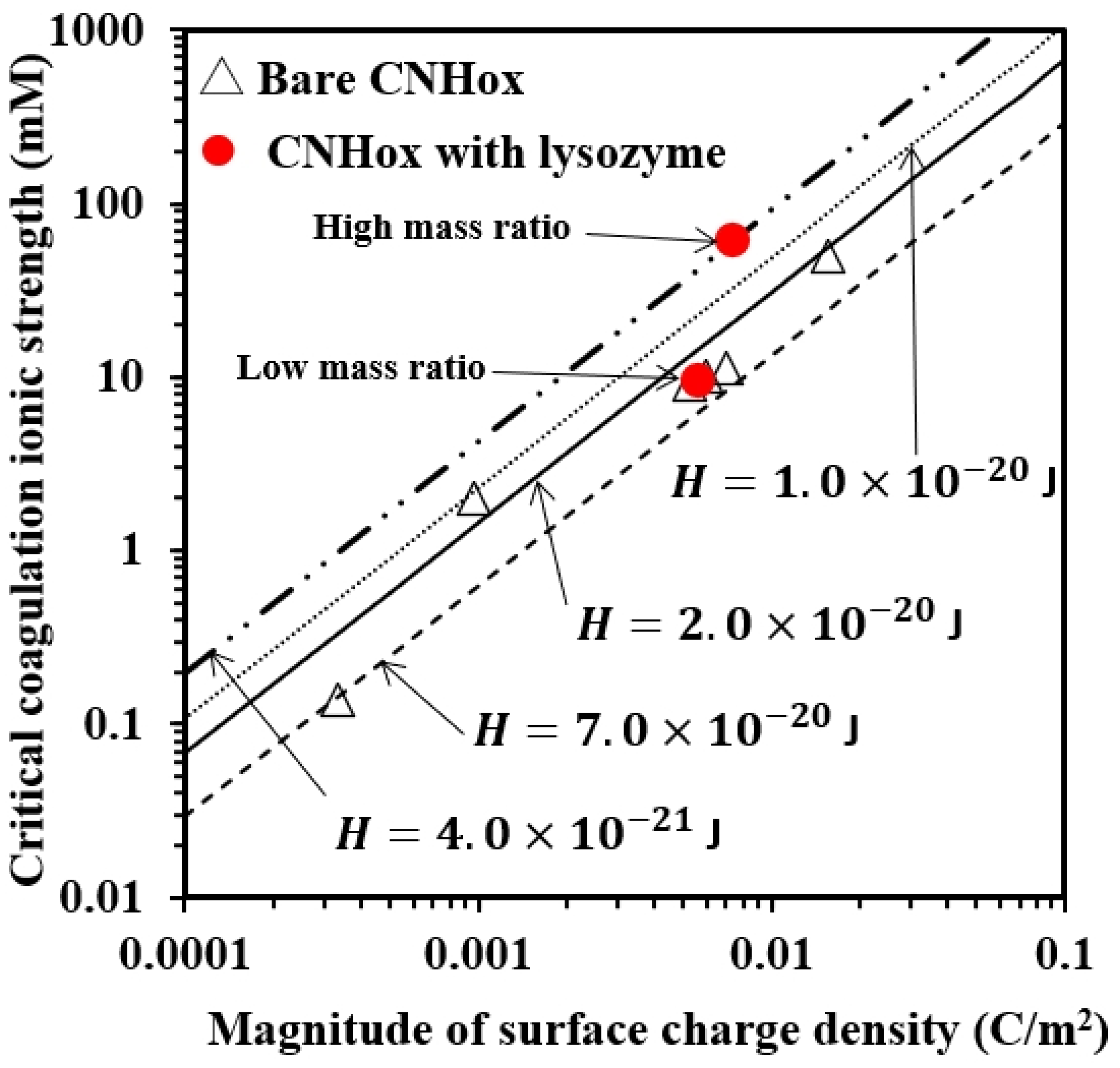
3.5. Comparison between Experimental Data and DLVO Prediction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, B.; Thomas, K.M. Adsorption of aqueous metal ions on oxygen and nitrogen functionalized nanoporous activated carbons. Langmuir 2005, 21, 3892–3902. [Google Scholar] [CrossRef]
- Ajima, K.; Yudasaka, M.; Murakami, T.; Maigné, A.; Shiba, K.; Iijima, S. Carbon nanohorns as anticancer drug carriers. Mol. Pharm. 2005, 2, 475–480. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Sonkar, S.K.; Sarkar, S. Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 2011, 3, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.H.; Decamp, D.L.; Sijbesma, R.P.; Srdanov, G.; Wudl, F.; Kenyon’j, G.L. Inhibition of the HIV-1 Protease by Fullerene Derivatives: Model Building Studies and Experimental Verification. J. Am. Chem. Soc. 1993, 115, 6506–6509. Available online: https://pubs.acs.org/doi/pdf/10.1021/ja00068a005 (accessed on 31 January 2024). [CrossRef]
- Praetorius, A.; Badetti, E.; Brunelli, A.; Clavier, A.; Gallego-Urrea, J.A.; Gondikas, A.; Hassellöv, M.; Hofmann, T.; Mackevica, A.; Marcomini, A.; et al. Strategies for determining heteroaggregation attachment efficiencies of engineered nanoparticles in aquatic environments. Environ. Sci. Nano 2020, 7, 351–367. [Google Scholar] [CrossRef]
- Peijnenburg, W.J.G.M.; Baalousha, M.; Chen, J.; Chaudry, Q.; Von der Kammer, F.; Kuhlbusch, T.A.J.; Lead, J.; Nickel, C.; Quik, J.T.K.; Renker, M.; et al. A Review of the Properties and Processes Determining the Fate of Engineered Nanomaterials in the Aquatic Environment. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2084–2134. [Google Scholar] [CrossRef]
- Lin, D.; Tian, X.; Wu, F.; Xing, B. Fate and Transport of Engineered Nanomaterials in the Environment. J. Environ. Qual. 2010, 39, 1896–1908. [Google Scholar] [CrossRef]
- Liao, P.; Pan, C.; Ding, W.; Li, W.; Yuan, S.; Fortner, J.D.; Giammar, D.E. Formation and Transport of Cr(III)-NOM-Fe Colloids upon Reaction of Cr(VI) with NOM-Fe(II) Colloids at Anoxic-Oxic Interfaces. Environ. Sci. Technol. 2020, 54, 4256–4266. [Google Scholar] [CrossRef]
- Ling, X.; Yan, Z.; Liu, Y.; Lu, G. Transport of nanoparticles in porous media and its effects on the co-existing pollutants. Environ. Pollut. 2021, 283, 117098. [Google Scholar] [CrossRef]
- Kumari, J.; Mathur, A.; Rajeshwari, A.; Venkatesan, A.; Satyavati, S.; Pulimi, M.; Chandrasekaran, N.; Nagarajan, R.; Mukherjee, A. Individual and co transport study of titanium dioxide NPs and zinc oxide NPs in porous media. PLoS ONE 2015, 10, e0134796. [Google Scholar] [CrossRef]
- Jaisi, D.P.; Saleh, N.B.; Blake, R.E.; Elimelech, M. Transport of single-walled carbon nanotubes in porous media: Filtration mechanisms and reversibility. Environ. Sci. Technol. 2008, 42, 8317–8323. [Google Scholar] [CrossRef]
- Li, X.; He, E.; Xia, B.; Van Gestel, C.A.; Peijnenburg, W.J.; Cao, X.; Qiu, H. Impact of CeO2 nanoparticles on the aggregation kinetics and stability of polystyrene nanoplastics: Importance of surface functionalization and solution chemistry. Water Res. 2020, 186, 116324. [Google Scholar] [CrossRef]
- Li, X.; He, E.; Jiang, K.; Peijnenburg, W.J.; Qiu, H. The crucial role of a protein corona in determining the aggregation kinetics and colloidal stability of polystyrene nanoplastics. Water Res. 2021, 190, 116742. [Google Scholar] [CrossRef]
- Omija, K.; Hakim, A.; Masuda, K.; Yamaguchi, A.; Kobayashi, M. Effect of counter ion valence and pH on the aggregation and charging of oxidized carbon nanohorn (CNHox) in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126552. [Google Scholar] [CrossRef]
- Li, M.; Kobayashi, M. The aggregation and charging of natural clay allophane: Critical coagulation ionic strength in the presence of multivalent counter-ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127021. [Google Scholar] [CrossRef]
- Ryan, J.N.; Elimelech, M. Colloid mobilization and transport in groundwater. Colloids Surf. A Physicochem. Eng. Asp. 1996, 107, 1–56. [Google Scholar] [CrossRef]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, K. Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Derjaguin, B.; Landau, L. Theory of the Stability of Strongly Charged Lyophobic Sols and of the Adhesion of Strongly Charged Particles in Solutions of Electrolytes. Prog. Surf. Sci. 1993, 43, 30–59. [Google Scholar] [CrossRef]
- Verwey, E.J.; Overbeek, J.T.G. Theory of the Stability of Lyophobic Colloids; Elsevier Publishing Company: Amsterdam, The Netherlands, 1948. [Google Scholar]
- Ohshima, H. Electrical Phenomena at Interfaces and Biointerfaces, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; Available online: https://books.google.co.jp/books?hl=en&lr=&id=OZzi9FkNiuEC&oi=fnd&pg=PR11&ots=9g91twSkvP&sig=Kwc9mHwCCcNGh2JNsJZ6YgKxV18&redir_esc=y#v=onepage&q&f=false (accessed on 8 February 2024).
- Sugimoto, T.; Cao, T.; Szilagyi, I.; Borkovec, M.; Trefalt, G. Aggregation and charging of sulfate and amidine latex particles in the presence of oxyanions. J. Colloid Interface Sci. 2018, 524, 456–464. [Google Scholar] [CrossRef]
- Huang, Y.; Yamaguchi, A.; Pham, T.D.; Kobayashi, M. Charging and aggregation behavior of silica particles in the presence of lysozymes. Colloid Polym. Sci. 2018, 296, 145–155. [Google Scholar] [CrossRef]
- Takeshita, C.; Masuda, K.; Kobayashi, M. The effect of monovalent anion species on the aggregation and charging of allophane clay nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 103–109. [Google Scholar] [CrossRef]
- Bharti, B.; Meissner, J.; Klapp, S.H.L.; Findenegg, G.H. Bridging interactions of proteins with silica nanoparticles: The influence of pH, ionic strength and protein concentration. Soft Matter 2014, 10, 718–728. [Google Scholar] [CrossRef]
- Adachi, Y.; Feng, L.; Kobayashi, M. Kinetics of flocculation of polystyrene latex particles in the mixing flow induced with high charge density polycation near the isoelectric point. Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 38–44. [Google Scholar] [CrossRef]
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Maroni, P.; Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar] [CrossRef]
- Gillies, G.; Lin, W.; Borkovec, M. Charging and aggregation of positively charged latex particles in the presence of anionic polyelectrolytes. J. Phys. Chem. B 2007, 111, 8626–8633. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kobayashi, S.; Kobayashi, M. Aggregation of colloidal silica particles in the presence of fulvic acid, humic acid, or alginate: Effects of ionic composition. Colloids Surf. A Physicochem. Eng. Asp. 2011, 379, 21–26. [Google Scholar] [CrossRef]
- Saleh, N.B.; Pfefferle, L.D.; Elimelech, M. Influence of biomacromolecules and humic acid on the aggregation kinetics of single-walled carbon nanotubes. Environ. Sci. Technol. 2010, 44, 2412–2418. [Google Scholar] [CrossRef]
- Zieba, W.; Czarnecka, J.; Rusak, T.; Zieba, M.; Terzyk, A.P. Nitric-acid oxidized single-walled carbon nanohorns as a potential material for bio-applications—Toxicity and hemocompatibility studies. Materials 2021, 14, 1419. [Google Scholar] [CrossRef]
- D’amora, M.; Camisasca, A.; Lettieri, S.; Giordani, S. Toxicity assessment of carbon nanomaterials in zebrafish during development. Nanomaterials 2017, 7, 414. [Google Scholar] [CrossRef]
- Zhang, M.; Yudasaka, M.; Ajima, K.; Miyawaki, J.; Iijima, S. Light-assisted oxidation of single-wall carbon nanohorns for abundant creation of oxygenated groups that enable Chemical modifications with proteins to enhance biocompatibility. ACS Nano 2007, 1, 265–272. [Google Scholar] [CrossRef]
- Yuge, R.; Manako, T.; Nakahara, K.; Yasui, M.; Iwasa, S.; Yoshitake, T. The production of an electrochemical capacitor electrode using holey single-wall carbon nanohorns with high specific surface area. Carbon 2012, 50, 5569–5573. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Kobayashi, M. Quantitative evaluation of shift of slipping plane and counterion binding to lysozyme by electrophoresis method. Colloid Polym. Sci. 2016, 294, 1019–1026. [Google Scholar] [CrossRef]
- Jachimska, B.; Kozłowska, A.; Pajor-Świerzy, A. Protonation of lysozymes and its consequences for the adsorption onto a mica surface. Langmuir 2012, 28, 11502–11510. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ahn, S.H.; Kang, S.T.; Yoon, B.J. Electrophoretic mobility equation for protein with molecular shape and charge multipole effects. J. Colloid Interface Sci. 2006, 299, 486–492. [Google Scholar] [CrossRef]
- Ohshima, H. Theory of Colloid and Interfacial Electric Phenomena, 1st ed.; Academic Press: Tokyo, Japan, 2006; Volume 12, Available online: https://books.google.co.jp/books?id=X__ekPRD-Z4C&printsec=frontcover&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false (accessed on 8 February 2024).
- Li, M.; Sugimoto, T.; Yamashita, Y.; Kobayashi, M. Aggregation and charging of natural allophane particles in the presence of oxyanions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129413. [Google Scholar] [CrossRef]
- Grolimund, D.; Elimelech, M.; Borkovec, M. Aggregation and Deposition Kinetics of Mobile Colloidal Particles in Natural Porous Media. Colloids Surf. A Physicochem. Eng. Asp. 2001, 191, 179–188. [Google Scholar] [CrossRef]
- Wei, T.; Carignano, M.A.; Szleifer, I. Molecular dynamics simulation of lysozyme adsorption/desorption on hydrophobic surfaces. J. Phys. Chem. B 2012, 116, 10189–10194. [Google Scholar] [CrossRef]
- Israelachvili, J.; Pashley, R. Measurement of the Hydrophobic Interaction between Two Hydrophobic Surfaces in Aqueous Electrolyte Solutions. J. Colloid Interface Sci. 1984, 98, 500–514. [Google Scholar] [CrossRef]
- Behrens, S.H.; Christl, D.I.; Emmerzael, R.; Schurtenberger, P.; Borkovec, M. Charging and Aggregation Properties of Carboxyl Latex Particles: Experiments verses DLVO Theory. Langmuir 2000, 16, 2566–2575. [Google Scholar] [CrossRef]
- Molina-Bolívar, J.A.; Ortega-Vinuesa, J.L. How proteins stabilize colloidal particles by means of hydration forces. Langmuir 1999, 15, 2644–2653. [Google Scholar] [CrossRef]
- Borkovec, M.; Papastavrou, G. Interactions between solid surfaces with adsorbed polyelectrolytes of opposite charge. Curr. Opin. Colloid Interface Sci. 2008, 13, 429–437. [Google Scholar] [CrossRef]
- Trefalt, G.; Szilagyi, I.; Téllez, G.; Borkovec, M. Colloidal Stability in Asymmetric Electrolytes: Modifications of the Schulze-Hardy Rule. Langmuir 2017, 33, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, E.; Claesson, P.M.; Froberg, J.C.; Tilton, R.D. Interaction between Adsorbed Layers of Lysozyme Studied with the Surface Force Technique. Langmuir 1994, 10, 2325–2334. [Google Scholar] [CrossRef]
- Lotito, V.; Zambelli, T. Approaches to self-assembly of colloidal monolayers: A guide for nanotechnologists. Adv. Colloid Interface Sci. 2017, 246, 217–274. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Li, M.; Sugimoto, T.; Kobayashi, M. The Effect of Lysozyme on the Aggregation and Charging of Oxidized Carbon Nanohorn (CNHox) in Aqueous Solution. Appl. Sci. 2024, 14, 2645. https://doi.org/10.3390/app14062645
Tian Z, Li M, Sugimoto T, Kobayashi M. The Effect of Lysozyme on the Aggregation and Charging of Oxidized Carbon Nanohorn (CNHox) in Aqueous Solution. Applied Sciences. 2024; 14(6):2645. https://doi.org/10.3390/app14062645
Chicago/Turabian StyleTian, Zhengjian, Maolin Li, Takuya Sugimoto, and Motoyoshi Kobayashi. 2024. "The Effect of Lysozyme on the Aggregation and Charging of Oxidized Carbon Nanohorn (CNHox) in Aqueous Solution" Applied Sciences 14, no. 6: 2645. https://doi.org/10.3390/app14062645
APA StyleTian, Z., Li, M., Sugimoto, T., & Kobayashi, M. (2024). The Effect of Lysozyme on the Aggregation and Charging of Oxidized Carbon Nanohorn (CNHox) in Aqueous Solution. Applied Sciences, 14(6), 2645. https://doi.org/10.3390/app14062645






