1. Introduction
Ultrasound guiding has become the standard method for regionally delivering anaesthesia due to its ability to provide the real-time visualization of anatomical structures and the needle trajectory [
1]. However, ultrasound has its limitations, particularly in accurately detecting the needle tip, especially in tissues with complex echogenicity, such as nerves [
2]. This can make it challenging to achieve precise needle placement and avoid nerve damage during regional anaesthesia interventions. In order purportedly to achieve satisfactory anaesthesia delivery and prevent nerve damage, it is recommended that a local anaesthetic is injected distantly from the epineurium [
3]. However, this approach fails to account for the myriad of circumneural collagen fibres and fat cells [
4] that reduce local anaesthetic delivery to the axons, according to Fick’s law of diffusion [
5]. Fat-filled circumneural fascial tissue is only visible via histology [
4] or experimental 40 MHz microultrasound [
6]. Accordingly, different authors [
2,
5,
6] have recommended that the nerve is touched gently [
6] so that the aperture of the block needle partially covers the epineurium and the injectate takes the path of least resistance away from the stiff epineurium to the elastic circumneural fat compartments [
7].
Although high intraneural pressures [
8] and ischemia are acknowledged causes of nerve damage, excessive forces may also cause harm. Steinfeldt et al. demonstrated that forceful needle–nerve contact is associated with intraneural haematoma [
9]. Furthermore, we recently postulated that forceful nerve damage activates the piezo ion channels, the release of intracellular calcium, and the inhibition of the natural repair mechanism of the nerves [
10]. Therefore, understanding the mechanics of needle–tissue interaction is helpful in practical surgical simulation, pre-operation planning, delivering therapeutic drugs, and using robot-assisted mechanical methods [
11]. The pressure that generates first in a syringe–tubing needle system at the beginning of fluid injection is called a bolus. This includes the pressure inside the needle, tubing and syringe at the start of the injection. Multiple variables influence the opening injection pressure, including the injection rate, needle size, tubing length and solution viscosity [
12]. Meanwhile, the pressure applied when injecting a fluid continuously into tissues with a needle is known as the continuous or inline pressure. It is affected by tissue resistance, the needle type and the injection rate [
13].
A puncture force is produced when the tissue surface is penetrated, and its magnitude and duration decrease with an increasing insertion velocity. Larger-diameter needles require higher forces due to increased friction and cutting forces. Increasing the insertion velocity can reduce the puncture force by increasing the energy release rate [
14]. Therefore, when monitoring a needle inserted into soft tissues, the interaction forces increase at the tip of the needle and are the same along the shaft of the needle. The needle tip force is generated due to the cutting of tissues, and the force from the needle shaft results from friction between the tissue and needle [
15]. Meltsner et al. studied needle rotation upon insertion. They argue that it might decrease the frictional force, while improving accuracy. However, needle rotations have been found to increase tissue damage because of the drilling nature of insertion [
11]. In contrast, highly forceful needle insertion can lead to tissue deformation, increased resistance and potential nerve injuries [
16].
A study examined the use of needles by investigating the insertion of a thin bevel-tipped needle, but a thin bevel led to more force and cutting of the tissue [
17]. A recent study used Fiber Bragg Gratings (FBGs) to monitor the real-time force during needle insertion. The FBGs were also incorporated into needles for epidural anaesthesia administration and loss-of-resistance detection. OCT imaging was used to visualise the tissue layers. However, optimizing FBG rubber systems and imaging adipose tissue with OCT was found to be challenging [
18]. The recent advancements in technology, including modifications to needle designs and enhancements in ultrasound machine capabilities, have aimed to enhance these procedures’ precision and efficiency. These advancements include improvements in needle visibility through techniques such as dimpling and coating and adjustments in ultrasound machine algorithms to optimise the needle–beam angles. Additionally, three-dimensional ultrasound offers spatial visualization and the ability to track the local anaesthetic spread. However, despite these technological advancements, challenges persist. Needle visibility enhancements may vary in effectiveness, particularly in superficial blocks, and slower frame rates and a reduced image quality limit three-dimensional ultrasound [
19]. Thus, understanding the forces and pressure involved in needle–tissue interaction and adopting techniques that minimise tissue trauma, while ensuring precise anaesthetic delivery and improving the patients’ outcomes, is essential [
20].
In this study, we aim to investigate needle–tissue interaction by comparing two injection techniques, bolus injection and continuous infusion, using an ex vivo model of fresh lamb sciatic nerves. By evaluating the forces exerted on the nerve tissue during needle–nerve interaction, we aim to determine the efficacy of each technique in minimizing tissue trauma and optimizing anaesthesia delivery. Our ultimate goal is to identify the most effective approach for reducing friction between the needle and tissue, thereby enhancing patients’ safety and procedural outcomes in regional anaesthesia interventions. The findings of this study have significant implications for improving the safety and efficacy of ultrasound-guided regional anaesthesia procedures. This study’s secondary objectives are to compare the force and fluid injection pressure profiles over a range of needle insertion angles (30°, 45° and 60°) and at different tissue sites.
2. Materials and Methods
2.1. Experiment Setup
As part of this research, we utilised a B Braun 20 g needle measuring 150 mm in length with a 30° bevel (BBraun (Melsungen, Germany)) passing through an annular force sensor (LC8100-200-10) from Omega in Manchester, UK to accurately measure the force exerted during each insertion. This sensor was connected to a data acquisition system (cDAQ-9174) from National Instruments in Austin, TX, USA, which communicated real-time force data with the LabVIEW 2017 program that displayed force in real time at (Newton) 10 Hz. To administer the infusion, we used an electromechanical infusion pump (Harvard Apparatus, Holliston, MA, USA, 2001 PHD 2000) to hold a 60 mL plastic syringe attached to a pressure sensor (Pendo TECH Pressure MAT
® (PMAT)) from Princeton, NJ, USA, that measures pressure at (Kpa) 10 Hz. To obtain the necessary data, we used a motor stage (TDC001, Thorlabs, Newton, NJ, USA) to drive the needle into the tissue at three different angles (30°, 45° and 60°) to ensure unbiased allocation and determine the impact of using more angles in a clinic, with a maximum velocity of 1 mm·s
−1 and acceleration of 1 mm·s
−². We utilised an L18-5 MHz linear ultrasound transducer (Phillips EPIQ 5G, Eindhoven, The Netherlands) to provide imaging throughout the procedure as shown in
Figure 1a,b.
2.2. Procedure
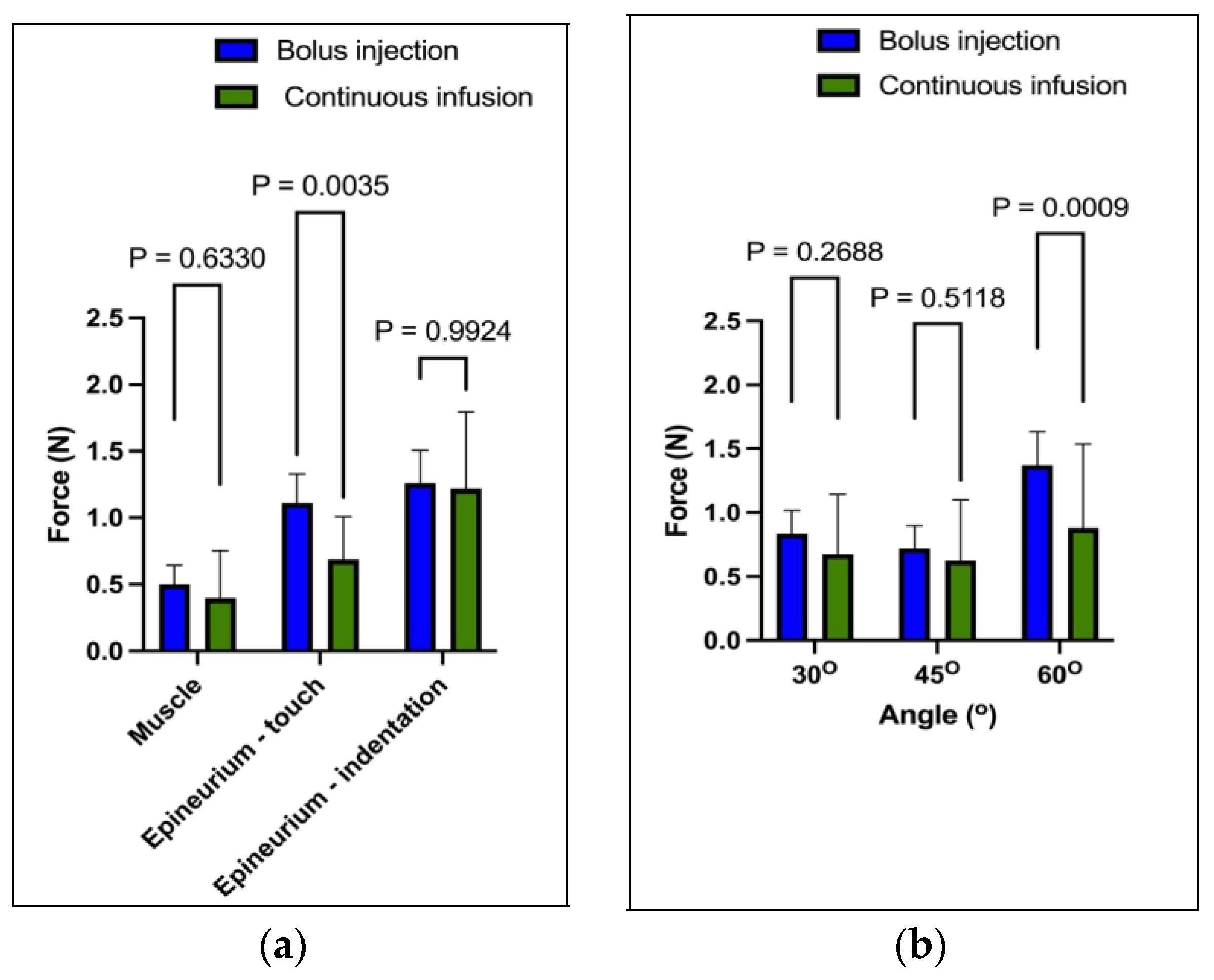
In this experiment, a total of six fresh legs from middle-aged lambs were used, which were placed in plastic trays and secured with a clamp. The experiment was conducted in the ultrasound laboratory of Ninewells Hospital. The experiment involved 162 insertions, which were divided into two types—simultaneous force bolus injections and simultaneous force continuous infusion experiments. Each experimental technique had 81 insertions, with 27 insertions made at each angle. The tissues targeted were muscle, the epineurium was gently touched, and epineurium indentations were made. A distance of 2.5 cm was maintained between each insertion. The motor stage targeted 9 equally spaced sites by driving the needle into the legs at a constant velocity of 1 mm·s−1.
The injections were block randomised to one of two techniques (bolus injection or infusion) at three anatomical locations (the muscle and epineurium, which was gently touched or intended). In order to perform the bolus injection, the needle tip was positioned at each anatomical location, and a 1.8 mL volume of saline (9 mg·mL−1) was delivered using an electromechanical pump (Harvard Instruments, MA) as a bolus over 6 s (18 mL·min−1). In contrast, the infusion was started at time zero before the needle had penetrated the skin and continued for the 60 s (1.8 mL·min−1), which totalled 0.03 mL/s At each site, force and pressure were measured at three angles (30°, 45° and 60°). All the needle insertions and fluid injections were guided using an L18-5 MHz linear transducer (Phillips EPIQ 5G, Eindhoven, The Netherlands). Needle insertion at extreme angles was practically impossible using our motor stage.
The force, pressure and ultrasound videos were time-synchronised, and calibrated instruments were used for each insertion to ensure accuracy and reliability. All the devices were calibrated to zero before each insertion. Time zero was regarded as when the procedure began and the motor stage started moving and the ultrasound video began recording. Then, the data were collected, synchronised and plotted in MATLAB R2023a.
2.3. Statistical Analysis
Dependent data were analysed using the paired t-test and linear mixed models. p-values < 0.05 were considered significant. Tukey–Kramer’s simultaneous confidence intervals are reported for multiple comparisons of all the pairs.
2.4. Power Analysis
As no studies were available to guide power analysis, we arbitrarily assumed an effect size of 0.3, α = 0.05, and β = 0.90. This gave a sample of 120. In order to account for any technical issues, we decided to perform 162 injections.
4. Discussion
In an ex vivo model of legs of lambs, it was observed that the application of a continuous infusion resulted in less needle force generated on the epineurium compared to that of the bolus injection. The difference in the magnitude of the force applied was found to be at the maximum at a 60-degree angle. Upon analysis, it was found that continuous infusion reduced the frictional forces, thus leading to the smooth flow of the injected substance. On the other hand, the bolus injection had to overcome the resistance of the surrounding tissue in order to initiate flow, resulting in a comparatively greater force being applied on the epineurium.
The infusion technique has a principal advantage over the bolus technique in that needle insertion and fluid injection are linked together. This technique is similar to the continuous hydrolocation technique used in clinical practice to detect circumneural spaces, where sustained pressure is applied to the syringe. However, in the bolus technique, the times between needle tip positioning, the initiation of injection, and the time to peak pressure are different. Our research has shown that the mean (SD) time between the initiation of injection and the attainment of peak pressure is 2.8 (1.0) seconds. It is important to note that the delay may be such that the needle may be removed before the peak pressure response has been attained (
Figure 2a,e). This pressure-monitoring facet has not been emphasised previously, but has important implications for clinical practice. If the pressure response is delayed sufficiently during injection, then an anaesthesiologist is powerless to intervene and prevent potential damage from excessively high pressures. Therefore, it is crucial to ensure that pressure monitoring is performed accurately and promptly to avoid potential harm to the patient.
During our injection experiments, we observed an interesting anomaly, which involved a sudden rise in pressure before the force of the injection at a 60-degree angle. This phenomenon occurred because the cutting edge of the needle was more perpendicular to the tissue surface at this angle, requiring a greater force to penetrate the tissue. As a general principle, we found that the greater the angle of the needle to the perpendicular fibres and the greater the stiffness of the tissues are, the greater the resistance during tissue penetration is. This increased resistance made the needles more likely to deflect and bend, and the injected liquid was more likely to flow retrograde along the path of least resistance into compliant adipose tissue.
This research has identified a wide range of forces and pressures that are measured in muscles when subjected to a gentle touch and the indentation of the epineurium. This variation in force can be attributed to the needle tip position and is consistent with the previous studies conducted on pigs [
6] and soft embalmed cadavers [
10,
21]. However, the standard ultrasound technology used in medical procedures does not provide a sufficient resolution to accurately determine the needle tip’s precise location. When gently touching the epineurium, the needle tip may partially reside within the epineurium and partly face the circumneural fascial fibres, making it difficult to determine the exact location. This scenario makes it highly likely that any injection will take the path of least resistance and flow around the nerve. Upon indentation of the epineurium, the needle tip may partially penetrate the epineurium, making the injection intraneural, but not visible via ultrasound. In contrast, when a needle is inserted into the muscle, the tip orifice may lie partially on collagenous fibres, such as the epimysium (surrounding the muscle), or partially within the perimysium. This information highlights the complexity of needle insertion and injection techniques and emphasises the importance of considering the physical properties of different tissues when performing medical procedures.
Based on the results obtained, we have formulated a hypothesis that a continuous infusion technique is more effective in identifying the subcirumneural spaces with a less force as compared to the standard bolus technique. Our hypothesis is supported by some evidence as well. In particular, researchers using an epidural needle-based system that relies on computerised injection pump technology (Compuflo, Livingston, NJ, USA) reported higher injection accuracy with fewer dural punctures [
22]. The system employs a constant infusion rate of approximately 3 mL/min and measures the changes in injection pressure at 4 Hz. Furthermore, an ex vivo study has suggested that this system is more accurate than the B-Smart (BS) in-line manometer and could be used for peripheral nerve blocking procedures [
23]. In our study of isolated nerves, we followed the same fundamental principles as in previous research. However, in our study, we infused saline at a rate of 1.8 mL per minute and measured the pressure and force at a frequency of 10 Hz. Our primary focus was examining the initial interaction between the needle and the nerve and the hypothetical separation of the circumneurium. At this stage, we cannot comment on the clinical applications of this technique, as further research is needed to investigate the subsequent dosing and other aspects. In order to gain a more detailed understanding of the changes at the epineurium and the subcircumneural compartments, we plan to conduct future studies using microultrasound on cadaver models.
The research findings presented a comprehensive understanding of needle–tissue interaction dynamics, directly impacting clinical practices in regional anaesthesia. Specifically, our study explains the biomechanical factors influencing tissue trauma and injection precision. By integrating these insights into clinical protocols, practitioners can tailor their approaches to minimise tissue trauma and optimise injection precision. This involves careful consideration of needle insertion angles and infusion techniques to reduce the force exertion on tissues during needle insertion. Additionally, the findings of the result underscore the significance of real-time visualization technologies in guiding needle placement with heightened accuracy and safety. Moreover, this study assesses the stage for future research to refine the existing techniques and develop creative technologies to enhance the efficacy and safety of ultrasound-guided regional anaesthesia procedures.
This study we conducted has two main impacts that are worth highlighting. Firstly, force is a physical entity used in bioengineering to identify and measure the interaction of trocars, needles and sutures with soft tissue. However, it has yet to be widely applied to regional anaesthesia procedures [
24]. Our research findings suggest that the forces generated during regional anaesthesia administration can be significant, but are rarely appreciated because they are not measured. We have performed ongoing work showing that forceful interaction with tissues is a crucial aspect of regional anaesthesia administration, which deserves further exploration. Secondly, using a liquid as a lubricant could potentially improve the ease and efficiency of medical procedures. Our study results can inform the understanding of the forces encountered during needle-based procedures in soft tissue. These findings have significant implications in the medical field as they may lead to the development of more effective techniques for carrying out procedures like anaesthesia- and other needle-based procedures.
The biomechanical factors that influence needle–tissue interaction during regional anaesthesia procedures are significant, as highlighted by this study. The study results emphasise the significance of the needle insertion angle and infusion rates in reducing tissue force exertion, which is aligned with the proposed needle–tissue interaction computation model. This model provides a comprehensive framework for predicting and planning needle insertion paths before surgery, utilising a Kriging-based friction force calculation model for accurate real-time friction estimation [
25]. Our study’s recommendations for optimising the needle insertion angles and techniques can help clinicians minimise tissue damage and enhance patients’ safety. This research confirms that using shallower angles during needle insertion results in lower force generation, which is attributed to the smoother traversal of the needle through tissue with reduced resistance. Therefore, using shallower insertion angles allows for a more controlled and gradual approach, which is crucial for minimising tissue trauma during regional anaesthesia procedures. In contrast, more vertical angles resulted in a shorter time for injection to reach peak pressure and higher force. Using a more vertical angle for injection means that the needle can penetrate the tissue more directly, reducing the distance it travels to reach the target site. This direct path allows the injected fluid to encounter more resistance within the tissue, facilitating quicker pressure buildup created by the vertical angle, leading to a shorter time for the injected fluid to reach its maximum pressure. More vertical angles require a greater force to penetrate the tissue than the shallower angles, resulting in increased force exertion. The needle’s perpendicular or near-perpendicular orientation relative to the tissue surface requires greater mechanical energy to overcome tissue resistance, which is why an increased force is needed. The combination of increased force and resistance leads to a quicker escalation in pressure, resulting in the more rapid attainment of peak pressure during injection. However, it is essential to note this study’s limitations. Firstly, this study used an ex vivo lamb leg model, and the results may need to be validated using in vivo tissue dynamics. Secondly, this study did not investigate the variations in needle bevel angles, which could have influenced the force dynamics and tissue response. Lastly, future research is needed to explore the impact of different infusion rates on needle–tissue interaction, providing a deeper understanding of the needle–tissue interaction and optimised needle insertion techniques for regional anaesthesia-related procedures.