The Antifungal Effect of Weissella confusa WIKIM51 (Wilac D001) on Vaginal Epithelial Cells Infected by Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal and Bacterial Strains
2.2. Culture of Vaginal Epithelial Cell
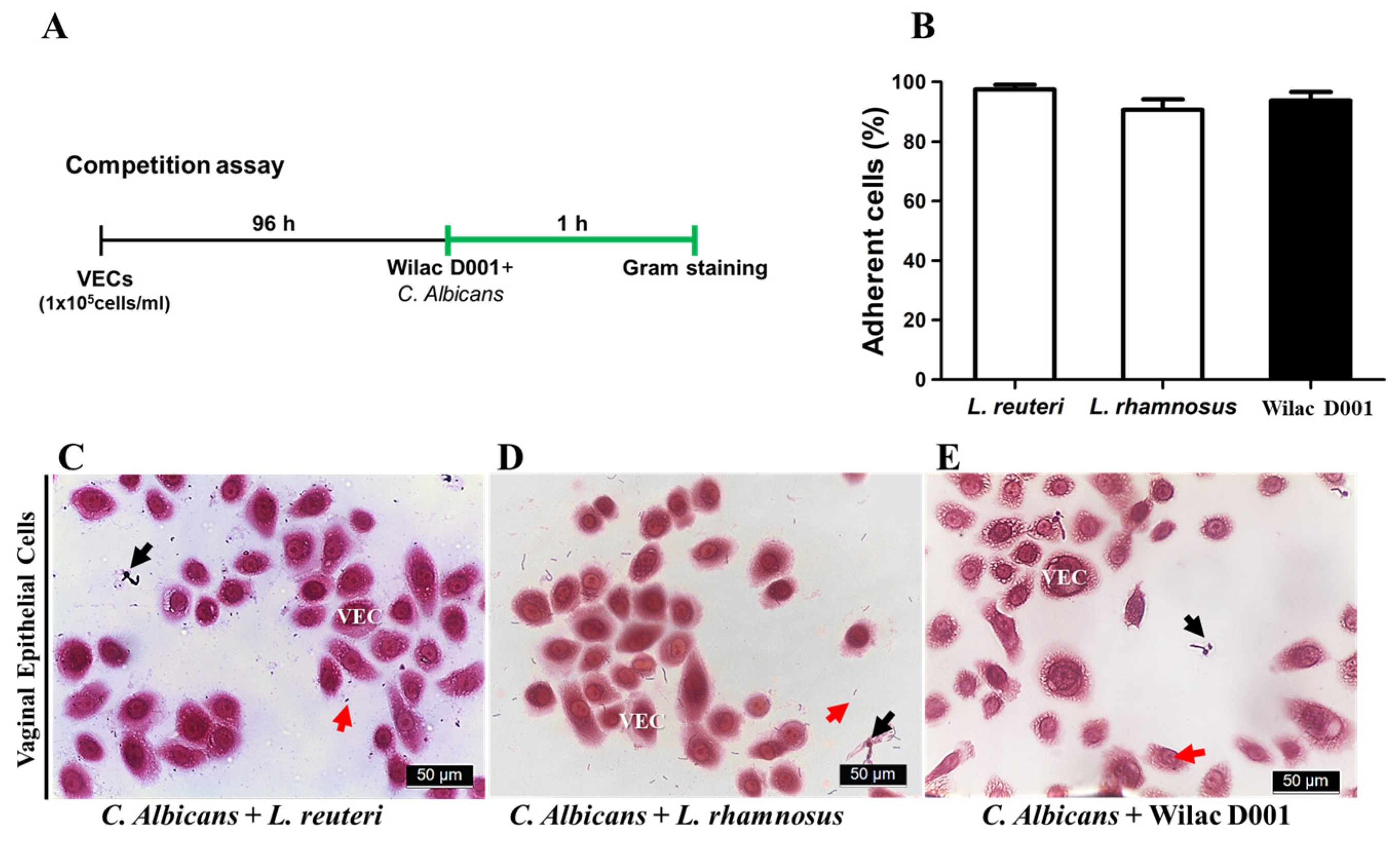
2.3. Adhesion Test
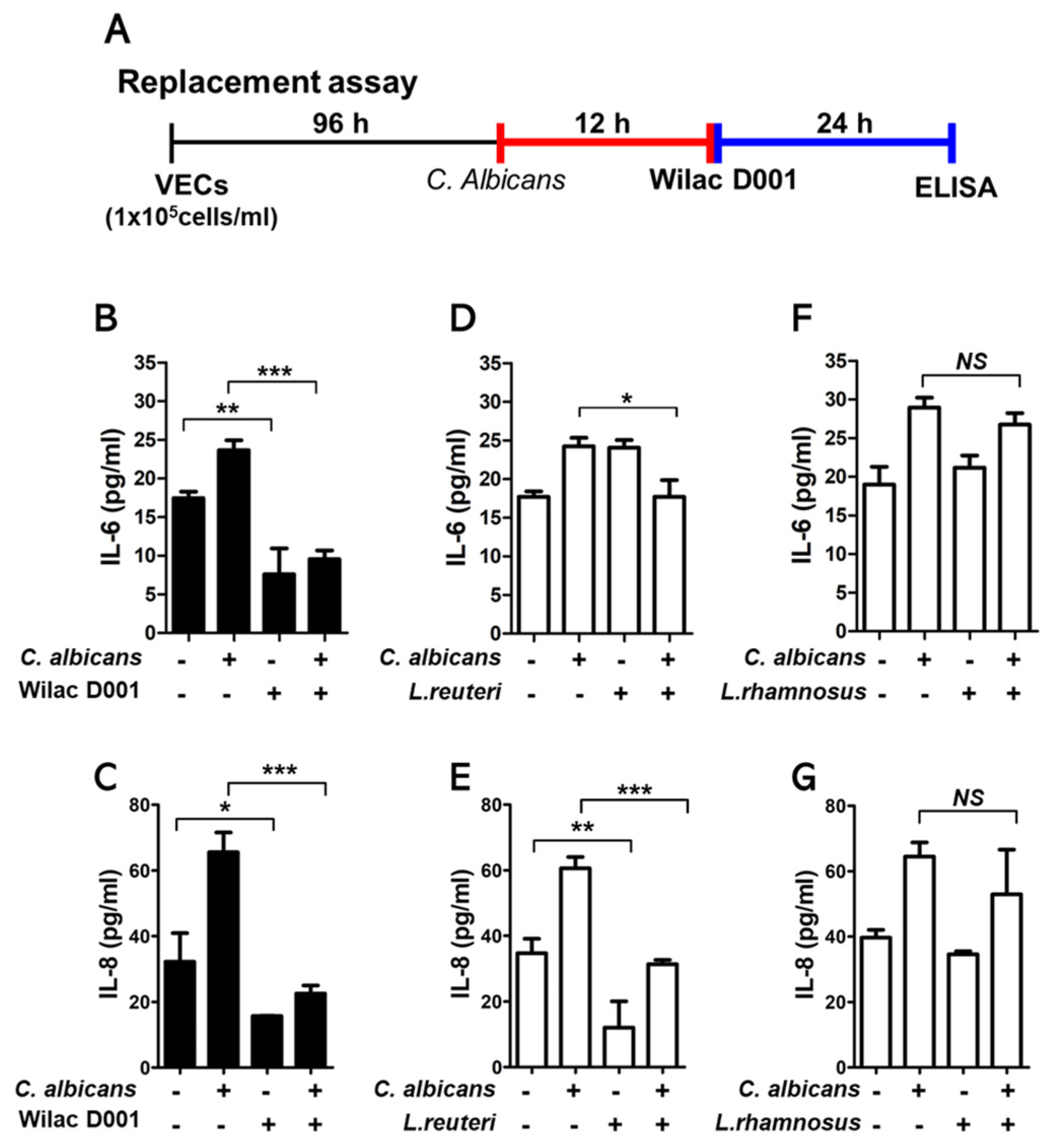
2.4. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
2.5. Disk Diffusion Assay
2.6. Statistical Analysis
3. Results
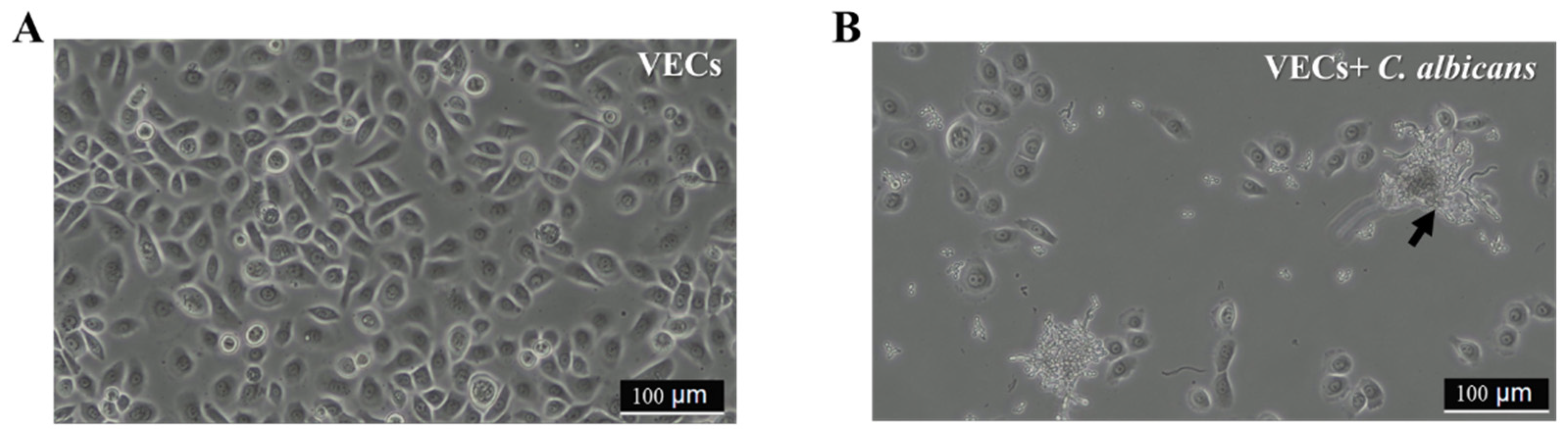
3.1. Protective Effect of Wilac D001 against C. albicans on VECs
3.2. The Inhibitory Effect of Pro−Inflammatory Cytokine Production
3.3. Susceptibility of Wilac D001 against C. alibcans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yano, J.; Sobel, J.D.; Nyirjesy, P.; Sobel, R.; Williams, V.L.; Yu, Q.; Noverr, M.C.; Fidel, P.L. Current patient perspectives of vulvovaginal candidiasis: Incidence, symptoms, management and post-treatment outcomes. BMC Womens Health 2019, 19, 48. [Google Scholar] [CrossRef]
- Sobel, J.D.; Faro, S.; Force, R.W.; Foxman, B.; Ledger, W.J.; Nyirjesy, P.R.; Reed, B.D.; Summers, P.R. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol. 1998, 178, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cerdeira, C.; Gregorio, M.C.; Molares-Vila, A.; López-Barcenas, A.; Fabbrocini, G.; Bardhi, B.; Sinani, A.; Sánchez-Blanco, E.; Arenas-Guzmán, R.; Hernandez-Castro, R. Biofilms and vulvovaginal candidiasis. Colloids Surf. B Biointerfaces 2019, 174, 110–125. [Google Scholar] [CrossRef]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Come, R.A.; Moench, T.R. Vaginal pH measured in vivo: Lactobacilli determine pH and lactic acid concentration. BMC Microbiol. 2019, 19, 13. [Google Scholar] [CrossRef]
- Xu, J.; Sobel, J.D. Antibiotic-associated vulvovaginal candidiasis. Curr. Infect. Dis. Rep. 2003, 5, 481–487. [Google Scholar] [CrossRef]
- Reed, B.D. Risk factors for Candida vulvovaginitis. Obstet. Gynecol. Surv. 1992, 47, 551–560. [Google Scholar] [CrossRef]
- De Gregorio, P.R.; Parolin, C.; Abruzzo, A.; Luppi, B.; Protti, M.; Mercolin, L.; Silva, J.A.; Giordani, B.; Marangoni, A.; Nader-Macías, M.E.F. Biosurfactant from vaginal Lactobacillus crispatus BC1 as a promising agent to interfere with Candida adhesion. Microb. Cell Factories 2020, 19, 133. [Google Scholar] [CrossRef]
- Bertarello, C.; Savio, D.; Morelli, L.; Bouzalov, S.; Davidova, D.; Bonetti, A. Efficacy and safety of Lactobacillus plantarum P 17630 strain soft vaginal capsule in vaginal candidiasis: A randomized non-inferiority clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 384–391. [Google Scholar]
- De Vuyst, L.; Callewaert, R.; Crabbé, K. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 1996, 142, 817–827. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O. The vaginal microenvironment: The physiologic role of lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Manuzak, J.A.; Hensley-McBain, T.; Zevin, A.S.; Miller, C.; Cubas, R.; Agricola, B.; Gile, J.; Richert-Spuhler, L.; Patilea, G.; Estes, J.D.; et al. Enhancement of Microbiota in Healthy Macaques Results in Beneficial Modulation of Mucosal and Systemic Immune Function. J. Immunol. 2016, 196, 2401–2409. [Google Scholar] [CrossRef]
- Martinez, R.; Franceschini, S.A.; Patta, M.C.; Quintana, S.M.; Candido, R.C.; Ferreira, J.C.; De Martinis, E.C.P.; Reid, G. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett. Appl. Microbiol. 2009, 48, 269–274. [Google Scholar] [CrossRef]
- Chew, S.Y.; Cheah, Y.K.; Seow, H.F.; Sandai, D.; Than, L.T.L. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J. Appl. Microbiol. 2015, 118, 1180–1190. [Google Scholar] [CrossRef]
- Köhler, G.A.; Assefa, S.; Reid, G. Probiotic Interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the Opportunistic Fungal Pathogen Candida albicans. Infect. Dis. Obstet. Gynecol. 2012, 2012, 636474. [Google Scholar] [CrossRef]
- Collins, M.D.; Samelis, J.; Metaxopoulos, J.; Wallbanks, S. Taxonomic studies on some leuconostoc-like organisms from fermented sausages: Description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 1993, 75, 595–603. [Google Scholar] [CrossRef]
- Choi, I.-K.; Jung, S.-H.; Kim, B.-J.; Park, S.-Y.; Kim, J.; Han, H.-U. Novel Leuconostoc citreum starter culture system for the fermentation of kimchi, a fermented cabbage product. Antonie Van Leeuwenhoek 2003, 84, 247–253. [Google Scholar] [CrossRef]
- Park, S.M.; Mok, J.Y.; Cho, H.-R.; Song, I.-b.; Shin, Y.J.; Lee, K.b.; Lee, B.W.; Bae, M.-J. Effects of Weissella confusa Wilac D001 Isolated from Dandelion Kimchi on Dextran Sulphate Sodium-Induced Colitis in Mice. Food Suppl. Biomater. Health 2021, 1, e25. [Google Scholar] [CrossRef]
- Jin, H.; Jeong, Y.; Yoo, S.-H.; Johnston, T.V.; Ku, S.; Ji, G.E. Isolation and characterization of high exopolysaccharide-producing Weissella confusa VP30 from young children’s feces. Microb. Cell Factories 2019, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Lucid, A.; Arendt, E.K.; Sleator, R.D.; Lucey, B.; Coffey, A. Genomics of Weissella cibaria with an examination of its metabolic traits. Microbiology 2015, 161, 914–930. [Google Scholar] [CrossRef] [PubMed]
- You, Y.A.; Kwon, E.J.; Choi, S.J.; Hwang, H.S.; Choi, S.K.; Lee, S.M.; Kim, Y.J. Vaginal microbiome profiles of pregnant women in Korea using a 16S metagenomics approach. Am. J. Reprod. Immunol. 2019, 82, e13124. [Google Scholar] [CrossRef]
- Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Han, K.-S.; Lee, J.; Wang, M.-H. Polysaccharides of Weissella cibaria Act as a Prebiotic to Enhance the Probiotic Potential of Lactobacillus rhamnosus. Appl. Biochem. Biotechnol. 2023, 195, 3928–3940. [Google Scholar] [CrossRef]
- Lim, S.K.; Kwon, M.-S.; Lee, J.; Oh, Y.J.; Jang, J.-Y.; Lee, J.-H.; Park, H.W.; Nam, Y.-D.; Seo, M.-J.; Roh, S.W. Weissella cibaria WIKIM28 ameliorates atopic dermatitis-like skin lesions by inducing tolerogenic dendritic cells and regulatory T cells in BALB/c mice. Sci. Rep. 2017, 7, 40040. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, R.; Hornby, J.M.; Nickerson, K.W. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob. Agents Chemother. 2004, 48, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Osset, J.; Bartolome, R.M.; Garcia, E.; Andreu, A. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J. Infect. Dis. 2001, 183, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Naidu, A.S.; Chen, J.; Martinez, C.; Tulpinski, J.; Pal, B.K.; Fowler, R.S. Activated lactoferrin’s ability to inhibit Candida growth and block yeast adhesion to the vaginal epithelial monolayer. J. Reprod. Med. 2004, 49, 859–866. [Google Scholar] [PubMed]
- Zárate, G.; Nader-Macias, M. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett. Appl. Microbiol. 2006, 43, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.-X.; Li, T.; Zhang, X.; Wang, S.-X.; Liu, Z.-H. Lactobacillus crispatus Modulates Vaginal Epithelial Cell Innate Response to Candida albicans. Chin. Med. J. 2017, 130, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Matevosyan, L.; Bazukyan, I.; Trchounian, A. Antifungal and antibacterial effects of newly created lactic acid bacteria associations depending on cultivation media and duration of cultivation. BMC Microbiol. 2019, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Hočevar, K.; Maver, A.; Vidmar Šimic, M.; Hodžić, A.; Haslberger, A.; Premru Seršen, T.; Peterlin, B. Vaginal microbiome signature is associated with spontaneous preterm delivery. Front. Med. 2019, 6, 201. [Google Scholar] [CrossRef]
- Elizaldi, S.R.; Verma, A.; Walter, K.A.; Rolston, M.; Dinasarapu, A.R.; Durbin-Johnson, B.P.; Settles, M.; Kozlowski, P.A.; Raeman, R.; Iyer, S.S. Rectal microbiome composition correlates with humoral immunity to HIV-1 in vaccinated rhesus macaques. Msphere 2019, 4, e00824-00819. [Google Scholar] [CrossRef]
- Hummelen, R.; Vos, A.P.; van’t Land, B.; van Norren, K.; Reid, G. Altered host-microbe interaction in HIV: A target for intervention with pro- and prebiotics. Int. Rev. Immunol. 2010, 29, 485–513. [Google Scholar] [CrossRef]
- Cheaito, R.A.; Awar, G.; Alkozah, M.; Cheaito, M.A.; El Majzoub, I. Meningitis due to Weissella confusa. Am. J. Emerg. Med. 2020, 38, 1298.e1–1298.e3. [Google Scholar] [CrossRef]
- Cupi, D.; Elvig-Jørgensen, S.G. Safety assessment of Weissella confusa–A direct-fed microbial candidate. Regul. Toxicol. Pharmacol. 2019, 107, 104414. [Google Scholar] [CrossRef]
- Ahmed, S.; Singh, S.; Singh, V.; Roberts, K.D.; Zaidi, A.; Rodriguez-Palacios, A. The Weissella genus: Clinically treatable bacteria with antimicrobial/probiotic effects on inflammation and cancer. Microorganisms 2022, 10, 2427. [Google Scholar] [CrossRef]
- Fatmawati, N.N.D.; Gotoh, K.; Mayura, I.P.B.; Nocianitri, K.A.; Suwardana, G.N.R.; Komalasari, N.L.G.Y.; Ramona, Y.; Sakaguchi, M.; Matsushita, O.; Sujaya, I.N. Enhancement of intestinal epithelial barrier function by Weissella confusa F213 and Lactobacillus rhamnosus FBB81 probiotic candidates in an in vitro model of hydrogen peroxide-induced inflammatory bowel disease. BMC Res. Notes 2020, 13, 489. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Pham, D.T.N.; Tabassum, N.; Khan, M.S.A.; Kim, Y.-M. Mixed biofilms of pathogenic Candida-bacteria: Regulation mechanisms and treatment strategies. Crit. Rev. Microbiol. 2021, 47, 699–727. [Google Scholar] [CrossRef]
- Ahmad Khalili, A.; Ahmad, M.R. A review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Coudeyras, S.; Jugie, G.; Vermerie, M.; Forestier, C. Adhesion of human probiotic Lactobacillus rhamnosus to cervical and vaginal cells and interaction with vaginosis-associated pathogens. Infect. Dis. Obstet. Gynecol. 2008, 2008, 549640. [Google Scholar] [CrossRef]
- Steele, C.; Fidel Jr, P.L. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect. Immun. 2002, 70, 577–583. [Google Scholar] [CrossRef]
- Spear, G.T.; Zariffard, M.R.; Cohen, M.H.; Sha, B.E. Vaginal IL-8 levels are positively associated with Candida albicans and inversely with lactobacilli in HIV-infected women. J. Reprod. Immunol. 2008, 78, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Hackett, A.; Trinick, R.; Rose, K.; Flanagan, B.; McNamara, P. Weakly acidic pH reduces inflammatory cytokine expression in airway epithelial cells. Respir. Res. 2016, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Xu, J.; Schwartz, K.; Bartoces, M.; Monsur, J.; Severson, R.K.; Sobel, J.D. Effect of Antibiotics on Vulvovaginal Candidiasis: A MetroNet Study. J. Am. Board. Fam. Med. 2008, 21, 261–268. [Google Scholar] [CrossRef]
- Hassanzadazar, H.; Ehsani, A.; Mardani, K.; Hesari, J. Investigation of antibacterial, acid and bile tolerance properties of lactobacilli isolated from Koozeh cheese. Proc. Vet. Res. Forum 2012, 3, 181–185. [Google Scholar]
| Negative | Positive Control | Wilac D001 | |||
|---|---|---|---|---|---|
| Blank | Sertaconazole Nitrate | L. rueteri | L. rhamnosus | ||
| ZoI (diameter, mm) | 6.0 ± 0.0 | 18.3 ± 0.6 | 10.8 ± 0.3 *,# | 11.2 ± 0.3 *,# | 11.2 ± 0.3 *,# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; You, Y.-A.; Ansari, A.; Go, Y.-Y.; Park, S.; Hur, Y.M.; Kim, S.-M.; Park, S.M.; Kim, Y.J. The Antifungal Effect of Weissella confusa WIKIM51 (Wilac D001) on Vaginal Epithelial Cells Infected by Candida albicans. Appl. Sci. 2024, 14, 2676. https://doi.org/10.3390/app14072676
Lee G, You Y-A, Ansari A, Go Y-Y, Park S, Hur YM, Kim S-M, Park SM, Kim YJ. The Antifungal Effect of Weissella confusa WIKIM51 (Wilac D001) on Vaginal Epithelial Cells Infected by Candida albicans. Applied Sciences. 2024; 14(7):2676. https://doi.org/10.3390/app14072676
Chicago/Turabian StyleLee, Gain, Young-Ah You, Abuzar Ansari, Yoon-Young Go, Sunwha Park, Young Min Hur, Soo-Min Kim, Sang Min Park, and Young Ju Kim. 2024. "The Antifungal Effect of Weissella confusa WIKIM51 (Wilac D001) on Vaginal Epithelial Cells Infected by Candida albicans" Applied Sciences 14, no. 7: 2676. https://doi.org/10.3390/app14072676
APA StyleLee, G., You, Y.-A., Ansari, A., Go, Y.-Y., Park, S., Hur, Y. M., Kim, S.-M., Park, S. M., & Kim, Y. J. (2024). The Antifungal Effect of Weissella confusa WIKIM51 (Wilac D001) on Vaginal Epithelial Cells Infected by Candida albicans. Applied Sciences, 14(7), 2676. https://doi.org/10.3390/app14072676





