Nutritional Composition and Safety Parameters of Mealworms (Tenebrio molitor) Reared on Substrates Derived from By-Products
Abstract
1. Introduction
2. Materials and Methods
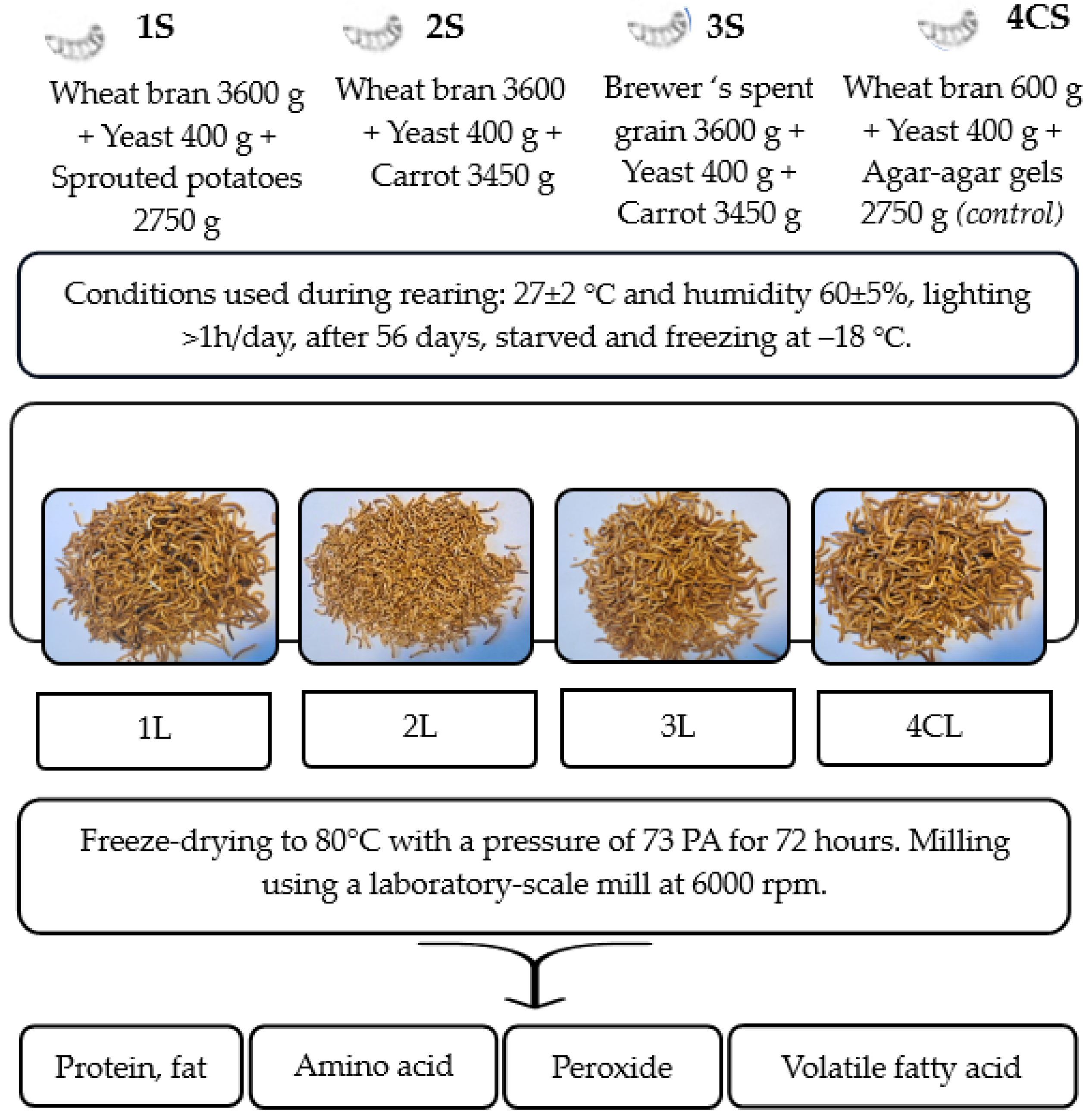
2.1. Rearing of Tenebrio molitor
2.2. Preparation of Samples
2.3. Determination of Protein and Fat Content
2.4. Determination of Amino Acids
2.5. Determination of Fatty Acids
2.6. Determination of Peroxide Value
2.7. Determination of Volatile Fatty Acid Content
2.8. Statistics
3. Results and Discussion
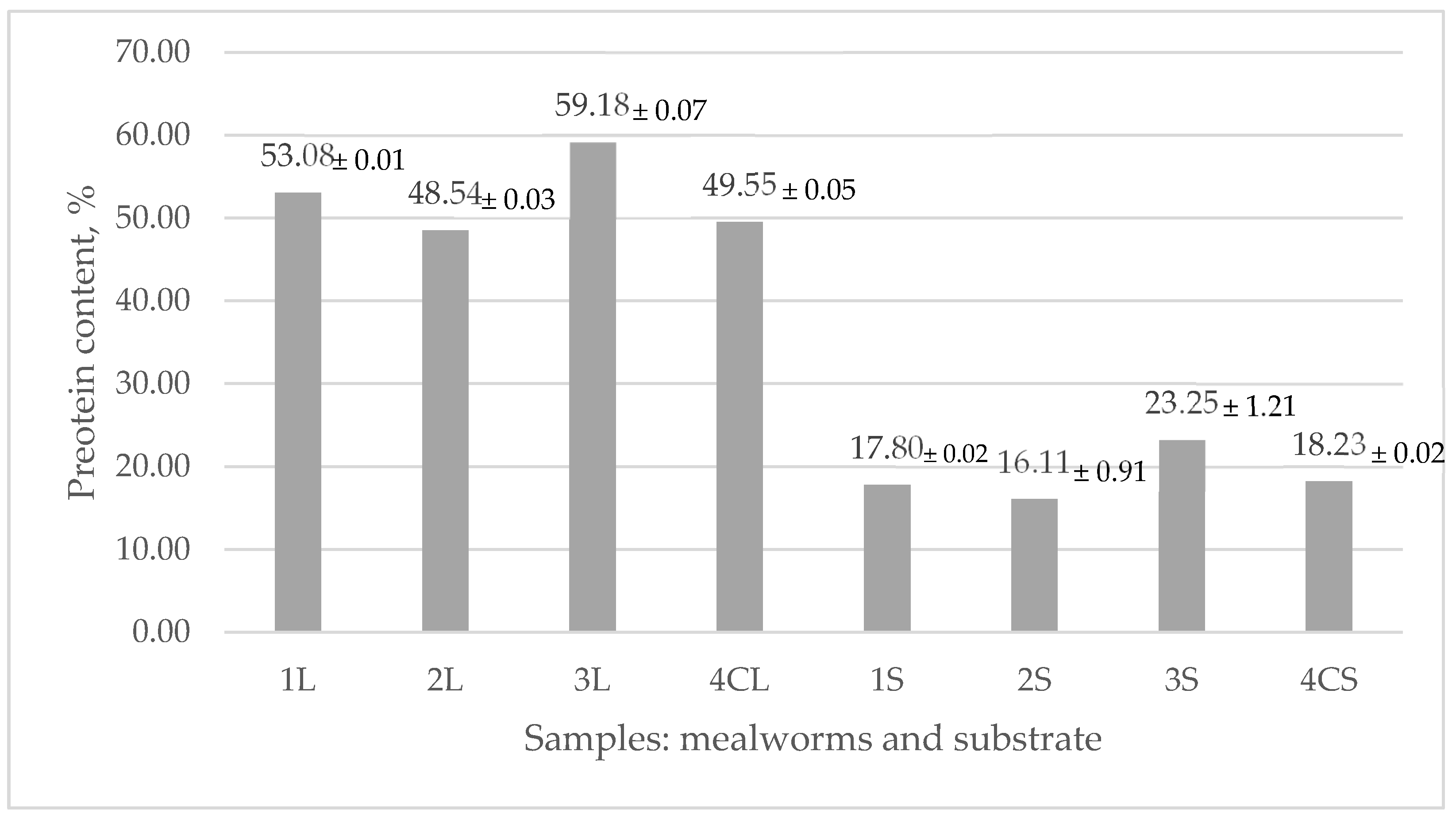
3.1. Protein Content
3.2. Composition of Amino Acids
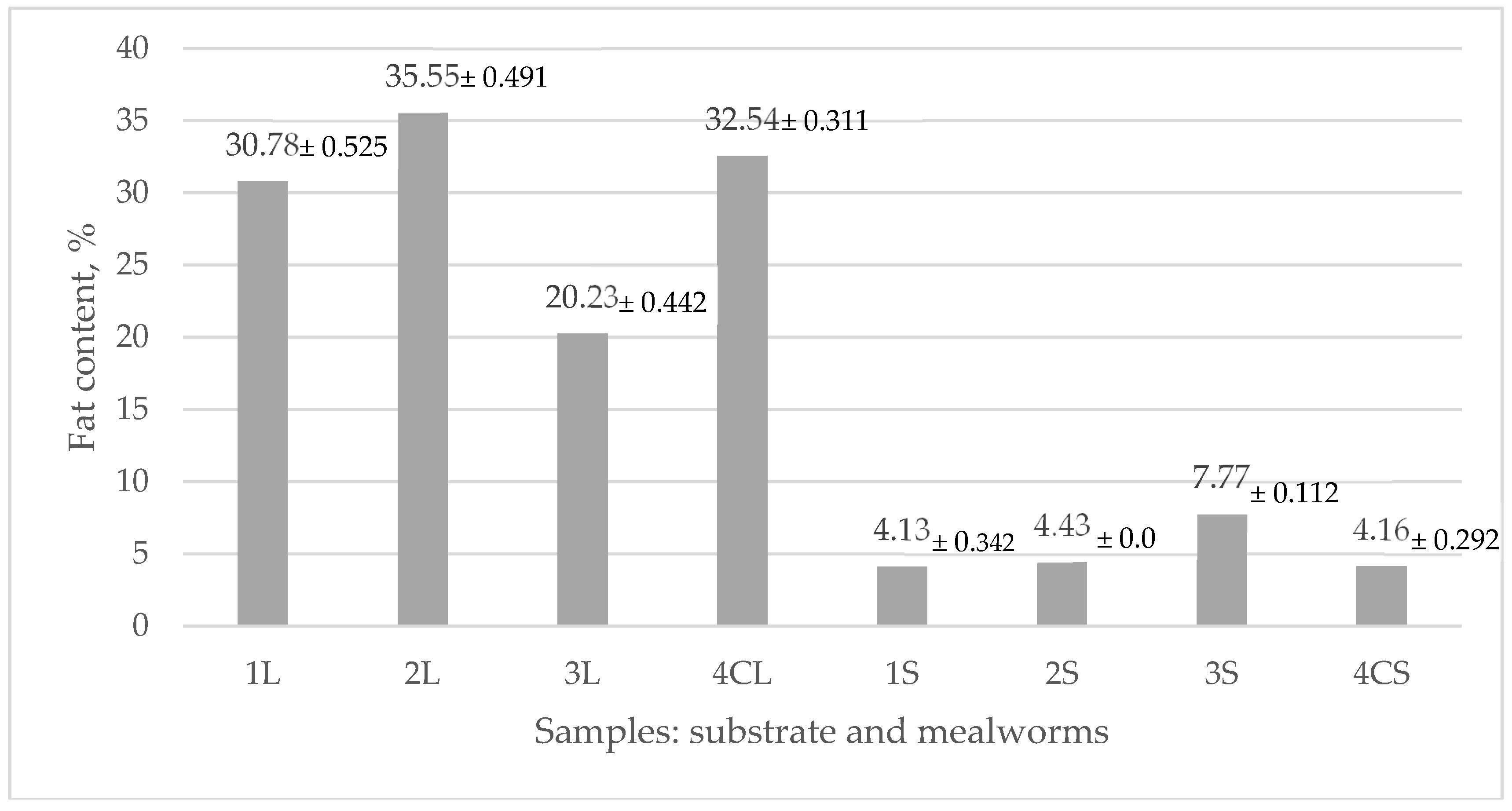
3.3. Fat Content
3.4. Fatty Acid Content
3.5. Peroxide Value and Volatile Fatty Acid Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imathiu, S. Benefits and Food Safety Concerns Associated with Consumption of Edible Insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Derler, H.; Lienhard, A.; Berner, S.; Grasser, M.; Posch, A.; Rehorska, R. Use Them for What They Are Good at: Mealworms in Circular Food Systems. Insects 2021, 12, 40. Available online: https://www.mdpi.com/2075-4450/12/1/40 (accessed on 4 January 2024). [CrossRef] [PubMed]
- Montalbán, A.; Sánchez, C.J.; Hernández, F.; Schiavone, A.; Madrid, J.; Martínez-Miró, S. Effects of Agro-Industrial Byproduct-Based Diets on the Growth Performance, Digestibility, Nutritional and Microbiota Composition of Mealworm (Tenebrio molitor L.). Insects 2022, 13, 323. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Pedro, S.; Lourenço, H.; Batista, I.; Teixeira, B.; Bandarra, N.M.; Murta, D.; Nunes, R.; Pires, C. Evaluation of Tenebrio molitor Larvae as an Alternative Food Source. NFS J. 2020, 21, 57–64. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.G.; Yoon, H.J.; Lee, K.Y.; Kim, N.J. Nutritional Analysis of Alternative Feed Ingredients and Their Effects on the Larval Growth of Tenebrio molitor (Coleoptera: Tenebrionidae). Entomol. Res. 2017, 47, 194–202. [Google Scholar] [CrossRef]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable Farming of the Mealworm Tenebrio molitor for the Production of Food and Feed. Z. Naturforschung C 2017, 72, 337–349. [Google Scholar] [CrossRef]
- Nations, U. World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100. Available online: https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 4 January 2024).
- Berners-Lee, M.; Kennelly, C.; Watson, R.; Hewitt, C.N. Current Global Food Production Is Sufficient to Meet Human Nutritional Needs in 2050 Provided There Is Radical Societal Adaptation. Elem. Sci. Anthr. 2018, 6, 52. [Google Scholar] [CrossRef]
- Hadi, J.; Brightwell, G. Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein. Foods 2021, 10, 1226. Available online: https://www.mdpi.com/2304-8158/10/6/1226 (accessed on 4 January 2024). [CrossRef]
- Kröncke, N.; Benning, R. Influence of Dietary Protein Content on the Nutritional Composition of Mealworm Larvae (Tenebrio molitor L.). Insects 2023, 14, 261. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Schop, M.; de Boer, I.J.M.; Huppertz, T. Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor Larvae) as an Alternative Protein Source for Monogastric Animal: A Review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Essential Amino Acids: Definition, Benefits, and Food Sources. Available online: https://www.healthline.com/nutrition/essential-amino-acids (accessed on 4 January 2024).
- Ravzanaadii, N.; Kim, S.-H.; Choi, W.-H.; Hong, S.-J.; Kim, N.-J. Nutritional Value of Mealworm, Tenebrio molitor as Food Source. Int. J. Ind. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef]
- Selaledi, L.; Mabelebele, M. The Influence of Drying Methods on the Chemical Composition and Body Color of Yellow Mealworm (Tenebrio molitor L.). Insects 2021, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Bordiean, A.; Krzyżaniak, M.; Aljewicz, M.; Stolarski, M.J. Influence of Different Diets on Growth and Nutritional Composition of Yellow Mealworm. Foods 2022, 11, 3075. [Google Scholar] [CrossRef]
- Avenue, 677 Huntington; Boston; Ma 02115 Omega-3 Fatty Acids: An Essential Contribution. Available online: https://www.hsph.harvard.edu/nutritionsource/what-should-you-eat/fats-and-cholesterol/types-of-fat/omega-3-fats/ (accessed on 4 January 2024).
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary Fatty Acids Influence the Growth and Fatty Acid Composition of the Yellow Mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the Human Health Effects of Chemical Mixtures. Environ. Health Perspect. 2002, 110, 25–42. [Google Scholar] [CrossRef]
- Jankauskienė, A.; Aleknavičius, D.; Antanaitis, Š.; Kiseliovienė, S.; Wedi, P.; Šumskienė, M.; Juknienė, I.; Gaižauskaitė, Ž.; Kabašinskienė, A. The Impact of Farm and Industrial Feed Waste on the Safety Parameters of Tenebrio molitor Larvae. Processes 2024, 12, 37. [Google Scholar] [CrossRef]
- Kröncke, N.; Grebenteuch, S.; Keil, C.; Demtröder, S.; Kroh, L.; Thünemann, A.F.; Benning, R.; Haase, H. Effect of Different Drying Methods on Nutrient Quality of the Yellow Mealworm (Tenebrio molitor L.). Insects 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Boguś, M. The Metabolism and Role of Free Fatty Acids in Key Physiological Processes in Insects of Medical, Veterinary and Forensic Importance. PeerJ 2021, 9, e12563. [Google Scholar] [CrossRef]
- Muangrat, R.; Pannasai, S. Exploring the potential of black soldier fly larvae oil: Supercritical CO2 extraction, physicochemical analysis, antioxidant properties, shelf life, and keratinocyte growth inhibition. J. Agric. Food Res. 2024, 15, 101008. Available online: https://www.sciencedirect.com/science/article/pii/S2666154324000450 (accessed on 2 March 2024). [CrossRef]
- Lipid Peroxidation—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/lipid-peroxidation (accessed on 2 March 2024).
- Kanner, J.; German, J.B.; Kinsella, J.E. Initiation of Lipid Peroxidation in Biological Systems. Crit. Rev. Food Sci. Nutr. 1987, 25, 317–364. [Google Scholar] [CrossRef]
- Jeon, Y.-H.; Son, Y.-J.; Kim, S.-H.; Yun, E.-Y.; Kang, H.-J.; Hwang, I.-K. Physicochemical Properties and Oxidative Stabilities of Mealworm (Tenebrio molitor) Oils under Different Roasting Conditions. Food Sci. Biotechnol. 2016, 25, 105–110. [Google Scholar] [CrossRef]
- Fasce, B.; Ródenas, L.; López, M.C.; Moya, V.J.; Pascual, J.J.; Cambra-López, M. Nutritive Value of Wheat Bran Diets Supplemented With Fresh Carrots and Wet Brewers’ Grains in Yellow Mealworm. J. Insect Sci. 2022, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Lienhard, A.; Rehorska, R.; Pöllinger-Zierler, B.; Mayer, C.; Grasser, M.; Berner, S. Future Proteins: Sustainable Diets for Tenebrio molitor Rearing Composed of Food By-Products. Foods 2023, 12, 4092. [Google Scholar] [CrossRef] [PubMed]
- Insect Protein Solutions. Available online: https://www.divaks.com/ (accessed on 23 October 2023).
- Kauno Technologijos Universitetas. Available online: https://en.ktu.edu/ (accessed on 23 October 2023).
- ISO 1443:1973; Meat and Meat Products Determination of Total Fat Content. International Organization for Standardization: Geneva, Switzerland, 1973. Available online: https://www.iso.org/standard/6038.html (accessed on 13 December 2023).
- Nacionalinis Maisto Ir Veterinarijos Rizikos Vertinimo Institutas—NMVRVI. Available online: https://nmvrvi.lrv.lt/lt/ (accessed on 23 October 2023).
- European Union. Komisijos Reglamentas (EB) Nr. 152/2009, Nustatantis Oficialiai Pašarų Kontrolei Taikytinus Bendrijos Ėminių Ėmimo ir Analizės Metodus (Tekstas Svarbus EEE); European Union: Brussels, Belgium, 2009; Volume 54. [Google Scholar]
- Juknienė, I.; Zaborskienė, G.; Jankauskienė, A.; Kabašinskienė, A.; Zakarienė, G.; Bliznikas, S. Effect of Lyophilization Process on Nutritional Value of Meat By-Products. Appl. Sci. 2022, 12, 12984. [Google Scholar] [CrossRef]
- Ichihara, K.; Kohsaka, C.; Yamamoto, Y. Determination of Proteinaceous Free Amino Acids by Gas Chromatography. Anal. Biochem. 2021, 633, 114423. [Google Scholar] [CrossRef] [PubMed]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils Gas Chromatography of Fatty Acid Methyl Esters Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/72142.html (accessed on 4 January 2024).
- ISO 27107:2008; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Potentiometric End-Point Determination. International Organization for Standardization: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/43999.html (accessed on 2 March 2024).
- 422 Dėl Mėsos Ir Paukštienos Šviežumo Įvertinimo Techninio Reglamento Patvirtinimo. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.192760/hkCgftsGEk?jfwid=4t02bucv7 (accessed on 2 March 2024).
- Regulation-853/2004-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2004/853/oj (accessed on 2 March 2024).
- Regulation-16/2012-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2012/16/oj (accessed on 2 March 2024).
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former Foodstuff Products in Tenebrio molitor Rearing: Effects on Growth, Chemical Composition, Microbiological Load, and Antioxidant Status. Animals 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.-E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and Physicochemical Characterization of Tenebrio molitor Proteins. Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.H.; Berger, L.L.; Drackley, J.K.; Fahey, G.C.; Hernot, D.C.; Parsons, C.M. 18—Nutritional Properties and Feeding Values of Soybeans and Their Coproducts. In Soybeans; Johnson, L.A., White, P.J., Galloway, R., Eds.; AOCS Press: Champaign, IL, USA, 2008; pp. 613–660. ISBN 978-1-893997-64-6. [Google Scholar]
- Relative Value of Fish Meal versus Solvent Soybean Meal for Lactating Dairy Cows Fed Alfalfa Silage as Sole Forage. Available online: https://www.researchgate.net/publication/43266635_Relative_value_of_fish_meal_versus_solvent_soybean_meal_for_lactating_dairy_cows_fed_alfalfa_silage_as_sole_forage (accessed on 2 March 2024).
- Mlček, J.; Adámek, M.; Adámková, A.; Matyáš, J.; Bučková, M.; Mrázková, M.; Vícha, R.; Vychodil, R.; Knížková, I.; Volek, Z. Feed Parameters Influencing the Breeding of Mealworms (Tenebrio molitor). Sustainability 2021, 13, 12992. [Google Scholar] [CrossRef]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Protein and Amino Acids. In Recommended Dietary Allowances, 10th ed.; National Academies Press (US): Washington, DC, USA, 1989. [Google Scholar]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Roth, E. Immune and Cell Modulation by Amino Acids. Clin. Nutr. 2007, 26, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Vianna, D.; Teodoro, G.F.R.; Torres-Leal, F.L.; Tirapegui, J. Protein Synthesis Regulation by Leucine. Braz. J. Pharm. Sci. 2010, 46, 29–36. [Google Scholar] [CrossRef]
- He, W.; Wu, G. Metabolism of Amino Acids in the Brain and Their Roles in Regulating Food Intake. In Amino Acids in Nutrition and Health: Amino Acids in Systems Function and Health; Wu, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 167–185. ISBN 978-3-030-45328-2. [Google Scholar]
- Flynn, N.E.; Shaw, M.H.; Becker, J.T. Amino Acids in Health and Endocrine Function. In Amino Acids in Nutrition and Health: Amino Acids in Systems Function and Health; Wu, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 97–109. ISBN 978-3-030-45328-2. [Google Scholar]
- Stull, V.J.; Kersten, M.; Bergmans, R.S.; Patz, J.A.; Paskewitz, S. Crude Protein, Amino Acid, and Iron Content of Tenebrio molitor (Coleoptera, Tenebrionidae) Reared on an Agricultural Byproduct from Maize Production: An Exploratory Study. Ann. Entomol. Soc. Am. 2019, 112, 533–543. [Google Scholar] [CrossRef]
- Dabbou, S.; Gasco, L.; Lussiana, C.; Brugiapaglia, A.; Biasato, I.; Renna, M.; Cavallarin, L.; Gai, F.; Schiavone, A. Yellow mealworm (Tenebrio molitor L.) larvae inclusion in diets for free-range chickens: Effects on meat quality and fatty acid profile. Renew. Agric. Food Syst. 2020, 35, 571–578. Available online: https://www.cambridge.org/core/journals/renewable-agriculture-and-food-systems/article/abs/yellow-mealworm-tenebrio-molitor-l-larvae-inclusion-in-diets-for-freerange-chickens-effects-on-meat-quality-and-fatty-acid-profile/D61760A2C148D22C6456131A4E1DE4F3 (accessed on 4 January 2024). [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of Dietary Odd-Chain Saturated Fatty Acid Pentadecanoic Acid Parallels Broad Associated Health Benefits in Humans: Could It Be Essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef] [PubMed]
- Czumaj, A.; Śledziński, T. Biological Role of Unsaturated Fatty Acid Desaturases in Health and Disease. Nutrients 2020, 12, 356. Available online: https://www.mdpi.com/2072-6643/12/2/356 (accessed on 4 January 2024). [CrossRef]
- Petermann, A.B.; Reyna-Jeldes, M.; Ortega, L.; Coddou, C.; Yévenes, G.E. Roles of the Unsaturated Fatty Acid Docosahexaenoic Acid in the Central Nervous System: Molecular and Cellular Insights. Int. J. Mol. Sci. 2022, 23, 5390. [Google Scholar] [CrossRef]
- Office of Dietary Supplements—Omega-3 Fatty Acids. Available online: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/ (accessed on 4 January 2024).
- Jones, P.; Kubow, S. Lipids, Sterols and Their Metabolites. In Modern Nutrition in Health and Disease; Williams & Wilkins a Waverly Company: Philadelphia, PA, USA, 2012. [Google Scholar]
- Aggett, P.; Ahnen, R.; Aydemir, T.; Bailey, L.; Bettendorff, L.; Blaner, W.; Borum, P.; Bruno, R.; Calder, P.; Caudill, M.; et al. Contributors to Volume 1. In Present Knowledge in Nutrition; Elsevier: Amsterdam, The Netherlands, 2020; pp. xi–xiii. ISBN 978-0-323-66162-1. [Google Scholar]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Wieczorek, T.; Drabińska, N.; Gould, O.; Osborne, A.; Costello, B.D.L. A Mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: An aid to understanding the origins of volatile organic compounds from the human body. J. Breath Res. 2020, 14, 034001. [Google Scholar] [CrossRef] [PubMed]
- Wąsowicz, E.; Gramza, A.; Hęś, M.; Jeleń, H.H.; Korczak, J.; Małecka, M.; Mildner-Szkudlarz, S.; Rudzińska, M.; Samotyja, U.; Zawirska-Wojtasiak, R. Oxidation of lipids in food. Pol. J. Food Nutr. Sci. 2004, 54, 87–100. [Google Scholar]
- Johnson, D.R.; Decker, E.A. The role of oxygen in lipid oxidation reactions: A review. Annu. Rev. Food Sci. Technol. 2015, 6, 171–190. [Google Scholar] [CrossRef] [PubMed]
- BS EN ISO 27107:2010; Animal and Vegetable Fats and Oils. Determination of Peroxide Value. Potentiometric End-Point Determination. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.en-standard.eu/bs-en-iso-27107-2010-animal-and-vegetable-fats-and-oils-determination-of-peroxide-value-potentiometric-end-point-determination/ (accessed on 2 March 2024).
- Jankauskienė, A.; Aleknavičius, D.; Kiseliovienė, S.; Antanaitis, Š.; Falkauskas, R.; Šumskienė, M.; Juknienė, I.; Kabašinskienė, A. The influence of different sustainable substrates on the nutritional value of Tenebrio molitor larvae. Foods 2024, 13, 365. [Google Scholar] [CrossRef]
| Larvae | Substrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Samples | 1L | 2L | 3L | 4CL (control) | 1S | 2S | 3S | 4CS (control) |
| Valine | 2.88 ± 0.134 a | 2.25 ± 0.013 b | 3.14 ± 0.130 a | 2.93 ± 0.174 a | 0.84 ± 0.089 a | 0.74 ± 0.061 a | 1.19 ± 0.025 b | 0.83 ± 0.038 a |
| Leucine | 3.54 ± 0.130 a | 3.02 ± 0.002 b | 3.79 ± 0.183 a | 3.45 ± 0.262 ab | 1.09 ± 0.060 a | 1.00 ± 0.071 a | 1.53 ± 0.009 b | 1.10 ± 0.041 a |
| Isoleucine | 2.01 ± 0.104 a | 1.61 ± 0.011 b | 2.14 ± 0.096 a | 2.02 ± 0.111 a | 0.63 ± 0.085 ac | 0.47 ± 0.059 b | 0.79 ± 0.007 c | 0.52 ± 0.042 ab |
| Threonine | 1.39 ± 0.082 | 1.27 ± 0.045 | 1.45 ± 0.002 | 1.34 ± 0.111 | 0.45 ± 0.004 a | 0.39 ± 0.019 b | 0.52 ± 0.013 c | 0.41 ± 0.017 b |
| Methionine | 0.60 ± 0.027 a | 0.46 ± 0.044 b | 0.62 ± 0.023 a | 0.57 ± 0.026 ab | 0.18 ± 0.011 a | 0.24 ± 0.001 b | 0.30 ± 0.017 c | 0.26 ± 0.012 b |
| Phenylalanine | 1.63 ± 0.065 ab | 1.63 ± 0.039 ab | 1.83 ± 0.097 a | 1.61 ± 0.089 b | 0.72 ± 0.034 a | 0.66 ± 0.045 a | 1.11 ± 0.010 b | 0.69 ± 0.034 a |
| Lysine | 2.73 ± 0.060 a | 2.46 ± 0.016 b | 2.92 ± 0.152 a | 2.80 ± 0.075 a | 0.80 ± 0.048 a | 0.87 ± 0.044 ab | 1.07 ± 0.001 c | 0.95 ± 0.042 b |
| Histidine | 1.60 ± 0.116 abc | 1.49 ± 0.032 b | 1.81 ± 0.116 cd | 1.89 ± 0.009 d | 0.83 ± 0.005 a | 0.61 ± 0.021 b | 0.71 ± 0.008 c | 0.67 ± 0.002 d |
| Aspartic acid | 3.92 ± 0.132 a | 3.21 ± 0.035 b | 4.30 ± 0.257 a | 3.78 ± 0.306 ab | 1.22 ± 0.056 a | 1.10 ± 0.051 a | 1.47 ± 0.048 b | 1.19 ± 0.060 a |
| Glutamic acid | 5.88 ± 0.238 a | 3.73 ± 0.121 b | 6.49 ± 0.903 a | 6.17 ± 0.046 a | 3.86 ± 0.254 a | 2.80 ± 0.199 b | 3.67 ± 0.171 a | 3.00 ± 0.081 b |
| Glycine | 2.41 ± 0.062 a | 2.87 ± 0.067 b | 2.66 ± 0.091 b | 2.43 ± 0.090 a | 0.83 ± 0.054 | 0.74 ± 0.061 | 0.81 ± 0.003 | 0.76 ± 0.03 |
| Serine | 1.55 ± 0.116 ab | 1.39 ± 0.041 a | 1.73 ± 0.002 b | 1.68 ± 0.111 b | 0.75 ± 0.003 a | 0.66 ± 0.033 b | 0.74 ± 0.013 a | 0.67 ± 0.004 b |
| Alanine | 3.35 ± 0.109 a | 3.38 ± 0.094 ab | 3.74 ± 0.166 b | 3.40 ± 0.148 ab | 0.80 ± 0.05 a | 0.72 ± 0.046 a | 0.99 ± 0.029 b | 0.79 ± 0.045 a |
| Proline | 2.94 ± 0.106 ab | 2.67 ± 0.020 a | 2.98 ± 0.143 ab | 3.09 ± 0.174 b | 1.12 ± 0.043 a | 1.06 ± 0.063 a | 1.88 ± 0.029 b | 1.13 ± 0.025 a |
| Tyrosine | 3.25 ± 0.037 a | 3.47 ± 0.144 a | 3.88 ± 0.160 b | 3.54 ± 0.070 a | 0.50 ± 0.013 a | 0.64 ± 0.034 b | 0.85 ± 0.026 c | 0.40 ± 0.020 d |
| Cystine | 0.38 ± 0.005 a | 0.20 ± 0.005 b | 0.37 ± 0.013 a | 0.38 ± 0.007 a | 0.34 ± 0.014 a | 0.50 ± 0.001 b | 0.50 ± 0.001 b | 0.48 ± 0.013 b |
| Larvae | Substrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Samples | 1L | 2L | 3L | 4CL | 1S | 2S | 3S | 4CS |
| C12:0 | 3.14 ± 0.001 a | 0.41 ± 0.001 b | 4.05 ± 0.001 c | 2.25 ± 0.001 d | - | - | 0.43 ± 0.008 b | - |
| C14:1 cis | - | - | 0.96 ± 0.001 b | - | - | - | - | - |
| C16:0 | 17.05 ± 0.002 a | 8.38 ± 0.003 b | 35.14 ± 0.113 c | 14.3 ± 0.665 d | 19.72 ± 0.083 a | 17.50 ± 0.062 b | 24.63 ± 0.003 c | 18.92 ± 0.044 d |
| C16:1 | 3.69 ± 0.011 a | 0.61 ± 0.001 b | 0.68 ± 0.001 c | 2.62 ± 0.114 d | - | - | 0.29 ± 0.001 b | - |
| C17:0 | 1.29 ± 0.002 | 9.60 ± 0.009 | 2.35 ± 0.001 | 0.34 ± 0.001 | - | - | - | - |
| C18:0 | 4.80 ± 0.012 a | 2.23 ± 0.001 b | 10.47 ± 0.023 c | 0.41 ± 0.012 d | 1.22 ± 0.001 a | 2.11 ± 0.003 b | 2.34 ± 0.012 c | 1.35 ± 0.094 d |
| C18:1 cis | 39.24 ± 0.002 a | 33.85 ± 0.001 b | 33.30 ± 0.067 c | 36.51 ± 0.654 d | 19.25 ± 0.043 a | 18.30 ± 0.072 b | 16.10 ± 0.073 c | 18.83 ± 0.063 d |
| C18:2 trans | - | - | 1.42 ± 0.002 b | 0.42 ± 0.033c | - | - | - | - |
| C18:2 cis W-6 | 26.45 ± 0.004 a | 12.40 ± 0.005 b | 6.70 ± 0.050 c | 22.83 ± 0.548 d | 53.99 ± 0.06 a | 49.54 ± 0.004 b | 49.54 ± 0.052 b | 53.91 ± 0.041 c |
| c18:3 alfa | - | - | 0.36 ± 0.001 b | - | - | - | - | - |
| c18:3 gama | - | 1.34 ± 0.001 b | 0.53 ± 0.006 c | 0.61 ± 0.003 d | - | - | 0.52 ± 0.042 b | - |
| C20:1 | 2.01 ± 0.001 a | 1.15 ± 0.001 b | 0.94 ± 0.001 c | 1.55 ± 0.021 d | 5.39 ± 0.001 a | 10.17 ± 0.051 b | 7.74 ± 0.032 c | 5.35 ± 0.032 d |
| C22:0 | 0.14 ± 0.003 a | 3.55 ± 0.003 b | 0.64 ± 0.014 c | 0.02 ± 0.001 d | - | 1.18 ± 0.001 b | 1.18 ± 0.001 c | - |
| C20:3 w6 | 0.35 ± 0.012 a | 3.72 ± 0.002 b | 0.90 ± 0.003 c | 1.33 ± 0.001 d | - | - | - | - |
| C22:1 w9 | 0.18 ± 0.002 a | 2.41 ± 0.001 b | - | 2.68 ± 0.003 d | - | - | - | - |
| C20:3w-3 | 1.03 ± 0.011 a | 13.46 ± 0.221 b | - | 2.06 ± 0.001 d | 0.39 ± 0.041 a | 0.39 ± 0.012 a | 1.18 ± 0.062 b | 0.14 ± 0.003 c |
| C22:4 w6 | 0.32 ± 0.012 a | 2.53 ± 0.011 b | 0.22 ± 0.004 c | 5.06 ± 0.085 d | - | - | 0.13 ± 0.001 b | - |
| C20:5 w3 | 0.32 ± 0.001 a | 2.80 ± 0.001 b | 0.05 ± 0.001 c | 2.81 ± 0.006 d | 0.03 ± 0.001 a | 0.29 ± 0.004 b | - | - |
| DHA | - | 1.59 ± 0.001 b | 1.28 ± 0.001 c | 4.19 ± 0.003 d | - | - | 2.7 ± 0.008 b | 1.53 ± 0.008 c |
| Fatty Acids | C12:0 | C16:1 | C17:0 | C18:0 | C18:1 cis | C18:2 trans | c18:3 gama | C20:1 | C20:3 w6 | C22:1 w9 | C20:3 w3 | C22:4 w6 | C20:5 w3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | ||||||||||||||
| Larvae | 2.46 ± 1.411 *** | 1.90 ± 1.373 *** | 3.39 ± 3.821 * | 4.48 ± 3.960 * | 35.72 ± 2.472 *** | 0.46 ± 0.613 *** | 0.62 ± 0.504 ** | 1.41 ± 0.431 *** | 1.58 ± 1.341 ** | 1.32 ± 1.294 ** | 4.14 ± 5.674 * | 2.03 ± 2.027 ** | 1.49 ± 1.371 ** | |
| Substrate | 0.11 ± 0.201 | 0.07 ± 0.132 | 0 ± 0 | 1.75 ± 0.51 | 18.12 ± 1.273 | 0 ± 0 | 0.14 ± 0.233 | 7.16 ± 2.084 | 0 ± 0 | 0 ± 0 | 0.53 ± 0.411 | 0.03 ± 0.062 | 0.08 ± 0.135 |
| Larvae | Substrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Samples | 1L | 2L | 3L | 4CL | 1S | 2S | 3S | 4CS |
| Total SFA | 26.42 a | 24.15 b | 52.64 c | 17.33 d | 20.94 a | 20.79 b | 28.59 c | 20.25 d |
| Total MUFA | 45.12 a | 38.02 b | 35.89 c | 43.37 d | 24.64 a | 28.47 b | 24.13 c | 24.18 d |
| Total PUFA | 28.47 a | 36.24 b | 10.18 c | 35.11 d | 54.42a | 50.74 b | 44.63 c | 54.05 d |
| Omega-6 FA | 27.12 a | 18.65 b | 7.82 c | 29.22 d | 54.00 a | 49.54 b | 42.92 c | 53.91 d |
| Omega-3 FA | 0.32 a | 4.39 b | 1.69 c | 6.99 d | 0.03 a | 0.29 b | 2.65 c | 1.53 d |
| Omega-6/3 FA | 85.05 a | 4.25 b | 4.632 c | 4.18 d | 1838.83 a | 170.51 b | 16.20 c | 35.34 d |
| Peroxide Value, mg | Volatile Fatty Acid Content, meq/kg of Fat | |
|---|---|---|
| 1L | 0.1 ± 0.0 a | 44.67 ± 1.33 a |
| 2L | 0.1 ± 0.0 a | 39 ± 1.0 a |
| 3L | 14.87 ± 0.3 b | 346 ± 4.0 b |
| 4CL | 0.17 ± 0.3 a | 43 ± 1.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankauskienė, A.; Aleknavičius, D.; Andrulevičiūtė, V.; Mockus, E.; Bartkienė, E.; Juknienė, I.; Kiseliovienė, S.; Zavistanavičiūtė, P.; Zaborskienė, G.; Kabašinskienė, A. Nutritional Composition and Safety Parameters of Mealworms (Tenebrio molitor) Reared on Substrates Derived from By-Products. Appl. Sci. 2024, 14, 2744. https://doi.org/10.3390/app14072744
Jankauskienė A, Aleknavičius D, Andrulevičiūtė V, Mockus E, Bartkienė E, Juknienė I, Kiseliovienė S, Zavistanavičiūtė P, Zaborskienė G, Kabašinskienė A. Nutritional Composition and Safety Parameters of Mealworms (Tenebrio molitor) Reared on Substrates Derived from By-Products. Applied Sciences. 2024; 14(7):2744. https://doi.org/10.3390/app14072744
Chicago/Turabian StyleJankauskienė, Agnė, Dominykas Aleknavičius, Vaida Andrulevičiūtė, Ernestas Mockus, Elena Bartkienė, Ignė Juknienė, Sandra Kiseliovienė, Paulina Zavistanavičiūtė, Gintarė Zaborskienė, and Aistė Kabašinskienė. 2024. "Nutritional Composition and Safety Parameters of Mealworms (Tenebrio molitor) Reared on Substrates Derived from By-Products" Applied Sciences 14, no. 7: 2744. https://doi.org/10.3390/app14072744
APA StyleJankauskienė, A., Aleknavičius, D., Andrulevičiūtė, V., Mockus, E., Bartkienė, E., Juknienė, I., Kiseliovienė, S., Zavistanavičiūtė, P., Zaborskienė, G., & Kabašinskienė, A. (2024). Nutritional Composition and Safety Parameters of Mealworms (Tenebrio molitor) Reared on Substrates Derived from By-Products. Applied Sciences, 14(7), 2744. https://doi.org/10.3390/app14072744







