Long-Term Effects of External Sulfate Attack on Low-Carbon Cementitious Materials at Early Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Mixes Design
2.2. Mixing, Casting, and Exposure Procedures
2.3. Characterizations Methods
2.3.1. Thermogravimetric Analysis
2.3.2. Fourier Transform Infrared Spectroscopy
2.3.3. Raman Spectroscopy
2.3.4. Water Porosity
3. Results
3.1. Expansion
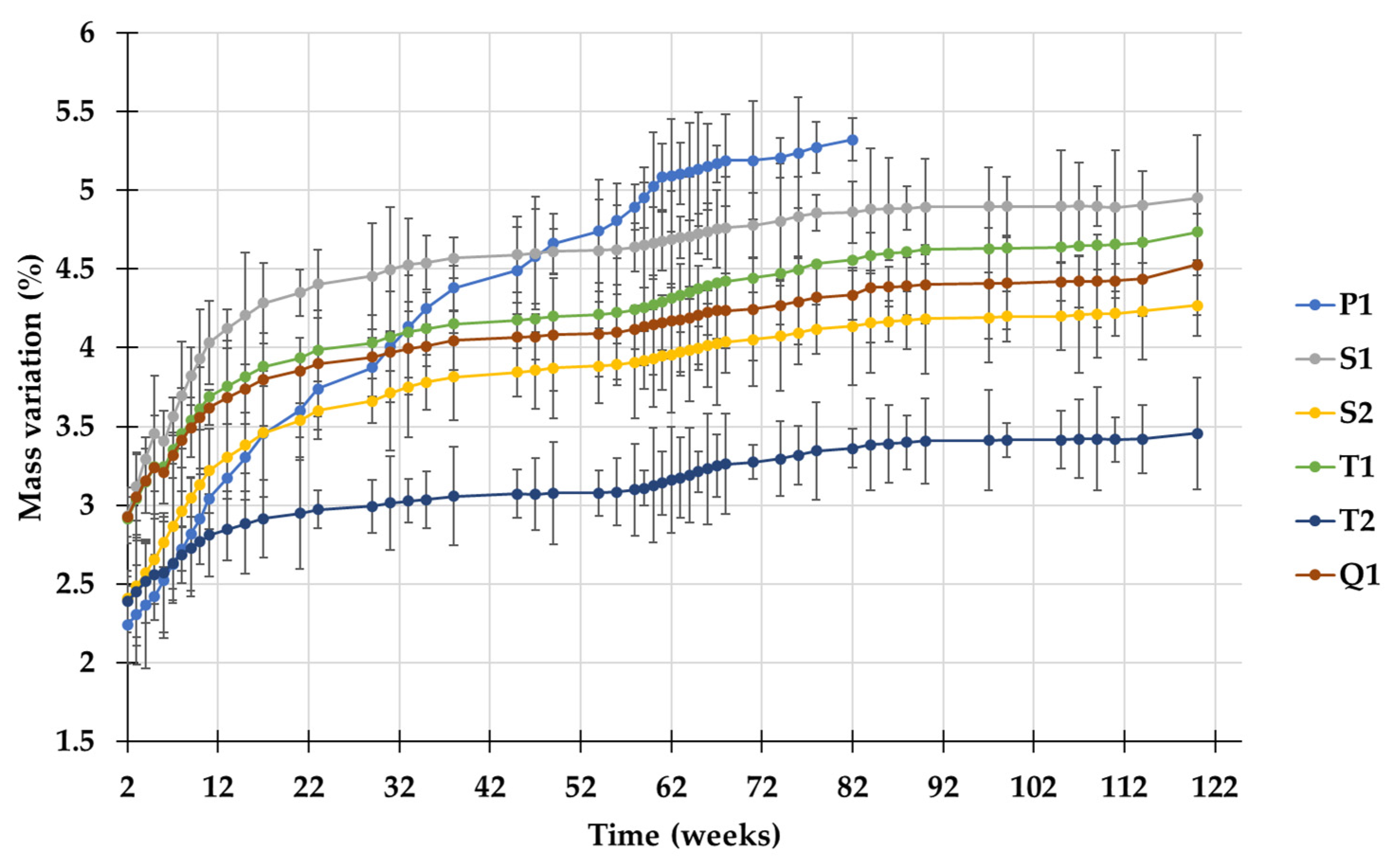
3.2. Mass Changes and Visual Inspection
3.3. Thermogravimetric Analysis
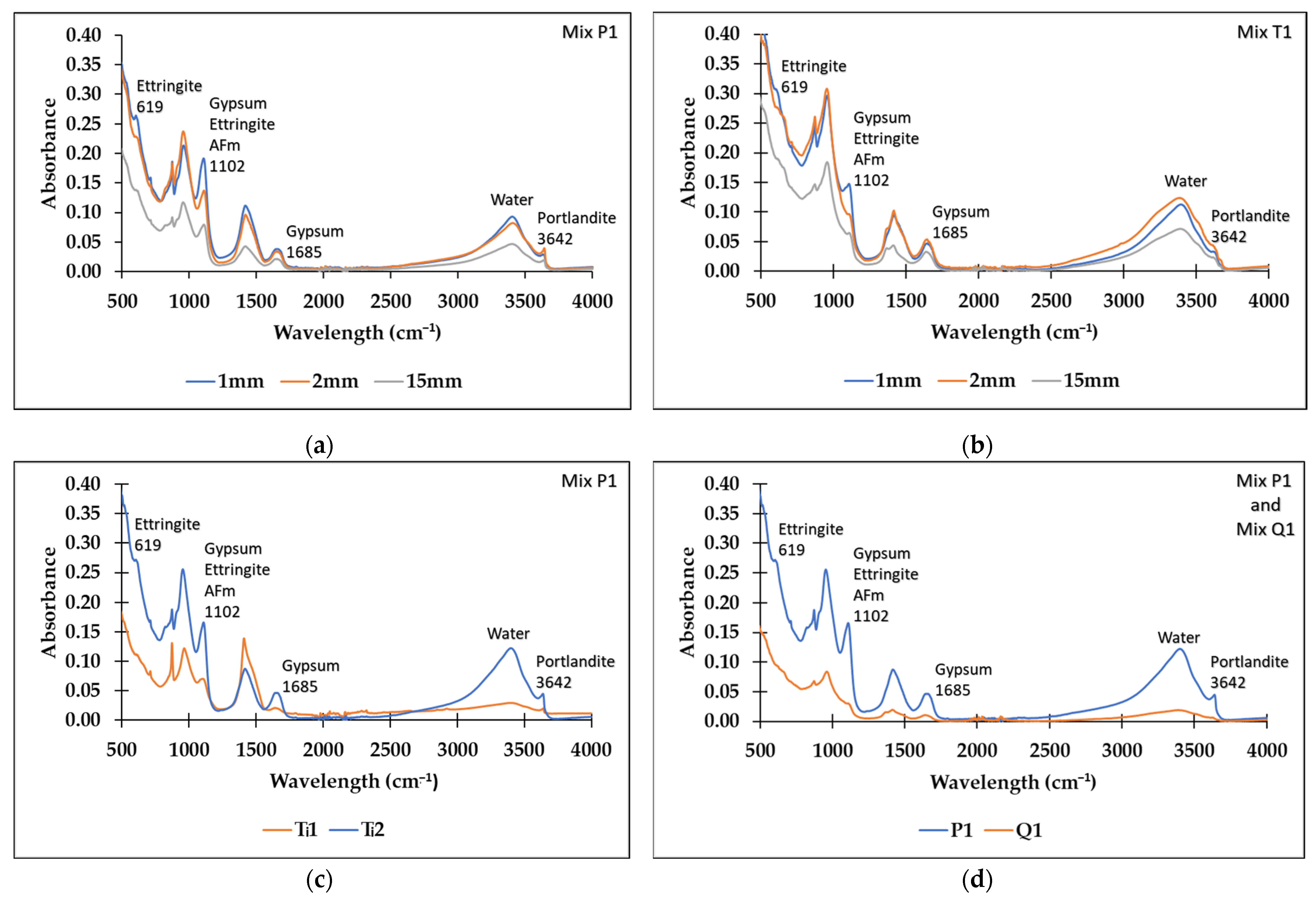
3.4. Fourier Transform Infrared Spectroscopy
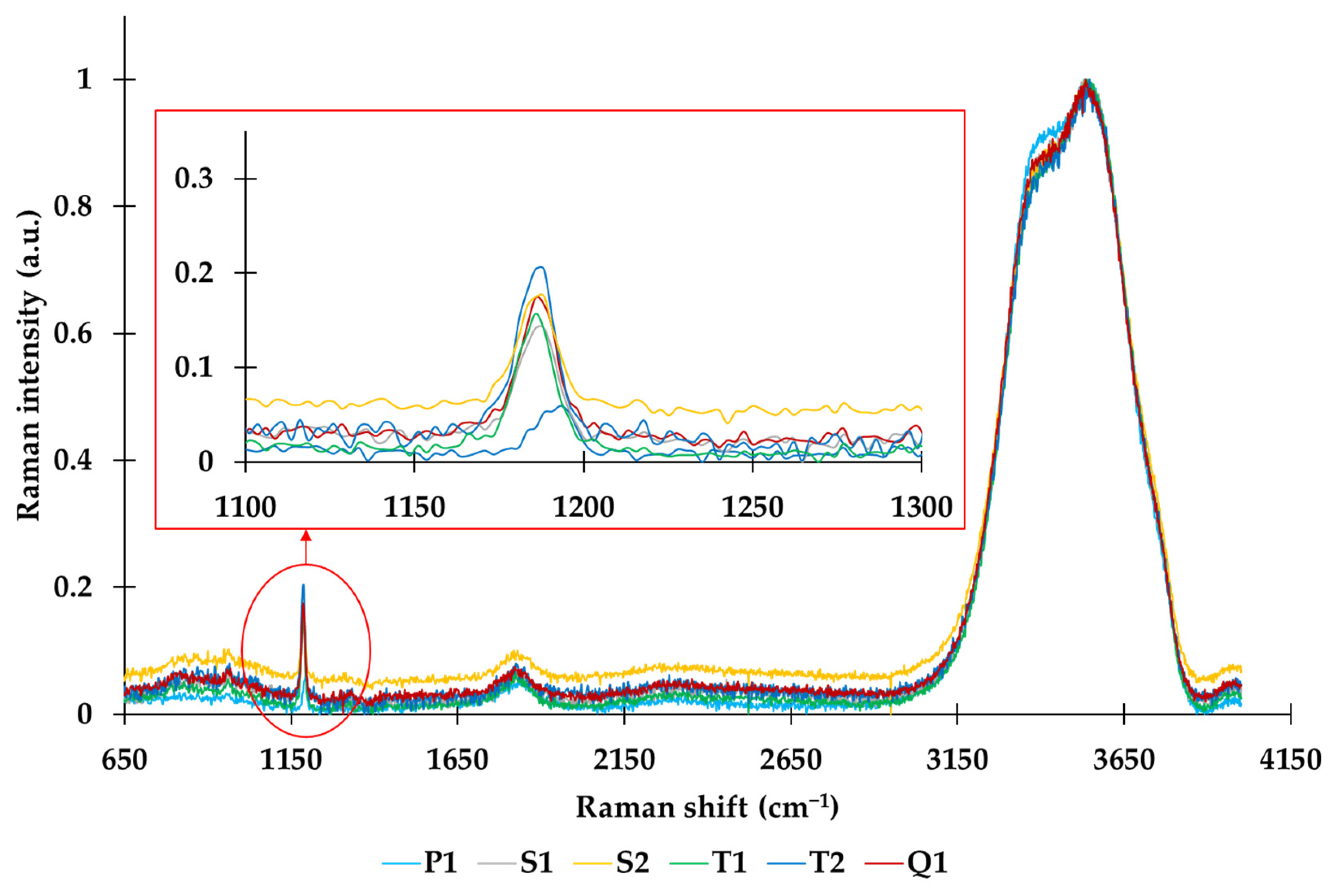
3.5. Raman Spectroscopy
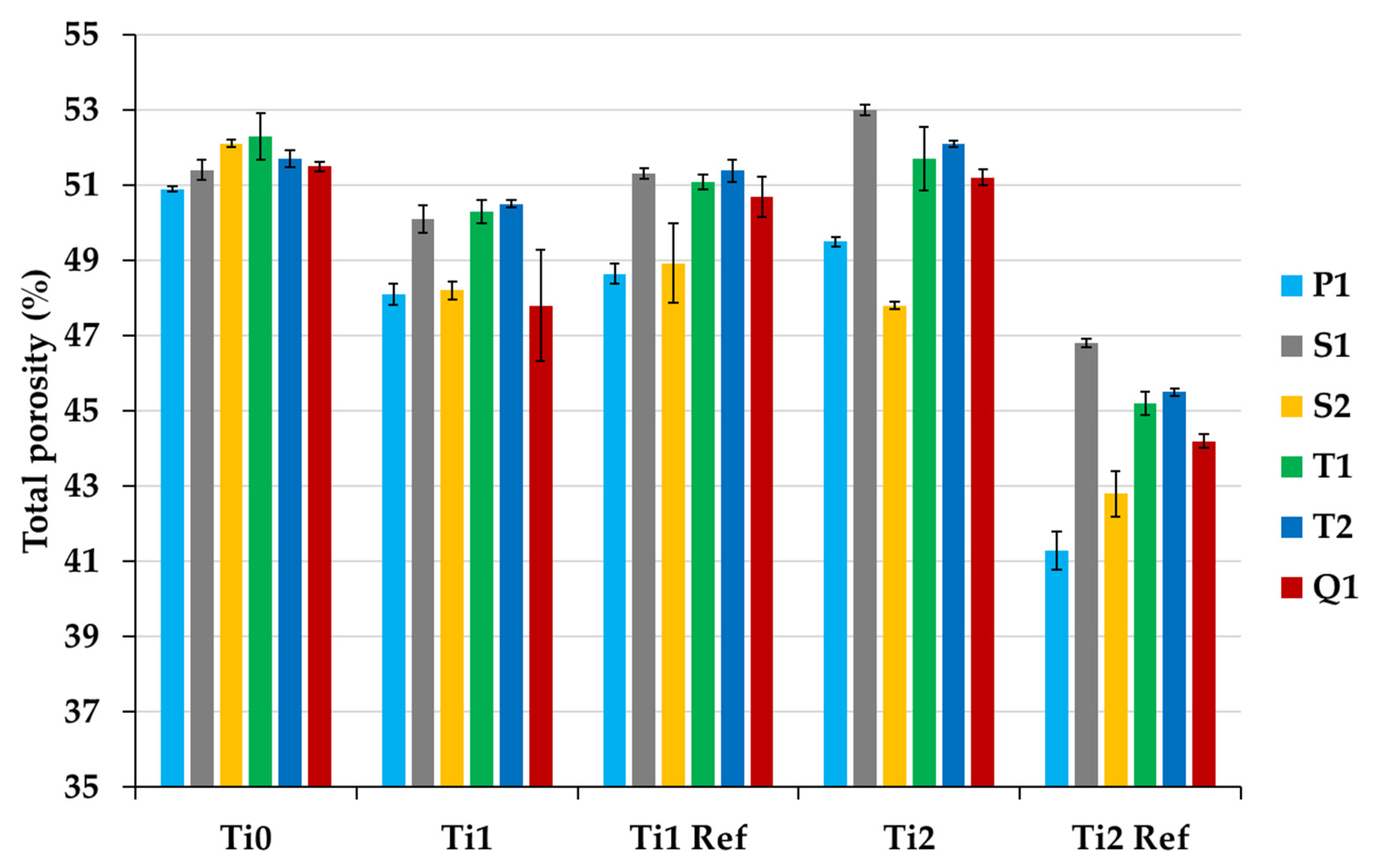
3.6. Water Porosity
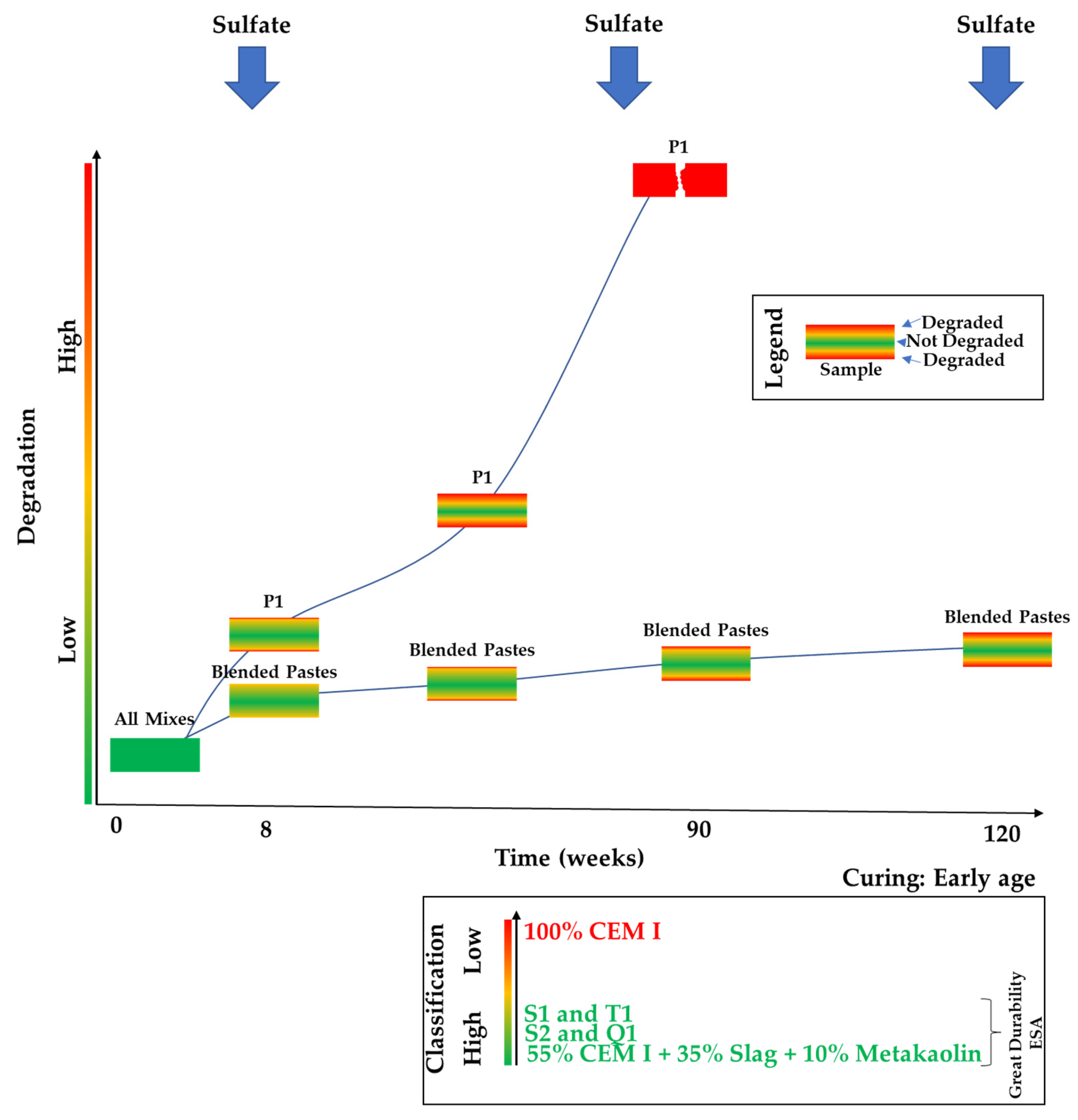
3.7. Results Overview Illustration
4. Conclusions
- CEM I exhibits the lowest resistance to ESA when compared to blended mixes. It shows the highest mass gain, expansion, formation of ettringite and gypsum, sulfate consumption from the solution, and microstructure alteration. Additionally, during the course of this experiment, it deteriorated only after 90 weeks, whereas the blended cements maintained their structural integrity even after 120 weeks;
- blended specimens demonstrated good durability, retaining structural integrity after 120 weeks of sulfate exposure from an early age;
- incorporating a low quantity (10%) of metakaolin along with blast furnace slag emerged as the most effective substitute for clinker, outperforming other combinations based on the observed behavior of various blended mixes in sulfate exposure;
- non-invasive Raman spectroscopy emerged as a reliable method for monitoring the ESA effect by quantifying the sulfate ions left in the attacking solutions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, G.; Li, J.; Shao, W. Effect of mixed chlorides on the degradation and sulfate diffusion of cast-in-situ concrete due to sulfate attack. Constr. Build. Mater. 2018, 181, 49–58. [Google Scholar] [CrossRef]
- Ouyang, C.; Nanni, A.; Chang, W.F. Internal and external sources of sulfate ions in portland cement mortar: Two types of chemical attack. Cem. Concr. Res. 1988, 18, 699–709. [Google Scholar] [CrossRef]
- Geng, J.; Easterbrook, D.; Li, L.; Mo, L. The stability of bound chlorides in cement paste with sulfate attack. Cem. Concr. Res. 2015, 68, 211–222. [Google Scholar] [CrossRef]
- Wongprachum, W.; Sappakittipakorn, M.; Sukontasukkul, P.; Chindaprasirt, P.; Banthia, N. Resistance to sulfate attack and underwater abrasion of fiber reinforced cement mortar. Constr. Build. Mater. 2018, 189, 686–694. [Google Scholar] [CrossRef]
- Skaropoulou, A.; Sotiriadis, K.; Kakali, G.; Tsivilis, S. Use of mineral admixtures to improve the resistance of limestone cement concrete against thaumasite form of sulfate attack. Cem. Concr. Compos. 2013, 37, 267–275. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 1: Summary of experimental results. Cem. Concr. Res. 2002, 32, 915–921. [Google Scholar] [CrossRef]
- Yu, X.; Chen, D.; Feng, J.; Zhang, Y.; Liao, Y. Behavior of mortar exposed to different exposure conditions of sulfate attack. Ocean Eng. 2018, 157, 1–12. [Google Scholar] [CrossRef]
- Ikumi, T.; Segura, I.; Cavalaro, S.H.P. Effects of biaxial confinement in mortars exposed to external sulfate attack. Cem. Concr. Compos. 2019, 95, 111–127. [Google Scholar] [CrossRef]
- El Inaty, F.; Marchetti, M.; Quiertant, M.; Omikrine Metalssi, O. Chemical Mechanisms Involved in the Coupled Attack of Sulfate and Chloride Ions on Low-Carbon Cementitious Materials: An In-Depth Study. Appl. Sci. 2023, 13, 11729. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Yu, C.; Sun, W.; Scrivener, K. Mechanism of expansion of mortars immersed in sodium sulfate solutions. Cem. Concr. Res. 2013, 43, 105–111. [Google Scholar] [CrossRef]
- Xiong, C.; Jiang, L.; Xu, Y.; Chu, H.; Jin, M.; Zhang, Y. Deterioration of pastes exposed to leaching, external sulfate attack and the dual actions. Constr. Build. Mater. 2016, 116, 52–62. [Google Scholar] [CrossRef]
- Shao, W.; Li, Q.; Zhang, W.; Shi, D.; Li, H. Numerical modeling of chloride diffusion in cement-based materials considering calcium leaching and external sulfate attack. Constr. Build. Mater. 2023, 401, 132913. [Google Scholar] [CrossRef]
- El Inaty, F.; Baz, B.; Aouad, G. Long-term durability assessment of 3D printed concrete. J. Adhes. Sci. Technol. 2022, 37, 1921–1936. [Google Scholar] [CrossRef]
- Al-Amoudi, O.S.B. Sulfate attack and reinforcement corrosion in plain and blended cements exposed to sulfate environments. Build. Environ. 1998, 33, 53–61. [Google Scholar] [CrossRef]
- Jabbour, M. Multi-Scales Study for the External Sulfatic Attack in Reinforced Concrete Structures. Ph.D. Thesis, Université Paris-Est, Paris, France, 2019. Available online: https://tel.archives-ouvertes.fr/tel-02956401 (accessed on 2 November 2023).
- Wu, M.; Zhang, Y.; Ji, Y.; She, W.; Yang, L.; Liu, G. A comparable study on the deterioration of limestone powder blended cement under sodium sulfate and magnesium sulfate attack at a low temperature. Constr. Build. Mater. 2020, 243, 118279. [Google Scholar] [CrossRef]
- Jabbour, M.; Metalssi, O.O.; Quiertant, M.; Baroghel-Bouny, V. A Critical Review of Existing Test-Methods for External Sulfate Attack. Materials 2022, 15, 7554. [Google Scholar] [CrossRef] [PubMed]
- Imre BICZOK. Concrete Corrosion and Concrete Protection; Chemical Publishing Compan: New York, NY, USA, 1967; Available online: https://scholar.google.com/scholar_lookup?title=Concrete+Corrosion+and+Concrete+Protection&author=Biczok,+I.&publication_year=1967 (accessed on 20 November 2023).
- Metalssi, O.O.; Ragoug, R.; Barberon, F.; d’Espinose de Lacaillerie, J.-B.; Roussel, N.; Divet, L.; Torrenti, J.-M. Effect of an Early-Age Exposure on the Degradation Mechanisms of Cement Paste under External Sulfate Attack. Materials 2023, 16, 6013. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Ma, H.; Hou, D. Advanced Concrete Technology; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Zhao, G.; Shi, M.; Guo, M.; Fan, H. Degradation Mechanism of Concrete Subjected to External Sulfate Attack: Comparison of Different Curing Conditions. Materials 2020, 13, 3179. [Google Scholar] [CrossRef]
- Pawar, Y.; Kate, S. Curing of Concrete: A Review. Int. Res. J. Eng. Technol. 2020, 7, 1820–1824. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Zhao, Y.; Yu, X.; Li, C.; Chen, D. Effect of initial curing period on the behavior of mortar under sulfate attack. Constr. Build. Mater. 2022, 326, 126852. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Zou, Y.-X.; Zuo, X.-B.; Zhang, H.-L.; Wang, S.-Q. Influence of fly ash and chlorides on the behavior of sulfate attack in blended cement pastes. Constr. Build. Mater. 2023, 394, 132231. [Google Scholar] [CrossRef]
- Elahi, M.M.A.; Shearer, C.R.; Naser Rashid Reza, A.; Saha, A.K.; Khan, M.N.N.; Hossain, M.M.; Sarker, P.K. Improving the sulfate attack resistance of concrete by using supplementary cementitious materials (SCMs): A review. Constr. Build. Mater. 2021, 281, 122628. [Google Scholar] [CrossRef]
- Ramyar, K.; İnan, G. Sodium sulfate attack on plain and blended cements. Build. Environ. 2007, 42, 1368–1372. [Google Scholar] [CrossRef]
- Al-Akhras, N.M. Durability of metakaolin concrete to sulfate attack. Cem. Concr. Res. 2006, 36, 1727–1734. [Google Scholar] [CrossRef]
- Shi, Z.; Ferreiro, S.; Lothenbach, B.; Geiker, M.R.; Kunther, W.; Kaufmann, J.; Herfort, D.; Skibsted, J. Sulfate resistance of calcined clay–Limestone–Portland cements. Cem. Concr. Res. 2019, 116, 238–251. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U.; Maslehuddin, M.; Al-Zahrani, M.M.; Sharif, A.M.; Shameem, M.; Ibrahim, M. Sulfate resistance of plain and blended cements exposed to varying concentrations of sodium sulfate. Cem. Concr. Compos. 2003, 25, 429–437. [Google Scholar] [CrossRef]
- Lee, S.T.; Moon, H.Y.; Swamy, R.N. Sulfate attack and role of silica fume in resisting strength loss. Cem. Concr. Compos. 2005, 27, 65–76. [Google Scholar] [CrossRef]
- Baghabra Al-Amoudi, O.S. Attack on plain and blended cements exposed to aggressive sulfate environments. Cem. Concr. Compos. 2002, 24, 305–316. [Google Scholar] [CrossRef]
- Miah, M.J.; Huaping, R.; Paul, S.C.; Babafemi, A.J.; Li, Y. Long-term strength and durability performance of eco-friendly concrete with supplementary cementitious materials. Innov. Infrastruct. Solut. 2023, 8, 255. [Google Scholar] [CrossRef]
- EN 197-1:2011; European Committee for Standardization. Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. iTeh, Inc.: Newark, DE, USA, 2011.
- NF EN 197-5; European Committee for Standardization. Ciment—Partie 5: Ciment Portland Composé CEM II/C-M et Ciment composé CEM VI. Afnor EDITIONS. AFNOR: Saint-Denis, France, 2021. Available online: https://www.boutique.afnor.org/fr-fr/norme/nf-en-1975/ciment-partie-5-ciment-portland-compose-cem-ii-cm-et-ciment-compose-cem-vi/fa200094/264804 (accessed on 18 October 2023).
- Bogue, R.H. The Chemistry of Portland Cement, 2nd ed.; LWW: Pennvania, PA, USA, 1955; Volume 79, p. 322. [Google Scholar]
- NF 196-1; Méthodes d’Essais des Ciments. AFNOR: Saint-Denis, France, 2006.
- NF P18-459; Béton-Essai pour Béton Durci—Essai de Porosité et de Masse Volumique. AFNOR: Saint-Denis, France, 2010. Available online: https://www.boutique.afnor.org/fr-fr/norme/nf-p18459/beton-essai-pour-beton-durci-essai-de-porosite-et-de-masse-volumique/fa160729/34961 (accessed on 9 January 2024).
- Metalssi, O.O.; Touhami, R.R.; Barberon, F.; d’Espinose de Lacaillerie, J.-B.; Roussel, N.; Divet, L.; Torrenti, J.-M. Understanding the degradation mechanisms of cement-based systems in combined chloride-sulfate attack. Cem. Concr. Res. 2023, 164, 107065. [Google Scholar] [CrossRef]
- Jiang, L.; Niu, D. Study of deterioration of concrete exposed to different types of sulfate solutions under drying-wetting cycles. Constr. Build. Mater. 2016, 117, 88–98. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, X.; Luo, W. Long-term behaviors of concrete under low-concentration sulfate attack subjected to natural variation of environmental climate conditions. Cem. Concr. Res. 2019, 116, 217–230. [Google Scholar] [CrossRef]
- Nosouhian, F.; Mostofinejad, D.; Hasheminejad, H. Concrete Durability Improvement in a Sulfate Environment Using Bacteria. J. Mater. Civ. Eng. 2016, 28, 04015064. [Google Scholar] [CrossRef]
- Haufe, J.; Vollpracht, A. Tensile strength of concrete exposed to sulfate attack. Cem. Concr. Res. 2019, 116, 81–88. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Effects of gypsum formation on the performance of cement mortars during external sulfate attack. Cem. Concr. Res. 2003, 33, 325–332. [Google Scholar] [CrossRef]
- Tian, B.; Cohen, M.D. Does gypsum formation during sulfate attack on concrete lead to expansion? Cem. Concr. Res. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Ma, X.; Çopuroğlu, O.; Schlangen, E.; Han, N.; Xing, F. Expansion and degradation of cement paste in sodium sulfate solutions. Constr. Build. Mater. 2018, 158, 410–422. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, X.; Lu, N.; Hou, S.; He, Z.; Liang, C. Effect of recycled powders on the mechanical properties and durability of fully recycled fiber-reinforced mortar. J. Build. Eng. 2022, 45, 103574. [Google Scholar] [CrossRef]
- Nochaiya, T.; Sekine, Y.; Choopun, S.; Chaipanich, A. Microstructure, characterizations, functionality and compressive strength of cement-based materials using zinc oxide nanoparticles as an additive. J. Alloys Compd. 2015, 630, 1–10. [Google Scholar] [CrossRef]
- Irbe, L.; Beddoe, R.E.; Heinz, D. The role of aluminium in C-A-S-H during sulfate attack on concrete. Cem. Concr. Res. 2019, 116, 71–80. [Google Scholar] [CrossRef]
- Fode, T.A.; Chande Jande, Y.A.; Kivevele, T. Effects of different supplementary cementitious materials on durability and mechanical properties of cement composite–Comprehensive review. Heliyon 2023, 9, e17924. [Google Scholar] [CrossRef]
- Farcas, F.; Touzé, P. La spectrométrie infrarouge à transformée de Fourier (IRTF). Bull. Lab. Ponts Chaussées 2001, 230, 77–88. [Google Scholar]
- Liu, P.; Chen, Y.; Wang, W.; Yu, Z. Effect of physical and chemical sulfate attack on performance degradation of concrete under different conditions. Chem. Phys. Lett. 2020, 745, 137254. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, J.J.; Basheer, P.A.M.; Bai, Y. Raman spectroscopic investigation of Friedel’s salt. Cem. Concr. Compos. 2018, 86, 306–314. [Google Scholar] [CrossRef]
- Tang, C.; Ling, T.-C.; Mo, K.H. Raman spectroscopy as a tool to understand the mechanism of concrete durability—A review. Constr. Build. Mater. 2021, 268, 121079. [Google Scholar] [CrossRef]
- Water Molecule Vibrations with Raman Spectroscopy. PhysicsOpenLab. Available online: https://physicsopenlab.org/2022/01/08/water-molecule-vibrations-with-raman-spectroscopy/ (accessed on 19 October 2023).
- Nehdi, M.L.; Suleiman, A.R.; Soliman, A.M. Investigation of concrete exposed to dual sulfate attack. Cem. Concr. Res. 2014, 64, 42–53. [Google Scholar] [CrossRef]
- Toutanji, H.; Delatte, N.; Aggoun, S.; Duval, R.; Danson, A. Effect of supplementary cementitious materials on the compressive strength and durability of short-term cured concrete. Cem. Concr. Res. 2004, 34, 311–319. [Google Scholar] [CrossRef]




| CEM I (%) | Fly Ash (%) | Blast Furnace Slag (%) | Metakaolin (%) | |
|---|---|---|---|---|
| P1 | 100 | - | - | - |
| S1 | 55 | 45 | - | - |
| S2 | 55 | - | 45 | - |
| T1 | 55 | 15 | 30 | - |
| T2 | 55 | - | 35 | 10 |
| Q1 | 55 | 15 | 20 | 10 |
| Components | CEM I (w%) 1 | Fly Ash (w%) 1 | Blast Furnace Slag (w%) 1 |
|---|---|---|---|
| SiO2 | 20.38 | 70.83 | 35.71 |
| Al2O3 | 4.30 | 24.36 | 10.65 |
| Fe2O3 | 3.80 | 2.24 | 0.45 |
| TiO2 | 0.24 | 1.48 | 0.73 |
| MnO | 0.08 | 0.05 | 0.23 |
| CaO | 62.79 | 0.06 | 43.32 |
| MgO | 1.25 | 0.23 | 3.97 |
| SO3 | 3.46 | - | 3.06 |
| K2O | 0.73 | 0.64 | 0.45 |
| Na2O | 0.35 | 0.1 | 0.16 |
| P2O5 | - | 0.05 | 0.02 |
| S2− | Traces | - | - |
| Cl− | 0.05 | - | - |
| Loss of ignition | 2.54 | - | - |
| Free lime | 1.39 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Inaty, F.; Aydin, B.; Houhou, M.; Marchetti, M.; Quiertant, M.; Omikrine Metalssi, O. Long-Term Effects of External Sulfate Attack on Low-Carbon Cementitious Materials at Early Age. Appl. Sci. 2024, 14, 2831. https://doi.org/10.3390/app14072831
El Inaty F, Aydin B, Houhou M, Marchetti M, Quiertant M, Omikrine Metalssi O. Long-Term Effects of External Sulfate Attack on Low-Carbon Cementitious Materials at Early Age. Applied Sciences. 2024; 14(7):2831. https://doi.org/10.3390/app14072831
Chicago/Turabian StyleEl Inaty, François, Bugra Aydin, Maryam Houhou, Mario Marchetti, Marc Quiertant, and Othman Omikrine Metalssi. 2024. "Long-Term Effects of External Sulfate Attack on Low-Carbon Cementitious Materials at Early Age" Applied Sciences 14, no. 7: 2831. https://doi.org/10.3390/app14072831
APA StyleEl Inaty, F., Aydin, B., Houhou, M., Marchetti, M., Quiertant, M., & Omikrine Metalssi, O. (2024). Long-Term Effects of External Sulfate Attack on Low-Carbon Cementitious Materials at Early Age. Applied Sciences, 14(7), 2831. https://doi.org/10.3390/app14072831









