State-of-All-the-Art and Prospective Hydrogel-Based Transdermal Drug Delivery Systems
Abstract
1. Introduction
2. Overview of Transdermal Drug Delivery
3. Fundamentals of Hydrogels
3.1. Hydrogel Composition
3.2. Types of Hydrogels
3.2.1. Classification Based on Source Origin
3.2.2. Classification of Hydrogels Based on Charge and Composition
3.2.3. Classification Based on External Stimuli Response
3.2.4. Classification Depending on Crystallinity
3.2.5. Classification Based on Appearance
3.2.6. Classification Based on Crosslinking
3.3. Preparation of Hydrogels
3.3.1. Physically Crosslinked Hydrogels
3.3.2. Chemically Crosslinked Type of Hydrogels
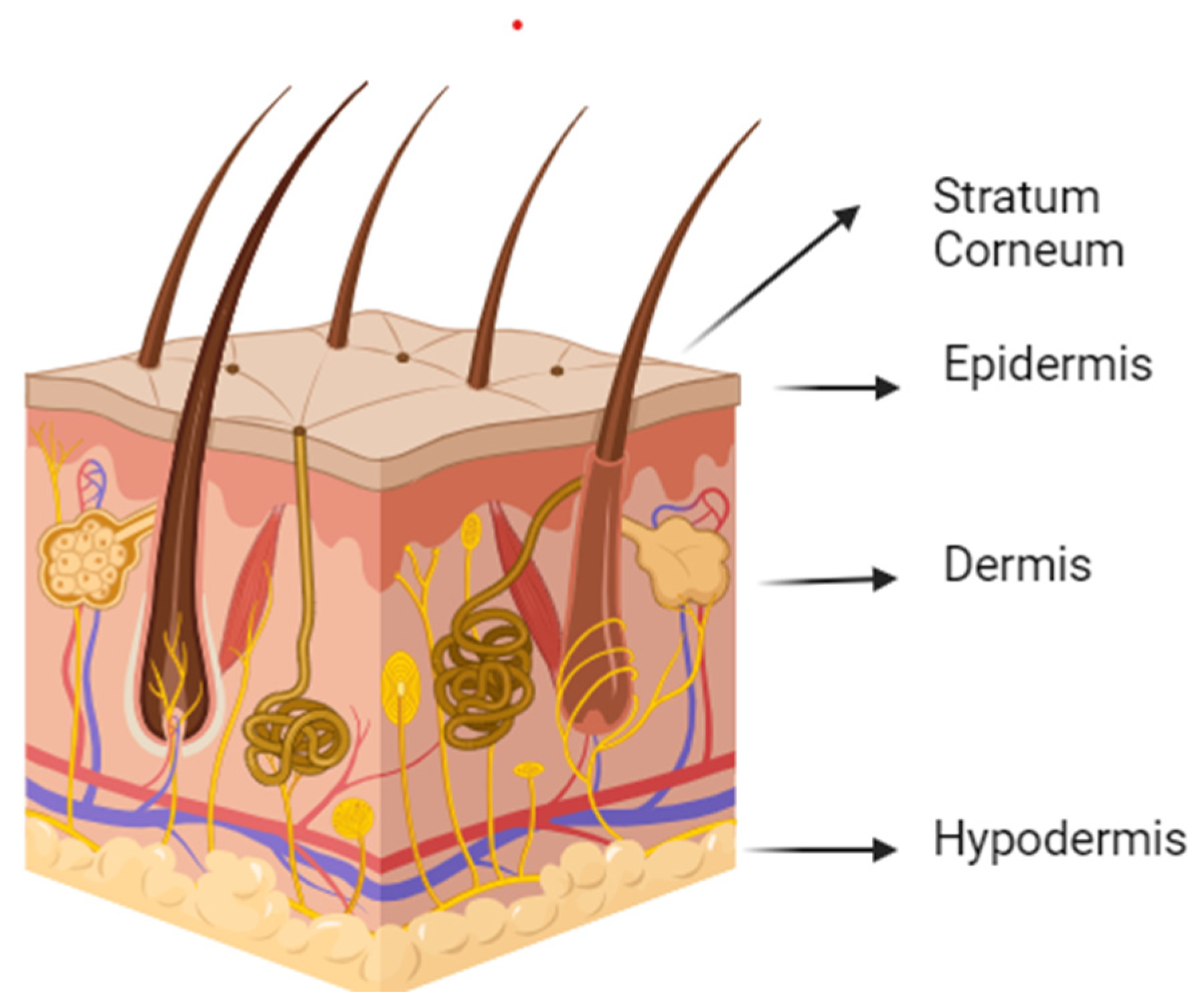
4. Skin and Diffusion through the Skin
4.1. Skin Structure
4.2. Factors Influencing Drug Diffusion through the Skin
4.2.1. The Physiological Factor
4.2.2. The Drug Absorption Factor
4.2.3. Drug Delivery Setting
4.3. Methods of Enhancing Drug Penetration
4.3.1. Active Methods for Permeation Enhancement
Iontophoresis
Sonophoresis
Electroporation
Photomechanical Waves
Microneedles (M.N.s)
Thermal Ablation
4.3.2. Passive Methods for Permeation Enhancement
4.4. Mechanism of Drug Release through Hydrogels
4.4.1. Diffusion-Controlled Release Mechanism
4.4.2. Swelling-Controlled Release
4.4.3. Chemically Controlled Release
5. Hydrogels in Transdermal Drug Delivery
6. Some Important Applications of Hydrogels in Transdermal Drug Delivery Systems
6.1. Cancer Therapy
6.2. Diabetes Treatment
6.3. Cardiovascular Treatment
6.4. Treatment of Hormone Deficiency
6.5. Wound Management
7. Challenges and Future Directions of Hydrogel-Based Transdermal Drug Delivery Systems
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| TDDSs | Transdermal drug delivery systems |
| DDS | Drug delivery system |
| GIT | Gastrointestinal tract |
| COOH | Carboxyl group |
| O.H. | Hydroxyl group |
| NH2 | Amino acid group |
| PVA | Polyvinyl alcohol |
| PEG | Polyethylene glycol |
| PEO | Polyethylene oxide |
| PHEMA | Poly(2-hydroxyethyl methacrylate) |
| PAA | Polyacrylic acid |
| PAAM | Polyacrylamide |
| PNIPAAm | Poly (N-isopropyl acrylamide) |
| PAA-co-PEG | Polyacrylic acid co-poly ethyl glycol |
| IPN | Interpenetrating polymer network |
| MAPTAC | Methacrylamidopropyltrimethylammonium chloride |
| SSS | Sodium styrene sulfonate |
| pKa | Acid dissociation constant |
| DNA | Deoxyribonucleic acid |
| S.C. | Stratum corneum |
| MN | Microneedle |
| R.F. | Radio Frequency |
| NLC | Nanostructured lipid carriers |
| PEGDA | Polyethylene glycol diacrylate |
| PAAm-g-CS | Polyacrylamide-grafted-chondroitin sulfate |
| Gel-PAAm | Gelatin polyacrylamide |
| CS-A2 | Chitosan azelaic acid |
| CMCS-SFP/OPL | Carboxymethyl chitosan silk fibroin peptide/oxidized pullulan |
| CmCHTpHEA | Carboxymethyl chitosan-grafted 2 hydroxyethyl acrylate |
| pH | Potential of hydrogen |
| FDA | Food and Drug Administration |
| PK | Pharmacokinetics |
| P.D. | Pharmacodynamics |
| P.H. | Pulmonary hypertension |
| GHD | Growth hormone deficiency |
| SC | Subcutaneous |
| rhGH | Recombinant human growth hormone |
References
- Nayak, A.K.; Ahmad, S.A.; Beg, S.; Ara, T.J.; Hasnain, M.S. Drug delivery: Present, past, and future of medicine. In Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing: Cambridge, UK, 2018; pp. 255–282. [Google Scholar] [CrossRef]
- Jain, K.K. An overview of drug delivery systems. Meth. Mol. Biol. 2020, 2059, 1–54. [Google Scholar] [CrossRef]
- Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomed-ical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, A.; Tian, W.-X.; Farooq, M.A.; Khan, D.H. An overview of hydrogels and their role in transdermal drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 574–584. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical Applications of Hydrogels in Drug Delivery Sys-tem: An Update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Santos, L.F.; Correia, I.J.; Silva, A.S.; Mano, J.F. Biomaterials for drug delivery patches. Eur. J. Pharm. Sci. 2018, 118, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Machekposhti, S.A.; Narayan, R.J. Evolution of Transdermal Drug Delivery Devices and Novel Microneedle Technologies: A Historical Perspective and Review. JID Innov. 2023, 3, 100225. [Google Scholar] [CrossRef]
- Wong, W.F.; Ang, K.P.; Sethi, G.; Looi, C.Y. Recent Advancement of Medical Patch for Transdermal Drug Delivery. Medicina 2023, 59, 778. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C.; Madhusudhan, A.; Azamal, H. Smart Nanomaterials in Biomedical Applications, 1st ed.; Kim, J.-C., Alle, M., Husen, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-84261-1. [Google Scholar]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Punagoti, R.A.; Vasam, M.; Mourya, R. Applications of Dendrimers in Drug Delivery Systems. In Smart Nanomaterials in Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 373–388. [Google Scholar]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Bird, D.; Ravindra, N.M. Transdermal drug delivery and patches—An overview. Med. Devices Sens. 2020, 3, e10069. [Google Scholar] [CrossRef]
- Wokovich, A.M.; Prodduturi, S.; Doub, W.H.; Hussain, A.S.; Buhse, L.F. Transdermal Drug Delivery System (TDDS) Adhe-sion as a Critical Safety, Efficacy and Quality Attribute. Eur. J. Pharm. Biopharm. 2006, 64, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Yahia, L. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classifica-tion According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as Intelligent Materials: A Brief Review of Syn-thesis, Properties and Applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, K.; Coelho, J.; Ferreira, P.; Pinto, I.; Lorenzetti, S.; Ferreira, E.; Higa, O.; Gil, M. Synthesis and characterization of membranes obtained by graft copolymerization of 2-hydroxyethyl methacrylate and acrylic acid onto chitosan. Int. J. Pharm. 2006, 310, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Aminabhavi, T.M. Novel interpenetrating network chitosan-poly(ethylene oxide-g-acrylamide) hydrogel microspheres for the controlled release of capecitabine. Int. J. Pharm. 2006, 324, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Sutar, P.B.; Mishra, R.K.; Pal, K.; Banthia, A.K. Development of pH sensitive polyacrylamide grafted pectin hydrogel for controlled drug delivery system. J. Mater. Sci. Mater. Med. 2008, 19, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Schwoerer, A.D.; Harling, S.; Scheibe, K.; Menzel, H.; Daniels, R. Influence of degree of substitution of HES–HEMA on the release of incorporated drug models from corresponding hydrogels. Eur. J. Pharm. Biopharm. 2009, 73, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Khansari, M.M.; Sorokina, L.V.; Mukherjee, P.; Mukhtar, F.; Shirdar, M.R.; Shahidi, M.; Shokuhfar, T. Classification of Hy-drogels Based on Their Source: A Review and Application in Stem Cell Regulation. JOM 2017, 69, 1340–1347. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–10. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Baker, J.P.; Blanch, H.W.; Prausnitz, J.M. Swelling properties of acrylamide-based ampholytic hydrogels: Comparison of experiment with theory. Polymer 1995, 36, 1061–1069. [Google Scholar] [CrossRef]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Soni, S.; Sachdev, A.; Vikas; Mishra, S. Near-infrared stimulated hydrogel patch for photothermal therapeutics and thermoresponsive drug delivery. J. Photochem. Photobiol. B: Biol. 2020, 210, 111960. [Google Scholar] [CrossRef] [PubMed]
- Rybak, D.; Su, Y.-C.; Li, Y.; Ding, B.; Lv, X.; Li, Z.; Yeh, Y.-C.; Nakielski, P.; Rinoldi, C.; Pierini, F.; et al. Evolution of nanostructured skin patches towards multifunctional wearable platforms for biomedical applications. Nanoscale 2023, 15, 8044–8083. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Jung, J.M.; Lee, D.S. Recent Strategies to Develop PH-Sensitive Injectable Hydrogels. Biomater. Sci. 2023, 11, 1948–1961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mu, X.; Xu, Y.; Li, S.; Liu, X.; Lei, Z. Popcorn-based dual-monomer copolymerized temperature/pH-sensitive core-shell hydrogels. J. Environ. Chem. Eng. 2023, 11, 109510. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, H.; Niu, Y.; Song, L.; Li, H.; Chen, H.; Cao, D. pH-Sensitive Microgels with Different Chemical Structures of Internal Curing of Cementitious Materials. J. Mater. Civ. Eng. 2023, 35, 04022375. [Google Scholar] [CrossRef]
- Tian, M.L.; Zhou, J.F.; Qi, X.; Shen, R. Thermo-sensitive hydrogel and their biomedical applications. IOP Conf. Series: Earth Environ. Sci. 2021, 714, 032062. [Google Scholar] [CrossRef]
- Duan, T.; Bian, Q.; Li, H. Light-Responsive Dynamic Protein Hydrogels Based on LOVTRAP. Langmuir 2021, 37, 10214–10222. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light responsive hydrogels for controlled drug delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef]
- Garcia, A.; Marquez, M.; Cai, T.; Rosario, R.; Hu, Z.; Gust, D.; Hayes, M.; Vail, S.A.; Park, C.-D. Photo-, Thermally, and pH-Responsive Microgels. Langmuir 2007, 23, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Usta, A.; Asmatulu, R. Synthesis and Analysis of Electrically Sensitive Hydrogels Incorporated with Cancer Drugs. J. Pharm. Drug Deliv. Res. 2016, 5, 2. [Google Scholar] [CrossRef]
- Dolai, S.; Leu, H.Y.; Magda, J.; Tabib-Azar, M. Metal-Oxide-Hydrogel Field-Effect Sensor. In Proceedings of the 2018 IEEE Sensors, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar]
- Islam, M.R.; Oyen, M.L. Mechanical Characterization of Hydrogels. In The Mechanics of Hydrogels: Mechanical Properties, Testing, and Applications; Woodhead Publishing: Cambridge, UK, 2022. [Google Scholar]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Intech Open: London, UK, 2011. [Google Scholar]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Shoukat, H.; Buksh, K.; Noreen, S.; Pervaiz, F.; Maqbool, I. Hydrogels as potential drug-delivery systems: Network design and applications. Ther. Deliv. 2021, 12, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Ravarino, P.; Giuri, D.; Faccio, D.; Tomasini, C. Designing a Transparent and Fluorine Containing Hydrogel. Gels 2021, 7, 43. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Yoshii, F.; Zhanshan, Y.; Isobe, K.; Shinozaki, K.; Makuuchi, K. Electron beam crosslinked PEO and PEO/PVA hydrogels for wound dressing. Radiat. Phys. Chem. 1999, 55, 133–138. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Yang, X.; Wu, X.F.; Fan, Y. Bin Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocar-riers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef]
- Yadav, P.R.; Nasiri, M.I.; Vora, L.K.; Larrañeta, E.; Donnelly, R.F.; Pattanayek, S.K.; Das, D.B. Super-swelling hydrogel-forming microneedle based transdermal drug delivery: Mathematical modelling, simulation and experimental validation. Int. J. Pharm. 2022, 622, 121835. [Google Scholar] [CrossRef]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming Skin Barriers through Advanced Trans-dermal Drug Delivery Approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef]
- Wissler, E.H.; Havenith, G. A simple theoretical model of heat and moisture transport in multi-layer garments in cool ambient air. Eur. J. Appl. Physiol. 2009, 105, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Tai, Z.; Shen, M.; Li, Y.; Yu, J.; Wang, J.; Zhu, Q.; Chen, Z. Advance and Challenges in the Treatment of Skin Dis-eases with the Transdermal Drug Delivery System. Pharmaceutics 2023, 15, 2165. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Dwivedi, S.; Ajazuddin; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for Breaking the Barriers of Drug Permeation through Transdermal Drug Delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2017. [Google Scholar]
- Benson, H.A.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]
- Kogure, K.; Fukuta, T. Transdermal drug delivery by iontophoresis. Drug Deliv. Syst. 2021, 36, 198–208. [Google Scholar] [CrossRef]
- Dhote, V.; Bhatnagar, P.; Mishra, P.K.; Mahajan, S.C.; Mishra, D.K. Iontophoresis: A Potential Emergence of a Transdermal Drug Delivery System. Sci. Pharm. 2012, 80, 1–28. [Google Scholar] [CrossRef]
- Huang, D.; Sun, M.; Bu, Y.; Luo, F.; Lin, C.; Lin, Z.; Weng, Z.; Yang, F.; Wu, D. Microcapsule-embedded hydrogel patches for ultrasound responsive and enhanced transdermal delivery of diclofenac sodium. J. Mater. Chem. B 2019, 7, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.A.; Ramos, D.N.; Lopez, R.F.V. Hydrogel increases localized transport regions and skin permeability during low frequency ultrasound treatment. Sci. Rep. 2017, 7, srep44236. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Sung, K.C.; Wang, J.-J.; Chu, C.-C.; Chen, K.-T. The effects of iontophoresis and electroporation on transdermal delivery of buprenorphine from solutions and hydrogels. J. Pharm. Pharmacol. 2010, 54, 1329–1337. [Google Scholar] [CrossRef]
- Dermol-Černe, J.; Pirc, E.; Miklavčič, D. Mechanistic view of skin electroporation – models and dosimetry for successful applications: An expert review. Expert Opin. Drug Deliv. 2020, 17, 689–704. [Google Scholar] [CrossRef]
- Lee, S.; McAuliffe, D.J.; Flotte, T.J.; Kollias, N.; Doukas, A.G. Photomechanical transdermal delivery: The effect of laser confinement. Lasers Surg. Med. 2001, 28, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Heimark, H.; Bertollo, N.; Tobin, D.J.; O’Cearbhaill, E.D.; Annaidh, A.N. Insights into the mechanics of solid conical microneedle array insertion into skin using the finite element method. Acta Biomater. 2021, 135, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, A.J.; McAlister, E.; McCrudden, M.T.; Vora, L.; Steiner, L.; Levin, G.; Levy-Nissenbaum, E.; Shterman, N.; Kearney, M.-C.; McCarthy, H.O.; et al. Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery. J. Control. Release 2020, 322, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCrudden, M.T.C.; Alkilani, A.Z.; Larrañeta, E.; McAlister, E.; Courtenay, A.J.; Kearney, M.-C.; Singh, T.R.R.; McCarthy, H.O.; Kett, V.L.; et al. Hydrogel-Forming Microneedles Prepared from “Super Swelling” Polymers Combined with Lyophilised Wafers for Transdermal Drug Delivery. PLOS ONE 2014, 9, e111547. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.Y.R.; Hasir, N.A.; Imran, N.B.P.; Hamdan, M.F.; Mahfufah, U.; Wafiah, N.; Arjuna, A.; Utami, R.N.; Permana, A.D. Development of hydrogel-forming microneedles for transdermal delivery of albendazole from liquid reservoir. J. Biomater. Sci. Polym. Ed. 2023, 34, 1101–1120. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R.; Mandru, A. Enhancement of Skin Permeability with Thermal Ablation Techniques: Concept to Commercial Products. Drug Deliv. Transl. Res. 2021, 11, 817–841. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Zhang, S.; Xin, P.; Ou, Q.; Hollett, G.; Gu, Z.; Wu, J. Poly(ester amide)-based hybrid hydrogels for efficient transdermal insulin delivery. J. Mater. Chem. B 2018, 6, 6723–6730. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Tran, L.; Parks, J.; Zhao, Y.; Hai, N.; Zhong, Y.; Ji, H. Highly stretchable gelatin-polyacrylamide hydrogel for potential transdermal drug release. Nano Sel. 2021, 2, 107–115. [Google Scholar] [CrossRef]
- Birajdar, R.P.; Patil, S.B.; Alange, V.V.; Kulkarni, R.V. Synthesis and characterization of electrically responsive poly(acrylamide)-grafted-chondroitin sulfate hydrogel for transdermal drug delivery application. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 148–157. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Janus, L.; Piątkowski, M.; Sierakowska, A.; Matysek, D. ZnO nanorods functionalized with chitosan hydrogels crosslinked with azelaic acid for transdermal drug delivery. Colloids Surfaces B: Biointerfaces 2020, 194, 111170. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liu, S.; Tong, Z.; Chen, T.; Yang, M.; Guo, Y.; Sun, H.; Wu, Y.; Chu, Y.; Fan, L. Hydrogel-based microneedles of chitosan derivatives for drug delivery. React. Funct. Polym. 2022, 172, 105200. [Google Scholar] [CrossRef]
- Ha, J.H.; Lim, J.H.; Lee, J.M.; Chung, B.G. Electro-Responsive Conductive Blended Hydrogel Patch. Polymers 2023, 15, 2608. [Google Scholar] [CrossRef]
- Esmaeili, J.; Barati, A.; Ai, J.; Nooshabadi, V.T.; Mirzaei, Z. Employing hydrogels in tissue engineering approaches to boost conventional cancer-based research and therapies. RSC Adv. 2021, 11, 10646–10669. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Qin, G.; Zhang, S.; Wu, Y.; Xu, B.; Gao, Y. Novel lyophilized hydrogel patches for convenient and effective administration of microneedle-mediated insulin delivery. Int. J. Pharm. 2012, 437, 51–56. [Google Scholar] [CrossRef]
- Fitri, A.M.N.; Elim, D.; Mahfud, M.A.S.; Sultan, N.A.F.; Saputra, M.D.; Afika, N.; Friandini, R.A.; Djide, N.J.N.; Permana, A.D. Polymeric hydrogel forming microneedle-mediated transdermal delivery of sildenafil citrate from direct-compressed tablet reservoir for potential improvement of pulmonary hypertension therapy. Int. J. Pharm. 2023, 631, 122549. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Wang, X.; Gao, N.; Li, X.; Chen, H.; Mei, L.; Zeng, X. Actively separated microneedle patch for sustained-release of growth hormone to treat growth hormone deficiency. Acta Pharm. Sin. B 2023, 13, 344–358. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- Sivaraman, A.; Banga, A.K. Novel in situ forming hydrogel microneedles for transdermal drug delivery. Drug Deliv. Transl. Res. 2017, 7, 16–26. [Google Scholar] [CrossRef]
- Pitakjakpipop, H.; Rajan, R.; Tantisantisom, K.; Opaprakasit, P.; Nguyen, D.D.; Ho, V.A.; Matsumura, K.; Khanchaitit, P. Facile Photolithographic Fabrication of Zwitterionic Polymer Microneedles with Protein Aggregation Inhibition for Transdermal Drug Delivery. Biomacromolecules 2022, 23, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, F.; Liu, J.; Fan, G.; Welsh, W.; Zhu, H.; Jin, T. Phase-Transition Microneedle Patches for Efficient and Accurate Transdermal Delivery of Insulin. Adv. Funct. Mater. 2015, 25, 4633–4641. [Google Scholar] [CrossRef]
- Chew, S.W.T.; Shah, A.H.; Zheng, M.; Chang, H.; Wiraja, C.; Steele, T.W.J.; Xu, C. A self-adhesive microneedle patch with drug loading capability through swelling effect. Bioeng. Transl. Med. 2020, 5, e10157. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Jung, H.Y.; Park, N.; Kim, J. Adhesive Composite Hydrogel Patch for Sustained Transdermal Drug Delivery To Treat Atopic Dermatitis. Chem. Mater. 2023, 35, 1209–1217. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, M.K.; Lee, J.Y.; Choi, S.W.; Kim, J. Adhesive Hydrogel Patch with Enhanced Strength and Adhesiveness to Skin for Transdermal Drug Delivery. Adv. Funct. Mater. 2020, 30, 2004407. [Google Scholar] [CrossRef]
- Thanakorphimol, K.; Paradee, N. Fabrication of Transdermal Patch from Freeze-dried Agarose Hydrogel for Electrically Controlled Release. In Proceedings of the ECTI-CON 2021—2021 18th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications, and Information Technology: Smart Electrical System and Technology, Chiang Mai, Thailand, 19–22 May 2021. [Google Scholar]
- An, Y.-H.; Lee, J.; Son, D.U.; Kang, D.H.; Park, M.J.; Cho, K.W.; Kim, S.; Kim, S.-H.; Ko, J.; Jang, M.-H.; et al. Facilitated Transdermal Drug Delivery Using Nanocarriers-Embedded Electroconductive Hydrogel Coupled with Reverse Electrodialysis-Driven Iontophoresis. ACS Nano 2020, 14, 4523–4535. [Google Scholar] [CrossRef]
- D’souza, A.; Marshall, L.R.; Yoon, J.; Kulesha, A.; Edirisinghe, D.I.U.; Chandrasekaran, S.; Rathee, P.; Prabhakar, R.; Makhlynets, O.V. Peptide hydrogel with self-healing and redox-responsive properties. Nano Converg. 2022, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wat, E.; Hui, P.C.L.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.B.S.; et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef] [PubMed]
- Krathumkhet, N.; Imae, T.; Paradee, N. Electrically controlled transdermal ibuprofen delivery consisting of pectin-bacterial cellulose/polypyrrole hydrogel composites. Cellulose 2021, 28, 11451–11463. [Google Scholar] [CrossRef]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Anis, A.; Kim, D.; Pal, K. Environment Sensitive Hydrogels for Drug Delivery Applications. Eur. Polym. J. 2019, 120, 109220. [Google Scholar] [CrossRef]
- Gao, W.; Vecchio, D.; Li, J.; Zhu, J.; Zhang, Q.; Fu, V.; Li, J.; Thamphiwatana, S.; Lu, D.; Zhang, L. Hydrogel Containing Nanoparticle-Stabilized Liposomes for Topical Antimicrobial Delivery. ACS Nano 2014, 8, 2900–2907. [Google Scholar] [CrossRef]
- Ziai, Y.; Lanzi, M.; Rinoldi, C.; Zargarian, S.S.; Zakrzewska, A.B.; Kosik-Kozioł, A.; Nakielski, P.; Pierini, F. Developing strategies to optimize the anchorage between electrospun nanofibers and hydrogels for multi-layered plasmonic biomaterials. Nanoscale Adv. 2024, 6, 1246–1258. [Google Scholar] [CrossRef]
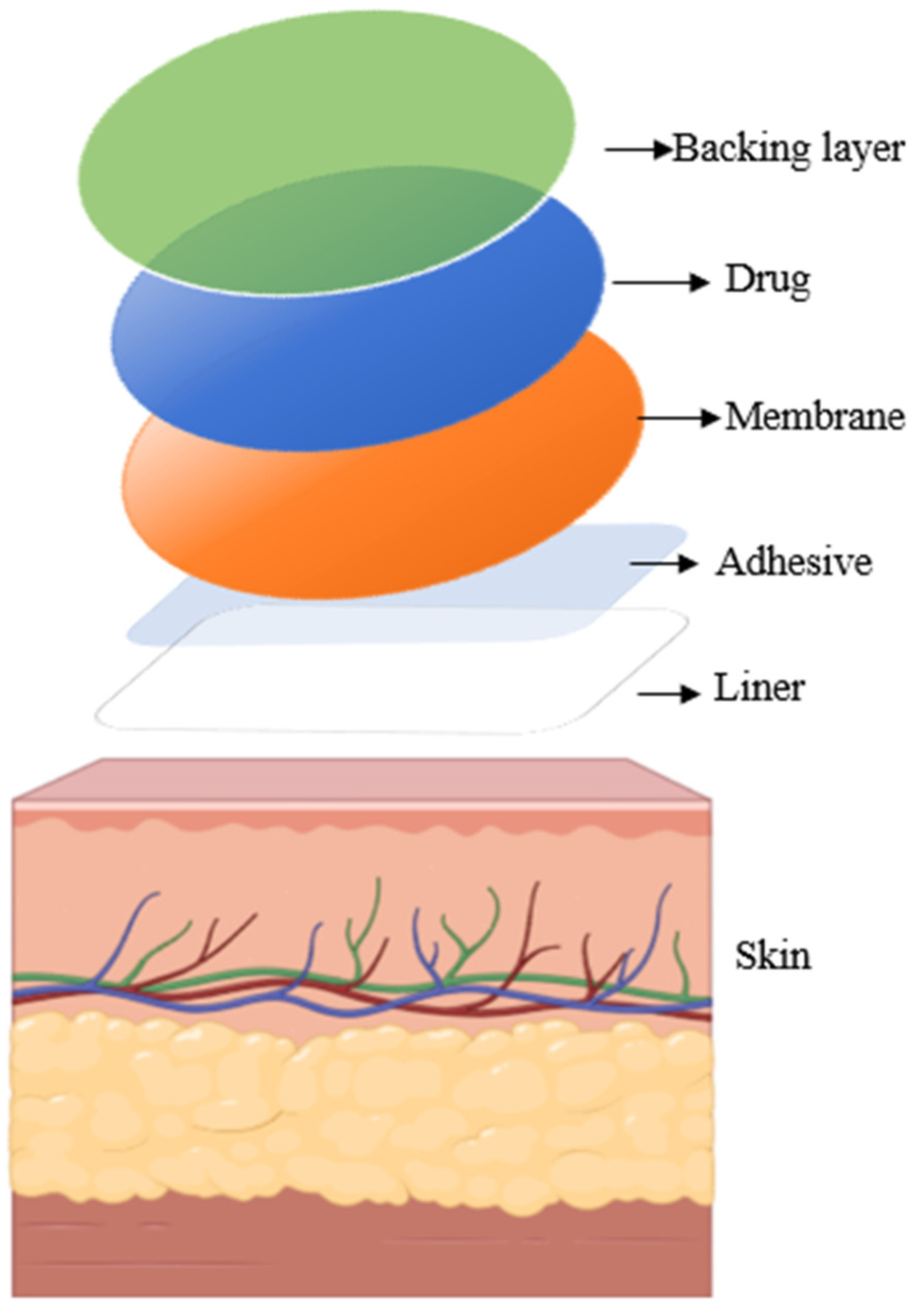
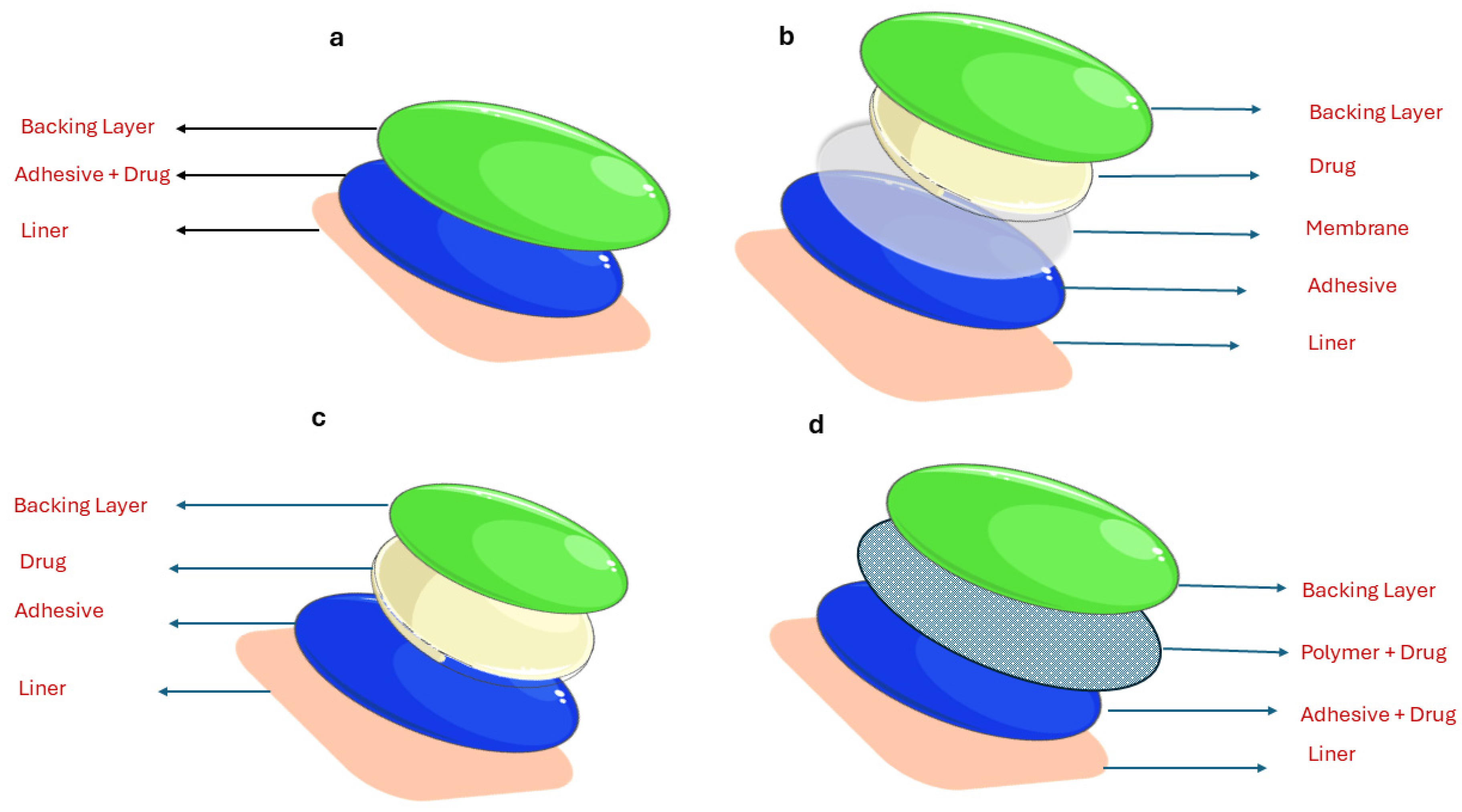
| Advantages | Disadvantages |
|---|---|
| Mitigate problems from the gastrointestinal tract (GIT) and hepatic metabolism [11]. | Poor permeability across the skin [12] |
| Noninvasive drug administration and less pain involved [13] | Few classes of drugs exhibit skin penetration [14] |
| Provide drug release for an extended period [9] | Slow drug release |
| Maintain a steady plasma level of the drug [15] | May cause local irritation |
| Increased patient compliance | Variation in barriersd [12] |
| Type of Composition | Synthetic Polymer Hydrogel | Natural Polymer Hydrogel | Natural& Synthetic Hydrogel |
|---|---|---|---|
|
|
| |
| Advantages |
|
|
|
| Disadvantage |
|
|
|
| Stimuli | Advantages | Disadvantages |
|---|---|---|
| pH | Response to change in environmental pH Controlled release of therapeutic drugs Minimal harm [38] | Poor reusability [39] Susceptible to rupture [40] |
| Temperature | Easy to formulate Deliver both hydrophilic and hydrophobic drugs [41] | Low sensitivity and Poor reusability [39] |
| Light | Easily controlled and triggered by light Precise and targeted drug delivery [42,43] | Ultraviolet light may have potential toxicity concerns. Side-reactivity and challenges in optimizing release mechanisms [44] |
| Electric Field | Allows controllable drug release under different voltages Lightweight, continuous deformability, and high environment adaptability [45] | May require specific conditions or stimuli to exhibit their electrically responsive behavior [46] |
| Physically Crosslinked Hydrogel | Chemically Crosslinked Hydrogel |
|---|---|
| Involve non-covalent interactions | Crosslinks formed by covalent bonds between polymer chains |
| Reversible | Irreversible |
| Used for stimuli-responsive behavior, injectability, or biodegradability. | Used for mechanical strength, stability, and resistance to degradation. |
| Product | Active Ingredients | Application | Reference |
|---|---|---|---|
| Lidoderm® | Sodium carboxymethylcellulose | Nerve Pain Relief | [88] |
| Astero® | Polyethylene Glycol (PEG) 400 | Burn/Irritation/Discomfort | [88] |
| Neutrogena® | Hyaluronic Acid | Collagen synthesis | [88] |
| Daytrana® | Methylphenidate | Hyperactivity Disorder (ADHD) | [89] |
| Emsam® | Selegiline | Mental Depression | [89] |
| Flector® | Diclofenac epolamine | Acute Pain | [89] |
| Ionsys® | Fentanyl | Severe and Persistent Pain | [89] |
| Secuado® | Asenapine | Schizophrenia | [89] |
| Twirla® | Ethinyl estradiol Levonorgestrel | Contraceptive | [89] |
| Zecuity® | Sumatriptan | Acute Migraine | [89] |
| Gel Type | Therapeutic/Drug | Application | Results | Reference |
|---|---|---|---|---|
| Hydrogel Forming Microneedle | Ibuprofen Sodium Ovalbumin | Clinically relevant non-potent drug delivery Pain management |
| [72] |
| Hydrogel Microneedle | Methotrexate | Treatment of solid tumors |
| [90] |
| Hydrogel Photo Crosslinked Microneedle | Rhodamine B | Suppress protein aggression |
| [91] |
| Phase Transition Microneedle Patch | Insulin | Diabetes Management |
| [92] |
| Hydrogel Microneedle Patch | Doxorubicin hydrochloride (Dox) | Controlled drug release |
| [93] |
| Adhesive Composite Hydrogel | Dexamethasone (DEX) | Atopic dermatitis (A.D.) |
| [94] |
| Bioadhesive Hydrogel | Wound Healing |
| [95] | |
| Adhesive Hydrogel | Rhodamine 6G | Local and systemic delivery of drugs |
| [96] |
| Hydrogel Patch | Ibuprofen | Pain management |
| [97] |
| Electro-responsive Hydrogel Patch | Doxorubicin | Wearable skin patch applications |
| [83] |
| Electroconductive Hydrogel | Rosiglitazone (Rosi) | Treat a diet-induced type-2 diabetic and obese mouse. |
| [98] |
| Metallo-Hydrogel | Antimicrobial Peptide F9 4ʹPyA | Wound dressing and wound healing applications. |
| [99] |
| Thermosensitive Poloxamer Hydrogel | Cortex Moutan (CM) | Treatment of atopic dermatitis (A.D.) |
| [100] |
| Microcapsules-embedded Hydrogel Patches | Diclofenac Sodium | Treating topical tissue injuries and skin diseases |
| [65] |
| Electrically Controlled Hydrogel Composite | Ibuprofen | Controlled anionic drug (ibuprofen) release. For pain management applications |
| [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alex, M.; Alsawaftah, N.M.; Husseini, G.A. State-of-All-the-Art and Prospective Hydrogel-Based Transdermal Drug Delivery Systems. Appl. Sci. 2024, 14, 2926. https://doi.org/10.3390/app14072926
Alex M, Alsawaftah NM, Husseini GA. State-of-All-the-Art and Prospective Hydrogel-Based Transdermal Drug Delivery Systems. Applied Sciences. 2024; 14(7):2926. https://doi.org/10.3390/app14072926
Chicago/Turabian StyleAlex, Meera, Nour M. Alsawaftah, and Ghaleb A. Husseini. 2024. "State-of-All-the-Art and Prospective Hydrogel-Based Transdermal Drug Delivery Systems" Applied Sciences 14, no. 7: 2926. https://doi.org/10.3390/app14072926
APA StyleAlex, M., Alsawaftah, N. M., & Husseini, G. A. (2024). State-of-All-the-Art and Prospective Hydrogel-Based Transdermal Drug Delivery Systems. Applied Sciences, 14(7), 2926. https://doi.org/10.3390/app14072926






