Assessment of the Effects of Enamel Remineralization After Treatment with Hydroxylapatite Active Substance: SEM Study
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Application of the Product
2.3. SEM Analyses
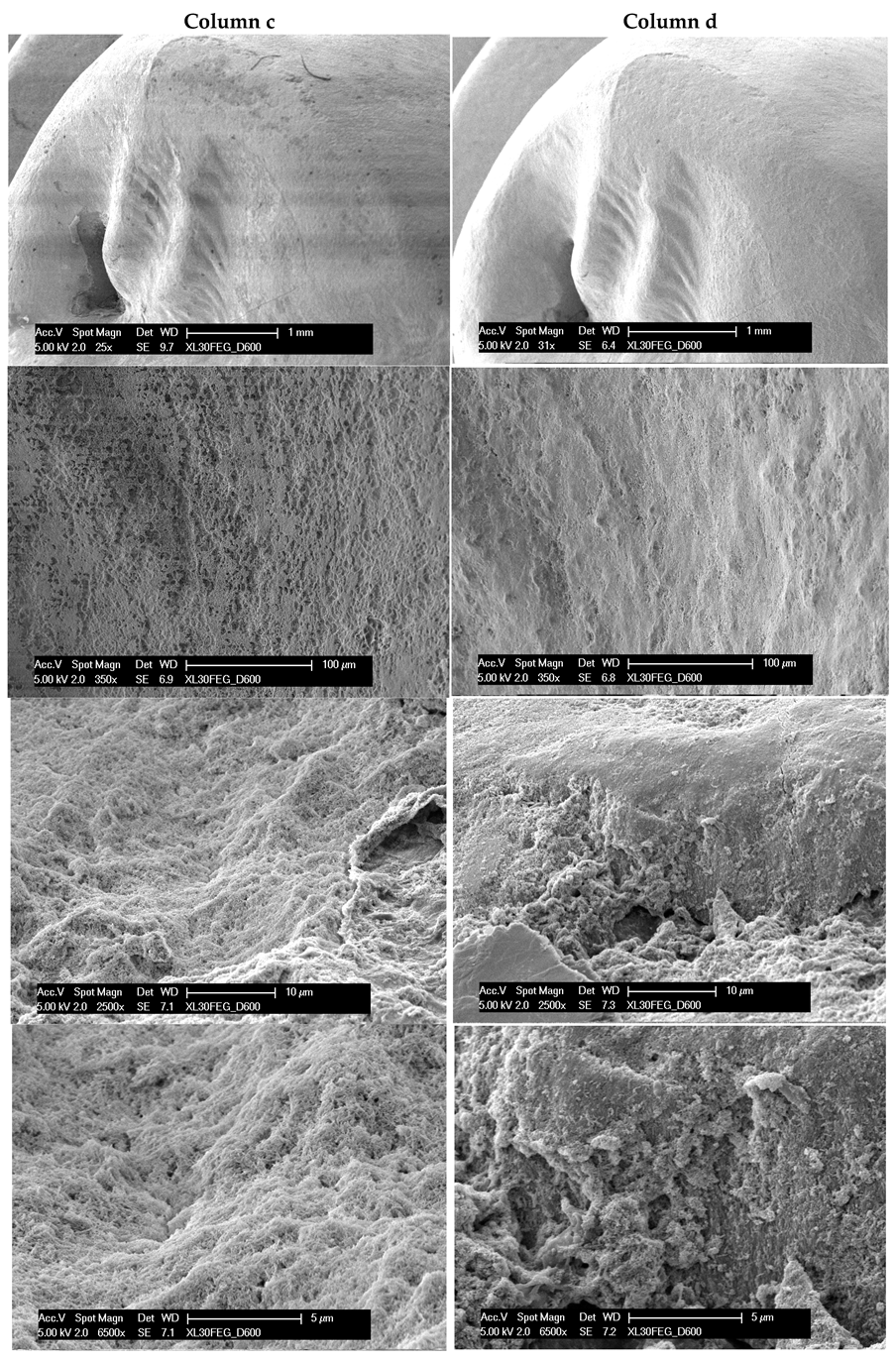
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arola, D.D.; Gao, S.; Zhang, H.; Masri, R. The Tooth. Dent. Clin. N. Am. 2017, 61, 651–668. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.E.; Teepe, J.D.; Hu, Y.; Smith, C.E.; Fajardo, R.J.; Chun, Y.-H.P. Estimating Mineral Changes in Enamel Formation by Ashing/BSE and MicroCT. J. Dent. Res. 2014, 93, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Simmer, J.P.; Fincham, A.G. Molecular Mechanisms of Dental Enamel Formation. Crit. Rev. Oral Biol. Med. 1995, 6, 84–108. [Google Scholar] [CrossRef]
- Smith, C.E.; Nanci, A. Protein dynamics of amelogenesis. Anat. Rec. 1996, 245, 186–207. [Google Scholar] [CrossRef]
- Robinson, C.; Weatherell, J.A.; Hallsworth, A.S. Variation in Composition of Dental Enamel Within Thin Ground Tooth Sections. Caries Res. 2009, 5, 44–57. [Google Scholar] [CrossRef]
- LeFevre, M.L.; Manly, R.S. Moisture, Inorganic and Organic Contents of Enamel and Dentin from Carious Teeth. J. Am. Dent. Assoc. Dent. Cosm. 1938, 25, 233–242. [Google Scholar] [CrossRef]
- Lasota, A.; Kuczumow, A.; Gorzelak, M.; Blicharski, T.; Niezbecka-Zając, J.; Turżańska, K.; Szabelska, A.; Łobacz, M.; Wiszumirska, K.; Wieruszewski, M.; et al. Contribution to Knowledge on Bioapatites: Does Mg Level Reflect the Organic Matter and Water Contents of Enamel? Int. J. Mol. Sci. 2023, 24, 15974. [Google Scholar] [CrossRef] [PubMed]
- Boyde, A. Microstructure of Enamel. In Novartis Foundation Symposia; Chadwick, D.J., Cardew, G., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 18–31. ISBN 978-0-471-96872-6. [Google Scholar]
- Li, X.; Wang, J.; Joiner, A.; Chang, J. The remineralisation of enamel: A review of the literature. J. Dent. 2014, 42, S12–S20. [Google Scholar] [CrossRef]
- Abou Neel, E.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.; Doméjean, S. The Role of Remineralizing and Anticaries Agents in Caries Management. Adv. Dent. Res. 2012, 24, 28–31. [Google Scholar] [CrossRef]
- Carvalho, T.S.; Lussi, A. Age-related morphological, histological and functional changes in teeth. J. Oral Rehabil. 2017, 44, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.I. Relationship between demineralization events in dental enamel and the pH and mineral content of plaque. Proc. Finn. Dent. Soc. Suom. Hammaslaakariseuran Toim. 1991, 87, 527–539. [Google Scholar]
- West, N.X.; Joiner, A. Enamel mineral loss. J. Dent. 2014, 42, S2–S11. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Morolla, R.; De Santis, M.; Piras, F.; Settanni, V.; Mancini, A.; Di Venere, D.; Inchingolo, F.; Inchingolo, A.D.; et al. Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons. Bioengineering 2023, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Grohe, B.; Mittler, S. Advanced non-fluoride approaches to dental enamel remineralization: The next level in enamel repair management. Biomater. Biosyst. 2021, 4, 100029. [Google Scholar] [CrossRef]
- Green, J.I. Prevention and Management of Tooth Wear: The Role of Dental Technology. Prim. Dent. J. 2016, 5, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, N.J.; Cai, F.; Huq, N.L.; Burrow, M.F.; Reynolds, E.C. New Approaches to Enhanced Remineralization of Tooth Enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef]
- Cury, J.A.; Tenuta, L.M.A. Enamel remineralization: Controlling the caries disease or treating early caries lesions? Braz. Oral Res. 2009, 23, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Untreated Caries: A Systematic Review and Metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Piao, Y.-Z.; Kim, S.-M.; Lee, Y.-K.; Kim, K.-N.; Kim, K.-M. Acid neutralizing, mechanical and physical properties of pit and fissure sealants containing melt-derived 45S5 bioactive glass. Dent. Mater. 2013, 29, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- Borjian, A.; Ferrari, C.C.F.; Anouf, A.; Touyz, L.Z.G. Pop-Cola Acids and Tooth Erosion: An In Vitro, In Vivo, Electron-Microscopic, and Clinical Report. Int. J. Dent. 2010, 2010, 957842. [Google Scholar] [CrossRef]
- Featherstone, J.D.B.; Chaffee, B.W. The Evidence for Caries Management by Risk Assessment (CAMBRA®). Adv. Dent. Res. 2018, 29, 9–14. [Google Scholar] [CrossRef]
- ten Cate, J.M. Models and Role Models. Caries Res. 2015, 49, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Lee, M.-J.; Kim, K.-M.; Yang, S.-Y.; Seo, J.-Y.; Choi, S.-H.; Kwon, J.-S. Enamel Demineralization Resistance and Remineralization by Various Fluoride-Releasing Dental Restorative Materials. Matesrials 2021, 14, 4554. [Google Scholar] [CrossRef] [PubMed]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A narrative review 2021. F1000Research 2021, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.W.; Swartz, M.L. Effect of Fluorides on Hardness of Tooth Enamel. J. Am. Dent. Assoc. 1948, 37, 1–13. [Google Scholar] [CrossRef]
- Nasution, A.I.; Zawil, C. The comparison of enamel hardness between fluoride and theobromine application. Int. J. Contemp. Dent. Med. Rev. 2024, 2014, 031214. [Google Scholar]
- Singh, S.; Sharma, S. Conquering the enemy within: Noncavitated carious lesions. J. Conserv. Dent. Endod. 2024, 27, 889–890. [Google Scholar] [CrossRef]
- Raghu, R.; Shetty, A.; Reddy, S.; Srinivasan, R.; Priyadarshini, S.; Gautham, P. Effect of organic versus inorganic fluoride on enamel microhardness: An in vitro study. J. Conserv. Dent. JCD 2013, 16, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.A.; Patel, M.P.; Hill, R.G.; Fleming, P.S. The effect of bioactive glasses on enamel remineralization: A systematic review. J. Dent. 2017, 67, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Heshmat, H.; Kazemi Yazdi, H.; Hoorizad Ganjkar, M.; Chaboki, F.; Shokri, M.; Kharazifard, M.J. Effect of Two Remineralizing Agents on Dentin Microhardness of Non-Caries Lesions. J. Dent. 2023, 24, 417–421. [Google Scholar] [CrossRef]
- Al Ghwainem, A. Impact of Various Remineralizing Agents on Artificial White Spot Lesion on Primary Teeth—A Comparative Study. J. Pharm. Bioallied Sci. 2023, 15, S426–S429. [Google Scholar] [CrossRef]
- Pepla, E. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- L’effetto del latte fermentato sullo smalto deciduo in presenza e in assenza di fluoro: Studio in vitro. Minerva Stomatol. 2013, 62, 289–294. Available online: https://www-minervamedica-it.insubria.idm.oclc.org/it/riviste/minerva-dental-and-oral science/articolo.php?cod=R18Y2013N07A0289 (accessed on 4 November 2024).
- Yu, O.Y.; Lam, W.Y.-H.; Wong, A.W.-Y.; Duangthip, D.; Chu, C.-H. Nonrestorative Management of Dental Caries. Dent. J. 2021, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Azinović, Z.; Keros, J.; Buković, D.; Azinović, A. SEM analysis of tooth enamel. Coll. Antropol. 2003, 27, 381–386. [Google Scholar]
- De Menezes Oliveira, M.A.H.; Torres, C.P.; Gomes-Silva, J.M.; Chinelatti, M.A.; De Menezes, F.C.H.; Palma-Dibb, R.G.; Borsatto, M.C. Microstructure and mineral composition of dental enamel of permanent and deciduous teeth. Microsc. Res. Tech. 2010, 73, 572–577. [Google Scholar] [CrossRef]
- Poggio, C.; Lombardini, M.; Vigorelli, P.; Ceci, M. Analysis of dentin/enamel remineralization by a CPP-ACP paste: AFM and SEM study. Scanning 2013, 35, 366–374. [Google Scholar] [CrossRef]
- Vitiello, F.; Tosco, V.; Monterubbianesi, R.; Orilisi, G.; Gatto, M.L.; Sparabombe, S.; Memé, L.; Mengucci, P.; Putignano, A.; Orsini, G. Remineralization Efficacy of Four Remineralizing Agents on Artificial Enamel Lesions: SEM-EDS Investigation. Materials 2022, 15, 4398. [Google Scholar] [CrossRef]
- Risnes, S.; Saeed, M.; Sehic, A. Scanning Electron Microscopy (SEM) Methods for Dental Enamel. Methods Mol. Biol. Clifton NJ 2019, 1922, 293–308. [Google Scholar] [CrossRef]
- Huang, S.B.; Gao, S.S.; Yu, H.Y. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed. Mater. 2009, 4, 034104. [Google Scholar] [CrossRef] [PubMed]
- Anjali, D.; Pathak, D.A.; Tiwana, D.J.K. Remineralizing agents in pediatric dentistry: A review of literature. Int. J. Curr. Res. 2021, 13, 18067–18070. [Google Scholar]
- Ostrovidov, S.; Ramalingam, M.; Bae, H.; Orive, G.; Fujie, T.; Shi, X.; Kaji, H. Bioprinting and biomaterials for dental alveolar tissue regeneration. Front. Bioeng. Biotechnol. 2023, 11, 991821. [Google Scholar] [CrossRef]
- Zecca, P.A.; Brambilla, A.; Reguzzoni, M.; Protasoni, M.; Raspanti, M. Enhancing SEM positioning precision with a LEGO®-based sample fitting system. Microsc. Res. Tech. 2024, 87, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Zecca, P.A.; Reguzzoni, M.; Protasoni, M.; Raspanti, M. The chondro-osseous junction of articular cartilage. Tissue Cell 2023, 80, 101993. [Google Scholar] [CrossRef] [PubMed]
- Wegehaupt, F.J.; Tauböck, T.T.; Attin, T. Influence of prophylaxis paste treatment on the abrasive wear of surface sealants. Acta Odontol. Scand. 2013, 71, 744–750. [Google Scholar] [CrossRef]
- Magalhães, A.C.; Wiegand, A.; Rios, D.; Buzalaf, M.A.R.; Lussi, A. Fluoride in dental erosion. Monogr. Oral Sci. 2011, 22, 158–170. [Google Scholar] [CrossRef]
- Ceci, S.; Berate, P.; Candrea, S.; Babtan, A.-M.; Azzollini, D.; Piras, F.; Curatoli, L.; Corriero, A.; Patano, A.; Valente, F.; et al. The oral and gut microbiota: Beyond a short communication. Balneo PRM Res. J. 2021, 12, 405–411. [Google Scholar] [CrossRef]
- Severe Tooth Wear: European Consensus Statement on Management Guidelines. J. Adhes. Dent. 2017, 19, 111–119. Available online: https://www.quintessence-publishing.com/deu/de/article/843339/the-journal-of-adhesive-dentistry/2017/02/severe-tooth-wear-european-consensus-statement-on-management-guidelines (accessed on 21 September 2024).
- Ali, S.; Farooq, I.; Al-Thobity, A.M.; Al-Khalifa, K.S.; Alhooshani, K.; Sauro, S. An in-vitro evaluation of fluoride content and enamel remineralization potential of two toothpastes containing different bioactive glasses. Biomed. Mater. Eng. 2019, 30, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Pinto de Souza, S.C.T.; de Araújo, K.C.; Barbosa, J.R.; Cancio, V.; Rocha, A.A.; Tostes, M.A. Effect of dentifrice containing fTCP, CPP-ACP and fluoride in the prevention of enamel demineralization. Acta Odontol. Scand. 2018, 76, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Vincent, S.; Thomas, A.M. Fluoride Levels in Saliva and Plaque following the Use of High Fluoride and Conventional Dentifrices- a Triple Blinded Randomised Parallel Group Trial. Sci. World J. 2019, 2019, 1636209. [Google Scholar] [CrossRef]
- Bartlett, D.; O’Toole, S. Tooth wear and aging. Aust. Dent. J. 2019, 64, S59–S62. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, A.; Holban, A.-M.; Păuna, M.-R.; Imre, M.; Farcașiu, A.-T.; Farcașiu, C. Review of Professionally Applied Fluorides for Preventing Dental Caries in Children and Adolescents. Appl. Sci. 2022, 12, 1054. [Google Scholar] [CrossRef]
- Larson, T.D. Why do we polish? Part one. Northwest Dent. 2011, 90, 17–22. [Google Scholar] [PubMed]
- Sawai, M.A.; Bhardwaj, A.; Jafri, Z.; Sultan, N.; Daing, A. Tooth polishing: The current status. J. Indian Soc. Periodontol. 2015, 19, 375–380. [Google Scholar] [CrossRef]
- Bizhang, M.; Kaleta-Kragt, S.; Singh-Hüsgen, P.; Altenburger, M.J.; Zimmer, S. Effect of 10% fluoride on the remineralization of dentin in situ. J. Appl. Oral Sci. 2015, 23, 562. [Google Scholar] [CrossRef]
- Gore, A.B.; Patel, S.P.; Gulve, M.N.; Aher, G.B. Comparative evaluation of the remineralizing potential of different calcium and fluoride-based delivery systems on artificially demineralized enamel surface; an in vitro study. J. Conserv. Dent. JCD 2022, 25, 292. [Google Scholar] [CrossRef] [PubMed]
- Chaiwat, A.; Chunhacheevachaloke, E.; Kidkhunthod, P.; Pakawanit, P.; Ajcharanukul, O. Enamel Remineralization and Crystallization after Fluoride Iontophoresis. J. Dent. Res. 2023, 102, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Thimmaiah, C.; Shetty, P.; Shetty, S.B.; Natarajan, S.; Thomas, N.-A. Comparative analysis of the remineralization potential of CPP–ACP with Fluoride, Tri-Calcium Phosphate and Nano Hydroxyapatite using SEM/EDX—An in vitro study. J. Clin. Exp. Dent. 2019, 11, e1120. [Google Scholar] [CrossRef]
- Wiegand, A.; Schlueter, N. The Role of Oral Hygiene: Does Toothbrushing Harm? In Erosive Tooth Wear; Karger Publishers: Basel, Switzerland, 2014; pp. 215–219. [Google Scholar] [CrossRef]
- Atsu, S.S.; Aka, P.S.; Kucukesmen, H.C.; Kilicarslan, M.A.; Atakan, C. Age-related changes in tooth enamel as measured by electron microscopy: Implications for porcelain laminate veneers. J. Prosthet. Dent. 2005, 94, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Young, D.A.; Featherstone, J.D.B.; Roth, J.R.; Anderson, M.; Autio-Gold, J.; Christensen, G.J.; Fontana, M.; Kutsch, V.K.; Peters, M.C.; Simonsen, R.J.; et al. Caries management by risk assessment: Implementation guidelines. J. Calif. Dent. Assoc. 2007, 35, 799–805. [Google Scholar] [PubMed]
- Anil, A.; Ibraheem, W.I.; Meshni, A.A.; Preethanath, R.S.; Anil, S. Nano-Hydroxyapatite (nHAp) in the Remineralization of Early Dental Caries: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 5629. [Google Scholar] [CrossRef]
- Harpenau, L.A.; Noble, W.H.; Kao, R.T. Diagnosis and management of dental wear. J. Calif. Dent. Assoc. 2011, 39, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Etiology and Pathogenesis of Dental Erosion. Available online: https://www.quintessence-publishing.com/deu/de/article/840922/quintessence-international/2016/04/etiology-and-pathogenesis-of-dental-erosion (accessed on 21 September 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reguzzoni, M.; Carganico, A.; Lo Presti, D.; Zecca, P.A.; Scurati, E.I.; Caccia, M.; Levrini, L. Assessment of the Effects of Enamel Remineralization After Treatment with Hydroxylapatite Active Substance: SEM Study. Appl. Sci. 2025, 15, 3. https://doi.org/10.3390/app15010003
Reguzzoni M, Carganico A, Lo Presti D, Zecca PA, Scurati EI, Caccia M, Levrini L. Assessment of the Effects of Enamel Remineralization After Treatment with Hydroxylapatite Active Substance: SEM Study. Applied Sciences. 2025; 15(1):3. https://doi.org/10.3390/app15010003
Chicago/Turabian StyleReguzzoni, Marcella, Andrea Carganico, Doriana Lo Presti, Piero Antonio Zecca, Eleonora Ivonne Scurati, Margherita Caccia, and Luca Levrini. 2025. "Assessment of the Effects of Enamel Remineralization After Treatment with Hydroxylapatite Active Substance: SEM Study" Applied Sciences 15, no. 1: 3. https://doi.org/10.3390/app15010003
APA StyleReguzzoni, M., Carganico, A., Lo Presti, D., Zecca, P. A., Scurati, E. I., Caccia, M., & Levrini, L. (2025). Assessment of the Effects of Enamel Remineralization After Treatment with Hydroxylapatite Active Substance: SEM Study. Applied Sciences, 15(1), 3. https://doi.org/10.3390/app15010003







