Quantitative 31P NMR Spectroscopy: Principles, Methodologies, and Applications in Phosphorus-Containing Compound Analysis
Abstract
:1. Introduction
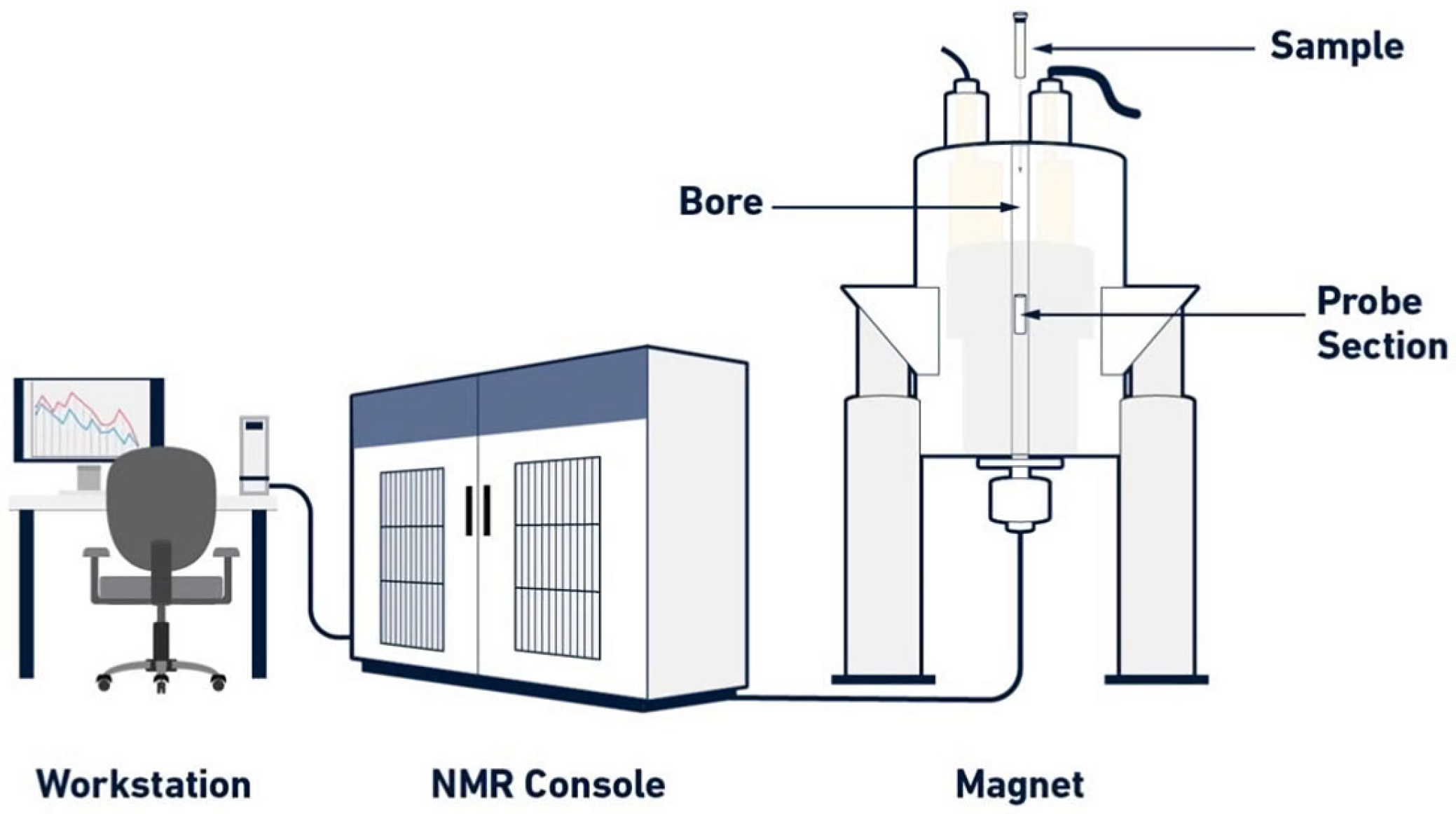
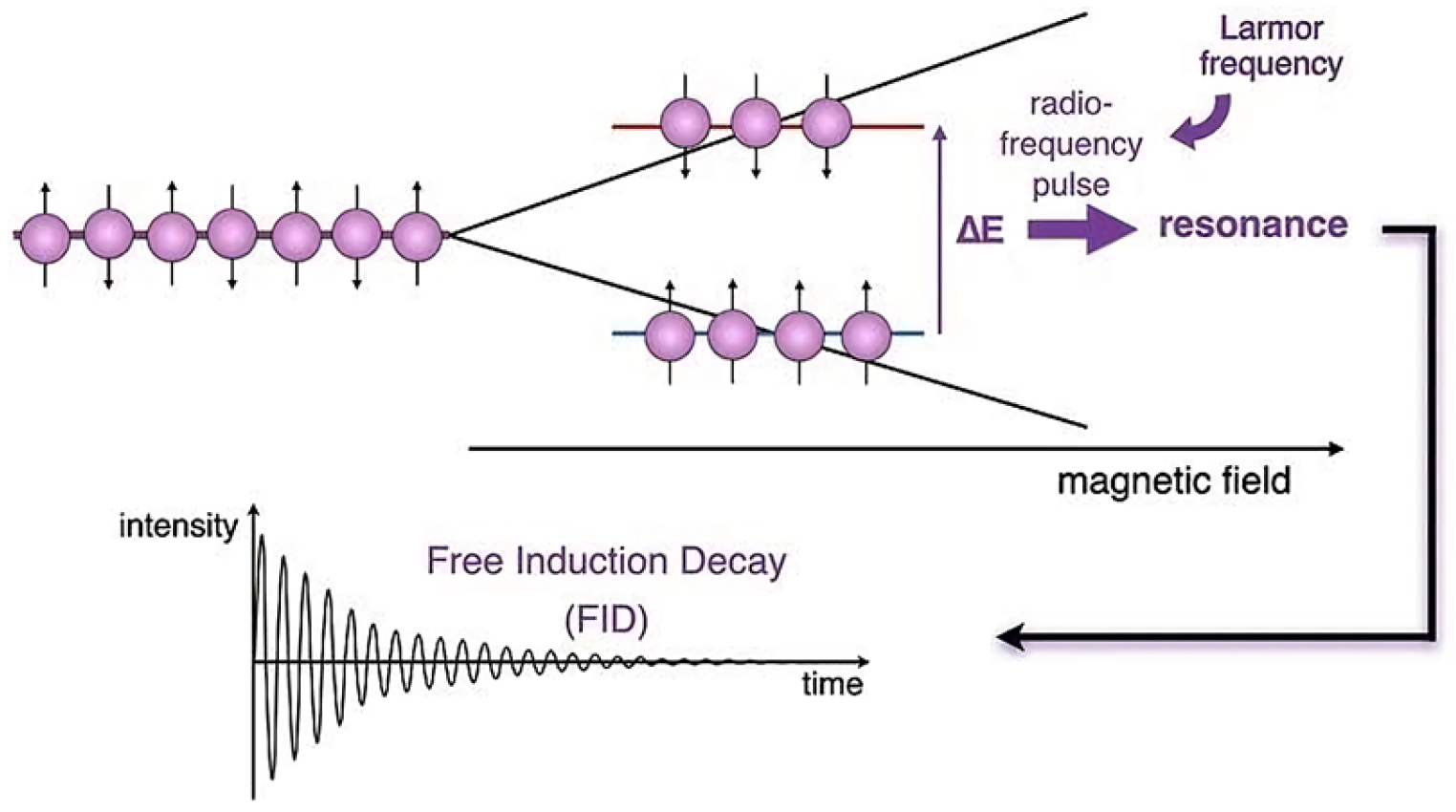
2. General Principles of NMR
3. Overview of 31P qNMR for Phosphorus Compounds
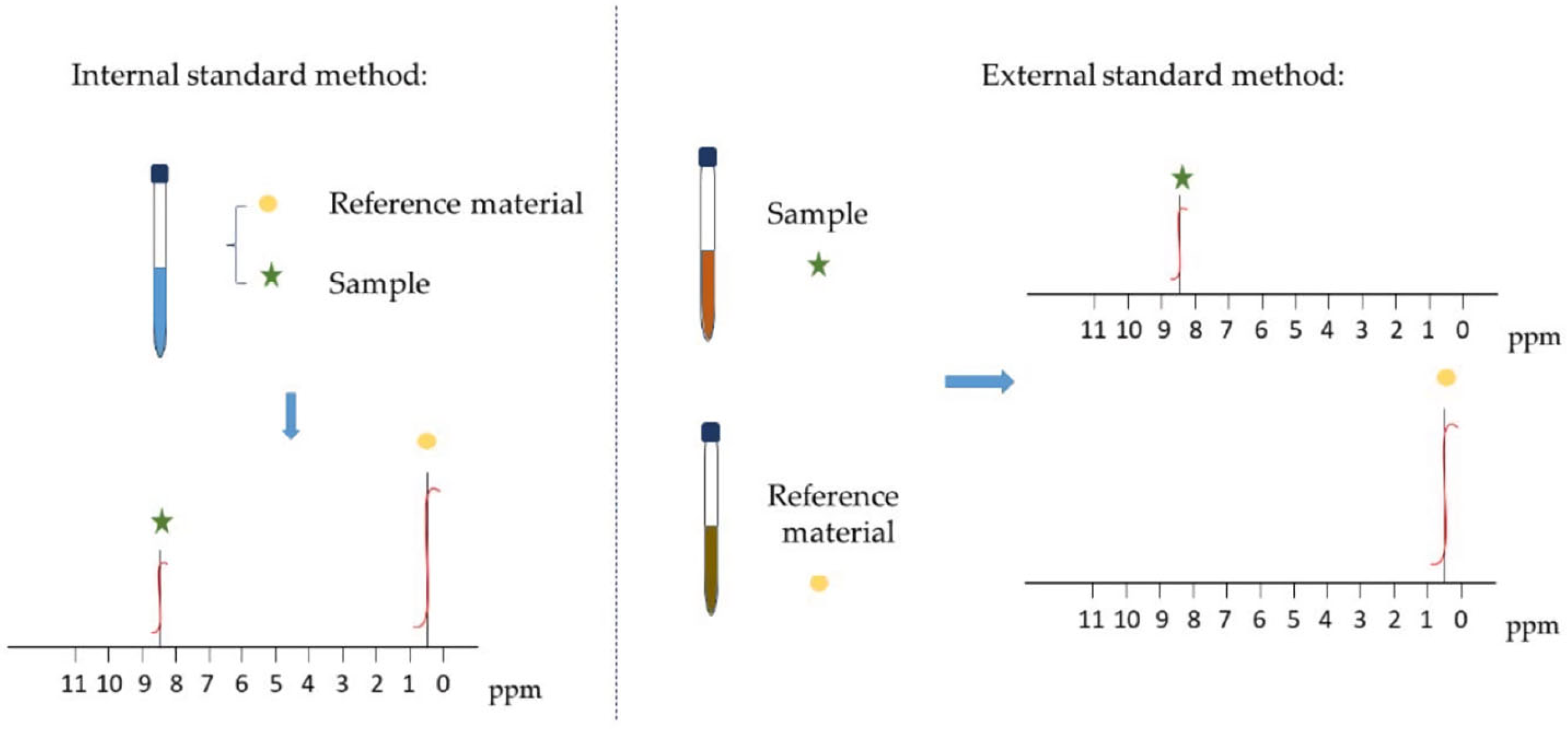
3.1. Internal Standard Method for qNMR
3.2. External Standard Method for qNMR
3.3. 31P Quantitative NMR Method
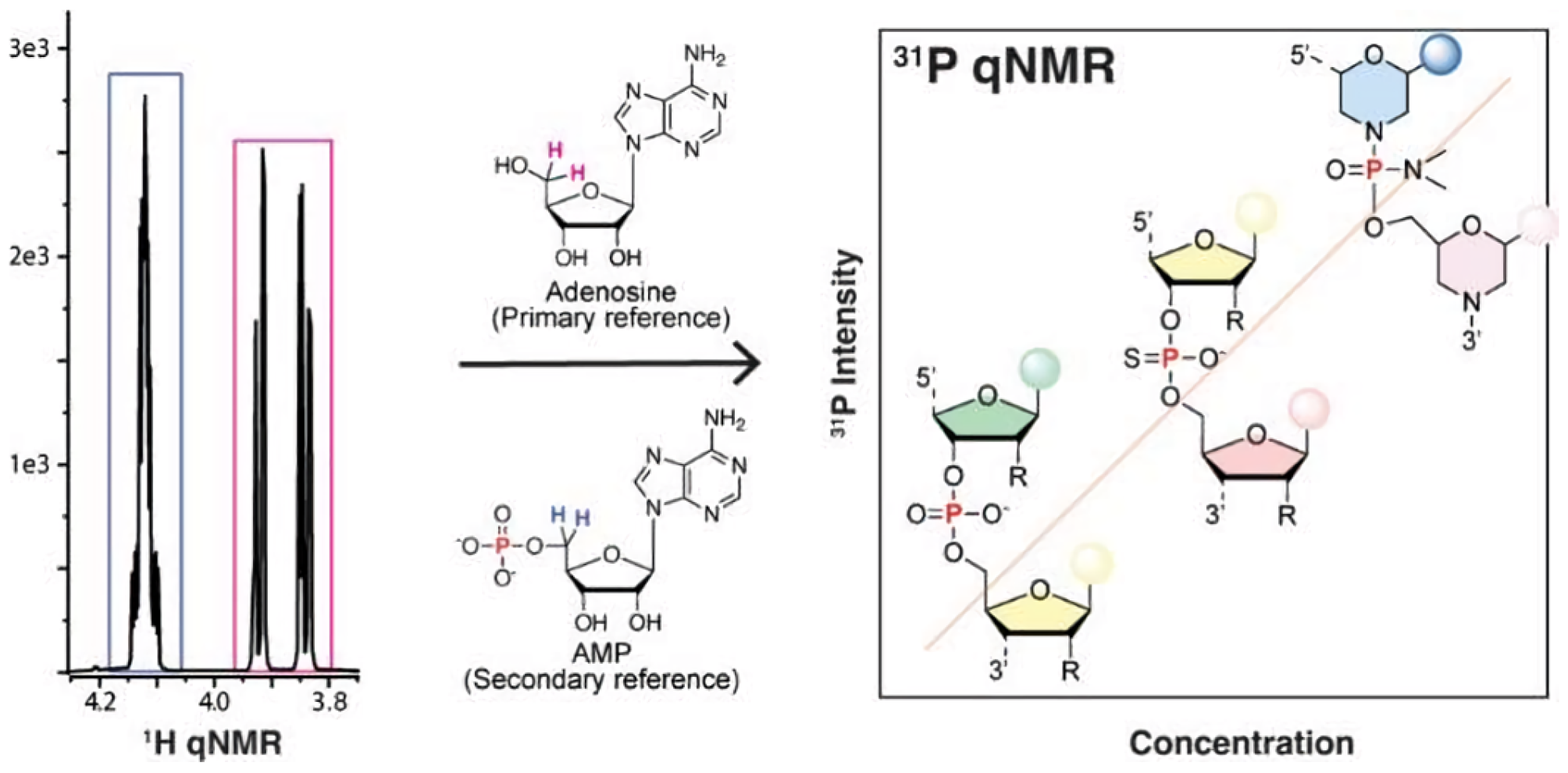
- Research on 31P qNMR standard substances. Weber et al. [39] investigated 31P qNMR internal standards with metrological traceability, such as triphenyl phosphate and phosphoacetic acid, and their practical applications as shown in Figure 5. The study assessed the purity of tris(2-chloroethyl) phosphate using different traceability schemes and solvent systems. The findings demonstrated consistent results within measurement uncertainty ranges, highlighting the reliability of the certification approach. The application of these certified reference materials was further exemplified through measurements of tris(2-chloroethyl) phosphate. Additionally, researchers have used the 31P nucleus for quantitative NMR, testing various phosphorus-containing standards based on solubility and application needs. Internal standards, such as triphenyl phosphate and sodium phosphate, or external standards like phosphoric acid, are commonly employed to prevent reactions with analytes [40].
- Development of Internal Standards for Coaxial Insertion. Henderson et al. [41] developed an absolute quantification method for 31P NMR using coaxial nuclear magnetic resonance tube inserts containing internal standards. This approach physically separates the reference material from the test sample, avoiding chemical interactions and mixing errors. The method demonstrated precision and accuracy exceeding 1% for purity determinations and detected impurities at concentrations as low as 25 µg/mL. Compared to traditional chromatographic methods, it eliminates the need for extensive sample preparation and reference standards, providing significant cost-effectiveness and operational simplicity.
- Integration of 31P qNMR with Other Analytical Techniques. Atanassova et al. [42] studied the interaction between two extractants in deuterated chloroform using 1H, 13C, and 31P nuclear magnetic resonance spectra, along with NOESY experiments. Derewinski et al. [43] applied quantitative NMR techniques, including 27Al, 31P, and 1H magic-angle spinning NMR, together with temperature-programmed desorption to study the impact of various treatment processes on materials. NMR spectroscopy played a key role in this study, offering detailed characterization of transformations in aluminum, phosphorus, and acid sites. The study revealed the mechanisms underlying phosphorus modification and its partial reversibility, offering critical insights for advancing the understanding and optimization of zeolite-based catalysts in industrial applications.
4. Applications of 31P qNMR for Phosphorus-Containing Compounds
4.1. In the Field of Food
4.2. In the Field of Pharmaceuticals
4.3. In the Field of Biology
4.4. Others
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMR | Nuclear magnetic resonance spectroscopy |
| 31P qNMR | Quantitative phosphorus nuclear magnetic resonance spectroscopy |
| FID | Free induction decay |
| RF | Radio frequency |
| PL | Phospholipid |
| AQ | Acquisition time |
References
- Rabi, I.I.; Millman, S.; Kusch, P.; Zacharias, J.R. The Magnetic Moments of 3Li6, 3Li7 and 9F19. Phys. Rev. 1939, 55, 495. [Google Scholar]
- Purcell, E.; Torrey, H.; Pound, R. Resonance Absorption by Nuclear Magnetic Moments in a Solid. Phys. Rev. 1946, 69, 37–38. [Google Scholar] [CrossRef]
- Bloch, F.; Hansen, W.; Packard, M. Nuclear Induction. Phys. Rev. 1946, 69, 127. [Google Scholar] [CrossRef]
- Malet-Martino, M.; Holzgrabe, U. NMR Techniques in Biomedical and Pharmaceutical Analysis. J. Pharm. Biomed. Anal. 2011, 55, 1–15. [Google Scholar] [CrossRef]
- Holzgrabe, U.; Deubner, R.; Schollmayer, C.; Waibel, B. Quantitative NMR Spectroscopy Applications in Drug Analysis. J. Pharm. Biomed. Anal. 2005, 38, 806–812. [Google Scholar] [CrossRef]
- Wood, J.S.; Poggetto, G.D.; Wang, X.; Reibarkh, M.; Williamson, R.T.; Cohen, R.D. Quantitative Nuclear Magnetic Resonance of Chloride by an Accurate Internal Standard Approach. Magn. Reson. Chem. 2023, 61, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Malz, F.; Jancke, H. Validation of Quantitative NMR. J. Pharm. Biomed. Anal. 2005, 38, 813–823. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, T.; Oyler, N.A.; Youan, B.B. Direct and Real-time Quantification of Tenofovir Release from pH-sensitive Microparticles into Simulated Biological Fluids using 1H Nuclear Magnetic Resonance. J. Pharm. Sci. 2014, 103, 1170–1177. [Google Scholar] [CrossRef]
- Agrahari, V.; Meng, J.; Sudhaunshu, S.; Nathan, A.; Youan, B.B. Real-Time Analysis of Tenofovir Release Kinetics using Quantitative Phosphorus (31P) Nuclear Magnetic Resonance Spectroscopy. J. Pharm. Sci. 2017, 106, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Asnani, A.; Gerszten, R.E. Application of Metabolomics to Cardiovascular Biomarker and Pathway Discovery. J. Am. Coll. Cardiol. 2008, 52, 117–123. [Google Scholar] [CrossRef]
- Bonilla, L.; Means, G.; Lee, K.; Patterson, S. The Evolution of Tools for Protein Phosphorylation Site Analysis: From Discovery to Clinical Application. Biotechniques 2008, 44, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Page, R.; Peti, W. Preparation of Phosphorylated Proteins for NMR Spectroscopy. Methods Enzymol. 2019, 614, 187–205. [Google Scholar]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Bernardo-Seisdedos, G.; Bilbao, J.; Fernández-Ramos, D.; Lopitz-Otsoa, F.; Gutierrez de Juan, V.; Bizkarguenaga, M.; Mateos, B.; Fondevila, M.F.; Abril-Fornaguera, J.; Millet, O.; et al. Metabolic Landscape of the Mouse Liver by Quantitative 31P Nuclear Magnetic Resonance Analysis of the Phosphorome. Hepatology 2021, 74, 148–163. [Google Scholar] [CrossRef]
- Fujiwara, S.; Wainai, T. Analysis of Tar Products by Nuclear Magnetic Resonance Spectrometry. Anal. Chem. 1961, 33, 1085–1087. [Google Scholar] [CrossRef]
- Jungnickel, J.L.; Forbes, J.W. Quantitative Measurement of Hydrogen Types by Integrated Nuclear Magnetic Resonance Intensities. Anal. Chem. 1963, 35, 938–942. [Google Scholar] [CrossRef]
- Hollis, D.P. Quantitative Analysis of Aspirin, Phenacetin, and Caffeine Mixtures by Nuclear Magnetic Resonance Spectrometry. Anal. Chem. 1963, 35, 1682–1684. [Google Scholar] [CrossRef]
- Wells, R.J.; Cheung, J.; Hook, J.M. Dimethylsulfone as a Universal Standard for Analysis of Organics by QNMR. Accred. Qual. Assur. 2004, 9, 450–456. [Google Scholar] [CrossRef]
- Barding, G.A.; Salditos, R.; Larive, C.K. Quantitative NMR for Bioanalysis and Metabolomics. Anal. Bioanal. Chem. 2012, 404, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Rigger, R.; Ruck, A.; Hellriegel, C.; Sauermoser, R.; Morf, F.; Breitruck, K.; Obkircher, M. Certified Reference Material for Use in 1H, 31P, and 19F Quantitative NMR, Ensuring Traceability to the International System of Units. J. Aoac. Int. 2017, 100, 1365–1375. [Google Scholar] [CrossRef]
- Maniara, G.; Rajamoorthi, K.; Rajan, S.; Stockton, G.W. Method Performance and Validation for Quantitative Analysis by 1H and 31P NMR Spectroscopy. Applications to Analytical Standards and Agricultural Chemicals. Anal. Chem. 1998, 70, 4921–4928. [Google Scholar] [CrossRef] [PubMed]
- Deen, T.S.A.; Hibbert, D.B.; Hook, J.M.; Wells, R.J. Quantitative Nuclear Magnetic Resonance Spectrometry. Anal. Chim. Acta 2002, 474, 125–135. [Google Scholar] [CrossRef]
- Pearce, J.M.; Komoroski, R.A.; Mrak, R.E. Phospholipid Composition of Postmortem Schizophrenic Brain by 31P NMR Spectroscopy. Magn. Reson. Med. 2010, 61, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q. Research Progress of NMR in Natural Product Quantification. Molecules 2021, 26, 6308. [Google Scholar] [CrossRef]
- Luan, J.; Feng, R.; Chen, Y.; Wu, X.; Su, M. Quantitative Assessment of the Absolute Purity of Thiopeptcin Reference Standard by 1H-NMR. Anal. Sci. 2018, 34, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Schleiff, M.; Sommers, C.; Yang, J.; Shen, X.; Rodriguez, J.D.; Shu, Q. Development and Validation of a Quantitative Proton NMR Method for the Analysis of Pregnenolone. J. Pharm. Biomed. Anal. 2023, 222, 115073. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.J.; Hook, J.M.; Al-Deen, T.S.; Hibbert, D.B. Quantitative Nuclear Magnetic Resonance (QNMR) Spectroscopy for Assessing the Purity of Technical Grade Agrochemicals: 2,4-dichlorophenoxyacetic Acid (2,4-D) and Sodium 2,2-dichloropropionate. J. Agric. Food Chem. 2002, 50, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bhavaraju, S.; Thibeault, M.P.; Melanson, J.; Blomgren, A.; Rundlöf, T.; Kilpatrick, E.; Swann, C.J.; Rudd, T.; Aubin, Y. Survey of Peptide Quantification Methods and Comparison of Their Reproducibility: A Case Study using Oxytocin. J. Pharm. Biomed. Anal. 2019, 166, 105–112. [Google Scholar] [CrossRef]
- Nishizaki, Y.; Lankin, D.C.; Chen, S.; Pauli, G.F. Accurate and Precise External Calibration Enhances the Versatility of Quantitative NMR (qNMR). Anal. Chem. 2011, 93, 2733–2741. [Google Scholar] [CrossRef]
- Castaing-Cordier, T.; Ladroue, V.; Besacier, F.; Bulete, A.; Jacquemin, D.; Giraudeau, P.; Farjon, J. High-field and Benchtop NMR Spectroscopy for the Characterization of New Psychoactive Substances. Forensic Sci. Int. 2021, 321, 110718. [Google Scholar] [CrossRef]
- Sun, L.; Fan, Y.; Wang, Q.; Xiang, L.; Han, H.; Chen, D. Validated Quantitative 31P NMR Spectroscopy for Positional Isomeric Impurity Determination in L-α-glycerylphosphorylcholine (L-α-GPC). J. Pharm. Biomed. Anal. 2022, 221, 115067. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Yoshida, A.; Tanaka, K. Comparison of Pig Cornea Preservation Solutions using Quantitative 31P NMR Spectroscopy. Magn. Reson. Med. 1993, 29, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, N.; Hosoe, J.; Komatsu, T.; Sugimoto, N.; Ishizuki, K.; Koide, T.; Murabayashi, M.; Shinozaki, T.; Kobayashi, K.; Fujimine, Y.; et al. Quantitative 31P NMR for the Purity Determination of the Organophosphorus Compound Brigatinib and Its Method Validation. Chem. Pharm. Bull. 2024, 72, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, H.; Cai, N.; Zou, J.; Ju, X. Quantitative 31P-NMR Spectroscopy for the Determination of Fosfomycin and Impurity A in Pharmaceutical Products of Fosfomycin Sodiumor Calcium. Magn. Reson. Chem. 2015, 53, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. Cardiac 31P MR Spectroscopy: Development of the Past Five Decades and Future Vision-will it Be of Diagnostic Use in Clinics? Heart Fail Rev. 2023, 28, 485–532. [Google Scholar] [CrossRef] [PubMed]
- Gard, D.R.; Burquin, J.C.; Gard, J.K. Quantitative Analysis of Short-Chain Phosphates by Phosphorus-31 Nuclear Magnetic Resonance and Interlaboratory Comparison with Infrared and Chromatographic Methods. Anal. Chem. 1992, 64, 557–561. [Google Scholar] [CrossRef]
- Holzgrabe, U. Quantitative NMR Spectroscopy in Pharmaceutical Applica-tions. Prog. Nucl. Mag. Res. Sp. 2010, 57, 229–240. [Google Scholar] [CrossRef]
- Martino, R.; Gilard, V.; Desmoulin, F.; Malet-Martino, M. Fluorine-19 or Phosphorus-31 NMR Spectroscopy: A Suitable Analytical Technique for Quantitative in Vitro Metabolic Studies of Fluorinated or Phosphorylated Drugs. J. Pharmaceut. Biomed. 2005, 38, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hellriegel, C.; Rueck, A.; Wuethrich, J.; Jenks, P.; Obkircher, M. Method Development in Quantitative NMR Towards Metrologically Traceable Organic Certified Reference Materials Used as 31P qNMR Standards. Anal. Bioanal. Chem. 2015, 407, 3115–3123. [Google Scholar] [CrossRef]
- Gauss, D.; Sanchez-Moreno, I.; Oroz-Guinea, G.J.I.; Wohlgemuth, R. Phosphorylation Catalyzed by Dihydroxyacetone Kinase. Eur. J. Org. Chem. 2018, 23, 2892–2895. [Google Scholar] [CrossRef]
- Henderson, T.J. Quantitative NMR Spectroscopy using Coaxial Inserts Containing a Reference Standard: Purity Determinations for Military Nerve Agents. Anal. Chem. 2002, 74, 191–198. [Google Scholar] [CrossRef]
- Derewinski, M.; Sarv, P.; Sun, X.; Muller, S.; Van Veen, A.C.; Lercher, J.A. Reversibility of the Modification of HZSM-5 with Phosphate Anions. J. Phys. Chem. C 2014, 118, 6122–6131. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V. Synergism in the Solvent Extraction of Europium(III) with Thenoyltrifluoroacetone and CMPO in Methylimidazolium Ionic liquids. J. Solution Chem. 2019, 48, 15–30. [Google Scholar] [CrossRef]
- Belmonte-Sanchez, J.R.; Aguilera-Saez, L.M.; Romero-Gonzalez, R.; Vidal, J.L.M.; Frenich, A.G. Determination of Etidronic Acid in Vegetable-washing Water by a Simple and Validated Quantitative 31P Nuclear Magnetic Resonance Method. Microchem. J. 2019, 150, 104083. [Google Scholar] [CrossRef]
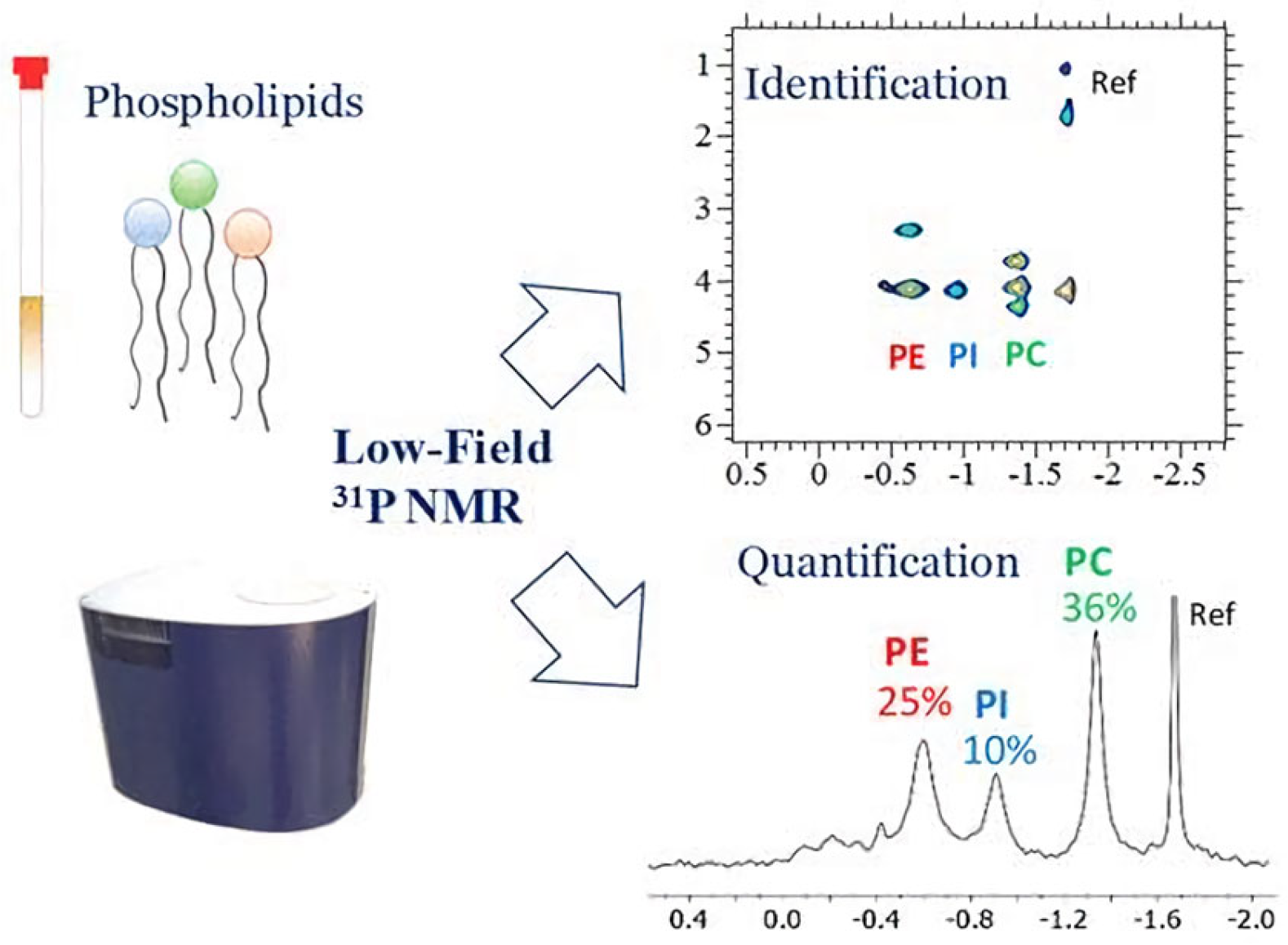
- Kato, T.; Nishimiya, M.; Kawata, A.; Kishida, K.; Suzuri, K.; Saito, M.; Fujita, K.; Igarashi, T.; Inagaki, M. Quantitative 31P NMR Method for Individual and Concomitant Determination of Phospholipid Classes in Polar Lipid Samples. J. Oleo Sci. 2018, 67, 1279–1289. [Google Scholar] [CrossRef]
- Loening, N.M.; Chamberlin, A.M.; Zepeda, A.G.; Gonzalez, R.G.; Cheng, L.L. Quantification of Phosphocholine and Glycerophosphocholine with 31P edited 1H NMR Spectroscopy. NMR Biomed. 2005, 18, 413–420. [Google Scholar] [CrossRef]
- Kovacs, D.; Sebak, F.; Szabo, C.L.; Bodor, A. Determination of Phosphorus Content in Cola Drinks by 31P NMR Spectroscopy using External Calibration and Standard Addition Approaches. J. Chem. Educ. 2024, 101, 2524–2532. [Google Scholar] [CrossRef]
- Khatun, R.; Hunter, H.N.; Sheng, Y.; Carpick, B.W.; Kirkitadze, M.D. 27Al and 31P NMR Spectroscopy Method Development to Quantify Aluminum Phosphate in Adjuvanted Vaccine Formulations. J. Pharm. Biomed. Anal. 2018, 159, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Ackerstaff, E.; Natarajan, K.; Artemov, D.; Bhujwalla, Z.M. Real-time Changes in 1H and 31P NMR Spectra of Malignant Human Mammary Epithelial Cells during Treatment with the Anti-inflammatory Agent Indomethacin. Magn. Reson. Med. 2010, 48, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, N.; Kiyota, K.; Hosoe, J.; Komatsu, T.; Sugimoto, N.; Ishizuki, K.; Koide, T.; Murabayashi, M.; Kobayashi, K.; Fujimine, Y. Quantitative 31P NMR for Purity Determination of Sofosbuvir and Method Validation. Chem. Pharm. Bull. 2022, 70, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Doyoon, P.; Seokyoon, K.; Hyeona, L.; Hyo-Yeon, Y.; Sangdoo, A. Development and Validation of a Method for the Simultaneous Quantitative Analysis of the Active Forms of Vitamins B1, B2, and B6 using 31P qNMR. Microchem. J. 2024, 196, 109704. [Google Scholar]
- Li, J.; Chen, F.; Zhang, D.; Wang, Y.; Kozak, D.; Chen, K. An Accurate and Fast 31P qNMR Assay Method for Oligonucleotide Therapeutics. Anal. Chem. 2024, 96, 16514–16519. [Google Scholar] [CrossRef]
- Bjorstorp, S.; Malmstrom, J. Quantitative 31P NMR Spectroscopy Platform Method for the Assay of Oligonucleotides as Pure Drug Substances and in Drug Product Formulations Using the Internal Standard Method. Anal. Chem. 2024, 96, 11198–11204. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.; Martin-Hidalgo, D.; Bilbao, J.; Bernardo-Seisdedos, G.; Millet, O.; Garcia-Marin, L.J.; Bragado, M.J. Quantitative Analysis of the Human Semen Phosphorometabolome by 31P-NMR. Int. J. Mol. Sci. 2024, 25, 1682. [Google Scholar] [CrossRef]
- Gouilleux, B.; Christensen, N.V.; Malmos, K.G.; Vosegaard, T. Analytical Evaluation of Low-Field 31P NMR Spectroscopy for Lipid Analysis. Anal. Chem. 2019, 91, 3035–3042. [Google Scholar] [CrossRef]
- Matsumi, R.; Hellriegel, C.; Schoenenberger, B.; Milesi, T.; Vanderoost, J.; Wohlgemuth, R. Biocatalytic Asymmetric Phosphorylation of Mevalonate. RSC Adv. 2014, 4, 12989–12994. [Google Scholar] [CrossRef]
- Bettjeman, B.I.; Hofman, K.A.; Burgess, E.J.; Perry, N.B.; Killeen, D.P. Seafood Phospholipids: Extraction Efficiency and Phosphorous Nuclear Magnetic Resonance Spectroscopy (31P NMR) Profiles. J. Am. Oil. Chem. Soc. 2018, 95, 779–786. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, B.; Wan, B.; Xi, X.; Zeng, Z.; Liu, E.; Wu, G.; Liu, Z.; Wang, W. Degradation of Black Phosphorus: A Real-Time 31P NMR Study. 2D Mater. 2016, 3, 035025. [Google Scholar] [CrossRef]
- Mazumder, A.; Kumar, A.; Purohit, A.K.; Dubey, D.K. A High Resolution Phosphorus-31p Nuclear Magnetic Resonance (NMR) Spectroscopic Method for the Non-phosphorus Markers of Chemical Warfare Agents. Anal. Bioanal. Chem. 2012, 402, 1643–1652. [Google Scholar] [CrossRef]
- Wan, Y.; Li, K.; Li, X.; Li, X.; Chu, H.; Zhang, Q. Purity Assessment of Tripropyl Phosphate through Mass Balance and 1H and 31P Quantitative Nuclear Magnetic Resonance. Molecules 2024, 29, 1975. [Google Scholar] [CrossRef] [PubMed]
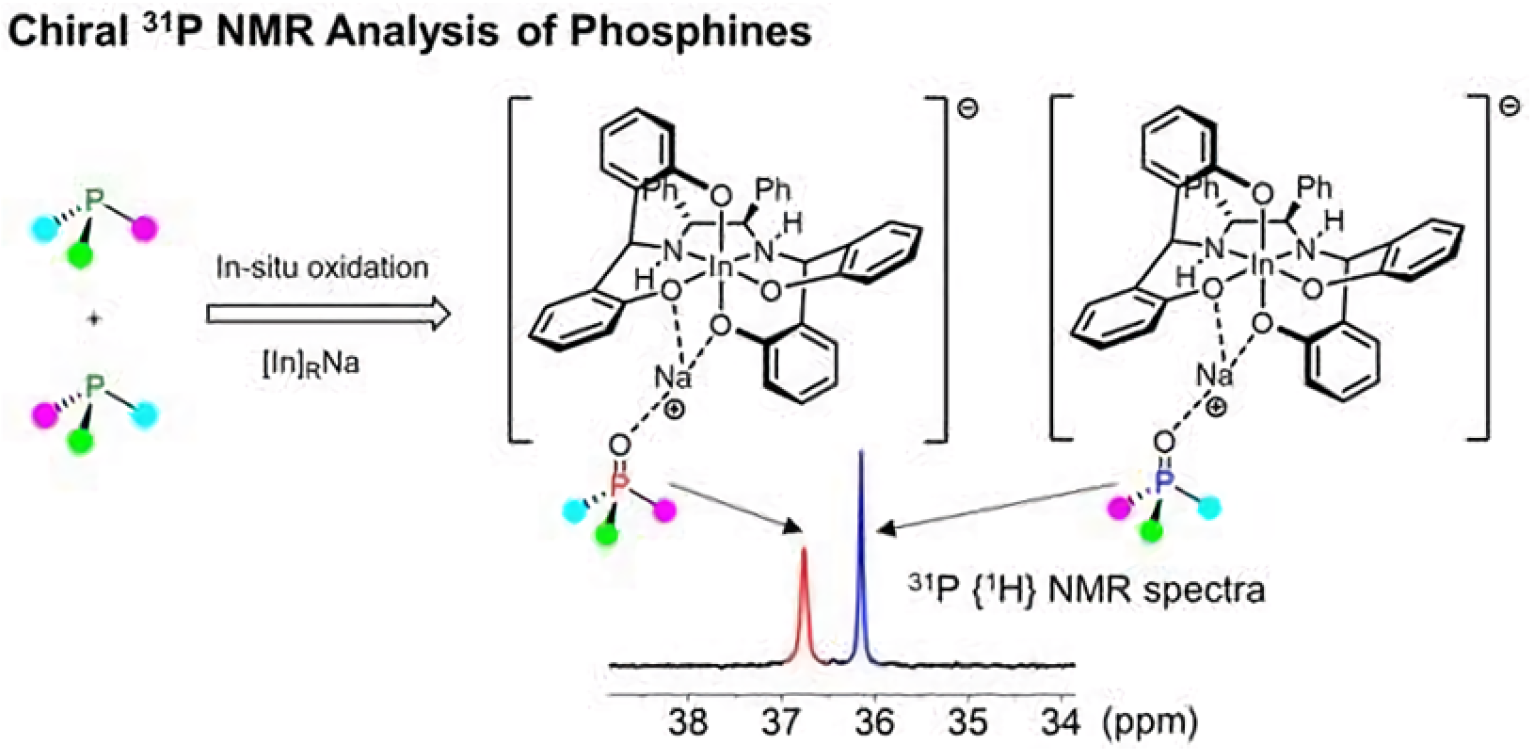
- Kwahk, E.J.; Jang, S.; Kim, H. Chiral Recognition of in Situ-Oxidized Phosphine Oxides with Octahedral Indium Complexes by 31P NMR Spectroscopy. Org. Lett. 2021, 23, 7829–7833. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Gao, L.; Yu, Z. Quantitative 31P NMR Spectroscopy: Principles, Methodologies, and Applications in Phosphorus-Containing Compound Analysis. Appl. Sci. 2025, 15, 323. https://doi.org/10.3390/app15010323
Liu Y, Gao L, Yu Z. Quantitative 31P NMR Spectroscopy: Principles, Methodologies, and Applications in Phosphorus-Containing Compound Analysis. Applied Sciences. 2025; 15(1):323. https://doi.org/10.3390/app15010323
Chicago/Turabian StyleLiu, Yaqin, Lina Gao, and Zeling Yu. 2025. "Quantitative 31P NMR Spectroscopy: Principles, Methodologies, and Applications in Phosphorus-Containing Compound Analysis" Applied Sciences 15, no. 1: 323. https://doi.org/10.3390/app15010323
APA StyleLiu, Y., Gao, L., & Yu, Z. (2025). Quantitative 31P NMR Spectroscopy: Principles, Methodologies, and Applications in Phosphorus-Containing Compound Analysis. Applied Sciences, 15(1), 323. https://doi.org/10.3390/app15010323




