Abstract
Glass ionomers are utilized extensively within the domain of dentistry, for instance, as provisional restorations, liners, or bases, in addition to their application as pit and fissure sealers. It is imperative that this type of material exhibits favorable physico-chemical and biological properties. The primary objective of the presented study is to modify commercial resin-modified glass ionomer (Riva Light Cure, RMGIC) by doping it with copper particles (RMGIC + Cu) and to evaluate its properties in terms of potential beneficial clinical applications. Susceptibility to adhesion of microbial species and potential antimicrobial activity was evaluated against the Candida albicans, Streptococcus mutans, and Lactobacillus rhamnosus strains. Antiviral properties were evaluated against two viruses: Herpes simplex virus type 1 and human Adenovirus 5. Cytotoxicity of the materials was assessed using Balb/3T3 mouse fibroblast cell line. Temporal fluoride release up to 168 h in water and artificial saliva of different pH levels were also measured and assessed using statistical analysis. Samples were also subjected to Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy and Fourier-Transform Raman Spectroscopy. The findings of the present study demonstrate that RMGIC + Cu displays reduced biofilm formation against the tested strains when compared to non-modified material. The influence of the Cu presence on fluoride release is most pronounced in artificial saliva with a low pH (4.5), where the difference is significantly higher in samples with Cu than in samples without it. No reduction in herpes simplex 1 titers under the influence of either material was observed, whereas both materials exhibited virucidal properties against human adenovirus 5. Commercial glass ionomer presented no cytotoxicity, while the modified biomaterial caused changes in the fibroblast culture only under the sample (slight cytotoxicity, grade 1). Considering all the acquired results, doping glass ionomer with copper may be an interesting modification enhancing antimicrobial properties of the biomaterial, but it requires further evaluation in terms of long-term cytotoxicity before further in vivo studies.
1. Introduction
The World Health Organization has communicated to member states the advantages inherent in the suitable utilization of fluoride [1]. Since its initial discovery as a caries-preventive agent in the mid-20th century, fluoride has played a crucial role in the development of dental public health. Fluoride ions have been demonstrated to reduce the susceptibility of the enamel surface to bacterial adhesion, to modify the metabolism of cariogenic bacteria, and to play a role in the process of enamel remineralization [2]. Fluoride is utilized in a variety of preventive measures, including those administered in dental clinics (e.g., fluoride varnish) and those employed in the domestic setting (e.g., toothpaste containing fluoride). Fluoride ions present in the environment of the oral cavity enable enamel remineralisation, and therefore are crucial for caries protection.
In the course of deliberations pertaining to the advantageous effects of fluoride in oral cavities, it is important to acknowledge its concomitant potential toxicity. It is evident from the extensive literature on the subject that the most detrimental effects of fluoride exposure manifest during development, including enamel fluorosis. Nevertheless, it has been reported that excessive consumption of this element can also have adverse effects on the health of adults. The process of long-term fluoride accumulation within the bones is medically termed “skeletal fluorosis”. Fluoride has been shown to have a nephrotoxic effect, the result of which is a deterioration of kidney filtration function and a disruption of cellular metabolism by means of inhibition of glycolytic enzyme activity. There is also a demonstrable link between hypothyroidism and long-term exposure to elevated fluoride concentrations [3]. A plethora of studies have been conducted to investigate the impact of fluoride on enamel and enamel-forming cells. Moreover, the investigation into the potential negative impact of fluoride on dentin is ongoing. The in vivo study by Okamoto et al. concluded that high doses of systemic fluoride (100–125 ppm in drinking water) can cause dentine dysplasia [4]. It is important to notice that the mentioned adverse effects of fluoride are noticed during the internal intake of fluoride in high doses.
Glass ionomer cements are biomaterials that have found wide application in dentistry due to their favorable biological and functional properties [5,6,7]. One of the most significant advantages of this product is its ability to release fluoride over an extended period of time. This property may contribute to enamel remineralisation, maintain a more balanced microbial activity in the oral cavity, and reduce the development of secondary caries [8,9]. The cements are frequently employed in routine clinical practice due to their strong chemical adhesion to dental tissues, biocompatibility, and ease of use [10,11]. The dental materials market offers a broad range of glass ionomer cements, including both conventional and resin-modified formulations, allowing for selection of the material best suited to specific applications and clinical conditions [12].
Riva (SDI Limited, Victoria, Australia) is a resin-modified glass ionomer cement (RMGIC), visible in X-rays. It is intended for small fillings class I, II, and III according to Black, as well as class V fillings. Moreover, it is recommended for deciduous teeth, geriatric and temporary restorations, and base or liner. Another potential application of the material is its use as core build-up, root surface restoration, and pit and fissure sealant. It is available in multiple colors (A1, A2, A3, A3,5, A4, B2, B3, B4, C2, C4, and bleach shades) and in the form of capsules or powder and liquid for manual preparation. The tooth surface requires no etching or bonding prior to glass ionomer placement; however, conditioning with polyacrylic acid (Riva conditioner) or 37% phosphoric acid prior to Riva application enables optimal adhesion of glass ionomer cement to hard tooth tissues [13]. Manufacturers recommend light-curing for 20 s using a visible light-curing device (470 nm wavelength) with the light source as close as possible to the cement surface. Instructions from manufacturers determine powder to liquid dosage as 1 flat scoop to 2 drops and mixing time—30 s. At 23 °C/74 °F working time should be about 120 s. As claimed in a manufacturer’s brochure, Riva contains ionglass filler, made of a unique blend of different sizes of ultrafine highly reactive glass particles [14].
Numerous studies have demonstrated that copper exerts an inhibitory effect on the formation of dental plaque [15,16,17]. The incorporation of copper in biomaterials intended for use in dental restoration is of significant benefit, owing to the material’s capacity to enhance the process of odontogenesis [18]. For example, researchers discovered that copper-containing titanium alloys may contribute to promotion of osteogenesis and angiogenesis by releasing copper ions [19]. The studies investigating antibacterial properties of dental materials doped with Cu are reported in the literature. Aguilar-Perez et al. reported on doping commercial glass ionomer and evaluating its properties. The authors concluded that biomaterial modified with copper nanoparticles, which is a mixture of both metallic copper and cuprous oxide, exhibits antimicrobial activity against strains present in oral cavity biofilm [20]. Another study regarding glass ionomer modification was applied by Renné et al., who incorporated polyacrylic acid-coated copper iodide nanoparticles into the ionomer matrix. The authors concluded that the antibacterial properties of modified biomaterials were significantly enhanced, whilst collagen degradation was reduced, without any detrimental effect on the mechanical properties of the glass ionomer materials [21]. The optimal form of Cu to be used universally in biomaterials is not unequivocally determined. However, preliminary studies have shown encouraging results in terms of its antimicrobial properties. The in vitro tests and biological studies, such as the assessment of effects on pulp cell healing presented in the study by Okamoto et al., are essential for evaluating the clinical potential of the new biomaterial [22]. Further research is required to ascertain copper’s addition potential toxicity and its adverse effects on biomaterial properties.
Dental plaque, being a complex biofilm that accumulates in the oral cavity, is a factor that promotes caries and periodontal disease development. Oral microbial biofilm is defined as bacterial communities that are structurally three-dimensional in nature. Lactobacillus sp. have been identified as the primary constituent cells of bacterial aggregates in subgingival plaque, whereas Streptococcus sp. and the C. albicans yeast have been observed to form corncob structures [23]. It is desirable for biomaterials utilized in the oral cavity to have properties that impede dental plaque accumulation. Therefore, affinity of biomaterials for adhesion of microorganisms is of vital clinical importance [24].
Viral infections have been proven to carry a risk of severe illness, and in extreme cases, even death. Oral viral infections generally have a favorable prognosis, with complete tissue recovery being the typical outcome. However, it should be noted that many viruses persist within the body and are frequently shed without the manifestation of additional clinical symptoms [25]. Antiviral, antibacterial, and antifungal properties are desired traits of novel, functional dental biomaterials [26,27].
It is widely recognized within scientific reports that copper (Cu) is an essential micronutrient for cells. However, it is equally well established that copper can exhibit cytotoxicity at excess concentrations. It has been demonstrated that copper can induce a state of protein or enzyme dysfunction. In the absence of appropriate remedial measures, this may result in subsequent cellular deterioration and death [28,29]. Exposure to excess copper has been demonstrated to induce imbalances in antioxidant defense and to disrupt the levels of free radicals and active non-radicals, resulting in increased levels of reactive oxygen species [30].
It is evident that glass ionomers exhibit notable advantages as filling materials, primarily characterized by their high fluoride release and capacity for cross-linking within the moist environment of the oral cavity. Conversely, composite materials exhibit numerous advantages over glass ionomers, predominantly in terms of aesthetics and resistance. The prospect of modifying glass ionomer materials may enhance their appeal for utilization in dentistry, including pit and fissure sealing. The trend of glass ionomer modifications aiming to improve both their physical-mechanical and antimicrobial properties has been noticed by Ching et al. in their review article from 2018 [31]. It has been observed that modified glass ionomers have the potential to be utilized as restorative materials in dentistry, thus broadening their application. Researchers attempt to utilize multiple groups of compounds as an addition to glass ionomers, e.g., quaternized chitosan-coated mesoporous silica nanoparticles [32], bioactive glass nanoparticles [33], or N-vinylpyrrolidone [34]. A modification of resin-based composite material with surface pre-reacted glass ionomer in scope of inhibition of biofilm adhesion and growth is also reported in the literature. In the study by Lee et al., the authors investigated the synergistic effect of a surface pre-reacted glass ionomer filler and 2-methacryloyloxyethyl phosphorylcholine in inhibiting multi-species biofilm development [35]. Therefore, modification of glass ionomers with bioactive fillers affecting their clinical properties is of vital clinical importance. The utilization of copper exhibits considerable potential as a promising avenue for further scientific exploration.
2. Materials and Methods
2.1. Sample Manufacturing and Sterilization
To obtain RMGIC samples, Riva Light-Cure (SDI Limited, A2, Lot no. 1236427) in the form of powder and liquid was mixed in accordance with the manufacturer’s recommendations (1 measuring spoon of powder per 2 droplets of liquid) and placed in a 6-well teflon mold. Photograph of the utilized Riva Light-Cure material is presented in Figure S1. Size of each sample in this study was 4 mm in diameter and 2 mm in height. Subsequent light-curing was performed using Flashmax P3 lamp (CMS Dental, Glyngøre, Denmark). The wavelength range of the lamp is 450–470 nm with an intensity peak at 460 nm. Irradiation of each sample lasted 20 s. To obtain RMGIC + Cu, 2 wt.% of copper powder (Carl Roth, Karlsruhe, Germany, Figure S1) was added during the mixing stage. The addition of 2 wt.% copper to the RMGIC matrix was selected based on a previously reported study on copper-doped ionomer cement [20]. It aimed to evaluate whether the inclusion of Cu particles influences the material’s biological and physico-chemical properties and is expected to establish a foundation for subsequent research endeavors. Light-curing procedure was the same for both specimens. The sample composition is described in Table 1, and its appearance is exhibited in Figure 1. Colorimetric analysis of the samples was conducted using the ImageJ software (version 1.54p; W.S. Rasband, National Institutes of Health, Bethesda, MD, USA). Based on a nine-point analysis (Figure S2), the RMGIC sample had an average RGB value of (155.56, 139.22, 102.33), while the RMGIC + Cu sample had an average RGB value of (138.11, 120.78, 96.33).

Table 1.
Samples designation.
Figure 1.
Obtained samples of RMGIC (A) and RMGIC + Cu (B).
Prior to biological assessment, the samples were sterilized for a period of 35 min. This process was conducted at a temperature of 134 °C and a pressure of 2.17 bar in the Vacuklav 44 B+ Evolution autoclave (Berlin, Germany).
2.2. Fluoride Release
Fluoride release was performed on the RMGIC and RMGIC + Cu samples according to the methodology published previously [36,37]. The fluoride concentration was assessed in µg/mm2 after 1, 3, 24, 48, 72, 96, and 168 h of incubation in 9 different solutions in 5 repetitions. The solutions included tap water, distilled water, demineralized water, 0.9% NaCl solution, and artificial saliva with pHs of 4.5; 5.5; 6.0; 7.0; and 7.5. NaCl and artificial saliva solutions were based on demineralized water. Composition of artificial saliva with addition of calcium ions was based on the formulation previously utilized by the authors [38]. The utilized substances included sodium chloride (NaCl), potassium chloride (KCl), sodium bisulfate (Na2S·9H2O), sodium hydrogen phosphate (NaH2PO4·2H2O), and urea (supplied by Chempur, Piekary Ślaskie, Poland). Calcium chloride dihydrate (CaCl2·2H2O) was supplied by Sigma Aldrich (St. Louis, MO, USA).
2.3. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR) and Fourier-Transform Raman Spectroscopy (FT-Raman)
The cured RMGIC and RMGIC + Cu samples were ground in an agate mortar to increase surface area and homogeneity, facilitating contact with the ATR crystal. The resulting powder was then subjected to ATR-FTIR analysis. The spectra of RMGIC and RMGIC + Cu were obtained using an FTIR Nicolet 6700 spectrometer (Thermo Scientific, Waltham, MA, USA), which was equipped with an ATR accessory containing a diamond crystal (Pike Technologies, Fitchburg, MA, USA). The spectra we acquired at 22 °C in 128 scans with a resolution of 4 cm−1 within the range of 4000–400 cm− 1. The obtained spectra were analyzed with the OriginPro 2025 software (Origin Lab Corporation, Northampton, MA, USA). The ATR-FTIR spectral processing included baseline subtraction and smoothing using the Savitzky–Golay filter (SG, polynomial order: 2 and points of window: 15).
FT-Raman spectra of RMGIC and RMGIC + Cu were recorded on a gold-coated glass slide using a Nicolet NXR 9650 FT-Raman spectrometer (Thermo Scientific, Waltham, MA, USA), which was equipped with an Nd:YAG laser operating at 1064 nm as the excitation source and an indium gallium arsenide (InGaAs) detector. The laser power was adjusted to 350 mW, and the Raman signal was collected in 128 scans with a spectral resolution of 4 cm−1 within the spectral range of 0–3700 cm−1. Spectra for each sample were recorded three times, and the obtained spectra were averaged. Photographs showing the precise locations of Raman spectra acquisition are presented in Figure S3 of the Supplementary Materials. The spectra were analyzed with the OriginPro 2025 software. Post-processing included baseline correction and smoothing (SG, polynomial order: 2 and points of window: 25).
2.4. Antimicrobial Properties
Experiments were conducted on reference strains from the American Type Culture Collection (ATCC): Candida albicans (ATCC 10231) and Streptococcus mutans (ATCC 25175), Lactobacillus rhamnosus (ATCC 9595). The biomaterials for analysis included RMGIC and RMGIC + Cu (Figure 1).
The evaluated microbial strains were deep frozen in −80 °C in liquid Tryptic Soy Broth with 15% of glycerol (Biomaxima S.A., Lublin, Poland) in several repetitions. Before each experimental cycle, strains were cultured on solid media:
- C. albicans: Sabouraud Dextrose Agar (Biomaxima) (37 °C, 24 h, aerobic);
- S. mutans: BHI Agar- Brain Heart Infusion Agar (Biomaxima) (37 °C, 24 h, increased level of CO2);
- L. rhamnosus: MRS Agar-de Man, Rogosa and Sharpe Agar (Biomaxima) (37 °C, 24 h, anaerobically).
2.4.1. Assessment of Antimicrobial Properties
Suspensions were prepared from fresh strain cultures of C. albicans, S. mutans, and L. rhamnosus. Suspensions had a density of 0.5 on the McFarland scale (1.5 × 106 CFU/mL for fungi and 1.5 × 108 CFU/mL for bacteria). For that procedure, liquid culture media were applied: Sabouraud Dextrose Broth (SDB) with 5% sucrose (Biomaxima) for C. albicans; Brain Heart Infusion Broth (BHI) with 5% sucrose (Biomaxima) for S. mutans; and Man-Rogosa-Sharpe Broth (MRS, Biomaxima) for L. rhamnosus. The subsequent step involved adding sterile biomaterial samples to a 1 mL mixture containing 900 µL of culture media and 100 µL of microbial suspension. Culture without biomaterial was used as a control. After 24 h incubation in 37 °C (C. albicans aerobically; S. mutans in increased CO2; L. rhamnosus anaerobically), 100 µL of suspension was taken and diluted tenfold in geometric progression and inoculated on according media. After incubation, the number of colonies was counted and expressed as colony-forming unit per milliliter (CFU/mL) in accordance with the following equation (Equation (1)).
CFU/mL = average number of colonies × reciprocal dilution × 10
2.4.2. Adhesion of Microorganisms and Biofilm Formation
Suspensions of fresh strain cultures were prepared with a density of 0.5 on the McFarland scale (1.5 × 106 CFU/mL for fungi and 1.5 × 108 CFU/mL for bacteria). Corresponding culture media were used: SDB with 5% sucrose (Biomaxima) for C. albicans; BHI with 5% sucrose (Biomaxima) for S. mutans; and MRS (Biomaxima) for L. rhamnosus.
Sterile biomaterial samples were introduced to a 1 mL mixture containing 900 µL of culture media and 100 µL of microbial suspension. Subsequently, the incubation stage took place: C. albicans—37 °C, 24 h, aerobically; S. mutans—37 °C, 24 h, increased CO2; and L. rhamnosus—37 °C, 24 h, anaerobically. After incubation, materials were washed thrice in NaCl and shaken for 1 min in 1 mL of 0.5% saponin solution (Sigma-Aldrich, St. Louis, MO, USA). The obtained suspension was eluted from the surface of the material, diluted in geometrical progression, and inoculated quantitatively on corresponding culture media [39,40,41,42,43]. After incubation, CFU/mL was calculated according to the formula presented in Equation (1). The experiment was conducted in three repetitions.
2.4.3. Confocal Microscopy
For microscopic analysis, the C. albicans and S. mutans biofilms were stained with fluorescent dyes: SYTO 9 and propidium iodide (PI). SYTO 9 labels DNA in cells, both with intact and damaged membranes. PI only penetrates the cells with damaged membranes, resulting in a reduction in the fluorescence of SYTO 9 when both dyes are present. After the staining procedure, the slides were fixed in 4% formaldehyde for 15 min, after which the formaldehyde was replaced with buffered saline. For imaging, the analyzed material with biofilms was removed from the saline, transferred onto a microscope slide, and covered on the biofilm side with a #1.5 coverslip. Imaging was performed using a Leica TCS SP8 confocal microscope (Leica Microsystems, Wetzlar, Germany) with a 20×/0.75 NA dry objective and two lasers: 488 nm (SYTO 9 excitation) and 552 nm (PI excitation). Fluorescence emissions were recorded sequentially on two spectral PMT detectors with set ranges: 495–537 nm (SYTO 9) and 575–681 nm (PI). Individual optical planes were collected at 0.684 µm intervals, and the imaging volume was 50–420 µm, depending on the curvature of the material. There were at least 3 representative fields of view for the experimental condition. The three-dimensional volume was rendered in Imaris 9.5.1 (Oxford Instruments, Abingdon, Great Britain, UK) using a maximum intensity projection (MIP) algorithm.
2.5. Antiviral Properties
Experiments The virucidal activity assessment was performed against two viruses: Herpes simplex virus type 1 (HSV-1, ATCC® VR-1493™) and human Adenovirus 5 (Ad-5 virus—strain Adenoid 75, ATCC® VR-5™). Two cell lines were utilized in the experimental setup: The A549 cell line (human lung carcinoma, ATCC® CCL-185™) and the HeLa cell line (human cervix carcinoma, ATCC® CCL-2™) were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The culture medium employed was Dulbecco’s Modified Eagle’s Medium (DMEM) (Lonza, Basel, Switzerland), which was enriched with 10% fetal bovine serum (FBS) (Biological Industries, Kibbutz Beit-Haemek, Israel), 4 mM L-glutamine (Biological Industries), and supplemented with 100 U/mL penicillin and 100 µg/mL streptomycin (Sigma-Aldrich, Munich, Germany).
The antiviral testing was conducted in accordance with the International Standard ISO 21702:2019 (Measurement of antiviral activity on plastics and other non-porous surfaces) [44]. The present guideline delineates the procedures for the assessment of the antiviral properties of treated non-porous materials in relation to specified viruses. In accordance with this standard, viruses other than influenza and feline calicivirus may be employed; therefore, human adenovirus 5, a non-enveloped virus commonly found in humans and known for its resistance to inactivation, was selected. This virus has been associated with common cold infections. The second virus examined in the study was HSV-1 (herpes simplex 1), an enveloped virus that is responsible for causing herpes in humans.
The capacity of selected viral strains to attain elevated titers under laboratory conditions facilitated the effective validation of the methods. Test samples, having previously undergone treatment with an SCDLP neutralizer, were placed into 48-well plates. Each sample was inoculated with 200 µL of the test virus and covered with a 2 × 3 cm polymer film. Incubation process was conducted at a temperature of 25 °C for a duration of 24 h, under conditions of 90% relative humidity. Subsequent to the incubation period, the viral suspension was recovered by means of pipetting. It is imperative to note that all experiments were conducted in triplicate. For the purpose of conducting a comparative analysis, a control sample, which was composed of RMGIC material devoid of any active additives, was subjected to the identical procedure.
In accordance with ISO 21702:2019, the TCID50 method was employed as an alternative to the plaque assay. Serial 10-fold dilutions (up to 10−12) were prepared from each sample. Fifty microliters of each dilution were added, in eight replicates, to wells of a microtiter plate containing 50 µL of a confluent monolayer of either A549 or HeLa cells. The plates were examined on a daily basis for a period of up to four days using an inverted microscope (Olympus Corp., Hamburg, Germany; Axio Observer, Carl Zeiss MicroImaging GmbH, Gerlingen, Germany) in order to observe the development of cytopathic effects.
In accordance with the stipulated ISO criteria [44], a material is deemed to demonstrate virucidal efficacy if it leads to a minimum of four log10 (log) reduction in viral titer (corresponding to an inactivation rate of at least 99.99%) within the stipulated contact time.
The TCID50/mL value was calculated using the Spearman–Kärber method, following Equation (2).
where
log10TCID50 = x0 − 0.5 + Σ (r/n)
x0—the log10 of the lowest dilution that shows a 100% positive response;
r—the total number of positive results at that and all higher dilutions;
n—the number of replicates per dilution step.
2.6. Cytotoxicity and Direct Cell Assay
2.6.1. Cell Lines
The studies were conducted using normal mouse fibroblasts (Balb/3T3, clone A31, American Type Culture Collection, ATCC® CCL-163™) as reference lines for the in vitro assessment of biomaterial cytotoxicity (PN-EN ISO 10993-5:2009, ‘Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity’). The Balb/3T3 cells were cultured in DMEM supplemented with 10% calf serum (CS). The medium was supplemented with 1% L-glutamine, streptomycin, and penicillin (all from Sigma Aldrich). The cell cultures were maintained under standard conditions (37 °C, 5% CO2, constant humidity) in a HERA Cell CO2 150i incubator (Thermo Scientific) and passaged when they reached 70% confluence using a trypsin-EDTA solution [45,46,47].
2.6.2. Direct Contact Assay
The Balb/3T3 cells were trypsinized (using 0.25% Trypsin-EDTA, Sigma-Aldrich®), suspended in complete culture medium, and seeded into a 6-well plate (TPP, Trasadingen, Switzerland) at a density of 1.5 × 105 cells per well. After 24 h, the test materials, which were approximately 5 mm in diameter, were placed on each plate. After a further 24 h of incubation with the materials, the morphology of the cells under the sample, near the sample, and in the whole well was evaluated using an inverted phase-contrast microscope (Olympus CKX53, Tokyo, Japan). A culture grown in complete medium under standard conditions, without contact with the investigated materials, was used as a control [46,48,49].
2.7. Statistical Analysis
Statistical analysis regarding fluoride release was conducted using multifactorial ANOVA to assess the impact of various factors on cumulative fluoride release. Pairwise comparisons were performed using Tukey’s HSD post hoc test. Given the large number of comparisons, the significance threshold was set at p = 0.01 to reduce the risk of type I error. Analysis was performed in the Jamovi software v.2.6 (https://www.jamovi.org (accessed on 4 August 2025)). Fluoride release over time was visualized using smoothed curves generated in the R statistical environment v.4.4 (https://cran.r-project.org (accessed on 4 August 2025)) with the ggplot2 package, applying the loess smoothing function.
An experiment regarding antimicrobial properties, adhesion of microorganisms, and biofilm formation was conducted in three independent repetitions. Descriptive statistics and the Kruskal–Wallis test (p = 0.05) were applied.
3. Results
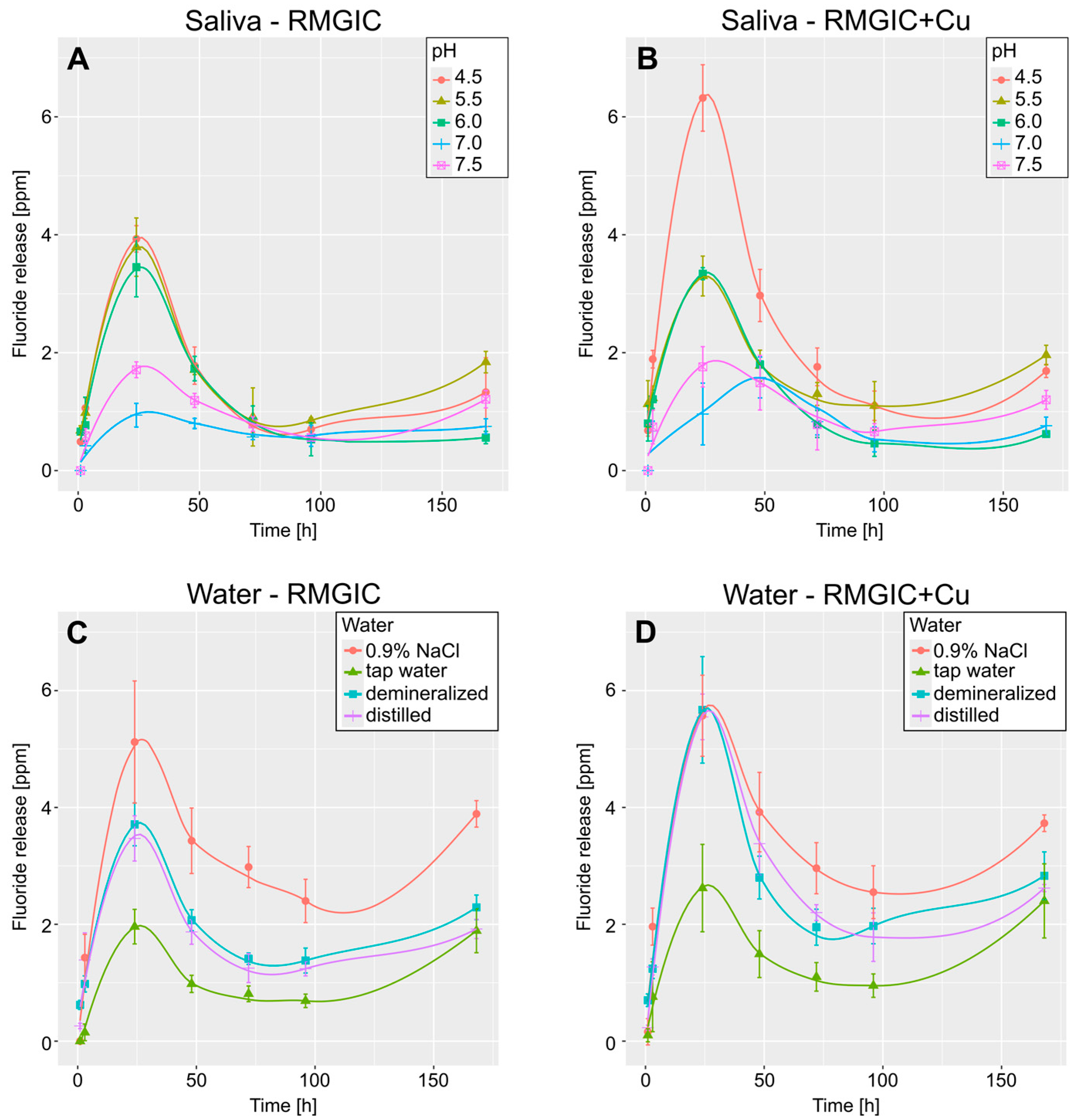
Figure 2 presents the smoothed curves, which illustrate the temporal variations in fluoride release (after 1, 3, 24, 48, 72, 96, and 168 h). The pattern that was observed was consistent across all the samples, with a sharp increase in fluoride release for a 24 h period, followed by a continuous decrease to approximately half of the recorded peak value, and subsequently, a slight increase at the final measurement. In contrast, artificial saliva exhibited a divergent pattern in samples with addition of Cu and pH = 7.0, with the peak fluoride release occurring after 48 h instead of 24.
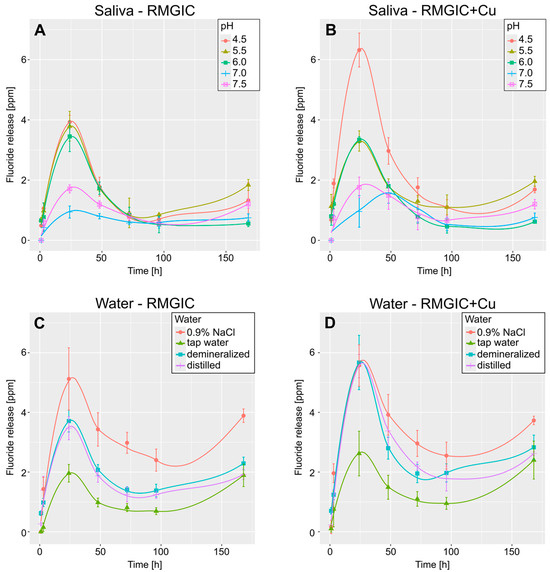
Figure 2.
Smoothed curves depicting mean fluoride release over time. (A,B)—curves for release in artificial saliva for RMGIC and RMGIC + Cu, respectively. (C,D)—curves for release in water for RMGIC and RMGIC + Cu. Whiskers represent standard deviation.
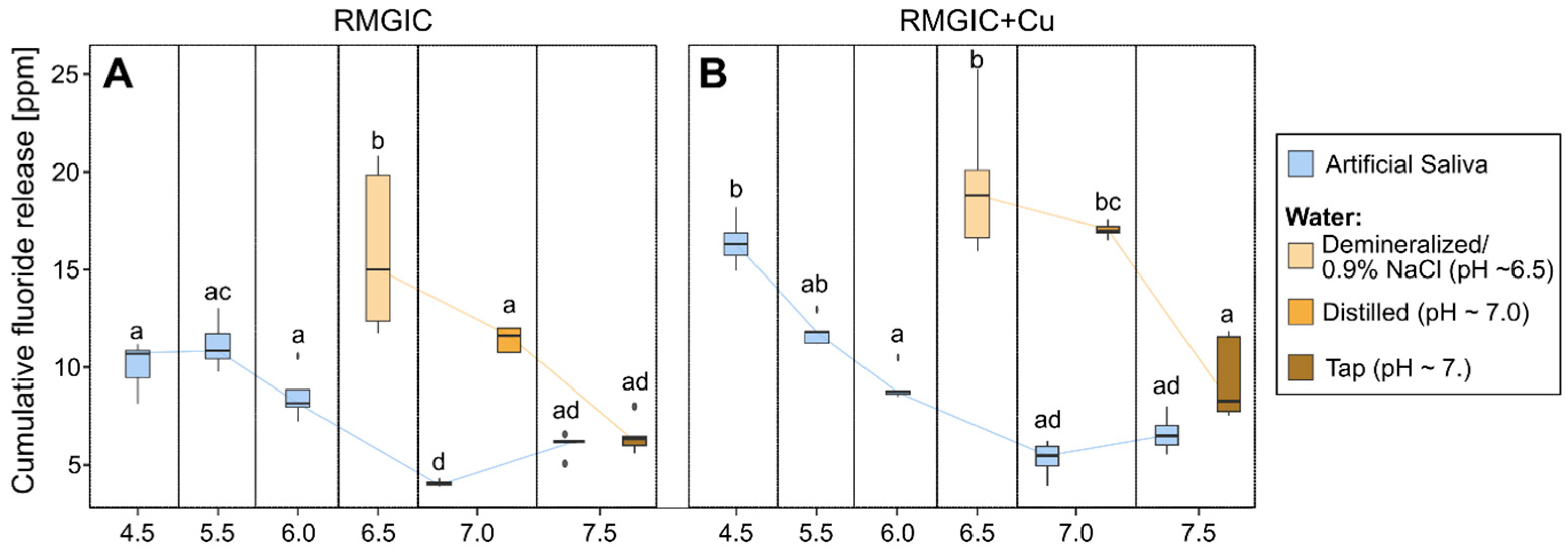
Due to the comparable release patterns over time exhibited by the majority of samples, cumulative fluoride release (summed from all measurement times) was utilized to facilitate a comparative analysis of their performance in fluoride release. The results of the multifactorial ANOVA analysis for artificial saliva are shown in Table 2. Figure 3 presents bar plots that illustrate the results for the various groups under consideration.

Table 2.
Results of multifactorial ANOVA analysis of cumulative fluoride release. Due to the incomplete range of pH in the water samples, comparisons were made for artificial saliva only. The ƞ2 statistic is a measure of effect size. This analysis indicates that pH is the variable that most influences fluoride release.
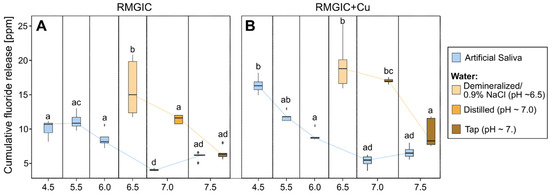
Figure 3.
Box plot depicting cumulative fluoride release after 168 h for samples with different pH levels. (A) RMGIC and (B) RMGIC + Cu. The boxes presenting the results for artificial saliva are blue, and the boxes presenting the results for water are orange. Boxes without any common letter are statistically significantly different (p < 0.01). The results were compared using a post hoc Tukey test.
Multifactorial analysis indicates that pH has the strongest influence on fluoride release (ƞ2 = 0.93), followed by the interaction of Cu presence*pH (ƞ2 = 0.62) and the presence of Cu alone (ƞ2 = 0.53). As demonstrated in Figure 3, the tendency identified in the multifactorial analysis is evident in the box plots. Furthermore, it has been demonstrated that fluoride release tends to decrease with increasing pH. The influence of Cu presence is most pronounced in artificial saliva with a low pH (4.5), where the difference is significantly higher in samples with Cu than in samples without it. Furthermore, water samples have been shown to exhibit a higher rate of fluoride release than that observed in artificial saliva.
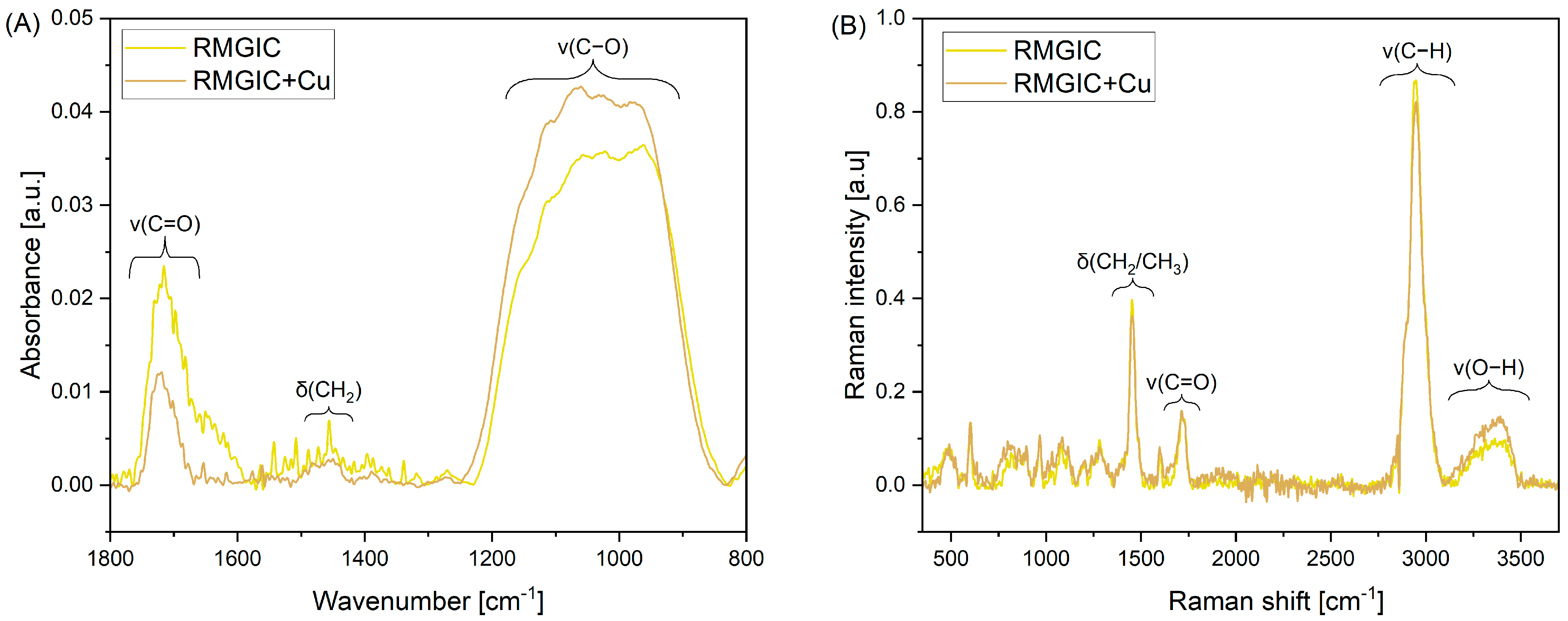
Acquired ATR-FTIR and FT-Raman spectra of RMGIC and RMGIC + Cu are presented in Figure 4. The ATR-FTIR spectra (Figure 4A) are characterized by bands that are indicative of organic polymer materials within the spectral range of 1800–800 cm−1. The band between 1770 and 1660 cm−1 is attributed to the stretching of the carbonyl bond (v(C=O)). A second distinctive band, located within the range of 1500–1420 cm−1, has been identified as being connected to scissoring vibrations of the CH2 group (δ(CH2)). The third band, observable at 1250–830 cm−1, has been shown to be associated with the stretching mode of the C-O bond (v(C-O)).
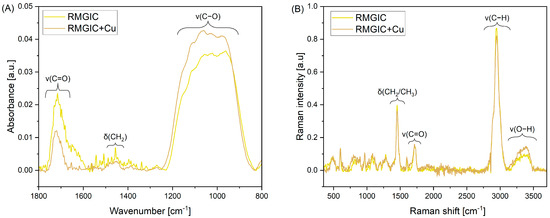
Figure 4.
ATR-FTIR (A) and Raman (B) spectra of RMGIC and RMGIC + Cu. ATR-FTIR spectra are presented in the 1800–800 cm−1 range.
Four distinctive assigned bands are visible in the full FT-Raman spectra (Figure 4B). Namely, the band located at 1454 cm−1 is associated with bending of CH2 and CH3 groups (δ(CH2/CH3)). The band at 1720 cm−1 is associated with stretching mode of the carbonyl bond (v(C=O)). Two other assignments are made for stretching of the C-H bond at 2945 cm−1 (v(C-H)) and O–H stretching vibrations at 3155–3503 cm−1 (v(O-H)).
It cannot be determined whether either of the tested materials, RMGIC or RMGIC + Cu, exhibits a statistically significant antimicrobial effect against the microorganisms analyzed in the study compared to the control group. Statistical analysis (Kruskal–Wallis test) revealed no statistically significant differences (p > 0.05). The results of the analysis are presented in Table 3.

Table 3.
Influence of evaluated biomaterials on viability of C. albicans, S. mutans, and L. rhamnosus strains.
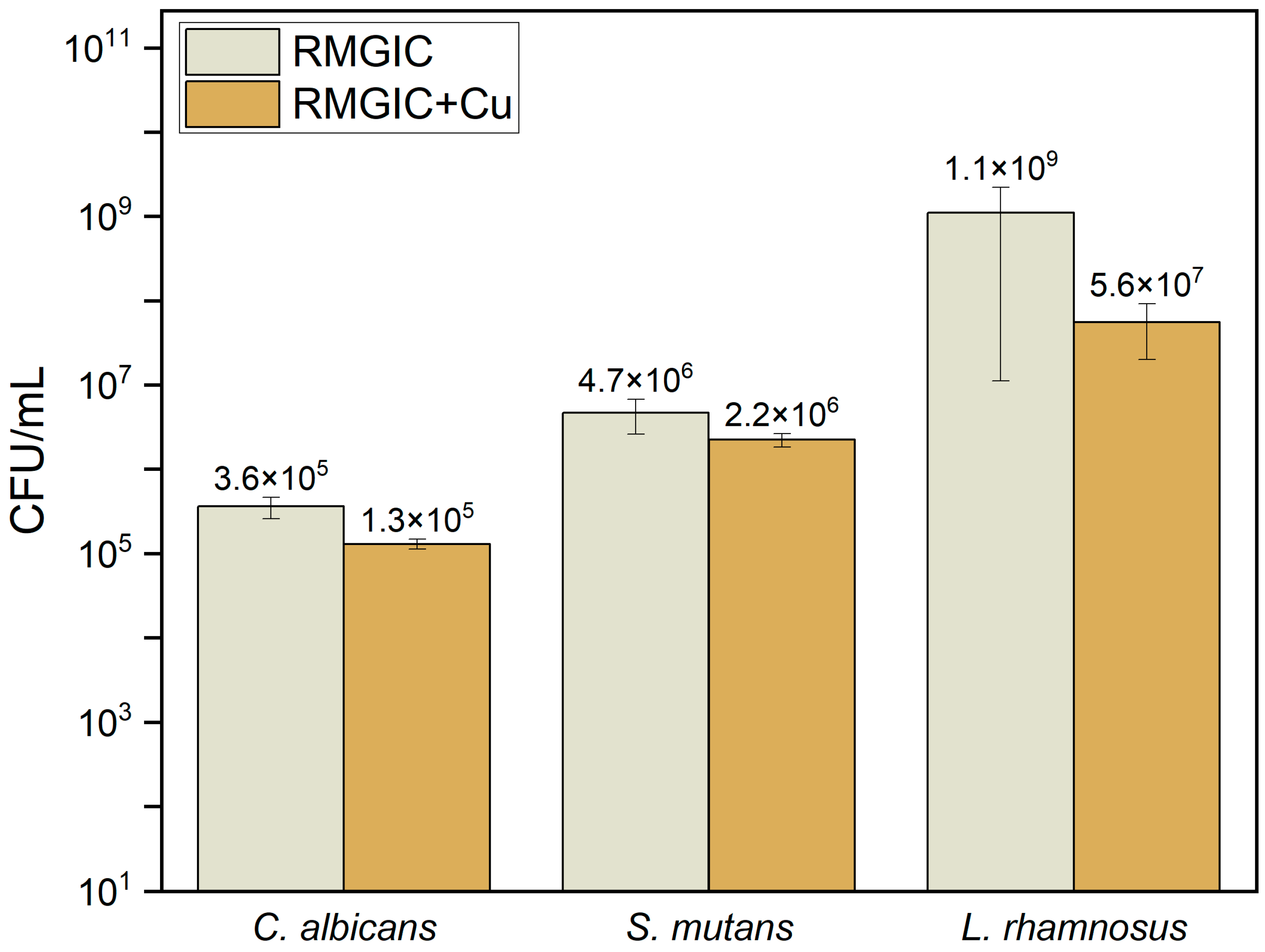
Addition of 2% of copper to RMGIC resulted in a statistically significant decrease in adhesion of all the microorganisms used in the study (C. albicans, S. mutans, and L. rhamnosus) in comparison with RMGIC. The p-values for all three evaluated strains are lower than the established level of significance (α = 0.05). The results are presented in Table 4 and in Figure 5.

Table 4.
Affinity of evaluated biomaterials for adhesion of microorganisms.
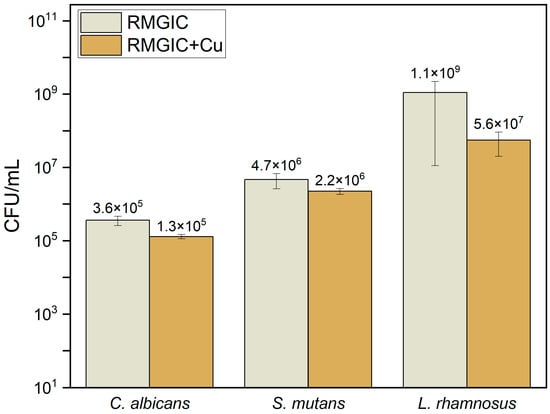
Figure 5.
Susceptibility of biomaterials for adhesion of microorganisms.
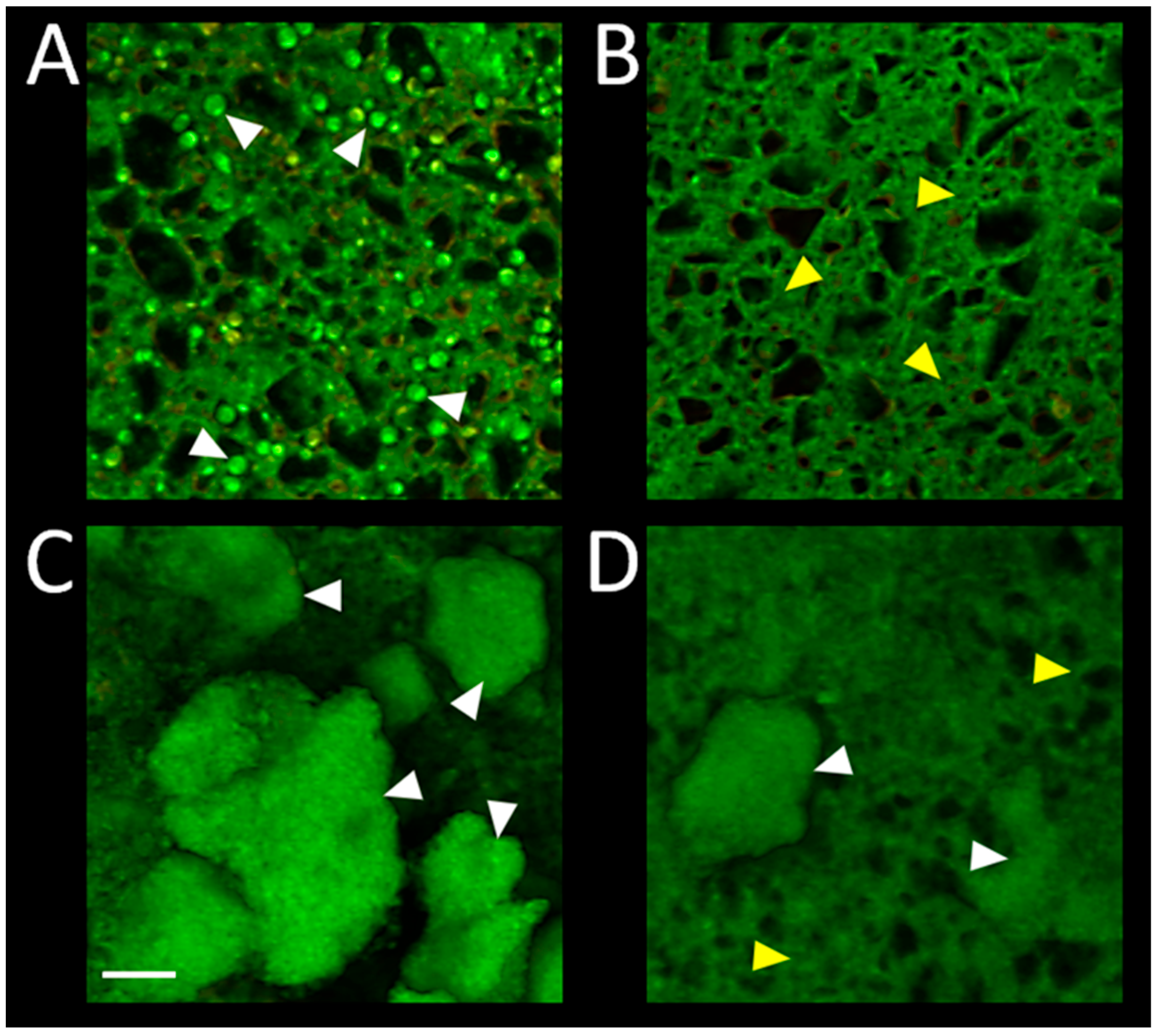
Confocal microscopy imaging of the biofilm on surface of RMGIC and RMGIC + Cu, corresponding with numerical results depicted in Figure 5, is presented in Figure 6. Viable C. albicans cells were present on RMGIC material, while they were less frequent or absent on RMGIC + Cu surface. S. mutans formed large aggregates of live biofilm covering the RMGIC material, which were markedly reduced on the RMGIC + Cu sample, exposing a biofilm-free surface.
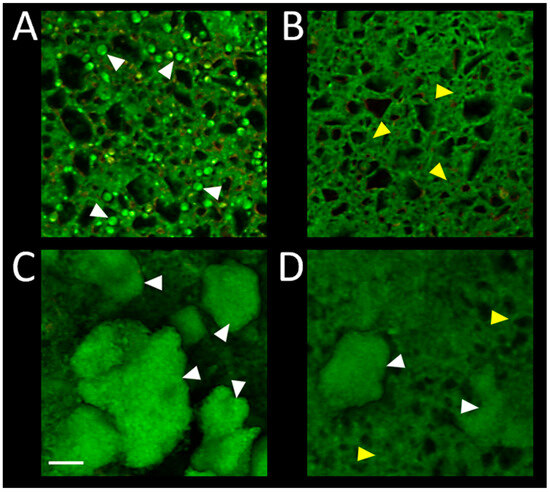
Figure 6.
Representative confocal microscopy images of biofilms developed on surface of the evaluated materials. RMGIC with C. albicans (A); RMGIC + Cu with C. albicans (B); RMGIC with S. mutans (C); RMGIC + Cu with S. mutans (D). Biofilms were stained with the LIVE/DEAD viability dye and imaged as whole-mounts. Green: live microorganisms, red: dead microorganisms. Material autofluorescence present in both channels. White triangles point to single yeast cells (A) or biofilm aggregates (C,D). Yellow triangles indicate exposed material surface. Scale bar = 20 µm.
The obtained RMGIC and RMGIC + Cu materials were tested for their potential virucidal properties (Table 5) against herpes simplex virus type 1 (HSV-1) and human adenovirus 5 (HAdV-5). We observed no reduction in HSV-1 (herpes simplex 1) titers under the influence of either material. In contrast, in the case of human adenovirus 5, pure RIVA showed stronger virucidal properties, at 99% (2 log), compared to RIVA with copper admixture, for which the reduction was 1.25 log, equivalent to a 90.25% reduction.

Table 5.
Comparison of the virucidal properties of the tested materials (expressed in the logarithmic scale and % reduction).
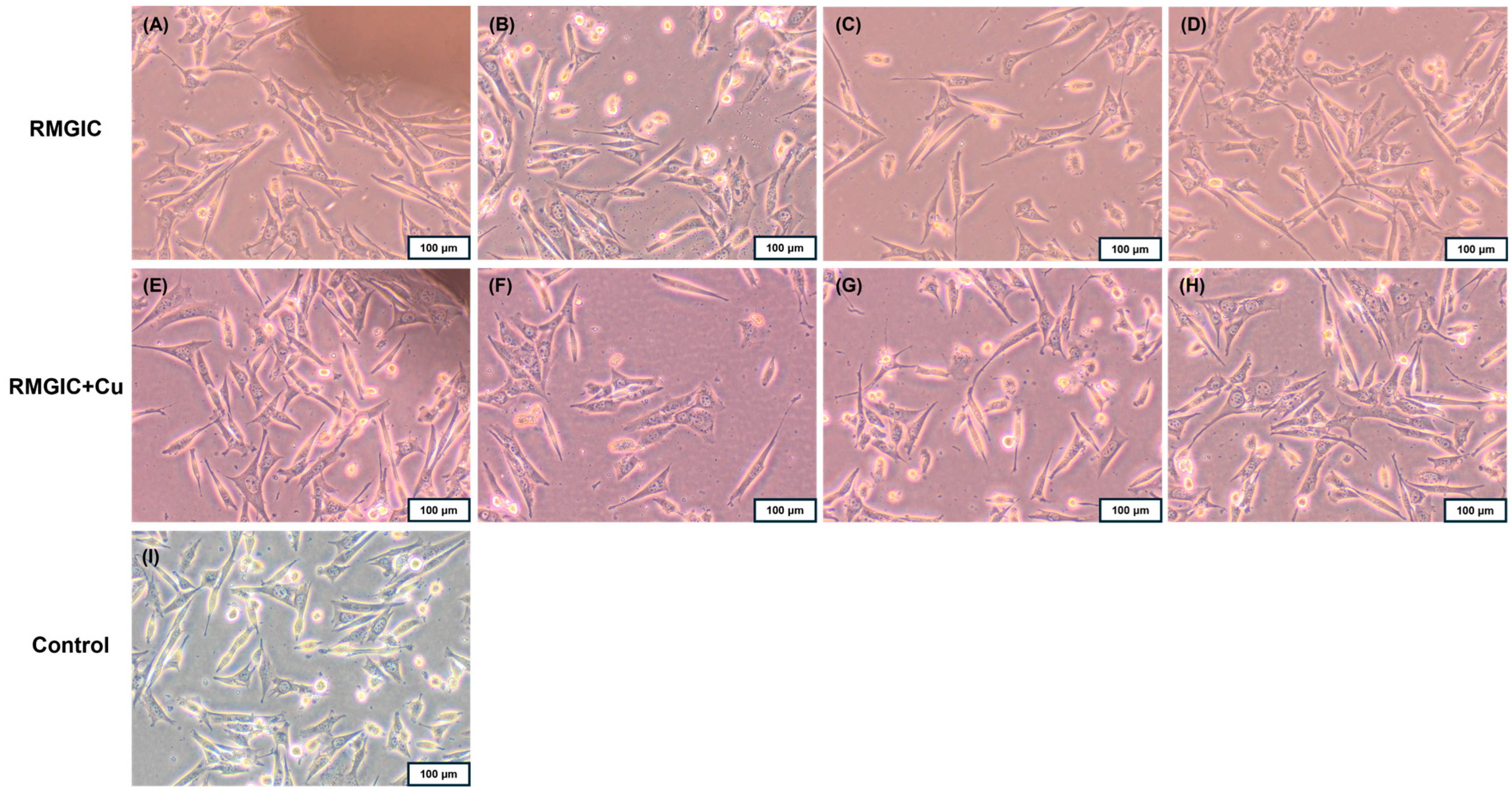
The degree of toxicity of RMGIC and RMGIC + Cu was assessed based on changes in Balb/3T3 fibroblast cell morphology using a 0–4 scale. Grade 0 (none), which is defined as no toxicity, is characterized by the absence of changes under or near the tested material. The toxicity gradation is as follows: Grade 1: slight (single cells degenerate or become distorted under the material); Grade 2: mild (the zone of altered cells is limited to the surface under the material); Grade 3: moderate (the zone of altered cells is limited to 1 cm around the material); Grade 4: severe (the zone of altered cells exceeds 1 cm around the material) [45]. RMGIC material exhibited no cytotoxic effect on cells (grade 0), whereas the RMGIC + Cu resulted in changes in the culture, including cell rounding and thinning, only under the sample (grade 1). Fibroblasts at the edge of the tested materials exhibited normal morphology (Figure 7). Furthermore, no other changes were observed in the cultures after contact with the tested materials compared to the control culture.
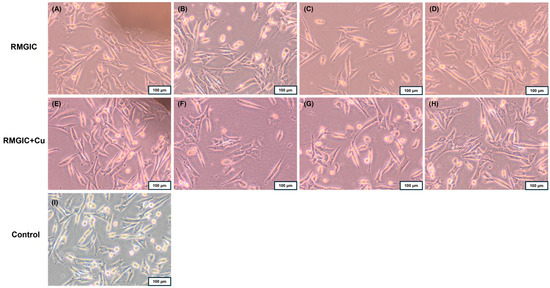
Figure 7.
Direct contact of Balb/3T3 fibroblasts after 24 h with the tested materials RMGIC and RMGIC + Cu; sample location: (A,E) near the sample, (B,F) under the sample, (C,G) 1 cm from the sample, (D,H) further from the sample; the control culture had no contact with the tested material (I). Magnification 100×.
4. Discussion
Research regarding the effect of pH on the fluoride release rate from dental materials, particularly glass ionomer cements, in various environments (such as deionized water or artificial saliva) is critical for several reasons [50]. The decrease in pH that occurs after the consumption of acidic foods leads to accelerated surface degradation of the cements, significantly impacting the initial fluoride release [50]. The utilization of pH-cycling simulations, which periodically modify conditions to emulate the environment following carbohydrate intake, frequently engenders elevated and more variable fluoride release [51]. This approach offers a more accurate reflection of the material’s actual ion release profile than tests conducted at constant pH. Furthermore, the composition of the testing medium exerts a substantial influence on the outcomes of fluoride release. It has been demonstrated that deionized water, devoid of buffers and mineral ions, frequently exhibits fluoride emission values that are several to over ten times higher than those obtained in artificial saliva containing remineralizing components [52].
As demonstrated in Figure 2B, one specimen (artificial saliva with a pH of 7.0 and copper) exhibited a divergent fluoride release time in comparison to the other specimens. It is conceivable that the other samples may also exhibit varying peak fluoride release times; however, this would necessitate the addition of measuring points between 24 and 48 h. However, it is important to note that different peak times cannot be excluded. Nevertheless, the present study has demonstrated that pH is the factor that exerts the greatest influence on fluoride release. However, the presence of copper has also been demonstrated to be a significant factor in this regard. The presence of Cu has been found to have the most significant impact on the lowest evaluated pH, which is 4.5. In other cases, no statistically significant differences are observed within the same pH ranges. It is notable that the samples released slightly less fluoride in artificial saliva solutions than in water-based solutions, which is an observation previously noted for fluoride gels [38].
Fluoride availability in the oral cavity may be increased by its release from biomaterials present in this environment. There are multiple factors affecting fluoride release that may affect the level of fluoride ions and, consequently, the balance between demineralization and remineralization processes. A systematic review by Tokarczuk et al. considered various restorative materials (composites, compomers, and glass ionomers) and concluded that surface coatings of biomaterials decrease fluoride release; however, their other benefits in caries prevention are evident [53]. Conversely, the article on compomers that release fluoride from biomaterial matrices may be potentiated by an acidic environment [54]. Light-curing glass ionomer cement modified with nanofluoroapatite was examined by Dobrzyński et al. in the scope of fluoride release. The authors observed that highest amounts of fluoride ions were released not only in artificial saliva with acidic pH (4.5) but also at pH at the level of 7.5 [55]. Another study discussing fluoride release from dental biomaterials over time proved higher amounts of fluoride ions at the acidic pH (4.5–5.5) compared between compomer and composite materials. The authors claim that the capacity to emit the greatest quantities of fluoride ions within an acidic environment is attributable to the deterioration of the superficial layer [56]. The presented study confirms that the pH of the environment exerts a significant influence on the release of fluoride. Elevated levels of fluoride release were observed in the presence of an acidic pH environment. Metal-modified glass ionomer cements were also evaluated in the scope of fluoride release. The systematic review by Klimas et al. demonstrated that fluoride release may be increased by addition of strontium, titanium oxide, silver nanoparticles, or zirconium oxide, while sintered silver reduces it [57]. The authors of this study noted that level of fluoride release is mostly affected by pH, but interaction of Cu presence and pH, and the presence of Cu alone, are also important factors affecting fluoride release.
In terms of ATR and FT-Raman spectra, representative peaks have been identified and assigned. Since the detailed composition of the Riva Light Cure material is not fully disclosed, we can suspect the presence of the organic polymer—most likely a polyacrylate or polymethacrylate derivative. Furthermore, the presence of v(O-H) band at lower values of Raman shift may indicate hydrogen-bonded hydroxyl groups, which may occur in cross-linked polymeric structures [58].
Biofilm accumulation is a primary cause of oral diseases like dental caries, periodontitis, peri-implantitis, and stomatitis due to their persistence and resistance to conventional treatments. Analysis of interaction of microorganisms with dental biomaterials helps to understand how the material surface and oral microbes interplay. It is vital for development of materials or coatings with antimicrobial properties that reduce biofilm formation, thus preventing infections and enhancing oral health [59,60].
A major concern pertains to the development of resistance among microorganisms to multiple antibiotics or disinfecting agents. It has been demonstrated that bulk copper materials exhibit notable antimicrobial properties, effective against a broad spectrum of microorganisms, including bacteria, fungi, algae, and viruses [61]. The review by Sidin et al. lists Cu among the most popular inorganic antibacterial agents. The authors notice that the antibacterial efficacy of each antibacterial agent is influenced by its mechanism of action and the species of bacterial strain [62]. In consideration of the aforementioned studies, the utilization of copper surfaces as antimicrobial agents to promote public hygiene appears to be a rational approach. The role of copper in antimicrobial mechanisms is undoubtable. However, numerous studies pay attention to morphology of the particles. A nanometric size has been appreciated in multiple studies. It has been demonstrated that copper nanoparticles possess a large surface area, which enables close interaction with cell membranes. This interaction is of significant importance in terms of the antimicrobial efficacy of the nanoparticles, which is concentration-dependent [63].
As depicted on Figure 5 and Figure 6, addition of copper to RMGIC resulted in statistically significantly lower adhesion of microorganisms in comparison to the reference formulation. The effect of copper on eradication of bacteria has been extensively studied, with cell envelope damage indicated as a main mechanism of action [64]. Therefore, this mechanism can be attributed to interaction with S. mutans and L. rhamnosus. For yeast (C. albicans), the mechanism may include disruption of cell membrane integrity as well as protein denaturation and enzyme inhibition due to accumulation of copper [65]. Utilization of metallic copper is particularly effective in eradicating bacteria, as most species are Cu sensitive [66]. On the other hand, higher concentrations of copper may pose a potential cytotoxic effect during longer exposure due to the release of copper ions [67,68]. However, the study was limited to assessing the impact of copper incorporation in a single formulation, without examining variations across multiple compositions. Nevertheless, cytotoxicity evaluation revealed grade 0 for RMGIC and grade 1 for RMGIC + Cu, which demonstrates slight effect of copper on cellular morphology. Moreover, the addition of copper may alter the color of biomaterial—the aesthetic aspect is important while discussing modern biomaterials for restorative and conservative dentistry. It has been noticed that size of metal plays an important role in terms of aesthetics, and nanoparticles are a preferable choice regarding that aspect [69]. Copper has also found application in dental implants covering—literature reports that it may contribute to better bone integration, proliferation, and viability [70]. As concluded in other systematic reviews regarding glass ionomer cement reinforced by metallic agents, fillers enhance antimicrobial properties, while any adverse effects are rarely reported [71]. Long-term studies and variations in composition need to be examined to determine the full impact of fillers on glass ionomer-based materials, particularly the effect on the cytotoxicity, shear strength, and adhesion [72]; marginal and internal adaptation [73]; and clinical aspects. In this aspect it is worth noticing the importance of normative ISO-based studies confirming various properties (e.g., cytotoxicity).
To date, the majority of research conducted on glass ionomer materials has centered on their antibacterial properties, with a particular emphasis on their efficacy against oral bacteria, including S. mutans and Lactobacillus spp. [74,75,76,77]. However, there is a paucity of data in the extant literature concerning their potential antiviral effects. To the best of our knowledge, this is the first study to assess the activity of glass ionomers against viruses, specifically HSV-1 and HAdV-5. The observed reduction in viral infectivity, ranging from 90.25% (RMGIC + Cu) to 99% (RMGIC), suggests an unexpected yet moderate antiviral potential. This finding opens new avenues for future research.
5. Conclusions
Based on our research, it is evident that RMGIC + Cu demonstrates potential antimicrobial activity against the tested strains of C. albicans, S. mutans, and L. rhamnosus when compared to the control group (RMGIC). The biomaterial supplemented with copper exhibits lower susceptibility to adhesion by all the microbial species used in the study compared to the reference material, as confirmed by both the mean values and statistical analysis results. Considering fluoride release, the influence of the Cu presence is most pronounced in artificial saliva with a low pH (4.5), where the difference is significantly higher in the samples with Cu than in the samples without it. No reduction in herpes simplex 1 titers was observed under the influence of either material, whereas both materials exhibited virucidal properties against human adenovirus 5. A collective consideration of the results obtained reveals the potential merits of doping glass ionomer with copper. This modification may be of interest due to the enhancement of the biomaterial’s antimicrobial properties. RMGIC + Cu may find a potential application in caries treatment and prevention as a restorative material or dental sealant. It can be beneficial in terms of caries prevention compared to commercially available RMGIC with copper addition. It is essential to emphasize that further long-term cytotoxicity evaluation is vital prior to further in vivo studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15179506/s1, Figure S1: Photograph of Riva Light Cure material – powder and liquid components of the RMGIC (A) and copper powder used for modification (B); Figure S2: Colorimetric analysis of RMGIC (A) and RMGIC+Cu (B). Analysis was conducted in ImageJ software. RGB mean values are presented on the histograms along with Standard Deviations (SD) and mode values; Figure S3: Photographs of RMGIC (A) and RMGIC+Cu (B) before registering FT-Raman spectra. Red marks indicate exact spots where spectra were acquired
Author Contributions
Conceptualization, A.P., P.J.P., M.S., and M.D.; methodology, A.P., P.J.P., M.J.K., M.P., J.N., A.C., A.R., G.C., and M.D.; software, P.J.P., M.J.K., M.P., J.N., A.C., A.R., and G.C.; validation, A.P., P.J.P., M.J.K., M.P., J.N., A.C., A.R., and G.C.; formal analysis, A.P., P.J.P., M.J.K., M.P., J.N., A.C., A.R., and G.C.; investigation, A.P., P.J.P., M.P., J.N., A.C., A.R., and G.C.; resources, P.J.P., M.J.K., M.P., J.N., A.C., A.R., G.C., and M.D.; data curation, A.P., P.J.P., M.J.K., M.P., J.N., A.C., A.R., and G.C.; writing—original draft preparation, A.P., M.J.K., P.J.P., M.P., J.N., A.C., A.R., and G.C.; writing—review and editing, A.P., P.J.P., M.P., J.N., G.C., M.S., and M.D.; visualization, P.J.P., M.J.K., A.R., and G.C.; supervision, M.S. and M.D.; project administration, A.P. and M.D.; funding acquisition, J.N. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a subsidy from Wroclaw Medical University, number SUBK.A130.24.058 and SUBZ.B180.25.091. The APC was co-financed by a general subsidy from Wroclaw Medical University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Sugars and Dental Caries. Available online: https://www.who.int/news-room/fact-sheets/detail/sugars-and-dental-caries (accessed on 7 July 2025).
- Naaman, R.; El-Housseiny, A.A.; Alamoudi, N. The Use of Pit and Fissure Sealants-a Literature Review. Dent. J. 2017, 5, 34. [Google Scholar] [CrossRef]
- Małyszek, A.; Zawiślak, I.; Kulus, M.; Watras, A.; Kensy, J.; Kotela, A.; Styczyńska, M.; Janeczek, M.; Matys, J.; Dobrzyński, M. Assessment of Fluoride Intake Risk via Infusions of Commercial Leaf Teas Available in Poland Using the Target Hazard Quotient Index Approach. Foods 2025, 14, 2944. [Google Scholar] [CrossRef]
- Okamoto, M.; Yamashita, S.; Mendonca, M.; Brueckner, S.; Achong-Bowe, R.; Thompson, J.; Kuriki, N.; Mizuhira, M.; Benjamin, Y.; Duncan, H.F.; et al. Ultrastructural Evaluation of Adverse Effects on Dentine Formation from Systemic Fluoride Application in an Experimental Mouse Model. Int. Endod. J. 2025, 58, 128–140. [Google Scholar] [CrossRef]
- Sidhu, S.; Nicholson, J. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Khoroushi, M.; Keshani, F. A Review of Glass-Ionomers: From Conventional Glass-Ionomer to Bioactive Glass-Ionomer. Dent. Res. J. 2013, 10, 411–420. [Google Scholar]
- Iranparvar, P.; Ghasemi, A.; Iranparvar, P. Adhesion of Glass Ionomer Cements to Primary Dentin Using a Universal Adhesive. Dent. Med. Probl. 2024, 61, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hafshejani, T.M.; Zamanian, A.; Venugopal, J.R.; Rezvani, Z.; Sefat, F.; Saeb, M.R.; Vahabi, H.; Zarrintaj, P.; Mozafari, M. Antibacterial Glass-Ionomer Cement Restorative Materials: A Critical Review on the Current Status of Extended Release Formulations. J. Control. Release 2017, 262, 317–328. [Google Scholar] [CrossRef]
- Ge, K.X.; Lam, W.Y.; Chu, C.-H.; Yu, O.Y. Updates on the Clinical Application of Glass Ionomer Cement in Restorative and Preventive Dentistry. J. Dent. Sci. 2024, 19, S1–S9. [Google Scholar] [CrossRef]
- Croll, T.P.; Nicholson, J.W. Glass Ionomer Cements in Pediatric Dentistry: Review of the Literature. Pediatr. Dent. 2002, 24, 423–429. [Google Scholar] [PubMed]
- Almuhaiza, M. Glass-Ionomer Cements in Restorative Dentistry: A Critical Appraisal. J. Contemp. Dent. Pract. 2016, 17, 331–336. [Google Scholar] [CrossRef]
- ResearchAndMarkets. Glass Ionomer Filling Market: Market Size, Trends, Opportunities and Forecast by Defect Class, End-User Type, Product Type, Region, By Country: 2020–2030; ResearchAndMarkets: Dublin, Ireland, 2024. [Google Scholar]
- SDI. Riva Conditioner Brochure. Available online: https://www.sdi.com.au/wp-content/uploads/instructions/instruction_PL/in_riva_con_pl.pdf (accessed on 7 July 2025).
- SDI. Riva Light Cure Brochure. Available online: https://www.sdi.com.au/images/stories/instructions/instructions_pdf/riva_lc/in_riva_lc_en.pdf (accessed on 7 July 2025).
- Oppermann, R.V.; Johansen, J.R. Effect of Fluoride and Non-fluoride Salts of Copper, Silver and Tin on the Acidogenicity of Dental Plaque in Vivo. Eur. J. Oral. Sci. 1980, 88, 476–480. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Nagay, B.E.; Dini, C.; Souza, J.G.S.; Rangel, E.C.; da Cruz, N.C.; Yang, F.; van den Beucken, J.J.J.P.; Barão, V.A.R. Copper Source Determines Chemistry and Topography of Implant Coatings to Optimally Couple Cellular Responses and Antibacterial Activity. Biomater. Adv. 2022, 134, 112550. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Malaquias, P.; Hass, V.; Matos, T.P.; Lourenço, L.; Reis, A.; Loguercio, A.D.; Farago, P.V. The Role of Copper Nanoparticles in an Etch-and-Rinse Adhesive on Antimicrobial Activity, Mechanical Properties and the Durability of Resin-Dentine Interfaces. J. Dent. 2017, 61, 12–20. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Vakhnovetsky, J.; Vakhnovetsky, A. Functional Role of Inorganic Trace Elements in Dentin Apatite—Part II: Copper, Manganese, Silicon, and Lithium. J. Trace Elem. Med. Biol. 2022, 72, 126995. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Liu, Y.; Lu, Y.; Geng, W.; Lin, J. Copper-Containing Titanium Alloys Promote the Coupling of Osteogenesis and Angiogenesis by Releasing Copper Ions. Biochem. Biophys. Res. Commun. 2023, 681, 157–164. [Google Scholar] [CrossRef]
- Aguilar-Perez, D.; Vargas-Coronado, R.; Cervantes-Uc, J.M.; Rodriguez-Fuentes, N.; Aparicio, C.; Covarrubias, C.; Alvarez-Perez, M.; Garcia-Perez, V.; Martinez-Hernandez, M.; Cauich-Rodriguez, J.V. Antibacterial Activity of a Glass Ionomer Cement Doped with Copper Nanoparticles. Dent. Mater. J. 2020, 39, 389–396. [Google Scholar] [CrossRef]
- Renné, W.G.; Lindner, A.; Mennito, A.S.; Agee, K.A.; Pashley, D.H.; Willett, D.; Sentelle, D.; Defee, M.; Schmidt, M.; Sabatini, C. Antibacterial Properties of Copper Iodide-Doped Glass Ionomer-Based Materials and Effect of Copper Iodide Nanoparticles on Collagen Degradation. Clin. Oral. Investig. 2017, 21, 369–379. [Google Scholar] [CrossRef]
- Okamoto, M.; Ali, M.; Komichi, S.; Watanabe, M.; Huang, H.; Ito, Y.; Miura, J.; Hirose, Y.; Mizuhira, M.; Takahashi, Y.; et al. Surface Pre-Reacted Glass Filler Contributes to Tertiary Dentin Formation through a Mechanism Different Than That of Hydraulic Calcium-Silicate Cement. J. Clin. Med. 2019, 8, 1440. [Google Scholar] [CrossRef] [PubMed]
- Zijnge, V.; van Leeuwen, M.B.M.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmür, R.; Harmsen, H.J.M. Oral Biofilm Architecture on Natural Teeth. PLoS ONE 2010, 5, e9321. [Google Scholar] [CrossRef] [PubMed]
- Elbahie, D.M.; Badawy, R.E.-S.; Ibrahim, S.A.M.; Hassan, M.; Habib, N.A. Assessment of the Antibacterial Activity of Glass Ionomer Cements Modified by Polyamidoamine and Bioactive Glass: An in Vitro Study. Dent. Med. Probl. 2025. Ahead of Print. [Google Scholar] [CrossRef] [PubMed]
- Dennison, D.K.; Meredith, G.M.; Shillitoe, E.J.; Caffesse, R.G. The Antiviral Spectrum of Listerine Antiseptic. Oral Pathol. Oral Radiol. Endodontology 1995, 79, 442–448. [Google Scholar] [CrossRef]
- Jaros, S.W.; Florek, M.; Bażanów, B.; Panek, J.; Krogul-Sobczak, A.; Oliveira, M.C.; Król, J.; Śliwińska-Hill, U.; Nesterov, D.S.; Kirillov, A.M.; et al. Silver Coordination Polymers Driven by Adamantoid Blocks for Advanced Antiviral and Antibacterial Biomaterials. ACS Appl. Mater. Interfaces 2024, 16, 13411–13421. [Google Scholar] [CrossRef]
- Shalaby, H.A.; Soliman, N.K.; Al–Saudi, K.W. Antibacterial and Preventive Effects of Newly Developed Modified Nano-Chitosan/Glass-Ionomer Restoration on Simulated Initial Enamel Caries Lesions: An in Vitro Study. Dent. Med. Probl. 2024, 61, 353–362. [Google Scholar] [CrossRef]
- O’Hern, C.I.Z.; Djoko, K.Y. Copper Cytotoxicity: Cellular Casualties of Noncognate Coordination Chemistry. mBio 2022, 13, e00434-22. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska-Deptuła, S.J.; Piktel, E.; Wolak, P.; Wollny, T.; Bucki, R. Metallic Nanosystems in the Development of Antimicrobial Strategies with High Antimicrobial Activity and High Biocompatibility. Int. J. Mol. Sci. 2023, 24, 2104. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Chen, X.; Jiang, T.; Xue, J.; Liber, K.; Liu, H.; Yang, J. Copper Induces Cytotoxicity in Freshwater Bivalve Anodonta Woodiana Hemocytes. Chemosphere 2024, 362, 142595. [Google Scholar] [CrossRef]
- Ching, H.S.; Luddin, N.; Kannan, T.P.; Ab Rahman, I.; Abdul Ghani, N.R.N. Modification of Glass Ionomer Cements on Their Physical-Mechanical and Antimicrobial Properties. J. Esthet. Restor. Dent. 2018, 30, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Elshenawy, E.A.; El-Ebiary, M.A.; Kenawy, E.R.; El-Olimy, G.A. Modification of Glass-Ionomer Cement Properties by Quaternized Chitosan-Coated Nanoparticles. Odontology 2023, 111, 328–341. [Google Scholar] [CrossRef]
- Valanezhad, A.; Odatsu, T.; Udoh, K.; Shiraishi, T.; Sawase, T.; Watanabe, I. Modification of Resin Modified Glass Ionomer Cement by Addition of Bioactive Glass Nanoparticles. J. Mater. Sci. Mater. Med. 2016, 27, 3. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Ansari, S.; Movasaghi, Z.; Billington, R.W.; Darr, J.A.; Rehman, I.U. Modification of Conventional Glass-Ionomer Cements with N-Vinylpyrrolidone Containing Polyacids, Nano-Hydroxy and Fluoroapatite to Improve Mechanical Properties. Dent. Mater. 2008, 24, 1381–1390. [Google Scholar] [CrossRef]
- Lee, M.J.; Kwon, J.S.; Kim, J.Y.; Ryu, J.H.; Seo, J.Y.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Bioactive Resin-Based Composite with Surface Pre-Reacted Glass-Ionomer Filler and Zwitterionic Material to Prevent the Formation of Multi-Species Biofilm. Dent. Mater. 2019, 35, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Kosior, P.; Dobrzyński, M.; Korczyński, M.; Herman, K.; Czajczyńska-Waszkiewicz, A.; Kowalczyk-Zając, M.; Piesiak-Pańczyszyn, D.; Fita, K.; Janeczek, M. Long-Term Release of Fluoride from Fissure Sealants—In Vitro Study. J. Trace Elem. Med. Biol. 2017, 41, 107–110. [Google Scholar] [CrossRef]
- Kosior, P.; Kaczmarek, U. Short-Term Fluoride Release from Conseal F Fissure Sealant in Some Media—An in Vitro Study. Ann. Acad. Med. Stetin. 2006, 52 (Suppl. S1), 61–65. [Google Scholar]
- Piszko, P.J.; Kulus, M.; Piszko, A.; Kiryk, J.; Kiryk, S.; Kensy, J.; Małyszek, A.; Michalak, M.; Dobrzyński, W.; Matys, J.; et al. The Influence of Calcium Ions and PH on Fluoride Release from Commercial Fluoride Gels in an In Vitro Study. Gels 2025, 11, 486. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-Review. Res. Rev. J. Eng. Technol. 2017, 6. [Google Scholar] [PubMed]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of Multiple Methods for Quantification of Microbial Biofilms Grown in Microtiter Plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef]
- Solis-Velazquez, O.A.; Gutiérrez-Lomelí, M.; Guerreo-Medina, P.J.; Rosas-García, M.d.L.; Iñiguez-Moreno, M.; Avila-Novoa, M.G. Nosocomial Pathogen Biofilms on Biomaterials: Different Growth Medium Conditions and Components of Biofilms Produced in Vitro. J. Microbiol. Immunol. Infect. 2021, 54, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.; Ranque, S.; Prêcheur, I. Oral Fungal-Bacterial Biofilm Models in Vitro: A Review. Med. Mycol. 2018, 56, 653–667. [Google Scholar] [CrossRef]
- ISO 21702:2019; Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Heise, T.; Sawyer, A.Y.; Hirai, T.; Schaible, S.; Sy, H.; Wickramasekara, S. Report on Investigation of ISO 10993–12 Extraction Conditions. Regul. Toxicol. Pharmacol. 2022, 131, 105164. [Google Scholar] [CrossRef]
- Galal, M.M.; Ismail, A.G.; Nashaat, Y.; Hamdy, T.M. Evaluation of the Cytotoxicity, Apoptotic Effects, and Remineralization Potential of Recent Bioceramic-Based Root Canal Sealers. J. Oral. Biol. Craniofac Res. 2025, 15, 757–762. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.
- Szymanski, L.; Kiernozek, M.; Gromadka, B.; Straszecka, W.; Wiktorek-Smagur, A.; Matak, D. Chemical Characterization in Medical Device Evaluation: Current Practices, Regulatory Requirements, and Future Directions. Ann. Biomed. Eng. 2025, 53, 1068–1079. [Google Scholar] [CrossRef]
- Kumari, P.D.; Khijmatgar, S.; Chowdhury, A.; Lynch, E.; Chowdhury, C.R. Factors Influencing Fluoride Release in Atraumatic Restorative Treatment (ART) Materials: A Review. J. Oral. Biol. Craniofac Res. 2019, 9, 315–320. [Google Scholar] [CrossRef]
- Garcez, R.M.V.d.B.; Buzalaf, M.A.R.; Araújo, P.A. de Fluoride Release of Six Restorative Materials in Water and PH-Cycling Solutions. J. Appl. Oral Sci. 2007, 15, 406–411. [Google Scholar] [CrossRef]
- El Mallakh, B.F.; Sarkar, N.K. Fluoride Release from Glass-Ionomer Cements in de-Ionized Water and Artificial Saliva. Dent. Mater. 1990, 6, 118–122. [Google Scholar] [CrossRef]
- Tokarczuk, D.; Tokarczuk, O.; Kiryk, J.; Kensy, J.; Szablińska, M.; Dyl, T.; Dobrzyński, W.; Matys, J.; Dobrzyński, M. Fluoride Release by Restorative Materials after the Application of Surface Coating Agents: A Systematic Review. Appl. Sci. 2024, 14, 4956. [Google Scholar] [CrossRef]
- Attin, T.; Buchalla, W.; Siewert, C.; Hellwig, E. Fluoride Release/Uptake of Polyacid-modified Resin Composites (Compomers) in Neutral and Acidic Buffer Solutions. J. Oral. Rehabil. 1999, 26, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, W.; Nikodem, A.; Diakowska, D.; Wiglusz, R.J.; Watras, A.; Dobrzyński, M.; Mikulewicz, M. Comparison of the Fluoride Ion Release from Nanofluoroapatite-Modified Orthodontic Cement under Different PH Conditions—An in Vitro Study. Acta Bioeng. Biomech. 2023, 25, 159–176. [Google Scholar] [CrossRef]
- Kosior, P.; Dobrzynski, M.; Zakrzewska, A.; Diakowska, D.; Nienartowicz, J.; Blicharski, T.; Nagel, S.; Sikora, M.; Wiglusz, K.; Watras, A.; et al. Comparison of the Fluoride Ion Release from Composite and Compomer Materials under Varying PH Conditions—Preliminary In Vitro Study. Appl. Sci. 2022, 12, 12540. [Google Scholar] [CrossRef]
- Klimas, S.; Kiryk, S.; Kiryk, J.; Kotela, A.; Kensy, J.; Michalak, M.; Rybak, Z.; Matys, J.; Dobrzyński, M. The Impact of Environmental and Material Factors on Fluoride Release from Metal-Modified Glass Ionomer Cements: A Systematic Review of In Vitro Studies. Materials 2025, 18, 3187. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ozaki, Y.; Nakashima, K. Infrared, Raman, and near-Infrared Spectroscopic Evidence for the Coexistence of Various Hydrogen-Bond Forms in Poly(Acrylic Acid). Macromolecules 1997, 30, 1111–1117. [Google Scholar] [CrossRef]
- Dobrzynski, M.; Pajaczkowska, M.; Nowicka, J.; Jaworski, A.; Kosior, P.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Bogucki, Z.A.; Filipiak, J.; et al. Study of Surface Structure Changes for Selected Ceramics Used in the CAD/CAM System on the Degree of Microbial Colonization, In Vitro Tests. Biomed. Res. Int. 2019, 2019, 9130806. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Nowicka, J.; Pajaczkowska, M.; Szymonowicz, M.; Targonska, S.; Sobierajska, P.; Wiglusz, K.; Dobrzynski, W.; Lubojanski, A.; et al. The Influence of Ozonated Olive Oil-Loaded and Copper-Doped Nanohydroxyapatites on Planktonic Forms of Microorganisms. Nanomaterials 2020, 10, 1997. [Google Scholar] [CrossRef]
- Longano, D.; Ditaranto, N.; Sabbatini, L.; Torsi, L.; Cioffi, N. Synthesis and Antimicrobial Activity of Copper Nanomaterials. In Nano-Antimicrobials; Springer: Berlin/Heidelberg, Germany, 2012; pp. 85–117. [Google Scholar]
- Saidin, S.; Jumat, M.A.; Mohd Amin, N.A.A.; Saleh Al-Hammadi, A.S. Organic and Inorganic Antibacterial Approaches in Combating Bacterial Infection for Biomedical Application. Mater. Sci. Eng. C 2021, 118, 111382. [Google Scholar] [CrossRef]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper Nanoparticles with High Antimicrobial Activity. Mater. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial Killing by Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Dupont, C.L.; Grass, G.; Rensing, C. Copper Toxicity and the Origin of Bacterial Resistance—New Insights and Applications. Metallomics 2011, 3, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Cortizo, M.C.; De Mele, M.F.L. Cytotoxicity of Copper Ions Released from Metal: Variation with the Exposure Period and Concentration Gradients. Biol. Trace Elem. Res. 2004, 102, 129–141. [Google Scholar] [CrossRef]
- Solangi, J.A.; Memon, T.F.; Umair, M.; Jabeen, S.; Kumar, H.; Ali, A.; Shaikh, S.H.; Phulpoto, A.H. Antimicrobial efficacy of copper nanoparticles: A comprehensive review. Insights J. Health Rehabil. 2024, 2, 243–254. [Google Scholar] [CrossRef]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications—A Systematic Review. Biol. Trace Elem. Res. 2019, 197, 70–88. [Google Scholar] [CrossRef]
- Abdulghafor, M.A.; Mahmood, M.K.; Tassery, H.; Tardivo, D.; Falguiere, A.; Lan, R. Biomimetic Coatings in Implant Dentistry: A Quick Update. J. Funct. Biomater. 2023, 15, 15. [Google Scholar] [CrossRef]
- Danelon, M.; Nunes, G.P.; Sterzenbach, T.; Hannig, C. Enhancing Antimicrobial Properties of Glass Ionomer Cement through Metallic Agent Reinforcement: A Systematic Review and Meta-Analysis. J. Dent. 2025, 160, 105892. [Google Scholar] [CrossRef]
- Kim, S.; Geryak, R.D.; Zhang, S.; Ma, R.; Calabrese, R.; Kaplan, D.L.; Tsukruk, V.V. Interfacial Shear Strength and Adhesive Behavior of Silk Ionomer Surfaces. Biomacromolecules 2017, 18, 2876–2886. [Google Scholar] [CrossRef]
- Sahli, A.; Daeniker, L.; Rossier, I.; Caseiro, L.; di Bella, E.; Krejci, I.; Bortolotto, T. Comparison of Class II Bulk-Fill, Self-Adhesive Composites, Alkasite, and High-Viscosity Glass Ionomer Restorations in Terms of Marginal and Internal Adaptation. Materials 2024, 17, 4373. [Google Scholar] [CrossRef]
- Pedrini, D.; Gaetti-Jardim Júnior, E.; de Vasconcelos, A.C. Retention of Oral Microorganisms on Conventional and Resin-Modified Glass-Ionomer Cements. Pesqui. Odontológica Bras. 2001, 15, 196–200. [Google Scholar] [CrossRef][Green Version]
- Plant, C.G.; Tobias, R.S.; Rippin, J.W.; Brooks, J.W.; Browne, R.M. A Study of the Relationship among Pulpal Response, Microbial Microleakage, and Particle Heterogeneity in a Glass-Ionomer-Base Material. Dent. Mater. 1991, 7, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Duque, C.; Negrini, T.D.C.; Sacono, N.T.; Spolidorio, D.M.P.; De Souza Costa, C.A.; Hebling, J. Clinical and Microbiological Performance of Resin-Modified Glass-Ionomer Liners after Incomplete Dentine Caries Removal. Clin. Oral. Investig. 2009, 13, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Palenik, C.J.; Behnen, M.J.; Setcos, J.C.; Miller, C.H. Inhibition of Microbial Adherence and Growth by Various Glass Ionomers in Vitro. Dent. Mater. 1992, 8, 16–20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).