Abstract
Based on molecular simulation methods, this paper constructs a molecular model of the CH4-drilling fluid system to conduct an in-depth analysis of the microscopic dissolution behavior of CH4 in drilling fluids. By utilizing key parameters such as molecular motion trajectories, interaction energies and solubility free energies, the mechanisms of CH4 dissolution and diffusion are revealed. The factors influencing CH4 solubility and their variation mechanisms are elucidated at the molecular level. Through gas solubility experiments, the variation patterns of CH4 solubility in drilling fluids under different temperature and pressure conditions are investigated, and optimized solubility models for CH4-drilling fluid systems are selected. The results indicate that the dissolution and diffusion behavior of CH4 in drilling fluids can be described using free volume, interaction energy and solubility free energy, with the degree of influence ranked as follows: interaction energy > free volume > solubility free energy. The interaction and free volume of CH4 in oil-based drilling fluids are both greater than those in water-based drilling fluids, suggesting a higher solubility of CH4 in oil-based drilling fluids. Solubility models of CH4 in drilling fluids under conditions of 30~120 °C and 10~60 MPa are obtained by regression. The research findings not only deepen the understanding of the dissolution and diffusion behavior of CH4 in drilling fluids for shale gas horizontal wells, but also provide crucial parameters for establishing wellbore pressure models in managed pressure drilling.
1. Introduction
As the difficulty in conventional natural gas exploration continues to increase and the demand for natural gas grows steadily, countries around the world are placing greater emphasis on the exploration and development of unconventional natural gas resources, with particular attention given to the exploitation and utilization of shale gas [1,2,3]. Currently, the primary countries achieving large-scale commercial extraction of shale gas are China and the US [4]. The main components of gases in shale gas reservoirs are listed in Table 1. In shale gas, CH4 accounts for approximately 70% to 95%, making it the primary constituent. During managed pressure drilling in shale gas horizontal wells, if gas influx occurs, formation gases will enter the wellbore annulus, altering the flow conditions within the annulus and subsequently leading to increased well control issues and safety risks. Therefore, studying the dissolution behavior of CH4 in drilling fluids and determining its solubility in drilling fluids hold significant engineering importance.

Table 1.
Main gas components of shale gas reservoir.
In the early stages, scholars conducted extensive research on the solubility characteristics of CH4 in water-based/oil-based drilling fluids. O’Bryan et al. [5] provided experimental data on the solubility of CH4 in oil-based drilling fluids over a certain temperature range and offered empirical correlation formulas. Duan et al. [6] developed a solubility model applicable for predicting the solubility of CH4 in brine under temperatures ranging from 0 to 250 °C and pressures ranging from 0 to 160 MPa. Fu et al. [7] proposed that the solubility mechanism of gases in water involves interstitial filling and hydration. They combined this with Henry’s law to derive a gas solubility equation, and analyzed the influence of hydration equilibrium constants and Henry’s constants under different conditions, based on molecular volume. Xue et al. [8] conducted tests on the solubility of CH4 in the oil phase, derived a solubility model for CH4 in crude oil, and determined the model parameters using experimental data. Fu et al. [9] established a theoretical model for calculating the solubility of gases in water-based and oil-based drilling fluids, based on solubility calculation models. They also analyzed the change in the gas solubility in water-based and oil-based drilling fluids in deep water environments. Xu [10] carried out experimental tests on the solubility of CH4 in different drilling fluid systems under various temperature and pressure conditions to study the differences in the solubility characteristics of CH4 in different drilling fluids. Su et al. [11] considered a transient-flow model incorporating gas solubility to investigate the impact of gas solubility on gas invasion during drilling with oil-based drilling fluids.
Overall, the existing models for calculating the gas solubility in drilling fluids primarily fall into two categories. The first category consists of fitting formulas derived from experimental data, which are used to obtain solubility models for different drilling fluid systems under the influence of temperature and pressure [12,13,14,15,16,17]. However, the main issue with these models is that the amount of experimental data is insufficient, and the temperature and pressure ranges covered are not broad. As a result, the empirical models obtained are difficult to apply to long-reach horizontal shale gas wells with high temperature and high pressure. The second category involves theoretical research, which establishes solubility models for gases in water-based and oil-based drilling fluids, based on molecular interaction theories and state equations [18,19,20]. Nevertheless, these solubility calculation models still have deficiencies in understanding the gas dissolution mechanisms at the molecular level.
Therefore, this paper employs molecular simulation methods to construct molecular models for the CH4-drilling fluid system. It conducts an in-depth analysis of the microscopic dissolution behavior of CH4 in drilling fluids, utilizing key parameters such as molecular motion trajectories, interaction energies and solubility free energies, to reveal the mechanisms of gas dissolution and diffusion. At the molecular level, it elucidates the factors influencing CH4 solubility and variation mechanisms. Through experimental research on CH4 solubility, this study explores the solubility patterns of CH4 in water-based and oil-based drilling fluids under different temperature and pressure conditions. It optimizes the solubility equations for CH4 in drilling fluids, providing crucial parameters for calculating wellbore pressure during managed pressure drilling in shale gas horizontal wells.
Section 2 details the methodology, including the molecular simulation framework, force field, drilling fluid models, and experimental setup. Section 3 presents the results and discussion, covering CH4 trajectories, diffusion (MSD), solubility free energy, free volume analysis, and the effects of temperature and pressure. Section 4 concludes with key findings and implications for well control and managed pressure drilling in shale gas horizontal wells.
2. Methodology
2.1. Molecular Simulation Method
Molecular simulation is a technical approach that investigates the structure, properties and behavior of molecular systems. It enables accurate predictions and analyses at the atomic and molecular levels, facilitating an in-depth understanding of the microscopic mechanisms of molecular systems, which are often challenging to observe directly using experimental techniques [21,22,23,24,25,26]. In this paper, molecular dynamics simulation technology is utilized to study the solubility variations of CH4 in water-based and oil-based drilling fluids under different temperature and pressure conditions, thereby revealing its microscopic mechanisms and relevant influencing factors.
2.1.1. Fundamental Theory of Molecular Dynamics
Molecular dynamics simulation is based on Newton’s laws, and solves the motion behavior of particles under mutual interactions through numerical integration. The basic steps of molecular dynamics simulation include the following: ① initializing the positions and velocities of particles; ② calculating the forces between particles through potential energy functions; and ③ using numerical methods to solve the equations of motion and updating the positions and velocities of particles.
In molecular simulations, Newton’s laws of motion are typically employed to calculate the trajectories of particles:
In the equation, F represents the force acting on the particle, N. m denotes the mass of the particle, and kg. a is the acceleration of the particle, m/s2.
The interaction forces between particles are described through potential energy functions. Common potential energy functions are shown in Table 2 [27]:

Table 2.
Common potential energy functions.
The Verlet algorithm exhibits good numerical stability, and is thus widely applied in molecular dynamics simulations. The equation is as follows:
where ri(t) is the position of particle i at time t, m. Fi(t) is the force acting on particle i at time t, N. mi is the mass of particle i, kg. Δt is the time step, s.
2.1.2. Related Parameter Calculation
Relevant parameters that can be calculated through molecular simulations include gas diffusion coefficients, radial distribution functions, free volume fractions and interaction energies, among others (shown in Table 3). The gas diffusion coefficient characterizes the thermal motion behavior of molecules by calculating the mean squared displacement (MSD) of characteristic structural units. The radial distribution function is a commonly used method for analyzing the interactions between gases and drilling fluids [28]. It reveals the degree of order in a substance, with peaks typically appearing at specific distances. The free volume fraction is generally defined as the ratio of the “void volume” between all molecules to the total volume [29]. The interaction energy reflects the interactions between particles, and is an important parameter for describing aspects such as the structure, stability, and reaction kinetics of substances [30]. The solubility free energy refers to the work required to transfer a solute from the gaseous state to a solution. It measures the driving force for gas molecules to dissolve into the liquid. When calculating the solubility free energy, it is usually divided into three main components: ideal gas interactions, van der Waals forces, and electrostatic interactions. These three components work together to determine the solubility of gases in the solution and its variation patterns.

Table 3.
Calculation of Related Parameters.
2.1.3. Model of Drilling Fluid System
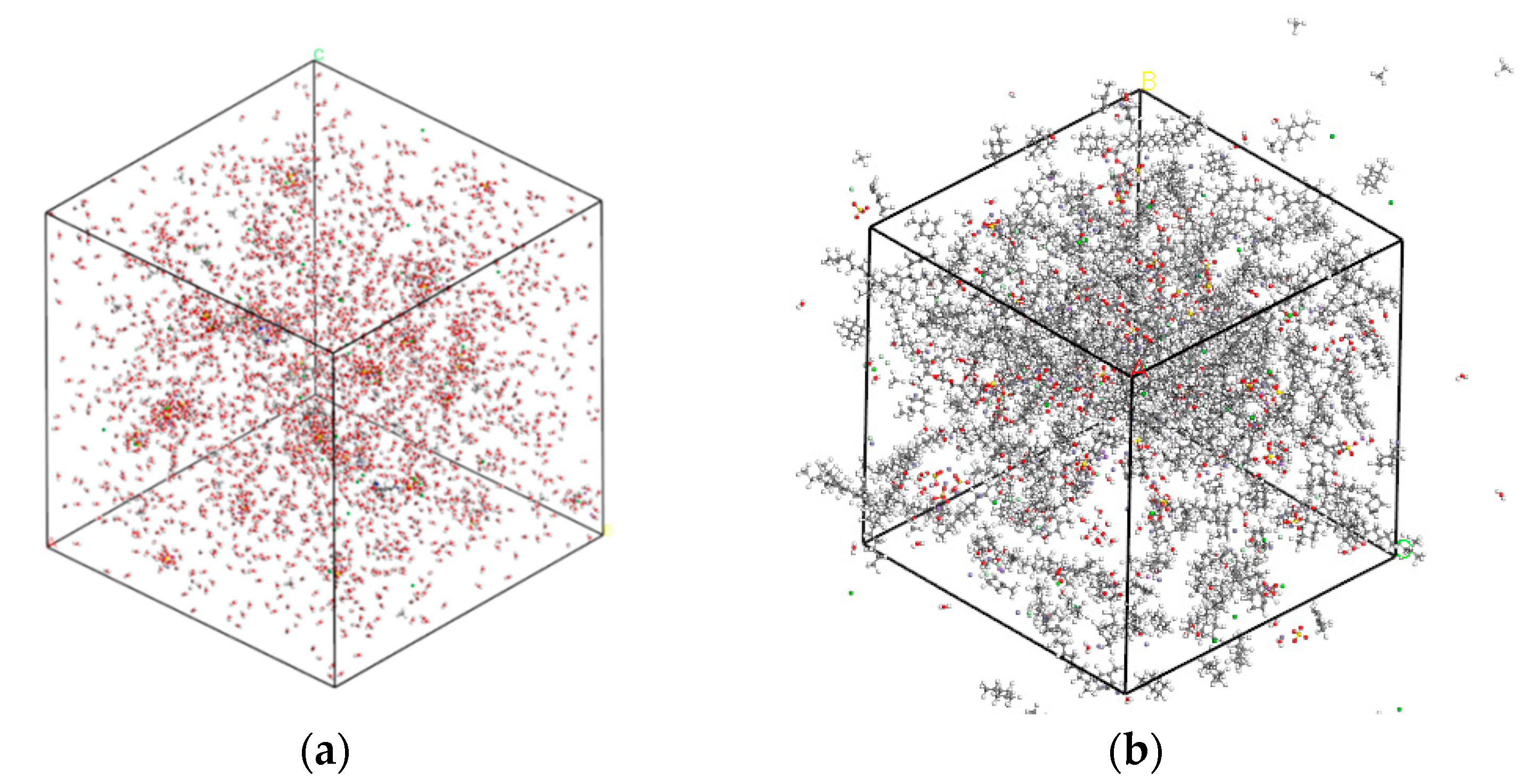
Before establishing the CH4-drilling fluid system, it is necessary to construct water-based/oil-based drilling fluid models. The configuration of the water-based drilling fluid is shown in Figure 1a. It is mainly composed of a water phase, BaSO4 (weighting agents), Fe2O3 (substituted by Fe2+ for modeling purposes), Sodium lignosulfonate (a dispersant, CHNa2OS2), Polyacrylamide (a viscosity-increasing agent, (C3H5NO)ₙ), and water, etc. In this water-based drilling fluid system, there are 10 CH4 molecules, and the total number of molecules is 1044. Figure 1b depicts the configuration of the oil-based drilling fluid, which consists of an oil phase (paraffinic, 0.85 g/cm3 at 20 °C), BaSO4 (weighting agents), Fe2O3 (substituted by Fe2+), Polyoxyethylene lauryl ether (an emulsifier, C12H25O(C2H4O)23H, average EO number 23, HLB 16.9), etc. In this oil-based drilling fluid system, there are 10 CH4 molecules, and the total number of molecules is 843.
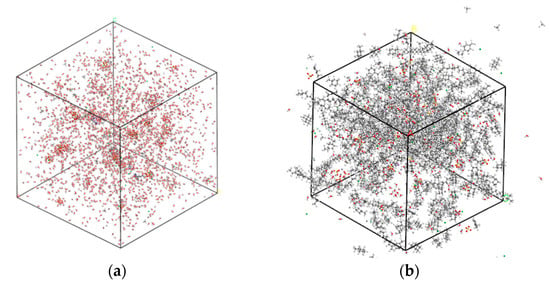
Figure 1.
Drilling fluid model: (a) water-based; (b) oil-based.
As shown in Figure 1, the water-based system is dominated by a polar, hydrogen-bonding continuous phase, where CH4 interacts primarily with structured water clusters; the oil-based system is a non-polar continuous phase where dispersion (van der Waals) interactions prevail. Consequently, the oil-based model will exhibit larger hydrophobic free volumes and weaker electrostatic contributions, whereas the water-based model will show stronger short-range constraints from the hydrogen-bond network. These intrinsic differences lead to the distinct CH4 mobility, diffusion coefficients, and solubility trends that will be reported in Section 3.
2.1.4. Simulation Calculation Process
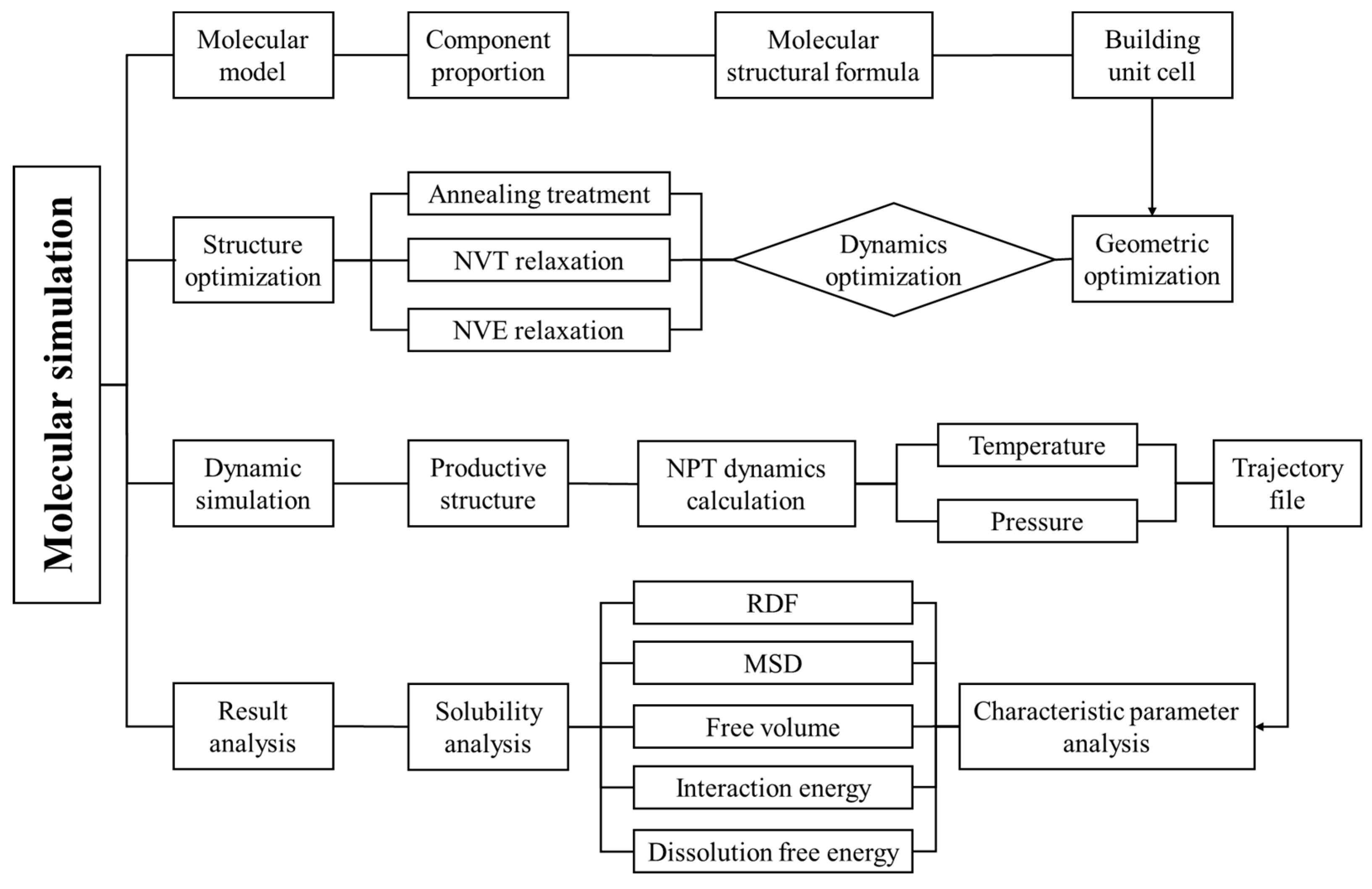
Figure 2 illustrates the molecular dynamics simulation process.
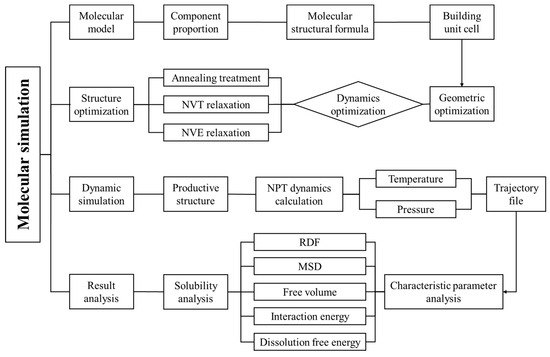
Figure 2.
Schematic diagram of simulation process.
(a) Construct gas–water-based drilling fluid and gas–oil-based drilling fluid unit cells. Perform structural optimization for 10,000 ps and energy minimization on these unit cells.
(b) Annealing treatment is then performed on the optimized structures. The annealing process involves a temperature range of 300–500 K with 5 cycles and a total of 5000 steps.
(c) NVT (constant number of particles, volume, and temperature) dynamic relaxation is conducted for 100 ps at a specified temperature, with one set of data output every 2000 steps.
(d) NVE (constant number of particles, volume, and energy) dynamic relaxation is used to eliminate the influence of the temperature-controlling function on the system.
(e) NPT (constant number of particles, pressure, and temperature) dynamic simulations are carried out for 1000 ps on the equilibrium configurations formed in steps (a)–(d) under different temperatures and pressures. The motion of molecules under different temperatures and pressures is calculated, with a time step of 1 fs and one set of data output every 4000 steps.
This duration of 1000 ps is selected based on convergence tests: (i) the running averages of density and total energy reach stationary plateaus (slope of the moving average over the last 100 ps < 1% of the corresponding grand mean; and (ii) the normalized autocorrelation of the solute–solvent interaction energy decays to the noise level, with an integrated autocorrelation time much shorter than 1000 ps. Production statistics—MSD, free volume, and interaction energy—are accumulated only over the final equilibrium window of the NPT trajectory.
In this research, the COMPASS Ⅱ universal force field was employed to simulate the interactions between gas and drilling fluid. The Nose thermostat method was utilized to ensure that the simulation process remained in thermal equilibrium. The Berendsen barostat method was adopted to stabilize the system pressure. The Ewald method was applied to calculate the long-range interactions between molecules. Additionally, the Particle–Particle Particle–Mesh (PPPM) method was used to compute the electrostatic interaction forces.
For the molecular simulation, the tested temperature range is 30~150 °C, and the pressure range is 10~70 MPa, which roughly corresponds to a maximum well depth of 7000 m and covers most of the conditions encountered during drilling. In the simulation, a total of 30 cases are conducted, with the combination of 5 temperature points (30 °C, 60 °C, 90 °C, 110 °C, 130 °C, 150 °C) and 6 pressure points (10 MPa, 25 MPa, 30 MPa, 40 MPa, 50 MPa, 60 MPa).
Molecular dynamics simulations are carried out using BIOVIA Materials Studio (Forcite MD module) with the COMPASS II force field. Long-range electrostatics are evaluated with the Ewald summation method, consistent with Section 2.1.4. Post-processing of trajectories (MSD, RDF, and free volume) employs the built-in analysis tools in Materials Studio, and figures are produced from the exported data.
2.2. Solubility Experiment
2.2.1. Experimental Device
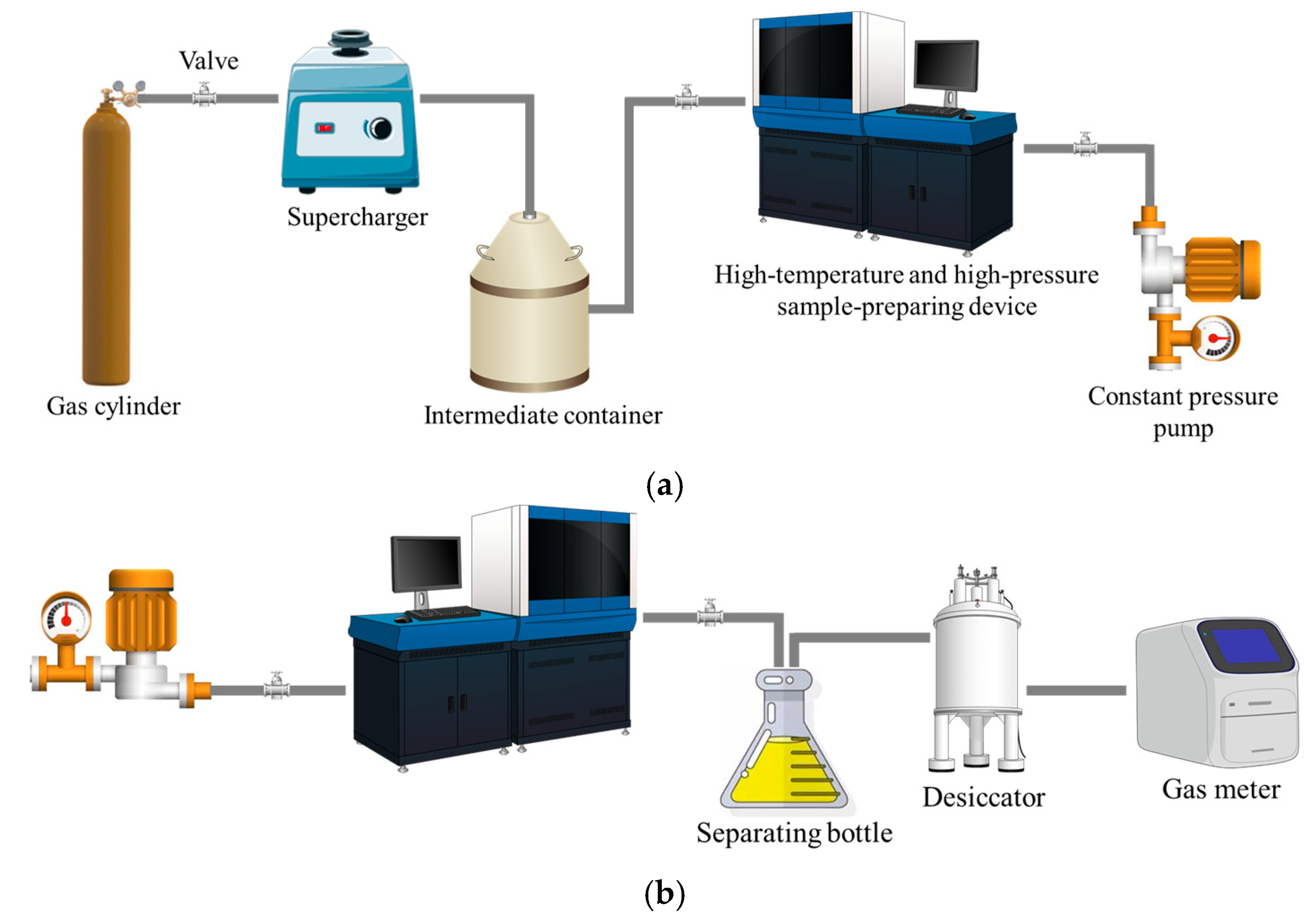
Figure 3 displays the experimental apparatus for measuring the solubility of CH4 in drilling fluids. The entire experimental system can be divided into a reaction unit and a measurement unit, and is composed of several key pieces of equipment, including an intermediate container, a high-pressure displacement pump, a high-temperature and high-pressure sample-preparing device and a gas meter, among others. The solubility experiment is mainly conducted within the high-temperature and high-pressure sample-preparing device. This device is designed to simulate the downhole high-temperature and high-pressure environment, in order to determine the gas solubility behavior under different conditions. The total volume of the sample-preparing device is 2000 mL, and it can withstand a maximum operating pressure of 80 MPa and a maximum temperature of 150 °C. The gas meter has a measurement range of 2000 mL and an accuracy of up to 1 mL. The temperature inside the vessel is maintained and measured using a calibrated PT100 resistance temperature detector (RTD), with an accuracy of ±0.1 °C. The sensor is directly immersed in the drilling fluid, to ensure the reading reflects the actual temperature of the fluid–gas mixture. Temperature is regulated by a proportional–integral–derivative (PID) control system, which stabilizes the setpoint temperature within a narrow range of ±0.5 °C throughout the 20 min equilibrium period. This high precision in temperature control and measurement is essential for ensuring the reliability of the solubility data.
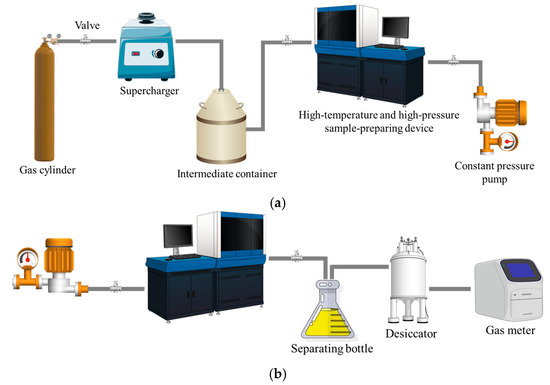
Figure 3.
Diagram of CH4 solubility experimental device: (a) reaction unit; (b) measurement unit.
During the experiment, the time for the gas–liquid mixture under pressure to achieve equilibrium is a key factor that affects the measurement reliability. We have tested the waiting time of 1, 5, 10, 15, 20, 25, 30, and 40 min and found that 20 min are enough to ensure a complete saturating of drilling fluid with CH4. Therefore, for each pressure and temperature condition, the gas and drilling fluid are mixed in the container for 20 min before the mixture is released for further measurement. Five repeating experiments are conducted for each condition, to validate the full saturation of drilling fluid. (If there is insufficient saturation, the underestimated measured solubility will vary.)
After saturation, the saturated gas in the sample-preparing device was released, along with the drilling fluid, and then entered a separation flask. In this process, the gas escaped from the liquid. The escaped gas passed through a drier to remove moisture and then flowed into a gas meter to measure its volume under normal temperature and pressure. Based on this measurement data, the solubility of the gas in the drilling fluid could be calculated (the volume ratio of solution gas to drilling fluid), enabling the analysis of gas solubility characteristics under different experimental conditions. The volume of drilling fluid is calculated by its measured mass and density.
The precision of the final solubility value (0.001 m3/m3) is derived from the combined accuracies of all key measuring instruments involved in the experimental process. The volume of gas liberated from the drilling fluid is measured by the gas meter with an accuracy of ±1 mL. The mass of the drilling fluid sample is determined using a high-precision electronic balance with an accuracy of ±0.01 g. The density of the drilling fluid, essential for converting the sample mass to volume, is measured prior to each experiment using a densitometer with an accuracy of ±0.005 g/cm3. The pressure and temperature within the high-temperature and high-pressure sample-preparing device are maintained and monitored by a high-accuracy pressure transducer (±0.1% FS) and a calibrated PT100 sensor (±0.1 °C), respectively. The high repeatability of the measurements, confirmed by five replicates for each data point, supports the resolution of the reported solubility data.
2.2.2. Experimental Scheme
The experiment tested the solubility of CH4 in water-based and oil-based drilling fluids under different temperature and pressure conditions. The drilling fluids in the experiment were prepared according to the formulations used at shale gas drilling sites. Oil-based drilling fluid was mainly composed of white oil (paraffinic, supplier Sinopec Sichuan Branch, Chengdu, China, 0.85 g/cm3 at 20 °C), weighting agents (barium sulfate and iron oxide, supplier PetroChina Chuanqing Drilling Company, Chengdu, China, purity > 99.9%), emulsifiers (Polyoxyethylene lauryl ether, C12H25O(C2H4O)23H, supplier Sinopec Sichuan Branch, Chengdu, China, average EO number 23, HLB 16.9, purity >99%), etc. Water-based drilling fluid was primarily composed of water, weighting agents, sodium lignosulfonate (CHNa2OS2, supplier Sinopec Sichuan Branch, Chengdu, China, purity >95%), polyacrylamide ((C3H5NO)ₙ, supplier Sinopec Sichuan Branch, Chengdu, China, purity >99.9%), etc. CH4 was sourced from industrial gas supplies, with a purity of 99.99%.
Taking into account the geological conditions of the target block, the actual wellbore conditions and the feasibility of experimental procedures, 7 sets of experimental temperatures (30 °C, 50 °C, 70 °C, 80 °C, 90 °C, 110 °C, 120 °C) and 8 sets of experimental pressures (10 MPa, 20 MPa, 30 MPa, 40 MPa, 45 MPa, 50 MPa, 55 MPa, 60 MPa) were tested. The study aimed to investigate the variation patterns of CH4 solubility in drilling fluids under varying temperature and pressure conditions.
3. Results and Discussion
3.1. Effect of Drilling Fluid System on CH4 Dissolution Characteristics
3.1.1. Molecular Motion Trajectory
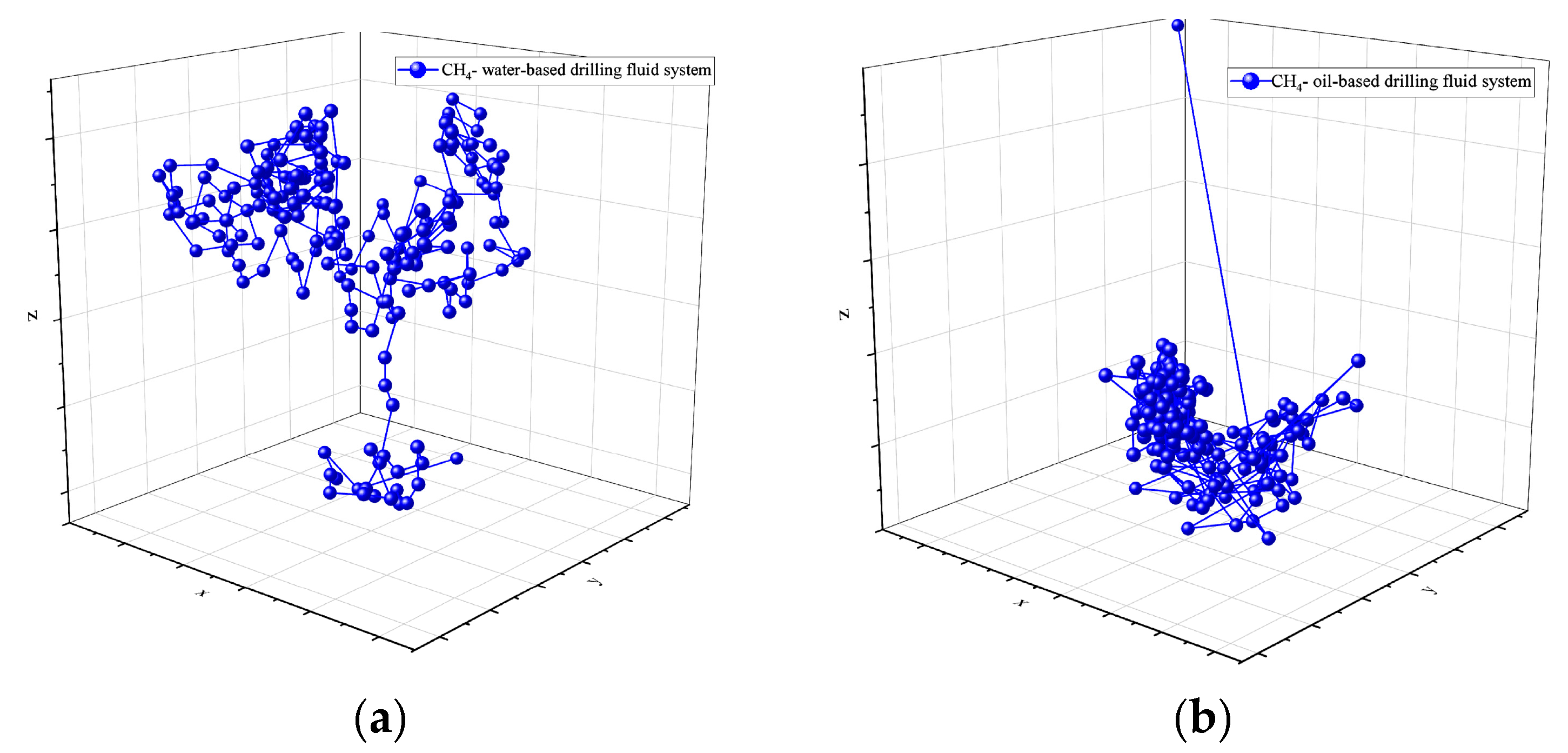
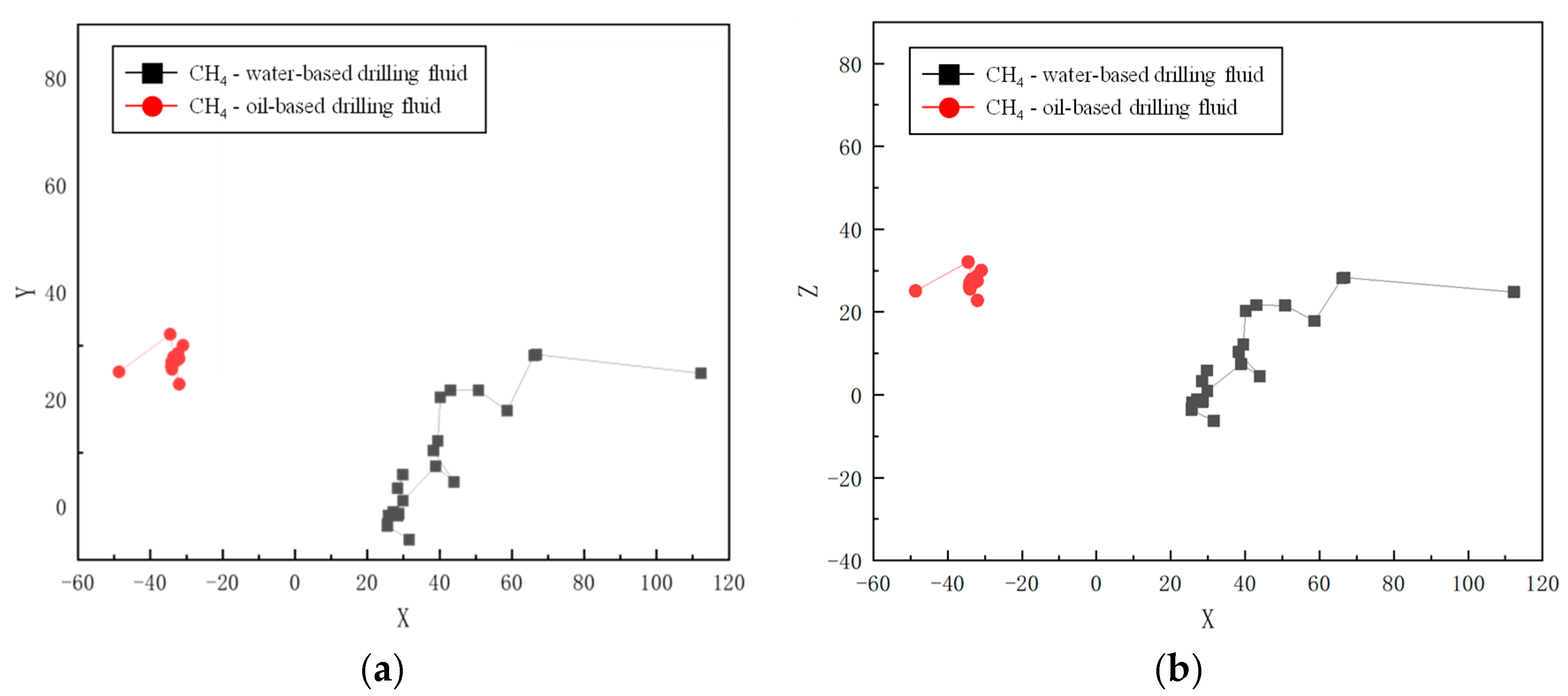
Molecular dynamics simulations offer a detailed analysis of the spatial motion trajectories of CH4 molecules in both water-based and oil-based drilling fluids. The simulation results at 30 °C and 70 MPa (as shown in Figure 4 and Figure 5) reveal an obvious “hopping” diffusion behavior from both planar (X-Y, X-Z) and spatial motion trajectories. That is, gas molecules repeatedly hop and diffuse through the voids in the solvent. This phenomenon reflects the interaction between the diffusion ability of gas molecules and the void distribution in the solvent. In water-based drilling fluids, CH4 molecules exhibit a relatively large diffusion range, indicating that the voids in the solution are relatively sparse and widely distributed. The larger voids allow gas molecules to migrate more freely, suggesting that the structural characteristics of the solvent contribute to enhancing the molecular mobility. In contrast, in oil-based drilling fluids, the diffusion range of CH4 molecules is restricted, indicating that the voids in the solvent are denser and more localized, thus limiting the free movement of CH4 molecules. These results emphasize the crucial role of the solvent’s microstructure in CH4 solubility and diffusion, and are of great significance for revealing the gas–liquid interactions in drilling fluids.
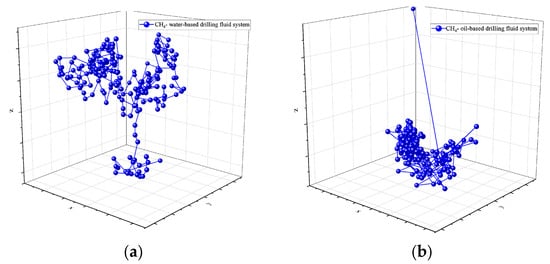
Figure 4.
Spatial trajectory of CH4 in drilling fluid: (a) water-based drilling fluid; (b) oil-based drilling fluid.
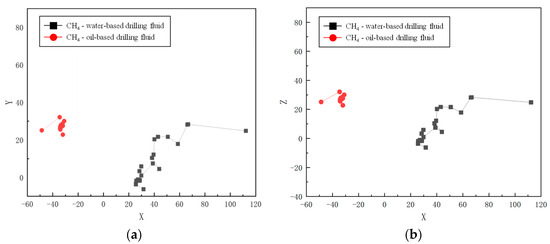
Figure 5.
Movement track of CH4 in drilling fluid: (a) X-Y plane; (b) X-Z plane.
3.1.2. Mean Squared Displacement
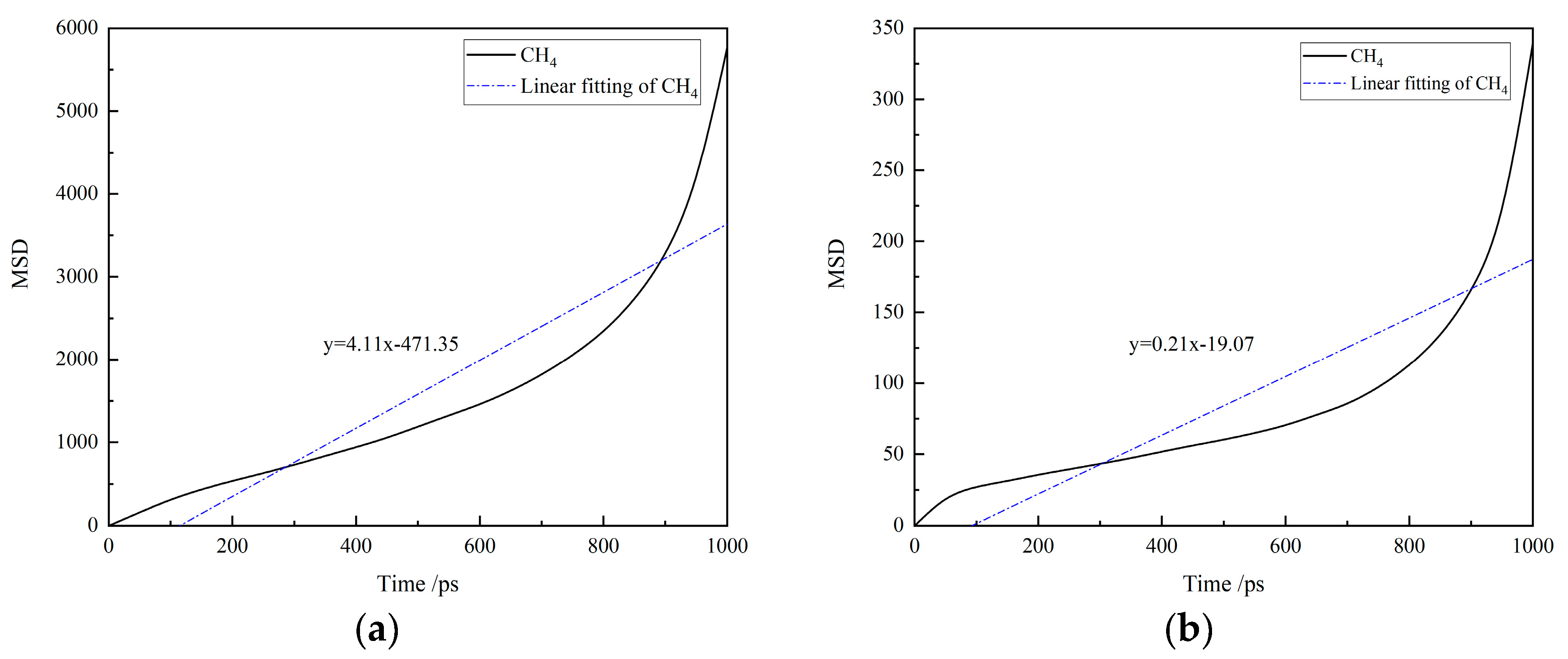
The MSD curves of CH4 in water-based and oil-based drilling fluids at 30 °C and 70 MPa are presented in Figure 6. The diffusion coefficients of CH4, which are the slopes of the fitted straight lines, are shown in Table 4. It can be observed that the diffusion coefficient of CH4 in water-based drilling fluids is significantly higher than that in oil-based drilling fluids. This indicates that CH4 molecules have a stronger diffusion ability in water-based drilling fluids, exhibiting a broader range of motion, which is closely related to its higher diffusion coefficient. Further, combined with the molecular motion trajectories revealed by molecular dynamics simulations, water-based drilling fluids provide a more expansive space for molecular movement, explaining why the diffusion range of CH4 is greater in the water-based system compared to the oil-based system. Moreover, due to the higher diffusion coefficient of water-based drilling fluids, under different temperature and pressure conditions, the system of CH4 and water-based drilling fluids can reach solubility equilibrium more rapidly in solubility experiments.
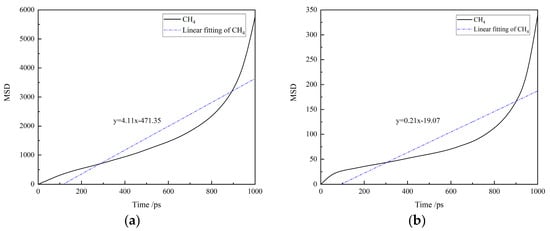
Figure 6.
The MSD curves of CH4 in drilling fluid: (a) water-based drilling fluid; (b) oil-based drilling fluid.

Table 4.
Diffusion coefficient of CH4 in water-based/oil-based drilling fluid.
3.1.3. Radial Distribution Function
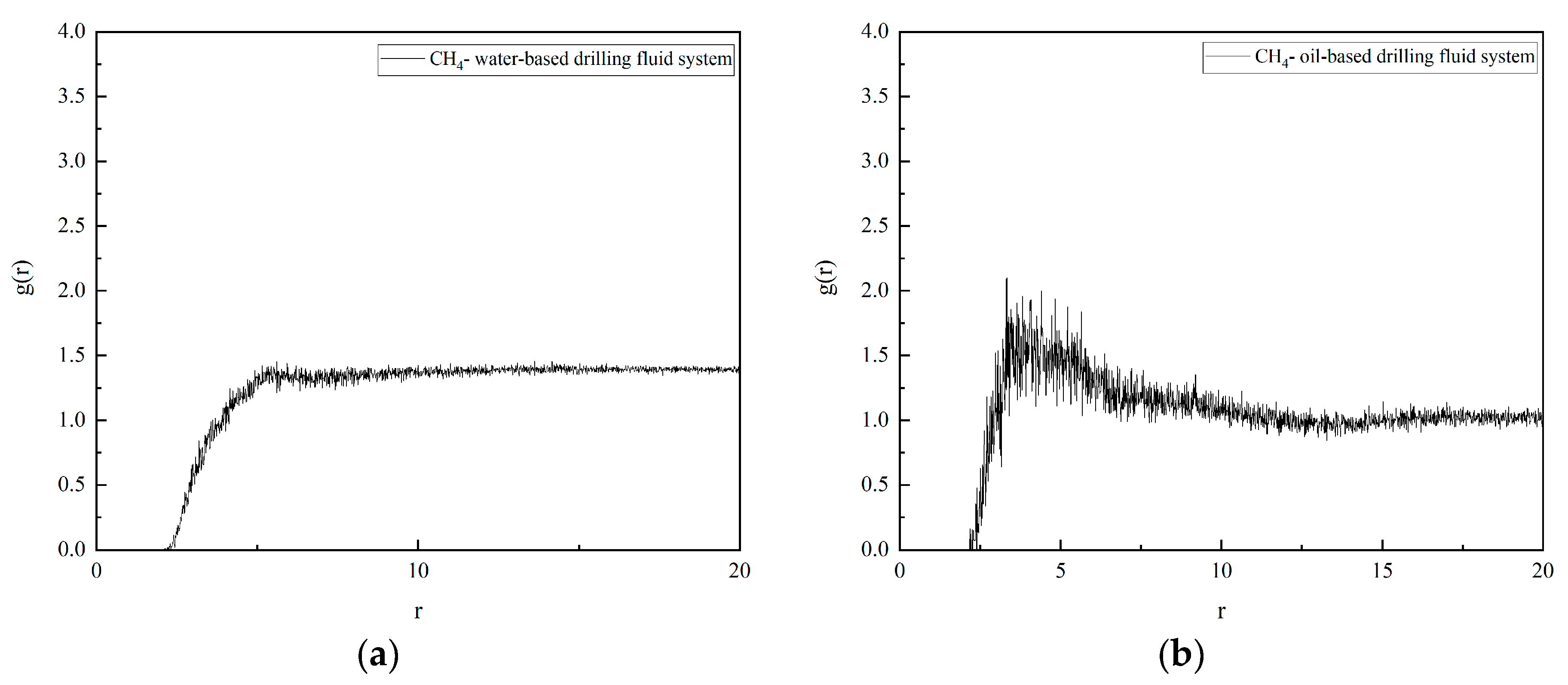
The radial distribution function (RDF) curves of CH4 and water molecules (H2O) in different systems are shown in Figure 7. By examining the RDF curves, it is evident that they exhibit a single-peak shape. This implies that in oil-based drilling fluids, an aggregation layer of water molecules forms around CH4 molecules, indicating the presence of a stable hydration layer. In contrast, the RDF curve in water-based drilling fluids shows no distinct peaks or troughs, suggesting that there is no significant interaction between CH4 molecules and water molecules in water-based drilling fluids, and no solvation structure is formed.
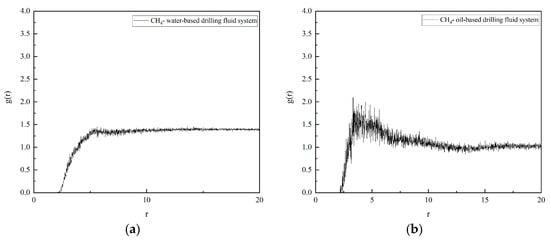
Figure 7.
PDF of CH4 in drilling fluid: (a) water-based drilling fluid; (b) oil-based drilling fluid.
3.1.4. Free Volume Fraction of CH4 in Different Drilling Fluid Systems
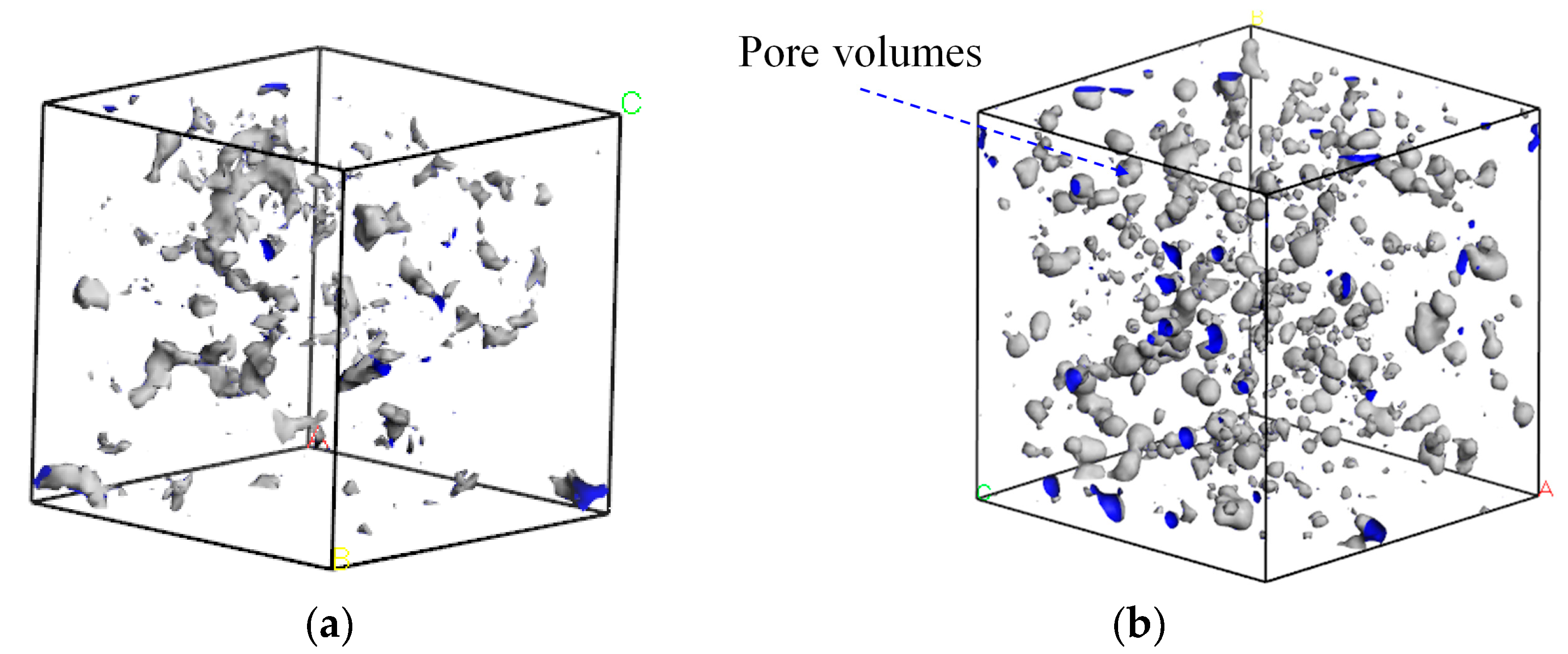
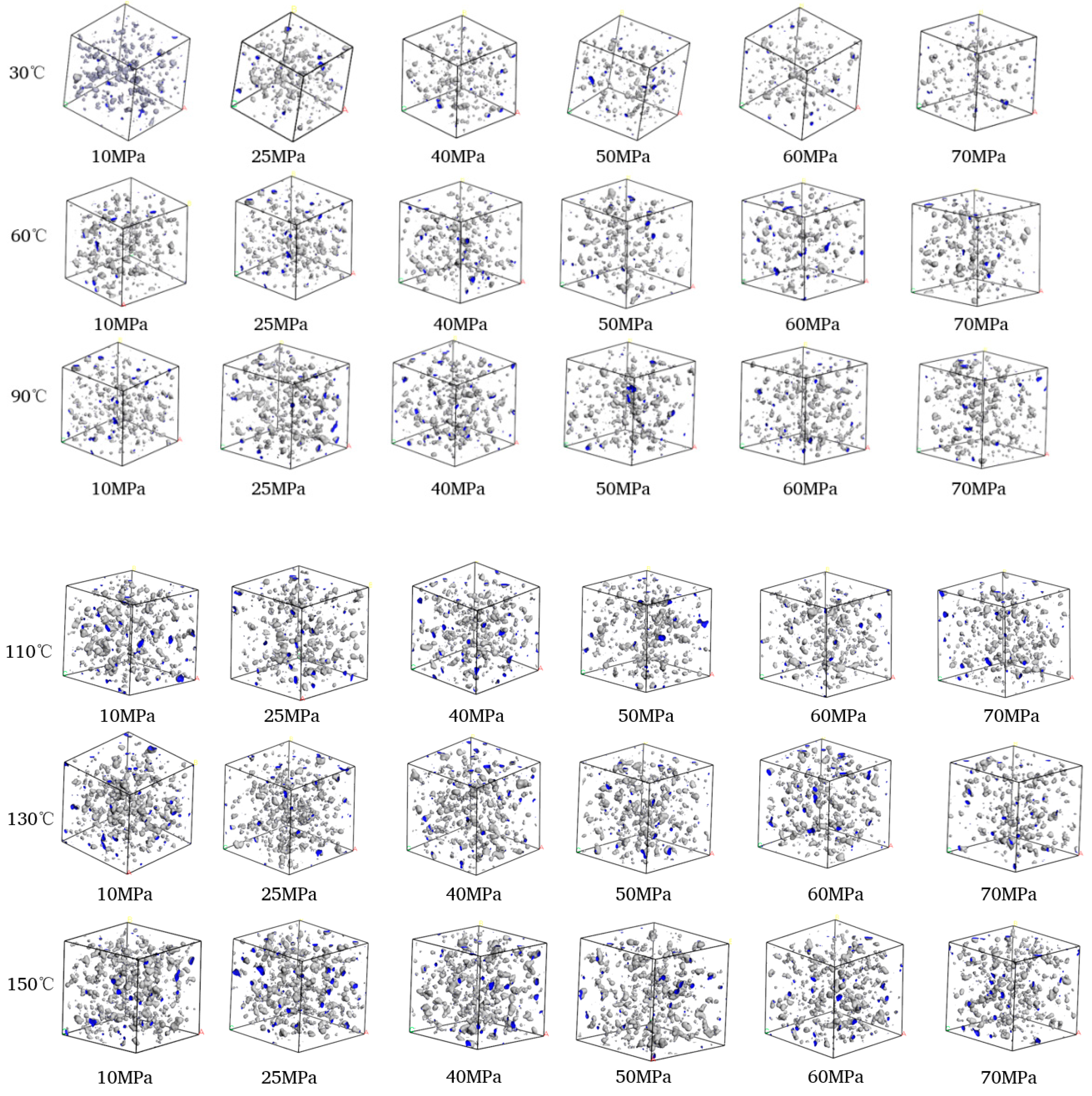
The CH4-drilling fluid model was scanned using the scanning probe method, with the kinetic radius of methane molecules (0.2–0.21 nm) serving as the hard-sphere probe (as shown in Figure 8). The free volume within each system was estimated to calculate the free volume fraction, thereby enabling a microscopic analysis of solubility. Based on the analysis results, it was found that there were fewer fillable voids in water-based drilling fluids, and these voids were more dispersed in distribution. In contrast, oil-based drilling fluids had more concentrated voids.
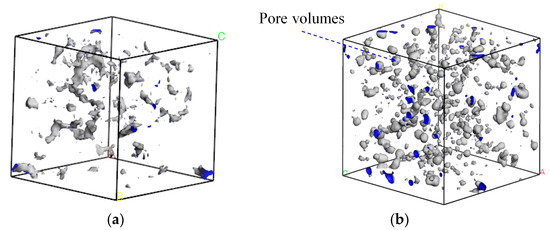
Figure 8.
Free volume distribution of CH4-drilling fluid: (a) water-based drilling fluid; (b) oil-based drilling fluid.
3.1.5. Interaction Energy of CH4 in Different Drilling Fluid Systems
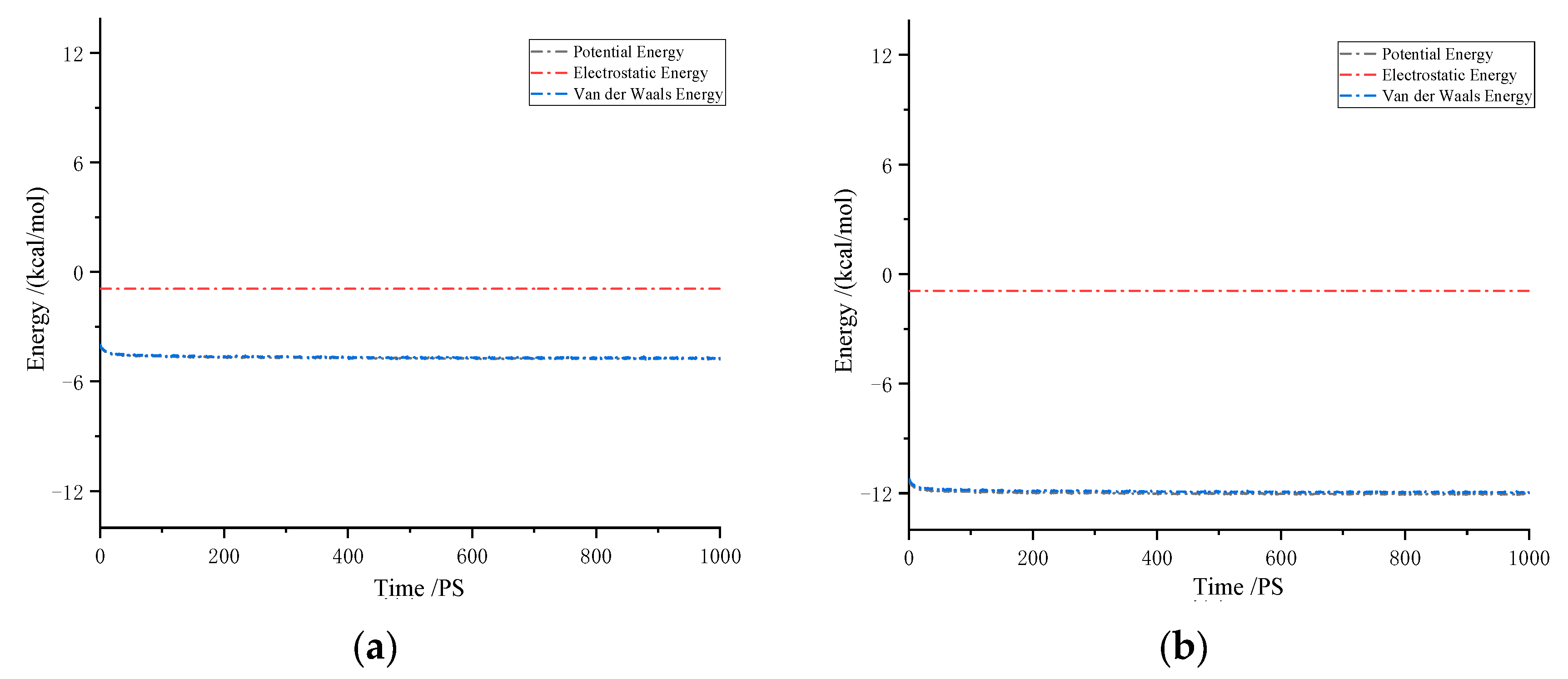
Figure 9 illustrates the interaction energies between CH4 molecules and solutes in drilling fluid. The results indicate that van der Waals forces are the dominant interactive forces, while the contribution of electrostatic forces is almost negligible. In the system, the interaction energies between CH4 molecules are negative, suggesting a significant attractive interaction between gas molecules and solute molecules. The more negative the interaction energy, the stronger the attraction. In particular, in oil-based drilling fluids, the interaction force between CH4 and the drilling fluid is more pronounced. This strong attraction effectively promotes an increase in gas solubility. The molecular structure and interactive forces in oil-based drilling fluids are more complex than those in water-based drilling fluids. This complexity enhances the attraction between gas molecules and liquid molecules, thereby improving the gas’s solubility in the liquid.
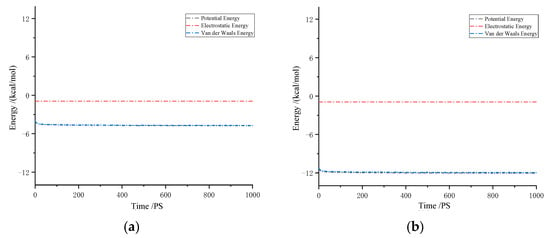
Figure 9.
Interaction energy of CH4 in drilling fluid: (a) water-based drilling fluid; (b) oil-based drilling fluid.
3.1.6. Dissolution Free Energy of CH4 in Different Drilling Fluid Systems
As shown in Table 5, the solubility free energy of CH4 molecules in the drilling fluid is predominantly governed by van der Waals interactions. The data indicate that the energy required for CH4 molecules to dissolve in water-based drilling fluids is the highest, while the energy required in oil-based solutions is lower. This suggests that the dissolution process of CH4 molecules is more favorable in oil-based drilling fluids than in water-based drilling fluids.

Table 5.
Dissolution free energy of single CH4 molecules (30 °C, 70 MPa).
3.2. Effect of Temperature and Pressure on CH4 Dissolution Characteristics
The aforementioned simulation results demonstrate that, under the same temperature and pressure, there are significant differences in motion trajectories, MSD, and RDF, between water-based and oil-based drilling fluids. These differences may be closely related to variations in temperature and pressure. Therefore, this paper will delve into the changes in free volume, interaction energy and solubility free energy of CH4 in drilling fluids at different temperatures and pressures.
3.2.1. Free Volume Variation of CH4 at Different Temperatures and Pressures
- (1)
- CH4-water based drilling fluid system
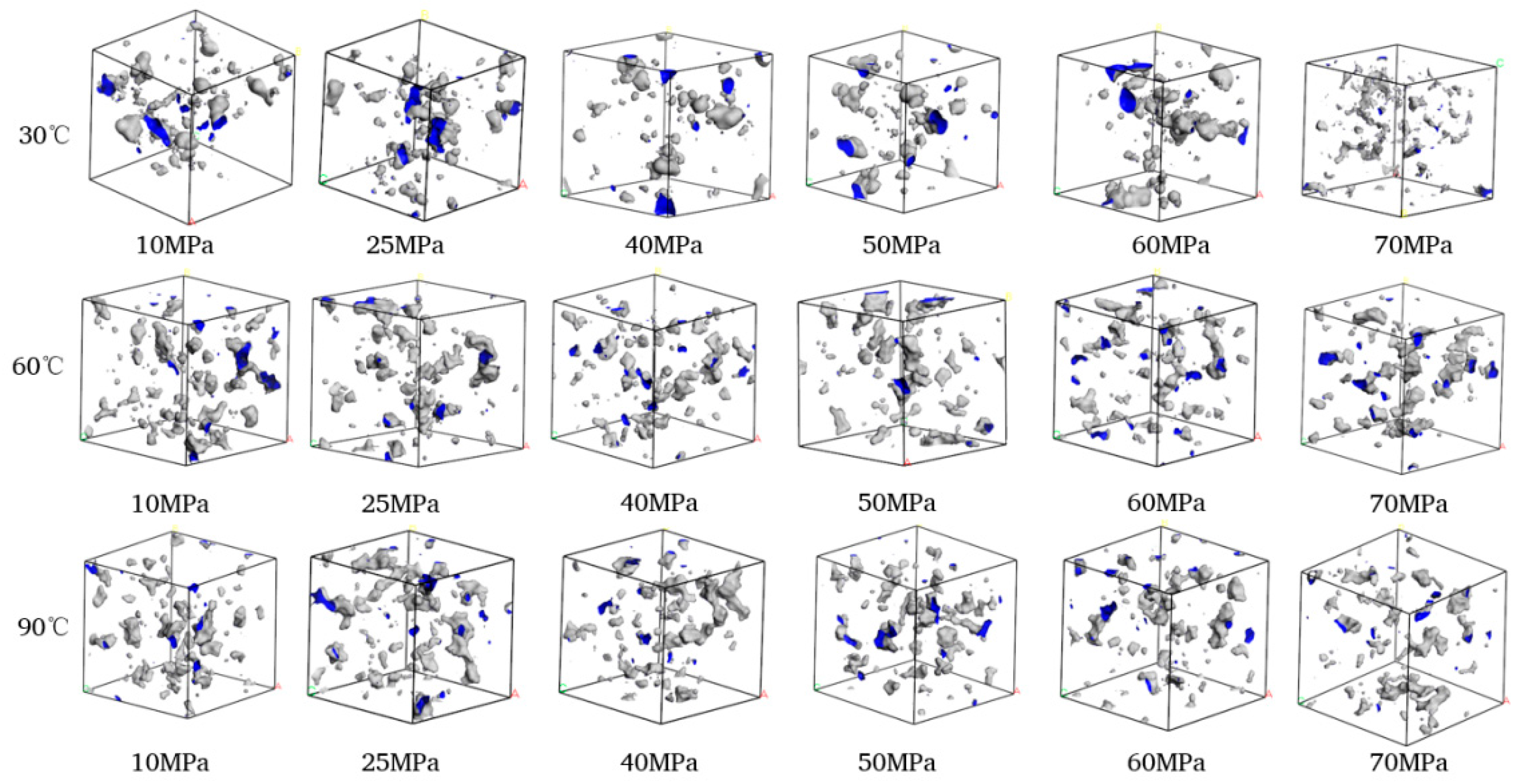
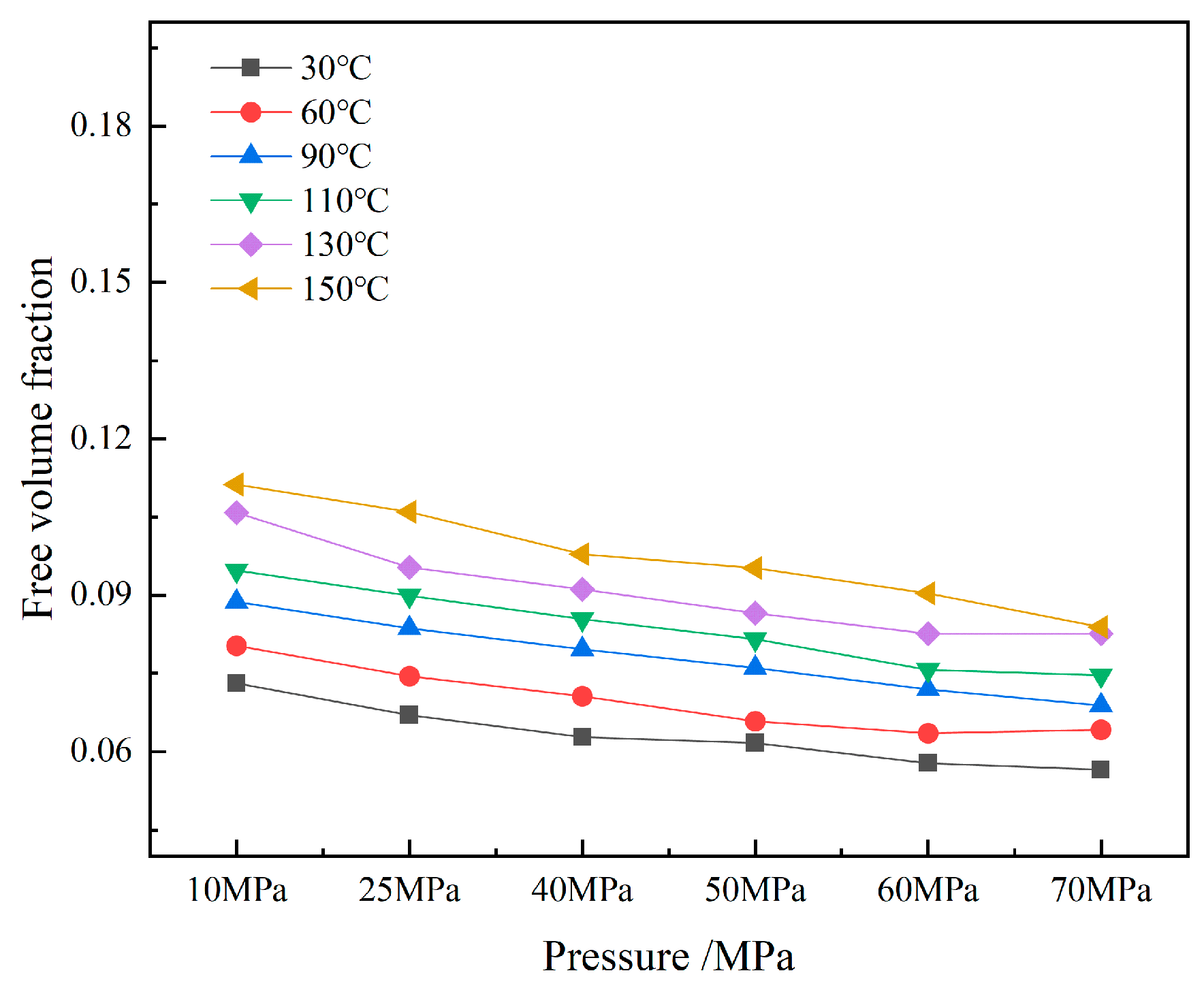
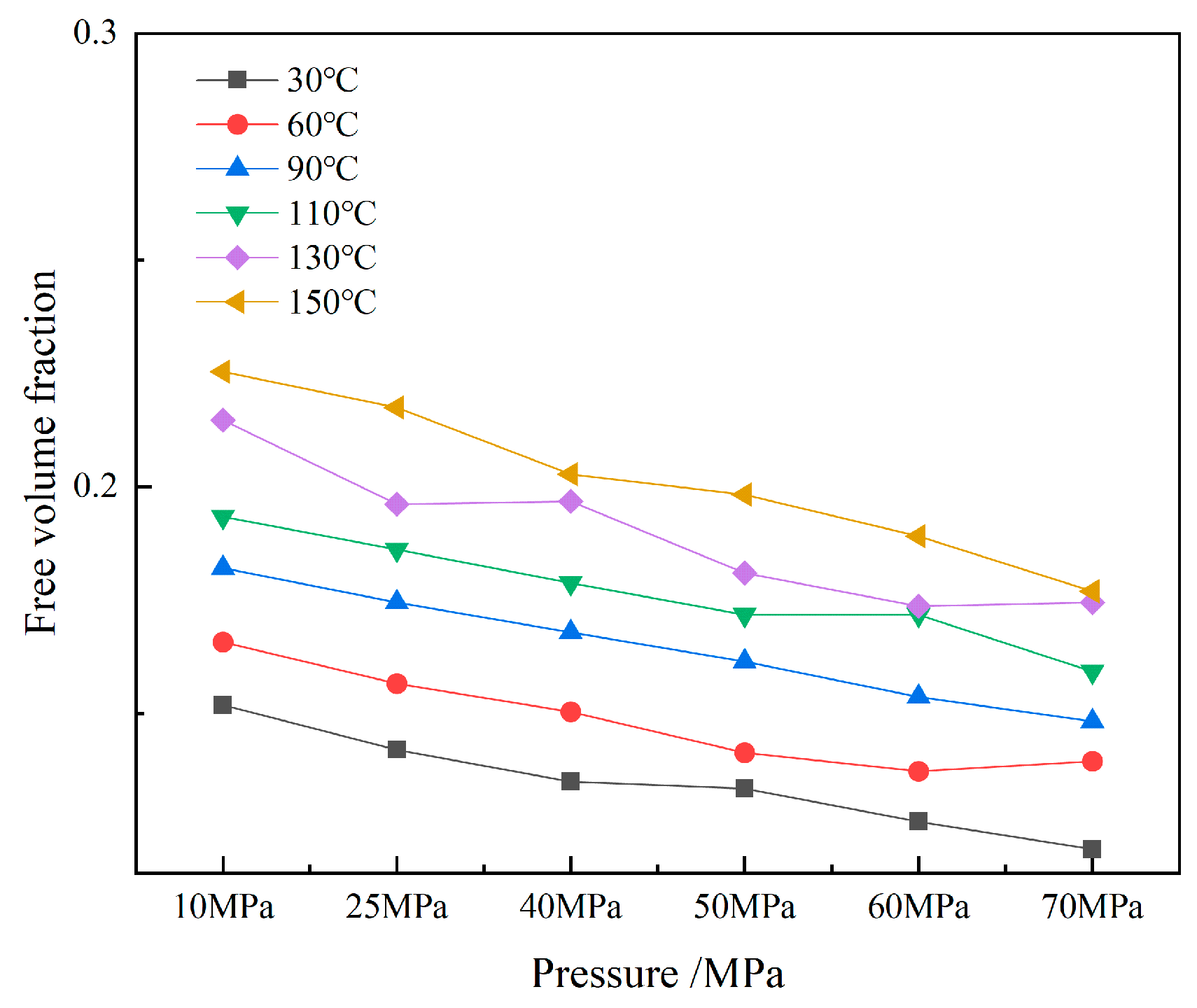
The volume distributions of various components in the CH4-water-based drilling fluid system unit under different temperature and pressure are presented in Table 6. Figure 10 displays the free volume distribution of the CH4-water-based drilling fluid system unit under varying pressure and temperature. Figure 11 shows the trend of changes in the free volume fraction. The results indicate that temperature has a particularly significant impact on the free volume fraction. As the temperature rises, the thermal motion of molecules intensifies, leading to an increase in the intermolecular spacing and a notable rise in the free volume fraction. Under high temperature, the intermolecular forces weaken, and the overall available space in the system increases, which is conducive to gas diffusion and solubility. In contrast, when the pressure increases, the distance between molecules decreases, and the system tends to be more closely packed, resulting in a slight decrease in the free volume fraction. The synergistic effect of temperature and pressure directly influences the microscopic structure of the system and the gas solubility behavior.

Table 6.
Volumes of CH4-water-based drilling fluid system units at different temperatures and pressures.
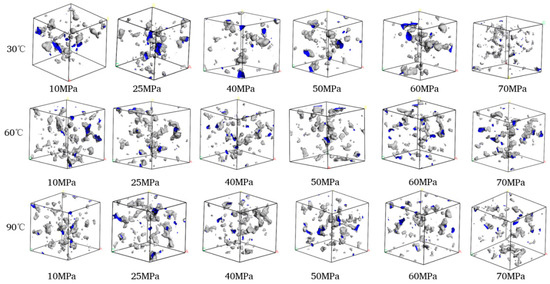
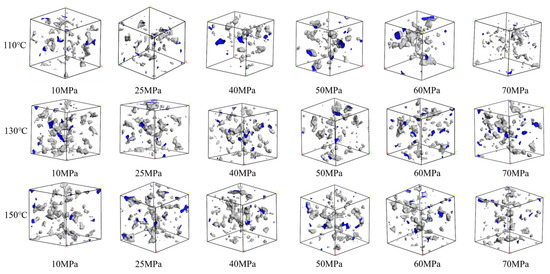
Figure 10.
Comparison diagram of unit free volume distribution of CH4-water based drilling fluid system at different pressures and temperatures.
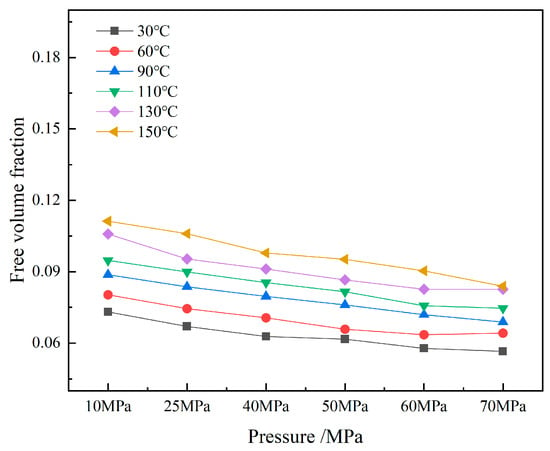
Figure 11.
Free volume fraction of CH4-water-based drilling fluid system unit.
- (2)
- CH4-oil based drilling fluid system
The volume distributions of various components in the CH4-oil-based drilling fluid system unit under different temperature and pressure are shown in Table 7. The data in the table indicate that, under a system model with the same number of atoms, changes in temperature and pressure significantly affect the volume distribution of the system unit, leading to variations in different volumes. These changes are mainly manifested in the different responses of the total volume, the occupied volume and the free volume. Specifically, when the temperature is held constant, as the pressure increases, the total volume, the occupied volume and the free volume of the system unit gradually decrease. This is because under high pressure, the distance between gas molecules shrinks, and the repulsive forces between molecules decrease, resulting in the compression of the overall volume. On the other hand, when the temperature is kept constant, the kinetic energy of gas molecules increases at high temperatures, and the intermolecular motion intensifies, causing the volume to gradually increase. Under constant pressure, when the temperature rises, the movement speed of gas molecules accelerates, leading to an increase in intermolecular interactions and the range of motion. As a result, the volume of the system unit gradually expands. This phenomenon is primarily due to the increase in intermolecular collisions and expansion, which leads to an increase in the free volume.

Table 7.
Volumes of CH4-oil-based drilling fluid system units at different temperatures and pressures.
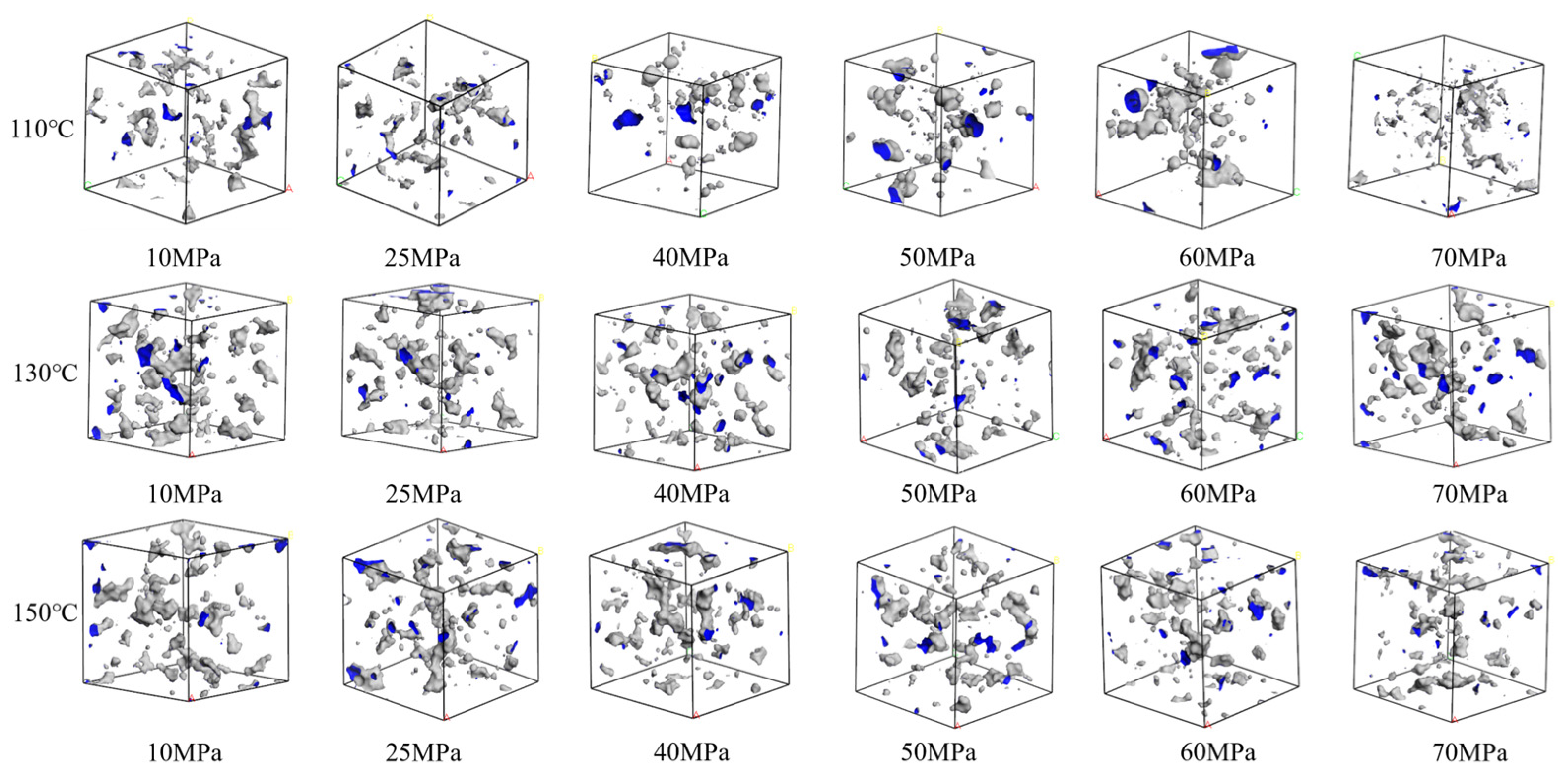
Figure 12 illustrates the free volume distribution of the CH4-oil-based drilling fluid system unit under varying pressure and temperature. These data indicate that changes in temperature and pressure have a highly significant impact on the free volume. At low temperatures, gas molecules are more stable, and the free space between molecules is relatively small. In contrast, at high temperatures, the motion of gas molecules intensifies, leading to an increase in the free volume. Figure 13 presents the trend of changes in the free volume fraction. Results shows that temperature has a particularly pronounced effect on the free volume fraction. As the temperature rises, the free volume fraction increases significantly. This suggests that when the temperature increases, molecular motion becomes more vigorous, and the intermolecular forces between gas molecules weaken, thereby increasing the free volume. Conversely, when the pressure rises, the free volume fraction decreases slightly, mainly because gas molecules are compressed under high pressure, resulting in a reduction in the free volume.
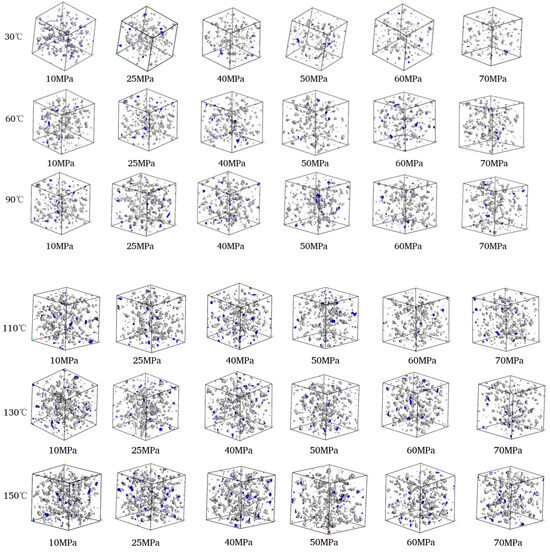
Figure 12.
Comparison diagram of unit free volume distribution of CH4-oil-based drilling fluid system at different pressures and temperatures.
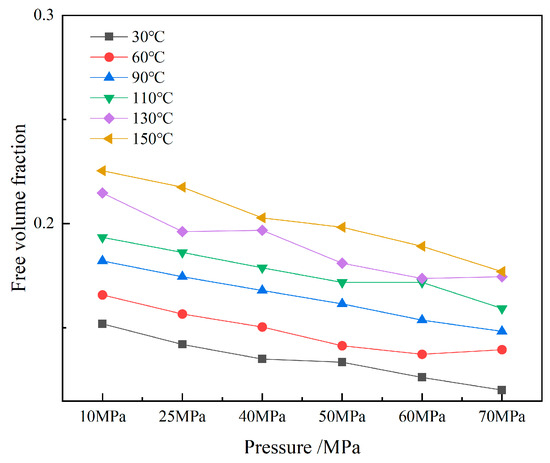
Figure 13.
Free volume fraction of CH4-oil-based drilling fluid system unit.
Temperature and pressure have a significant impact on the free volume of the CH4-drilling fluid system. Although there are certain differences in the solubility of CH4 molecules in different types of drilling fluids, the mechanisms are basically the same. Results indicate that temperature has a more sensitive effect on the solubility of CH4 molecules in drilling fluids compared to pressure. Generally, as the temperature rises, the solubility tends to increase. This is primarily because when the temperature increases, the thermal motion of molecules intensifies, enhancing the diffusion ability of gas molecules in the liquid. Meanwhile, the binding effect of the liquid on gas molecules is reduced, thereby improving the gas’s solubility. In contrast, the influence of pressure changes on gas solubility is relatively small. Especially under low temperature conditions, the response of gas solubility to pressure changes is rather weak. However, under high temperature conitions, as the pressure increases, the intermolecular forces among gas molecules strengthen, making it easier for some gas molecules to aggregate, which leads to a slight decrease in solubility.
An analysis of the variation patterns of free volume reveals that free volume is not the primary factor determining the differences in gas solubility. Free volume generally refers to all the available void spaces in a solution that are larger than, or equal to, the size of gas molecules, but this does not directly determine the actual solubility of gases. Although free volume fluctuates with changes in temperature and pressure, its influence on gas solubility behavior is relatively limited. This indicates that the dissolution process of gases in liquids is not only affected by free volume, but also jointly influenced by factors such as solvent–solute interactions, molecular polarity, van der Waals forces, and hydrogen bonds.
In conclusion, although changes in free volume can have a certain impact on gas diffusion and solubility, it is not the core factor determining gas solubility. The gas solubility is mainly influenced by a combination of factors, including intermolecular forces and the solubilizing capacity of the liquid, as well as the temperature and pressure of the system.
3.2.2. Interaction Energy Variation in CH4 at Different Temperatures and Pressures
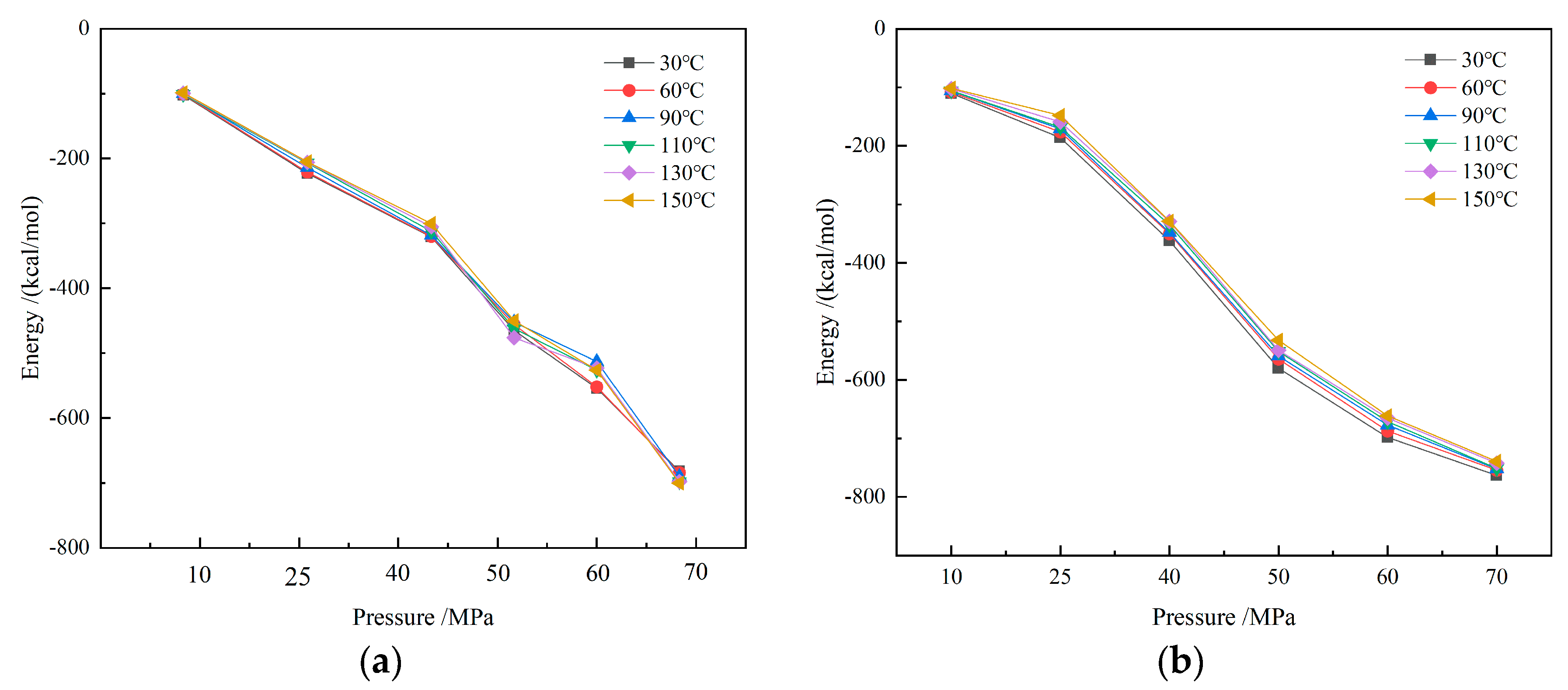
Under different temperature and pressure conditions, the interaction energies of the CH4-drilling fluid system are shown in Figure 14. It can be observed that the interaction energies of CH4-drilling fluid are negative values, indicating that there is a significant attractive force between gas molecules and drilling fluid molecules. The more negative the interaction energy, the stronger the attractive force, which helps to increase the gas solubility in the drilling fluid. As the pressure increases, gas molecules are further compressed, and the intermolecular spacing decreases, leading to an enhanced interaction between gas molecules and drilling fluid molecules and an increase in the absolute value of the interaction energy. The strengthening of the attractive force encourages more gas molecules to dissolve in the drilling fluid, so the solubility increases significantly with rising pressure. This trend suggests that under high pressure, the drilling fluid’s ability to dissolve gases is significantly enhanced. Especially in deep-well and ultra-deep-well drilling operations, the high-pressure environment causes most of the methane to dissolve in the oil-based drilling fluid, increasing the difficulty of overflow monitoring.
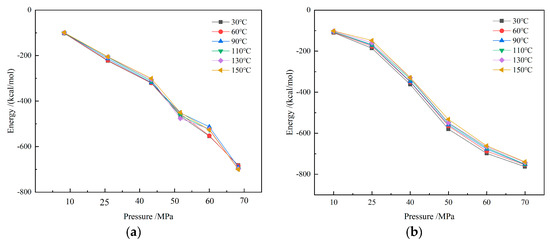
Figure 14.
Interaction energy of CH4 drilling fluid system: (a) water-based drilling fluid; (b) oil-based drilling fluid.
In contrast, temperature has a relatively minor impact on the interaction energy. When the temperature rises, the thermal motion of molecules intensifies, and some gas molecules gain sufficient energy to overcome the intermolecular attractive forces, resulting in a slight decrease in the interaction energy. However, this effect is relatively weak, and does not significantly alter the overall solubility trend of gases. Therefore, in actual drilling processes, although changes in temperature may affect the diffusion rate and flow characteristics of gases, pressure remains the primary factor influencing gas solubility.
3.2.3. Dissolution Free Energy Variation of CH4 at Different Temperatures and Pressures
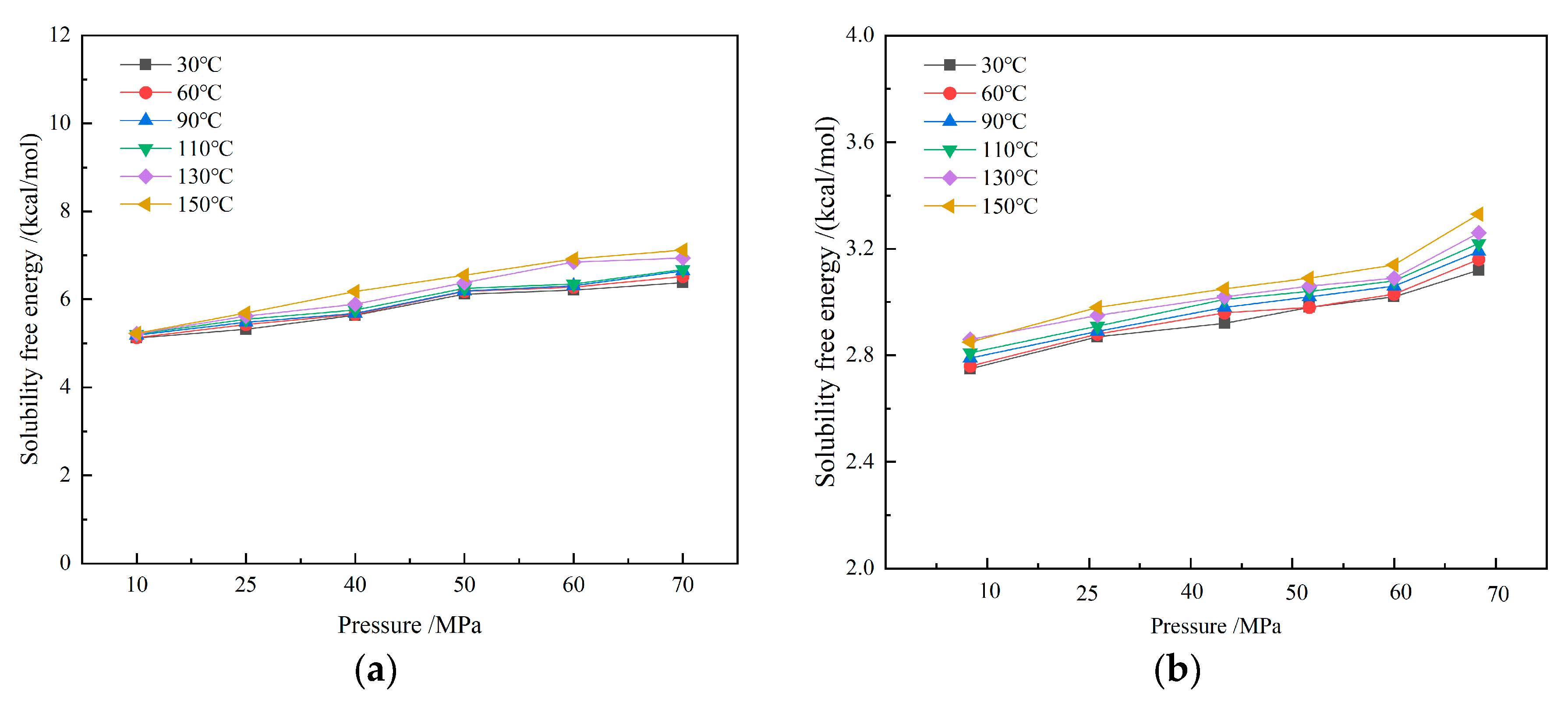
The solubility free energy of CH4 molecules in drilling fluid increases with rising pressure, while the influence of temperature is relatively small, showing an overall slight upward trend. Solubility free energy reflects the energy required for a gas to transition from the gaseous state to the dissolved state. As both temperature and pressure increase, the solubility free energy rises, indicating that the dissolution process becomes more difficult. However, the gas solubility still increases significantly with rising pressure (as shown in Figure 15). Therefore, solubility free energy is not the main factor contributing to differences in gas solubility.
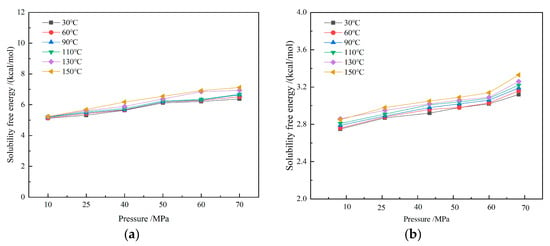
Figure 15.
Dissolution free energy of CH4-drilling fluid system: (a) water-based drilling fluid; (b) oil-based drilling fluid.
At the microscopic level, pressure enhances the interaction energy between solute and solvent molecules, thereby increasing the driving force for dissolution. When pressure rises, the attractive forces strengthen, significantly improving gas solubility. The influence of temperature on gas solubility is mainly realized through changes in free volume. As temperature increases, the free volume expands. Although the fluctuations in free volume have a limited impact on solubility, their effect becomes more pronounced under high solubility. At low pressures, solubility is not sensitive to temperature changes. At high pressures, an increase in temperature promotes an increase in solubility. Despite the fact that the solubility free energy increases with rising temperature and pressure, and the work required for dissolution also rises, the change in solubility free energy is relatively small, compared to the interaction energy and free volume. Therefore, its impact on the gas dissolution process is limited.
3.3. Test Results of CH4 Solubility in Drilling Fluid
3.3.1. Water-Based Drilling Fluid
The solubility of CH4 in water-based drilling fluid at different temperatures and pressures is shown in Table 8.

Table 8.
Solubility of CH4 in water-based drilling fluid at different temperatures and pressures/(m3/m3).
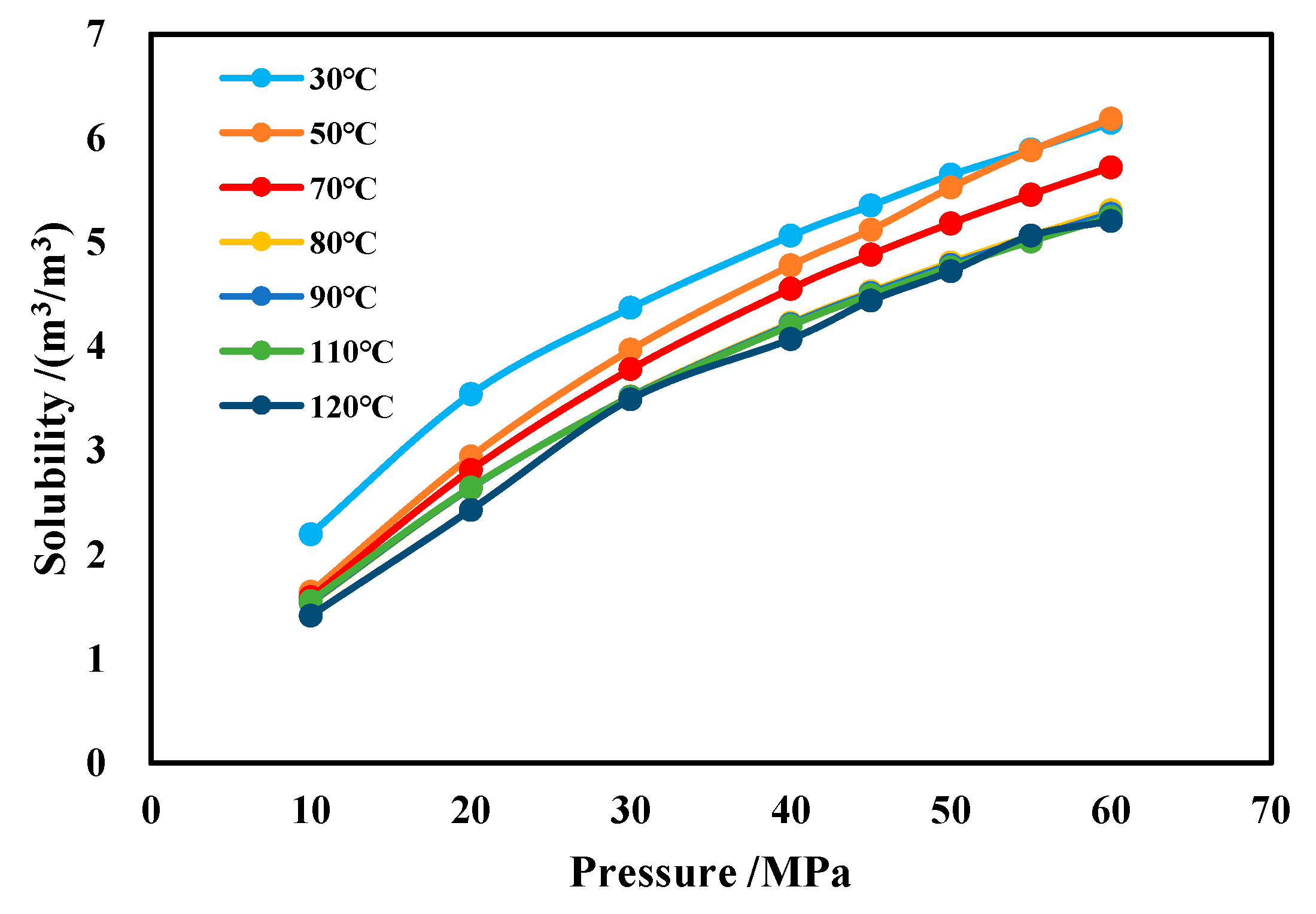
The effects of temperature and pressure on CH4 solubility are illustrated in Figure 16. It can be observed that, under the same conditions, the solubility of CH4 increases with rising pressure and decreases slightly with increasing temperature.
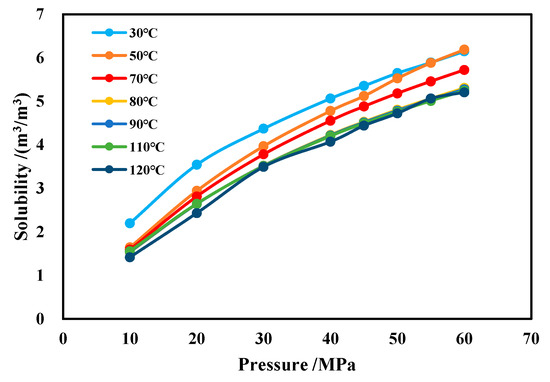
Figure 16.
Solubility of CH4 in water-based drilling fluid.
The methane solubility formula in water-based drilling fluid obtained through polynomial nonlinear fitting is shown in Equation (10). When applying this formula, the temperature (in °C) must first be input to determine the polynomial coefficients a, b, and c. These coefficients are then substituted into the formula, along with the pressure (in MPa), to calculate methane solubility under specific temperature–pressure conditions. This formula demonstrates an agreement rate exceeding 97.7% with experimental results.
where y is methane solubility, m3/m3; P is ambient pressure, MPa; and T is ambient temperature, °C.
3.3.2. Oil-Based Drilling Fluid
The solubility of CH4 in oil-based drilling fluid at different temperatures and pressures is shown in Table 9.

Table 9.
Solubility of CH4 in oil-based drilling fluid at different temperatures and pressures/(m3/m3).
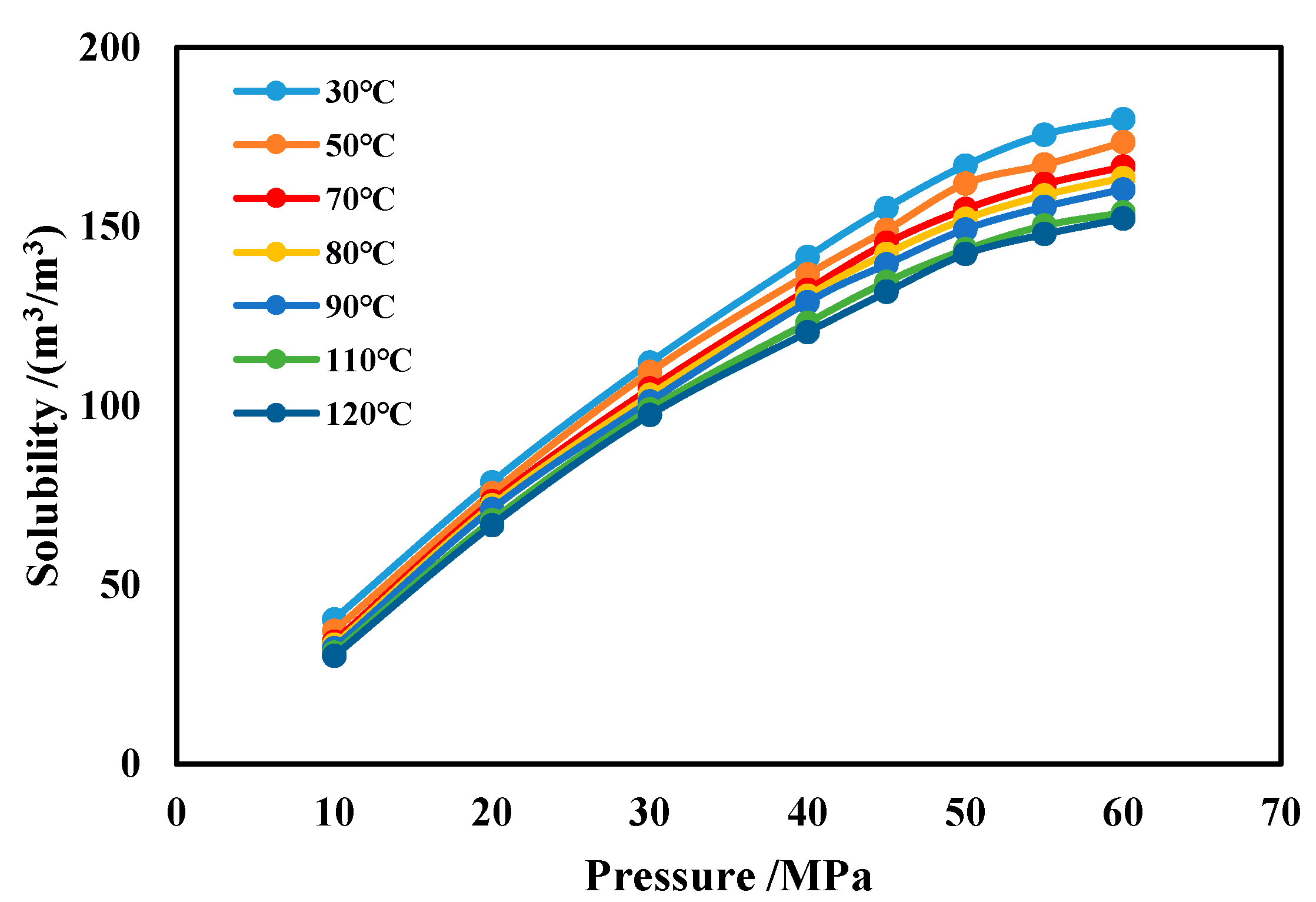
The effects of temperature and pressure on CH4 solubility are shown in Figure 17. It can be seen that, under the same conditions, the CH4 solubility increases with rising pressure and decreases slightly with increasing temperature. After the pressure exceeds 40 MPa, if the pressure continues to increase, the rate of solubility increase keeps diminishing. Comparing to water-based drilling fluid, methane has a much greater solubility in oil-based drilling fluid.
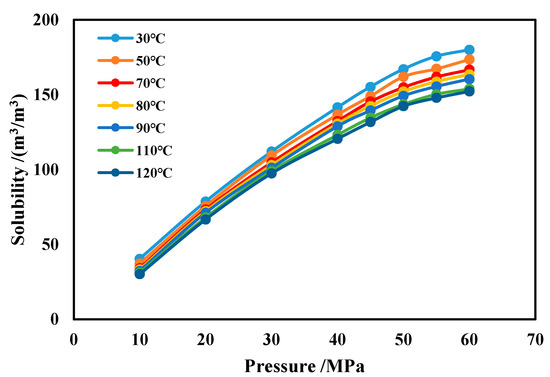
Figure 17.
Solubility of CH4 in oil-based drilling fluid.
The methane solubility formula in oil-based drilling fluid obtained through polynomial nonlinear fitting is shown in Equation (11). This formula demonstrates an agreement rate exceeding 99% with experimental results.
At a given temperature, the influence of pressure on methane solubility varies significantly. To accurately capture the solubility trends of methane in drilling fluids, it is essential to employ the solubility models of Equations (10) and (11). This provides critical foundational data for precisely characterizing methane dissolution behavior in downhole environments.
The empirical correlations (Equations (10) and (11)) are compared with the framework of cubic equations of state (e.g., Peng–Robinson). For multicomponent drilling fluids, EOS-based predictions require component-wise parameterization and mixing rules and direct numerical comparison depends on detailed fluid composition. Methodologically, both approaches enforce fugacity equality between phases. Our quadratic pressure form with temperature-dependent coefficients can be interpreted as a second-order Taylor expansion of the chemical potential (or Henry’s constant) with respect to pressure around a reference state, with temperature entering via enthalpy/entropy terms. This rationalizes the observed direct pressure dependence and inverse temperature dependence over the studied range. We outline how EOS predictions could be generated for our systems, given full compositional data and binary interaction parameters, and leave a full numerical benchmark to future work.
The second-degree polynomial form of Equations (10) and (11) is selected empirically for its high fidelity in regressing the experimental data within the tested temperature and pressure ranges. While this functional form effectively captures the nonlinear solubility trends, it is primarily a mathematical representation rather than one derived explicitly from thermodynamic first principles. The molecular simulation results presented in Section 3.1 and Section 3.2 provide the physical justification for the observed behavior, identifying the fact that the interaction energy and free volume are the dominant factors governing solubility. The development of a constitutive solubility model that directly incorporates these microscopic parameters (e.g., interaction energy, free volume fraction) presents a significant opportunity for future work. Such a model would bridge the gap between the molecular-scale mechanisms and macroscopic engineering predictions, further enhancing its predictive power and physical transparency.
3.4. Discussion
Based on molecular simulation results and solubility experimental data, it can be observed that temperature and pressure exert certain influences on the free volume of gas-drilling fluid mixed systems, but this is not the primary determinant of gas solubility differences. Specifically, the available space for gas dissolution in the solution far exceeds the actual solubility, indicating that free volume has limited reference value for understanding temperature and pressure effects on gas solubility.
Among the three factors—free volume, interaction energy, and solvation free energy—the influence on gas solubility follows the order interaction energy > free volume > solvation free energy. Therefore, the interaction energy between gas and drilling fluid can be used to interpret solubility behavior. As illustrated in Figure 14, the attractive binding energy between gas and drilling fluid gradually strengthens with increasing pressure, meaning methane experiences enhanced binding energy. Pressure acts as a driving force that promotes gas–solvent binding. Macroscopically, at constant temperature, gas solubility increases with rising pressure.
Regarding temperature, interaction energy weakens as temperature increases, facilitating gas–solvent separation. Under tested pressure conditions (10~70 MPa), solubility decreases with temperature elevation, and temperature exerts a less significant influence on solubility than pressure. Experimental solubility results confirm that methane solubility increases with rising pressure and methane solubility decreases with increasing temperature. At the microscopic level, changes in interaction energy constitute the fundamental cause of methane solubility variations in drilling fluids under different temperature and pressure conditions. It is important to note that the high agreement rate (R2 > 0.97) between the fitted solubility models (Equations (10) and (11)) and the experimental data indicates a good descriptive capability within the tested temperature and pressure ranges. However, the models were developed and validated solely on the available dataset, and no external validation on an independent dataset or sensitivity analysis was performed. Therefore, while the models accurately represent the experimental conditions under study, their predictive robustness and generalizability to broader operational conditions—particularly beyond the tested ranges of 30~120 °C and 10~60 MPa—remain to be further verified. In future work, we intend to expand the experimental database and employ cross-validation techniques, along with sensitivity analysis, to enhance the model’s reliability and applicability in field scenarios.
While data-driven approaches (e.g., neural networks) have shown success in predicting properties of multicomponent materials, this work intentionally does not employ neural networks. The present dataset is sized for mechanistic interpretation, rather than for training a generalizable model; moreover, well-controlled applications require interpretable relations that respect thermodynamic constraints (e.g., fugacity equality and Henry-type behavior), which our physics-motivated regressions provide explicitly. We therefore regard neural networks as a complementary, future direction, once a larger, composition-rich dataset is available—preferably in physics-informed form to preserve monotonicities and constraints.
4. Conclusions
This paper conducts a microscopic study on the solubility of CH4 in drilling fluids, based on molecular dynamics. Research into microscopic parameters such as free volume fraction, interaction energy and solubility free energy is carried out under different temperatures and pressures. Meanwhile, experimental tests are conducted on the solubility of CH4 in drilling fluids to quantitatively analyze the effects of temperature and pressure on the solubility of CH4. Based on the gas–liquid phase equilibrium theory, a solubility model for CH4 in drilling fluids is fitted, and the following insights are obtained:
(1) Analysis of motion trajectories, RDF, and MSD, all reveal the diffusion behavior of CH4 molecules in drilling fluids. Free volume, interaction energy and solubility free energy can be used to describe the solubility and diffusion capabilities of CH4 molecules in drilling fluids. Under different temperatures and pressures, the magnitudes of their effects are interaction energy > free volume > solubility free energy.
(2) The greater the interaction energy and free volume between CH4 and drilling fluids, the more capable CH4 is of dissolving in the drilling fluids. Moreover, the stronger the interaction, the higher the solubility. The interaction energy and free volume of CH4 in oil-based drilling fluids are both greater than those in water-based drilling fluids, indicating that CH4 has a higher solubility in oil-based drilling fluids.
(3) The solubility of CH4 in oil-based and water-based drilling fluids under temperatures of 30~120 °C and pressures of 10~60 MPa are obtained through experiments. Under identical conditions, methane solubility in both oil-based and water-based drilling fluids increases with rising pressure and decreases with elevating temperature. Empirically derived solubility equations for methane in these drilling fluids have been fitted across varying temperature–pressure combinations, with accuracy over 96%. These equations provide critical parameters for borehole pressure calculations in drilling horizontal shale gas wells.
(4) The results also suggest two field-oriented areas of guidance: a) the stronger dispersion-dominated affinity and larger free volume in oil-based fluids explain their higher CH4 solubility, enabling more accurate gas-influx estimates during managed pressure drilling; and (b) the quantified temperature–pressure sensitivity of solubility can be embedded into well-control models to refine real-time pit-gain interpretation and to support mud-program optimization (e.g., switching base fluids or adjusting emulsifier ratios) for gas-bearing shale formations.
Author Contributions
Conceptualization, H.L. and C.P.; methodology, L.G.; software, D.C.; validation, H.L., L.G. and Z.L.; formal analysis, X.W.; investigation, Y.Z.; resources, D.C.; data curation, X.W.; writing—original draft preparation, H.L. and L.G.; writing—review and editing, C.P.; visualization, Z.L.; supervision, D.C.; project administration, H.L.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sichuan Science and Technology Program grant number 2025ZNSFSC0373 and the Technology Cooperation Project of the CNPC-SWPU Innovation Alliance grant number No. 2020CX040202.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Huaqing Liu, Linyan Guo, Dejun Cai, Xiansi Wang, Zhigang Li, Yongsheng Zhang were employed by the China National Petroleum Corporation Offshore Engineering Co., Ltd. The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Nomenclature
| F | the force acting on the particle, N |
| m | the mass of the particle, kg |
| a | the acceleration of the particle, m/s2 |
| r | the distance between two particles, m |
| σ | the distance between two particles without interaction, m |
| q1, q2 | the charge between particles, C |
| ϵ0 | vacuum dielectric constant, 8.854187817 × 10−12 F/m |
| De | potential well, J |
| re | the most stable distance between two atoms, m |
| ri(t) | the position of particle i at time t, m |
| Fi(t) | the force acting on particle i at time t, N |
| mi | the mass of particle i, kg |
| Δt | the time step, s |
| Vfree | the free volume, 10−10 m3 |
| Vtotal | the total volume in the system, 10−10 m3 |
| IE | interaction energy of a single solute molecule, kcal/mol |
| n | number of solute molecules |
| Esolute | total energy of all solute molecules, kcal/mol |
| Etotal | total energy of solution, kcal/mol |
| Esolvent | total energy of solvent, kcal/mol |
References
- Ma, Y.S.; Cai, X.Y.; Zhao, P.R. China’s shale gas exploration and development: Understanding and practice. Pet. Explor. N Dev. 2018, 45, 561–574. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, Q.; Cong, L.; Wang, H.; Shi, Z.; Wu, J.; Pan, S. Development progress, potential and prospect of shale gas in China. Nat. Gas Ind. 2021, 41, 1–14. [Google Scholar]
- Dong, D.; Wang, Y.; Li, X.; Zou, C.; Guan, Q.; Zhang, C.; Huang, J.; Wang, S.; Wang, H.; Liu, H.; et al. Breakthrough and prospect of shale gas exploration and development in China. Nat. Gas Ind. 2016, 36, 19–32. [Google Scholar] [CrossRef]
- Guo, X.S.; Wang, R.R.; Shen, B.J.; Wang, G.; Wan, C.; Wang, Q. Geological characteristics, resource potential, and developmentdirection of shale gas in China. Pet. Explor. Dev. 2025, 52, 15–28. [Google Scholar] [CrossRef]
- O’Bryan, P.L.; Bourgoyne, A.T., Jr.; Monger, T.G.; Kopcso, D.P. An Experimental Study of Gas Solubility in Oil-Based Drilling Fluids. SPE Drill. Eng. 1988, 3, 33–42. [Google Scholar] [CrossRef]
- Duan, Z.; Møller, N.; Greenberg, J.; Weare, J.H. The prediction of methane solubility in natural waters to high ionic strength from 0 to 250 °C and from 0 to 1600 bar. Geochim. Cosmochim. Acta 1992, 56, 1451–1460. [Google Scholar] [CrossRef]
- Fu, X.T.; Wang, Z.P.; Lu, S.F. Dissolution mechanism and solubility equation of gas in water. Sci. Sin. 1996, 26, 124–130. [Google Scholar]
- Xu, H.T.; Liu, L.Z. Solubility of natural gas in Qaqing crude oil. J. Northeast. Pet. Univ. 2001, 25, 12–15+103. [Google Scholar]
- Fu, J.H.; Xu, C.; Zhang, Z. Gas solubility calculation in oil based drilling fluid during deepwater drilling. Drill. Prod. Technol. 2012, 35, 85–87+127–128. [Google Scholar]
- Su, Y.; Ma, H.; Guo, J.; Shen, X.; Yang, Z.; Wu, J. The behaviors of gas-liquid two-phase flow under gas kick during horizontal drilling with oil-based muds. Petroleum 2024, 10, 49–67. [Google Scholar] [CrossRef]
- Xu, C. Analysis of air phase dissolution characteristics of deepwater overflow ring. West-China Explor. Eng. 2019, 31, 72–74. [Google Scholar]
- Thomas, D.C.; Lea, J.F.; Turek, E.A. Gas solubility in oil-based drilling fluids: Effects on kick detection. J. Pet. Technol. 1984, 36, 959–968. [Google Scholar] [CrossRef]
- Berthezene, N.; De Hemptinne, J.C.; Audibert, A.; Argillier, J.F. Methane solubility in synthetic oilbased drilling muds. J. Pet. Sci. Eng. 1999, 23, 71–81. [Google Scholar] [CrossRef]
- Ribeiro, P.R.; Pessôa-Filho, P.A.; Lomba, R.F. Measurement and modeling of methanedis solution in synthetic liquids applied to drilling fluid formulation for deep and ultradeep water wells. J. Pet. Sci. Eng. 2006, 51, 37–44. [Google Scholar] [CrossRef]
- Kim, N.R.; Ribeiro, P.R.; Pessôa-Filho, P.A. PVT behavior of methane and ester-baseddrilling emulsions. J. Pet. Sci. Eng. 2015, 135, 360–366. [Google Scholar] [CrossRef]
- Flatabø, G.Ø.; Torsvik, A.; Oltedal, V.M.; Bjørkvik, B.; Grimstad, A.A.; Linga, H. Experimental gas absorption in petroleumfluids at HPHT conditions. In Proceedings of the SPE Bergen One Day Seminar, Bergen, Norway, 22 April 2015; Society of Petroleum Engineers: Bellingham, WA, USA, 2015. [Google Scholar]
- Marques, D.C.; Ribeiro, P.R.; Santos, O.L.; Lomba, R.F. Thermodynamic behavior of olefin/methane mixtures applied to synthetic-drilling-fluid well control. SPE Drill. Complet. 2018, 33, 230–240. [Google Scholar] [CrossRef]
- O’Bryan, P.L.; Bourgoyne, A.T. Swelling of oil-based drilling fluids resulting from dissolved gas. SPE Drill. Eng. 1990, 5, 149–155. [Google Scholar] [CrossRef]
- Ghosh, A.; Chapman, W.G.; French, R.N. Gas solubility in hydrocarbons-a SAFT-based approach. Fluid Phase Equilibria 2003, 209, 229–243. [Google Scholar] [CrossRef]
- Chen, X.R.; Wang, Y.P.; He, Z.H.; Fan, Q. Solubility models of CH4, CO2 and noble gases and their geological applications. Nat. Gas Geosci. 2023, 34, 707–718. [Google Scholar] [CrossRef]
- Wu, X.P.; Liu, Z.P. Molecular Dynamics Simulation of Gas Solubility in Room Temperature Ionic Liquids. Acta Phys.-Chim. Sin. 2005, 21, 1138–1142. [Google Scholar]
- Zhang, Y.; Yang, J.C.; Yu, Y.X.; Li, Y. Application of molecular simulation in the field of supercritical fluid. Prog. Chem. 2005, 17, 9–16. [Google Scholar]
- Zhang, W.N.; Yu, F.; Zhao, S.L.; Zhang, Z.Q.; He, Y.P. Progress in Molecular Dynamics and Hansen Solubility Parameters of Low Molecular Weight Gels. Chin. J. Appl. Chem. 2022, 39, 1803–1817. [Google Scholar]
- Jiang, Y.P. Molecular dynamics simulation and microscopic mechanism of CO2 composite flooding. Pet. Geol. Exp. 2019, 41, 274–279. [Google Scholar]
- Li, H.Y. Molecular Dynamics Simulation for Supercritical Carbon Dioxide Extraction of Heavy Oil Components in Deep Reservoir. Sci. Technol. Eng. 2021, 24, 12543–12550. [Google Scholar]
- Li, B.C.; Liu, G.; Chen, L. Study on the influence mechanism of CH4 dissolution on the intermolecular interaction between crude oil molecules based on molecular dynamics simulation. CIESC J. 2021, 72, 1253–1263. [Google Scholar]
- Hu, W.F. Molecular Dynamics Study on the Microscopic Characteristics of Natural Gas Hydrate Phase Transformation. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2021. [Google Scholar]
- Tang, J.; Ahmadi, A.; Alizadeh, A.; Abedinzadeh, R.; Abed, A.M.; Smaisim, G.F.; Hadrawi, S.K.; Nasajpour-Esfahani, N.; Toghraie, D. Investigation of the mechanical properties of different amorphous composites using the molecular dynamics simulation. J. Mater. Res. Technol. 2023, 24, 1390–1400. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Zhang, Y.; Lin, P.; Apostolidis, P.; Erkens, S.; Li, M.; Xu, J. Multi-scale characterization of lignin modified bitumen using experimental and molecular dynamics simulation methods. Constr. Build. Mater. 2021, 287, 123058. [Google Scholar] [CrossRef]
- Ma, Q.; Zhu, W.; Song, Z.; Zhang, J.; Li, B.; Bu, W.; Pan, B. Influences and mechanisms of imidazolium-based ionic liquids on oil-water interfacial tension and quartz wettability: Experiment and molecular dynamics simulations. Fuel 2023, 352, 129053. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).