Linking Analysis to Atmospheric PFAS: An Integrated Framework for Exposure Assessment, Health Risks, and Future Management Strategies
Abstract
1. Introduction
2. Definition and Substances of PFASs
3. PFAS Analysis Methods
4. Environmental Behavior and Levels of PFASs
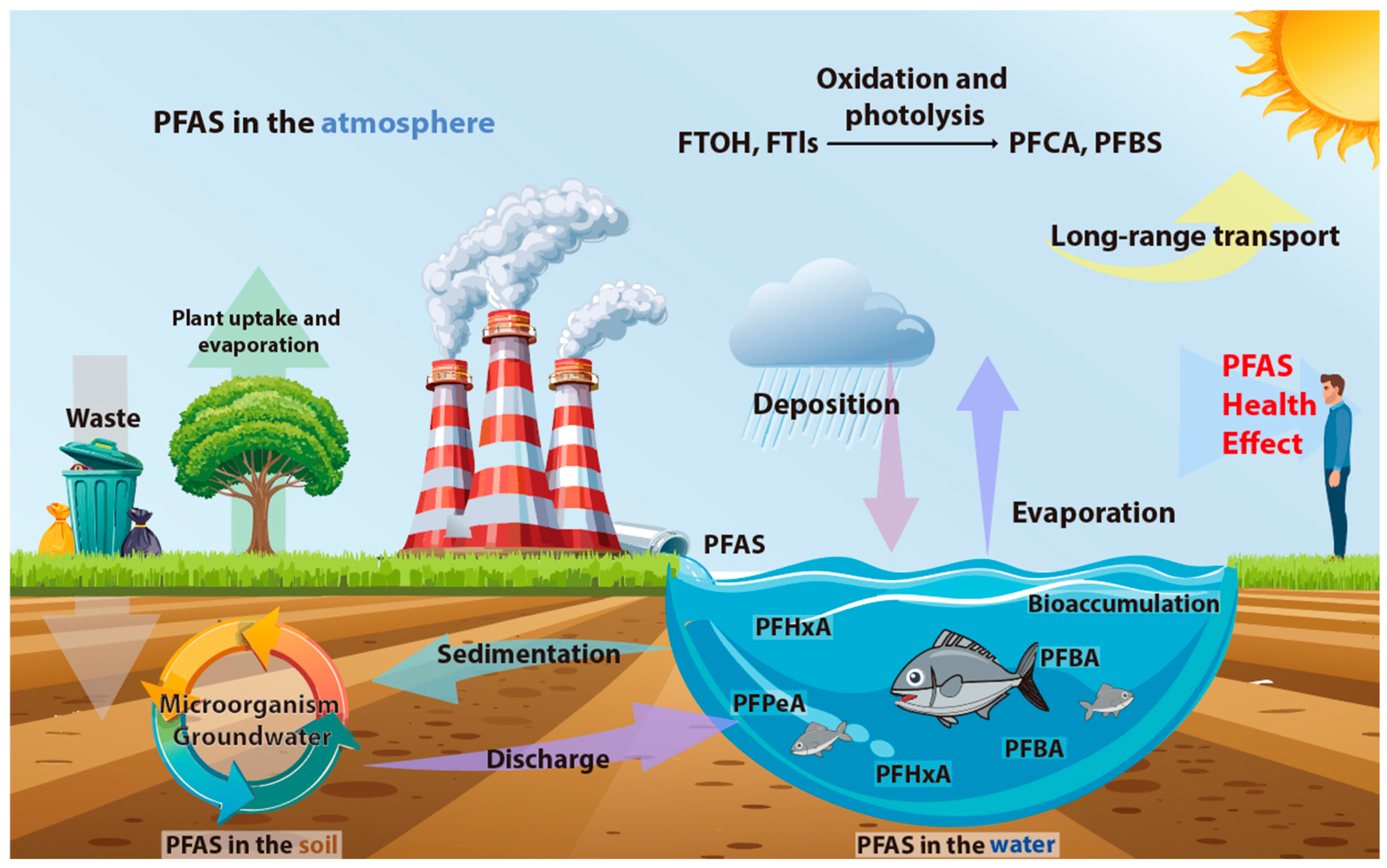
4.1. Direct and Indirect Emissions Define PFAS Presence in the Environment
4.2. PFAS Transport and Partitioning Between Water, Sediment, and Atmosphere
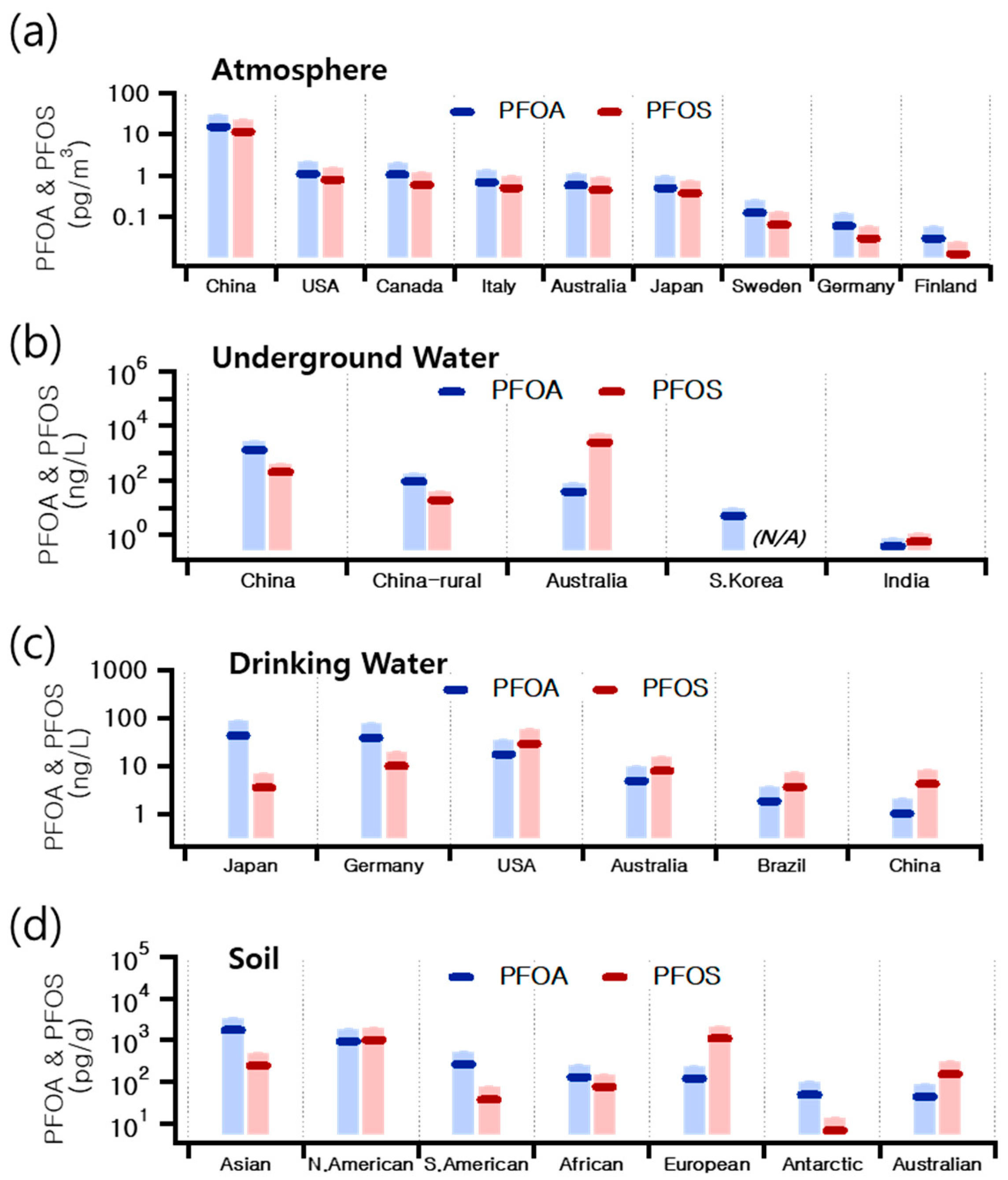
4.3. Global Variations in PFAS Concentrations Across Water, Soil, and Atmospheric Environments
5. Health Risks of PFAS
6. Importance of PFAS in the Atmospheric Environment
7. Future PFAS Management Strategies and Review Conclusions
- Comprehensive surveillance of atmospheric PFASs: A unified and globally harmonized monitoring network is urgently needed to capture PFASs across gaseous and particle-bound phases, precipitation, and deposition pathways. This includes not only legacy PFAS but also precursors, transformation products, and substitutes. Enhanced laboratory capacity, standardized analytical protocols, and greater access to reference standards will ensure comparability and accuracy of data. Such infrastructure will enable researchers and regulators to track spatiotemporal patterns, identify emission hotspots, and quantify long-range transport mechanisms.
- Mechanistic understanding of transformation and transport: atmospheric PFAS research must go beyond occurrence data to unravel the kinetics and mechanisms of photochemical, oxidative, and heterogeneous reactions that generate new PFAS species in situ. This includes linking emission inventories to transformation pathways and assessing how chain length, functional groups, and atmospheric conditions influence mobility and deposition. Integrating atmospheric models with field data will clarify exposure scenarios and inform predictive risk assessments.
- Human health and ecosystem risk integration: a holistic risk framework is required to capture inhalation and dermal uptake as critical exposure routes alongside ingestion. Research should quantify internal doses, biotransformation, and the bioaccumulation potential of atmospheric PFASs, especially emerging short-chain and ether-based compounds. This risk integration must also address sensitive ecosystems such as the Arctic, where atmospheric PFASs act as cumulative indicators of global emissions and climate-driven transport processes.
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance; Organisation for Economic Co-Operation and Development: Paris, France, 2021; Available online: https://www.oecd.org/en/publications/reconciling-terminology-of-the-universe-of-per-and-polyfluoroalkyl-substances_e458e796-en.html (accessed on 23 August 2025).
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Kissa, E. Fluorinated Surfactants and Repellents; Marcel Dekker AG: New York, NY, USA, 2001. [Google Scholar]
- Johansson, J.H.; Berger, U.; Cousins, I.T. Can the use of deactivated glass fibre filters eliminate sorption artefacts associated with active air sampling of perfluorooctanoic acid? Environ. Pollut. 2017, 224, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Bundschuh, M. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Martin, J.W.; Smithwick, M.M.; Braune, B.M.; Hoekstra, P.F.; Muir, D.C.G.; Mabury, S.A. Identification of long-chain perfluorinated acids in biota from the Canadian Arctic. Environ. Sci. Technol. 2004, 38, 373–380. [Google Scholar] [CrossRef]
- Houde, M.; Czub, G.; Small, J.M.; Backus, S.; Wang, X.; Alaee, M.; Muir, D.C.G. Fractionation and bioaccumulation of perfluorooctane sulfonate (PFOS) isomers in a Lake Ontario food web. Environ. Sci. Technol. 2008, 42, 9397–9403. [Google Scholar] [CrossRef]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef]
- Loos, R.; Locoro, G.; Huber, T.; Wollgast, J.; Christoph, E.H.; de Jager, A.; Manfred Gawlik, B.; Hanke, G.; Umlauf, G.; Zaldívar, J.-M. Analysis of perfluorooctanoate (PFOA) and other perfluorinated compounds (PFCs) in the River Po watershed in N-Italy. Chemosphere 2008, 71, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Powley, C.R.; George, S.W.; Russell, M.H.; Hoke, R.A.; Buck, R.C. Polyfluorinated chemicals in a spatially and temporally integrated food web in the Western Arctic. Chemosphere 2008, 70, 664–672. [Google Scholar] [CrossRef]
- Yamashita, N.; Taniyasu, S.; Petrick, G.; Wei, S.; Gamo, T.; Lam, P.K.S.; Kannan, K. Perfluorinated acids as novel chemical tracers of global circulation of ocean waters. Chemosphere 2008, 70, 1247–1255. [Google Scholar] [CrossRef]
- OECD. OECD, Summary Report on Updating the OECD 2007 List of Per- and Polyfluorinated Substances (PFASs); OECD Environment, Health and Safety Publications, Series on Risk Management, No. 39, Report ENV/JM/MONO(2018)7, Paris, France. 2018. Available online: https://one.oecd.org/document/ENV/JM/MONO(2018)7/en/pdf (accessed on 23 August 2025).
- TSCA. Toxic Substances Control Act Reporting and Recordkeeping Requirements for Perfluoroalkyl and Polyfluoroalkyl Substances; TSCA Substances. 2020. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/tsca-section-8a7-reporting-and-recordkeeping (accessed on 23 August 2025).
- National Defense Authorization. National Defense Authorization Act for Fiscal Year 2020; Public Law No. 116–92, 2019. Available online: https://www.congress.gov/bill/116th-congress/senate-bill/1790 (accessed on 23 August 2025).
- EPA. Per- and Polyfluoroalkyl Substances (PFAS): Basic Information and Effects; U.S. Environmental Protection Agency: Washington, DC, USA, 2021. Available online: https://www.epa.gov/pfas/basic-information-pfas (accessed on 23 August 2025).
- Kourtchev, I.; Sebben, B.G.; Bogush, A.; Godoi, A.F.L.; Godoi, R.H.M. Per- and polyfluoroalkyl substances (PFASs) in urban PM2.5 samples from Curitiba, Brazil. Atmos. Environ. 2023, 309, 119911. [Google Scholar] [CrossRef]
- Ford, L.C.; Lin, H.-C.; Zhou, Y.-H.; Wright, F.A.; Gombar, V.K.; Sedykh, A.; Shah, R.R.; Chiu, W.A.; Rusyn, I. Characterizing PFAS hazards and risks: A human population-based in vitro cardiotoxicity assessment strategy. Hum. Genom. 2024, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Al Amin, M.; Sobhani, Z.; Liu, Y.; Dharmaraja, R.; Chadalavada, S.; Naidu, R.; Chalker, J.M.; Fang, C. Recent advances in the analysis of per- and polyfluoroalkyl substances (PFAS)—A review. Environ. Technol. Innov. 2020, 19, 100879. [Google Scholar] [CrossRef]
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef]
- IARC. Evaluation of the Carcinogenicity of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonic Acid (PFOS); International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://publications.iarc.fr/588 (accessed on 23 August 2025).
- UNEP. Environmental Risks of Per- and Polyfluoroalkyl Substances; United Nations Environment Programme: Nairobi, Kenya, 2019; Available online: https://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-PUB-factsheet-PFAS-2022.English.pdf (accessed on 23 August 2025).
- Place, B.J.; Field, J.A. Identification of novel fluorochemicals in aqueous film-forming foams used by the US military. Environ. Sci. Technol. 2012, 46, 7120–7127. [Google Scholar] [CrossRef] [PubMed]
- Podder, A.; Sadmani, A.H.M.A.; Reinhart, D.; Chang, N.-B.; Goel, R. Per- and polyfluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects. J. Hazard. Mater. 2021, 419, 126361. [Google Scholar] [CrossRef]
- Zarębska, M.; Bajkacz, S. Poly- and perfluoroalkyl substances (PFAS)—Recent advances in the aquatic environment analysis. TrAC Trends Anal. Chem. 2023, 163, 117062. [Google Scholar] [CrossRef]
- Barber, J.L.; Berger, U.; Chaemfa, C.; Huber, S.; Jahnke, A.; Temme, C.; Jones, K.C. Analysis of per- and polyfluorinated alkyl substances in air samples from Northwest Europe. J. Environ. Monit. 2007, 9, 530–541. [Google Scholar] [CrossRef]
- Fiedler, H.; de Boer, J.; Abad, E. Persistent organic pollutants in air across the globe using a comparative passive air sampling method. TrAC Trends Anal. Chem. 2024, 171, 117494. [Google Scholar] [CrossRef]
- Jahnke, A.; Ahrens, L.; Ebinghaus, R.; Berger, U.; Barber, J.L.; Temme, C. An improved method for the analysis of volatile polyfluorinated alkyl substances in environmental air samples. Anal. Bioanal. Chem. 2007, 387, 965–975. [Google Scholar] [CrossRef]
- Kubwabo, C.; Stewart, B.; Zhu, J.; Marro, L. Occurrence of perfluorosulfonates and other perfluorochemicals in dust from selected homes in the city of Ottawa, Canada. J. Environ. Monit. 2005, 7, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Del Vento, S.; Schuster, J.; Zhang, G.; Chakraborty, P.; Kobara, Y.; Jones, K.C. Perfluorinated Compounds in the Asian Atmosphere. Environ. Sci. Technol. 2011, 45, 7241–7248. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, H.; Takata, Y.; Arakawa, R. Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in vacuum cleaner dust collected in Japanese homes. J. Environ. Monit. 2003, 5, 753–757. [Google Scholar] [CrossRef]
- Padilla-Sánchez, J.A.; Papadopoulou, E.; Poothong, S.; Haug, L.S. Investigation of the best approach for assessing human exposure to poly- and perfluoroalkyl substances through indoor air. Environ. Sci. Technol. 2017, 51, 12836–12843. [Google Scholar] [CrossRef]
- Saini, A.; Chinnadurai, S.; Schuster, J.K.; Eng, A.; Harner, T. Per- and polyfluoroalkyl substances and volatile methyl siloxanes in global air: Spatial and temporal trends. Environ. Pollut. 2023, 323, 121291. [Google Scholar] [CrossRef]
- Shoeib, M.; Harner, T.; Ikonomou, M.; Kannan, K. Indoor and outdoor air concentrations and phase partitioning of perfluoroalkyl sulfonamides and polybrominated diphenyl ethers. Environ. Sci. Technol. 2004, 38, 1313–1320. [Google Scholar] [CrossRef]
- Shoeib, M.; Harner, T.; Lee, S.C.; Lane, D.; Zhu, J. Sorbent-impregnated polyurethane foam disk for passive air sampling of volatile fluorinated chemicals. Anal. Chem. 2008, 80, 675–682. [Google Scholar] [CrossRef]
- Shoeib, T.; Hassan, Y.; Rauert, C.; Harner, T. Poly- and perfluoroalkyl substances (PFASs) in indoor dust and food packaging materials in Egypt: Trends in developed and developing countries. Chemosphere 2016, 144, 1573–1581. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Y.; Sun, H.; Chang, S.; Zhu, L.; Alder, A.C.; Kannan, K. Per- and polyfluoroalkyl substances (PFASs) in indoor air and dust from homes and various microenvironments in China: Implications for human exposure. Environ. Sci. Technol. 2018, 52, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Backe, W.J.; Day, T.C.; Field, J.A. Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film-forming foam formulations and groundwater from US military bases by nonaqueous large-volume injection HPLC-MS/MS. Environ. Sci. Technol. 2013, 47, 5226–5234. [Google Scholar] [CrossRef]
- Boiteux, V.; Dauchy, X.; Bach, C.; Colin, A.; Hemard, J.; Sagres, V.; Rosin, C.; Munoz, J.-F. Concentrations and patterns of perfluoroalkyl and polyfluoroalkyl substances in a river and three drinking water treatment plants near and far from a major production source. Sci. Total Environ. 2017, 583, 393–400. [Google Scholar]
- Boulanger, B.; Vargo, J.; Schnoor, J.L.; Hornbuckle, K.C. Detection of perfluorooctane surfactants in Great Lakes water. Environ. Sci. Technol. 2004, 38, 4064–4070. [Google Scholar] [CrossRef]
- Deng, Z.-H.; Cheng, C.-G.; Wang, X.-L.; Shi, S.-H.; Wang, M.-L.; Zhao, R.-S. Preconcentration and determination of perfluoroalkyl substances (PFASs) in water samples by bamboo charcoal-based solid-phase extraction prior to liquid chromatography–tandem mass spectrometry. Molecules 2018, 23, 902. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Martínez-Carballo, E.; Sitka, A.; Scharf, S.; Gans, O. Method optimization for determination of selected perfluorinated alkylated substances in water samples. Anal. Bioanal. Chem. 2006, 386, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.J.; Johnson, H.; Eldridge, J.; Butenhoff, J.; Dick, L. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ. Sci. Technol. 2002, 36, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Choi, Y.J.; Deeb, R.A.; Strathmann, T.J.; Higgins, C.P. Application of hydrothermal alkaline treatment for destruction of per- and polyfluoroalkyl substances in contaminated groundwater and soil. Environ. Sci. Technol. 2022, 56, 6647–6657. [Google Scholar] [CrossRef]
- Hebert, G.N.; Odom, M.A.; Craig, P.S.; Dick, D.L.; Strauss, S.H. Method for the determination of sub-ppm concentrations of perfluoroalkylsulfonate anions in water. J. Environ. Monit. 2002, 4, 90–95. [Google Scholar] [CrossRef]
- Janda, J.; Nödler, K.; Brauch, H.-J.; Zwiener, C.; Lange, F.T. Robust trace analysis of polar (C2-C8) perfluorinated carboxylic acids by liquid chromatography-tandem mass spectrometry: Method development and application to surface water, groundwater and drinking water. Environ. Sci. Pollut. Res. 2019, 26, 7326–7336. [Google Scholar] [CrossRef]
- Kaboré, H.A.; Duy, S.V.; Munoz, G.; Méité, L.; Desrosiers, M.; Liu, J.; Sory, T.K.; Sauvé, S. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci. Total Environ. 2018, 616, 1089–1100. [Google Scholar]
- Loewen, M.; Halldorson, T.; Wang, F.; Tomy, G. Fluorotelomer carboxylic acids and PFOS in rainwater from an urban center in Canada. Environ. Sci. Technol. 2005, 39, 2944–2951. [Google Scholar] [CrossRef]
- Loos, R.; Tavazzi, S.; Mariani, G.; Suurkuusk, G.; Paracchini, B.; Umlauf, G. Analysis of emerging organic contaminants in water, fish and suspended particulate matter (SPM) in the Joint Danube Survey using solid-phase extraction followed by UHPLC-MS-MS and GC–MS analysis. Sci. Total Environ. 2017, 607, 1201–1212. [Google Scholar] [CrossRef]
- Miyake, Y.; Yamashita, N.; Rostkowski, P.; So, M.K.; Taniyasu, S.; Lam, P.K.S.; Kannan, K. Determination of trace levels of total fluorine in water using combustion ion chromatography for fluorine: A mass balance approach to determine individual perfluorinated chemicals in water. J. Chromatogr. A 2007, 1143, 98–104. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Nguyen, D.; Fang, Y.; Gonda, N.; Zhang, C.; Shea, S.; Higgins, C.P. PFAS Porewater concentrations in unsaturated soil: Field and laboratory comparisons inform on PFAS accumulation at air-water interfaces. J. Contam. Hydrol. 2024, 264, 104359. [Google Scholar] [CrossRef]
- Schultz, M.M.; Barofsky, D.F.; Field, J.A. Quantitative determination of fluorotelomer sulfonates in groundwater by LC MS/MS. Environ. Sci. Technol. 2004, 38, 1828–1835. [Google Scholar] [CrossRef]
- Wilson, S.R.; Malerød, H.; Holm, A.; Molander, P.; Lundanes, E.; Greibrøkk, T. On-line SPE–Nano-LC–Nanospray-MS for rapid and sensitive determination of perfluorooctanoic acid and perfluorooctane sulfonate in river water. J. Chromatogr. Sci. 2007, 45, 146–152. [Google Scholar] [CrossRef]
- Yamashita, N.; Kannan, K.; Taniyasu, S.; Horii, Y.; Okazawa, T.; Petrick, G.; Gamo, T. Analysis of perfluorinated acids at parts-per-quadrillion levels in seawater using liquid chromatography-tandem mass spectrometry. Environ. Sci. Technol. 2004, 38, 5522–5528. [Google Scholar] [CrossRef]
- Baqar, M.; Saleem, R.; Zhao, M.; Zhao, L.; Cheng, Z.; Chen, H.; Yao, Y.; Sun, H. Combustion of high-calorific industrial waste in conventional brick kilns: An emerging source of PFAS emissions to agricultural soils. Sci. Total Environ. 2024, 906, 167612. [Google Scholar] [CrossRef]
- Higgins, C.P.; Field, J.A.; Criddle, C.S.; Luthy, R.G. Quantitative determination of perfluorochemicals in sediments and domestic sludge. Environ. Sci. Technol. 2005, 39, 3946–3956. [Google Scholar] [CrossRef]
- Müller, V.; Andrade Costa, L.C.; Rondan, F.S.; Matic, E.; Mesko, M.F.; Kindness, A.; Feldmann, J. Per- and polyfluoroalkylated substances (PFAS) target and EOF analyses in ski wax, snowmelts, and soil from skiing areas. Environ. Sci. Process. Impacts 2023, 25, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Powley, C.R.; George, S.W.; Ryan, T.W.; Buck, R.C. Matrix effect-free analytical methods for determination of perfluorinated carboxylic acids in environmental matrices. Anal. Chem. 2005, 77, 6353–6358. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.F. Determination of fluorinated surfactants and their metabolites in sewage sludge samples by liquid chromatography with mass spectrometry and tandem mass spectrometry after pressurized liquid extraction and separation on fluorine-modified reversed-phase sorbents. J. Chromatogr. A 2003, 1020, 131–151. [Google Scholar]
- Semerád, J.; Hatasová, N.; Grasserová, A.; Černá, T.; Filipová, A.; Hanč, A.; Innemanová, P.; Pivokonský, M.; Cajthaml, T. Screening for 32 per- and polyfluoroalkyl substances (PFAS) including GenX in sludges from 43 WWTPs located in the Czech Republic—Evaluation of potential accumulation in vegetables after application of biosolids. Chemosphere 2020, 261, 128018. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, E.; Kannan, K. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ. Sci. Technol. 2006, 40, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Wang, J.; Hou, J.; Han, B.; Liu, W. Spatial distribution, compositional characteristics, and source apportionment of legacy and novel per- and polyfluoroalkyl substances in farmland soil: A nationwide study in mainland China. J. Hazard. Mater. 2024, 470, 134238. [Google Scholar] [CrossRef]
- Washington, J.W.; Ellington, J.J.; Jenkins, T.M.; Evans, J.J. Analysis of perfluorinated carboxylic acids in soils: Detection and quantitation issues at low concentrations. J. Chromatogr. A 2007, 1154, 111–120. [Google Scholar] [CrossRef]
- Washington, J.W.; Henderson, W.M.; Ellington, J.J.; Jenkins, T.M.; Evans, J.J. Analysis of perfluorinated carboxylic acids in soils II: Optimization of chromatography and extraction. J. Chromatogr. A 2008, 1181, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Schlummer, M.; Möller, A.; Gruber, L.; Wolz, G.; Ungewiss, J.; Böhmer, S.; Dekant, W.; Mayer, R.; Liebl, B. Exposure of an adult population to perfluorinated substances using duplicate diet portions and biomonitoring data. Environ. Sci. Technol. 2007, 41, 7928–7933. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Park, J.; Kim, H.-J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Kim, S.; Choi, K.; et al. Perfluoroalkyl substances (PFASs) in breast milk from Korea: Time-course trends, influencing factors, and infant exposure. Sci. Total Environ. 2018, 612, 286–292. [Google Scholar] [CrossRef]
- Llorca, M.; Farré, M.; Picó, Y.; Teijón, M.L.; Álvarez, J.G.; Barceló, D. Infant exposure of perfluorinated compounds: Levels in breast milk and commercial baby food. Environ. Int. 2010, 36, 584–592. [Google Scholar] [CrossRef]
- Luque, N.; Ballesteros-Gómez, A.; van Leeuwen, S.; Rubio, S. Analysis of perfluorinated compounds in biota by microextraction with tetrahydrofuran and liquid chromatography/ion isolation-based ion-trap mass spectrometry. J. Chromatogr. A 2010, 1217, 3774–3782. [Google Scholar] [CrossRef]
- Nania, V.; Pellegrini, G.E.; Fabrizi, L.; Sesta, G.; De Sanctis, P.; Lucchetti, D.; Di Pasquale, M.; Coni, E. Monitoring of perfluorinated compounds in edible fish from the Mediterranean Sea. Food Chem. 2009, 115, 951–957. [Google Scholar] [CrossRef]
- Squadrone, S.; Ciccotelli, V.; Prearo, M.; Favaro, L.; Scanzio, T.; Foglini, C.; Abete, M. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA): Emerging contaminants of increasing concern in fish from Lake Varese, Italy. Environ. Monit. Assess. 2015, 187, 1–7. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Pepper, K.; Seymour, C.; Moisey, J.; Bronson, R.; Cao, X.-L.; Dabeka, R.W. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J. Agric. Food Chem. 2007, 55, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Zabaleta, I.; Bizkarguenaga, E.; Iparragirre, A.; Navarro, P.; Prieto, A.; Fernández, L.Á.; Zuloaga, O. Focused ultrasound solid–liquid extraction for the determination of perfluorinated compounds in fish, vegetables and amended soil. J. Chromatogr. A 2014, 1331, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Dinglasan-Panlilio, M.J.A.; Mabury, S.A. Significant residual fluorinated alcohols present in various fluorinated materials. Environ. Sci. Technol. 2006, 40, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, E.; Kim, S.K.; Akinleye, H.B.; Kannan, K. Quantitation of gas-phase perfluoroalkyl surfactants and fluorotelomer alcohols released from nonstick cookware and microwave popcorn bags. Environ. Sci. Technol. 2007, 41, 1180–1185. [Google Scholar] [CrossRef]
- Zabaleta, I.; Bizkarguenaga, E.; Bilbao, D.; Etxebarria, N.; Prieto, A.; Zuloaga, O. Fast and simple determination of perfluorinated compounds and their potential precursors in different packaging materials. Talanta 2016, 152, 353–363. [Google Scholar] [CrossRef]
- Zabaleta, I.; Negreira, N.; Bizkarguenaga, E.; Prieto, A.; Covaci, A.; Zuloaga, O. Screening and identification of per- and polyfluoroalkyl substances in microwave popcorn bags. Food Chem. 2017, 230, 497–506. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Costopoulou, D.; Vassiliadou, I.; Bakeas, E.; Leondiadis, L. Determination of perfluorinated compounds (PFCs) in various foodstuff packaging materials used in the Greek market. Chemosphere 2014, 94, 169–176. [Google Scholar] [CrossRef]
- Liu, W.; Wu, J.; He, W.; Xu, F. A review on perfluoroalkyl acids studies: Environmental behaviors, toxic effects, and ecological and health risks. Ecosyst. Health Sustain. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, fate, and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef]
- Andrews, D.Q.; Hayes, J.; Stoiber, T.; Brewer, B.; Campbell, C.; Naidenko, O.V. Identification of point source dischargers of per- and polyfluoroalkyl substances in the United States. AWWA Water Sci. 2021, 3, e1252. [Google Scholar] [CrossRef]
- Ellis, D.A.; Martin, J.W.; De Silva, A.O.; Mabury, S.A.; Hurley, M.D.; Sulbaek Andersen, M.P.; Nielsen, O.J. Degradation of fluorotelomer alcohols: A likely atmospheric source of perfluorinated carboxylic acids. Environ. Sci. Technol. 2004, 38, 3316–3321. [Google Scholar] [CrossRef]
- Dinglasan, M.J.A.; Ye, Y.; Edwards, E.A.; Mabury, S.A. Aerobic biotransformation of 14C-labeled 8-2 telomer alcohol by activated sludge from a domestic sewage treatment plant. Environ. Sci. Technol. 2004, 38, 1225–1231. [Google Scholar]
- Butt, C.M.; Berger, U.; Bossi, R.; Tomy, G.T. Review: Spatial trends of perfluoroalkyl substances in wildlife. Chemosphere 2010, 78, 933–941. [Google Scholar]
- Martin, J.W.; Asher, B.J.; Beesoon, S.; Benskin, J.P.; Ross, M.S. Biodegradation and metabolism of polyfluorinated compounds. Rev. Environ. Contam. Toxicol. 2010, 208, 53–71. [Google Scholar]
- Begley, T.H.; White, K.; Honigfort, P.; Twaroski, M.L.; Neches, R.; Walker, R.A. Perfluorochemicals: Potential sources of and migration from food packaging. Food Addit. Contam. 2005, 22, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, B.; Peck, A.M.; Schnoor, J.L.; Hornbuckle, K.C. Mass budget of perfluorooctane surfactants in Lake Ontario. Environ. Sci. Technol. 2005, 39, 74–79. [Google Scholar] [CrossRef]
- D’Eon, J.C.; Mabury, S.A. Production of perfluorinated carboxylic acids (PFCAs) from polyfluoroalkyl phosphate diesters (PAPs) in the rat. Environ. Sci. Technol. 2007, 41, 956–962. [Google Scholar]
- Webster, E.; Ellis, D.A.; McLachlan, M.S. Modeling the fate of perfluorocarboxylates and their precursors in the atmosphere. Environ. Sci. Technol. 2006, 40, 6123–6130. [Google Scholar]
- Chiappero, M.S.; Malanca, F.E.; Argüello, G.A.; Wooldridge, S.T.; Hurley, M.D.; Ball, J.C.; Wallington, T.J.; Waterland, R.L.; Buck, R.C. Atmospheric chemistry of perfluoroaldehydes and fluorotelomer aldehydes: Quantification of the important role of photolysis. J. Phys. Chem. A 2006, 110, 11944–11953. [Google Scholar]
- D’Eon, J.C.; Hurley, M.D.; Wallington, T.J.; Mabury, S.A. Atmospheric chemistry of N-methyl perfluorobutane sulfonamidoethanol: Kinetics and mechanism of reaction with OH. Environ. Sci. Technol. 2006, 40, 1862–1868. [Google Scholar] [CrossRef]
- Conder, J.M.; Hoke, R.A.; Wolf, W.d.; Russell, M.H.; Buck, R.C. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Corsolini, S.; Falandysz, J.; Oehme, G.; Focardi, S.; Giesy, J.P. Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas. Environ. Sci. Technol. 2002, 36, 3210–3216. [Google Scholar] [CrossRef]
- Kumar, K.S.; Zushi, Y.; Masunaga, S.; Gilligan, M.; Pride, C.; Sajwan, K.S. Perfluorinated organic contaminants in sediment and aquatic wildlife, including sharks, from Georgia, USA. Mar. Pollut. Bull. 2009, 58, 621–629. [Google Scholar] [CrossRef]
- Toms, L.; Bräunig, J.; Vijayasarathy, S.; Phillips, S.; Hobson, P.; Aylward, L.; Kirk, M.; Mueller, J. Per-and polyfluoroalkyl substances (PFAS) in Australia: Current levels and estimated population reference values for selected compounds. Int. J. Hyg. Environ. Health 2019, 222, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Bossi, R.; Skjøth, C.A.; Skov, H. Three years (2008–2010) of measurements of atmospheric concentrations of organochlorine pesticides (OCPs) at Station Nord, North-East Greenland. Environ. Sci. Process Impacts 2013, 15, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, A.; Matthias, V.; Temme, C.; Ebinghaus, R. Annual Time Series of Air Concentrations of Polyfluorinated Compounds. Environ. Sci. Technol. 2009, 43, 4029–4036. [Google Scholar] [CrossRef]
- Jahnke, A.; Berger, U.; Ebinghaus, R.; Temme, C. Latitudinal Gradient of Airborne Polyfluorinated Alkyl Substances in the Marine Atmosphere between Germany and South Africa (53° N−33° S). Environ. Sci. Technol. 2007, 41, 3055–3061. [Google Scholar] [CrossRef]
- Shimizu, M.S.; Mott, R.; Potter, A.; Zhou, J.; Baumann, K.; Surratt, J.D.; Turpin, B.; Avery, G.B.; Harfmann, J.; Kieber, R.J.; et al. Atmospheric Deposition and Annual Flux of Legacy Perfluoroalkyl Substances and Replacement Perfluoroalkyl Ether Carboxylic Acids in Wilmington, NC, USA. Environ. Sci. Technol. Lett. 2021, 8, 366–372. [Google Scholar] [CrossRef]
- Wang, Q.; Ruan, Y.; Lin, H.; Lam, P.K.S. Review on perfluoroalkyl and polyfluoroalkyl substances (PFASs) in the Chinese atmospheric environment. Sci. Total Environ. 2020, 737, 139804. [Google Scholar]
- Shoeib, M.; Harner, T.; M. Webster, G.; Lee, S.C. Indoor Sources of Poly- and Perfluorinated Compounds (PFCS) in Vancouver, Canada: Implications for Human Exposure. Environ. Sci. Technol. 2011, 45, 7999–8005. [Google Scholar] [PubMed]
- Valsecchi, S.; Conti, D.; Crebelli, R.; Polesello, S.; Rusconi, M.; Mazzoni, M.; Preziosi, E.; Carere, M.; Lucentini, L.; Ferretti, E.; et al. Deriving environmental quality standards for perfluorooctanoic acid (PFOA) and related short chain perfluorinated alkyl acids. J. Hazard. Mater. 2017, 323, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiao, X.-C.; Gai, N.; Li, X.-J.; Wang, X.-C.; Lu, G.-H.; Piao, H.-T.; Rao, Z.; Yang, Y.-L. Perfluorinated compounds in soil, surface water, and groundwater from rural areas in eastern China. Environ. Pollut. 2016, 211, 124–131. [Google Scholar] [CrossRef]
- Bao, J.; Yu, W.-J.; Liu, Y.; Wang, X.; Jin, Y.-H.; Dong, G.-H. Perfluoroalkyl substances in groundwater and home-produced vegetables and eggs around a fluorochemical industrial park in China. Ecotoxicol. Environ. Saf. 2019, 171, 199–205. [Google Scholar] [CrossRef]
- Hepburn, E.; Madden, C.; Szabo, D.; Coggan, T.L.; Clarke, B.; Currell, M. Contamination of groundwater with per- and polyfluoroalkyl substances (PFAS) from legacy landfills in an urban re-development precinct. Environ. Pollut. 2019, 248, 101–113. [Google Scholar] [CrossRef]
- Sharma, B.M.; Bharat, G.K.; Tayal, S.; Larssen, T.; Bečanová, J.; Karásková, P.; Whitehead, P.G.; Futter, M.N.; Butterfield, D.; Nizzetto, L. Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: Emissions and implications for human exposure. Environ. Pollut. 2016, 208, 704–713. [Google Scholar] [CrossRef]
- Yong, Z.Y.; Kim, K.Y.; Oh, J.-E. The occurrence and distributions of per- and polyfluoroalkyl substances (PFAS) in groundwater after a PFAS leakage incident in 2018. Environ. Pollut. 2021, 268, 115395. [Google Scholar] [CrossRef] [PubMed]
- Mak, Y.L.; Taniyasu, S.; Yeung, L.W.Y.; Lu, G.; Jin, L.; Yang, Y.; Lam, P.K.S.; Kannan, K.; Yamashita, N. Perfluorinated compounds in tap water from China and several other countries. Environ. Sci. Technol. 2009, 43, 4824–4829. [Google Scholar] [CrossRef]
- Quinete, N.; Wu, Q.; Zhang, T.; Yun, S.H.; Moreira, I.; Kannan, K. Specific profiles of perfluorinated compounds in surface and drinking waters and accumulation in mussels, fish, and dolphins from southeastern Brazil. Chemosphere 2009, 77, 863–869. [Google Scholar] [CrossRef]
- Quiñones, O.; Snyder, S.A. Occurrence of perfluoroalkyl carboxylates and sulfonates in drinking water utilities and related waters from the United States. Environ. Sci. Technol. 2009, 43, 9089–9095. [Google Scholar] [CrossRef]
- Takagi, S.; Adachi, F.; Miyano, K.; Koizumi, Y.; Tanaka, H.; Mimura, M.; Watanabe, I.; Tanabe, S.; Kannan, K. Perfluorooctanesulfonate and perfluorooctanoate in raw and treated tap water from Osaka, Japan. Chemosphere 2008, 72, 1409–1412. [Google Scholar] [CrossRef]
- Thompson, J.; Eaglesham, G.; Mueller, J. Concentrations of PFOS, PFOA and other perfluorinated alkyl acids in Australian drinking water. Chemosphere 2011, 83, 1320–1325. [Google Scholar] [CrossRef]
- Wilhelm, M.; Bergmann, S.; Dieter, H.H. Occurrence of perfluorinated compounds (PFCs) in drinking water of North Rhine-Westphalia, Germany, and new approach to assess drinking water contamination by shorter-chained C4-C7 PFCs. Int. J. Hyg. Environ. Health 2010, 213, 224–232. [Google Scholar] [CrossRef]
- Rankin, K.; Mabury, S.A.; Jenkins, T.M.; Washington, J.W. A North American and global survey of perfluoroalkyl substances in surface soils: Distribution patterns and mode of occurrence. Chemosphere 2016, 161, 333–341. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Meng, J.; Liu, S.; Lu, Y.; Khim, J.S.; Giesy, J.P. A review of sources, multimedia distribution and health risks of perfluoroalkyl acids (PFAAs) in China. Chemosphere 2015, 129, 87–99. [Google Scholar] [CrossRef]
- Zareitalabad, P.; Siemens, J.; Hamer, M.; Amelung, W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater—A review on concentrations and distribution coefficients. Chemosphere 2013, 91, 725–732. [Google Scholar] [CrossRef]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A community data resource for environmental chemistry. J. Cheminform. 2017, 9, 61. [Google Scholar] [CrossRef]
- Patlewicz, G.; Richard, A.M.; Williams, A.J.; Grulke, C.M.; Sams, R.; Lambert, J.; Noyes, P.D.; DeVito, M.J.; Hines, R.N.; Strynar, M.; et al. A chemical category-based prioritization approach for selecting 75 per- and polyfluoroalkyl substances (PFAS) for tiered toxicity and toxicokinetic testing. Environ. Health Perspect. 2019, 127, 014501. [Google Scholar] [CrossRef]
- Patlewicz, G.; Richard, A.M.; Williams, A.J.; Judson, R.S.; Thomas, R.S. Towards reproducible structure-based chemical categories for PFAS to inform and evaluate toxicity and toxicokinetic testing. Comput. Toxicol. 2022, 24, 100250. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [PubMed]
- Cousins, I.T.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A. PFAS: Forever chemicals—Persistent, bioaccumulative and mobile. Reviewing the status and the need for their phase-out and remediation of contaminated sites. Environ. Sci. Eur. 2023, 35, 1–18. [Google Scholar]
- Koshy, T.T.; Attina, T.M.; Ghassabian, A.; Gilbert, J.; Burdine, L.K.; Marmor, M.; Honda, M.; Chu, D.B.; Han, X.; Shao, Y.; et al. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environ. Int. 2017, 109, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 147–168. [Google Scholar] [CrossRef]
- Grandjean, P.; Andersen, E.W.; Budtz-Jørgensen, E.; Nielsen, F.; Mølbak, K.; Weihe, P.; Heilmann, C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 2012, 307, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Rice, N.; Depledge, M.H.; Henley, W.E.; Galloway, T.S. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the US National Health and Nutrition Examination Survey. Environ. Health Perspect. 2010, 118, 686–692. [Google Scholar]
- Fei, C.; McLaughlin, J.K.; Lipworth, L.; Olsen, J. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod. 2009, 24, 1200–1205. [Google Scholar] [CrossRef]
- Eriksen, K.T.; Sørensen, M.; McLaughlin, J.K.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Perfluorooctanoic acid and perfluorooctane sulfonate plasma levels and risk of cancer in the general Danish population. J. Natl. Cancer Inst. 2009, 101, 605–609. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Wang, Z.; Wang, X.; Cai, Y.; Shi, Y. Atmospheric Emission of Per- and Polyfluoroalkyl Substances (PFAS) from a Fluoropolymer Manufacturing Facility: Focus on Emerging PFAS and the Potential Contribution of Condensable PFAS on their Atmospheric Partitioning. Environ. Sci. Technol. 2025, 59, 9709–9720. [Google Scholar] [CrossRef]
- Ahrens, L.; Rakovic, J.; Ekdahl, S.; Kallenborn, R. Environmental distribution of per- and polyfluoroalkyl substances (PFAS) on Svalbard: Local sources and long-range transport to the Arctic. Chemosphere 2023, 345, 140463. [Google Scholar] [CrossRef]
- Hartz, W.F.; Björnsdotter, M.K.; Yeung, L.W.Y.; Humby, J.D.; Eckhardt, S.; Evangeliou, N.; Ericson Jogsten, I.; Kärrman, A.; Kallenborn, R. Sources and seasonal variations of per- and polyfluoroalkyl substances (PFAS) in surface snow in the Arctic. Environ. Sci. Technol. 2024, 58, 21817–21828. [Google Scholar] [CrossRef]
- Cousins, I.T.; Johansson, J.H.; Salter, M.E.; Sha, B.; Scheringer, M. Outside the safe operating space of a new planetary boundary for per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Technol. 2022, 56, 11172–11179. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef]
- EPA. Method 537; Version 1.1. Determination of Selected Perfluorinated Alkyl Acids in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS); U.S. Environmental Protection Agency: Washington, DC, USA, 2008; EPA/600/R-08/092.
- EPA. Method 533; Determination of Per-and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry; U.S. Environmental Protection Agency: Washington, DC, USA, 2019; EPA Document No. 815-B-19-020, 1–52.
- EPA. Method 537.1; Version 2.0. Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS); U.S. Environmental Protection Agency: Washington, DC, USA, 2020.
- EPA. SW-846 Method 8327; Per-and Polyfluoroalkyl Substances (PFAS) Using External Standard Calibration and Multiple Reaction Monitoring (MRM) Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS); U.S. Environmental Protection Agency: Washington, DC, USA, 2019; Revision 0.
- ISO 21675; Water Quality—Determination of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in Water—Method Using Solid Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 25101; Water Quality—Determination of Perfluorooctanesulfonate (PFOS) and Perfluorooctanoate (PFOA)—Method for Unfiltered Samples Using Solid Phase Extraction and Liquid Chromatography/Mass Spectrometry. International Organization for Standardization: Geneva, Switzerland, 2009.
- ASTM D7979-20; Standard Test Method for Determination of Per-and Polyfluoroalkyl Substances in Water, Sludge, Influent, Effluent, and Wastewater by Liquid Chromatography Tandem Mass Spectrometry (LC/MS/MS). ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D7968-17a; Standard Test Method for Determination of Polyfluorinated Compounds in Soil by Liquid Chromatography Tandem Mass Spectrometry (LC/MS/MS). ASTM International: West Conshohocken, PA, USA, 2017.
- Alzaga, R.; Salgado-Petinal, C.; Jover, E.; Bayona, J. Development of a procedure for the determination of perfluorocarboxylic acids in sediments by pressurised fluid extraction, headspace solid-phase microextraction followed by gas chromatographic–mass spectrometric determination. J. Chromatogr. A 2005, 1083, 1–6. [Google Scholar]
- Rogatsky, E.; O’Hehir, C.; Daly, J.; Tedesco, A.; Jenny, R.; Aldous, K. Development of high throughput LC/MS/MS method for analysis of perfluorooctanoic acid from serum, suitable for large-scale human biomonitoring. J. Chromatogr. B 2017, 1049–1050, 24–29. [Google Scholar]
- Kuklenyik, Z.; Reich, J.A.; Tully, J.S.; Needham, L.L.; Calafat, A.M. Automated Solid-Phase Extraction and Measurement of Perfluorinated Organic Acids and Amides in Human Serum and Milk. Environ. Sci. Technol. 2004, 38, 3698–3704. [Google Scholar] [CrossRef] [PubMed]
- Takino, M.; Daishima, S.; Nakahara, T. Determination of perfluorooctane sulfonate in river water by liquid chromatography/atmospheric pressure photoionization mass spectrometry by automated on-line extraction using turbulent flow chromatography. Rapid Commun. Mass Spectrom. 2003, 17, 383–390. [Google Scholar] [PubMed]
- EPA. PFAS Drinking Water Advisory Levels. U.S. Environmental Protection Agency. 2024. Available online: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas (accessed on 23 August 2025).
- NYSDEC. New York State PFAS Regulations for Drinking Water; New York State Department of Environmental Conservation: Albany, NY, USA, 2024. Available online: https://dec.ny.gov/environmental-protection/site-cleanup/pfas (accessed on 23 August 2025).
- MassDEP. Final PFAS Drinking Water Standard and Cleanup Standards; Massachusetts Department of Environmental Protection: Boston, MA, USA, 2024. Available online: https://www.mass.gov/info-details/per-and-polyfluoroalkyl-substances-pfas-in-drinking-water (accessed on 23 August 2025).
- EEA. European PFAS Drinking Water Directive Guidelines. European Environment Agency. 2023. Available online: https://www.eea.europa.eu/en/european-zero-pollution-dashboards/indicators/treatment-of-drinking-water-to-remove-pfas-signal (accessed on 23 August 2025).
- UBA. PFAS Regulations in German Drinking Water. German Federal Environment Agency. 2023. Available online: https://www.umweltbundesamt.de/en/topics/water/drinking-water (accessed on 23 August 2025).
- NHMRC. PFAS in Australian Drinking Water. National Health and Medical Research Council. 2023. Available online: https://www.nhmrc.gov.au/health-advice/environmental-health/water/PFAS-review/questions-and-answers (accessed on 23 August 2025).
- MOE Japan. PFAS Drinking Water Standards in Japan. Ministry of the Environment Japan. 2023. Available online: https://www.env.go.jp/en/water/standard/pfas.html (accessed on 23 August 2025).
- NHC. PFAS Guidelines for Drinking Water in China. National Health Commission of China. 2023. Available online: http://www.nhc.gov.cn/ (accessed on 23 August 2025).
- DWS RSA. South Africa PFAS Drinking Water Guidelines. Department of Water and Sanitation, South Africa. 2023. Available online: https://www.dws.gov.za/ (accessed on 23 August 2025).
- IBAMA. PFAS Regulations in Brazilian Drinking Water. Brazilian Institute of Environment and Renewable Natural Resources. 2023. Available online: https://www.ibama.gov.br/ (accessed on 23 August 2025).
| References | Definitions |
|---|---|
| Buck et al. (2011) [6] | -Definition: PFAS are aliphatic substances where all hydrogen atoms in the carbon chain are replaced by fluorine atoms, including the “perfluoroalkyl moiety (−CnF2n+1−).” -Note: The moiety implies a fully fluorinated terminal carbon, but the textual definition does not explicitly require it. |
| OECD (2018) [13] | -Definition: PFAS are chemicals with a perfluoroalkyl moiety containing at least three carbons (–CnF2n−, n ≥ 3) or a perfluoroalkyl ether moiety with at least two carbons (–CnF2nOCmF2m−, n, m ≥ 1). -Note: Expanded the perfluoroalkyl moiety from Buck et al.’s “(CnF2n+1−)” to “–CnF2n–” including cases where both ends of the moiety are attached to functional groups. |
| TSCA (2020) [14] | -Definition: Any chemical substance or mixture containing the structural unit R-(CF2)-C(F)(R′)R″. -Both CF2 and CF moieties are saturated carbons, and none of the R groups (R, R′, or R″) can be hydrogen. -Application: Proposed rule for TSCA reporting and recordkeeping requirements and the 2021 Draft Drinking Water Contaminant Candidate List. |
| National Defense Authorization (2020) [15] | -Definition: Man-made chemicals with at least one fully fluorinated carbon atom. -Note: A simplified definition to encompass a broad range of PFAS. |
| OECD (2021) [1] | -Definition: Fluorinated substances containing at least one fully fluorinated methyl (–CF3) or methylene (–CF2–) carbon atom without any H/Cl/Br/I attached. -Note: Removes the requirement for entirely aliphatic structures, only requiring a minimally fully fluorinated carbon group. |
| EPA (2021) [16] | -PFASMASTER List: Initially contained over 5000 unique PFASs, including substances without defined chemical structures, polymers, and mixtures. -PFASSTRUCT List: Structure-based definitions to clearly delineate PFAS chemical space for research and regulatory purposes. |
| Sample Matrix | Analytical Technique | Extraction Approach | Reference |
|---|---|---|---|
| Air and air particles | GC-MS 1, GC-MS/MS 2, LC-MS/MS 3 | ASE 10, cold column extraction, concentration after solvent capture, SLE 11, Soxhlet extraction, SPE 12 | [26,27,28,29,30,31,32,33,34,35,36,37] |
| Water | GC-MS/MS, LC-MS/MS, LC-HRMS 4, 19F-NMR 5, Nano-LC-MS 6 | Automated solid-phase extraction, LLE 13, micro-LLE 14, Soxhlet extraction, SPE, SPME 15, turbulent flow chromatograph-based online extraction | [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] |
| Soil and sediment | Flow injection-MS/MS 7, LC-HRMS, LC-MS/MS, LC-QToF-MS 8 | FUSLE 16, hot vapor/Soxhelt extraction and PLE 17, PLE, SLE, SPE | [55,56,57,58,59,60,61,62,63,64] |
| Foods | LC-MS/MS, LC-QqLIT-MS 9 | FUSLE, IPE 18, LLE, microextraction, PLE, SLE, SPE | [65,66,67,68,69,70,71,72] |
| Packaging materials | GC-MS, LC-MS/MS, LC-QToF-MS | FUSLE, PLE, SLE, SPE, UPAE 19, XAD extracted with EtOAc 20 | [73,74,75,76,77] |
| Reference | Exposure Pathway | Biological Sample | Target Organ | Exposure Duration | Health Outcome | Measured PFAS Concentration |
|---|---|---|---|---|---|---|
| Cousins et al., 2023 [120] | Food, drinking water, environment | Not reported | Liver, kidney, thyroid, immune system | Chronic | Hepatotoxicity, immune suppression, endocrine disruption | Frequently exceeded EFSA TWI 1 of 4.4 ng/kg bw/week |
| Koshy et al., 2017 [121] | Environmental (disaster-related) | Serum | Metabolic system (lipids, insulin) | Adolescence | Dyslipidemia, insulin resistance | Serum PFASs (PFOA, PFHxS, PFNA) in WTC-exposed 2 adolescents; PFOA positively associated with cholesterol and triglycerides |
| Sunderland et al., 2018 [122] | Seafood, drinking water, food packaging, indoor environment | Not reported | Immune, metabolic, nervous systems | Long-term, chronic | Immune suppression, metabolic disorders, neurodevelopmental issues | Global biomonitoring: PFOS, PFOA, PFNA, PFHxS stable or increasing in serum |
| Fenton et al., 2021 [119] | Multiple environmental sources | Not reported | Liver, immune system | Long-term | Liver dysfunction, reduced immune response, developmental impairment | Doubling of serum PFOS/PFOA associated with ~39–49% reduction in vaccine antibody levels |
| Grandjean et al., 2012 [123] | Maternal exposure (placenta, child environment) | Maternal blood, cord blood, child serum | Immune system | Birth to childhood | Reduced vaccine antibody response, impaired immune function | Two-fold increase in PFOS/PFOA linked to ~39–49% lower antibody concentrations |
| Melzer et al., 2010 [124] | General environmental exposure | Serum | Thyroid | Chronic | Altered thyroid hormone levels | NHANES 3 data: serum PFOA/PFOS detected; associated with thyroid disease |
| Fei et al., 2009 [125] | Maternal PFAS levels | Maternal plasma | Fetus | Pregnancy | Reduced birth weight, developmental effects | Danish cohort 4: higher maternal PFOS/PFOA levels linked to reduced birth weight |
| Eriksen et al., 2009 [126] | General environmental exposure | Plasma | Liver | Chronic | Increased liver cancer risk | Danish cohort: plasma PFOA/PFOS associated with ~30–40% higher liver cancer risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Jeon, H.; Bae, M.-S. Linking Analysis to Atmospheric PFAS: An Integrated Framework for Exposure Assessment, Health Risks, and Future Management Strategies. Appl. Sci. 2025, 15, 10540. https://doi.org/10.3390/app151910540
Song M, Jeon H, Bae M-S. Linking Analysis to Atmospheric PFAS: An Integrated Framework for Exposure Assessment, Health Risks, and Future Management Strategies. Applied Sciences. 2025; 15(19):10540. https://doi.org/10.3390/app151910540
Chicago/Turabian StyleSong, Myoungki, Hajeong Jeon, and Min-Suk Bae. 2025. "Linking Analysis to Atmospheric PFAS: An Integrated Framework for Exposure Assessment, Health Risks, and Future Management Strategies" Applied Sciences 15, no. 19: 10540. https://doi.org/10.3390/app151910540
APA StyleSong, M., Jeon, H., & Bae, M.-S. (2025). Linking Analysis to Atmospheric PFAS: An Integrated Framework for Exposure Assessment, Health Risks, and Future Management Strategies. Applied Sciences, 15(19), 10540. https://doi.org/10.3390/app151910540







