A New Approach for the Utilization of Technical Egg Albumen Based on Acid–Thermal Coagulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Raw Materials
TA Extraction
Proximate Composition
Protein Separation and Identification
Amino Acid Profiling
2.2.2. Physicochemical Characteristics
pH Determination
Color Characteristic Determination
Water Binding Capacity (WBC)
Water Activity (aW)
2.2.3. Microbiological Status
2.2.4. Production Parameters
Pressing Efficiency (PE%)
Yield (Y)
2.2.5. Hydrolytic and Oxidative Changes
Oxidative Changes in Lipid Fraction
Hydrolytic and Oxidative Changes in Protein Fraction
2.2.6. Data Processing
3. Results
3.1. Raw Materials Characterization
3.2. Processing of the Technical Albumen
3.3. Characterization and Changes During Storage of Dried Technical Albumen Products
3.3.1. Proximate Composition and Aminoacidic Profile
3.3.2. Changes in Physicochemical Properties During Storage
3.3.3. Changes in Protein Fraction During Storage
3.3.4. Changes in Lipid Fraction During Storage
3.3.5. Changes in Microbial Status During Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitkova, T.; Enikova, R.; Karcheva, M.; Saraliev, P. Eggs in the human diet-facts and challenges. J. IMABs 2024, 30, 5314–5322. [Google Scholar] [CrossRef]
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Modifying the functional properties of egg proteins using novel processing techniques: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 986–1002. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Wu, L.; Younes, M.; Hincke, M. 2021. Biotechnological applications of eggshell: Recent advances. Front. Bioeng. Biotechnol. 2021, 9, 675364. [Google Scholar] [CrossRef]
- Lechevalier-Datin, V.; Guérin-Dubiard, C.; Pasco, M.; Gillard, A.; Le Gouar, Y.; Musikaphun, N.; Nau, F. Technological advances in egg product processing with reference to allergenicity. In Proceedings of the XVIIth European Symposium on the Quality of Eggs and Egg Products, Bergamo, Italy, 15–19 September 2013; Available online: https://hal.science/hal-01595727 (accessed on 20 November 2024).
- Nwadi, O.M.M.; Uchegbu, N.N.; Okonkwo, T.M. Use of Cereals and Egg Products in Extruded Foods: A Review. Pak. J. Nutr. 2023, 22, 1–10. [Google Scholar] [CrossRef]
- Vitkova, T.G.; Enikova, R.K.; Stoynovska, M.R. Medical evaluation of the potential biological and chemical dangers in high-risk foods. J. IMABs 2021, 27, 3924–3929. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Benelli, P.; Amante, E.R. A literature review on adding value to solid residues: Egg shells. J. Clean. Prod. 2013, 46, 42–47. [Google Scholar] [CrossRef]
- Hughes, R. The EU circular economy package–life cycle thinking to life cycle law? Procedia Cirp 2017, 61, 10–16. [Google Scholar] [CrossRef]
- Mignardi, S.; Archilletti, L.; Medeghini, L.; De Vito, C. Valorization of eggshell biowaste for sustainable environmental remediation. Sci. Rep. 2020, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, L.; Jha, H.; Sarkar, T.; Sarangi, P.K. Food waste utilization for reducing carbon footprints towards sustainable and cleaner environment: A review. Int. J. Environ. Res. Public Health 2023, 20, 2318. [Google Scholar] [CrossRef]
- Budžaki, S.; Velić, N.; Ostojčić, M.; Stjepanović, M.; Rajs, B.B.; Šereš, Z.; Strelec, I. Waste management in the agri-food industry: The conversion of eggshells, spent coffee grounds, and brown onion skins into carriers for lipase immobilization. Foods 2022, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Strelec, I.; Ostojčić, M.; Brekalo, M.; Hajra, S.; Kim, H.J.; Stanojev, J.; Budžaki, S. Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder. Green Process. Synth. 2023, 12, 20228151. [Google Scholar] [CrossRef]
- Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 Laying Down Health Rules as Regards Animal by-Products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 (Animal by-Products Regulation). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32009R1069&qid=1728306205351 (accessed on 20 November 2024).
- Commission Regulation (EU) No. 68/2013 of 16 January 2013 on the Catalogue of Feed Materials. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013R0068&qid=1728306278989 (accessed on 20 November 2024).
- Shurson, G.C. “What a waste”—Can we improve sustainability of food animal production systems by recycling food waste streams into animal feed in an era of health, climate, and economic crises? Sustainability 2020, 12, 7071. [Google Scholar] [CrossRef]
- Schmidt, L.D.; Blank, G.; Boros, D.; Slominski, B.A. The nutritive value of egg by-products and their potential bactericidal activity: In vitro and in vivo studies. J. Sci. Food Agric. 2007, 87, 378–387. [Google Scholar] [CrossRef]
- El-Deek, A.A.; Al-Harthi, M.A.; Bamarouf, A.O. The use of dried whole processed eggs as a feed additive to maintain broiler performance. In Proceeding of the 3rd International Poultry Conference, Hurghada, Egypt, 4–7 April 2005. [Google Scholar]
- James, B.W.; Sparks, J.C.; Jurgens, M.H.; Zimmerman, D.R. Comparison of Inedible Egg Product and Spray-Dried Plasma as Sources of Protein for Weanling Pigs. Iowa State Univ. Anim. Ind. Rep. 2000, 1, 1. [Google Scholar]
- Banožić, M.; Vladić, J.; Banjari, I.; Velić, D.; Aladić, K.; Jokić, S. Spray Drying as a Method of Choice for Obtaining High Quality Products from Food Wastes–A Review. Food Rev. Int. 2023, 39, 1953–1985. [Google Scholar] [CrossRef]
- Saraliev, P.; Balev, D.; Vlahova-Vangelova, D.; Kolev, N.; Dragoev, S. Examining coagulation conditions for the utilization of technical albumen. A preliminary study. BIO Web Conf. 2024, 102, 01004. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No. 142/2011 of 25 February 2011 Implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council Laying Down Health Rules as Regards Animal by-Products and Derived Products Not Intended for Human Consumption and Implementing Council Directive 97/78/EC as Regards Certain Samples and Items Exempt from Veterinary Checks at the Border Under That Directive. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32011R0142&qid=1732736923714 (accessed on 20 November 2024).
- AOAC. Official Methods of Analysis of AOAC International. AOAC 984.13–1994 Protein (Crude) in Animal Feed and Pet Food; AOAC: Rockville, MD, USA, 1996. [Google Scholar]
- ISO 1444:1996; Meat and Meat Products—Determination of Free Fat Content. ISO: Rome, Italy, 1996.
- ISO 1442:2023; Meat and Meat Products—Determination of Moisture Content—Reference method. ISO: Rome, Italy, 2023.
- ISO 936:1998; Meat and Meat Products—Determination of Total Ash. ISO: Rome, Italy, 1998.
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Uysal, R.S.; Acar-Soykut, E.; Boyaci, I.H. Determination of yolk: White ratio of egg using SDS-PAGE. Food Sci. Biotechnol. 2020, 29, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Heinrikson, R.L.; Meredith, S.C. Amino acid analysis by reverse-phase high-performance liquid chromatography: Precolumn derivatization with phenylisothiocyanate. Anal. Biochem. 1984, 136, 65–74. [Google Scholar] [CrossRef]
- Yust, M.M.; Pedroche, J.; Girón-Calle, J.; Vioque, J.; Millán, F.; Alaiz, M. Determination of tryptophan by high-performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection. Food Chem. 2004, 85, 317–320. [Google Scholar] [CrossRef]
- Opienska-Blauth, J.; Charȩziński, M.; Berbeć, H. A new, rapid method of determining tryptophan. Anal. Biochem. 1963, 6, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Dong, J.; Peng, Y.; Tang, X. Comparative study of albumen pH and whole egg pH for the evaluation of egg freshness. Spectrosc. Lett. 2017, 50, 463–469. [Google Scholar] [CrossRef]
- Zang, J.; Qing, M.; Chi, Y.; Chi, Y. Extending the shelf-life of whole egg powder with different packaging: Based on the multivariate accelerated shelf-life test model. Food Chem. 2004, 460, 140602. [Google Scholar] [CrossRef] [PubMed]
- Sze, W.K.; Huda, N.; Dewi, M.; Hashim, H. Physicochemical properties of egg white powder from eggs of different types of bird. Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 384–389. [Google Scholar] [CrossRef]
- ISO 6887-4:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. ISO: Rome, Italy, 2017. Available online: https://www.iso.org/standard/63338.html (accessed on 20 November 2024).
- EN ISO 4833-1:2013/A1:2022; Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. 2022. Available online: https://www.iso.org/standard/73329.html (accessed on 20 November 2024).
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, K.; Ivanov, G.; Balev, D.; Dobrev, G. Protein changes of chicken light and dark muscles during chilled storage. J. EcoAgriTourism 2012, 8, 263–268. [Google Scholar]
- Mercier, Y.; Gatellier, P.; Renerre, M. Lipid and protein oxidation in vitro, and antioxidant potential in meat from Charolais cows finished on pasture or mixed diet. Meat Sci. 2004, 66, 467–473. [Google Scholar] [CrossRef]
- Regulation (EC) No 999/2001 of the European Parliament and of the Council of 22 May 2001 Laying Down Rules for the Prevention, Control and Eradication of Certain Transmissible Spongiform Encephalopathies. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32001R0999&qid=1732736575679 (accessed on 20 November 2024).
- Jedrejek, D.; Lević, J.; Wallace, J.; Oleszek, W. Animal by-products for feed: Characteristics, European regulatory framework, and potential impacts on human and animal health and the environment. J. Anim. Feed Sci. 2016, 25, 189–202. [Google Scholar] [CrossRef]
- Saraliev, P.; Kolev, N. Valorization of egg processing by-products. In Proceedings of the Youth forums “Science, Technology, Innovation, Business”, Plovdiv, Bulgaria, 27–28 April 2023; p. 35. [Google Scholar]
- Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32004R0853&qid=1732736738799 (accessed on 20 November 2024).
- Song, M.; Kim, S.; Kim, Y.; Park, J.; Kim, Y. Value of spray-dried egg in pig nursery diets. Korean J. Agric. Sci. 2015, 42, 207–213. [Google Scholar] [CrossRef][Green Version]
- Norberg, S.E.; Dilger, R.N.; Dong, H.; Harmon, B.G.; Adeola, O.; Latour, M.A. Utilization of energy and amino acids of spray-dried egg, plasma protein, and soybean meal by ducks. Poult. Sci. 2004, 83, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Matumoto-Pintro, P.T.; Murakami, A.E.; Vital, A.C.P.; Croge, C.; da Silva, D.F.; Ospina-Roja, I.C.; Guerra, A.F.Q.G. Effects of storage time and temperature on lipid oxidation of egg powders enriched with natural antioxidants. Food Chem. 2017, 228, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Keerthirathne, T.P.; Ross, K.; Fallowfield, H.; Whiley, H. A review of temperature, pH, and other factors that influence the survival of Salmonella in mayonnaise and other raw egg products. Pathogens 2016, 5, 63. [Google Scholar] [CrossRef] [PubMed]
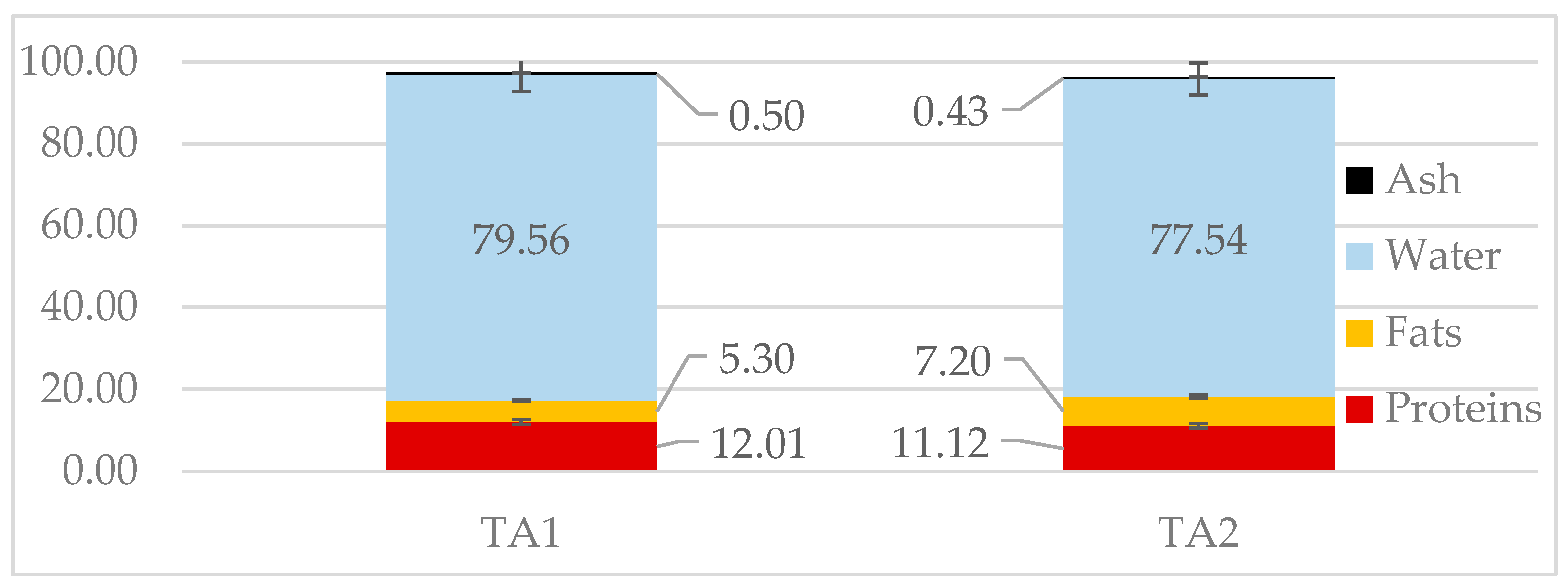


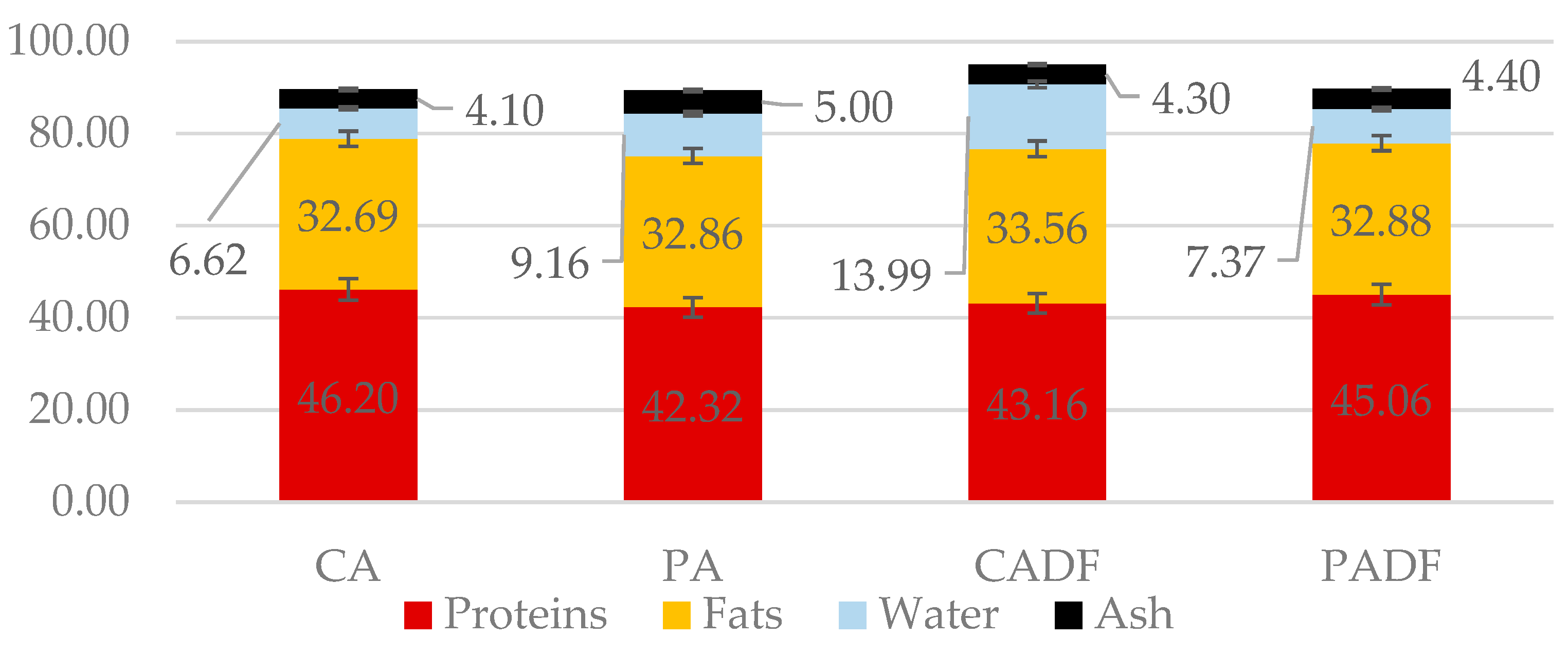
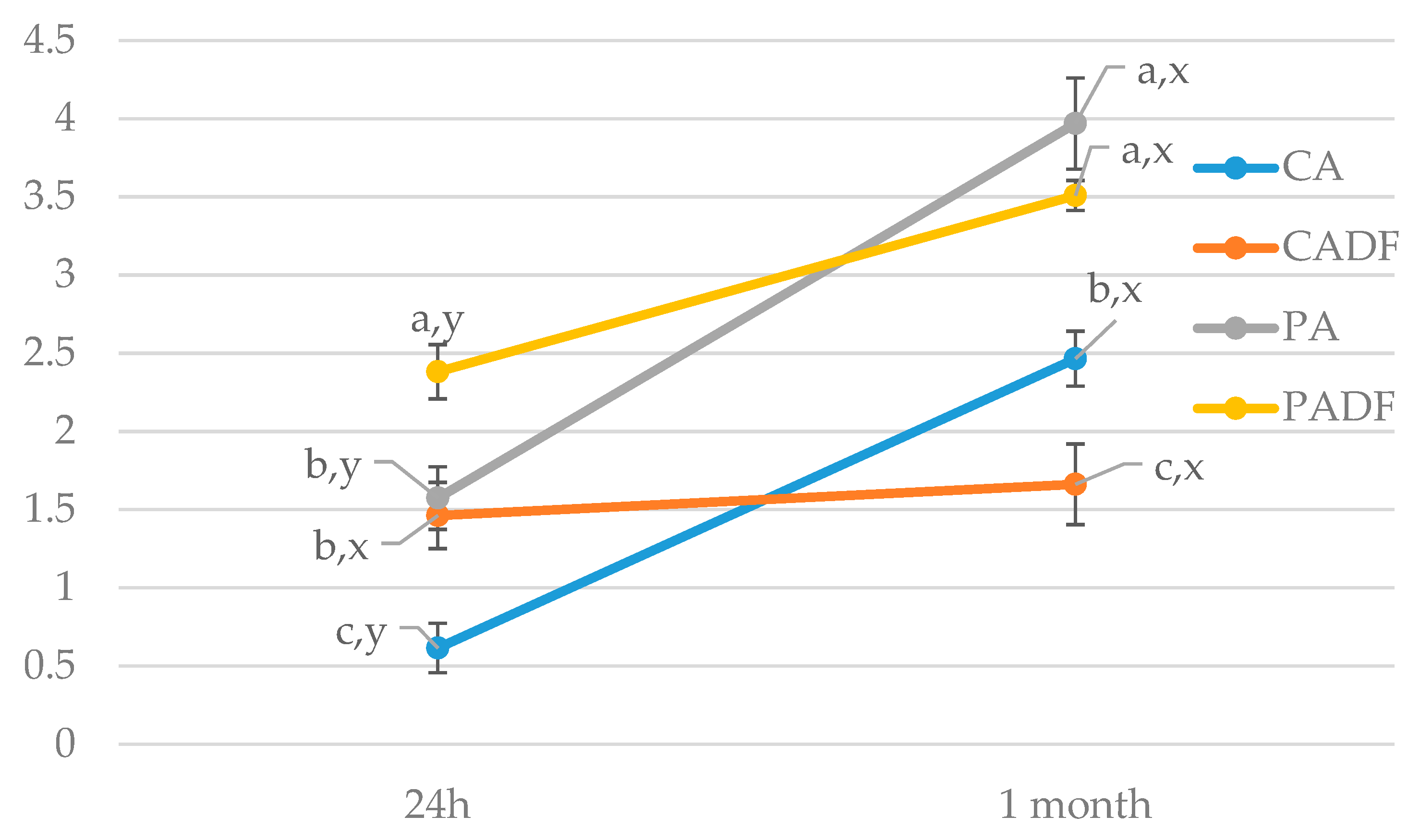
| Time, min | Buffer—A, % | AN (60%), % | Flow, mL/min |
|---|---|---|---|
| 0 | 100 | 0 | 0.9 |
| 0.5 | 98 | 2 | 0.9 |
| 18 | 93 | 7 | 0.9 |
| 22 | 90 | 10 | 0.9 |
| 33 | 67 | 33 | 0.8 |
| 34 | 65 | 35 | 0.8 |
| 36 | 50 | 50 | 0.8 |
| 38 | 0 | 100 | 0.8 |
| 40 | 100 | 0 | 0.8 |
| mg/mL | TA1 | TA2 | p-Value |
|---|---|---|---|
| Aspartic acid | 6.63 a ± 0.39 | 7.16 a ± 0.41 | 0.827886 |
| Serine | 4.05 a ± 0.43 | 4.37 a ± 0.45 | 0.712457 |
| Glutamic acid | 3.62 a ± 0.39 | 3.91 a ± 0.40 | 0.844731 |
| Glycine | 1.67 a ± 0.24 | 1.80 a ± 0.25 | 0.75009 |
| Histidine | 2.47 a ± 0.33 | 2.66 a ± 0.34 | 0.842366 |
| Arginine | 13.18 a ± 0.19 | 14.24 a ± 0.19 | 0.802974 |
| Threonine | 3.06 a ± 0.44 | 3.30 a ± 0.45 | 0.61783 |
| Alanine | 7.56 a ± 0.47 | 8.16 a ± 0.48 | 0.80066 |
| Proline | 3.77 a ± 0.42 | 4.07 a ± 0.43 | 0.700507 |
| Cysteine | 0.08 a ± 0.08 | 0.09 a ± 0.08 | 0.823836 |
| Tyrosine | 3.17 a ± 0.54 | 3.42 a ± 0.56 | 0.588348 |
| Valine | 2.56 a ± 0.32 | 2.76 a ± 0.33 | 0.732944 |
| Methionine | 1.62 a ± 0.25 | 1.75 a ± 0.26 | 0.722243 |
| Lysine | 6.44 a ± 0.38 | 6.95 a ± 0.39 | 0.793566 |
| Isoleucine | 2.18 a ± 0.29 | 2.35 a ± 0.31 | 0.727851 |
| Leucine | 3.63 a ± 0.45 | 3.92 a ± 0.47 | 0.687175 |
| Phenylalanine | 1.91 a ± 0.45 | 2.06 a ± 0.47 | 0.380355 |
| Tryptophan | 3.00 ± 0.21 | 3.55 a ± 0.19 | 0.935071 |
| TA1 | TA2 | p-Value | |
|---|---|---|---|
| pH value | 7.91 a ± 0.02 | 7.73 b ± 0.01 | 0.0002 |
| L* | 66.50 a ± 0.05 | 65.70 b ± 0.17 | 0.0008 |
| a* | 10.72 b ± 0.03 | 11.62 a ± 0.03 | 2.41446 × 10−6 |
| b* | 39.31 a ± 0.12 | 36.36 b ± 0.12 | 7.33241 × 10−6 |
| C | 40.75 a ± 0.11 | 38.17 b ± 0.11 | 8.1569 × 10−6 |
| h | 74.76 a ± 0.07 | 72.29 b ± 0.08 | 2.5518 × 10−6 |
| CFU/g | TA1 | TA2 | p-Value |
|---|---|---|---|
| TPC | 6.90 a ± 0.06 | 7.13 a ± 0.20 | 0.129065 |
| Coliforms | 4.70 b ± 0.16 | 5.35 a ± 0.30 | 0.029619 |
| Enterobacteriaceae | 20 b | 60 a | 0.003448 |
| Salmonella spp. | N.F. | N.F. | - |
| mg/g Protein | CA | CADF | PA | PADF | p-Value |
|---|---|---|---|---|---|
| Aspartic acid | 74.34 b ± 0.52 | 109.65 a ± 2.02 | 51.46 c ± 0.51 | 38.78 d ± 1.74 | 0.000295 |
| Serine | 48.00 b ± 0.66 | 68.04 a ± 1.72 | 29.74 c ± 0.88 | 33.70 c ± 1.41 | 0.00355 |
| Glutamic acid | 70.79 a ± 1.00 | 88.38 a ± 1.83 | 65.56 a ± 0.72 | 70.34 a ± 1.26 | 0.183027 |
| Glycine | 30.19 b ± 0.99 | 34.98 a ± 1.19 | 24.34 c ± 0.41 | 17.92 d ± 1.25 | 0.0073 |
| Histidine | 57.55 a ± 0.55 | 61.24 a ± 0.83 | 48.88 a ± 1.22 | 47.02 a ± 1.00 | 0.068277 |
| Arginine | 298.35 a ± 1.38 | 309.28 a ± 1.60 | 218.83 b ± 3.31 | 22.21 c ± 0.24 | 0.018412 |
| Threonine | 63.99 a ± 2.16 | 78.63 a ± 2.43 | 41.64 a ± 1.22 | 52.77 a ± 1.15 | 0.063141 |
| Alanine | 154.59 a ± 3.63 | 212.98 a ± 4.59 | 121.46 a ± 2.66 | 135.03 a ± 2.51 | 0.185893 |
| Proline | 79.51 b ± 1.31 | 98.50 a ± 2.26 | 59.70 c ± 1.86 | 44.13 d ± 1.56 | 0.003679 |
| Cysteine | 2.49 a ± 0.27 | 1.89 a ± 0.61 | 3.70 a ± 0.35 | 1.88 a ± 0.66 | 0.087418 |
| Tyrosine | 69.85 a ± 1.97 | 72.51 a ± 2.12 | 31.48 b ± 0.62 | 38.40 b ± 0.72 | 0.003063 |
| Valine | 31.31 b ± 0.89 | 46.36 a ± 1.59 | 26.28 c ± 0.83 | 21.03 d ± 0.24 | 0.001462 |
| Methionine | 30.88 b ± 1.08 | 38.90 a ± 1.47 | 19.57 c ± 0.70 | 23.14 c ± 1.25 | 0.009944 |
| Lysine | 143.08 b ± 2.53 | 185.54 a ± 3.51 | 82.18 c ± 1.44 | 93.25 c ± 1.00 | 0.004116 |
| Isoleucine | 27.67 b ± 0.90 | 42.11 a ± 1.55 | 22.03 c ± 0.37 | 19.34 c ± 0.42 | 0.00144 |
| Leucine | 34.21 b ± 1.33 | 63.87 a ± 2.22 | 20.61 c ± 0.64 | 16.74 c ± 0.26 | 0.001309 |
| Phenylalanine | 22.95 b ± 1.45 | 36.64 a ± 1.84 | 8.73 c ± 0.40 | 10.29 c ± 0.45 | 0.005354 |
| Tryptophan | 20.29 a ± 0.81 | 19.62 a ± 0.30 | 19.41 a ± 0.40 | 22.21 a ± 0.24 | 0.086523 |
| CA | CADF | PA | PADF | p-Value | |
|---|---|---|---|---|---|
| 24 h after production | |||||
| pH value | 5.32 a,x ± 0.01 | 5.15 c,x ± 0.01 | 5.22 b,x ± 0.01 | 5.12 d,x ± 0.02 | 3.77 × 10−8 |
| L* | 80.72 a,x ± 1.23 | 78.00 b,x ± 0.25 | 80.88 a,x ± 1.33 | 80.15 a,x ± 1.00 | 0.0315 |
| a* | 4.17 b,x ± 0.41 | 5.96 a,x ± 0.12 | 4.09 b,x ± 0.42 | 4.41 b,x ± 0.38 | 0.0374 |
| b* | 39.49 b,x ± 0.54 | 43.81 a,x ± 0.33 | 39.45 b,x ± 0.60 | 39.64 b,x ± 0.59 | 0.0165 |
| WBC, % | 10.73 a,y ± 0.95 | 4.86 d,y ± 0.12 | 7.93 c,y ± 0.37 | 9.47 b,y ± 0.22 | 0.0134 |
| aW | 0.541 b,x ± 0.004 | 0.655 a,x ± 0.001 | 0.500 c,x ± 0.010 | 0.512 c,x ± 0.004 | 3.44 × 10−9 |
| 1 month after production | |||||
| pH value | 5.27 a,x ± 0.04 | 5.17 b,x ± 0.04 | 5.25 a,x ± 0.06 | 5.11 c,x ± 0.01 | 0.0008 |
| L* | 81.04 a,x ± 0.09 | 78.62 c,x ± 0.13 | 80.33 b,x ± 0.15 | 80.35 b,x ± 0.14 | 0.9452 |
| a* | 3.52 d,y ± 0.08 | 5.27 a,y ± 0.09 | 4.13 b,x ± 0.09 | 3.82 c,y ± 0.09 | 0.3968 |
| b* | 37.69 c,y ± 0.14 | 41.83 a,y ± 0.17 | 39.81 b,x ± 0.38 | 37.92 c,y ± 0.22 | 0.2705 |
| WBC, % | 16.40 c,x ± 0.58 | 20.59 a,x ± 0.41 | 18.61 b,x ± 1.00 | 18.69 b,x ± 0.46 | 9.18 × 10−9 |
| aW | 0.461 b,y ± 0.001 | 0.464 a,y ± 0.002 | 0.462 a,y ± 0.002 | 0.463 a,y ± 0.002 | 0.0002 |
| CA | CADF | PA | PADF | p-Value | |
|---|---|---|---|---|---|
| 24 h after production | |||||
| SP, mg BSA/g | 211.79 a,x ± 0.58 | 163.53 b,x ± 0.48 | 218.85 a,x ± 0.85 | 204.89 a,x ± 0.67 | 0.001165 |
| FAN, mg alanine/g | 117.55 a,x ± 0.25 | 118.69 a,x ± 0.38 | 111.24 a,x ± 0.28 | 118.97 a,x ± 0.49 | 0.176337 |
| PC, nmol/g protein | 0.35 a,y ± 0.04 | 0.40 a,x ± 0.08 | 0.26 a,y ± 0.13 | 0.43 a,y ± 0.08 | 0.017052 |
| 1 month after production | |||||
| SP, mg BSA/g | 166.85 b,y ± 0.70 | 161.23 b,x ± 0.65 | 182.85 a,y ± 0.67 | 150.60 c,y ± 0.85 | 0.0007 |
| FAN, mg alanine/g | 102.72 a,y ± 0.41 | 102.33 a,y ± 0.45 | 110.24 a,x ± 0.31 | 98.78 a,y ± 0.38 | 3.28 × 10−6 |
| PC, nmol/g protein | 0.73 a,x ± 0.16 | 0.33 b,x ± 0.07 | 0.80 a,x ± 0.14 | 0.73 a,x ± 0.16 | 0.0084 |
| CFU/g | CA | CADF | PA | PADF | p-Value/ Significance F |
|---|---|---|---|---|---|
| 24 h after production | |||||
| TPC | 4.92 a,x ± 0.47 | 4.73 a,x ± 1.33 | 4.81 a,x ± 0.39 | 5.37 a,x ± 0.35 | 0.808425 |
| Coliforms | 4.00 a ± 1.00 | 3.00 b ± 0.420 | 4.15 a ± 1.15 | 3.83 a ± 0.62 | 0.768197 |
| E. coli | N.F. | N.F. | N.F. | N.F. | - |
| Enterobacteriaceae | <10 | <10 | <10 | <10 | - |
| Salmonella spp. | N.F. | N.F. | N.F. | N.F. | - |
| 1 month after production | |||||
| TPC | 3.96 a,x ± 0.41 | 4.65 a,x ± 0.37 | 4.41 a,x ± 0.37 | 4.40 a,x ± 0.38 | 0.1670 |
| Coliforms | N.F. | N.F. | N.F. | N.F. | |
| E. coli | N.F. | N.F. | N.F. | N.F. | - |
| Enterobacteriaceae | <10 | <10 | <10 | <10 | - |
| Salmonella spp. | N.F. | N.F. | N.F. | N.F. | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraliev, P.; Balev, D.; Vlahova-Vangelova, D.; Kolev, N.; Dragoev, S. A New Approach for the Utilization of Technical Egg Albumen Based on Acid–Thermal Coagulation. Appl. Sci. 2025, 15, 510. https://doi.org/10.3390/app15020510
Saraliev P, Balev D, Vlahova-Vangelova D, Kolev N, Dragoev S. A New Approach for the Utilization of Technical Egg Albumen Based on Acid–Thermal Coagulation. Applied Sciences. 2025; 15(2):510. https://doi.org/10.3390/app15020510
Chicago/Turabian StyleSaraliev, Plamen, Desislav Balev, Desislava Vlahova-Vangelova, Nikolay Kolev, and Stefan Dragoev. 2025. "A New Approach for the Utilization of Technical Egg Albumen Based on Acid–Thermal Coagulation" Applied Sciences 15, no. 2: 510. https://doi.org/10.3390/app15020510
APA StyleSaraliev, P., Balev, D., Vlahova-Vangelova, D., Kolev, N., & Dragoev, S. (2025). A New Approach for the Utilization of Technical Egg Albumen Based on Acid–Thermal Coagulation. Applied Sciences, 15(2), 510. https://doi.org/10.3390/app15020510







