Featured Application
The phytochemical sulforaphane has been safely consumed in cruciferous vegetables, wild and cultivated, for centuries. There are multiple applications and copious mechanistic documentation supporting its use in both female- and male-specific conditions, syndromes, diseases, and cancers. All of these relate to the body’s hormonal axes in one way or another. More awareness of the specific benefits of sulforaphane on female and male healthspan is warranted for both scientists and the general public.
Abstract
The health-promoting, preventive, protective, and therapeutic applications of the natural compound sulforaphane (SF) produced from its biogenic precursor in broccoli, glucoraphanin, are extremely well established. SF has been the subject of thousands of studies and over 125 clinical trials. The many mechanisms of action of SF in mammalian systems have been extensively documented. SF is the most potent naturally occurring inducer of the Keap1/Nrf2 pathway, which is most well-known for its upregulation of antioxidant and detoxification mechanisms and activation of pathways resulting in the inhibition of inflammation. Much of this regulation involves the various hormonal axes of the body. However, the influence of SF on hormone-mediated health conditions remains unexplored in recent scholarly reviews. This review aims to address this gap by exploring many of these interactions, with a focus on the health and wellness issues specific to both females and males.
1. Introduction
An area that has yet to be the subject of recent scholarly review is that of the effects of sulforaphane (SF) on hormone-mediated health conditions, specifically those that are sex-related. This review aims to address this gap. In so doing, we have conducted extensive literature searches on both the SCOPUS® and PubMed databases as well as thoroughly scouring the reference sections of all recent papers cited herein. Although the underlying mechanisms (see Figure 1) overlap significantly with those affecting other organ systems, distinguishing them by sex may initially seem arbitrary or trivial. However, the dominant paradigm for the practice of medicine (in the USA)—centered on an organ- and disease-centric approach—makes this distinction relevant and necessary. This review, therefore, intersects with gynecologic, urologic, endocrine, and oncologic fields and also speaks to preventive and healthspan-enhancing practices. It focuses specifically on the interactions between a phytochemical and endogenously produced hormones within the context of biologically assigned sex, as determined by chromosomal and physiological characteristics.
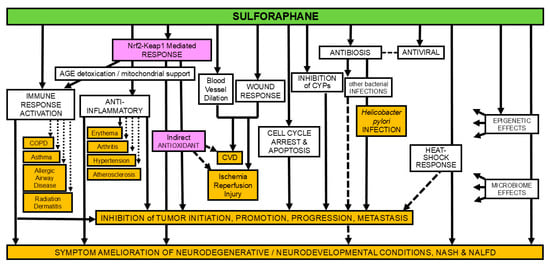
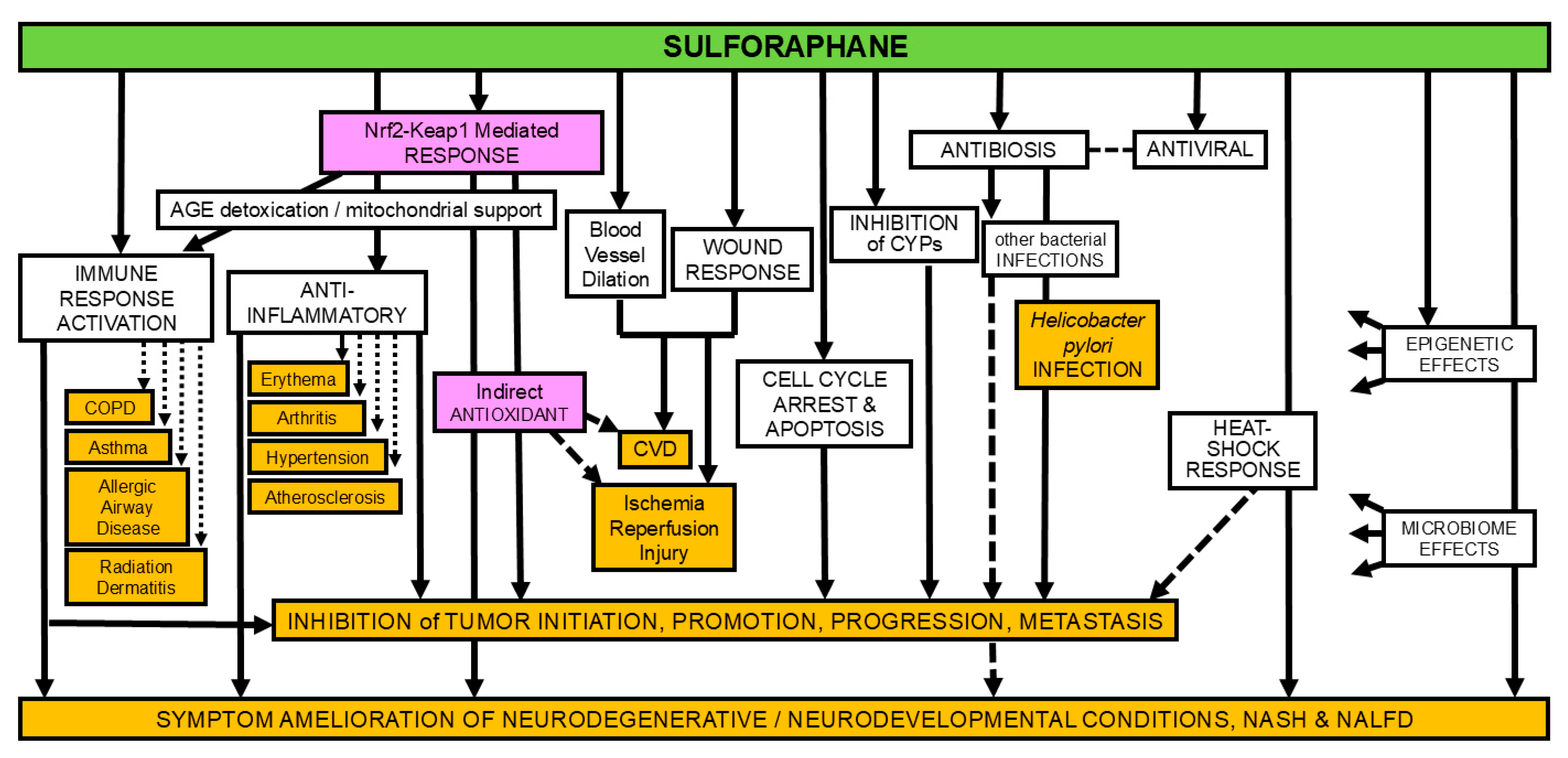
Figure 1.
The many mechanisms (not colored) of action of sulforaphane and some of the the diseases or conditions in which effects of sulforaphane have been examined experimentally (orange); magenta shading for emphasis of mode of action most targeted by clinical studies. (adapted from [1]).
The most readily identified sex-specific health conditions relate to the so-called secondary sex characteristics—the sex organs, reproductive systems, and breasts, which are distinguishing features of the two biologically defined genders. Pregnancy and its associated complications are exclusive to the female sex. Beyond these distinctions, we should note that women are more likely to experience morbidity-driven conditions such as lower back pain, depressive disorders, headache disorders, dementia, and strokes. Men, in contrast, are more likely to experience mortality-driven conditions like COVID-19 infections and ischemic heart disease, as well as chronic illnesses such as gout, kidney stones, bladder cancer, emphysema, inguinal hernias, aortic aneurysms, and STDs like chlamydia and gonorrhea, and they are much more likely to lose hair as they age.
Additionally, many conditions discussed in this review may directly impact both male and female fertility, though specific outcomes are not explicitly called out.
The connection between sex-specific health and cruciferous vegetable consumption was first highlighted in the early 1990s. The Iowa Women’s Health Study [2] revealed an inverse correlation between broccoli consumption and lung cancer risk in women, while subsequent studies showed the inverse correlation between prostate and bladder cancer and cruciferous vegetable consumption in men [3]. In the past three decades, beginning with the discovery of sulforaphane and identification of its role in cancer prevention [4] and the potency of broccoli sprouts as sources of sulforaphane [5], knowledge of its biomedical benefits has burgeoned, including its effect on all-cause mortality [6]. The growing volume of experimental data and clinical evidence has blossomed to the point that one can now focus on specific aspects of healthspan that sulforaphane and related isothiocyanates impact, explicating benefits that are specific or unique to each gender [7,8]. In this review, we aim to explore sex-specific health and wellness issues, highlighting distinctions where possible.
2. The Outsized Role of Hormonal Axes in Female and Male Health
Hormonal axes are integral to the regulation of physiological functions and overall health in both males and females. These axes encompass complex networks of interactions between the endocrine glands, working in concert to maintain homeostasis and respond to internal and external stimuli. Among the most critical systems are the hypothalamic–pituitary–gonadal (HPG) axis, the hypothalamic–pituitary–adrenal (HPA) axis, and the hypothalamic–pituitary–thyroid (HPT) axis, each contributing to a key aspect of health, including growth, metabolism, reproduction, and stress response [9].
The HPG axis is fundamental to reproductive health and development. In females, it regulates the menstrual cycle, ovulation, and fertility through the coordinated secretion of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and the sex hormones estrogen and progesterone [10]. In males, this axis controls the production of testosterone and spermatogenesis, which are essential for sexual function and secondary male characteristics [11].
The HPA axis is a key mediator of the body’s response to stress and influences immune function, energy metabolism, and emotional regulation. Dysregulation of the HPA axis is implicated in health conditions such as chronic stress, anxiety, depression, and metabolic syndrome [12]. This system operates through the release of corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and cortisol, which together modulate the stress response and influence numerous physiological systems [13].
The HPT axis plays a vital role in metabolic control and energy homeostasis. Thyroid hormones, primarily thyroxine (T4) and triiodothyronine (T3), regulate basal metabolic rate, thermogenesis, and protein synthesis [14]. Disruption of the HPT axis can lead to conditions such as hypothyroidism and hyperthyroidism, impacting cardiovascular health, cognitive function, and overall quality of life [15].
Emerging evidence spotlights the microbiota’s influence on these hormonal axes [16]. Prebiotics and probiotics are being widely prescribed to address some of the sex-specific conditions that are discussed herein. It is not a stretch of the imagination to posit that the effects of phytochemicals, like sulforaphane, on the gut microbiome [17] might impact far more than inflammatory bowel disease and diseases of the gastrointestinal system.
The neuroprotective roles of estrogen and testosterone naturally decline with age. In women, estrogen levels drop significantly after menopause, which typically occurs in the late 40s or early 50s. This decline correlates with increased risks of neurodegenerative conditions like Alzheimer’s disease and other cognitive impairments. Estrogen contributes to neural protection by reducing oxidative stress, modulating inflammation, and enhancing synaptic plasticity. Reduced levels diminish these protective effects. Research has shown that hormone replacement therapy (HRT) may provide some neuroprotective benefits, but its effectiveness depends on the timing; the “critical window” hypothesis suggests greater benefits if initiated close to menopause [18,19,20]. In men, testosterone levels decrease gradually after the age of 30 at a rate of approximately 1% per year, a process sometimes referred to as andropause. Testosterone is converted into estrogen in the brain by aromatase, which can exert protective effects. It also directly influences neurogenesis, reduces β-amyloid accumulation (a hallmark of Alzheimer’s), and modulates neurotransmitter systems. Reduced testosterone levels have been linked to increased risks of neurodegenerative diseases, mood disorders, and cognitive decline. Testosterone replacement therapy has shown mixed results, with some studies indicating cognitive and mood benefits in older men, while others highlight potential risks, including cardiovascular complications [18,21]. Importantly, both hormones play significant roles in maintaining brain health, and their age-related decline is a contributing factor to the increased incidence of neurodegenerative conditions in older adults. Both hormone replacement therapies and the judicious application of dietary and supplementation strategies are an area of active research to mitigate these effects.
In Successful Aging, neuroscientist Daniel Levitin emphasizes the integral relationship between hormone levels and balance and inflammation and aging. He notes that, “for many, testosterone for men and estrogen for women can lead to increased mental clarity, ability to focus, and improved memory”, [22]. Understanding the functions and interactions of these hormonal axes provides insight into the physiological differences and similarities between male and female health. It also underscores the critical importance of maintaining hormonal balance to support optimal well-being, explicating how disruptions in this balance can lead to various health conditions and/or pathologies.
3. Sulforaphane
Plants from sixteen different families of higher plants produce and contain compounds called glucosinolates. The Brassica family, which contains over 350 genera and 3000 species, most of them edible, is by far the most thoroughly researched of these families. Brassica vegetables include broccoli, cauliflower, arugula, Brussels sprouts, cabbage, and many others familiar to those who frequent the produce aisles of supermarkets [23]. The broccoli plant—whether it be the ungerminated seed, broccoli sprouts, or any byproduct, including the broccoli crowns you can buy in a supermarket—will contain glucoraphanin, the precursor to sulforaphane [5]. Glucosinolates such as glucoraphanin are “activated” or converted to isothiocyanates such as sulforaphane by an enzyme called myrosinase, which is present in that same plant tissue (e.g., seed, sprout, broccoli head, or microgreen) and/or in bacteria that all humans possess in their gastrointestinal tracts (the gut) [24]. Scientists refer to this population of bacteria resident within as the microbiome. In raw broccoli the active myrosinase enzyme is present and facilitates this conversion of glucoraphanin to sulforaphane. Cooking, however, will denature the enzyme, rendering it inactive. If this happens, the conversion to sulforaphane is accomplished entirely by the existing myrosinase from gut bacteria [5,25,26].
In this paper, we focus on sulforaphane (SF), the bioactive compound derived from this process, and its significant implications for health and disease.
Sulforaphane is the most potent naturally occurring inducer of the body’s cytoprotective response. Its modes of action include antioxidant, anti-inflammatory, antibiotic, cancer preventive, and many more [1]. From the perspective of enhancing human healthspan and reducing the burden of chronic disease, it is one of the most important phytochemicals or phytonutrients—defined as non-nutritive compounds made by plants in small quantities—that we know of [27]. Glucoraphanin is present at much higher levels in broccoli seeds and sprouts than in broccoli plants (or in the heads that are commonly eaten). Sulforaphane itself is not present in any appreciable amounts in any parts of fresh, healthy broccoli plants [5]. Broccoli seeds or sprouts can be used to produce glucoraphanin-rich supplements as sources of sulforaphane. All cruciferous vegetables (also known as. cole crops or brassicas) contain glucosinolates, compounds similar to glucoraphanin, many of which also have beneficial properties [24]. However, only broccoli is rich in glucoraphanin, and it is thus the primary source of sulforaphane in diets and for the production of supplements.
3.1. Health Effects of SF
The breadth of health-promoting, preventive, protective, and therapeutic applications of SF is widely acknowledged in the practitioner, scientific and dietary supplement sectors. Indeed, sulforaphane has been the subject of thousands of studies and well over 125 clinical trials. Unlike many other natural products, SF’s many mechanisms of action in mammalian systems have been well documented (Figure 1). Also noteworthy is that fact that, by and large, the SF precursor glucoraphanin is not itself biologically active, but must first be converted to SF in order to be useful to the body. There are many recent general reviews covering the role of GR and SF, including refs. [6,7] alongside publications on specific indications, diseases, syndromes, and/or fields of study. Several recent reviews include a specific focus on cancer prevention and therapy [28,29,30], neurodevelopmental and neurodegenerative conditions [8], diabetes [31], ophthalmic conditions [32], kidney disease [33], diseases of the liver [34], intestinal inflammation and GI disorders [17,35], and cardiovascular disease [36].
3.2. Keap1-Nrf2 Mediation of the Antioxidant and Detoxification Effects of SF
This now classic pathway is widely known for its upregulation of antioxidant and detoxification mechanisms, which also results in the inhibition of inflammation. Sulforaphane (SF) is recognized as the most potent naturally occurring inducer of the Keap1/Nrf2 pathway. Early work focused primarily on sulforaphane’s ability to induce antioxidant and cytoprotective pathways, including those involved in the synthesis of glutathione (GSH), drug metabolism and transport, metabolic (energetics) enzymes, heme and iron metabolism, and a variety of transcription factors [37,38,39]. As such, we now have a much greater understanding of the role that the Keap1/Nrf2 pathway plays in immune function, mitochondrial health, and neurodevelopmental and neurodegenerative conditions.
Glutathione is the body’s most abundant endogenous antioxidant and is essential for maintaining cellular health and defense. It alternates between two states: reduced (GSH) which carries out antioxidant and detoxification functions, and oxidized (GSSG), which forms after these critical processes. Extensive evidence supports this vital role, one that maintains a balance by both inducing production of GSH and promoting the synthesis of enzymes that recycle GSSG back to its active, GSH state [40].
Early work by Paul Talalay’s group at the Cullman Chemoprotection Center at Johns Hopkins School of Medicine identified NAD(P)H quinone dehydrogenase 1 (also referred to as NAD(P)H:quinone oxidoreductase 1, NQO1, or quinone reductase in the older scientific literature) as a key and highly responsive Phase 2 enzyme, the measurement of which has been a reliable and highly predictive indicator of the cytoprotective potential of both natural and synthetic compounds. Recently, expression of NQO1 in peripheral blood mononuclear cells has been used as a proxy for the expression of the whole suite of Nrf2/Keap1 regulated protective enzymes in human clinical studies [41]. NQO1 has critical cellular antioxidant activity, directly scavenging the superoxide radical, for example, as well as exerting indirect antioxidant effects, regenerating the antioxidants GSH, CoenzymeQ10, and alpha-tocopherol, all of which play critical roles in neuroprotection, together contributing to the maintenance of the redox balance of the brain and central nervous system. In addition to its enzymatic activities, NQO1 is involved in the recognition, repair, and removal of damaged proteins. NQO1 plays a protective role against the genotoxicity of the procarcinogen benzene and its benzoquinone metabolites. SF increases NQO1 gene and protein expression through the Nrf2 signaling pathway in most tissues examined, including within the CNS [42].
In reviewing the effects of SF on female and male health, it is helpful to attempt to separate “normal” age-related symptoms or issues experienced by each gender from what are clearly diseases (Table 1) and to examine the effects of SF, if any, in both prevention and treatment.

Table 1.
Potential sex-related targets of sulforaphane.
The roles of SF in supporting healthy metabolism are extremely diverse and far-reaching, exceeding the scope of this review. However, certain effects which relate to sex-specific and primarily hormone-mediated conditions will be discussed in subsequent sections. For example: (a) the potent cytoprotective and detoxication functions of SF aid in ameliorating the effects of liver damage (e.g., NAFLD and NASH) and bolstering detoxification in healthy livers. These pathways are critical for estrogen metabolism. (b) SF offsets tissue damage due to type 2 diabetes (T2D) and aging, for example. (c) SF can directly inhibit cancer initiation, tumor cell growth, and perhaps metastasis and angiogenesis, which supports tumor expansion. Hormonal balance is intimately linked with the cancers that are uniquely prevalent in either females or males. (d) SF aids in the neutralization of xenoestrogens via various detoxification modalities.
4. Female Health
4.1. Estrogen Metabolism
Estrogen is a generic term used for the body’s main estrogens: estradiol (more correctly termed 17β-estradiol; the dominant form produced by ovaries during reproductive years), estriol (produced placentally during pregnancy), and estrone (the dominant form produced post-menopause). Both the hormones estrogen and testosterone are steroids that are synthesized from cholesterol. The adrenal glands produce the hormone dehydroepiandrosterone (DHEA) that is then transformed into both testosterone and estrogen. In females, the process starts in the ovaries with androgen synthesis catalyzed by the enzyme aromatase. It converts testosterone to estradiol and converts androstenedione to estrone. In males, estrogens are also synthesized in a variety of testicular cells. In both genders, estradiol is also produced in the brain, adrenal glands, adipose tissue, pancreas, and even skin. It is both biosynthesized and further metabolized in the liver. Women have more circulating estrogen in the bloodstream than men.
Xenoestrogens are estrogen mimicking compounds which are not naturally produced by the body that disrupt the body’s normal hormonal balance. Their environmental presence, e.g., phthalates, bisphenol A (BPA), dichlorodiphenyltrichloroethane (DDT), and polybrominated diphenyl ethers (BDEs), raise large concerns due to their potential effects on all stages of human growth and development—particularly on developing fetuses, children, and female health. In addition to the general detoxification effects of SF, primarily via the Keap1-Nrf2 pathway, there are also now many credible studies demonstrating the ability of SF to reduce potential damage from specific xenoestrogens, in particular, BPA [43,44,45,46,47,48].
The estrogen metabolite 16α-hydroxyestrone (16α-OHE1) acts as a breast tumor promoter, whereas the alternate metabolite, 2-hydroxyestrone (2-OHE1), does not. Consumption of brassica vegetables (e.g., broccoli) shifts estrogen metabolism towards 2-OHE1 [49]. Decades of research now confirms that SF, indole-3-carbinol (I3C) and its metabolite diindolylmethane (DIM) all can play a helpful role in this effect (See Section 8).
4.2. Perimenopause and Menopause
Perimenopause, the transitional period before menopause when a woman’s body begins to undergo hormonal changes, is characterized by unpredictable fluctuations in levels of estrogen and progesterone. It can last several years, typically starting in a woman’s 40s, and is characterized by irregular menstrual cycles, hot flashes, night sweats, mood swings, sleep disturbances, changes in libido, vaginal dryness, and declining fertility. Menopause is a natural transition in a woman’s life, typically occurring by the early 50s, and is defined as that point at which a woman has not had a menstrual period for 12 consecutive months. It can be marked by loss of bone density and increased risk of osteoporosis, long-term cardiovascular risks due to decreased estrogen levels, and persistence of many of the symptoms of perimenopause. Estrogen and progesterone levels will have dropped significantly and stabilized at low levels and ovulation/fertility ends [50]. The search for natural and effective treatments for menopause-related symptoms has increasingly been directed towards phytochemicals like sulforaphane [27]. The central function of the Nrf2 pathway to modulate oxidative stress has been established along with the highly capable function of SF to activate this pathway. The complex relationship of this Nrf2-mediated reduction in oxidative stress and ovarian aging, including common factors exacerbated by the hormonal shifts occurring during menopause, has recently been reviewed [51], highlighting several important realities that ovarian aging drives, in addition to the obvious depletion of function: (a) development of menopausal syndromes; (b) increased risk of age-related diseases including osteoporosis, cognitive impairment, cardiovascular disease, and cancer; (c) epidemiologic associations between increased all-cause mortality and rates of cancer with premature ovarian failure compared to a normal menopausal age group; and (d) as a pacemaker for other organs aging during the lifespan. The review by [51] specifically links ovarian aging to the Nrf2-mediated antioxidant responses, including induction of GSH and the antioxidant response, apoptosis, and ferroptosis, which have been shown to be key factors in premature aging.
The anti-inflammatory effects of sulforaphane may also play a crucial role in alleviating menopausal symptoms. Chronic inflammation is associated with metabolic syndrome, cardiovascular disease, and other conditions that are more prevalent post-menopause due to changes in estrogen levels. By down-regulating pro-inflammatory cytokines and supporting cellular defense mechanisms, SF could potentially reduce the risk of these menopause-related health issues [52].
Additionally, the influence of SF on estrogen metabolism has been investigated. It has been suggested that sulforaphane may support the detoxification of estrogen metabolites through the upregulation of phase 2 detoxification enzymes, thereby contributing to a more favorable balance of estrogen metabolites [53]. This may be particularly relevant during menopause and perimenopause, when estrogen production shifts predominantly to peripheral tissues, altering the hormonal landscape [54].
One further relationship between SF and menopausal changes relates to the capacity of SF to influence gut health (the microbiome) [17]. The microbiome has been documented to have an important impact upon the progression and quality of life in menopause [55]. Thus, the incorporation of SF as a dietary intervention offers significant promise for improving health outcomes in menopausal women.
4.3. Breast Health
Breastfeeding and Lactation
Cabbage leaves have historically been used to help resolve breast engorgement and stimulate milk flow in lactating mothers. In a similar historical setting, “mustard plasters” made from mustard seeds and water were used to treat bronchitis and croup. Modern scientists have assumed that this activity is functionally linked to the glucosinolate contents of the cabbage and mustard seed (converted to compounds closely related to SF), and that these compounds were responsible for bronchodilation and enhanced blood flow that led to symptom amelioration.
Recent clinical and preclinical (rodent) studies [56,57] have evaluated the effects of broccoli or broccoli sprouts on lactation. In an elegant murine study, broccoli sprouts given during late gestation and lactation conferred protection against developmental delay induced by maternal inflammation [58].
One of the more compelling arguments that this author has seen for the powerful epigenetic, transgenerational effects of any phytochemical or synthetic compound was made by the Canadian scientist Bernhard Juurlink’s team. This group has long used spontaneously hypertensive stroke-prone rats (SHRsp) to study the interaction of oxidative stress and hypertension. They first showed that SF up-regulated the impaired glutathione (GSH) system in vascular smooth muscle cells from SHRsp rats [59]. They next demonstrated that a high GR diet (broccoli sprouts) reduced oxidative stress and associated problems in male SHRsp rats, including increases in GSH and GSH-dependent antioxidant enzymes in the blood vessels, heart, and kidneys [60,61], and they showed that endothelial function was improved and hypertension was thus reduced in these animals. Their follow-up and underappreciated experiments placed female SHRsp rats on a similar GR-rich diet and evaluated their health and that of their offspring (2nd generation) [61]. Similar results to those in the previous studies with male rats were obtained. They then found that female rats fed a high GR diet during pregnancy and weaning influenced the blood pressures of both their male and female offspring, with significantly lower systolic blood pressure through adulthood (more than 17 weeks of age) in the 2nd generation, in addition to positive changes in a variety of other oxidative stress and inflammation-associated biomarkers. They conclude that “normalizing the redox status of tissues and normalizing blood pressures in the pregnant female SHRsp using a dietary approach positively impacts the adult health of the offspring”, [61]. They performed follow-up experiments with a similar rat model in which SF (not GR) was given to the rats by daily gavage for ca. 3 months. The evidence demonstrated both reduced blood pressure of the animals as well as inhibition of vascular remodeling, which is often associated with hypertension. Moreover, changes in protein nitration and genomic DNA methylation indicated a pronounced epigenetic effect [62]. Finally, there has been much research on the preventive and therapeutic effects of SF on breast cancer, which is discussed in a later section of this review.
4.4. Other Non-Cancer
The effects of SF on a number of other female-specific conditions are less well-studied, but merit mention herein.
4.4.1. Polycystic Ovary Syndrome (PCOS)
PCOS is a medical condition that affects 8–13% of women of reproductive age and is associated with a wide range of unpleasant, if not debilitating, symptoms. Some of these symptoms can include irregular menstrual cycles, excess facial or body hair (hirsutism), acne, weight gain, insulin resistance, and infertility. Many women can also experience mood disorders like anxiety and depression because PCOS has been linked to elevated androgens. Reports of the antidepressant effects of SF, 13 in vivo and 1 RCT, have examined the effects of SF and other isothiocyanates on anxiety and depression [63,64,65,66,67,68,69], and these were most recently reviewed by Ramakrishnan et al. [8]. Research aimed directly at the depression associated with PCOS and on the syndrome itself is limited. However, recent findings have highlighted the potential of sulforaphane (SF) in restoring the energy sensors liver kinase B1 (LKB1)/adenosine monophosphate kinase (AMPK), in the nuclear translocation of SIRT1, and in the repression of FOXO1, NF-кB, and TNF-α production in a rat model of PCOS. These effects are interpreted as a “salvage pathway” to reduce or treat depression and other psychiatric symptoms associated with PCOS by suppressing the neuroinflammation mediated by NF-кB [70]. Supporting this, other recent ex vivo clinical research utilized cultured granulosa-lutein cells from patients with PCOS to demonstrate SF-induced lowering of ROS and apoptosis via activation of AMPK, AKT, and NRF2 [71,72]. These findings underscore SF’s potential to address both the physiological and psychological aspects of PCOS.
4.4.2. Endometriosis
Endometriosis affects 5–10% of women of childbearing age, causing chronic pain due to ectopic proliferation of tissue. A rat model of sciatic endometriosis demonstrated anti-nociception (alleviation of pain) and the anti-inflammatory activity of SF. Furthermore, SF inhibited ectopic endometrial tissue growth (lesion size and reduced VEGF levels) as well as reducing IL-6, IL-1β, and TNF-α levels, inducing DOX2, upregulating the Keap1-Nrf2 pathway and suppressing iNOS [73]. Some of these effects were echoed in contemporaneously published study [74], in which SF inhibited levels of IL-6, IL-10, TNF-α, IFN-γ, and VEGF by inhibiting the PI3K/Akt pathway.
4.4.3. Preeclampsia
Preeclampsia is a condition affecting 3–4% of pregnant women in the USA and 5–7% of them worldwide [75]. It poses a significant risk of maternal and perinatal morbidity. Preeclampsia is understood to be the result of reduced blood flow, inadequate placentation, or sustained ischemic-reperfusion placental injury. SF has been shown to improve endothelial activation and dysfunction, and to mitigate hypoxic and hyperoxic injury in a human umbilical vein endothelial cell (HUVEC) model [76]. SF improved HUVEC viability and protected against arterial dysfunction caused by placental antiangiogenic factors by facilitating arterial relaxation [77]. SF mitigated hyperoxic and superoxide-induced changes in syncytiotrophoblast mitochondrial function in vitro and improved mitochondrial respiration in trophoblast cells from preeclamptic placentae [78]. This research group then performed a clinical study in which they administered an SF-rich broccoli extract to 12 women with pregnancy hypertension using non-pregnant women as controls. Women with preeclampsia experienced a modest effect on decreasing diastolic blood pressure and soluble fms-like tyrosine kinase-1 (an indicator of preeclampsia that is employed in blood tests for the condition), thus suggesting that SF improves endothelial function and blood pressure in women with pregnancy hypertension [79]. A number of members of this Australian research team have drawn compelling analogies between the pathological and clinical manifestation of preeclampsia and COVID-19 with regard to maternal inflammation and subsequent fetal neuro-inflammation, suggesting that SF may offer a more effective therapeutic route for preeclampsia than existing antihypertensive treatments [80].
4.5. Cancer (Prevention and Treatment)
The modes of action of SF that are specifically pertinent to cancer cells generally are manifold and have been reviewed extensively. Among them are cancer-cell-specific inhibition of tumor initiation, promotion, progression, and metastasis as well as angiogenesis and histone deacetylation. In many cases these outcomes are a result of the cancer-cell-specific promotion of apoptosis and autophagy as well as cell cycle arrest. The mechanisms include but are not limited to inhibition of NFкB, HDACs, Pgp, MRP-1, BCRP, STAT3, and MEKK1 activity, AP-1 DNA binding, and tubulin polymerization; degradation of α and β tubulin; down-regulation of cycline B1, cdk1, cdc25B, cdc25C, HIF, VEGF, VEGF receptor, MMP-2, and MMP-9; modulation of Bcl-2 family proteins; and activation of caspases [1]. Although there are many gynecologic cancers, this review will only examine what is known about the effects of SF on the three most prevalent—those of the breast, cervix, and ovaries. Worldwide these cancers resulted in 2,300,000, 604,000, and 314,000 cases, respectively, in 2020, and in 685,000, 342,000, and 207,000 deaths [30].
4.5.1. Breast Cancer Prevention
There is a powerful and expansive bibliography underlying the potential of SF for both the treatment and prevention of breast cancer, as well as enhancing the activity of aromatase-inhibiting drugs commonly used in breast cancer treatment and prevention in post-menopausal women. Epidemiologic evidence for over 40 years has demonstrated a correlation between reduced breast cancer incidence and cruciferous vegetable consumption, which has recently been summarized [30,81]. For example, in one study of 54 women with abnormal mammogram findings who were scheduled for breast biopsy, total cruciferous vegetable intake was linked to a decrease in the cell proliferation marker Ki-67 in breast ductal carcinoma in situ (DCIS) tissue [82].
The first clinical examination of orally delivered SF reaching breast tissue and demonstrating changes in biomarkers of SF activity was conducted at Johns Hopkins University [83]. Early research on breast cancer preventive mechanisms was also performed at Johns Hopkins, where scientists analyzed the effects of SF on human breast cancer cell lines representing a wide range of tumor phenotypes [84]. They showed that SF inhibited cell growth, blocked proper cell cycling, and induced apoptosis by two distinct pathways of activation in a cell type-specific manner. They also showed that SF exposure inhibited HDAC activity and decreased the expression of certain proteins that are critical to breast cancer growth, such as estrogen receptor (ER)-a, epidermal growth factor receptor (EGFR), and human EGFR-2 (HER-2). However, in an intervention in which 54 women received either glucoraphanin or placebo for 2 to 8 weeks prior to having a breast biopsy, there was no effect on HDAC activity [85]. In a further example, mammary tumor formation in rats exposed to 17β-estradiol was diminished in rats treated with SF [86].
Although there are well over 350 peer-reviewed papers (most recently reviewed by Kuran and colleagues [87]) no clinical studies on the use of SF in primary prevention of breast cancer have yet been published.
4.5.2. Breast Cancer Treatment
In addition to a plethora of data on the cancer preventive potencies of SF and other ITCs, there is now emerging evidence that suggests that SF may serve as a helpful component of cancer treatment regimens.
The drug Exemestane is a potent inhibitor of aromatase (the enzyme that converts androgens to estrogens), which is widely used in the treatment of estrogen-driven breast cancer. Sulforaphane potently synergizes Exemestane in some of its modes of action, thus raising the potential of sulforaphane to enhance efficacy of this and other similar pharmaceuticals, as well as to reduce the dose of these drugs required, and to have a central role in preventive strategies [88]. Unfortunately, there have been no clinical trials following up on these observations.
Wang and colleagues [89] recently demonstrated trends towards increases in caspase-3 and tumor-infiltrating lymphocytes and towards decreases in Ki-67 and nuclear to cytoplasm ratio of estrogen receptor-α in post-menopausal breast cancer patients taking 200 µmol per day of ITC (primarily SF) from broccoli sprout extract. In this study, the expression of a variety of biomarkers of breast cancer changed from pre- to post-intervention, and the evaluation of global urinary proteomic profiles revealed significant alteration of 116 proteins, 55 of which involved signaling pathways, many of which were known to be related to isothiocyanate functions. Top predicted networks indicated activation of 3 functions downstream of the Nrf2-mediated oxidative stress response—apoptosis induction, lymphocyte activation, and neutrophil activation [89]. SF also suppressed the metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway [90].
4.5.3. Cervical Cancer
Considering its public health impact, there are relatively few studies investigating the effects of SF on cervical cancer, and all of them are molecular biology studies dealing with cultured cell lines—no preclinical or clinical studies. Thus, it has been shown that SF targets MAPK signaling [91], arrests cell cycle at G2/M [92], reverses tumor suppressor gene expression [93], induces apoptosis and is anti-inflammatory [94,95], and acts synergistically with eugenol (the major essential oil of cloves [96]).
4.5.4. Ovarian Cancer
Ovarian cancer is one of the leading causes of cancer deaths among women. In the USA, it is estimated that 20,000 women will receive a new diagnosis of ovarian cancer in 2024 and approximately 13,000 will die from ovarian cancer within a twelve-month cycle [97]. As with breast cancer, there is abundant epidemiologic evidence demonstrating a correlation between increased consumption of fruits and vegetables—particularly cruciferous vegetables—and reduced risk of ovarian cancer. To date, there are no published clinical studies, but there are now multiple studies which have evaluated the preventive effects of SF on cultured ovarian cancer cells and in rodent models, that are summarized as follows:
SF had potent antiproliferative effects on both human and mouse ovarian cancer cell lines [98]. SF treatment resulted in decreased total Akt protein, phosphoinositide-3-kinase (PI3K), and active phosphorylated levels of Akt (key members of the Akt pathway which are upregulated in ovarian cancer) in these cells. SF reduced the clonogenic ability of ovarian cancer cell lines, and it down-regulated critical cell cycle markers, inducing cell cycle arrest and apoptosis in them. Similar results were obtained with SF on the growth inhibition of ovarian cancer cells, increase apoptosis, reduced expression of hTERT (the main regulatory subunit of telomerase), and down-regulation of Bcl-2 (a gene involved in thwarting apoptosis) [99]. This group also identified synergy with epigallocatechin gallate (EGCG) on a number of these parameters. They further showed an enhancement of cisplatin-mediated apoptosis in ovarian cancer cells via potentiating cell cycle arrest and upregulation of the cell cycle inhibitor p21 by a combination of EGCG and SF [100]. SF enhanced the cisplatin sensitivity of ovarian carcinomas in a mouse xenograft model vis up-regulation of the cancer suppressor miR-30a, thus inducing DNA damage due to intracellular accumulation of cisplatin [101]. The retinoblastoma protein (RB) is a well-known regulator of the cell cycle. When under-phosphorylated, RB binding to the transcription factor E2F-1 is reduced, thus inhibiting AKT and both inhibiting cancer cells’ invasive ability and reducing their migration. SF induced growth arrest and apoptosis in ovarian cancer cells via inhibition of RB phosphorylation [102]. In a separate system, ovarian cancer cells accumulated in metaphase and could not divide (irreversible cell cycle arrest) due to the down-regulation of two cell cycle checkpoints when treated with SF [103]. Most recently, SF was shown to synergize with cisplatin to suppress ovarian cancer cell proliferation and enhance apoptosis [104], and this study also showed that SF suppressed tumor growth and inhibited proliferation xenografts of human ovarian cancer cells onto nude mice.
5. Male Health
As with female health, there are several conditions that are unique to males, often addressed by urologists, oncologists, and psychiatrists. The potential for SF to alleviate or ameliorate these conditions is discussed herein and relates primarily to its anti-inflammatory and antioxidant properties. Extensive research using cell culture and animal models has highlighted the ability of SF to support or upregulate the Nrf2 pathway and inhibition of epigenetic modifications, as with histone deacetylation.
5.1. Sperm Health and Testicular Damage
Sperm health is linked with testicular function and the hormonal axes but is frequently given separate attention in scientific studies. Most direct studies of sperm health have focused on metal- and other primarily diet-induced toxicities and on cryopreservation, which of course has immediate relevance to the breeding of domesticated animals and to fertility support in humans. More broadly, testicular health associated with oxidative stress and the Nrf2 pathway and their effects on endocrine disruption has been explored.
5.1.1. Toxicities Associated with Diet and Environmental Exposures
A variety of environmental stresses cause reductions in fertility and have been examined from the perspective of sperm and testicular health. Prime among them are metal (aluminum and cadmium) exposures and pesticides and plasticizers. The effects of SF in counteracting some of these toxicities has been evaluated.
Aluminum-induced oxidative stress alterations in sperm characterization and testicular histomorphometry of rats were reported after 4 weeks of daily gavage with SF [105]. Testicular histoarchitecture (the macro- and microstructure of the tissue) was normalized by SF treatment compared to control animals, as was seminiferous tubule shrinkage, spermatogenesis disruption, lipid peroxidation, and hormone and antioxidant status.
SF prevented testicular damage in cadmium-exposed mice via activation of the Nrf2 pathway and thus resulted in less degradation in sperm quality, as well as improvement in a variety of relevant biomarkers, e.g., serum testosterone, antioxidant levels, and expression of genes for glutathione peroxidase (GSH-Px), gamma glutamyl cysteine synthetase (g-GCS), heme oxygenase-1 (HO-1), and NAD(P)H:quinone oxidoreductase-1 (NQO1) [106]. Separately, protective effects against cadmium toxicity were shown in the testes and prostate tissues of rats [107].
SF reversed ferroptosis and reversed or attenuated oligospermia (reduced sperm count) in mice via Nrf2 activation [108]. Di-2-ethylhexyl phthalate (DEHP; a common plasticizer) induced testicular injury by ferroptosis and those effects were reduced by SF, also via the Nrf2 pathway, in rats [109]. Di-n-butylphthalate (DBP; a common human endocrine disrupter) induced testicular oxidative stress injury, which was reduced via Nrf2 activation in mice [110]. In mouse in testicular Leydig cells, SF-ameliorated oxidative stress was induced by DBP via Nrf2 induction—this was manifest by the enhanced activity of stress related genes, cell proliferation, and testosterone secretion [111].
SF supplementation significantly ameliorated multiple obesity-related effects on the reproductive systems of male mice that were induced by a high-fat diet. These effects included reduced testes weights, epididymis weights, sperm counts, sperm motility, and testosterone levels and increased leptin and estradiol [43]. In a separate study, subcutaneously injected SF attenuated a range of spermatogenic deficiencies (e.g., sperm viability and mobility) in mice fed a high fat diet [112].
5.1.2. Oxidative Stress
Administration of SF to mice activated testicular Nrf2 expression and function along with a marked attenuation in the angiotensin II (AngII)-induced testicular oxidative stress, inflammation, endoplasmic reticulum stress, and apoptotic cell death [113,114]. Other work with a mouse testes model demonstrated that SF provided protection from type 1 diabetes-induced (oxidative stress) testicular apoptosis and that this was associated with up-regulation of Nrf2 expression [115]. More recently, in a rat model, SF ameliorated testicular ischemia–reperfusion injury and partially reversed the effects of superoxide dismutase, catalase, and testicular reproductive function, suppressing reactive oxygen species content in the testicles of treated rats [116].
5.1.3. Semen Cryopreservation
Cryopreserved sperm cells are viable, functional cells. Although the mechanism(s) by which SF has been shown to mediate sperm health in vitro may not be identical to those in cryopreserved cells (ex vivo), the indirect antioxidant functions of SF are likely central to the demonstrations that SF is beneficial in semen cryopreservation. For example, the inclusion of SF in human semen samples subjected to cryopreservation improved all measures of sperm quality, including viability, motility, and morphology, following the freeze–thaw process. Additionally, evidence of reducing intracellular hydrogen peroxide and superoxide anion, increasing the percentage of viable sperm cells with an intact plasma membrane, and decreasing the level of lipid peroxidation post freeze–thaw was noted [117]. Similarly, the inclusion of SF in buffalo (Bubalis bubalis) semen cryopreservation freezing medium augmented motilities-related parameters (% motility, velocity), functional parameters (membrane functionality, mitochondrial potential and acrosome integrity), preserved biochemical features (calcium concentration, total antioxidant capacity, lactate dehydrogenase, reactive oxygen species and lipid peroxidation) and augmented the fertility rate of buffalo sperm compared to controls post-thawing [118].
5.2. Erectile Dysfunction (ED)
Vascular impairment, characterized by reduced capacity for vasodilation, is a major determinant of erectile dysfunction (ED). Recent research suggests that SF has the potential to address vasodilation associated with ED. In aged rats, SF improved endothelium-dependent vasodilation in aorta (RA), mesenteric arteries (RMA), coronary arteries (RCA), and corpus cavernosum (RCC; penile tissue) [119]. This research group also demonstrated that SF improves endothelium-dependent and H2O2-induced relaxations in human penile resistance arteries and human corpus cavernosum from patients with ED and from organ donors without ED. Furthermore, they demonstrated that SF treatment increased Nrf2 content, decreased oxidative stress markers, and increased heme oxygenase (HO-1) expression in human vascular tissues from ED patients, thus suggesting that the pharmacologic activation of the Nrf2 pathway might be a potential therapeutic target for managing ED.
5.3. Hair Loss/Growth
The vast majority of cases of hair loss in both men and women, though more pronounced in men, are attributed to androgenic alopecia, or AGA. This results from the enzymatic activity of the enzyme 5α-reductase (5α-R) that releases dihydrotestoterone (DHT) from testosterone and then binds to the so-called androgenic receptor (AR). Furthermore, DHT stimulates TGF-β, a growth factor, in the papillary cells, which ultimately causes the death of the hair follicles [120]. The 5α-R inhibitor finasteride is now widely used to treat AGA but is not without substantial side-effects.
SF promotes hair growth in mice by accelerating DHT degradation [121]. More recent work has supported this observation, showing increased expression of 3α-hydroxysteroid dehydrogenase in the liver, which accelerates DHT degradation [122]. This group also included a clinical study with 23 subjects, in which the number of hairs increased by 6.7% following 18 weeks of treatment with SF [122]. The ability of SF in a broccoli extract to prevent testosterone-induced inhibition of dermal papilla cell viability, up-regulate cytokeratin gene expression, prevent the increase in Bax gene levels induced by testosterone in dermal papilla cells, and promote the growth of hair follicles in mice was recently described [123].
5.4. Prostate Cancer and Benign Prostate Hyperplasia (BPH)
The connection between brassica vegetable consumption and both prostate and bladder health in men has long been a subject of epidemiologic research. Among the most notable was a review of the epidemiologic evidence from Alan Kristal and Johanna Lampe [3]. An early study of fruit and vegetable consumption in almost 48,000 men identified only those who reported diets containing the cruciferous vegetables broccoli and cabbage as having a protective effect against bladder cancer [124]. Much research has been in vitro, because animal models of prostate cancer were not available during the earliest years of prostate cancer research.
Some of the earliest in vitro work on the effects of SF on the prostate focused on epigenetics. These studies identified a dose-dependent inhibition of histone deacetylation (HDAC) in both prostate cancer cells and benign prostate hyperplasia (BPH) cells [125,126]. SF was later shown to destabilize the androgen receptor in prostate cells by inactivating HDAC6 [127]. Dozens of subsequent studies have examined the inhibition of HDAC using small molecules, including sulforaphane (reviewed by Ganai [128]). Investigation of the role of SF in inhibiting or preventing prostate cancer has continued to this day, with citations too numerous to recount in this review. Many of those investigations have been solidly mechanism-based and are reviewed more critically by numerous other investigators (e.g., [129,130,131]); here, we highlight a few of the more compelling:
SF attenuated the expression of prostate cancer-associated long noncoding RNAs (lncRNA) using an lncRNA that is overexpressed in prostate cancer and which, upon knockdown, results in decreased proliferation of prostate cancer cells [132].
SF downregulated: (a) galectin-1 (a protein that binds carbohydrates, with wide ranging biological functions) [133] and (b) phosphoglucomutase-3, which mediates both glycogen formation and utilization [134] and impacts the expression of the androgen receptor-responsive gene, prostate specific antigen (PSA), enhancing the efficiency of the pharmaceutical anti-androgens bicalutamide and enzalutamide, two mainstays of androgen deprivation therapy [135].
SF reversed: (a) many of the cancer-associated promoter methylation alterations, including aberrantly methylated genes that are dysregulated or are highly involved in cancer progression [136]; (b) the formation of lysosomal-associated membrane protein 2 (LAMP2), which assists in transporting materials into lysosomes and is involved in autophagy [137]; (c) the formation of specificity protein 1 (Sp1), a transcription factor regulating gene expression including those involved in cell growth, differentiation, and immune response [138]; (d) the Warburg phenomenon, which is a hallmark of cancer in which cancer cells use glycolysis to generate energy, even when oxygen is abundant, thus fermenting most of the glucose they consume into lactate, rather than oxidizing it via respiration [139].
SF increased tissue invasion, a cell culture predictor of metastatic capacity, by regulating E-cadherin and CD44 [140].
SF inhibited: (a) the growth of prostate cancer by down-regulating miRNAs, the noncoding RNAs that induce target genes’ mRNA degradation or inhibit the initiation of translation and protein synthesis, as well as the expression of the oncogenic gene DJ-1 [141]; (b) the synthesis of a variety of essential proteins in prostate cancer cells [142]; (c) fatty acid metabolism [143]; (d) autophagy [144], and was synergistic with chloroquine in doing so [145]; (e) c-Myc-mediated traits [146]; (f) activation of the essential-to-life protein STAT3, which is essential to viability [137,147]; (g) prostate cancer cell migration [144].
SF activated Notch signaling, which is frequently constitutively activated in many human cancers, including prostate. But this activation was not coupled with the effect of SF in inhibiting cell migration and is thus viewed as a therapeutic advantage [148].
SF modulated gene expression and alternative gene splicing [149] as well as the cdk-cyclin axis and expression of CD44 variants [150].
SF induced apoptosis, both itself and in synergy with paclitaxel [151].
The earliest clinical work on SF and prostate cancer pointed to potential modes of action and examined global gene expression in the human prostate before, during, and after a 12-month broccoli-rich diet [152]. Among the differences between men on treatment and control diets were TGFβ1, EGF, insulin, and androgen signaling. Furthermore, GSTM1 genotypes were found to be significant modulators of this signaling—consistent with the long-known effects of sulforaphane on multiple enzymes involved in the glutathione cycle.
Two clinical studies in which sulforaphane was administered to men were published in 2015. In the first study, 20 weeks of treatment with 200 µmol/d of SF rich extracts resulted in a significant lengthening of the on-treatment PSA doubling time compared to pre-treatment, and safety was exemplary even at this rather high SF dosing [153]. The second study was a double-blinded multicenter RCT with 78 patients in which Cippola and colleagues [154] examined serum PSA levels following radical prostatectomy. Patients were given 339 µmol SF/d for 6 months followed by a 2-month washout. PSA doubling time—a strong indicator of a higher risk of metastasis and prostate cancer-specific death—was 86% longer in the SF than in the placebo group (28.9 vs. 15.5 mo., respectively); SF effects were observed as soon as 3 months on intervention. Following treatment, the rates of PSA increase in the placebo arm remained unchanged, but that of the men treated with SF declined significantly.
A study in the UK employed a 12-month intervention with men on active surveillance for prostate cancer and identified hundreds of changes in potentially oncogenic pathways in men on the control arm vs. the attenuation of those effects in the men on the high glucoraphanin arm [155]. Inverse associations with clinical progression were also observed, though this study was not designed to assess these differences.
A study of 98 men scheduled for prostate biopsy randomized them to either a SF-rich broccoli sprout extract or placebo. Forty genes were identified that were differentially expressed between SF and placebo control arms, including two down-regulated genes that had been previously identified as contributing to prostate cancer development [156].
The duration of intervention was between 4 and 8 weeks, dependent upon timing of actual biopsy. This study also confirmed the presence of higher amounts of SF and its metabolites in the urine and blood of those randomized to receive SF, though they saw no differences in either HDAC activity or prostate tissue biomarkers.
A 39-person proof-of concept study demonstrated that prostate tissues accumulate SF and that it can be detected in the prostate. Livingstone et al. [157] performed prostate biopsies on men following ingestion of a high glucoraphanin dietary supplement and found significant levels of SF and its primary metabolite (SF-NAC) in those biopsies. Clinical (as well as pre-clinical studies) were last critically reviewed approximately 6 years ago [158].
6. SF Synergies with Cancer Treatments: Breast, Ovarian, Cervical, and Prostate
An abundance of peer reviewed studies very credibly document synergies between SF and: (a) existing cancer treatment drugs, (b) pharmaceuticals under development but not yet on the market or in the regulatory pipeline, (c) loosely characterized proprietary mixes such as extracts of fern leaves, and (d) cell lines that are resistant to various cancer drugs and but made more susceptible to them by treating with SF. Notably, the majority of these findings pertain to cancer therapies.
Seventy-six papers (and counting) provide evidence in cell lines. Many provide convincing mechanistic evidence for the effects, and some of them use the gold-standard Chou-Talalay technique [159] for demonstrating true synergy. Examples of those pertaining specifically to breast, cervical, ovarian and prostate cancers are listed in Table 2.

Table 2.
Synergy of sulforaphane with drug or treatment (e.g., phytochemical).
Li et al. [161] describe synergy between SF + biochanin A (BCA), which is an isoflavone found in Trifolium pratense (red clover). The combination suppressed cancer in an in vivo breast cancer system by reducing cell proliferation and promoting apoptosis, causing cell cycle arrest and down-regulating ERK-1/2 and other cell proliferation factors.
Pogorzelska et al. [174] examined the effects of SF + doxorubicin on triple-negative breast cancer in an animal model. Doxorubicin is a long-used chemotherapy drug that arrests or slows cancer cell growth by blocking the enzyme topoisomerase 2. The synergistic combination reduced primary mammary tumors in a mouse model, increased doxorubicin nuclear accumulation, and exhibited cardio-, nephro- and hepato-protective effects.
An in vitro study by Sharma and Tollefsbol [167] targeted epigenetic mechanisms in breast cancer. Sodium butyrate (NaB) is a short-chain fatty acid produced by gut bacteria which has a variety of antineoplastic properties, including the inhibition of histone deacetylase (HDAC). The phytochemical genistein (GE) is a potent inhibitor of DNA methyl transferases (DNMTs), which are epigenetic enzymes controlling DNA methylation and hence its transcriptional activity. SF, among its many other mechanisms, is an HDAC inhibitor. The triple combination (SF + GE + NaB) had the greatest impact followed by SF + GE, SF + NaB, and GE + NaB, which were integrally more efficacious than singly administered compounds or control in suppressing cell proliferation, promoting apoptosis and inducing cell cycle arrest. Additionally, the combination treatments administered at dietary levels had enhanced effects in modulating epigenetic chemistry via the down-regulation of key players of DNA methylation, histone deacetylation, histone methylation, and histone acetylation, along with global enzymatic activity changes.
Epithelial–mesenchymal transition (EMT) allows cancer cells of an epithelial origin to acquire mesenchymal properties, leading to the progression and development of drug resistance. Use of gefitinib has been associated with the development of EMT as a method of the cells escaping the drug toxicity. Wang and colleagues [182] demonstrated that the addition of SF could overcome gefitinib resistance in a lung adenocarcinoma cell line by inducing dose-dependent antiproliferative effects in both non-resistant and gefitinib-resistant cells, whereas induced cell cycle arrest and cell apoptosis occurred only in the gefitinib-resistant cells. The synergistic effect was mechanistically documented to result from a variety of known SF targets leading to EMT. Other systems have similarly shown the potential of SF to down-regulate the transition to EMT. In a triple negative breast cancer line, a SF-cisplatin combination inhibited stemness (reversion to pluripotent stem call characteristics) and metastatic potential by down-regulating sirtuin-mediated EMT signaling [173]. The combination was strongly synergistic and multifunctional.
Among the more mechanistically intriguing synergies is that of an antibiotic ionophore and SF. Salinomycin, a decades-old potassium ionophore isolated from Streptomyces spp. which has long been used in veterinary medicine. Over a decade ago, it was found to be 100 times more potent than paclitaxel against breast cancer stem cells, and then also effective against many other types of cancer, and it also enhances the cytotoxic effects of many conventional chemotherapeutic agents. Liu et al. [183] demonstrated that SF and salinomycin synergistically inhibited proliferation, induced apoptosis, and decreased migration and invasion of human colorectal adenocarcinoma cells.
SF and 5-fluorouracil were shown to act synergistically in a highly invasive, triple negative breast cancer cell line by inducing autophagy and premature senescence [172,184].
SF acted synergistically with cisplatin to inhibit ovarian cancer cell proliferation and promote apoptosis in vitro. SF by itself inhibited xenograft tumor growth and progression in vivo in a dose-dependent manner [104].
7. Conclusions
In this review, we have explored sex-specific health and wellness issues, improvements in which have been addressed experimentally by the phytochemical sulforaphane. We have included in vitro and in vivo studies—animal model systems as well as whatever human clinical evidence exists. We document the increasingly robust evidence of the beneficial effects of SF on a variety of both male- and female-specific conditions—most of them are hormone-related, and many of these improvements are related to SF’s powerful indirect antioxidant, chemoprotective, anti-inflammatory, and detoxification properties. Thus, with attention to both preventive and treatment strategies, we have covered knowledge to date on the effects of SF on estrogen metabolism as well as breast feeding and lactation, menopause and perimenopause, polycystic ovarian syndrome, preeclampsia, and endometriosis. We examined SF’s effects on sperm health from the perspective of cryopreservation (ex vivo) as well as sperm viability, quality, and sperm toxicities related to environmental exposures, hair loss, and erectile dysfunction. Finally, we have reviewed whatever work has been performed on cells, animal models, and clinical trials of the effects of SF on breast, ovarian, cervical, prostate, and testicular cancers, and the large and developing body of evidence on how SF can synergize with pharmaceuticals and other natural products used in reproductive cancer treatment and prevention.
8. Afterword: I3C and DIM
Cruciferous vegetables contain a wide array of glucosinolates—some 120 different but closely related compounds. Glucoraphanin is the predominant glucosinolate in broccoli sprouts and seeds, but, as the plant develops and matures, the composition of these glucosinolates (defensive compounds for the plants) changes, and in market stage broccoli, there is a preponderance of indole glucosinolates. It may be reasonable to assume that the presence of both glucoraphanin and indole glucosinolates in a plant matrix may be beneficial to humans, because the epidemiologic evidence for the benefit of broccoli to provide protection against disease is very strong [1,185].
Indole glucosinolates are unique among the glucosinolates in that they do not form stable isothiocyanates upon enzymatic treatment with myrosinase, but rather, form indole-3-carbinol (I3C) and its biologically active dimer (3,3′-diindolylmethane, di-indolyl methane, or DIM). I3C and DIM are thus often referred to together in both the scientific and general literature (e.g., I3C/DIM). As detailed in our earliest work on broccoli sprouts [5], “hydrolysis of indole glucosinolates by myrosinase gives rise to bifunctional inducers, such as indole-3-carbinol and indole-3-nitrile, and to condensation products, such as 3,3′-diindolylmethane and indole-3-carbazole, which bind to the aryl hydrocarbon receptor [186]. I3C is both an inhibitor and an enhancer of tumor formation in animals, depending upon the experimental system and the timing of administration in relation to exposure to carcinogen (see references within [187]). Consequently, there are potential limitations to the use of indole glucosinolates as chemoprotectors in humans because they (i) are weak inducers of phase 2 enzymes, (ii) are bifunctional inducers that activate phase 1 enzymes, (iii) may have estrogen receptor binding activity [188], and (iv) are potential tumor promoters”.
Much of this early work on these indole GS metabolites focused on the fact that they can form tetrameric polymerization products that extremely closely resemble dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)) and have both estrogenic and anti-estrogenic activity. A significant part of our discovery of the cancer preventive effects of broccoli sprouts was that, in addition to being a source of high levels of glucoraphanin, broccoli sprouts had next to no indole glucosinolates, and were therefore not a significant potential source of I3C or DIM [5]. This is in direct contrast to market stage broccoli florets, which are relatively rich in indole glucosinolates.
Much work has been conducted with I3C and DIM in subsequent years. Of particular relevance to this review, both additive effects and true synergies in the induction of Nrf2-regulated detoxication enzymes with reduced cytotoxicity have been reported between the SF and these two compounds [189]. Presently, there are 149 clinical studies on DIM and 11 on I3C listed on clinicaltrials.gov, suggesting a good safety profile—potential efficacy and mode of action in humans are a subject of intense current investigation, though definitive answers will not come for some time. Recently published human clinical work is suggestive of a protective effect, since one year of supplementation with DIM led to reduced breast density in BRCA positive women [190]. This is consistent with the many reports of DIM altering estrogen metabolism in healthy post-menopausal women and preventing cancer initiation and tumor development.
Other roles of I3C/DIM include, most notably, their effects as negative regulators of estrogen metabolism, favoring a shift from the 16-hydroxy to the 2-hydroxy form of estrone (estrone 2-hydroxylation) [191]. This reduction in estrogen metabolites is known to activate the estrogen receptor, thus changing from a tumor-promoting to a non-tumor-promoting form, and has been long known [192]. This shift (sometimes termed a shift from “good” to “bad” estrogen) has been invoked as the mechanism for the use of I3C/DIM to support perimenopausal issues in dietary supplements, though clinical trial evidence is lacking. Additionally, these compounds have been implicated in protection against oxidative damage, inhibition of cancer invasion, angiogenesis, proliferation [193], radioprotection—protection against some of the extremely negative effects of radiation therapy [194], inhibition of estrogen-inducible gene expression, and inhibition of androgen receptors (important in prostate cancer) [193,195].
Author Contributions
Conceptualization, J.W.F. and M.R.; validation, J.W.F. and M.R.; formal analysis, J.W.F. and M.R.; investigation, J.W.F. and M.R.; resources, J.W.F. and M.R.; data curation, J.W.F. and M.R.; writing—original draft preparation, J.W.F. and M.R.; writing—review and editing, J.W.F. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable. No new data were created or analyzed in this review.
Acknowledgments
The authors thank several colleagues for their comments on a preliminary draft of this manuscript.
Conflicts of Interest
Both authors advise several food and ingredient companies.
References
- Fahey, J.W.; Kensler, T.W. The Challenges of Designing and Implementing Clinical Trials with Broccoli Sprouts… and Turning Evidence Into Public Health Action. Front. Nutr. 2021, 8, 648788. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.A. Vegetables, Fruit, and Lung Cancer in the Iowa Women’s Health Study. Cancer Res. 1993, 53, 536–543. [Google Scholar] [PubMed]
- Kristal, A.; Lampe, J. Brassica Vegetables and Prostate Cancer Risk: A Review of the Epidemiological Evidence. Nutr. Cancer 2002, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Liu, X.; Chang, Y.; Li, Y.; Zhang, X.; Li, F.; Song, J.; Shi, H.; Chen, X.; Cui, J. Prospective cohort study of broccoli consumption frequency and all-cause and cause-specific mortality risks. Front. Nutr. 2024, 10, 1286658. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Ramakrishan, M.; Fahey, J.W.; Zimmerman, A.W.; Zhou, X.; Panjwani, A. The Role of Isothiocyanate-Rich Plants and Supplements in Neuropsychiatric Disorders: A Review and Update. Front. Nutr. 2024, 11, 1448130. [Google Scholar] [CrossRef]
- Nussey, S.; Whitehead, S. Endocrinology: An Integrated Approach; BIOS Scientific Publishers: Oxford, UK, 2001. [Google Scholar] [PubMed]
- Dólleman, M.; Verschuren, W.M.M.; Eijkemans, M.J.C.; Dollé, M.E.T.; Jansen, E.H.J.M.; Broekmans, F.J.M.; van der Schouw, Y.T. Reproductive and Lifestyle Determinants of Anti-Müllerian Hormone in a Large Population-Based Study. J. Clin. Endocrinol. Metabolism. 2013, 98, 2106–2115. [Google Scholar] [CrossRef]
- Meinhardt, U.; Mullis, P.E. The essential role of the aromatase/p450arom. Semin. Reprod. Med. 2002, 20, 277–284. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef]
- Gannesen, A.V.; Mart’yanov, S.V.; Plakunov, V.K. How human hormones regulate human microbiota: Where are we in the middle of this terra incognita? Curr. Opin. Endocr. Metab. Res. 2024, 36, 100537. [Google Scholar] [CrossRef]
- Holman, J.; Hurd, M.; Moses, P.L.; Mawe, G.M.; Zhang, T.; Ishaq, S.L.; Li, Y. Interplay of broccoli/broccoli sprout bioactives with gut microbiota in reducing inflammation in inflammatory bowel diseases. J. Nutr. Biochem. 2023, 113, 109238. [Google Scholar] [CrossRef]
- Vegeto, E.; Villa, A.; Della Torre, S.; Crippa, V.; Rusmini, P.; Cristofani, R.; Galbiati, M.; Maggi, A.; Poletti, A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr. Rev. 2020, 41, 273–319. [Google Scholar] [CrossRef]
- Brann, D.W.; Dhandapani, K.; Wakade, C.; Mahesh, V.B.; Khan, M.M. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids 2007, 72, 381–405. [Google Scholar] [CrossRef]
- Brinton, R.D. Estrogen-induced plasticity from cells to circuits: Predictions for cognitive function. Trends Pharmacol. Sci. 2009, 30, 212–222. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Lee, H.; Burnett-Bowie, S.A.; Pallais, J.C.; Yu, E.W.; Borges, L.F.; Jones, B.F.; Barry, C.V.; Wulczyn, K.E.; Thomas, B.J.; et al. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 2013, 369, 1011–1022. [Google Scholar] [CrossRef]
- Levitin, D.J. Successful Aging: A Neuroscientist Explores the Power and Potential of Our Lives; The New York Times: New York, NY, USA, 2020; p. 498. [Google Scholar]
- Fahey, J. Brassica: Characteristics and Properties. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Johnson, T.L.; Dinkova-Kostova, A.T.; Fahey, J.W. Glucosinolates from the Brassica Vegetables and Their Health Effects. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: New York, NY, USA, 2016; pp. 248–255. ISBN 9780123849533. [Google Scholar]
- Fahey, J.W.; Holtzclaw, W.D.; Wehage, S.L.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase. PLoS ONE 2015, 10, e0140963. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Panjwani, A.A.; Liu, H.; Cornblatt, G.; Cornblatt, B.S.; Ownby, S.L.; Fuchs, E.; Holtzclaw, W.D.; et al. Bioavailability of Sulforaphane Following Ingestion of Glucoraphanin-Rich Broccoli Sprout and Seed Extracts with Active Myrosinase: A Pilot Study of the Effects of Proton Pump Inhibitor Administration. Nutrients 2019, 11, 1489. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Kensler, T.W. Phytochemicals: Do they belong on our plate for sustaining healthspan? Food Front. 2021, 2, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef]
- Wang, Q.; Li, D.; Liu, L.; Shan, Y.; Bao, Y. Dietary isothiocyanates and anticancer agents: Exploring synergism for improved cancer management. Front. Nutr. 2024, 11, 1386083. [Google Scholar] [CrossRef]
- Shoaib, S.; Khan, F.B.; Alsharif, M.A.; Malik, M.S.; Ahmed, S.A.; Jamous, Y.F.; Uddin, S.; Tan, C.S.; Ardianto, C.; Tufail, S.; et al. Reviewing the Prospective Pharmacological Potential of Isothiocyanates in Fight against Female-Specific Cancers. Cancers 2023, 15, 2390. [Google Scholar] [CrossRef]
- Mthembu, S.X.H.; Mazibuko-Mbeje, S.E.; Moetlediwa, M.T.; Muvhulawa, N.; Silvestri, S.; Orlando, P.; Nkambule, B.B.; Muller, C.J.F.; Ndwandwe, D.; Basson, A.K.; et al. Sulforaphane: A nutraceutical against diabetes-related complications. Pharmacol. Res. 2023, 196, 106918. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Liu, Y.; Yang, X. Sulforaphane and ophthalmic diseases. Food Sci. Nutr. 2024, 12, 5296–5311. [Google Scholar] [CrossRef]
- Monteiro, E.B.; Ajackson, M.; Stockler-Pinto, M.B.; Guebre-Egziabher, F.; Daleprane, J.B.; Soulage, C.O. Sulforaphane exhibits potent renoprotective effects in preclinical models of kidney diseases: A systematic review and meta-analysis. Life Sci. 2023, 322, 121664. [Google Scholar] [CrossRef]
- Yan, L.; Yan, Y. Therapeutic potential of sulforaphane in liver diseases: A review. Front. Pharmacol. 2023, 29, 1256029. [Google Scholar] [CrossRef]
- Wei, Y.; Chang, L.; Liu, G.; Wang, X.; Yang, Y.; Hashimoto, K. Long-lasting beneficial effects of maternal intake of sulforaphane glucosinolate on gut microbiota in adult offspring. J. Nutr. Biochem. 2022, 109, 109098. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxidative Med. Cell Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 2017, 69 Pt B, 257–269. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef]
- Sedlak, T.W.; Nucifora, L.G.; Koga, M.; Shaffer, L.S.; Higgs, C.; Tanaka, T.; Wang, A.M.; Coughlin, J.M.; Barker, P.; Fahey, J.W.; et al. Sulforaphane augments glutathione and influences brain metabolites in human subjects: A clinical pilot study. Mol. Neuropsychiatry 2018, 3, 214–222. [Google Scholar] [CrossRef]
- Brown, R.H.; Reynolds, C.; Brooker, A.; Talalay, P.; Fahey, J.W. Sulforaphane improves the bronchoprotective response in asthmatics through Nrf2-mediated gene pathways. Respir. Res. 2015, 16, 106. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, T.; Mao, L.; Zhang, F. Sulforaphane Protects against Brain Diseases: Roles of Cytoprotective Enzymes. Austin J. Cerebrovasc. Dis. Stroke 2017, 4, 1054. [Google Scholar]
- Huo, L.; Su, Y.; Xu, G.; Zhai, L.; Zhao, J. Sulforaphane Protects the Male Reproductive System of Mice from Obesity-Induced Damage: Involvement of Oxidative Stress and Autophagy. Int. J. Environ. Res. Public Health 2019, 16, 3759. [Google Scholar] [CrossRef]
- Hong, L.; Xu, Y.; Wang, D.; Zhang, Q.; Li, X.; Xie, C.; Wu, J.; Zhong, C.; Fu, J.; Geng, S. Sulforaphane ameliorates bisphenol A-induced hepatic lipid accumulation by inhibiting endoplasmic reticulum stress. Sci. Rep. 2023, 13, 1147. [Google Scholar] [CrossRef]
- Baralić, K.; Živančević, K.; Marić, Đ.; Bozic, D.; Buha Djordjevic, A.; Antonijević Miljaković, E.; Ćurčić, M.; Bulat, Z.; Antonijević, B.; Đukić-Ćosić, D. Testing sulforaphane as a strategy against toxic chemicals of public health concern by toxicogenomic data analysis: Friend or foe at the gene level—Colorectal carcinoma case study. Environ. Res. 2023, 227, 115818. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Han, X.; Lee, S.-J.; Oh, G.; Park, K.-T.; Han, J.-K.; Choi, S.-I.; Lee, O.-H. Anti-Obesogenic Effects of Sulforaphane-Rich Broccoli (Brassica oleracea var. italica) Sprouts and Myrosinase-Rich Mustard (Sinapis alba L.) Seeds In Vitro and In Vivo. Nutrients 2022, 14, 3814. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Cho, B.-Y.; Choi, S.-H.; Jung, T.-D.; Choi, S.-I.; Lim, J.-H.; Lee, O.-H. Sulforaphane attenuates bisphenol A-induced 3T3-L1 adipocyte differentiation through cell cycle arrest. J. Funct. Foods 2018, 44, 17–23. [Google Scholar] [CrossRef]
- Shimpi, P.C.; More, V.R.; Paranjpe, M.; Donepudi, A.C.; Goodrich, J.M.; Dolinoy, D.C.; Rubin, B.; Slitt, A.L. Hepatic Lipid Accumulation and Nrf2 Expression following Perinatal and Peripubertal Exposure to Bisphenol A in a Mouse Model of Nonalcoholic Liver Disease. Environ. Health Perspect. EHP 2017, 125, 087005. [Google Scholar] [CrossRef]
- Fowke, J.H.; Longcope, C.; Hebert, J.R. Brassica vegetable consumption shifts estrogen metabolism in healthy postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2000, 9, 773–779. [Google Scholar] [PubMed]
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Gao, X.; Wang, B.; Huang, Y.; Wu, M.; Li, Y.; Li, Y.; Zhu, X.; Wu, M. Role of the Nrf2 Signaling Pathway in Ovarian Aging: Potential Mechanism and Protective Strategies. Int. J. Mol. Sci. 2023, 24, 13327. [Google Scholar] [CrossRef]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef]
- Atwell, L.L.; Zhang, Z.; Mori, M.; Farris, P.E.; Vetto, J.T.; Naik, A.M.; Oh, K.Y.; Thuillier, P.; Ho, E.; Shannon, J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev. Res. 2015, 8, 1184–1191. [Google Scholar] [CrossRef]
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal Symptoms and Their Management. Endocrinol. Metab. Clin. N. Am. 2015, 44, 497–515. [Google Scholar] [CrossRef]
- Peters, B.A.; Santoro, N.; Kaplan, R.C.; Qi, Q. Spotlight on the Gut Microbiome in Menopause: Current Insights. Int. J. Womens Health 2022, 14, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Daniels, L.M.; Reiter, A.R. Learning to like vegetables during breastfeeding: A randomized clinical trial of lactating mothers and infants. Am. J. Clin. Nutr. 2017, 106, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Fujita, A.; Ishima, T.; Hirai, A.; Suzuki, S.; Suganuma, H.; Hashimoto, K. Dietary intake of glucoraphanin during pregnancy and lactation prevents the behavioral abnormalities in the offspring after maternal immune activation. Neuropsychopharmacol. Rep. 2020, 40, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Bahry, A.M.; Shen, K.Q.; Armstrong, E.A.; Yager, J.Y. Consumption of broccoli sprouts during late gestation and lactation confers protection against developmental delay induced by maternal inflammation. Behav. Brain Res. 2016, 307, 239–249. [Google Scholar] [CrossRef]
- Wu, L.Y.; Juurlink, B.H.J. The Impaired Glutathione System and Its Up-Regulation by Sulforaphane in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats. J. Hypertens. 2001, 19, 1819–1825. [Google Scholar] [CrossRef]
- Wu, L.; Noyan-Ashraf, M.H.; Facci, M.; Wang, R.; Paterson, P.G.; Ferrie, A.; Juurlink, B.H. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc. Natl. Acad. Sci. USA 2004, 101, 7094–7099. [Google Scholar] [CrossRef]
- Noyan-Ashraf, M.H.; Sadeghinejad, Z.; Juurlink, B.H.J. Dietary approach to decrease aging-related CNS inflammation. Nutr. Neurosci. 2005, 8, 101–110. [Google Scholar] [CrossRef]
- Senanayake, G.V.; Banigesh, A.; Wu, L.; Lee, P.; Juurlink, B.H. The Dietary Phase 2 Protein Inducer Sulforaphane Can Normalize the Kidney Epigenome and Improve Blood Pressure in Hypertensive Rats. Am. J. Hypertens. 2012, 25, 229–235. [Google Scholar] [CrossRef]
- Ghazizadeh-Hashemi, F.; Bagheri, S.; Ashraf-Ganjouei, A.; Moradi, K.; Shahmansouri, N.; Mehrpooya, M.; Noorbala, A.; Akhondzadeh, S. Efficacy and safety of sulforaphane for treatment of mild to moderate depression in patients with history of cardiac interventions: A randomized, double-blind, placebo-controlled clinical trial. Psychiatry Clin. Neurosci. 2021, 75, 250–255. [Google Scholar] [CrossRef]
- Wu, S.; Gao, Q.; Zhao, P.; Gao, Y.; Xi, Y.; Wang, X.; Liang, Y.; Shi, H.; Ma, Y. Sulforaphane produces antidepressant-and anxiolytic-like effects in adult mice. Behav. Brain Res. 2016, 301, 55–62. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Wu, J.; Ushida, Y.; Suganuma, H.; et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J. Nutr. Biochem. 2017, 39, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Chamorro, P.; Redondo, A.; Riego, G.; Leánez, S.; Pol, O. Sulforaphane inhibited the nociceptive responses, anxiety-and depressive-like behaviors associated with neuropathic pain and improved the anti-allodynic effects of morphine in mice. Front. Pharmacol. 2018, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, C.; Quan, M.; Li, T.; Jia, J. Sulforaphane reverses the amyloid-β oligomers induced depressive-like behavior. J. Alzheimers Dis. 2020, 78, 127–137. [Google Scholar] [CrossRef]
- Tucci, P.; Bove, M.; Sikora, V.; Dimonte, S.; Morgese, M.G.; Schiavone, S.; Mannelli, L.D.C.; Ghelardini, C.; Trabace, L. Glucoraphanin triggers rapid antidepressant responses in a rat model of beta amyloid induced depressive-like behaviour. Pharmaceuticals 2022, 15, 1054. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, J.-C.; Ishima, T.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Wu, J.; Suganuma, H.; et al. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci. Rep. 2016, 6, 30659. [Google Scholar] [CrossRef]
- Kamel, A.S.; El-Sayed, S.S.; EL Sayed, N.S. Sulforaphane’s Role in Redefining Autophagic Responses in Depression Associated with Polycystic Ovarian Syndrome: Unveiling the SIRT1/AMPK/LKB1 Pathway Connection. Eur. J. Pharmacol. 2024, 969, 176477. [Google Scholar] [CrossRef]
- Taheri, M.; Roudbari, N.H.; Amidi, F.; Parivar, K. The Protective Effect of Sulforaphane against Oxidative Stress in Granulosa Cells of Patients with Polycystic Ovary Syndrome (PCOS) through Activation of AMPK/AKT/NRF2 Signaling Pathway. Reprod. Biol. 2021, 21, 100563. [Google Scholar] [CrossRef]
- Esfandyari, S. The Protective Effect of Sulforaphane against Oxidative Stress through Activation of NRF2/ARE Pathway in Human Granulosa Cells. Cell J. 2021, 23, 692–700. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Lu, X.; Meng, J.; Qin, X.; Jiang, J. Anti-Nociceptive and Anti-Inflammatory Effects of Sulforaphane on Sciatic Endometriosis in a Rat Model. Neurosci. Lett. 2020, 723, 134858. [Google Scholar] [CrossRef]
- Zhou, A.; Hong, Y.; Lv, Y. Sulforaphane Attenuates Endometriosis in Rat Models Through Inhibiting PI3K/Akt Signaling Pathway. Dose-Response Publ. Int. Hormesis Soc. 2019, 17, 1559325819855538. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; Costa, F.d.S.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.G.; Gurusinghe, S.; Rahman, R.A.; Leaw, B.; Chan, S.T.; Mockler, J.C.; Murthi, P.; Marshall, S.A.; Lim, R.; Wallace, E.M. Sulforaphane Improves Endothelial Function and Reduces Placental Oxidative Stress in Vitro. Pregnancy Hypertens. 2019, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Langston-Cox, A.; Leo, C.H.; Tare, M.; Wallace, E.M.; Marshall, S.A. Sulforaphane Improves Vascular Reactivity in Mouse and Human Arteries after ‘Preeclamptic-like’ Injury. Placenta 2020, 101, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Langston-Cox, A.; Muccini, A.; Marshall, S.; Yap; Palmer, K.; Wallace, E.; Ellery, S. Sulforaphane Improves Syncytiotrophoblast Mitochondrial Function after In Vitro Hypoxic and Superoxide Injury. Placenta 2020, 96, 44–54. [Google Scholar] [CrossRef]
- Langston-Cox, A.G.; Anderson, D.; Creek, D.J.; Palmer, K.R.; Marshall, S.A.; Wallace, E.M. Sulforaphane Bioavailability and Effects on Blood Pressure in Women with Pregnancy Hypertension. Reprod. Sci. 2021, 28, 1489–1497. [Google Scholar] [CrossRef]
- Fields, N.J.; Palmer, K.R.; Nisi, A.; Marshall, S.A. Preeclampsia to COVID-19: A Journey towards Improved Placental and Vascular Function Using Sulforaphane. Placenta 2023, 141, 84–93. [Google Scholar] [CrossRef]
- Farvid, M.S.; Chen, W.Y.; Rosner, B.A.; Tamimi, R.M.; Willett, W.C.; Eliassen, A.H. Fruit and vegetable consumption and breast cancer incidence: Repeated measures over 30 years of follow-up. Int. J. Cancer 2019, 144, 1496–1510. [Google Scholar] [CrossRef]
- Zhang, Z.; Atwell, L.L.; Farris, P.E.; Ho, E.; Shannon, J. Associations between cruciferous vegetable intake and selected biomarkers among women scheduled for breast biopsies. Public Health Nutr. 2016, 19, 1288–1295. [Google Scholar] [CrossRef]
- Cornblatt, B.S.; Ye, L.; Dinkova-Kostova, A.T.; Erb, M.; Fahey, J.W.; Singh, N.K.; Chen, M.-S.A.; Stierer, T.; Garrett-Mayer, E.; Argani, P.; et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 2007, 28, 1485–1490. [Google Scholar] [CrossRef]
- Pledgie-Tracy, A.; Sobolewski, M.D.; Davidson, N.E. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol. Cancer Ther. 2007, 6, 1013–1021. [Google Scholar] [CrossRef]
- Atwell, L.L.; Beaver, L.M.; Shannon, J.; Williams, D.E.; Dashwood, R.H.; Ho, E. Epigenetic Regulation by Sulforaphane: Opportunities for Breast and Prostate Cancer Chemoprevention. Curr. Pharmacol. Rep. 2015, 1, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Yang, L.; Chartoumpekis, D.V.; Wendell, S.G.; Fazzari, M.; Skoko, J.J.; Liao, Y.; Oesterreich, S.; Michalopoulos, G.K.; Kensler, T.W. Sulforaphane Diminishes the Formation of Mammary Tumors in Rats Exposed to 17β-Estradiol. Nutrients 2020, 30, 2282. [Google Scholar] [CrossRef]
- Kuran, D.; Pogorzelska, A.; Wiktorska, K. Breast Cancer Prevention-Is there a Future for Sulforaphane and Its Analogs? Nutrients 2020, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Talalay, P. Relevance of anti-inflammatory and antioxidant activities of exemestane and synergism with sulforaphane for disease prevention. Proc. Natl. Acad. Sci. USA 2013, 110, 19065–19070. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, C.; Pratt, R.; Khoury, T.; Qu, J.; Fahey, J.W.; McCann, S.E.; Zhang, Y.; Wu, Y.; Hutson, A.D.; et al. A Presurgical-Window Intervention Trial of Isothiocyanate-Rich Broccoli Sprout Extract in Patients with Breast Cancer. Mol. Nutr. Food Res. 2022, 66, e2101094. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Q.; Li, N.; Xu, M.; Miyamoto, T.; Liu, J. Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway. NPJ Breast Cancer 2022, 8, 40. [Google Scholar] [CrossRef]
- Luo, M. Anticancer Effect of Natural Product Sulforaphane by Targeting MAPK Signal through miRNA-1247-3p in Human Cervical Cancer Cells. Biointerface Res. Appl. Chem. 2020, 11, 7943–7972. [Google Scholar]
- Cheng, Y.-M.; Tsai, C.-C.; Hsu, Y.-C. Sulforaphane, a Dietary Isothiocyanate, Induces G2/M Arrest in Cervical Cancer Cells through CyclinB1 Downregulation and GADD45β/CDC2 Association. Int. J. Mol. Sci. 2016, 17, 1530. [Google Scholar] [CrossRef]
- Ali Khan, M.; Kedhari, S.M.; Hamza, A.; Quaraishi, U.; Gunasekera, D.; Ramesh, L.; Goala, P.; Al Alami, U.; Ansari, M.Z.; Rizvi, T.A.; et al. Sulforaphane Reverses the Expression of Various Tumor Suppressor Genes by Targeting DNMT3B and HDAC1 in Human Cervical Cancer Cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Sharma, C.; Sadrieh, L.; Priyani, A.; Ahmed, M.; Hassan, A.H.; Hussain, A. Anti-Carcinogenic Effects of Sulforaphane in Association with Its Apoptosis-Inducing and Anti-Inflammatory Properties in Human Cervical Cancer Cells. Cancer Epidemiol. 2011, 35, 272–278. [Google Scholar] [CrossRef]
- Park, S.; Kim, G.; Bae, S.-J.; Young, Y.; Choi, Y. Induction of Apoptosis by Isothiocyanate Sulforaphane in Human Cervical Carcinoma HeLa and Hepatocarcinoma HepG2 Cells through Activation of Caspase-3. Oncol. Rep. 2007, 18, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Priyuani, A.; Sadrieh, L.; Brahmbhatt, K.; Ahmed, M.; Sharma, C. Concurrent Sulforaphane and Eugenol Induces Differential Effects on Human Cervical Cancer Cells. Integr. Cancer Ther. 2012, 11, 154–165. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. 2024. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8773.00.pdf (accessed on 8 December 2024).
- Chaudhuri, D.; Orsulic, S.; Ashok, B.T. Antiproliferative Activity of Sulforaphane in Akt-Overexpressing Ovarian Cancer Cells. Mol. Cancer Ther. 2007, 6, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Landen, C.N.; Li, Y.; Alvarez, R.D.; Tollefsbol, T.O. Epigallocatechin Gallate and Sulforaphane Combination Treatment Induce Apoptosis in Paclitaxel-Resistant Ovarian Cancer Cells through hTERT and Bcl-2 down-Regulation. Exp. Cell Res. 2013, 319, 697–706. [Google Scholar] [CrossRef]
- Chen, H.; Landen, C.N.; Li, Y.; Alvarez, R.D.; Tollefsbol, T.O. Enhancement of Cisplatin-Mediated Apoptosis in Ovarian Cancer Cells through Potentiating G2/M Arrest and P21 Upregulation by Combinatorial Epigallocatechin Gallate and Sulforaphane. J. Oncol. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Gong, T.-T.; Liu, X.-D.; Zhan, Z.-P.; Wu, Q.-J. Sulforaphane Enhances the Cisplatin Sensitivity through Regulating DNA Repair and Accumulation of Intracellular Cisplatin in Ovarian Cancer Cells. Exp. Cell Res. 2020, 393, 112061. [Google Scholar] [CrossRef]
- Bryant, C.S.; Kumar, S.; Chamala, S.; Shah, J.; Pal, J.; Haider, M.; Seward, S.; Qazi, A.M.; Morris, R.; Semaan, A.; et al. Sulforaphane induces cell cycle arrest by protecting RB-E2F-1 complex in epithelial ovarian cancer cells. Mol. Cancer 2010, 9, 47. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hung, C.-M.; Yang, Y.-R.; Lee, M.-J.; Hsu, Y.-C. Sulforaphane induced cell cycle arrest in the G2/M phase via the blockade of cyclin B1/CDC2 in human ovarian cancer cells. J. Ovarian Res. 2013, 6, 41. [Google Scholar] [CrossRef]
- Kan, S.; Sun, G. Sulforaphane regulates apoptosis- and proliferation-related signaling pathways and synergizes with cisplatin to suppress human ovarian cancer. Int. J. Mol. Med. 2018, 42, 2447. [Google Scholar] [CrossRef]
- Ogunlade, B.; Adelakun, S.; Iteire, K. Sulforaphane response on aluminum-induced oxidative stress, alterations in sperm characterization and testicular histomorphometry in wistar rats. Int. J. Reprod. Biomed. 2020, 18, 611–624. [Google Scholar] [CrossRef]
- Yang, S.H.; Long, M.; Yu, L.H.; Li, L.; Li, P.; Zhang, Y.; Guo, Y.; Gao, F.; Liu, M.D.; He, J.B. Sulforaphane prevents testicular damage in kunming mice exposed to cadmium via activation of Nrf2/ARE signaling pathways. Int. J. Mol. Sci. 2016, 17, 1703. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Munawar, A.; Razak, S.; Anam, S.; Ain, Q.U.; Ullah, H.; Afsar, T.; Abulmeaty, M.; Almajwal, A. Ameliorative effects of rutin against cisplatin-induced reproductive toxicity in male rats. BMC Urol. 2018, 18, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Z.; Gao, J.; Li, H.; Wang, X.; Li, Y.; Sun, F. Inhibition of ferroptosis attenuates busulfan-induced oligospermia in mice. Toxicology 2020, 440, 152489. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Harb, N.G.; Ali, A.A.; El-Aziz, R.M.A.; Elrashidy, R.A. Sulforaphane substantially impedes testicular ferroptosis in adult rats exposed to di-2-ethylhexyl phthalate through activation of NRF-2/SLC7A11/GPX-4 trajectory. In Naunyn-Schmiedeberg’s Archives of Pharmacology; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Qin, Z.; Tang, J.; Han, P.; Jiang, X.; Yang, C.; Li, R.; Tang, M.; Shen, B.; Wang, W.; Qin, C.; et al. Protective effects of sulforaphane on di-n-butylphthalateinduced testicular oxidative stress injury in male mice offsprings via activating Nrf2/ARE pathway. Oncotarget 2017, 8, 82956–82967. [Google Scholar] [CrossRef]
- Shen, B.; Wang, W.; Ma, L.; Wang, S.; Ding, L.; Chen, Z.; Sao, Y.; Shen, H.; Wei, Z.; Zhang, W. Sulforaphane restores oxidative stress induced by di-n-butylphthalate in testicular leydig cells with low basal reactive oxygen species levels. Urology 2014, 84, 850–856. [Google Scholar] [CrossRef]
- Mu, Y.; Yin, T.L.; Huang, X.X.; Hu, X.; Yin, L.; Yang, J. Sulforaphane ameliorates high-fat diet-induced spermatogenic deficiency in mice. Biol. Reprod. 2019, 101, 223–234. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Xin, Y.; Bai, Y.; Kong, L.; Tan, Y.; Liu, F.; Cai, L. Sulforaphane Prevents Angiotensin II-Induced Testicular Cell Death via Activation of NRF2. Oxidative Med. Cell. Longev. 2017, 2017, 5374897. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Guo, W.; Sun, W.; Miao, X.; Wu, H.; Cong, X.; Wintergerst, K.A.; Kong, X.; Cai, L. Sulforaphane reduction of testicular apoptotic cell death in diabetic mice is associated with the upregulation of Nrf2 expression and function. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E14–E23. [Google Scholar] [CrossRef]
- Jiang, X.; Bai, Y.; Zhang, Z.; Xin, Y.; Cai, L. Protection by sulforaphane from type 1 diabetes-induced testicular apoptosis is associated with the up-regulation of Nrf2 expression and function. Toxicol. Appl. Pharmacol. 2014, 279, 198–210. [Google Scholar] [CrossRef]
- Wei, S.M.; Huang, Y.M. Effect of sulforaphane on testicular ischemia-reperfusion injury induced by testicular torsion-detorsion in rats. Sci. Rep. 2024, 14, 23420. [Google Scholar] [CrossRef]
- Valipour, J.; Nashtaei, M.S.; Khosravizadeh, Z.; Mahdavinezhad, F.; Nekoonam, S.; Esfandyari, S.; Amidi, F. Effect of sulforaphane on apoptosis, reactive oxygen species and lipids peroxidation of human sperm during cryopreservation. Cryobiology 2021, 99, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Andrabi, S.M.H.; Jahan, S. Semen quality parameters as fertility predictors of water buffalo bull spermatozoa during low-breeding season. Theriogenology 2016, 86, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; El Assar, M.; Sevilleja-Ortiz, A.; Fernández, A.; Sánchez-Ferrer, A.; Romero-Otero, J.; Martínez-Salamanca, J.I.; La Fuente, J.M.; Rodríguez-Mañas, L. Short-term pharmacological activation of Nrf2 ameliorates vascular dysfunction in aged rats and in pathological human vasculature. A potential target for therapeutic intervention. Redox Biol. 2019, 26, 101271. [Google Scholar] [CrossRef] [PubMed]
- Hibino, T.; NIshiyama, T. Role of TGF-β2 in the human hair cycle. J. Dermatol. Sci. 2004, 35, 9–18. [Google Scholar] [CrossRef]
- Sasaki, M.; Shinozaki, S.; Shimokado, K. Sulforaphane promotes murine hair growth by accelerating the degradation of dihydrotestosterone. Biochem. Biophys. Res. Commun. 2016, 472, 250–254. [Google Scholar] [CrossRef]
- Park, Y.; Choi, K.; Kim, H.; Lee, J.; Park, G.; Kim, J. Sulforaphane, L-Menthol, and Dexpanthenol as a Novel Active Cosmetic Ingredient Composition for Relieving Hair Loss Symptoms. Cosmetics 2021, 8, 63. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, X. Brassica oleracea extract, glucosinlates, and sulforaphane promote hair growth in vitro and ex vivo. J. Cosmet. Dermatol. 2022, 21, 1178–1184. [Google Scholar] [CrossRef]
- Michaud, D.S.; Spiegelman, D.; Clinton, S.K.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Fruit and Vegetable Intake and Incidence of Bladder Cancer in a Male Prospective Cohort. JNCI J. Natl. Cancer Inst. 1999, 91, 605–613. [Google Scholar] [CrossRef]
- Myzak, M.C.; Hardin, K.; Wang, R.; Dashwood, R.H.; Ho, E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis 2006, 27, 811–819. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Yu, Z.; Dashwood, R.H.; Ho, E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol. Nutr. Food Res. 2011, 55, 999–1009. [Google Scholar] [CrossRef]
- Gibbs, A.; Schwartzman, J.; Deng, V.; Alumkal, J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc. Natl. Acad. Sci. USA 2009, 106, 16663–16668. [Google Scholar] [CrossRef] [PubMed]
- Ganai, S.A. Histone deacetylase inhibitor sulforaphane: The phytochemical with vibrant activity against prostate cancer. Biomed. Pharmacother. 2016, 81, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.H.; Melchini, A.; Mithen, R.F. Sulforaphane and prostate cancer interception. Drug Discov. Today 2014, 19, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Kong, A.H.T. Pharmacometrics of nutraceutical sulforaphane and its implications in prostate cancer prevention. J. Chin. Pharm. Sci. 2016, 25, 12–22. [Google Scholar]
- Mordecai, J.; Ullah, S.; Ahmad, I. Sulforaphane and Its Protective Role in Prostate Cancer: A Mechanistic Approach. Int. J. Mol. Sci. 2023, 24, 6979. [Google Scholar] [CrossRef]
- Beaver, L.M.; Kuintzle, R.; Buchanan, A.; Wiley, M.W.; Glasser, S.T.; Wong, C.P.; Johnson, G.S.; Chang, J.H.; Löhr, C.V.; Williams, D.E.; et al. Long noncoding RNAs and sulforaphane: A target for chemoprevention and suppression of prostate cancer. J. Nutr. Biochem. 2017, 42, 72–83. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, Y.; Yang, G.; Geng, Y.; Wu, S.; Hu, Y.; Lin, K.; Wu, W. Sulforaphane-cysteine suppresses invasion via downregulation of galectin-1 in human prostate cancer DU145 and PC3 cells. Oncol. Rep. 2016, 36, 1361–1368. [Google Scholar] [CrossRef]
- Lee, C.H.; Jeong, S.-J.; Yun, S.-M.; Kim, J.-H.; Ahn, K.S.; Won, S.-H.; Kim, H.S.; Lee, H.-J.; Zhu, S.; Chen, C.-Y.; et al. Down-regulation of phosphoglucomutase 3 mediates sulforaphane-induced cell death in LNCaP prostate cancer cells. Proteome Sci. 2010, 8, 67. [Google Scholar] [CrossRef]
- Khurana, N.; Talwar, S.; Chandra, P.K.; Sharma, P.; Abdel-Mageed, A.B.; Mondal, D.; Sikka, S.C. Sulforaphane increases the efficacy of anti-androgens by rapidly decreasing androgen receptor levels in prostate cancer cells. Int. J. Oncol. 2016, 49, 1609–1619. [Google Scholar] [CrossRef]
- Wong, C.P.; Hsu, A.; Buchanan, A.; Palomera-Sanchez, Z.; Beaver, L.M.; Houseman, E.A.; Williams, D.E.; Dashwood, R.H.; Ho, E. Effects of sulforaphane and 3,3′-diindolylmethane on genome-wide promoter methylation in normal prostate epithelial cells and prostate cancer cells. PLoS ONE 2014, 9, e86787. [Google Scholar] [CrossRef]
- Hahm, E.R.; Singh, K.B.; Kim, S.-H.; Powolny, A.A.; Singh, S.V. The role of lysosome-associated membrane protein 2 in prostate cancer chemopreventive mechanisms of sulforaphane. Cancer Prev. Res. 2020, 13, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Beaver, L.M.; Buchanan, A.; Sokolowski, E.I.; Riscoe, A.N.; Wong, C.P.; Chang, J.H.; Löhr, C.V.; Williams, D.E.; Dashwood, R.H.; Ho, E. Transcriptome analysis reveals a dynamic and differential transcriptional response to sulforaphane in normal and prostate cancer cells and suggests a role for Sp1 in chemoprevention. Mol. Nutr. Food Res. 2014, 58, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Hahm, E.-R.; Alumkal, J.J.; Foley, L.M.; Hitchens, T.K.; Shiva, S.S.; A Parikh, R.; Jacobs, B.L.; Singh, S.V. Reversal of the Warburg phenomenon in chemoprevention of prostate cancer by sulforaphane. Carcinogenesis 2019, 40, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, Y.; Tian, H.; Yang, G.; Li, C.; Geng, Y.; Wu, S.; Wu, W. Sulforaphane inhibits invasion by phosphorylating ERK1/2 to regulate E-cadherin and CD44v6 in human prostate cancer DU145 cells. Oncol. Rep. 2015, 34, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wan, X.; Zhan, J.; Shen, M.; Li, R. Sulforaphane inhibits the growth of prostate cancer by regulating the microRNA-3919/DJ-1 axis. Front. Oncol. 2024, 14, 1361152. [Google Scholar] [CrossRef]
- Wiczk, A.; Hofman, D.; Konopa, G.; Herman-Antosiewicz, A. Sulforaphane, a cruciferous vegetable-derived isothiocyanate, inhibits protein synthesis in human prostate cancer cells. Biochim. Biophys. Acta-Mol. Cell Res. 2012, 1823, 1295–1305. [Google Scholar] [CrossRef]
- Singh, K.B.; Kim, S.-H.; Hahm, E.-R.; Pore, S.K.; Jacobs, B.L.; Singh, S.V. Prostate cancer chemoprevention by sulforaphane in a preclinical mouse model is associated with inhibition of fatty acid metabolism. Carcinogenesis 2018, 39, 826–837. [Google Scholar] [CrossRef]
- Vyas, A.R.; Hahm, E.-R.; Arlotti, J.A.; Watkins, S.; Stolz, D.B.; Desai, D.; Amin, S.; Singh, S.V. Chemoprevention of prostate cancer by D,L-sulforaphane is augmented by pharmacological inhibition of autophagy. Cancer Res. 2013, 73, 5985–5995. [Google Scholar] [CrossRef]
- Watson, G.W.; Wickramasekara, S.; Fang, Y.; Palomera-Sanchez, Z.; Maier, C.S.; Williams, D.E.; Dashwood, R.H.; Perez, V.I.; Ho, E. Analysis of autophagic flux in response to sulforaphane in metastatic prostate cancer cells. Mol. Nutr. Food Res. 2015, 59, 1954–1961. [Google Scholar] [CrossRef]
- Vyas, A.R.; Moura, M.B.; Hahm, E.R.; Singh, K.B.; Singh, S.V. Sulforaphane Inhibits c-Myc-Mediated Prostate Cancer Stem-Like Traits. J. Cell. Biochem. 2016, 117, 2482–2495. [Google Scholar] [CrossRef]
- Koh, W.; Ahn, K.S.; Jeong, S.J.; Lee, H.J.; Kim, M.; Lee, H.J.; Lee, E.O.; Kim, S.H. Reactive oxygen species involved in sulforaphane-induced STAT3 inactivation and apoptosis in DU145 prostate cancer cells. Chin. Sci. Bull. 2010, 55, 3922–3928. [Google Scholar] [CrossRef]
- Hahm, E.R.; Chandra-Kuntal, K.; Desai, D.; Amin, S.; Singh, S.V. Notch Activation Is Dispensable for D, L-Sulforaphane-Mediated Inhibition of Human Prostate Cancer Cell Migration. PLoS ONE 2012, 7, e44957. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.H.; Spinks, C.A.; Doleman, J.F.; Melchini, A.; Ball, R.Y.; Mills, R.D.; Mithen, R.F. The dietary isothiocyanate sulforaphane modulates gene expression and alternative gene splicing in a PTEN null preclinical murine model of prostate cancer. Mol. Cancer 2010, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Rutz, J.; Thaler, S.; Maxeiner, S.; Chun, F.K.H.; Blaheta, R.A. Sulforaphane reduces prostate cancer cell growth and proliferation in vitro by modulating the cdk-cyclin axis and expression of the CD44 variants 4, 5, and 7. Int. J. Mol. Sci. 2020, 21, 8724. [Google Scholar] [CrossRef] [PubMed]
- Habib, T.N.; Altonsy, M.O.; Ghanem, S.A.; Salama, M.S.; Hosny, M.A. Optimizing combination therapy in prostate cancer: Mechanistic insights into the synergistic effects of Paclitaxel and Sulforaphane-induced apoptosis. BMC Mol. Cell Biol. 2024, 25, 5. [Google Scholar] [CrossRef]
- Traka, M.; Gasper, A.V.; Melchini, A.; Bacon, J.R.; Needs, P.W.; Frost, V.; Chantry, A.; Jones, A.M.E.; Ortori, C.A.; Barrett, D.A.; et al. Broccoli Consumption Interacts with GSTM1 to Perturb Oncogenic Signalling Pathways in the Prostate. PLoS ONE 2008, 3, e2568. [Google Scholar] [CrossRef]
- Alumkal, J.J.; Slottke, R.; Schwartzman, J.; Cherala, G.; Munar, M.; Graff, J.N.; Beer, T.M.; Ryan, C.W.; Koop, D.R.; Gibbs, A.; et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Investig. New Drugs 2015, 33, 480–489. [Google Scholar] [CrossRef]
- Cipolla, B.G.; Mandron, E.; Lefort, J.M.; Coadou, Y.; Della Negra, E.; Corbel, L.; Le Scodan, R.; Azzouzi, A.R.; Mottet, N. Effect of Sulforaphane in Men with Biochemical Recurrence after Radical Prostatectomy. Cancer Prev. Res. 2015, 8, 712–719. [Google Scholar] [CrossRef]
- Traka, M.H.; Melchini, A.; Coode-Bate, J.; Kadhi, O.A.; Saha, S.; Defernez, M.; Troncoso-Rey, P.; Kibblewhite, H.; O’Neill, C.M.; Bernuzzi, F.; et al. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention-results from the Effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1133–1144. [Google Scholar] [CrossRef]
- Zhang, Z.; Garzotto, M.; Davis, E.W.; Mori, M.; Stoller, W.A.; Farris, P.E.; Wong, C.P.; Beaver, L.M.; Thomas, G.V.; Williams, D.E.; et al. Sulforaphane Bioavailability and Chemopreventive Activity in Men Presenting for Biopsy of the Prostate Gland: A Randomized Controlled Trial. Nutr. Cancer 2020, 72, 74–87. [Google Scholar] [CrossRef]
- Livingstone, T.L.; Saha, S.; Bernuzzi, F.; Savva, G.M.; Troncoso-Rey, P.; Traka, M.H.; Mills, R.D.; Ball, R.Y.; Mithen, R.F. Accumulation of Sulforaphane and Alliin in Human. Prostate Tissue Nutr. 2022, 14, 3263. [Google Scholar]
- Ferreira, P.M.P.; Rodrigues, L.A.R.L.; de Alencar Carnib, L.P.; de Lima Sousa, P.V.; Nolasco Lugo, L.M.; Nunes, N.M.F.; do Nascimento Silva, J.; da Silva Araûjo, L.; de Macêdo Gonçalves Frota, K. Cruciferous vegetables as antioxidative, chemopreventive and antineoplasic functional foods: Preclinical and clinical evidences of sulforaphane against prostate cancers. Curr. Pharm. Des. 2018, 24, 4779–4793. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, A.; Slominska-Wojewodzka, M.; Herman-Antosiewicz, A. Sensitization of estrogen receptor-positive breast cancer cell lines to 4-hydroxytamoxifen by isothiocyanates present in cruciferous plants. Eur. J. Nutr. 2016, 55, 1165–1180. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Sun, Y.; Fu, R.; Ye, D. An Insight on Synergistic Anti-cancer Efficacy of Biochanin A and Sulforaphane Combination Against Breast Cancer. Appl. Biochem. Biotechnol. 2024, 196, 992–1007. [Google Scholar] [CrossRef]
- Lubecka-Pietruszewska, K.; Kaufman-Szymczyk, A.; Stefanska, B.; Cebula-Obrzut, B.; Smolewski, P.; Fabianowska-Majewska, K. Sulforaphane alone and in combination with clofarabine epigenetically regulates the expression of DNA methylation-silenced tumour suppressor genes in human breast cancer cells. J. Nutr. Nutr. 2015, 8, 91–101. [Google Scholar] [CrossRef]
- Burnett, J.P.; Lim, G.; Li, Y.; Shah, R.B.; Lim, R.; Paholak, H.J.; McDermott, S.P.; Sun, L.; Tsume, Y.; Bai, S.; et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017, 394, 52–64. [Google Scholar] [CrossRef]
- Bose, C.; Awasthi, S.; Sharma, R.; Beneš, H.; Hauer-Jensen, M.; Boerma, M.; Singh, S.P. Sulforaphane potentiates anticancer effects of doxorubicin and attenuates its cardiotoxicity in a breast cancer model. PLoS ONE 2018, 13, e0193918. [Google Scholar] [CrossRef]
- Hussain, A.; Mohsin, J.; Prabhu, S.A.; Begum, S.; Nusri, Q.A.; Harish, G.; Javed, E.; Khan, M.A.; Sharma, C. Sulforaphane inhibits growth of human breast cancer cells and augments the therapeutic index of the chemotherapeutic drug, gemcitabine. Asian Pac. J. Cancer Prev. 2013, 14, 5855–5860. [Google Scholar] [CrossRef]
- Paul, B.; Li, Y.; Tollefsbol, T.O. The Effects of Combinatorial Genistein and Sulforaphane in Breast Tumor Inhibition: Role in Epigenetic Regulation. Int. J. Mol. Sci. 2018, 19, 1754. [Google Scholar] [CrossRef]
- Sharma, M.; Tollefsbol, T.O. Combinatorial epigenetic mechanisms of sulforaphane, genistein and sodium butyrate in breast cancer inhibition. Exp. Cell Res. 2022, 416, 113160. [Google Scholar] [CrossRef] [PubMed]
- Kaczynska, A.; Swierczynska, J.; Herman-Antosiewicz, A. Sensitization of HER2 Positive Breast Cancer Cells to Lapatinib Using Plants-Derived Isothiocyanates. Nutr. Cancer 2015, 67, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Combating breast cancer using combination therapy with 3 phytochemicals: Piperine, sulforaphane, and thymoquinone. Cancer 2019, 125, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Royston, K.J.; Paul, B.; Nozell, S.; Rajbhandari, R.; Tollefsbol, T.O. Withaferin A and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms. Exp. Cell Res. 2018, 368, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, L.; Krug, P.; Mazur, M.; Milczarek, M.; Chilmonczyk, Z.; Wiktorska, K. In the triple-negative breast cancer MDA-MB-231 cell line, sulforaphane enhances the intracellular accumulation and anticancer action of doxorubicin encapsulated in liposomes. Int. J. Pharm. 2019, 558, 311–318. [Google Scholar] [CrossRef]
- Milczarek, M.; Mielczarek, L.; Lubelska, K.; Dąbrowska, A.; Chilmonczyk, Z.; Matosiuk, D.; Wiktorska, K. In vitro evaluation of sulforaphane and a natural analog as potent inducers of 5-fluorouracil anticancer activity. Molecules 2018, 23, 3040. [Google Scholar] [CrossRef]
- Sinha, S.; Sharma, S.; Sharma, A.; Vora, J.; Shrivastava, N. Sulforaphane-cisplatin combination inhibits the stemness and metastatic potential of TNBCs via down regulation of sirtuins-mediated EMT signaling axis. Phytomedicine 2021, 84, 153492. [Google Scholar] [CrossRef]
- Pogorzelska, A.; Mazur, M.; Świtalska, M.; Wietrzyk, J.; Sigorski, D.; Fronczyk, K.; Wiktorska, K. Anticancer effect and safety of doxorubicin and nutraceutical sulforaphane liposomal formulation in triple-negative breast cancer (TNBC) animal model. Biomed. Pharmacother. 2023, 161, 114490. [Google Scholar] [CrossRef]
- Kallifatidis, G.; Labsch, S.; Rausch, V.; Mattern, J.; Gladkich, J.; Moldenhauer, G.; Büchler, M.W.; Salnikov, A.V.; Herr, I. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol. Ther. 2011, 19, 188–195. [Google Scholar] [CrossRef]
- Labsch, S.; Liu, L.; Bauer, N.; Zhang, Y.; Aleksandrowicz, E.; Gladkich, J.; Schönsiegel, F.; Herr, I. Sulforaphane and TRAIL induce a synergistic elimination of advanced prostate cancer stem-like cells. Int. J. Oncol. 2014, 44, 1470–1480. [Google Scholar] [CrossRef]
- Khurana, N.; Kim, H.; Chandra, P.K.; Talwar, S.; Sharma, P.; Abdel-Mageed, A.B.; Sikka, S.C.; Mondal, D. Multimodal actions of the phytochemical sulforaphane suppress both AR and AR-V7 in 22Rv1 cells: Advocating a potent pharmaceutical combination against castration-resistant prostate cancer. Oncol. Rep. 2017, 38, 2774–2786. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Barve, A.; Khor, T.O.; Shen, G.X.; Lin, W.; Chan, J.Y.; Cai, L.; Kong, A.N. Regulation of Nrf2-and AP-1-mediated gene expression by epigallocatechin-3-gallate and sulforaphane in prostate of Nrf2-knockout or C57BL/6J mice and PC-3 AP-1 human prostate cancer cells. Acta Pharmacol. Sin. 2010, 31, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Tuttis, K.; Machado, A.R.T.; Santos, P.W.D.S.; Antunes, L.M.G. Sulforaphane Combined with Vitamin D Induces Cytotoxicity Mediated by Oxidative Stress, DNA Damage, Autophagy, and JNK/MAPK Pathway Modulation in Human Prostate Tumor Cells. Nutrients 2023, 15, 2742. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wu, B.; Cao, Q.; Wu, L.; Yang, G. Hydrogen sulfide mqediates the anti-survival effect of sulforaphane on human prostate cancer cells. Toxicol. Appl. Pharmacol. 2011, 257, 420–428. [Google Scholar] [CrossRef]
- Shankar, S.; Ganapathy, S.; Srivastava, R.K. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin. Cancer Res. 2008, 14, 6855–6866. [Google Scholar] [CrossRef]
- Wang, F.; Meng, F.; Wong, S.C.C.; Cho, W.C.S.; Yang, S.; Chan, L.W.C. Combination therapy of gefitinib and miR-30a-5p may overcome acquired drug resistance through regulating the PI3K/AKT pathway in non-small cell lung cancer. Ther. Adv. Respir. Dis. 2020, 14, 1753466620915156. [Google Scholar] [CrossRef]
- Liu, F.; Lv, R.B.; Liu, Y.; Hao, Q.; Liu, S.J.; Zheng, Y.Y.; Li, C.; Zhu, C.; Wang, M. Salinomycin and Sulforaphane Exerted Synergistic Antiproliferative and Proapoptotic Effects on Colorectal Cancer Cells by Inhibiting the PI3K/Akt Signaling Pathway in Vitro and in Vivo. OncoTargets Ther. 2020, 13, 4957–4969. [Google Scholar] [CrossRef]
- Milczarek, M.; Wiktorska, K.; Mielczarek, L.; Koronkiewicz, M.; Dąbrowska, A.; Lubelska, K.; Matosiuk, D.; Chilmonczyk, Z. Autophagic cell death and premature senescence: New mechanism of 5-fluorouracil and sulforaphane synergistic anticancer effect in MDA-MB-231 triple negative breast cancer cell line. Food Chem. Toxicol. 2018, 111, 1–8. [Google Scholar] [CrossRef]
- Zareba, G. Chemoprotective Effects of Broccoli and Other Brassica Vegetables. Drugs Future 2004, 29, 1097–1104. [Google Scholar] [CrossRef]
- Bjeldanes, L.F.; Kim, J.-Y.; Grose, K.R.; Bartholomew, J.C.; Bradfield, C.A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. USA 1991, 88, 9543–9547. [Google Scholar] [CrossRef]
- Kim, D.J.; Han, B.S.; Ahn, B.; Hasegawa, R.; Shirai, T.; Ito, N.; Tsuda, H. Enhancement by indole-3-carbinol of liver and thyroid gland neoplastic development in a rat medium-term multiorgan carcinogenesis model. Carcinogenesis 1997, 18, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wormke, M.; Safe, S.H.; Bjeldanes, L.F. Indolo [3, 2-b]carbazole: A Dietary-Derived Factor That Exhibits Both Antiestrogenic and Estrogenic Activity. J. Natl. Cancer Inst. 1994, 86, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.-L.; Cintrón, M.; Wu, T.-Y.; Guo, Y.; Huang, Y.; Jeong, W.-S.; Kong, A.-N.T. Pharmacodynamics of dietary phytochemical indoles I3C and DIM: Induction of Nrf2-mediated phase II drug metabolizing and antioxidant genes and synergism with isothiocyanates. Biopharm. Drug Dispos. 2011, 32, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmi, R.; Bargil, S.; Ber, Y.; Ozlavo, R.; Sivan, T.; Rapson, Y.; Pomerantz, A.; Tsoref, D.; Sharon, E.; Caspi, O.; et al. 3,3-Diindolylmethane (DIM): A nutritional intervention and its impact on breast density in healthy BRCA carriers. A prospective clinical trial, Carcinogenesis 2020, 41, 1395–1401. [Google Scholar] [CrossRef]
- Auborn, K.J.; Fan, S.; Rosen, E.M.; Goodwin, L.; Chandraskaren, A.; Williams, D.E.; Chen, D.; Carter, T.H. Indole-3-carbinol is a negative regulator of estrogen. J. Nutr. 2003, 133 (Suppl. S7), 2470S–2475S. [Google Scholar] [CrossRef]
- Michnovicz, J.J.; Adlercreutz, H.; Bradlow, H.L. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J. Natl. Cancer Inst. 1997, 89, 718–723. [Google Scholar] [CrossRef]
- Banerjee, S.; Kong, D.; Wang, Z.; Bao, B.; Hillman, G.G.; Sarkar, F.H. Attenuation of multi-targeted proliferation-linked signaling by 3,3′-diindolylmethane (DIM): From bench to clinic. Mutat. Res. 2011, 728, 47–66. [Google Scholar] [CrossRef]
- Fan, S.; Meng, Q.; Xu, J.; Jiao, Y.; Zhao, L.; Zhang, X.; Sarkar, F.H.; Brown, M.L.; Dritschilo, A.; Rosen, E.M. DIM (3,3′-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18650–18655. [Google Scholar] [CrossRef]
- Le, H.T.; Schaldach, C.M.; Firestone, G.L.; Bjeldanes, L.F. Plant-derived 3,3′-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J. Biol. Chem. 2003, 278, 21136–21145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).