Experimental and Modeling Study for the Solar-Driven CO2 Electrochemical Reduction to CO
Abstract
:1. Introduction
2. Materials and Methods
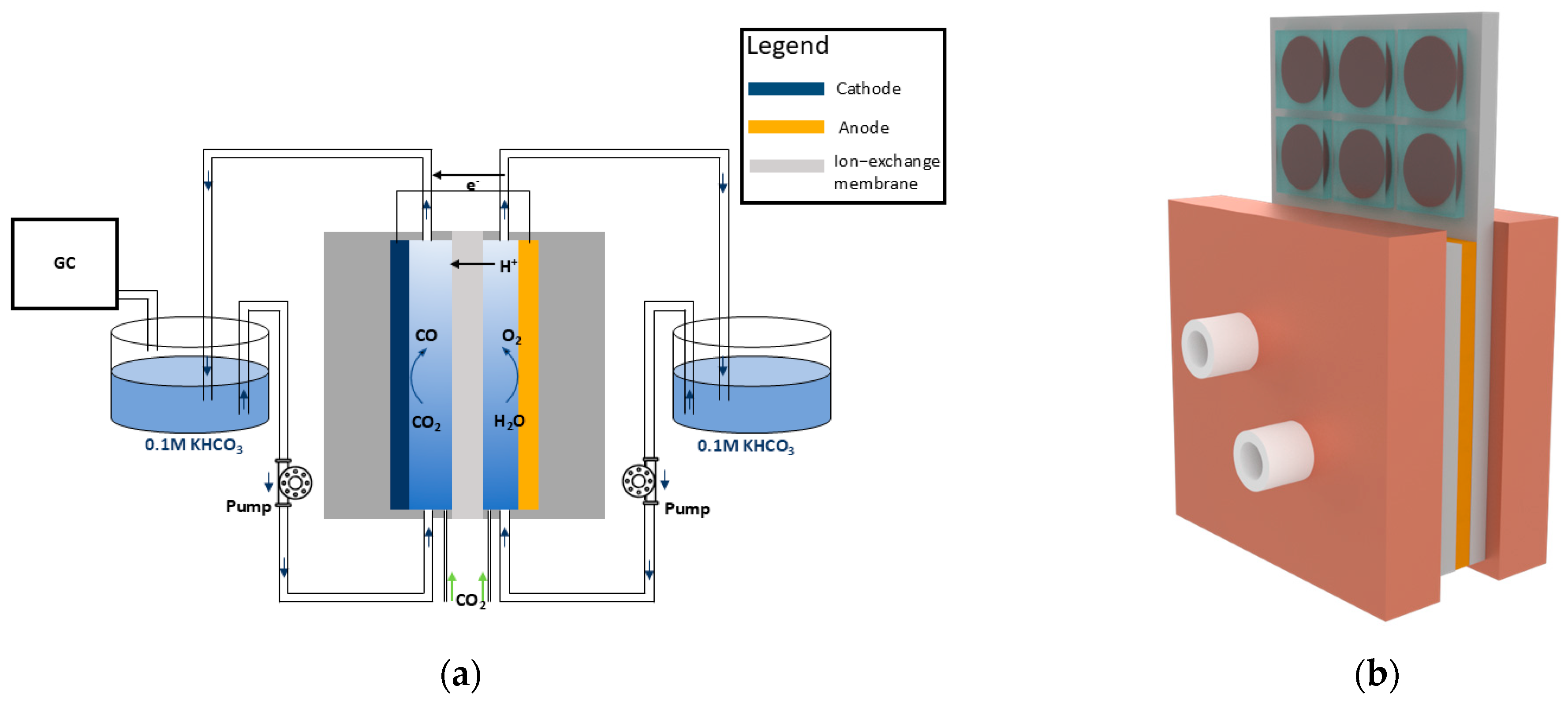
2.1. Electrochemical Cell
2.2. Photovoltaic Module
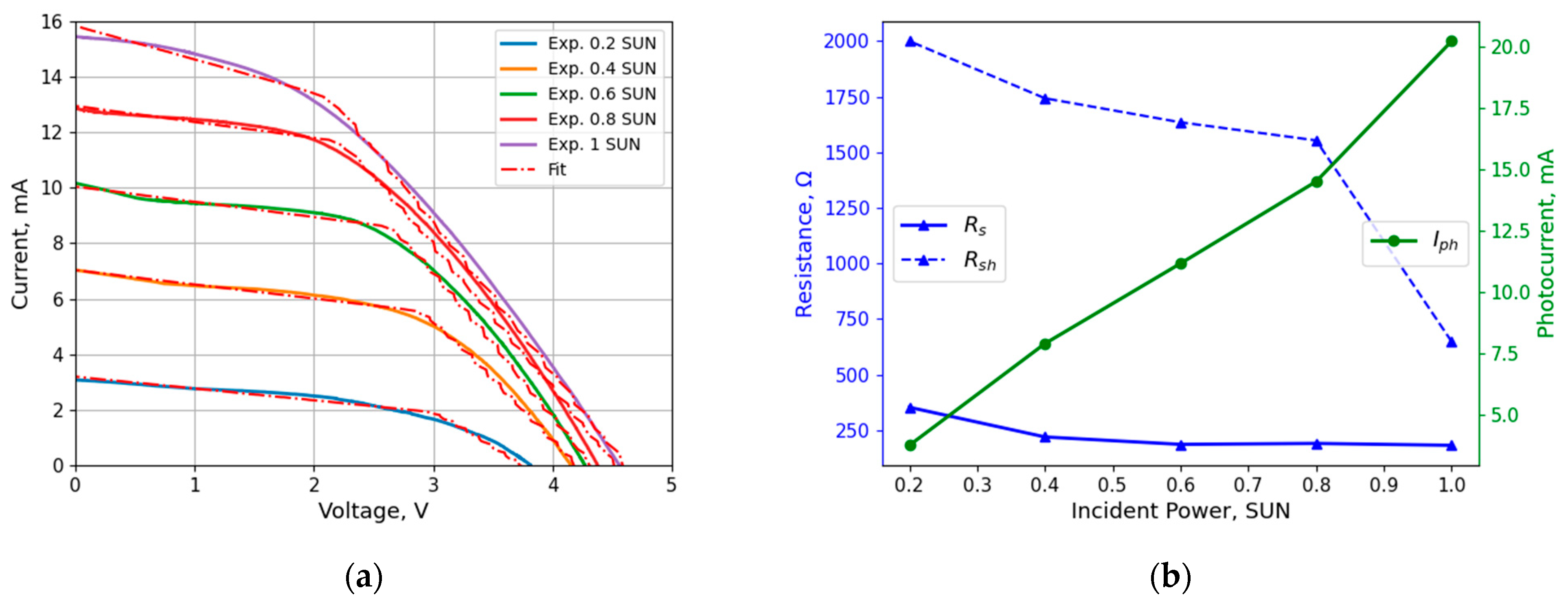
2.3. Numerical Model
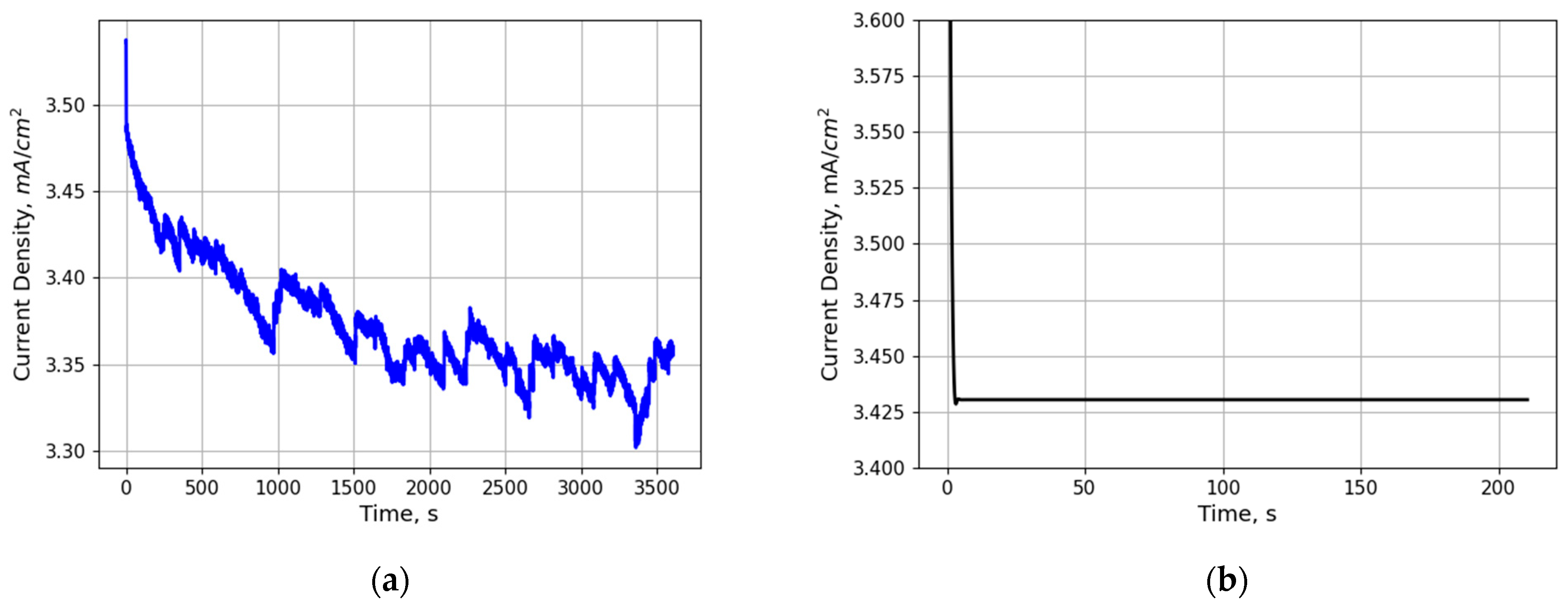
2.3.1. EC Model
- Exchange current density: Describes the reaction rate at equilibrium (zero overpotential).
- Charge-transfer coefficients: Reflect the fraction of the overpotential driving the anodic or cathodic reactions.
- Concentration dependencies: Influence the kinetics, particularly for CO2RR, where the surface CO2 concentration significantly affects the reaction rates.
2.3.2. Time-Dependent Simulation
2.3.3. PV Model
3. Results and Discussion
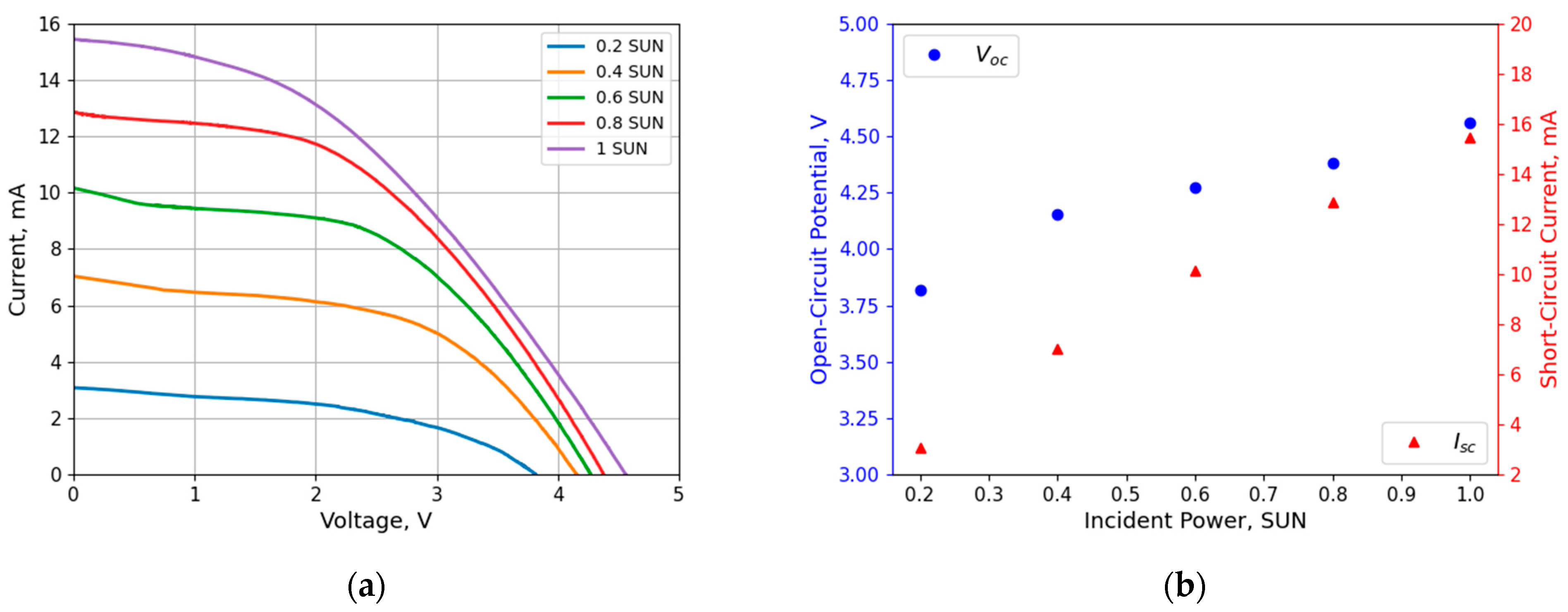
3.1. PV Characteristics
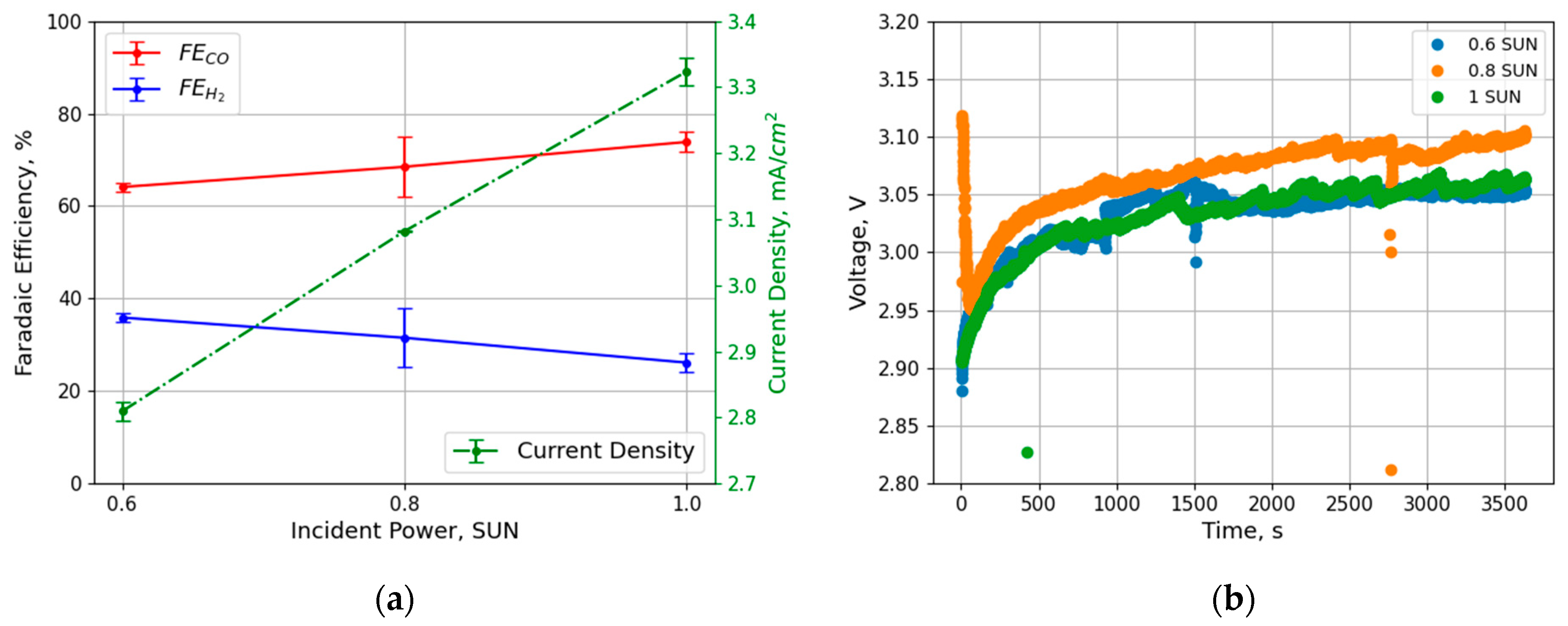
3.2. PV–EC Integration
4. Conclusions and Practical Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Overa, S.; Ko, B.H.; Zhao, Y.; Jiao, F. Electrochemical Approaches for CO2 Conversion to Chemicals: A Journey toward Practical Applications. Acc. Chem. Res. 2022, 55, 638–648. [Google Scholar] [CrossRef]
- Lin, R.; Guo, J.; Li, X.; Patel, P.; Seifitokaldani, A. Electrochemical Reactors for CO2 Conversion. Catalysts 2020, 10, 473. [Google Scholar] [CrossRef]
- Agliuzza, M.; Mezza, A.; Sacco, A. Solar-driven integrated carbon capture and utilization: Coupling CO2 electroreduction toward CO with capture or photovoltaic systems. Appl. Energy 2023, 334, 120649. [Google Scholar] [CrossRef]
- Creissen, C.E.; Fontecave, M. Solar-Driven Electrochemical CO2 Reduction with Heterogeneous Catalysts. Adv. Energy Mater. 2021, 11, 2002652. [Google Scholar] [CrossRef]
- Gurudayal; Bullock, J.; Srankó, D.F.; Towle, C.M.; Lum, Y.; Hettick, M.; Scott, M.C.; Javey, A.; Ager, J. Efficient solar-driven electrochemical CO2 reduction to hydrocarbons and oxygenates. Energy Environ. Sci. 2017, 10, 2222–2230. [Google Scholar]
- Kim, J.; Jeong, S.; Beak, M.; Park, J.; Kwon, K. Performance of photovoltaic-driven electrochemical cell systems for CO2 reduction. Chem. Eng. J. 2022, 428, 130259. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Abdelghafar, A.A.; Mahmoud, M.; Sayed, E.T.; Mahmoud, M.S.; Alami, A.H.; Agha, M.M.A.; Olabi, A.G. Optimized solar photovoltaic-powered green hydrogen: Current status, recent advancements, and barriers. Sol. Energy 2023, 265, 112072. [Google Scholar] [CrossRef]
- Chatterjee, P.; Ambati, M.S.K.; Chakraborty, A.K.; Chakrabortty, S.; Biring, S.; Ramakrishna, S.; Wong, T.K.S.; Kumar, A.; Lawaniya, R.; Dalapati, G.K. Photovoltaic/photo-electrocatalysis integration for green hydrogen: A review. Energy Convers. Manag. 2022, 261, 115648. [Google Scholar] [CrossRef]
- He, H.; Zhang, Q.; Wang, Z.; Pan, S.; Zhao, Y.; Zhang, X. Advances and Practical Prospects for Bias-Free Photovoltaic-Driven Electrochemical Water Splitting Systems. Adv. Energy Mater. 2024, 14, 2303713. [Google Scholar] [CrossRef]
- Sacco, A.; Speranza, R.; Savino, U.; Zeng, J.; Farkhondehfal, M.A.; Lamberti, A.; Chiodoni, A.; Pirri, C.F. An Integrated Device for the Solar-Driven Electrochemical Conversion of CO2 to CO. ACS Sustain. Chem. Eng. 2020, 8, 7563–7568. [Google Scholar] [CrossRef]
- Wang, C.; Ren, H.; Wang, Z.; Guan, Q.; Liu, Y.; Li, W. A promising single-atom Co-N-C catalyst for efficient CO2 electroreduction and high-current solar conversion of CO2 to CO. Appl. Catal. B Environ. 2022, 304, 120958. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, Y.; Chen, S.; Hu, Y.; Yang, S.; Tie, Z.; Jin, Z. Single-Atom Metal Anchored Zr6-Cluster-Porphyrin Framework Hollow Nanocapsules with Ultrahigh Active-Center Density for Electrocatalytic CO2 Reduction. Nano Lett. 2022, 22, 3340–3348. [Google Scholar] [CrossRef]
- Lee, W.H.; Lim, C.; Ban, E.; Bae, S.; Ko, J.; Lee, H.-S.; Min, B.K.; Lee, K.-Y.; Yu, J.S.; Oh, H.-S. W@Ag dendrites as efficient and durable electrocatalyst for solar-to-CO conversion using scalable photovoltaic-electrochemical system. Appl. Catal. B Environ. 2021, 297, 120427. [Google Scholar] [CrossRef]
- Gupta, N.; Gattrell, M.; MacDougall, B. Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J. Appl. Electrochem. 2006, 36, 161–172. [Google Scholar] [CrossRef]
- Burdyny, T.; Graham, P.J.; Pang, Y.; Dinh, C.-T.; Liu, M.; Sargent, E.H.; Sinton, D. Nanomorphology-Enhanced Gas-Evolution Intensifies CO2 Reduction Electrochemistry. ACS Sustain. Chem. Eng. 2017, 5, 4031–4040. [Google Scholar] [CrossRef]
- Kotb, Y.; Fateen, S.-E.K.; Albo, J.; Ismail, I. Modeling of a Microfluidic Electrochemical Cell for the Electro-Reduction of CO2 to CH3OH. J. Electrochem. Soc. 2017, 164, E391. [Google Scholar] [CrossRef]
- Wu, K.; Birgersson, E.; Kim, B.; Kenis, P.J.A.; Karimi, I.A. Modeling and Experimental Validation of Electrochemical Reduction of CO2 to CO in a Microfluidic Cell. J. Electrochem. Soc. 2015, 162, F23. [Google Scholar] [CrossRef]
- Bader, S.; Ma, X.; Oelmann, B. One-diode photovoltaic model parameters at indoor illumination levels—A comparison. Sol. Energy 2019, 180, 707–716. [Google Scholar] [CrossRef]
- Chegaar, M.; Hamzaoui, A.; Namoda, A.; Petit, P.; Aillerie, M.; Herguth, A. Effect of Illumination Intensity on Solar Cells Parameters. Energy Procedia 2013, 36, 722–729. [Google Scholar] [CrossRef]
- Kassis, A.; Saad, M. Analysis of multi-crystalline silicon solar cells at low illumination levels using a modified two-diode model. Sol. Energy Mater. Sol. Cells 2010, 94, 2108–2112. [Google Scholar] [CrossRef]
- Agliuzza, M.; Pirri, C.F.; Sacco, A. A comprehensive modeling for the CO2 electroreduction to CO. J. Phys. Energy 2024, 6, 015004. [Google Scholar] [CrossRef]
- Goss, B.; Cole, I.R.; Koubli, E.; Palmer, D.; Betts, T.R.; Gottschalg, R. 4—Modelling and prediction of PV module energy yield. In The Performance of Photovoltaic (PV) Systems; Pearsall, N., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 103–132. [Google Scholar]
- Zaky, A.A.; Sergeant, P.; Stathatos, E.; Falaras, P.; Ibrahim, M.N. Employing Dye-Sensitized Solar Arrays and Synchronous Reluctance Motors to Improve the Total Cost and Energy Efficiency of Solar Water-Pumping Systems. Machines 2022, 10, 882. [Google Scholar] [CrossRef]
- Guo, X.-Z.; Luo, Y.-H.; Li, C.-H.; Qin, D.; Li, D.-M.; Meng, Q.-B. Can the incident photo-to-electron conversion efficiency be used to calculate short-circuit current density of dye-sensitized solar cells. Curr. Appl. Phys. 2012, 12, e54–e58. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Richter, M.H.; Sullivan, I.; Larson, D.M.; Xiang, C.; Brunschwig, B.S.; Atwater, H.A. CO2 Reduction to CO with 19% Efficiency in a Solar-Driven Gas Diffusion Electrode Flow Cell under Outdoor Solar Illumination. ACS Energy Lett. 2020, 5, 470–476. [Google Scholar] [CrossRef]
- Sriramagiri, G.M.; Ahmed, N.; Luc, W.; Dobson, K.D.; Hegedus, S.S.; Jiao, F. Toward a Practical Solar-Driven CO2 Flow Cell Electrolyzer: Design and Optimization. ACS Sustain. Chem. Eng. 2017, 5, 10959–10966. [Google Scholar] [CrossRef]
- Monti, N.B.D.; Fontana, M.; Sacco, A.; Chiodoni, A.; Lamberti, A.; Pirri, C.F.; Zeng, J. Facile Fabrication of Ag Electrodes for CO2-to-CO Conversion with Near-Unity Selectivity and High Mass Activity. ACS Appl. Energy Mater. 2022, 5, 14779–14788. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agliuzza, M.; Speranza, R.; Lamberti, A.; Pirri, C.F.; Sacco, A. Experimental and Modeling Study for the Solar-Driven CO2 Electrochemical Reduction to CO. Appl. Sci. 2025, 15, 549. https://doi.org/10.3390/app15020549
Agliuzza M, Speranza R, Lamberti A, Pirri CF, Sacco A. Experimental and Modeling Study for the Solar-Driven CO2 Electrochemical Reduction to CO. Applied Sciences. 2025; 15(2):549. https://doi.org/10.3390/app15020549
Chicago/Turabian StyleAgliuzza, Matteo, Roberto Speranza, Andrea Lamberti, Candido Fabrizio Pirri, and Adriano Sacco. 2025. "Experimental and Modeling Study for the Solar-Driven CO2 Electrochemical Reduction to CO" Applied Sciences 15, no. 2: 549. https://doi.org/10.3390/app15020549
APA StyleAgliuzza, M., Speranza, R., Lamberti, A., Pirri, C. F., & Sacco, A. (2025). Experimental and Modeling Study for the Solar-Driven CO2 Electrochemical Reduction to CO. Applied Sciences, 15(2), 549. https://doi.org/10.3390/app15020549








