Biodiesel and Biolubricant Production from Waste Cooking Oil: Transesterification Reactor Modeling
Abstract
Featured Application
Abstract
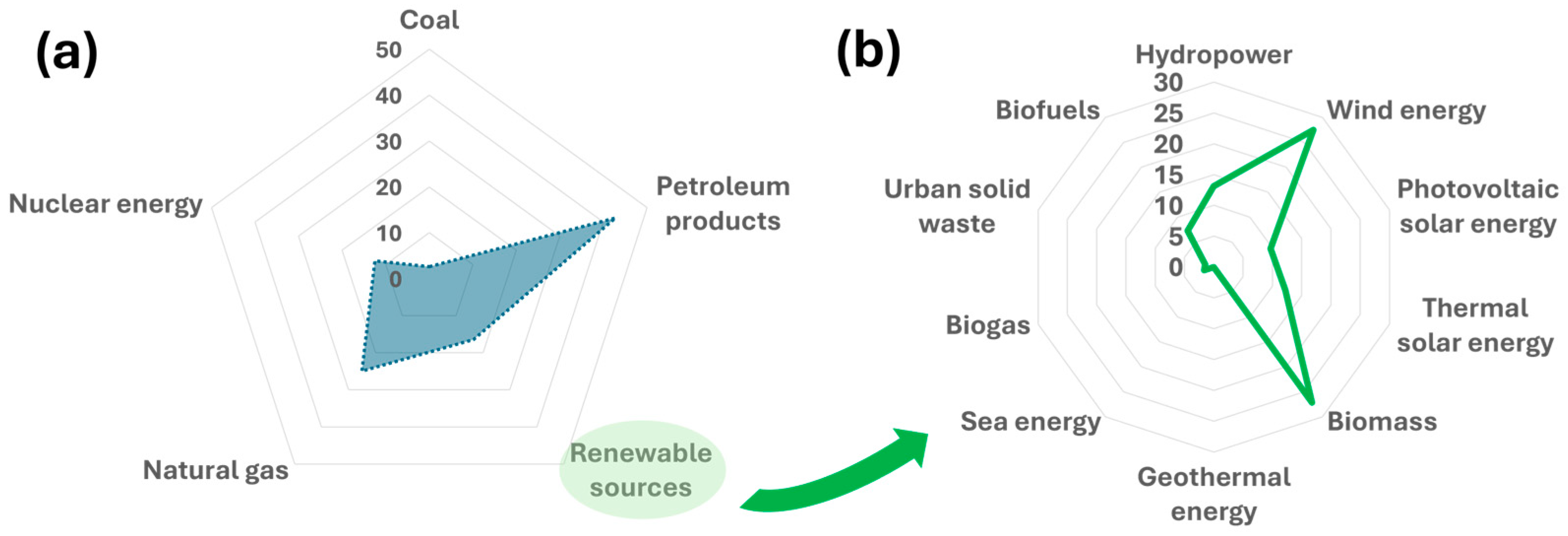
1. Introduction
- Fatty acid methyl ester (biodiesel) production using a first transesterification with methanol and waste cooking oil, including its characterization according to the UNE-EN 14214 standard;
- Biolubricant production through double transesterification of fatty acid methyl esters with 2-ethyl-2-hydroxymethyl-1,3-propanediol, with a study about the effects of the temperature, pressure, and catalyst concentration on the conversion. The viscosity and oxidation stability, among others, were determined;
- Improvement of the oxidation stability of the biolubricant by antioxidant addition;
- Kinetic study of the second transesterification of FAMEs;
- Preliminary design and economic feasibility study of an industrial plant for biodiesel and biolubricant production, with a special focus on the reactor design.
2. Materials and Methods
2.1. Waste Cooking Oil
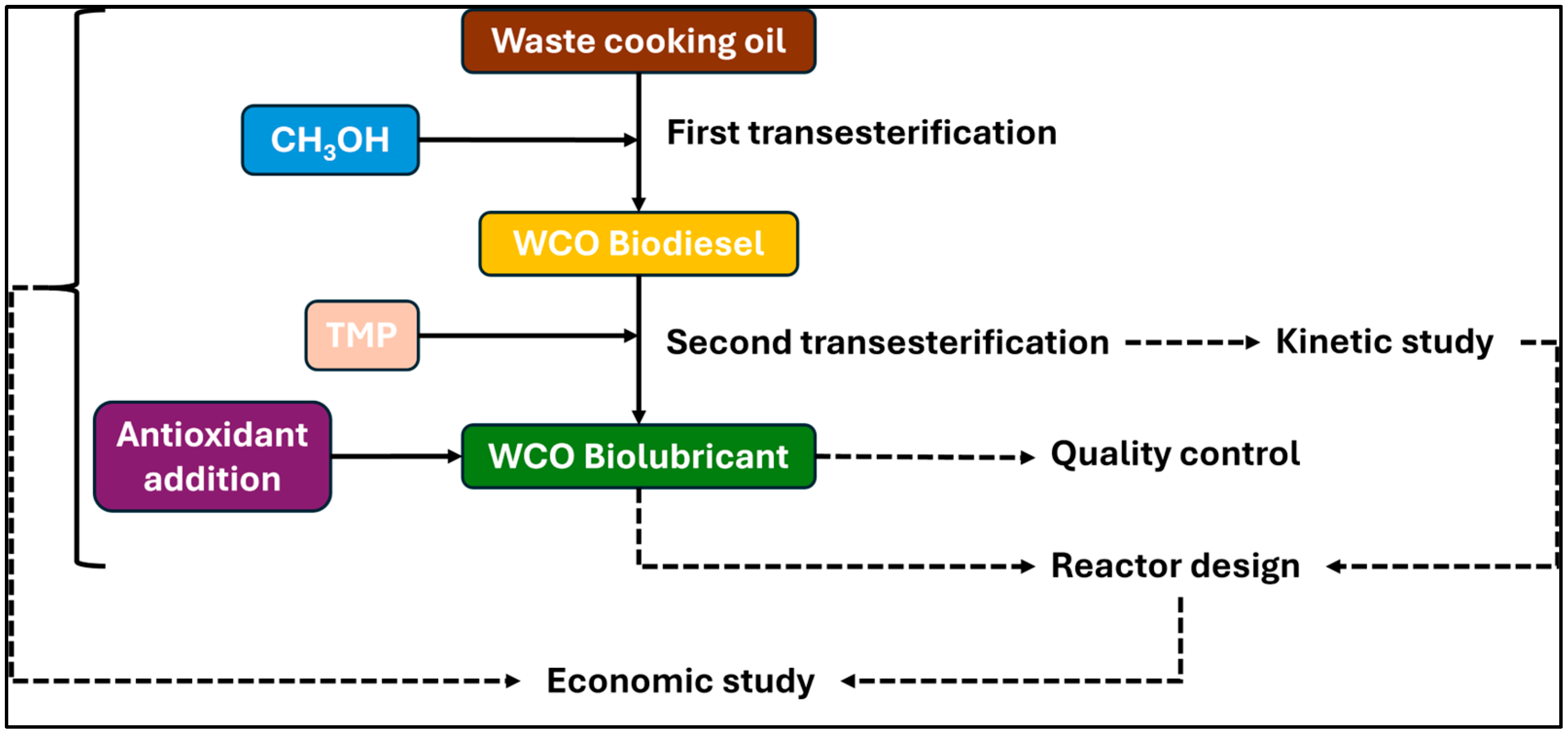
2.2. Biodiesel and Biolubricant Production
2.3. Biodiesel and Biolubricant Characterization
2.4. Antioxidant Addition
2.5. Kinetic Study: Foundations
3. Results and Discussion
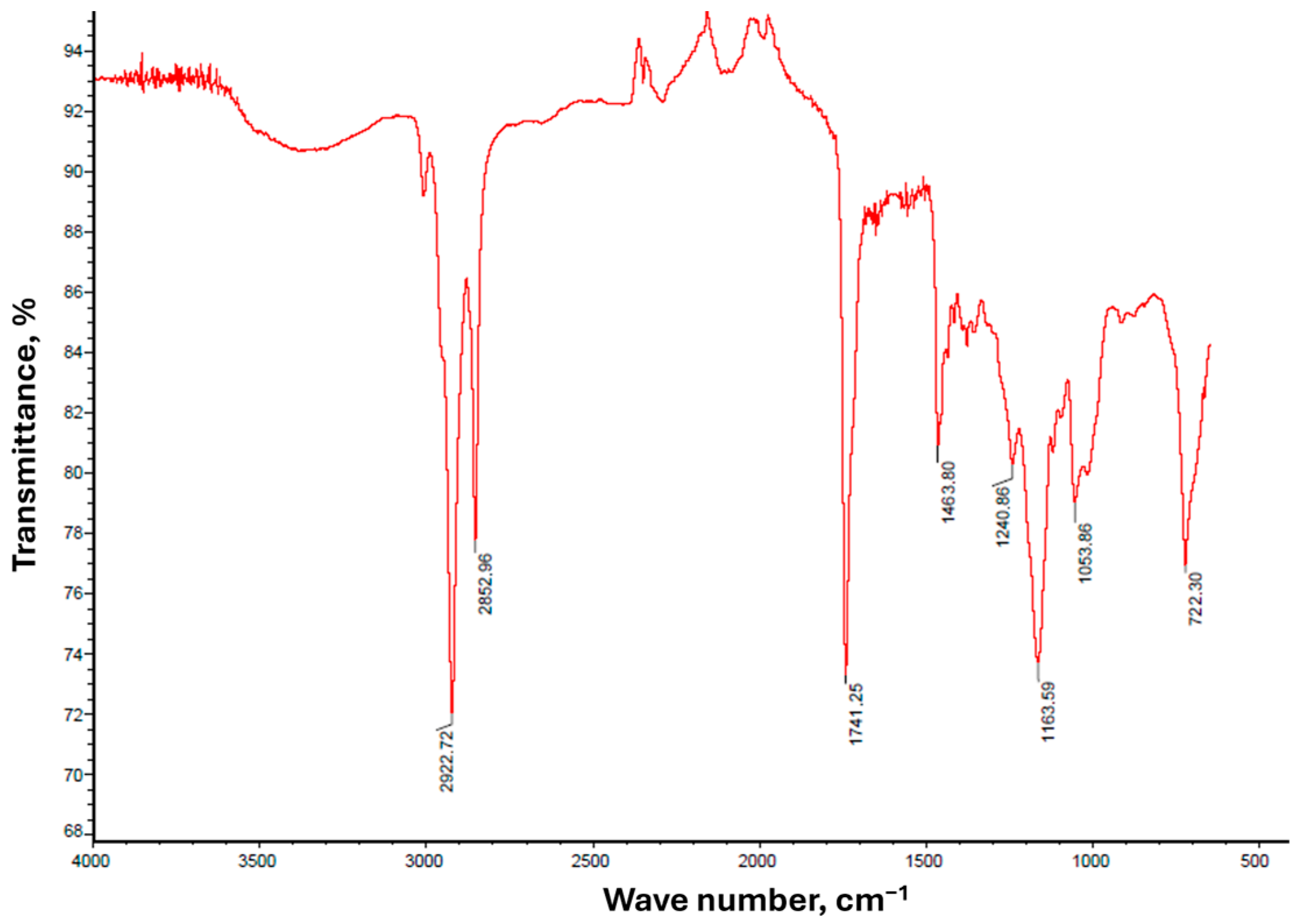
3.1. WCO-FAME Characteristics
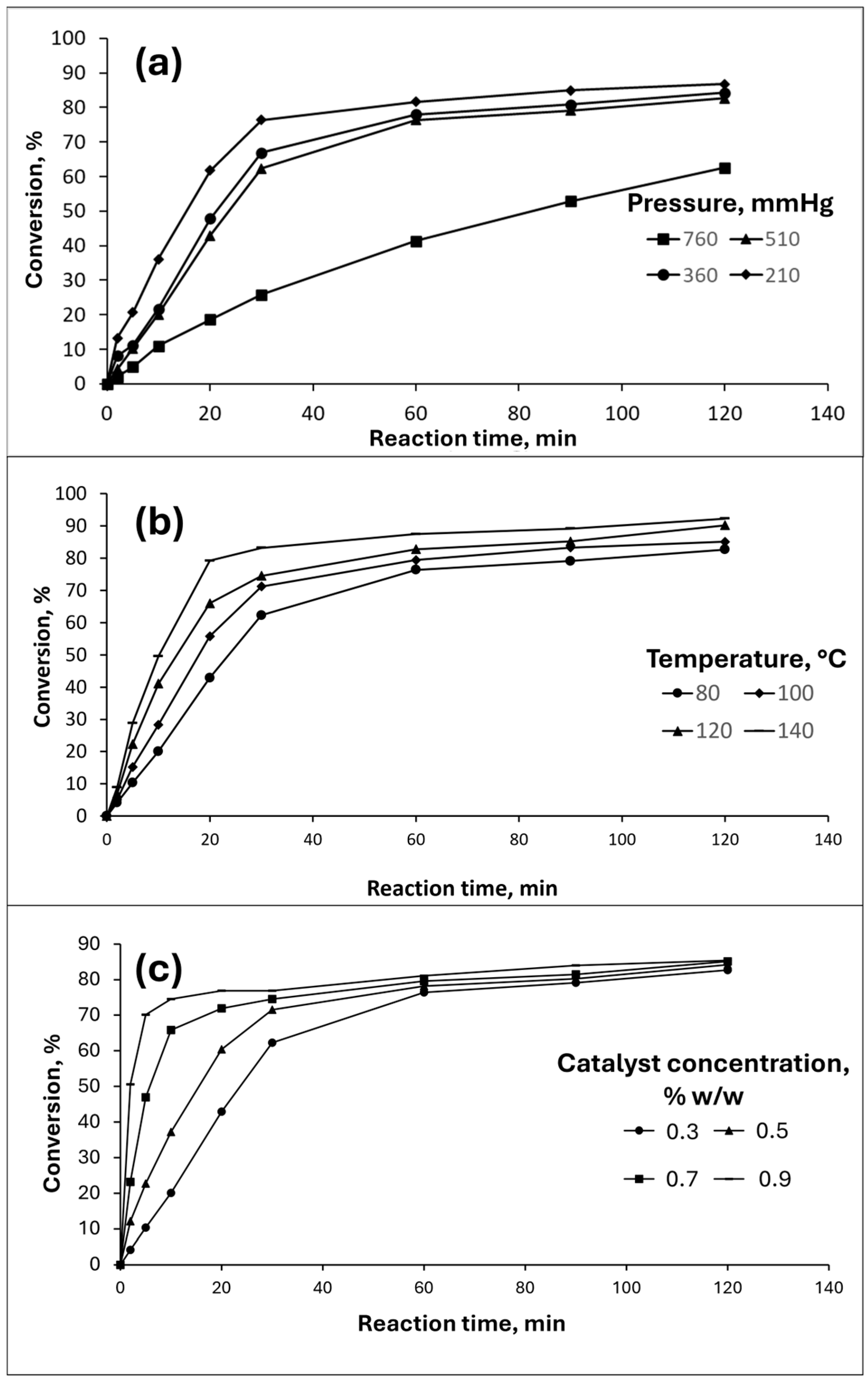
3.2. Effects of Pressure, Temperature, and Catalyst Concentration on WCO-TMP Production
3.3. WCO-TMP and TBHQ Addition
3.4. Kinetic Study
3.5. Industrial Equipment, Reactor Design, and Economic Feasibility Study
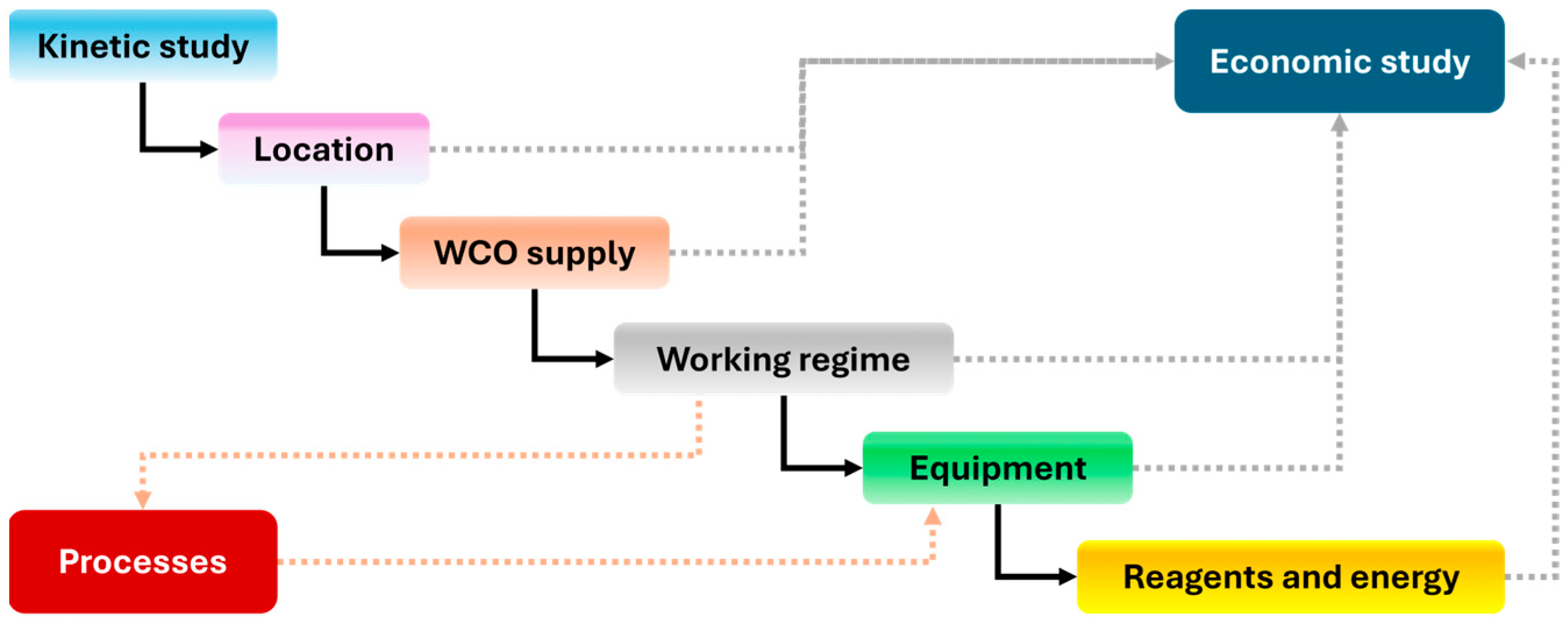
3.5.1. Preliminary Considerations: Site Location, Production Regime, and WCO Processing Capacity of the Plant
3.5.2. Main Equipment
3.5.3. Reactor Design
3.5.4. Economic Feasibility Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN Sustainable Development Goals. 2019. Available online: https://sdgs.un.org/goals (accessed on 15 December 2024).
- United Nations Framework Convention on Climate Change. Available online: https://unfccc.int (accessed on 15 December 2024).
- Ministerio para la Transición Ecológica y el Reto Demográfico Balances Energéticos. Available online: https://www.miteco.gob.es/es/energia/estrategia-normativa/balances/balances.html (accessed on 15 December 2024).
- Di Serio, M.; Ledda, M.; Cozzolino, M.; Minutillo, G.; Tesser, R.; Santacesaria, E. Transesterification of Soybean Oil to Biodiesel by Using Heterogeneous Basic Catalysts. Ind. Eng. Chem. Res. 2006, 45, 3009–3014. [Google Scholar] [CrossRef]
- Parente, E.J.; Marques, J.P.C.; Rios, I.C.; Cecilia, J.A.; Rodríguez-Castellón, E.; Luna, F.M.T.; Cavalcante, C.L. Production of Biolubricants from Soybean Oil: Studies for an Integrated Process with the Current Biodiesel Industry. Chem. Eng. Res. Des. 2021, 165, 456–466. [Google Scholar] [CrossRef]
- Mazanov, S.V.; Gabitova, A.R.; Usmanov, R.A.; Gumerov, F.M.; Labidi, S.; Amar, M.B.; Passarello, J.P.; Kanaev, A.; Volle, F.; Neindre, B. Le Continuous Production of Biodiesel from Rapeseed Oil by Ultrasonic Assist Transesterification in Supercritical Ethanol. J. Supercrit. Fluids 2016, 118, 107–118. [Google Scholar] [CrossRef]
- Koutsouki, A.A.; Tegou, E.; Badeka, A.; Kontakos, S.; Pomonis, P.J.; Kontominas, M.G. In Situ and Conventional Transesterification of Rapeseeds for Biodiesel Production: The Effect of Direct Sonication. Ind. Crops Prod. 2016, 84, 399–407. [Google Scholar] [CrossRef]
- Veljković, V.B.; Biberdžić, M.O.; Banković-Ilić, I.B.; Djalović, I.G.; Tasić, M.B.; Nježić, Z.B.; Stamenković, O.S. Biodiesel Production from Corn Oil: A Review. Renew. Sustain. Energy Rev. 2018, 91, 531–548. [Google Scholar] [CrossRef]
- Gülüm, M.; Bilgin, A. Density, Flash Point and Heating Value Variations of Corn Oil Biodiesel-Diesel Fuel Blends. Fuel Process. Technol. 2015, 134, 456–464. [Google Scholar] [CrossRef]
- Nongbe, M.C.; Ekou, T.; Ekou, L.; Yao, K.B.; Le Grognec, E.; Felpin, F.X. Biodiesel Production from Palm Oil Using Sulfonated Graphene Catalyst. Renew. Energy 2017, 106, 135–141. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N. Epoxidized Malaysian Elaeis Guineensis Palm Kernel Oil Trimethylolpropane Polyol Ester as Green Renewable Biolubricants. Biomass Bioenergy 2023, 175, 106883. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M.; Ozsezen, A.N.; Turkcan, A.; Sanli, H. Using Waste Animal Fat Based Biodiesels-Bioethanol-Diesel Fuel Blends in a Di Diesel Engine. Fuel 2015, 157, 245–254. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Stojković, I.J.; Stamenković, O.S.; Veljkovic, V.B.; Hung, Y.T. Waste Animal Fats as Feedstocks for Biodiesel Production. Renew. Sustain. Energy Rev. 2014, 32, 238–254. [Google Scholar] [CrossRef]
- Behçet, R.; Oktay, H.; Çakmak, A.; Aydin, H. Comparison of Exhaust Emissions of Biodiesel-Diesel Fuel Blends Produced from Animal Fats. Renew. Sustain. Energy Rev. 2015, 46, 157–165. [Google Scholar] [CrossRef]
- Ahmad, U.; Naqvi, S.R.; Ali, I.; Naqvi, M.; Asif, S.; Bokhari, A.; Juchelková, D.; Klemeš, J.J. A Review on Properties, Challenges and Commercial Aspects of Eco-Friendly Biolubricants Productions. Chemosphere 2022, 309, 136622. [Google Scholar] [CrossRef]
- Barbera, E.; Hirayama, K.; Maglinao, R.L.; Davis, R.W.; Kumar, S. Recent Developments in Synthesizing Biolubricants—A Review. Biomass Convers. Biorefin 2024, 14, 2867–2887. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación. Series de Datos de Consumo Alimentario En Hogares. Available online: https://www.mapa.gob.es/es/alimentacion/temas/consumo-tendencias/panel-de-consumo-alimentario/series-anuales (accessed on 15 December 2024).
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. A Review on Biolubricants Based on Vegetable Oils through Transesterification and the Role of Catalysts: Current Status and Future Trends. Catalysts 2023, 13, 1299. [Google Scholar] [CrossRef]
- Chen, J.; Bian, X.; Rapp, G.; Lang, J.; Montoya, A.; Trethowan, R.; Bouyssiere, B.; Portha, J.F.; Jaubert, J.N.; Pratt, P.; et al. From Ethyl Biodiesel to Biolubricants: Options for an Indian Mustard Integrated Biorefinery toward a Green and Circular Economy. Ind. Crops Prod. 2019, 137, 597–614. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Guiberteau Cabanillas, A.; Moro, J.P.; Encinar Martín, J.M. Use of Propyl Gallate in Cardoon Biodiesel to Keep Its Main Properties during Oxidation. Clean. Technol. 2023, 5, 569–583. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Cabanillas, A.G.; Romero, Á.G.; Encinar Martín, J.M. Monitoring Tert-Butylhydroquinone Content and Its Effect on a Biolubricant during Oxidation. Molecules 2022, 27, 8931. [Google Scholar] [CrossRef]
- Silva, E.T.; Spacino, K.R.; Silva, L.R.C.; Romagnoli, É.S.; Angilelli, K.G.; Borsato, D. Modelling of Relative Protection Factor of Antioxidants TBHQ, BHT and BHA in Mixture with Biodiesel. Acta Sci. Technol. 2018, 40. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, P.; Porwal, J.; Porwal, S.K. Evaluation of Multifunctional Green Copolymer Additives–Doped Waste Cooking Oil–Extracted Natural Antioxidant in Biolubricant Formulation. Biomass Convers. Biorefin 2024, 14, 761–770. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, P.; Porwal, S.K. Natural Antioxidant Extracted Waste Cooking Oil as Sustainable Biolubricant Formulation in Tribological and Rheological Applications. Waste Biomass Valoriz. 2022, 13, 3127–3137. [Google Scholar] [CrossRef] [PubMed]
- Abed, K.A.; El Morsi, A.K.; Sayed, M.M.; Shaib, A.A.E.; Gad, M.S. Effect of Waste Cooking-Oil Biodiesel on Performance and Exhaust Emissions of a Diesel Engine. Egypt. J. Pet. 2018, 27, 985–989. [Google Scholar] [CrossRef]
- Joshi, J.R.; Bhanderi, K.K.; Patel, J.V.; Karve, M. Chemical Modification of Waste Cooking Oil for the Biolubricant Production through Transesterification Process. J. Indian. Chem. Soc. 2023, 100, 100909. [Google Scholar] [CrossRef]
- Wang, E.; Ma, X.; Tang, S.; Yan, R.; Wang, Y.; Riley, W.W.; Reaney, M.J.T. Synthesis and Oxidative Stability of Trimethylolpropane Fatty Acid Triester as a Biolubricant Base Oil from Waste Cooking Oil. Biomass Bioenergy 2014, 66, 371–378. [Google Scholar] [CrossRef]
- Sun, G.; Li, Y.; Cai, Z.; Teng, Y.; Wang, Y.; Reaney, M.J.T. K2CO3-Loaded Hydrotalcite: A Promising Heterogeneous Solid Base Catalyst for Biolubricant Base Oil Production from Waste Cooking Oils. Appl. Catal. B 2017, 209, 118–127. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, H.; Song, Y.; Ma, S.; Xiong, W.; Chen, C.; Chen, B.; Zhang, X. Green Preparation of Branched Biolubricant by Chemically Modifying Waste Cooking Oil with Lipase and Ionic Liquid. J. Clean. Prod. 2020, 274, 122918. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Miranda, L.P.; Fernandez-Lafuente, R.; Tardioli, P.W. Immobilization of Eversa® Transform via CLEA Technology Converts It in a Suitable Biocatalyst for Biolubricant Production Using Waste Cooking Oil. Molecules 2021, 26, 193. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, H.; Adhikari, S.; Roy, P.; Shelley, M.; Hassani, E.; Oh, T.S. Synthesis of Novel Biolubricants from Waste Cooking Oil and Cyclic Oxygenates through an Integrated Catalytic Process. ACS Sustain. Chem. Eng. 2021, 9, 13424–13437. [Google Scholar] [CrossRef]
- Chowdhury, A.; Chakraborty, R.; Mitra, D.; Biswas, D. Optimization of the Production Parameters of Octyl Ester Biolubricant Using Taguchi’s Design Method and Physico-Chemical Characterization of the Product. Ind. Crops Prod. 2014, 52, 783–789. [Google Scholar] [CrossRef]
- Chowdhury, A.; Mitra, D.; Biswas, D. Biolubricant Synthesis from Waste Cooking Oil via Enzymatic Hydrolysis Followed by Chemical Esterification. J. Chem. Technol. Biotechnol. 2013, 88, 139–144. [Google Scholar] [CrossRef]
- Hussein, R.Z.K.; Attia, N.K.; Fouad, M.K.; ElSheltawy, S.T. Experimental Investigation and Process Simulation of Biolubricant Production from Waste Cooking Oil. Biomass Bioenergy 2021, 144, 105850. [Google Scholar] [CrossRef]
- Abdel Hamid, E.M.; Amer, A.M.; Mahmoud, A.K.; Mokbl, E.M.; Hassan, M.A.; Abdel-Monaim, M.O.; Amin, R.H.; Tharwat, K.M. Box-Behnken Design (BBD) for Optimization and Simulation of Biolubricant Production from Biomass Using Aspen plus with Techno-Economic Analysis. Sci. Rep. 2024, 14, 21769. [Google Scholar] [CrossRef] [PubMed]
- Awogbemi, O.; Onuh, E.I.; Inambao, F.L. Comparative Study of Properties and Fatty Acid Composition of Some Neat Vegetable Oils and Waste Cooking Oils. Int. J. Low-Carbon. Technol. 2019, 14, 417–425. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Pinilla, A. Biolubricant Production through Double Transesterification: Reactor Design for the Implementation of a Biorefinery Based on Rapeseed. Processes 2021, 9, 1224. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Guiberteau, A.; Encinar, J.M. Effect of Tert-Butylhydroquinone on Biodiesel Properties during Extreme Oxidation Conditions. Fuel 2022, 310, 122339. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Álvez-Medina, C.M. High Oleic Safflower Biolubricant through Double Transesterification with Methanol and Pentaerythritol: Production, Characterization, and Antioxidant Addition. Arab. J. Chem. 2022, 15, 103796. [Google Scholar] [CrossRef]
- UNE-EN 14214; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Biodiesel Engines and Heating Applications—Requirements and Test Methods. Asociación Española de Normalización: Madrid, Spain, 2013.
- UNE-EN ISO 3104/AC:1999; Petroleum Products Transparent and Opaque Liquids. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity (ISO 3104:1994). Asociación Española de Normalización: Madrid, Spain, 1999.
- UNE-EN-ISO 3675; Crude Petroleum and Liquid Petroleum Products. Laboratory Determination of Density. Hydrometer Method. Asociación Española de Normalización: Madrid, Spain, 1999.
- UNE-EN 14112; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Oxidation Stability (Accelerated Oxidation Test). Asociación Española de Normalización: Madrid, Spain, 2017.
- UNE-EN 14104:2003; Oil and Fat Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Acid Value. Asociación Española de Normalización: Madrid, Spain, 2003.
- UNE-EN 51023:1990; Petroleum Products. Determination of Flash and Fire Points. Cleveland Open Cup Method. Asociación Española de Normalización: Madrid, Spain, 1990.
- UNE-EN 116:2015; Diesel and Domestic Heating Fuels—Determination of Cold Filter Plugging Point-Stepwise Cooling Bath Method. Asociación Española de Normalización: Madrid, Spain, 2015.
- Focke, W.W.; Van der Westhuizen, I.; Oosthuysen, X. Biodiesel Oxidative Stability from Rancimat Data. Thermochim. Acta 2016, 633, 116–121. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification Kinetis of Soybean Oil; Springer: Berlin/Heidelberg, Germany, 1981; Volume 58. [Google Scholar]
- Khan, E.; Ozaltin, K.; Spagnuolo, D.; Bernal-ballen, A.; Piskunov, M.V.; Martino, A. Di Biodiesel from Rapeseed and Sunflower Oil: Effect of the Transesterification Conditions and Oxidation Stability. Energies 2023, 16, 657. [Google Scholar] [CrossRef]
- Bencheikh, K.; Atabani, A.E.; Shobana, S.; Mohammed, M.N.; Uğuz, G.; Arpa, O.; Kumar, G.; Ayanoğlu, A.; Bokhari, A. Fuels Properties, Characterizations and Engine and Emission Performance Analyses of Ternary Waste Cooking Oil Biodiesel–Diesel–Propanol Blends. Sustain. Energy Technol. Assess. 2019, 35, 321–334. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Hari, A.; Inayat, A.; Yousef, L.A.; Alarab, S.; Abdallah, M.; Shanableh, A.; Ghenai, C.; Shanmugam, S.; Kikas, T. Recent Advances on Biodiesel Production from Waste Cooking Oil (WCO): A Review of Reactors, Catalysts, and Optimization Techniques Impacting the Production. Fuel 2023, 348, 128514. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Guiberteau Cabanillas, A.; Catela Rodríguez, A. Combined Effect of Propyl Gallate and Tert-Butyl Hydroquinone on Biodiesel and Biolubricant Based on Waste Cooking Oil. Appl. Sci. 2024, 14, 9767. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. An Overview of Biodiesel Oxidation Stability. Renew. Sustain. Energy Rev. 2012, 16, 5924–5950. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Masjuki, H.H.; Kalam, M.A.; Hazrat, M.A.; Masum, B.M.; Imtenan, S.; Ashraful, A.M. Effect of Antioxidants on Oxidation Stability of Biodiesel Derived from Vegetable and Animal Based Feedstocks. Renew. Sustain. Energy Rev. 2014, 30, 356–370. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar Martín, J.M.; Sánchez Ocaña, M. Use of Mild Reaction Conditions to Improve Quality Parameters and Sustainability during Biolubricant Production. Biomass Bioenergy 2022, 161, 106456. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D.S. Screening of Antioxidant Additives for Biodiesel Fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; Guiberteau, A.; Márquez, S. The Effect of Antioxidants on Corn and Sunflower Biodiesel Properties under Extreme Oxidation Conditions. JAOCS J. Am. Oil Chem. Soc. 2019, 97, 201–212. [Google Scholar] [CrossRef]
- Souza, A.G.; Medeiros, M.L.; Cordeiro, A.M.M.T.; Queiroz, N.; Soledade, L.E.B.; Souza, A.L. Efficient Antioxidant Formulations for Use in Biodiesel. Energy Fuels 2014, 28, 1074–1080. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. Safflower Biodiesel: Improvement of Its Oxidative Stability by Using BHA and TBHQ. Energies 2019, 12, 1940. [Google Scholar] [CrossRef]
- Xin, J.; Imahara, H.; Saka, S. Kinetics on the Oxidation of Biodiesel Stabilized with Antioxidant. Fuel 2009, 88, 282–286. [Google Scholar] [CrossRef]
- Bizkaia Garbiker Recogida de Residuos. Available online: https://garbiker.bizkaia.eus/es/recogida-de-residuos (accessed on 15 December 2024).
- Boletín Oficial del Estado, XX Convenio Colectivo General de La Industria Química. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2021-12038 (accessed on 15 December 2024).
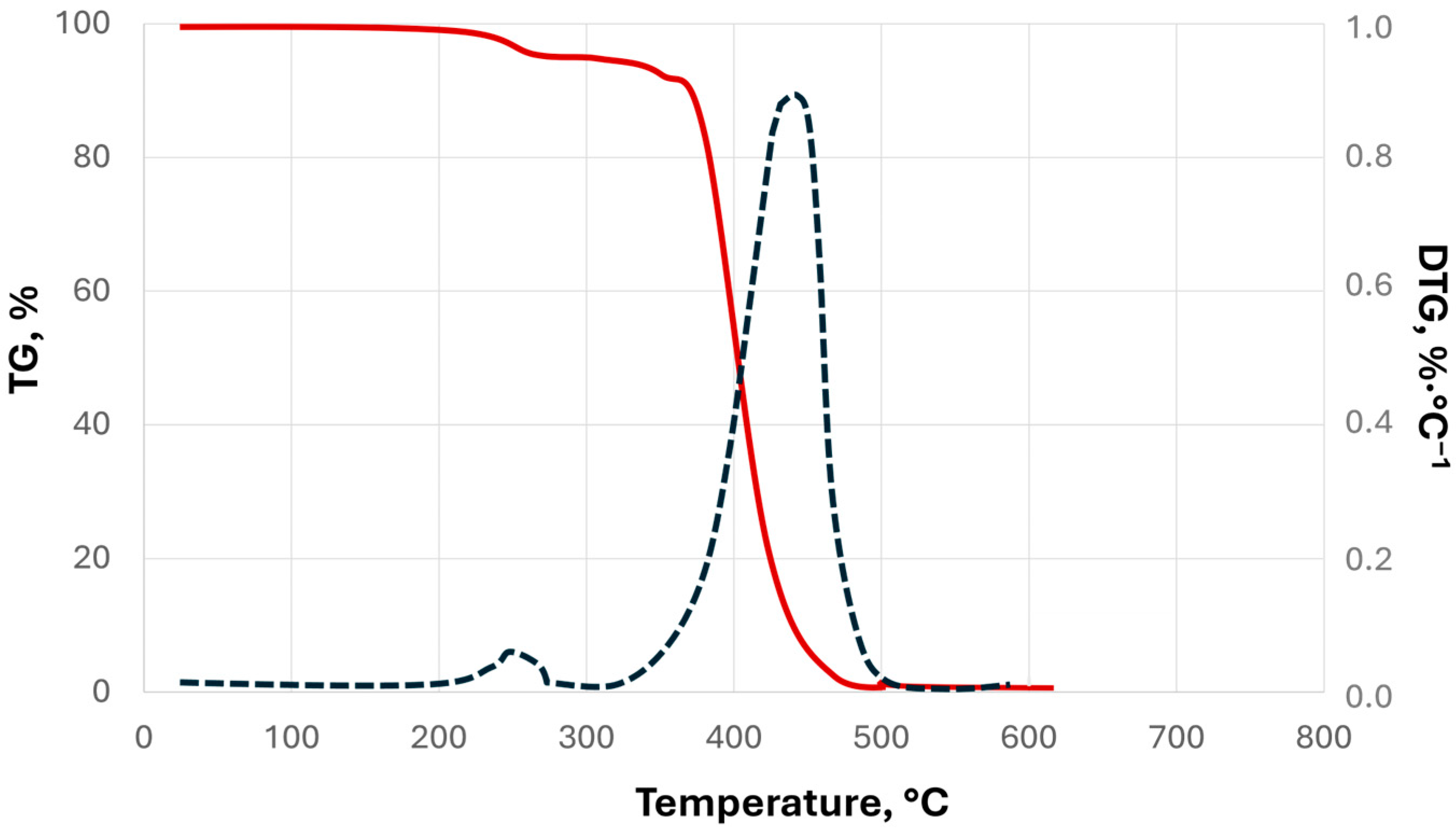
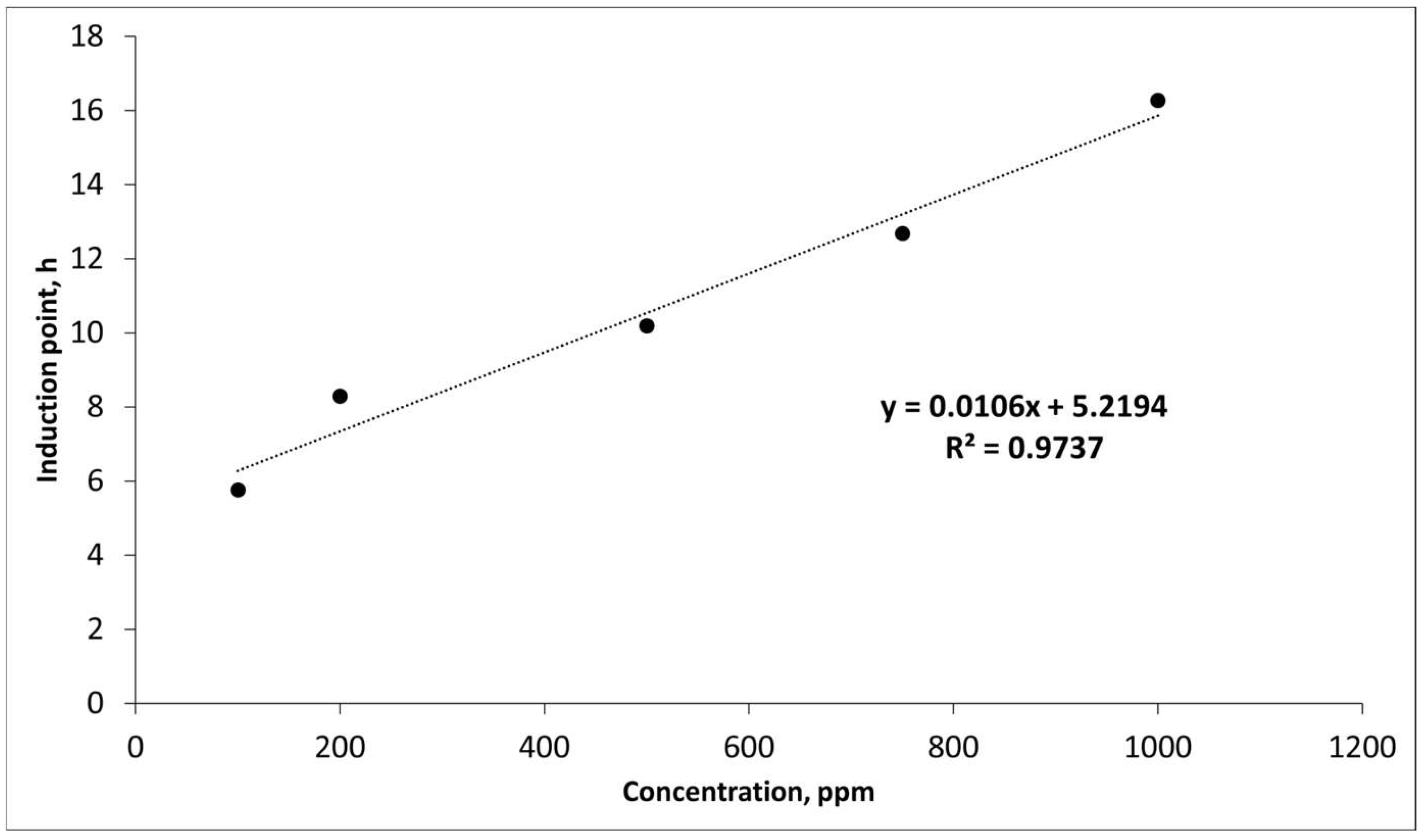

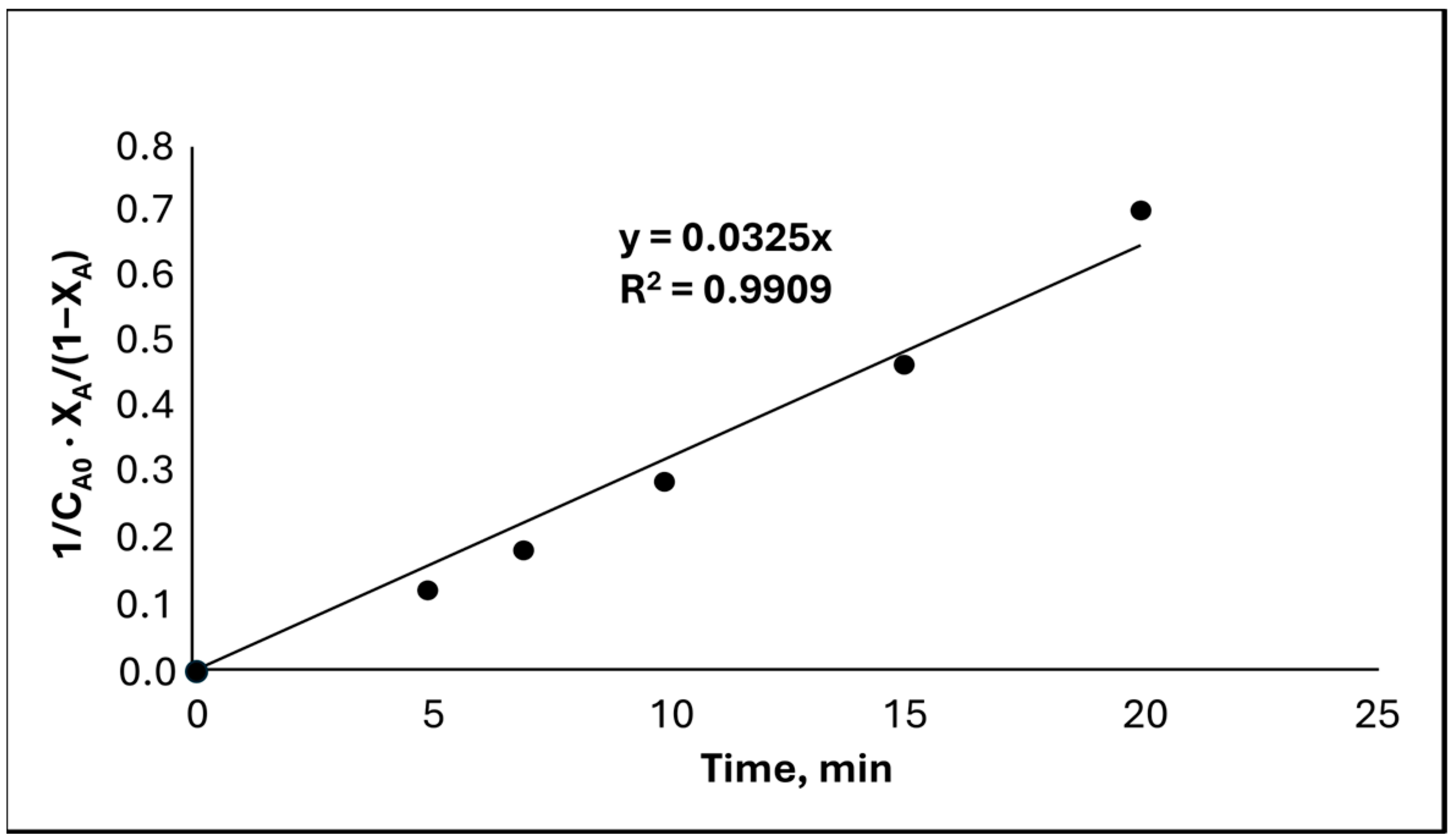

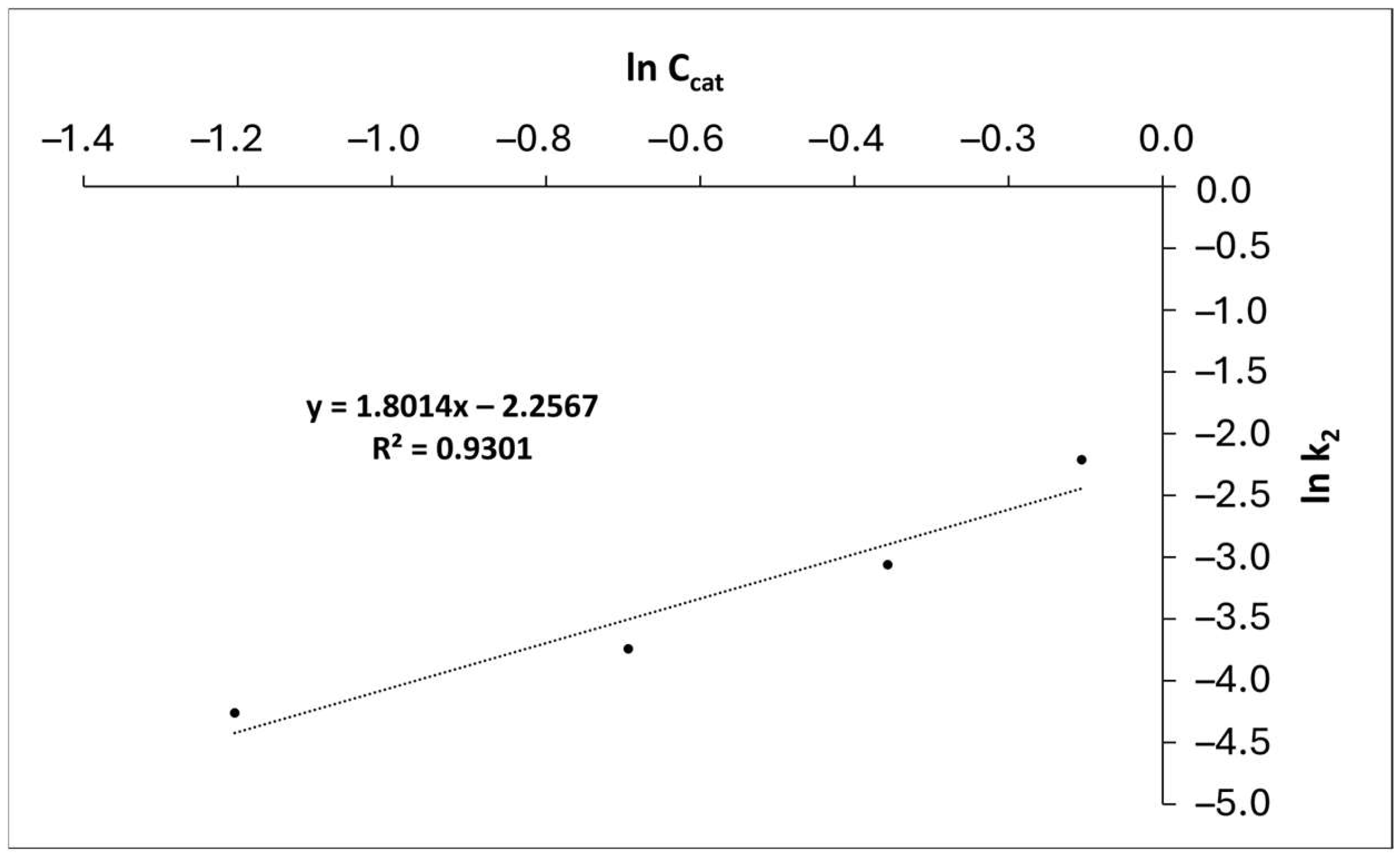
| Parameter | Value |
|---|---|
| WCO FAME/TMP ratio | 3 |
| Catalyst concentration, % | 0.3–1.0 |
| Reaction time, min | 120 |
| Reaction temperature, °C | 80–140 |
| Stirring rate, rpm | 350 |
| Working pressure, mmHg | 210–760 |
| Parameter | WCO-FAME | WCO-TMP | Details |
|---|---|---|---|
| FAME content | Yes | No | [40] |
| Viscosity | Yes | Yes | [41] |
| Density | Yes | Yes | [42] |
| Oxidation stability | Yes | Yes | [43] |
| Acidity value | Yes | Yes | [44] |
| Flash and combustion points | Yes | Yes | [45] |
| Cold filter plugging point | Yes | No | [46] |
| T, °C | T, K | k’ |
|---|---|---|
| 80 | 353 | 0.0141 |
| 100 | 373 | 0.0176 |
| 120 | 393 | 0.0245 |
| 140 | 413 | 0.0339 |
| Catalyst Concentration, % w/w | k’2 |
|---|---|
| 0.3 | 0.0141 |
| 0.5 | 0.0237 |
| 0.7 | 0.0468 |
| 0.9 | 0.1096 |
| Parameter | Value |
|---|---|
| Working hours, h·y−1 | 2112 |
| Collected WCO, kg·y−1 | 423,823 |
| Processing capacity, kg·h−1 | 206.03 |
| Processing capacity, kg·d−1 | 1648 |
| Methanol required *, kg·d−1 | 357.44 |
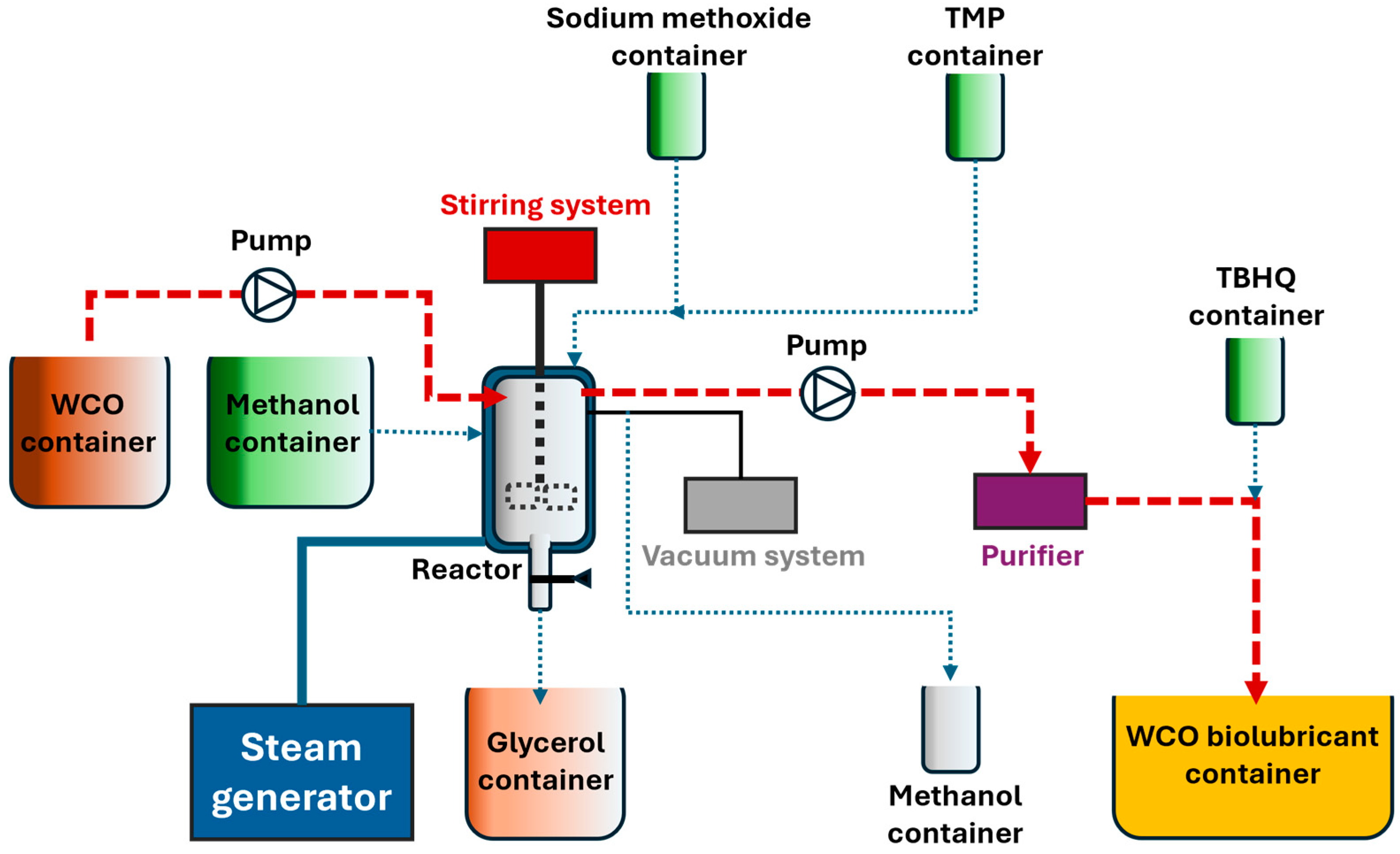
| Step | Process/Equipment | Details |
|---|---|---|
| WCO supply and preconditioning | Shipment (tank trucks), filtering and storage in tanks | Steel tank for WCO and TMP storage. Bulk containers (HDPEs) were used for methanol and sodium methoxide. |
| Pumping | Pump system | Pumps materials through the plant. |
| Steam generation | Electric steam generator | Provides steam to the jacketed batch reactor to control the temperature at desired values. |
| First transesterification | Jacketed batch reactor | WCO and methanol are mixed in the reactor. Once the catalyst is added, the reaction progresses. |
| First separation (decantation) | Jacketed batch reactor | Once the previous step is finished, the agitation system is stopped to let the glycerol phase settle and be removed from the bottom of the reactor. |
| Second transesterification | Jacketed batch reactor | TMP and catalyst are added to FAMEs generated in the previous transesterification, providing steam to retain the isothermal regime of the reaction. Methanol and the biolubricant are obtained as products. |
| Vacuum | Vacuum system | Vacuum is used to recover methanol, thus accelerating the second transesterification. |
| Purification | Pumping system and purifier | Once the reaction finishes, the resulting biolubricant is cooled down and purified (removing moisture, particles, and gas), and the resulting biolubricant is placed in a storage tank. |
| Antioxidant addition | TBHQ supply | A suitable amount of TBHQ was added to obtain the final product. |
| Equipment | Size | Details |
|---|---|---|
| WCO container | Volume = 2 m3; heigh = 2.8 m; diameter = 1.15 m; wall thickness = 1.2 mm | The container (stainless steel) is oversized to ensure WCO collection. |
| Methanol container | Volume = 0.6 m3; size = 1.2 × 0.8 × 1 m | HDPE containers, a smaller container (V = 0.3 m3), is used for the collection of methanol after vacuum filtration. |
| Sodium methoxide container | Volume = 0.3 m3; size = 1.2 × 0.8 × 1 m | HDPE container to supply 100 L of catalyst on a daily basis. |
| Glycerol container | Volume = 0.6 m3; size = 1.2 × 0.8 × 1 m | HDPE container to store 495 kg of glycerol obtained as a byproduct. |
| TMP container | Volume = 0.3 m3; height = 1.7 m; diameter = 0.74 m; wall thickness = 1 mm | Stainless-steel container to provide TMP. |
| Biolubricant container | Volume = 2 m3; height = 2.9 m; diameter = 1.15 m; wall thickness = 1.2 mm | Stainless-steel container to store WCO-TMP and to include TBHQ. A cooling and temperature control system is included. |
| TBHQ container | Volume = 150 L; height = 0.975 m; diameter = 0.48 m | HDPE container to store and supply TBHQ when necessary. |
| Condition | First Reaction | Second Reaction |
|---|---|---|
| Initial temperature, °C | 15 | 60 |
| Final (reaction) temperature, °C | 60 | 120 |
| Heating time, min | 35 | 40 |
| Saturated steam, kg | 136.1 | 112.5 |
| Flow, kg·min−1 | 3.9 | 2.82 |
| Reagent | Molecular Weight, g·mol−1 | Density, kg·m−3 | Mass Flow, kg·h−1 | Inlet Mass, kg | Inlet Volume, m3 |
|---|---|---|---|---|---|
| WCO | 900 | 920 | 209.2 | 1673.80 | 1.82 |
| Methanol | 32.04 | 792 | 44.68 | 357.44 | 0.45 |
| Sodium methoxide | 54.03 | 970 | 6.28 | 50.2 | 0.05 |
| Reagent | Molecular Weight, g·mol−1 | Density, kg·m−3 | Mass Flow, kg·h−1 | Inlet Mass, kg | Inlet Volume, m3 |
|---|---|---|---|---|---|
| FAMEs | 284.52 | 880 | 198.37 | 1586.96 | 1.8 |
| TMP | 134.17 | 1080 | 22.33 | 178.66 | 0.17 |
| Sodium methoxide | 54.03 | 970 | 5.95 | 47.62 | 0.05 |
| Parameter | Size |
|---|---|
| Reactor volume, m3 | 3 |
| Reactor surface area, m2 | 29.49 |
| Inner diameter, m | 1.563 |
| Height, m | 1.562 |
| Wall thickness, mm | 6 |
| Weight, kg | 168.53 |
| Da | H | J | E | W | L |
|---|---|---|---|---|---|
| 0.521 | 1.563 | 0.130 | 0.521 | 0.104 | 0.130 |
| Transesterification Process | Reagent | Price, €·T−1 | Amount, T·y−1 | Annual Cost, €·y−1 |
|---|---|---|---|---|
| First | WCO | 82 | 435.12 | 35,679.84 |
| CH3OH | 265 | 92.93 | 24,626.45 | |
| Sodium methoxide | 1800 | 13.05 | 23,490 | |
| Total | -- | 541.1 | 83,796.29 | |
| Second | TMP | 150 | 46.45 | 6967.5 |
| Sodium methoxide | 1800 | 12.38 | 22,284 | |
| Total | -- | 58.83 | 29,251.5 |
| Job Post | Number of Workers | Salary | Total Salary + Social Charges |
|---|---|---|---|
| Plant manager | 1 | 44,658.05 | 60,288.37 |
| Chemist | 1 | 28,985.65 | 39,130.63 |
| Qualified worker | 2 | 21,739.23 | 51,087.19 |
| Total | 4 | 95,382.93 | 150,506.19 |
| Step | Power, kW | Daily Working Time | Yearly Energy Consumption, kWh | Annual Cost, €·y−1 |
|---|---|---|---|---|
| Heating | 25.54 | 1.3 | 8632.52 | 1553.85 |
| Stirring | 22.25 | 2.51 | 14,520.35 | 2613.66 |
| Vacuum | 2.2 | 0.67 | 383.24 | 68.98 |
| Purification | 44 | 0.7 | 8008.00 | 1441.44 |
| Total | 91.87 | -- | 31,544.11 | 5677.94 |
| Equipment | Cost (VAT Included), € |
|---|---|
| WCO container | 2660 |
| Methanol container | 235.95 |
| Recovered-methanol container | 179.95 |
| Sodium methoxide container | 179.95 |
| Glycerol container | 235.95 |
| TMP container | 1180 |
| Biolubricant container | 4500 |
| TBHQ container | 42.35 |
| Steam generator | 3194 |
| Vacuum pump | 2360.60 |
| Purifier | 4100 |
| Reactor | 33,741 |
| Stirrer | 35,639 |
| Annual Production Costs | Annual Income | ||||
|---|---|---|---|---|---|
| Production | Cost, € | Product | Production, L·y−1 | Selling Price, €·L −1 | Annual Income, € |
| Raw materials | 150,808.43 | Biolubricant | 446,424 | 2.89 | 1,290,165.36 |
| Energy | 5677.94 | Production, T·y−1 | Selling price, €·T −1 | Annual income, € | |
| Water | 588.02 | Glycerol | 128.56 | 250 | 32,140 |
| Annual Profit | |||||
| Total annual costs (production), € | 157,074.39 | ||||
| Total annual incomes, € | 1,322,305.36 | ||||
| Annual gross profit, € | 1,165,230.97 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, P.M.; Collado Contreras, J.; Nogales-Delgado, S. Biodiesel and Biolubricant Production from Waste Cooking Oil: Transesterification Reactor Modeling. Appl. Sci. 2025, 15, 575. https://doi.org/10.3390/app15020575
Álvarez PM, Collado Contreras J, Nogales-Delgado S. Biodiesel and Biolubricant Production from Waste Cooking Oil: Transesterification Reactor Modeling. Applied Sciences. 2025; 15(2):575. https://doi.org/10.3390/app15020575
Chicago/Turabian StyleÁlvarez, Pedro M., Javier Collado Contreras, and Sergio Nogales-Delgado. 2025. "Biodiesel and Biolubricant Production from Waste Cooking Oil: Transesterification Reactor Modeling" Applied Sciences 15, no. 2: 575. https://doi.org/10.3390/app15020575
APA StyleÁlvarez, P. M., Collado Contreras, J., & Nogales-Delgado, S. (2025). Biodiesel and Biolubricant Production from Waste Cooking Oil: Transesterification Reactor Modeling. Applied Sciences, 15(2), 575. https://doi.org/10.3390/app15020575







