Uranium Mineral Transport in the Peña Blanca Desert: Dissolution or Fragmentation? Simulation in Sediment Column Systems
Abstract
1. Introduction
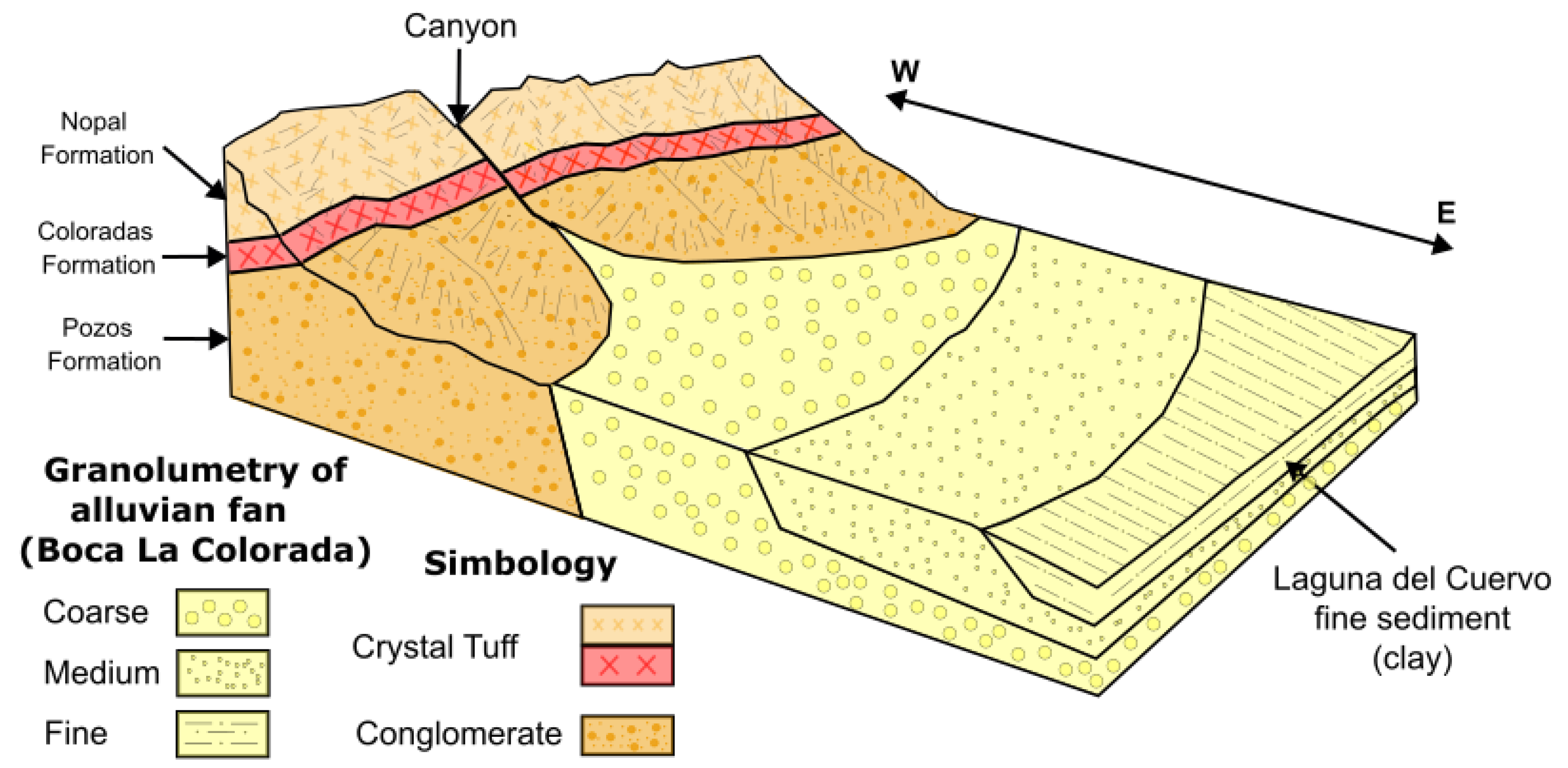
Sierra Peña Blanca, Deserts and Streams
2. Materials and Methods
2.1. Sampling and Conditioning of Minerals and Sediments
2.2. Elemental, Mineralogical, and Morphological Characterization
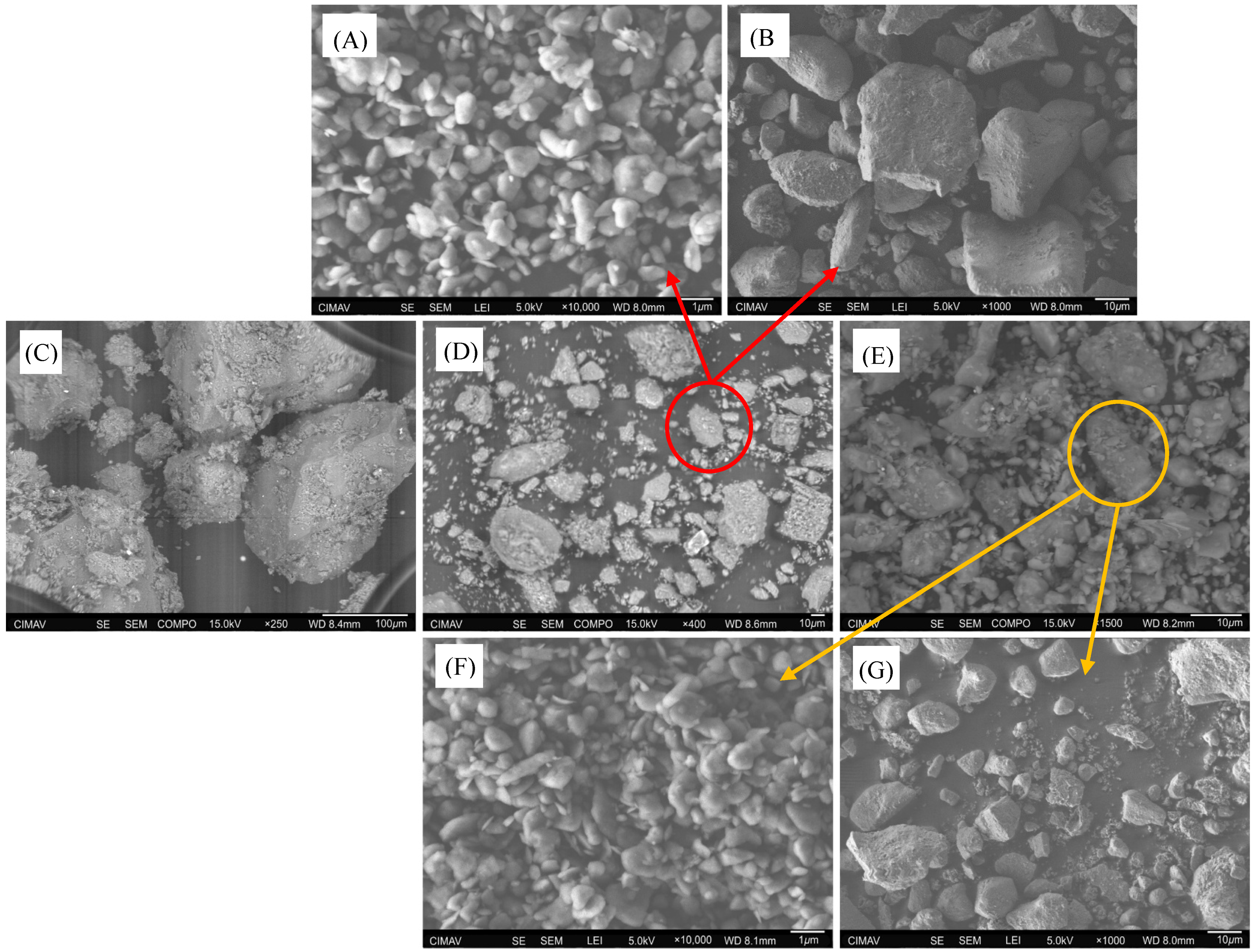
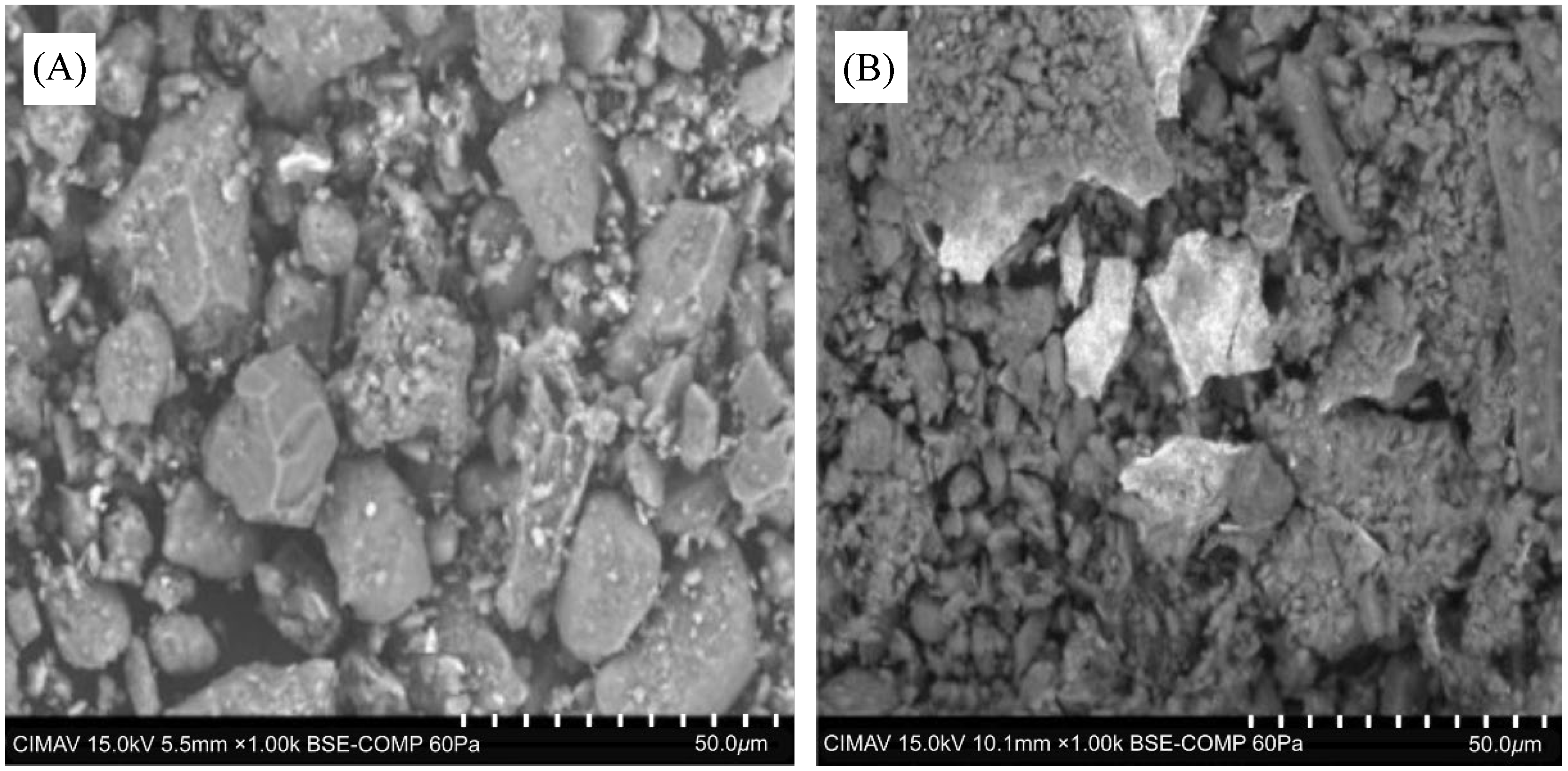
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. X-Ray Diffraction (XRD) and Elemental Characterization
2.3. Uranium Transport and Adsorption Experiments
2.3.1. Simulation of Ideal Adsorption by Fine Sediments
2.3.2. Simulation of Uranium Transport in the SPB Profile
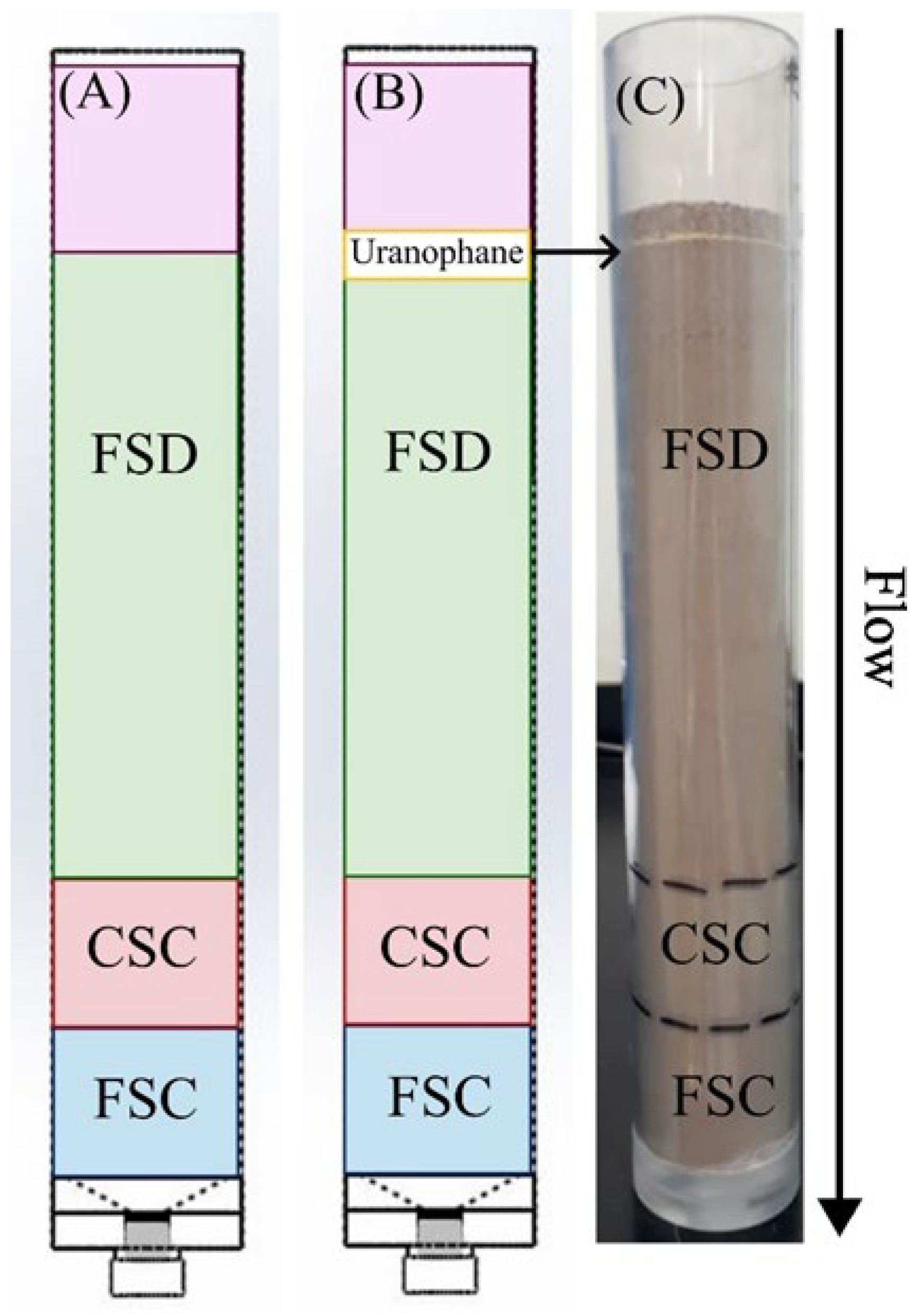
Column Design for Simulation
Implementation of the Experiment
Characterization of Packed Columns
Uranium Determination
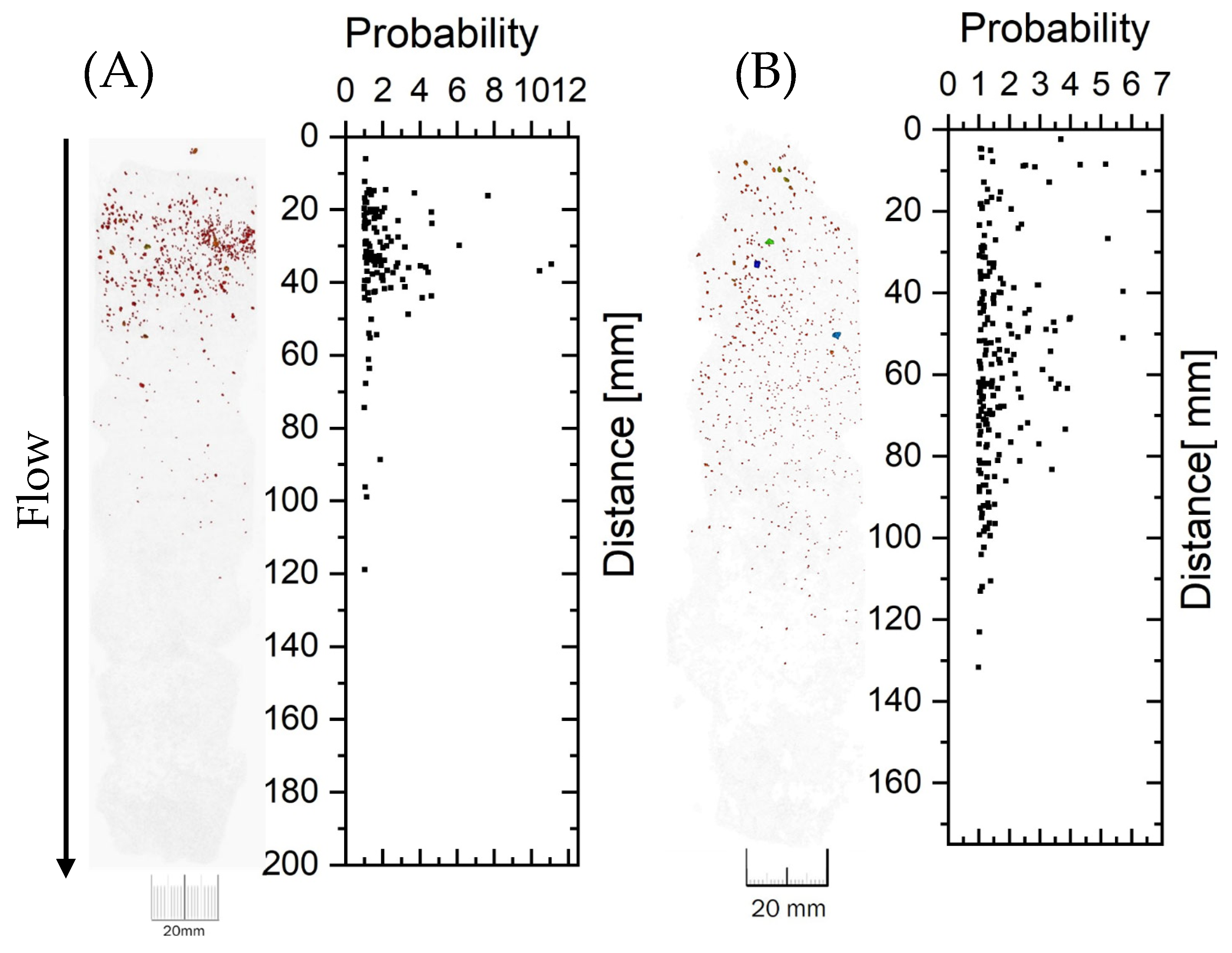
X-Ray Tomography
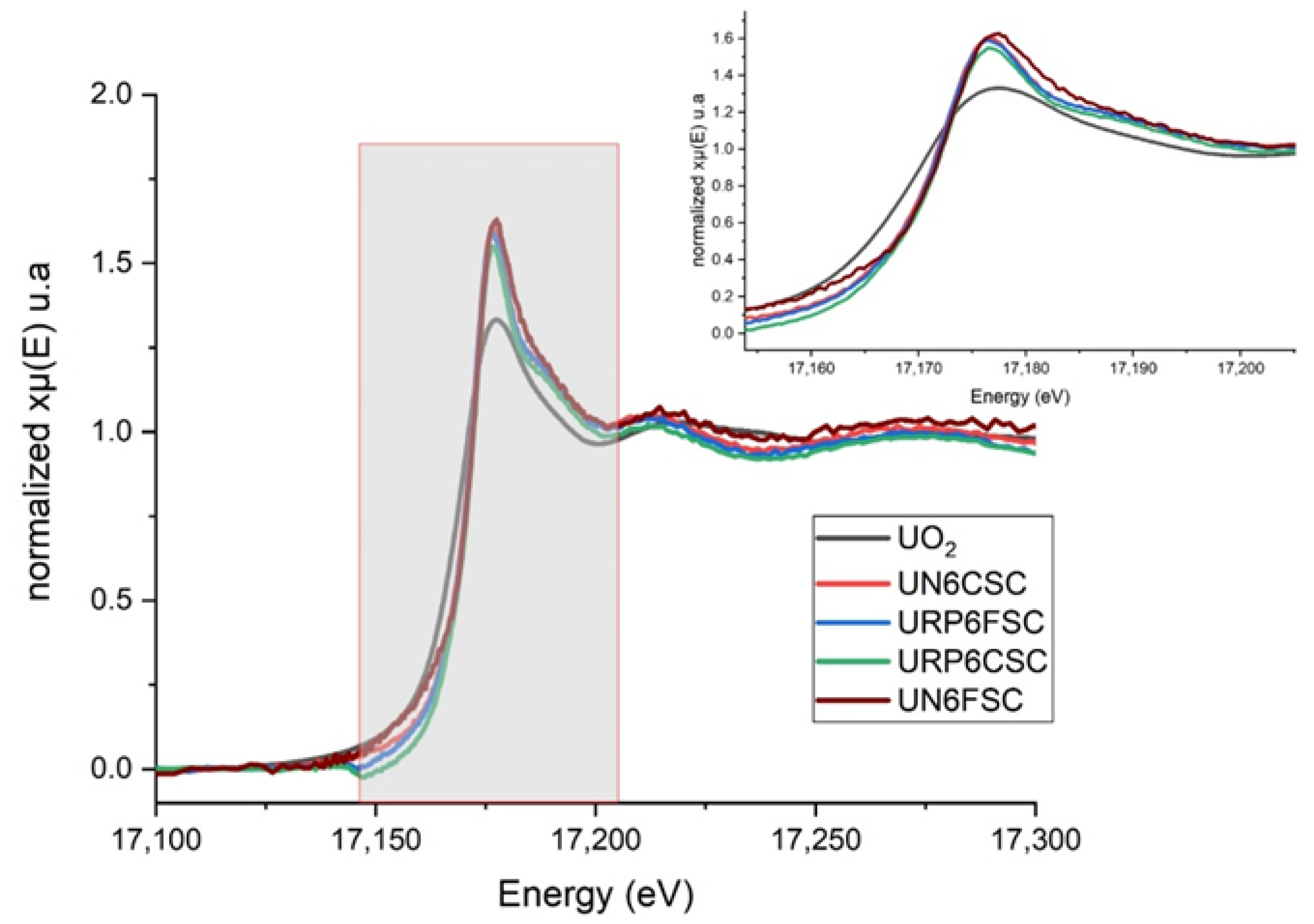
X-Ray Absorption Fine Structure (XAFS)
3. Results
3.1. Sediment Characterization for Batch and Transport Experiments
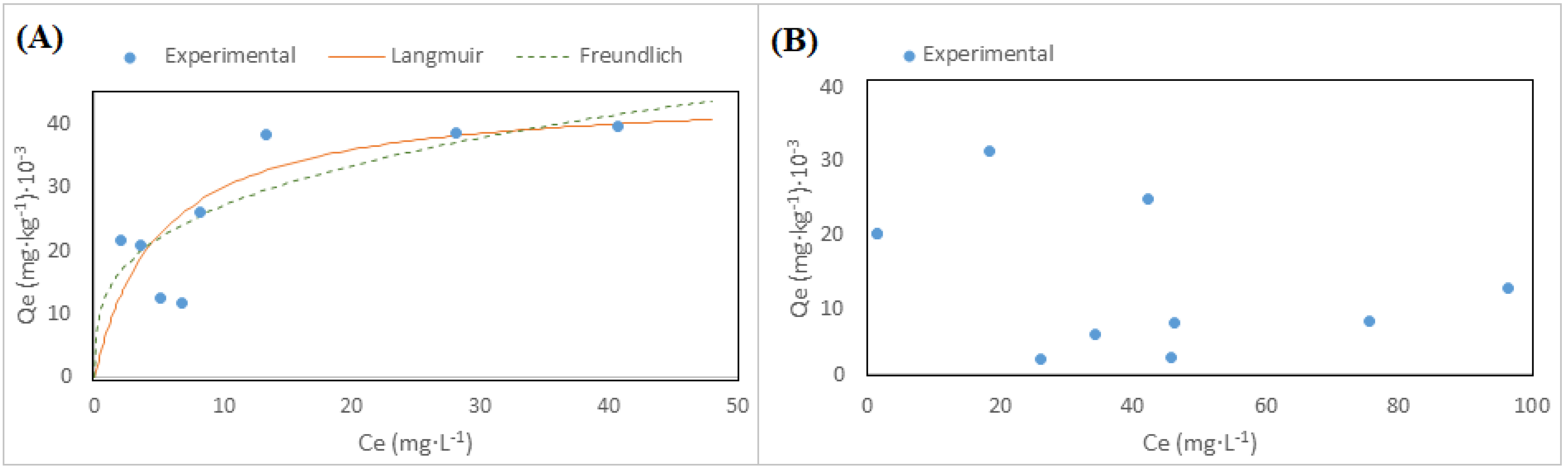
3.2. Modeling Adsorption from Uranium in Solution
3.2.1. BATCH Experiment
3.2.2. Transport Modeling with Uranium UN Solutions
3.2.3. Transport Modeling in URP Columns
Tomography of URP6 and URP12 Column Profiles
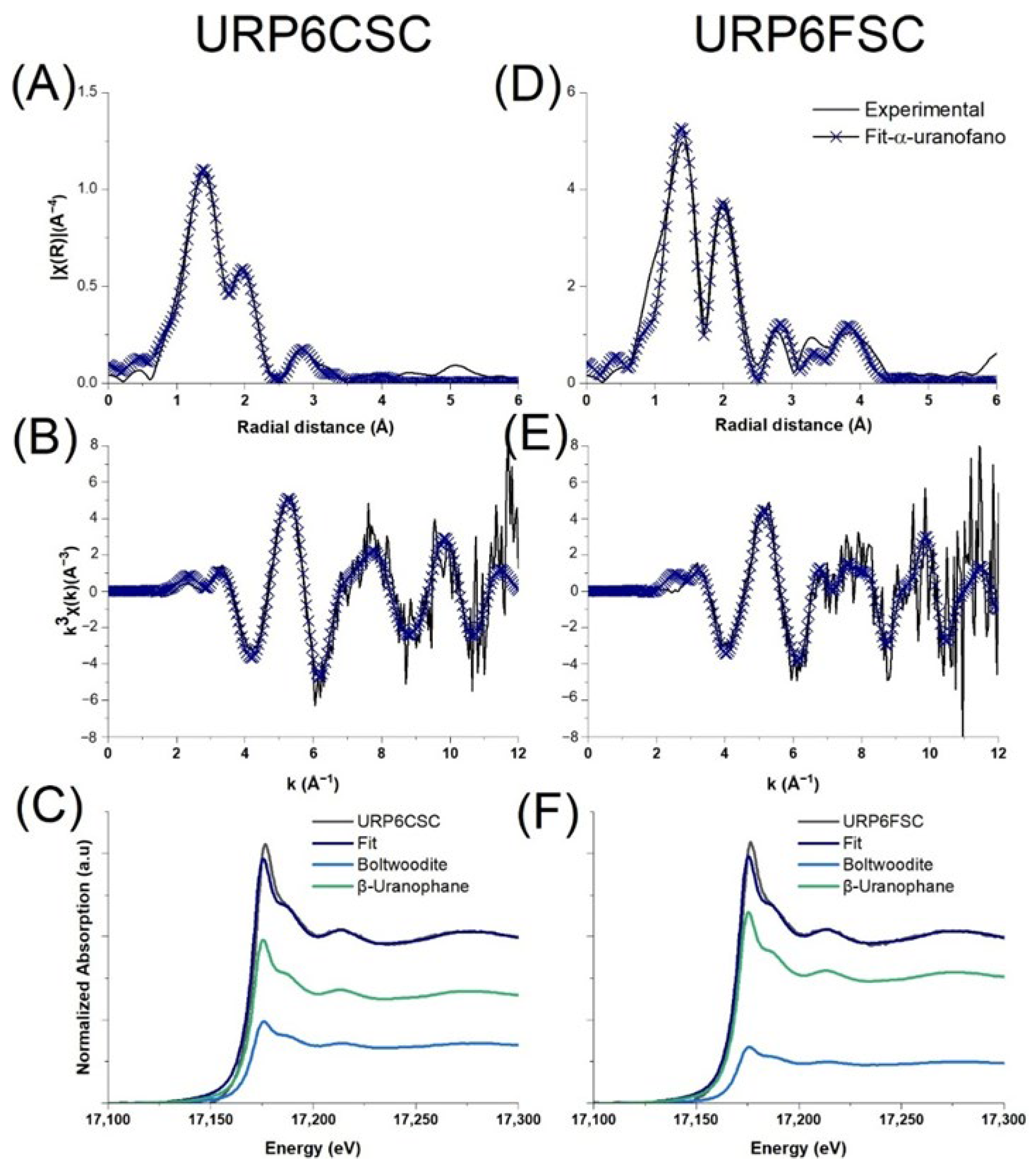
XAFS
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Separation of Silt and Clay Fractions
Appendix A.2. Pore Volume of Columns
Appendix B
Appendix B.1. Sediments and Effluent Characterization
Appendix B.1.1. Powder X-Ray Diffraction (XRD)
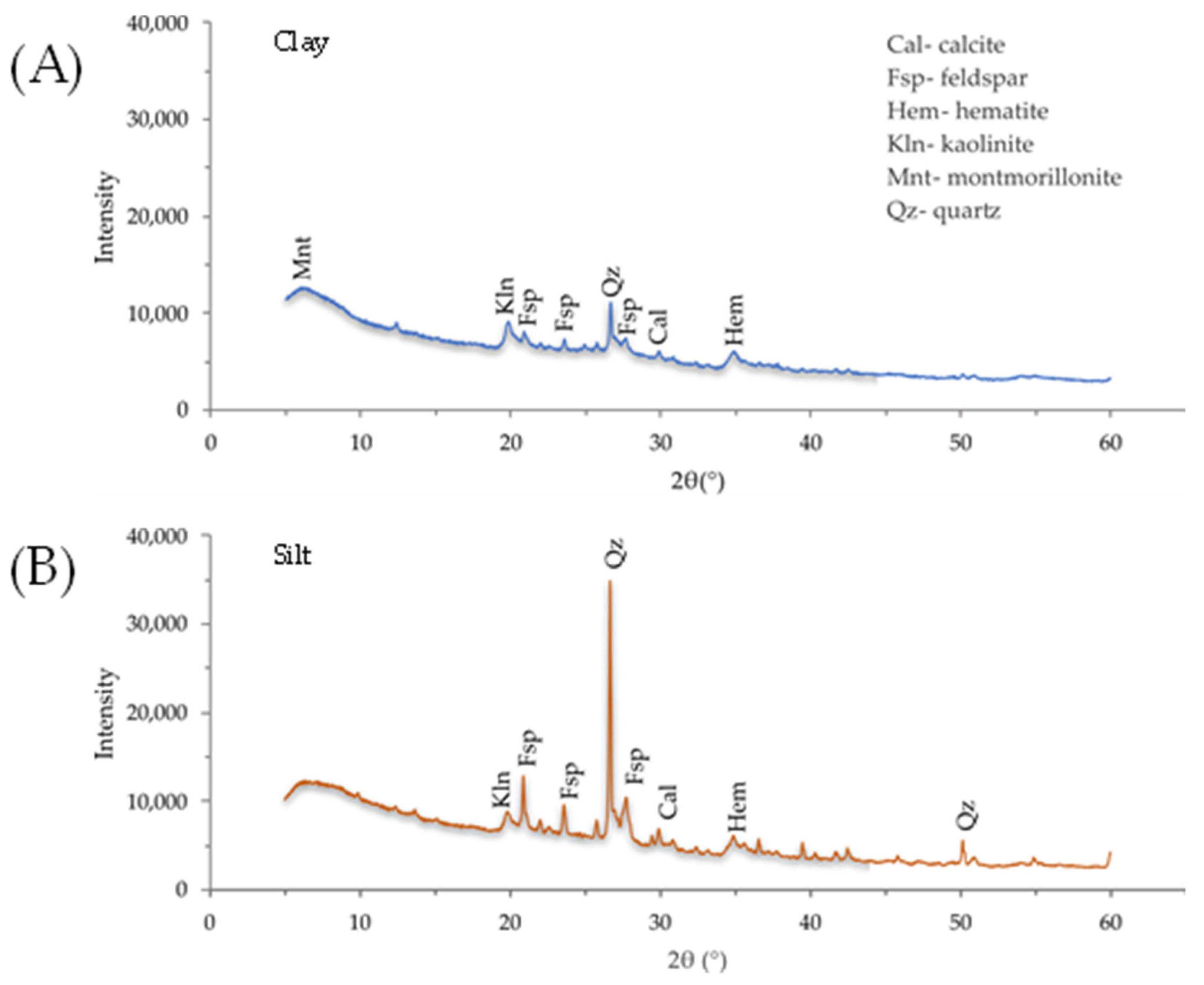
Appendix B.1.2. Microwave Plasma Atomic Emission Spectrometry (MP-AES)
| Sample ID | Element (mg kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Al | Na | K | Mg | Ca | Fe | Cu | Si * | |
| Detection limit | 6.7 | 1.5 | 12 | 1.3 | 3 | 7 | 0.23 | 12 |
| CSC | 9655 | 10 | 2675 | 2961 | 4828 | 7733 | 7 | 53 |
| FSC | 21,900 | 32 | 6269 | 7055 | 10,224 | 16,959 | 17 | LOD |
| UN6CSC | 12,695 | 7 | 3300 | 4005 | 6658 | 10,325 | 9 | 65 |
| UN6FSD | 15,795 | 11 | 4144 | 4933 | 7431 | 12,378 | 12 | 16 |
| UN12CSC | 20,358 | 19 | 5324 | 6406 | 9736 | 16,322 | 11 | <LD |
| UN12FSD | 21,233 | 29 | 5349 | 6454 | 9191 | 15,698 | 14 | <LD |
| URP6CSC | 17,622 | 22 | 4639 | 5514 | 8420 | 14,225 | 11 | LOD |
| URP6FSD | 13,729 | 12 | 4035 | 4271 | 6344 | 10,600 | 12 | 17 |
| URP12CSC | 18,768 | 30 | 5468 | 6127 | 9261 | 15,071 | 14 | LOD |
| URP12FSD | 15,154 | 22 | 4049 | 6979 | 10,713 | 13,907 | 11 | LOD |
| ID Sample | Element mg L−1 | |||
|---|---|---|---|---|
| Na | K | Mg | Ca | |
| Detection limit | 0.15 | 0.13 | 0.21 | 0.18 |
| WUN6_1 | 101.36 | 57.86 | 22.81 | 108.3 |
| WUN6_2 | 58.07 | 45.08 | 26.95 | 62.56 |
| WUN6_3 | 50.58 | 47.58 | 25.23 | 26.33 |
| WUN6_4 | 8.93 | 6.86 | 5.49 | 23.88 |
| WUN6_5 | 6.97 | 5.2 | 3.07 | 19.07 |
| WUN6_6 | <LOD | 1.41 | 1.05 | 5.88 |
| WUN6_7 | 4.33 | 2.89 | 1.97 | 12 |
| WUN12_1 | 45.5 | 49.63 | 27.24 | 79.83 |
| WUN12_2 | 39.05 | 40.76 | 21.68 | 63.72 |
| WUN12_3 | 47.38 | 37.95 | 16.09 | 32.11 |
| WUN12_4 | 41.1 | 26.22 | 16.41 | 74.32 |
| WUN12_5 | 39.6 | 25.75 | 11.07 | 57.48 |
| WUN12_6 | 17.48 | 12.75 | 7.6 | 41.52 |
| WUN12_7 | 36.7 | 21.85 | 6.07 | 41.36 |
| WUN12_8 | 18.09 | 14.4 | 4.14 | 28.03 |
| WUN12_9 | 15.99 | 11.36 | 3 | 21.63 |
| WUN12_10 | 43.78 | 33.04 | 8.7 | 72 |
| WUN12_11 | 24.71 | 21.97 | 6.07 | 55.22 |
| WUN12_12 | 16.42 | 23.35 | 6.72 | 61.13 |
| WURP6_1 | 103.65 | 62.64 | 23.93 | 114.23 |
| WURP6_2 | 88.09 | 70.53 | 60.7 | 295.89 |
| WURP6_3 | 43.82 | 64.47 | 33.77 | 57.55 |
| WURP6_4 | 41.32 | 35.66 | 17.84 | 114.06 |
| WURP6_5 | 31.81 | 25.16 | 12.34 | 79.09 |
| WURP6_6 | 27.24 | 19.68 | 9.47 | 66.42 |
| WURP6_7 | 22.46 | 18.77 | 7.88 | 63.28 |
| WURP6_8 | 29.25 | 17.72 | 6.76 | 43.58 |
| WURP12_3 | 62.24 | 71 | 35.14 | 50.84 |
| WURP12_7 | 45.82 | 26.46 | 9.46 | 55.49 |
| WURP12_8 | 9.82 | 8.9 | 4.42 | 28.28 |
| WURP12_9 | 16.92 | 12.83 | 7.09 | 46.51 |
| WURP12_10 | 13.49 | 9.06 | 4.61 | 34.36 |
| WURP12_11 | 25.44 | 19.43 | 6.07 | 44.7 |
| WURP12_12 | 9.84 | 10.68 | 5.26 | 39.86 |
| WURP12_13 | 7.54 | 6.07 | 3.18 | 23.84 |
Appendix B.2. Batch Experiment
Appendix B.2.1. EXAFS Results for pH = 7 C2 Type Clay Fraction
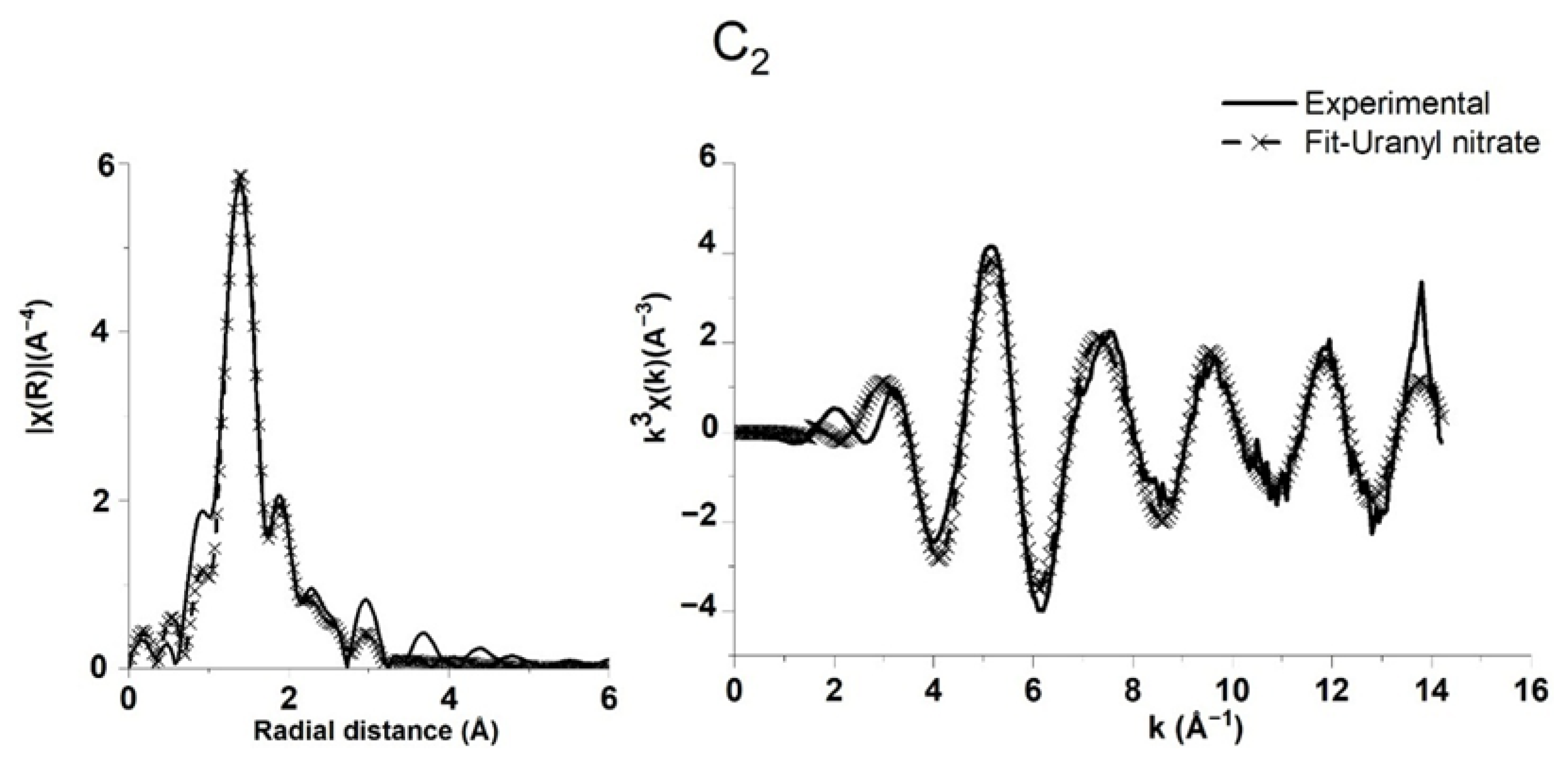
| Name | N | σ2 (Å2) | ΔR | Reff (Å) | Reff + ΔR (Å) |
|---|---|---|---|---|---|
| U_Oax | 2 | 0.0031 (6) | 0.059 ± 0.005 | 1.7602 | 1.819 |
| U_Oeq1 | 2 | 0.005 (3) | −0.09 ± 0.02 | 2.3972 | 2.31 |
| U_Oeq2 | 2 | 0.010 (4) | −0.04 ± 0.02 | 2.5038 | 2.47 |
| U_Oeq3 | 2 | 0.010 (4) | −0.04 ± 0.02 | 2.5475 | 2.51 |
| U_N1 | 1 | 0.007 (5) | −0.10 ± 0.04 | 2.9506 | 2.85 |
| U_N2 | 1 | 0.007 (5) | −0.10 ± 0.04 | 2.9845 | 2.88 |
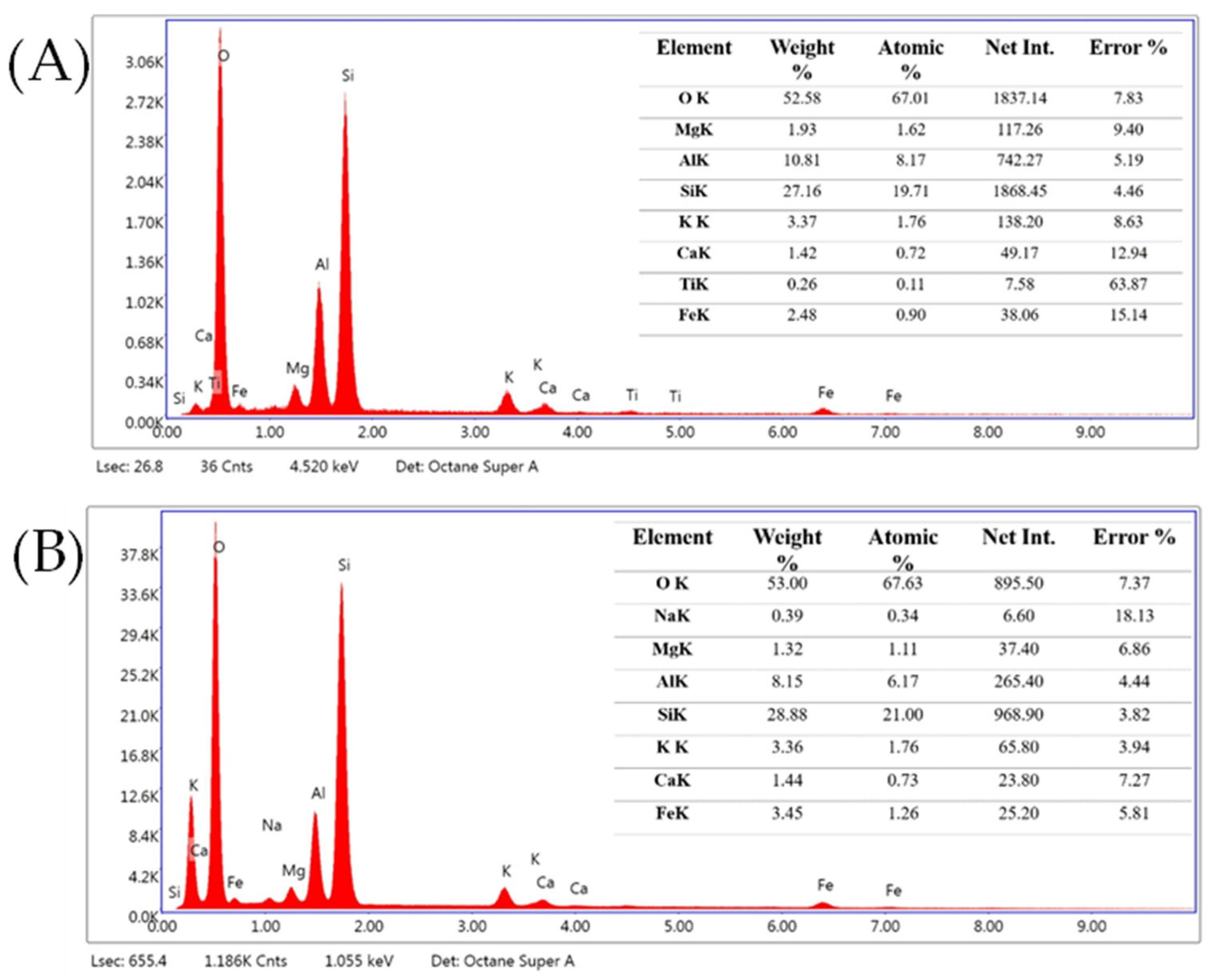
Appendix B.2.2. SEM-EDS Images for FSC pH = 5 and FSC pH = 7


Appendix B.3. Modeling Transport in URP Columns
Appendix B.3.1. URP6 Column–End of Experiment (SEM-EDS)

Appendix B.3.2. URP12 Column-End of Experiment

Appendix B.3.3. URP Experiment–Tomography
Calibration
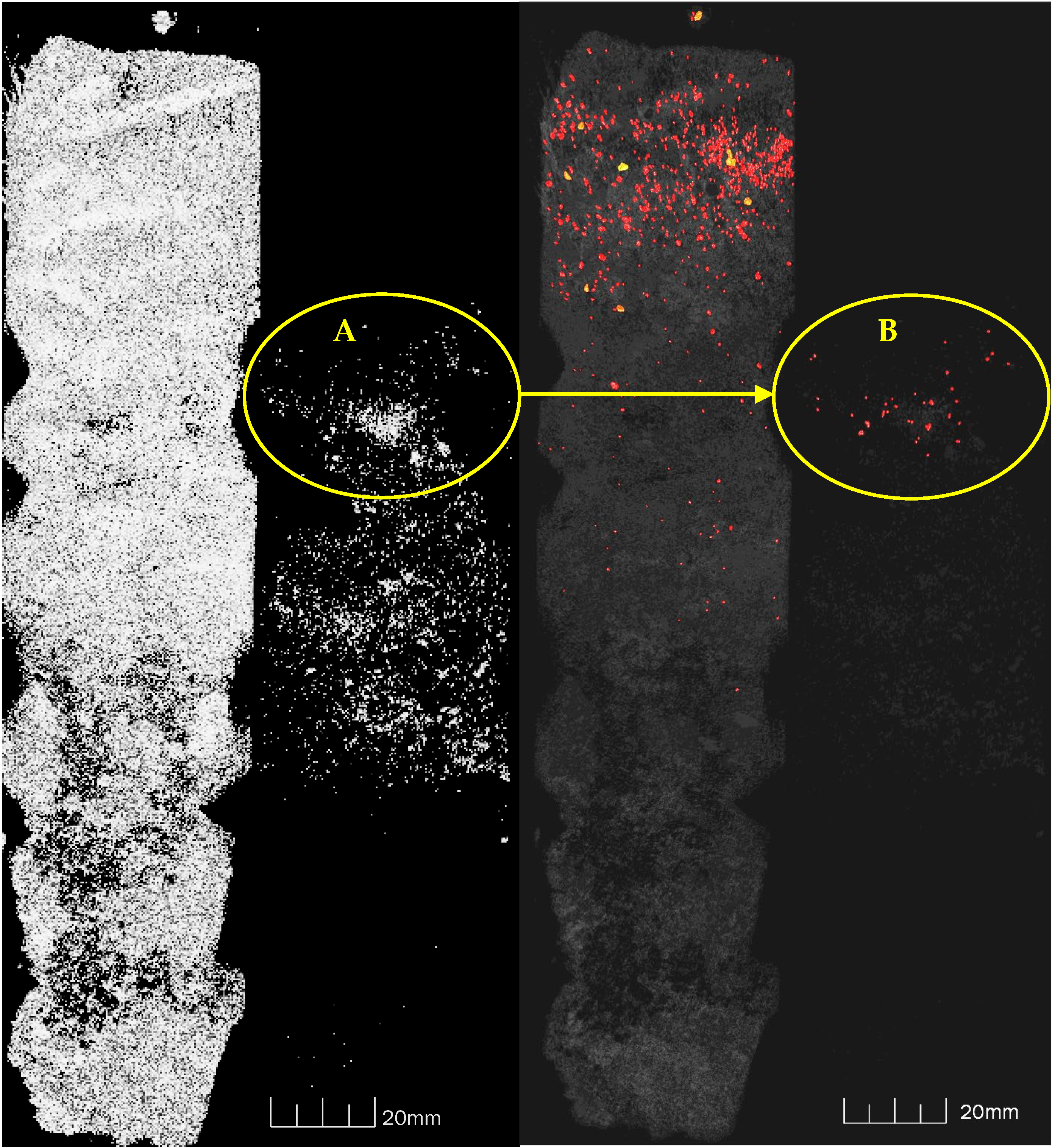
Comparison of Tomography-UV Light-Visible Light
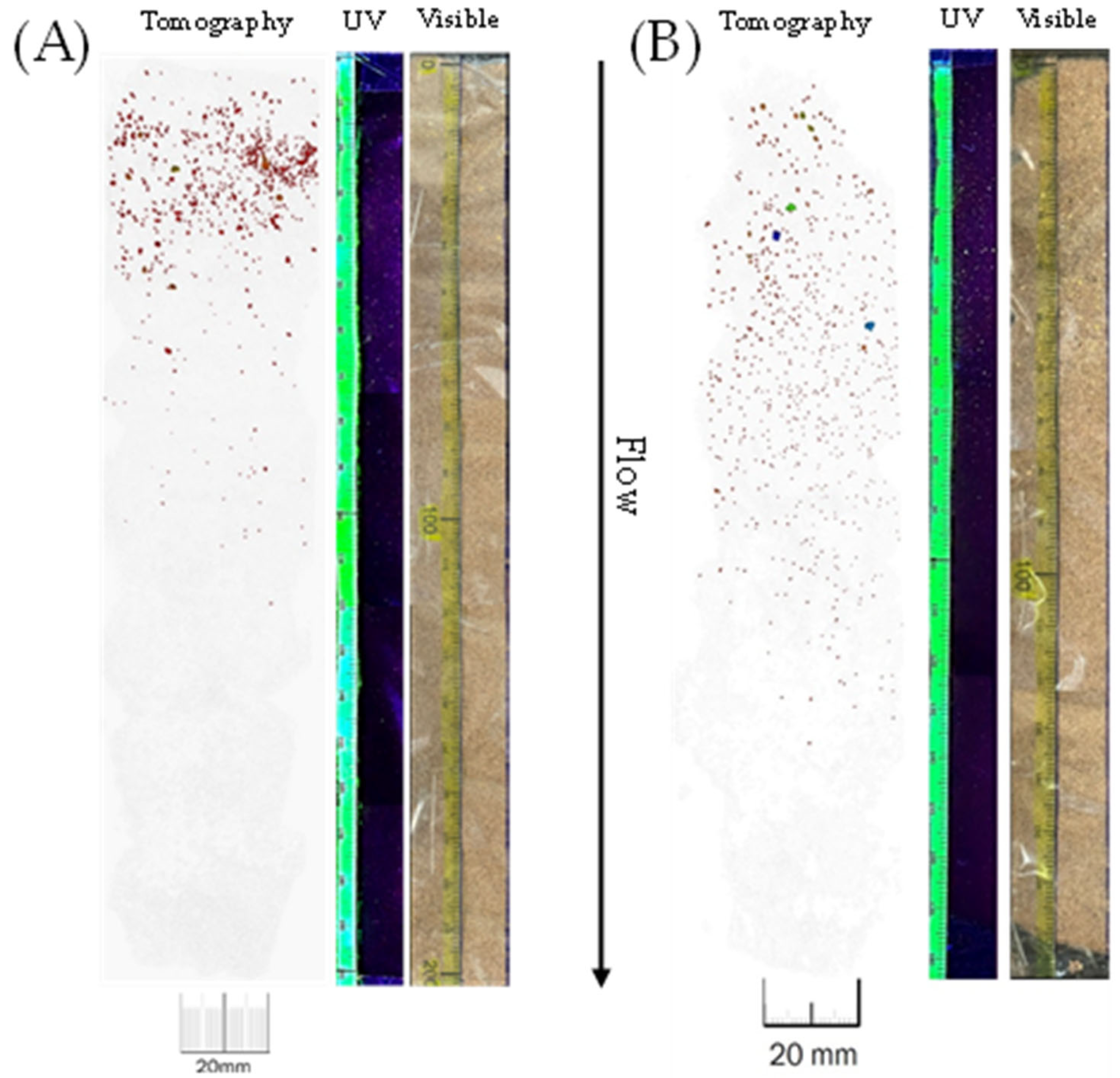
Appendix B.4. Uranium Concentrations in Effluents from Experiments
Appendix B.4.1. Transport Modeling with Uranium Solutions (UN Columns)
| UN6 | |
|---|---|
| Sample ID | U Total (mg L−1) |
| WUN6_1 | 0.7 |
| WUN6_2 | 0.1 |
| WUN6_3 | 0.1 |
| WUN6_4 | 0.1 |
| WUN6_5 | 0.1 |
| UN12 | ||
|---|---|---|
| Sample ID | U Total mg L−1 LSC | U mg L−1 MP-AES |
| WUN12_1 | 0.2 | 0.09 |
| WUN12_2 | 0.21 | 0.07 |
| WUN12_3 | 1.25 | 0.15 |
| WUN12_4 | 0.54 | 0.1 |
| WUN12_5 | 0.86 | 0.09 |
| WUN12_6 | 2.37 | 0.13 |
| WUN12_7 | 0.46 | 0.1 |
| WUN12_8 | 3.68 | 0.64 |
| WUN12_9 | 5.55 | 2.5 |
| WUN12_10 | 9.02 | 9.01 |
| WUN12_11 | 16.42 | 16.82 |
| WUN12_12 | 29.09 | 25.99 |
Appendix B.4.2. Modeling Transport in URP Columns
| URP6 | ||||
|---|---|---|---|---|
| ID Sample | 234U Bq L−1 | 238U Bq L−1 | RQ (%) | 238U mg L−1 |
| WURP6_2 | 2.1 ± 0.1 | 2.3 ± 0.1 | 23 | 0.18 |
| WURP6_3 | 2.1 ± 0.1 | 2.6 ± 0.1 | 88 | 0.21 |
| WURP6_4 | 6.3 ± 0.1 | 6.7 ± 0.1 | 60 | 0.55 |
| WURP6_6 | 19.0 ± 0.4 | 34.4 ± 0.7 | 69 | 2.79 |
References
- Smedley, P.L.; Kinniburgh, D.G. Uranium in Natural Waters and the Environment: Distribution, Speciation and Impact. Appl. Geochem. 2023, 148, 105534. [Google Scholar] [CrossRef]
- Bourdon, B.; Turner, S.; Henderson, G.M.; Lundstrom, C.C. Introduction to U-Series Geochemistry. Miner. Geochem. 2003, 52. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. The Long Term Stabilization of Uranium Mill Tailings; TECDOC Series; IAEA: Vienna, Austria, 2004; ISBN 92-0-108904-X. [Google Scholar]
- Liu, C.; Zachara, J.M.; Qafoku, O.; McKinley, J.P.; Heald, S.M.; Wang, Z. Dissolution of Uranyl Microprecipitates in Subsurface Sediments at Hanford Site, USA. Geochim. Cosmochim. Acta 2004, 68, 4519–4537. [Google Scholar] [CrossRef]
- Wang, Z.; Zachara, J.M.; Gassman, P.L.; Liu, C.; Qafoku, O.; Yantasee, W.; Catalano, J.G. Fluorescence Spectroscopy of U(VI)-Silicates and U(VI)-Contaminated Hanford Sediment. Geochim. Cosmochim. Acta 2005, 69, 1391–1403. [Google Scholar] [CrossRef]
- Pidchenko, I.; Bauters, S.; Sinenko, I.; Hempel, S.; Amidani, L.; Detollenaere, D.; Vinze, L.; Banerjee, D.; Van Silfhout, R.; Kalmykov, S.N.; et al. A Multi-Technique Study of Altered Granitic Rock from the Krunkelbach Valley Uranium Deposit, Southern Germany. RSC Adv. 2020, 10, 25529–25539. [Google Scholar] [CrossRef]
- Tayal, A.; Conradson, S.D.; Kanzari, A.; Lahrouch, F.; Descostes, M.; Gerard, M. Uranium Speciation in Weathered Granitic Waste Rock Piles: An XAFS Investigation. RSC Adv. 2019, 9, 11762–11773. [Google Scholar] [CrossRef]
- deLemos, J.L.; Bostick, B.C.; Quicksall, A.N.; Landis, J.D.; George, C.C.; Slagowski, N.L.; Rock, T.; Brugge, D.; Lewis, J.; Durant, J.L. Rapid Dissolution of Soluble Uranyl Phases in Arid, Mine-Impacted Catchments near Church Rock, NM. Environ. Sci. Technol. 2008, 42, 3951–3957. [Google Scholar] [CrossRef] [PubMed]
- Lottermoser, B.G.; Ashley, P.M.; Costelloe, M.T. Contaminant Dispersion at the Rehabilitated Mary Kathleen Uranium Mine, Australia. Environ. Geol. 2005, 48, 748–761. [Google Scholar] [CrossRef]
- Rodriguez, V.G.; Majumdar, A.; Meza, I.; Corcoran, L.; Pierson, A.; Gagnon, K.; Cano, C.; Ali, A.-M.S.; Shuey, C.M.; Jojola, G.; et al. Radiological Analyses of 226Ra and 238U in Surface Water and Sediments from the Jackpile Member of the Morrison Formation, Pueblo of Laguna, New Mexico. Environ. Sci. Technol. 2024, 58, 15138–15146. [Google Scholar] [CrossRef]
- Brown, C.F.; Serne, R.J.; Catalano, J.G.; Krupka, K.M.; Icenhower, J.P. Mineralization of Contaminant Uranium and Leach Rates in Sediments from Hanford, Washington. Appl. Geochem. 2010, 25, 97–104. [Google Scholar] [CrossRef]
- Bower, W.R.; Morris, K.; Livens, F.R.; Mosselmans, J.F.W.; Fallon, C.M.; Fuller, A.J.; Natrajan, L.; Boothman, C.; Lloyd, J.R.; Utsunomiya, S.; et al. Metaschoepite Dissolution in Sediment Column Systems—Implications for Uranium Speciation and Transport. Environ. Sci. Technol. 2019, 53, 9915–9925. [Google Scholar] [CrossRef]
- Johnson, R.H.; Tigar, A.D.; Richardson, C.D. Column-Test Data Analyses and Geochemical Modeling to Determine Uranium Reactive Transport Parameters at a Former Uranium Mill Site (Grand Junction, Colorado). Minerals 2022, 12, 438. [Google Scholar] [CrossRef]
- Fallon, C.M.; Bower, W.R.; Powell, B.A.; Livens, F.R.; Lyon, I.C.; McNulty, A.E.; Peruski, K.; Mosselmans, J.F.W.; Kaplan, D.I.; Grolimund, D.; et al. Vadose-Zone Alteration of Metaschoepite and Ceramic UO2 in Savannah River Site Field Lysimeters. Sci. Total Environ. 2023, 862, 160862. [Google Scholar] [CrossRef]
- Bam, W.; Teyssié, J.-L.; Metian, M.; Oberhaensli, F.; Maiti, K.; Swarzenski, P.W. An Experimental Approach to Assess the Post-Depositional Mobility of 134Cs. J. Environ. Radioact. 2021, 240, 106753. [Google Scholar] [CrossRef] [PubMed]
- Alba, L.A.; Chávez, R. K-Ar Ages of Volcanic Rocks from the Central Sierra Peha Blanca, Chihuahua, Mexic. Isochron/West 1974, 10, 21–23. [Google Scholar]
- George-Aniel, B.; Leroy, J.L.; Poty, B. Volcanogenic Uranium Mineralizations in the Sierra Pena Blanca District, Chihuahua, Mexico; Three Genetic Models. Econ. Geol. 1991, 86, 233–248. [Google Scholar] [CrossRef]
- Goodell, P.C. Chihuahua City Uranium Province, Chihuahua, Mexico. In Uranium Deposits in Volcanic Rocks, Proceedings of the Technical Committee Meeting on Uranium Deposits in Volcanic Rocks, El Paso, TX, USA, 2–5 April 1984; IAEA: Vienna, Austria, 1985; pp. 97–124. ISBN 92-0-041085-5. [Google Scholar]
- Dobson, P.F.; Fayek, M.; Goodell, P.C.; Ghezzehei, T.A.; Melchor, F.; Murrell, M.T.; Oliver, R.; Reyes-Cortés, I.A.; De La Garza, R.; Simmons, A. Stratigraphy of the PB-1 Well, Nopal I Uranium Deposit, Sierra Peña Blanca, Chihuahua, Mexico. Int. Geol. Rev. 2008, 50, 959–974. [Google Scholar] [CrossRef]
- Pearcy, E.C.; Prikryl, J.D. Alteration of Uraninite from the Nopai I Deposit, Pefia Blanca District, Chihuahua, Mexico, Compared to Degradation of Spent Nuclear Fuel in the Proposed U.S. High-Level Nuclear Waste Repository at Yucca Mountain, Nevada. Appl. Geochem. 1994, 9, 713–732. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2004; ISBN 970-32-1010-4. [Google Scholar]
- INEGI. Anuario Estadístico y Geográfico de Chihuahua; Instituto Nacional de Estadística y Geografía: Mexico City, Mexico, 2017; Available online: https://www.inegi.org.mx/app/biblioteca/ficha.html?upc=702825092139 (accessed on 5 September 2024).
- Schmidt, R.H. Chihuahuan Climate. Proceedings, Second Symposium on Resources of the Chihuahuan Desert, Chihuahuan; Chihuahuan Desert Research Institute: Alpine, TX, USA, 1986; pp. 40–63. Available online: https://www.utep.edu/leb/pdf/chihuahuanclimate.pdf (accessed on 5 September 2024).
- INEGI. Síntesis de Información Geográfica Del Estado de Chihuahua; Instituto Nacional de Estadística y Geografía: Mexico City, Mexico, 2003; ISBN 970-13-4166-X. [Google Scholar]
- Reineck, H.-E.; Singh, I.B. Depositional Sedimentary Environments with Reference to Terrigenous Clastics, Springer Study ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-540-10189-5. [Google Scholar]
- Denton, J.S.; Goldstein, S.J.; Paviet, P.; Nunn, A.J.; Amato, R.S.; Hinrichs, K.A. A Record of Uranium-Series Transport at Nopal I, Sierra Peña Blanca, Mexico: Implications for Natural Uranium Deposits and Radioactive Waste Repositories. Chem. Geol. 2016, 434, 12–27. [Google Scholar] [CrossRef]
- Dobson, P.F.; Ghezzehei, T.A.; Cook, P.J.; Rodríguez-Pineda, J.A.; Villalba, L.; De La Garza, R. Heterogeneous Seepage at the Nopal I Natural Analogue Site, Chihuahua, Mexico. Hydrogeol. J. 2012, 20, 155–166. [Google Scholar] [CrossRef]
- Goldstein, S.J.; Abdel-Fattah, A.I.; Murrell, M.T.; Dobson, P.F.; Norman, D.E.; Amato, R.S.; Nunn, A.J. Uranium-Series Constraints on Radionuclide Transport and Groundwater Flow at the Nopal I Uranium Deposit, Sierra Peña Blanca, Mexico. Environ. Sci. Technol. 2010, 44, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.L.; Luo, S.; Goldstein, S.J.; Murrell, M.T.; Chu, W.L.; Dobson, P.F. Modeling Non-Steady State Radioisotope Transport in the Vadose Zone—A Case Study Using Uranium Isotopes at Peña Blanca, Mexico. Geochim. Cosmochim. Acta 2009, 73, 6052–6064. [Google Scholar] [CrossRef]
- Pickett, D.A.; Murphy, W.M. Unsaturated Zone Waters From the Nopal I Natural Analog, Chihuahua, Mexico—Implications for Radionuclide Mobility at Yucca Mountain. MRS Proc. 1999, 556, 809. [Google Scholar] [CrossRef]
- Hernández-Hernández, D. Transporte y Equilibrio Radioactivo Del Uranio Desde El Yacimiento de Peña Blanca Hasta Laguna Del Cuervo, Chihuahua. Master’s Thesis, Centro de Investigación en Materiales Avanzados, Chihuahua, Mexico, 2019. [Google Scholar]
- Rodríguez-Guerra, Y. Transporte de Isótopos Radiactivos de La Serie Del Uranio Desde Peña Blanca Hasta Laguna Del Cuervo a Través Del Arroyo El Tigre, Chihuahua. Master’s Thesis, Centro de Investigación en Materiales Avanzados, Chihuahua, Mexico, 2023. [Google Scholar]
- Salas, G.; Castillo Nieto, F. Geology of Uranium Deposits in Mexico. In Economic Geology, Mexico; Geological Society of America: Boulder, CA, USA, 1991; Volume P-3, ISBN 978-0-8137-5470-3. [Google Scholar]
- Pérez-Reyes, V.; Cabral-Lares, R.M.; Méndez-García, C.G.; Caraveo-Castro, C.D.R.; Reyes-Cortés, I.A.; Carrillo-Flores, J.; Montero-Cabrera, M.E. Transport and Concentration of Uranium Isotopes in the Laguna Del Cuervo, Chihuahua, Mexico. Supl. Rev. Mex. Física 2022, 3, 010606-1. [Google Scholar] [CrossRef]
- Nash, T. Volcanogenic Uranium Deposits: Geology, Geochemical Processes, and Criteria for Resource Assessment; Open-File Report; United States Geological Survey: Reston, VA, USA, 2010. Available online: https://pubs.usgs.gov/of/2010/1001/pdf/OF10-1001.pdf (accessed on 5 September 2024).
- Hernández-Herrera, C.; Canche-Tello, J.; Reyes-Cortés, I.; Rodríguez-Guerra, Y.; Pérez-Reyes, V.; Faudoa-Goméz, F.; Cabral-Lares, M.; Hernández-Cruz, D.; Loredo-Portales, R.; Montero-Cabrera, M.E. Interpretación de Los Espectros de Absorción de Rayos-X Por Radiación Sincrotrón de Las Especies Minerales Uraníferas Transportadas Por El Arroyo “El Tigre”, En Peña Blanca, Chihuahua, México. In Proceedings of the LXVI Congreso Nacional de Física, Morelia, Mexico, 8–13 October 2023; Suplemento del Boletín Sociedad Mexicana de Física: Morelia, Mexico, 2023; p. 360. [Google Scholar]
- Kelly, S.D. Uranium Chemistry in Soils and Sediments. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2010; Volume 34, pp. 411–466. ISBN 978-0-444-53261-9. [Google Scholar]
- Koningsberger, D.C.; Prins, R. X-ray Absorption: Principles, Applications, Techniques of EXAFS, SEXAFS and XANES; John Wiley and Sons: New York, NY, USA, 1987. Available online: https://www.osti.gov/biblio/6748791 (accessed on 3 October 2024).
- ISO 18400-102; Soil Quality-Sampling-Part 102: Selection and Application of Sampling Techniques. International Organization for Standardization (ISO): Geneva, Switzerland, 2017; p. 71ISBN 978 0 580 80925 5.
- Switzer, A.D. Measuring and Analyzing Particle Size in a Geomorphic Context. In Treatise on Geomorphology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 14, pp. 224–242. ISBN 978-0-08-088522-3. [Google Scholar]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination Neutron Powder Diffraction. Phys. B Phys. Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Ehiomogue, P.; Ahuchaogu, I.I.; Ahaneku, I.E. Review of adsorption isotherms models. Acta Tech. Corviniensis-Bull. Eng. 2021, 14, 87–96. [Google Scholar]
- Tuli, J.K. Nuclear Wallet Cards. 2011. Available online: https://www.nndc.bnl.gov/walletcards/doc/wallet-cards-large-2011-10.pdf (accessed on 1 October 2024).
- Tang, Y.Q.; Jarvis, K.E.; Williams, J.G. Determination of Trace Elements in 11 Chinese Geological Reference Materials by ICP-MS. Geostand. Newsl. 1992, 16, 61–70. [Google Scholar] [CrossRef]
- McDowell, W.J. Isotopic Uranium by PERALS; Reference SOP #AM-792.0; ORDELA, Inc.: Oak Ridge, TN, USA, 1994. [Google Scholar]
- del Rocío, C.-C.C.; Elena, M.-C.M.; Grisel, M.-G.C.; Aurora, M.-M.; Marusia, R.-V.; Magaly, C.-L.R. Determination of 234U and 238U Activities in Soil by Liquid Scintillation and High-Resolution Alpha Spectrometry. J. Nucl. Phys. Mater. Sci. Radiat. Appl. 2021, 8, 115–120. [Google Scholar] [CrossRef]
- Villalobos, R. Influencia Del Hierro En La Remoción de Uranio Por Membranas de Triacetato de Celulosa Con Carbón Activado. Ph.D. Thesis, Centro de Investigación en Materiales Avanzados, Chihuahua, Mexico, 2012. Available online: http://cimav.repositorioinstitucional.mx/jspui/handle/1004/690 (accessed on 26 September 2024).
- Werth, C.J.; Zhang, C.; Brusseau, M.L.; Oostrom, M.; Baumann, T. A Review of Non-Invasive Imaging Methods and Applications in Contaminant Hydrogeology Research. J. Contam. Hydrol. 2010, 113, 1–24. [Google Scholar] [CrossRef]
- Catalano, J.G.; Brown, G.E. Analysis of Uranyl-Bearing Phases by EXAFS Spectroscopy: Interferences, Multiple Scattering, Accuracy of Structural Parameters, and Spectral Differences. Am. Mineral. 2004, 89, 1004–1021. [Google Scholar] [CrossRef]
- Denecke, M.A.; Janssens, K.; Proost, K.; Rothe, J.; Noseck, U. Confocal Micrometer-Scale X-ray Fluorescence and X-Ray Absorption Fine Structure Studies of Uranium Speciation in a Tertiary Sediment from a Waste Disposal Natural Analogue Site. Environ. Sci. Technol. 2005, 39, 2049–2058. [Google Scholar] [CrossRef]
- Bargar, J.R.; Williams, K.H.; Campbell, K.M.; Long, P.E.; Stubbs, J.E.; Suvorova, E.I.; Lezama-Pacheco, J.S.; Alessi, D.S.; Stylo, M.; Webb, S.M.; et al. Uranium Redox Transition Pathways in Acetate-Amended Sediments. Proc. Natl. Acad. Sci. USA 2013, 110, 4506–4511. [Google Scholar] [CrossRef]
- Bone, S.E.; Dynes, J.J.; Cliff, J.; Bargar, J.R. Uranium (IV) Adsorption by Natural Organic Matter in Anoxic Sediments. Proc. Natl. Acad. Sci. USA 2017, 114, 711–716. [Google Scholar] [CrossRef]
- Diaz-Moreno, S.; Hayama, S.; Amboage, M.; Freeman, A.; Sutter, J.; Duller, G. I20; the Versatile X-Ray Absorption Spectroscopy Beamline at Diamond Light Source. J. Phys. Conf. Ser. 2009, 190, 012038. [Google Scholar] [CrossRef]
- Faudoa, F. Modelo de La Evolución de Las Especies Minerales Superficiales de Uranio de La Sierra Peña Blanca, Chihuahua, México. Master’s Thesis, Centro de Investigación en Materiales Avanzados, Chihuahua, Mexico, 2023. [Google Scholar]
- Dent, A.J.; Cibin, G.; Ramos, S.; Smith, A.D.; Scott, S.M.; Varandas, L.; Pearson, M.R.; Krumpa, N.A.; Jones, C.P.; Robbins, P.E. B18: A Core XAS Spectroscopy Beamline for Diamond. J. Phys. Conf. Ser. 2009, 190, 012039. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Reyes-Cortés, M.; Fuentes-Cobas, L.; Torres-Moye, E.; Esparza-Ponce, H.; Montero-Cabrera, M.E. Uranium Minerals from the San Marcos District, Chihuahua, Mexico. Mineral. Petrol. 2010, 99, 121–132. [Google Scholar] [CrossRef]
- XAS Database (XASDB). Canadian Light Source. 2024. Available online: http://xasdb.lightsource.ca (accessed on 26 September 2024).
- Cárdenas-Flores, D. Volcanic Stratigraphy and U-Mo Mineralization of the Sierra de Peña District, Chihuahua, Mexico. In Uranium Deposits in Volcanic Rocks, Proceedings of a Technical Committee Meeting on Uranium Deposits in Volcanic Rocks, El Paso, TX, USA, 2–5 April 1984; IAEA: Vienna, Austria, 1985; pp. 125–136. [Google Scholar]
- Adamson, A. A Textbook of Physical Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-12-044262-1. [Google Scholar]
- Gao, X.; Bi, M.; Shi, K.; Chai, Z.; Wu, W. Sorption Characteristic of Uranium(VI) Ion onto K-Feldspar. Appl. Radiat. Isot. 2017, 128, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Meece, D.E.; Kohler, M.; Curtis, G.P. Approaches to Surface Complexation Modeling of Uranium(VI) Adsorption on Aquifer Sediments. Geochim. Cosmochim. Acta 2004, 68, 3621–3641. [Google Scholar] [CrossRef]
- Baqer, Y.; Thornton, S.; Stewart, D.I.; Norris, S.; Chen, X. Analysis of Uranium Sorption in a Laboratory Column Experiment Using a Reactive Transport and Surface Complexation Model. Transp. Porous Media 2023, 149, 423–452. [Google Scholar] [CrossRef]
- Fuller, A.J.; Leary, P.; Gray, N.D.; Davies, H.S.; Mosselmans, J.F.W.; Cox, F.; Robinson, C.H.; Pittman, J.K.; McCann, C.M.; Muir, M.; et al. Organic Complexation of U(VI) in Reducing Soils at a Natural Analogue Site: Implications for Uranium Transport. Chemosphere 2020, 254, 126859. [Google Scholar] [CrossRef] [PubMed]
- Bès, R.; Rivenet, M.; Solari, P.-L.; Kvashnina, K.O.; Scheinost, A.C.; Martin, P.M. Use of HERFD–XANES at the U L3- and M4-Edges To Determine the Uranium Valence State on [Ni(H2O)4]3[U(OH,H2O)(UO2)8O12(OH)3]. Inorg. Chem. 2016, 55, 4260–4270. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.; Nayak, C.; Babu, P.V.; Jha, S.N.; Bhattacharyya, D. Chemical Shift of U L3 Edges in Different Uranium Compounds Obtained by X-Ray Absorption Spectroscopy with Synchrotron Radiation. Bull. Mater. Sci. 2014, 37, 643–647. [Google Scholar] [CrossRef]
- Ginderow, D. Structure de l’uranophane alpha, Ca(UO2)2(SiO3OH)2.5H2O. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1988, 44, 421–424. [Google Scholar] [CrossRef]
- Refaei, H.; Rabea, F.; Elnona, M.; Morci, A. Sorption of Uranium on Some Natural Modified Clay Mineral Deposits. Arab. Univ. J. Agric. Sci. 2019, 27, 2329–2340. [Google Scholar] [CrossRef]
- Slukovskii, Z.I.; Guzeva, A.V.; Dauvalter, V.A.; Udachin, V.N.; Denisov, D.B. Uranium Anomalies in Recent Sediments of Lakes from the Northern Part of the Murmansk Region, Arctic. Geochem. Int. 2020, 58, 1374–1378. [Google Scholar] [CrossRef]
- Carvalho, F.P.; Oliveira, J.M.; Lopes, I.; Batista, A. Radionuclides from Past Uranium Mining in Rivers of Portugal. J. Environ. Radioact. 2007, 98, 298–314. [Google Scholar] [CrossRef]
- Pérez-Reyes, V. Estudio de La Distribución de Uranio Proveniente Del Yacimiento de Peña Blanca En La Laguna Del Cuervo. Master’s Thesis, Centro de Investigación en Materiales Avanzados, Chihuahua, Mexico, 2020. [Google Scholar]
- Shang, J.; Liu, C.; Wang, Z.; Zachara, J.M. Effect of Grain Size on Uranium (VI) Surface Complexation Kinetics and Adsorption Additivity. Environ. Sci. Technol. 2011, 45, 6025–6031. [Google Scholar] [CrossRef]
- Shuey, C.; Taylor, L.; Siskin, J. Church Rock Revisited: The Tailings Spill Three Years Later. Mine Talk 1982, 2, 7–26. Available online: http://sric.org/churchrock/Church%20Rock_Mine%20Talk_1982_64.pdf (accessed on 1 October 2024).
- Soil Survey Staf Kellogg Soil Survey Laboratory Methods Manual. Soil Survey Investigations Report No. 42, Version 6.0. 2022. Available online: https://www.nrcs.usda.gov/sites/default/files/2023-01/SSIR42.pdf (accessed on 26 September 2024).
- Nimmo, J.R. Porosity and Pore Size Distribution. Encycl. Soils Environ. 2004, 3, 295–303. [Google Scholar] [CrossRef]
- Reich, T.; Drebert, J.; Boulyga, S. EXAFS Study of Uranium (VI) Sorption on Kaolinite; Speciation Techniques and Facilities for Radioactive Materials at Synchrotron Light Sources; Organization for Economic Cooperation and Development (OECD): Berkeley, CA, USA, 2004; pp. 61–67. [Google Scholar]
- Thompson, H.A.; Parks, G.A.; Brown, G.E. Spectroscopic Studies of U(VI) Sorption at the Kaolinite-Water Interface LANL Grant 9-X42-6947E-1; Los Alamos National Lab: Los Alamos, NM, USA, 1994. [Google Scholar] [CrossRef][Green Version]

| ID Column | Experiment | Carrier Solution | Retention Time (Months) |
|---|---|---|---|
| UN6 | Exploration of adsorption of dissolved uranium by sediments. | (569 mgU L−1) | 6 |
| UN12 | 12 | ||
| URP6 | Exploration of mineral transport through sediments. | Distilled water | 6 |
| URP12 | 12 |
| Sediment Sample | ID Name |
|---|---|
| Fine sand | FSD |
| Coarse silt + clay | CSC |
| Fine silt + clay | FSC |
| Coarse silt | CS |
| Fine silt | FS |
| Clay from CSC | C1 |
| Clay from FSC | C2 |
| Sample | Mineral Phase (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | Calcite | Albite | Anorthite | Sanidine | Hematite | Natrolite | Kaolinite | Montmorillonite | Uranophane | Weeksite | |
| Sand a | 42.2 | 7.6 | - | 13.5 | 25 | - | 7.1 | 4.6 | LOD | - | - |
| Silt b | 36 | - | 16 | - | 44.3 | 2 | - | 2.1 | LOD | - | - |
| Clay b | 14.6 | - | 20 | - | 24.1 | 6.3 | - | 34.4 | <1 | - | - |
| Uranium mineral b | 84 | ||||||||||
| pH | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|
| Qm/mg·kg−1 | KL/L·mg−1 | R2 | KF/(mg·g−1) (L·mg−1)1/n | 1/n | R2 | |
| 5.0 | 44.8 × 103 | 0.20 | 0.94 | 13.4 | 0.30 | 0.59 |
| 7.0 | 9.7 × 103 | 0.04 | 0.22 | 20.5 | −0.26 | 0.17 |
| UN6 | |||||
|---|---|---|---|---|---|
| Sample Type | ID Sample | 234U (Bq kg−1) | 238U (Bq kg−1) | RQ (%) | 238U mg kg−1 |
| Solid | UN6CSC | 641 ± 17 | 1015 ± 26 | 90 | 82 |
| UN6FSC | 101 ± 1 | 171 ± 2 | X | 14 | |
| UN6CS | 364 ± 7 | 796 ± 13 | 82 | 64 | |
| UN6FS | 50 ± 1 | 109 ± 2 | 67 | 9 | |
| UN6C1 | 742 ± 15 | 1573 ± 29 | 94 | 127 | |
| UN6C2 | 189 ± 3 | 329 ± 5 | 93 | 27 | |
| Supernatant solution | 234U (Bq L−1) | 238U (Bq L−1) | RQ (%) | 238U mg L−1 | |
| WUN6CSC | 0.96 ± 0.03 | 2.07 ± 0.05 | 78 | 0.17 | |
| WUN6FSC | 0.086 ± 0.003 | 0.18 ±0.01 | 58 | 0.014 | |
| UN12 | |||||
|---|---|---|---|---|---|
| Sample Type | ID Sample | 234U Bq kg−1 | 238U Bq kg−1 | RQ (%) | 238U mg kg−1 |
| Solid | UN12CSC | 1104 ± 21 | 2269 ± 40 | 90 | 184 |
| UN12FSC | 803 ± 17 | 1532 ± 30 | 85 | 124 | |
| UN12CS | 1151 ± 21 | 2222 ± 37 | 74 | 180 | |
| UN12FS | 811 ± 16 | 1810 ± 32 | 98 | 147 | |
| UN12C1 | 1816 ± 25 | 3736 ± 48 | 80 | 303 | |
| UN12C2 | 1148 ± 22 | 2149 ± 39 | 92 | 174 | |
| Supernatant solution | 234U Bq L−1 | 238U Bq L−1 | RQ (%) | 238U mg L−1 | |
| WUN12CSC | 4.5 ± 0.1 | 8.9 ± 0.2 | 58 | 0.72 | |
| WUN12FSC | 2.68 ± 0.04 | 5.5 ± 0.1 | X | 0.45 | |
| URP6 | |||||
|---|---|---|---|---|---|
| Sample Type | ID Sample | 234U Bq kg−1 | 238U Bq kg−1 | RQ (%) | 238U mg kg−1 |
| Solid | URP6CSC | 1058 ± 21 | 1124 ± 22 | 78 | 91 |
| URP6FSC | 918 ± 9 | 865 ± 9 | X | 70 | |
| URP6CS | 782 ± 17 | 772 ± 17 | 78 | 63 | |
| URP6FS | 620 ± 14 | 628 ± 14 | 81 | 51 | |
| URP6C1 | 1324 ± 24 | 1362 ± 25 | 100 | 110 | |
| URP6C2 | 1304 ± 23 | 1272 ± 23 | 79 | 103 | |
| Supernatant solution | 234U Bq L−1 | 238U Bq L−1 | RQ (%) | 238U mg L−1 | |
| WURP6CSC | 1.19 ± 0.01 | 1.29 ± 0.01 | X | 0.10 | |
| WURP6FSC | 1.30 ± 0.01 | 1.41 ± 0.02 | X | 0.11 | |
| URP12 | |||||
|---|---|---|---|---|---|
| Sample Type | ID Sample | 234U Bq kg−1 | 238U Bq kg−1 | RQ (%) | 238U mg kg−1 |
| Solid | URP12CSC | 234 ± 3 | 244 ± 3 | X | 20 |
| URP12FSC | 79 ± 1 | 82 ± 1 | X | 7 | |
| URP12CS | 253 ± 4 | 305 ± 5 | 78 | 25 | |
| URP12FS | 95 ± 2 | 99 ± 2 | 81 | 8 | |
| URP12C1 | 464 ± 7 | 451 ± 7 | 98 | 37 | |
| URP12C2 | 169 ± 3 | 172 ± 3 | 94 | 14 | |
| Supernatant solution | 234U Bq L−1 | 238U Bq L−1 | 238U mg L−1 | ||
| WURP12CSC | 0.46 ± 0.01 | 0.58 ± 0.01 | 80 | 0.05 | |
| WURP12FSC | 0.33 ± 0.005 | 0.43 ± 0.01 | X | 0.03 | |
| URP6CSC | |||||||
|---|---|---|---|---|---|---|---|
| Name | N | S02 | σ2 (Å2) | E0 (eV) | ΔR | Reff (Å) | Reff + ΔR (Å) |
| U_Oax | 2 | 1.6 ± 0.3 | 0.006 (1) | 7.4 ± 2.7 | 0.000 ± 0.009 | 1.8045 | 1.805 |
| U_Oeq1 | 1 | 1.6 ± 0.3 | 0.017 (6) | 7.4 ± 2.7 | −0.04 ± 0.02 | 2.2411 | 2.20 |
| U_Oeq2 | 2 | 1.6 ± 0.3 | 0.017 (6) | 7.4 ± 2.7 | −0.04 ± 0.02 | 2.2952 | 2.26 |
| U_Oeq3 | 2 | 1.6 ± 0.3 | 0.004 (1) | 7.4 ± 2.7 | −0.022 ± 0.013 | 2.4498 | 2.428 |
| U_Si | 1 | 1.6 ± 0.3 | 0.009 (3) | 7.4 ± 2.7 | 0.05 ± 0.03 | 3.1444 | 3.19 |
| URP6FSC | |||||||
| U_Oax | 2 | 1.35 | 0.006 (1) | 9.6 ± 2.2 | 0.019 ± 0.009 | 1.8045 | 1.824 |
| U_Oeq1 | 1 | 1.35 | 0.039 (6) | 9.6 ± 2.2 | −0.003 ± 0.05 | 2.2411 | 2.24 |
| U_Oeq2 | 2 | 1.35 | 0.039 (6) | 9.6 ± 2.2 | −0.003 ± 0.05 | 2.2952 | 2.29 |
| U_Oeq3 | 2 | 1.35 | 0.003 (1) | 9.6 ± 2.2 | 0.006 ± 0.013 | 2.4498 | 2.456 |
| U_Si | 1 | 1.35 | 0.006 (4) | 9.6 ± 2.2 | 0.06 ± 0.03 | 3.1444 | 3.21 |
| Experiment | U Conc. (mg kg−1) | Distinctiveness, Singularity, Evidence |
|---|---|---|
| Batch | 44.8 × 103 | Upper limit |
| Mud “M2” | 141 | Maximum value at the study site a |
| UN6C1 | 127 | Initial transport and adsorption in CSC |
| UN6C2 | 27 | Initial transport and adsorption in FSC |
| UN12C1 | 303 | Maximum value of adsorption in CSC |
| UN12C2 | 174 | Maximum value of adsorption in FSC |
| Tomography | ||
| URP6C1 | 110 | Mineral particles close to the horizon. Dispersion up to 55 mm |
| URP6C2 | 103 | |
| URP12C1 | 37 | Mineral particles are dispersed by liquid flow. Dispersion up to 110 mm |
| URP12C2 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Reyes, V.; Cabral-Lares, R.M.; Canche-Tello, J.G.; Rentería-Villalobos, M.; González-Sánchez, G.; Carmona-Lara, B.P.; Hernández-Herrera, C.; Faudoa-Gómez, F.; Rodríguez-Guerra, Y.; Vázquez-Olvera, G.; et al. Uranium Mineral Transport in the Peña Blanca Desert: Dissolution or Fragmentation? Simulation in Sediment Column Systems. Appl. Sci. 2025, 15, 609. https://doi.org/10.3390/app15020609
Pérez-Reyes V, Cabral-Lares RM, Canche-Tello JG, Rentería-Villalobos M, González-Sánchez G, Carmona-Lara BP, Hernández-Herrera C, Faudoa-Gómez F, Rodríguez-Guerra Y, Vázquez-Olvera G, et al. Uranium Mineral Transport in the Peña Blanca Desert: Dissolution or Fragmentation? Simulation in Sediment Column Systems. Applied Sciences. 2025; 15(2):609. https://doi.org/10.3390/app15020609
Chicago/Turabian StylePérez-Reyes, Victoria, Rocio M. Cabral-Lares, Jesús G. Canche-Tello, Marusia Rentería-Villalobos, Guillermo González-Sánchez, Blanca P. Carmona-Lara, Cristina Hernández-Herrera, Fabián Faudoa-Gómez, Yair Rodríguez-Guerra, Gregorio Vázquez-Olvera, and et al. 2025. "Uranium Mineral Transport in the Peña Blanca Desert: Dissolution or Fragmentation? Simulation in Sediment Column Systems" Applied Sciences 15, no. 2: 609. https://doi.org/10.3390/app15020609
APA StylePérez-Reyes, V., Cabral-Lares, R. M., Canche-Tello, J. G., Rentería-Villalobos, M., González-Sánchez, G., Carmona-Lara, B. P., Hernández-Herrera, C., Faudoa-Gómez, F., Rodríguez-Guerra, Y., Vázquez-Olvera, G., Carrillo-Flores, J., Reyes-Cortés, I. A., Hernández-Cruz, D., Loredo-Portales, R., & Montero-Cabrera, M. E. (2025). Uranium Mineral Transport in the Peña Blanca Desert: Dissolution or Fragmentation? Simulation in Sediment Column Systems. Applied Sciences, 15(2), 609. https://doi.org/10.3390/app15020609





