Abstract
Dementia, including Alzheimer’s disease, is a neurodegenerative illness characterized by the progressive impairment of cognitive functions, posing a significant global health threat. Physical exercise is widely recognized for its preventive role, providing benefits for both the body composition and brain health. This study aimed to explore the relationship between physical exercise, the body composition, and the progression of dementia. The analysis used clinical and neuroradiology data from 42 patients enrolled in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Our study mainly focused on crucial parameters such as the body mass index (BMI), skeletal muscle index (SMI), and MRI biomarkers, including the hippocampal volume and white matter integrity. We grouped the participants according to the similarities of their body compositions through clustering techniques. Then, atrophy-related changes in the brain structures were computed using the Kullback–Leibler divergence. Our findings suggest that a higher BMI and greater muscle mass may slow down brain atrophy, suggesting a protective effect on the brain. Based on these results, preserving muscle mass and metabolic health through resistance and aerobic exercise appears crucial in reducing the risk of dementia. Body composition interventions may slow neurodegenerative changes and promote brain health. This is an essential piece of information about prevention strategies, especially for individuals at risk of dementia who may benefit from following structured physical activity strategies.
1. Introduction
Physical activity is widely acknowledged as a key factor in preventing and managing neurodegenerative conditions such as dementia and Alzheimer’s disease. Among these, Alzheimer’s disease (AD) represents the most prevalent neurodegenerative disorder, characterized by progressive cognitive impairment and the eventual development of dementia [1]. Epidemiological studies have consistently demonstrated that regular physical activity delays the progression of dementia-related symptoms and enhances cognitive performance [2,3]. Evidence from randomized controlled trials indicates that aerobic exercise improves mental performance in individuals with Alzheimer’s disease, particularly in domains such as working memory and spatial abilities, while also promoting neuroplasticity in brain regions, including the hippocampus [4,5]. Furthermore, research suggests that sustained physical activity mitigates degenerative alterations in the white matter integrity among older adults. This effect is particularly evident in APOE-4 allele carriers, a genetic variant associated with a higher risk of developing Alzheimer’s disease [6].
The body mass index (BMI) and body composition, intrinsically linked to physical activity levels, also represent significant determinants of dementia risk. Empirical evidence indicates that individuals with a higher BMI and greater fat mass are at an elevated risk of cognitive impairment and neurodegeneration. The findings from [7] highlight that individuals with an elevated BMI and increased body fat percentages often exhibit reduced levels of physical activity, further exacerbating their susceptibility to metabolic and neurodegenerative disorders [8,9]. Conversely, studies suggest that overweight or obese individuals who engage in regular physical activity experience notable cognitive benefits, likely attributable to enhanced cerebral perfusion and reduced systemic inflammation, both of which are exercise-induced effects [10,11].
The evidence further suggests that low muscle and high fat masses are linked to diminished cognitive function, likely due to the metabolic and inflammatory dysregulation associated with such a body composition [12,13,14]. The European Review for Medical and Pharmacological Sciences findings emphasize that enhancing the body composition through regular physical activity, particularly by increasing muscle mass and reducing adiposity, may be a critical factor in dementia prevention. This improvement supports better metabolic health and enhances neurovascular function, potentially delaying the onset and progression of neurodegenerative conditions [15].
Our study aimed to evaluate the relationship between physical activity and the progression of dementia by employing clustering techniques and a Kullback–Leibler divergence analysis. Applied to MRI data, these methods enabled the identification of structural brain changes, such as hippocampal atrophy and reduced white matter integrity, associated with varying levels of physical activity.
2. Materials and Methods
2.1. Method Overview
This examination was concerned with determining the association between physical exercise and the body composition and how dimensional adjacencies impact the progression of dementia using clustering mechanisms and probabilistic divergence measures. To this end, an integrated methodological framework was applied to combine clinical, demographic, and neuroimaging datasets to understand these interrelationships better. The data were sourced from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a publicly accessible resource offering high-resolution MRI scans and clinical evaluations. The key variables included the following:
- –
- Body Mass Index (BMI): Calculated as the weight in kilograms divided by the height in square meters, the BMI is a standard measure for assessing the body composition and classifying individuals into weight categories.
- –
- Skeletal Muscle Index (SMI): Defined as the skeletal muscle mass normalized by the height squared, the SMI provides a standardized metric for evaluating muscle mass relative to the body size. The complementary nature of the BMI and SMI enabled a detailed analysis of the influence of the body composition on neurodegenerative processes, particularly dementia progression.
These parameters were selected for their relevance in differentiating physical profiles and their potential to elucidate the relationship between the body composition and health outcomes.
Data preprocessing included z-score normalization to ensure comparability, multiple imputations to address missing data, and generating probability distributions for neuroimaging biomarkers, such as the hippocampal volume and white matter integrity. Clustering methods were applied to stratify participants based on the BMI and SMI, facilitating the identification of subgroups with distinct physical profiles. The k-means algorithm, optimized through k-means++ initialization to improve the centroid placement and convergence, was chosen for its robustness and computational efficiency in handling continuous variables like the BMI and SMI. To determine the optimal number of clusters, the elbow method was used to plot the within-cluster sum of squares (WCSS) against the number of clusters, identifying the point of diminishing returns for optimal data compactness and interpretability. The resulting clusters provided a foundation for exploring the association between physical profiles and neuroimaging outcomes.
To further analyze inter-cluster differences, the Kullback–Leibler divergence was employed as a measure of dissimilarity between the probability distributions of neuroimaging biomarkers across clusters. This probabilistic approach quantified variations in the brain structure and elucidated the potential role of physical activity in modulating neurodegenerative changes.
Cross-validation techniques were used to test the robustness of clustering results, while external metrics such as the Adjusted Rand Index assessed the cluster stability. Differences in the neuroimaging and clinical outcomes across clusters were statistically evaluated using Analysis of Variance (ANOVA) and post hoc tests for the rigorous interpretation of findings. Statistical analyses were conducted using Python version 3.10.11, incorporating the Pandas, NumPy, and SciPy libraries, as well as Statistica version 13.3.0, developed by TIBCO Software Inc. (Palo Alto, CA, USA) [16].
2.2. Dataset Characteristics
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset is a publicly accessible resource available through the ADNI website [17]. This dataset provides a comprehensive collection of data on individuals involved in Alzheimer’s disease research, encompassing healthy controls, individuals with mild cognitive impairment (MCI), and patients diagnosed with Alzheimer’s disease. The dataset includes high-resolution neuroimaging data (such as structural MRI scans), clinical assessments (including cognitive and diagnostic tests), and demographic information (e.g., age, gender, and education level). The present dataset has been painstakingly preprocessed for research purposes, handling missing values and converting categorical variables into numerical representations to enhance data analysis capabilities. Each subject is assigned a unique anonymous ID to ensure participant privacy and allow for the longitudinal evaluation of change over time and across visits and assessments. The ADNI dataset provides a tool for scientific research, leading to a better understanding of the progression of Alzheimer’s disease and its relationship with demographic, clinical, and imaging markers.
The dataset encompasses a variety of features that provide comprehensive information about the health status of participants. These include the following:
- –
- Magnetic Resonance Imaging (MRI) data that provide high-resolution scans of the brain, visualizing its structure and the detailed anatomical regions used to assess the brain volume, cortical thickness, and other neurodegenerative changes characteristic of Alzheimer’s disease;
- –
- The body mass index (BMI), calculated as the weight in kilograms divided by the height in square meters, which is a standard measure for assessing the body composition and classifying individuals into weight categories, including underweight, normal weight, overweight, and obese;
- –
- Muscle mass estimates (1—low; 2—high) that refer to inferred values derived from physical examinations, imaging proxies, or other relevant clinical indicators, as direct muscle mass measurements are not explicitly provided;
- –
- Demographic characteristics such as age, gender, education, and ethnicity enable analyses of how these factors influence the progression of Alzheimer’s disease;
- –
- Clinical assessments that include cognitive tests, medical histories, and diagnostic evaluations offer valuable insights into participants’ mental health and the severity of neurodegenerative conditions.
Our analysis utilized data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), focusing on a cohort of 42 participants who underwent repeated MRI assessments. By comparing the initial and follow-up scans, we aimed to quantify structural brain alterations and explore their relationship with activity factors, including the BMI and muscle mass estimates. This cohort comprised 24 men and 18 women, ensuring a comparable distribution between sexes.
As the ADNI dataset has been meticulously curated and maintained, the subset used in our analysis contained no missing values. Therefore, the need for multiple imputations or other techniques to handle missing data was eliminated. Moreover, the ADNI dataset has been developed and maintained through collaboration with neurologists and neuroscientists, ensuring the clinical relevance and accuracy of the data. Detailed documentation regarding the data collection procedures, including the completeness of the dataset, can be found in the ADNI documentation [18]. Consequently, the integrity of our analysis was preserved, as the results were based on fully available data, ensuring that no potential biases were introduced through imputation methods.
Participants were selected based on the availability of longitudinal neuroimaging data and comprehensive clinical assessments pertinent to our research objectives. The inclusion criteria encompassed individuals aged between 62 and 90 years, with varying cognitive statuses ranging from cognitively normal to a mild cognitive impairment (MCI) and mild Alzheimer’s disease (AD). The exclusion criteria included significant neurological conditions other than AD, contraindications to MRI, and severe systemic illnesses that could confound neuroimaging results [18].
Our study specifically aimed to assess the impact of physical activity on neurodegenerative changes. Therefore, our analyses did not include variables not directly related to physical activity. Nonetheless, we acknowledge that factors such as age, sex, family history, educational background, and comorbidities can influence dementia progression. The analysis of the demographic characteristics of our cohort confirmed a balanced sex distribution and a comparable age range, contributing to the generalizability of our findings.
2.3. Clustering Analysis
Clustering algorithms are essential in unsupervised machine learning, enabling the grouping of data points based on patterns or inherent structures without predefined labels [19]. This study used clustering to identify subgroups within the dataset that shared similar physical and neuroimaging characteristics. In principle, all clustering methods differ, with unique approaches and assumptions. Yet, most clustering techniques can also be tuned to different datasets with the relevant preprocessing and careful adjustments of parameters. Clustering analysis can be divided into four main approaches:
- Hierarchical clustering;
- Density-based clustering;
- Probabilistic clustering;
- Partitioning clustering.
Hierarchical clustering forms one nested hierarchy of clusters using agglomerative (bottom-up) or divisive (top-down) strategies, enabling data analysis at many different granularity levels. Such an approach makes the DBSCAN effective for datasets with non-linear cluster boundaries, though its performance can degrade with varying cluster densities [20].
Density-based methods such as the DBSCAN (Density-Based Spatial Clustering of Applications with Noise) group points based on dense regions in the feature space, identifying clusters of arbitrary shapes while isolating noise as outliers. This makes the DBSCAN effective for datasets with non-linear cluster boundaries, though its performance can degrade with varying cluster densities [21]. Model-based clustering assumes that data are generated from a mixture of probability distributions, often Gaussian, and seeks to optimize these models to represent the data best. This approach accommodates overlapping clusters but relies on accurate model assumptions [22].
Probabilistic methods assume that data points are generated from a mixture of probability distributions, typically Gaussian. The clustering process involves estimating distribution parameters to represent the dataset accurately. The Expectation–Maximization (EM) algorithm is an example of a probabilistic approach. It alternates between two steps: the Expectation (E) step, where data points are probabilistically assigned to clusters based on current model estimates, and the Maximization (M) step, where the distribution parameters are updated to maximize the likelihood of the observed data.
Partitioning methods such as the k-means direct the data into a fixed number of clusters by minimizing the within-cluster sum of squares; the spatial compactness of the resulting clusters ensures that the partition is suitable. Due to its effectiveness and simplicity, the k-means is commonly used in applications dealing with large continuous numerical datasets. However, the algorithm operates on the assumption of spherical clusters of an equal size. It is susceptible to the initial placement of centroids, a limitation that can be alleviated through advanced initialization methods such as k-means++ [23]. Despite these limitations, the scalability and interpretability of the k-means make it a popular choice in many domains, including medical and health-related research.
K-means clustering was selected for this study due to its ability to efficiently handle the continuous features: the body mass index (BMI) and skeletal muscle index (SMI). The algorithm’s reliance on minimizing within-cluster variance aligned group participants based on similar physical characteristics. The k-means++ initialization method enhanced the algorithm’s robustness by reducing the sensitivity to random centroid initialization, ensuring more consistent convergence.
The k-means algorithm operates by iteratively partitioning the dataset , comprising n data points in k clusters . The algorithm minimizes the within-cluster sum of squares (WCSS) to optimize the compactness of the clusters, with the objective function defined as Formula (1) [24]:
where: represents the centroid of cluster , computed as the mean of all data points assigned to that cluster according to Formula (2):
The algorithm alternates between two steps:
- –
- Assigning each data point, , to the nearest cluster centroid based on the Euclidean distance;
- –
- Updating the cluster centroids by recalculating the mean of all assigned points.
The process iterates until the centroids stabilize or a predefined maximum number of iterations is reached.
This study’s clustering was based on two key physical characteristics: the BMI and SMI. The BMI, calculated as the weight in kilograms divided by the height in square meters, is a standard measure used to assess the body composition and categorize individuals into underweight, normal weight, overweight, and obese groups. The skeletal muscle mass index (SMI) is a measure that has been defined as the skeletal muscle mass normalized by the height squared; it is a standard measure of muscle mass relative to the body size and provides information regarding the body composition. This cluster of features was chosen because of its differentiating use in physical profiles and possible consequences in health outcomes, including dementia progression.
Normalization was performed using z-score transformation, standardizing the features to have a mean of zero and a standard deviation of one to ensure comparability between the BMI and SMI, as depicted in Formula (3):
where is the feature value, is the mean, and is the standard deviation.
The normalization step was applied as k-means clustering relies on the Euclidean distance, which is sensitive to the scale of the features.
The optimal number of clusters k was determined using the elbow method. This method involves plotting the WCSS for different values of k and identifying the point where the rate of decrease slows significantly, indicating a balance between compactness and interpretability. The silhouette score was calculated to further validate the optimal number of clusters, providing an additional measure of cluster separation and cohesion. Based on this analysis, different cluster schemas were selected, each representing distinct patterns in the BMI and SMI. These schemas provided a meaningful stratification of participants, facilitating the subsequent analysis of their relationship with dementia progression.
2.4. Kullback–Leibler Divergence Measure
The Kullback–Leibler (KL) divergence was first introduced in [25]. It is also known as the Kullback–Leibler distance, cross-entropy, information divergence, and information for discrimination [26]. The Kullback–Leibler (KL) divergence measures the difference between two probability distributions, i.e., it quantifies how one distribution, P, diverges from another, Q [27,28,29]. The KL divergence is defined as shown in Equation (4).
where represents the probability of observing a specific feature (e.g., hippocampal volume) in the target distribution, and denotes the corresponding probability in the reference distribution.
The KL divergence quantifies the relative entropy between these distributions, with larger values indicating more significant divergence. The measure is non-negative (), with a zero value when the distributions are identical. It is asymmetric, meaning that , which emphasizes the importance of choosing an appropriate reference distribution. Unlike summary metrics such as the mean or variance, the KL divergence evaluates the entire shape of the distributions, capturing nuanced variations in atrophy patterns across groups. This comprehensive evaluation was particularly relevant for detecting the effects of physical activity on brain structures like the hippocampus, where the impact may manifest as small but significant differences in atrophy progression.
The distributions were extracted from preprocessed MRI scans, where voxel intensities or regional volumes were normalized to create valid probability distributions that summed to one. In the context of this study, the KL divergence was applied to analyze the following:
- (A)
- The intersubject variability;
- (B)
- The intrasubject variability.
(A) The intersubject variability was analyzed to evaluate differences in hippocampal atrophy distributions between groups with varying physical activity levels. The target distribution, P, represented individuals with higher physical activity levels, while the reference distribution, Q, corresponded to sedentary individuals. By computing , the divergence highlighted the extent to which physical activity mitigated brain atrophy.
(B) The intrasubject variability was analyzed to compare MRI images of the same patient to analyze dementia progression and track how the divergence between active and passive groups evolved. The target distribution, P, represented the current state of the brain (the MRI taken at a later time point). In contrast, the reference distribution, Q, corresponded to the baseline state of the brain (the MRI taken at an earlier time point). A higher value indicated greater divergence, suggesting more significant structural changes, which may correspond to disease progression. Conversely, a lower value indicated minimal divergence, suggesting stability in the brain structure over time.
As a result, our study offers more profound insights into the temporal dynamics of dementia progression and neuroprotection.
3. Results and Discussion
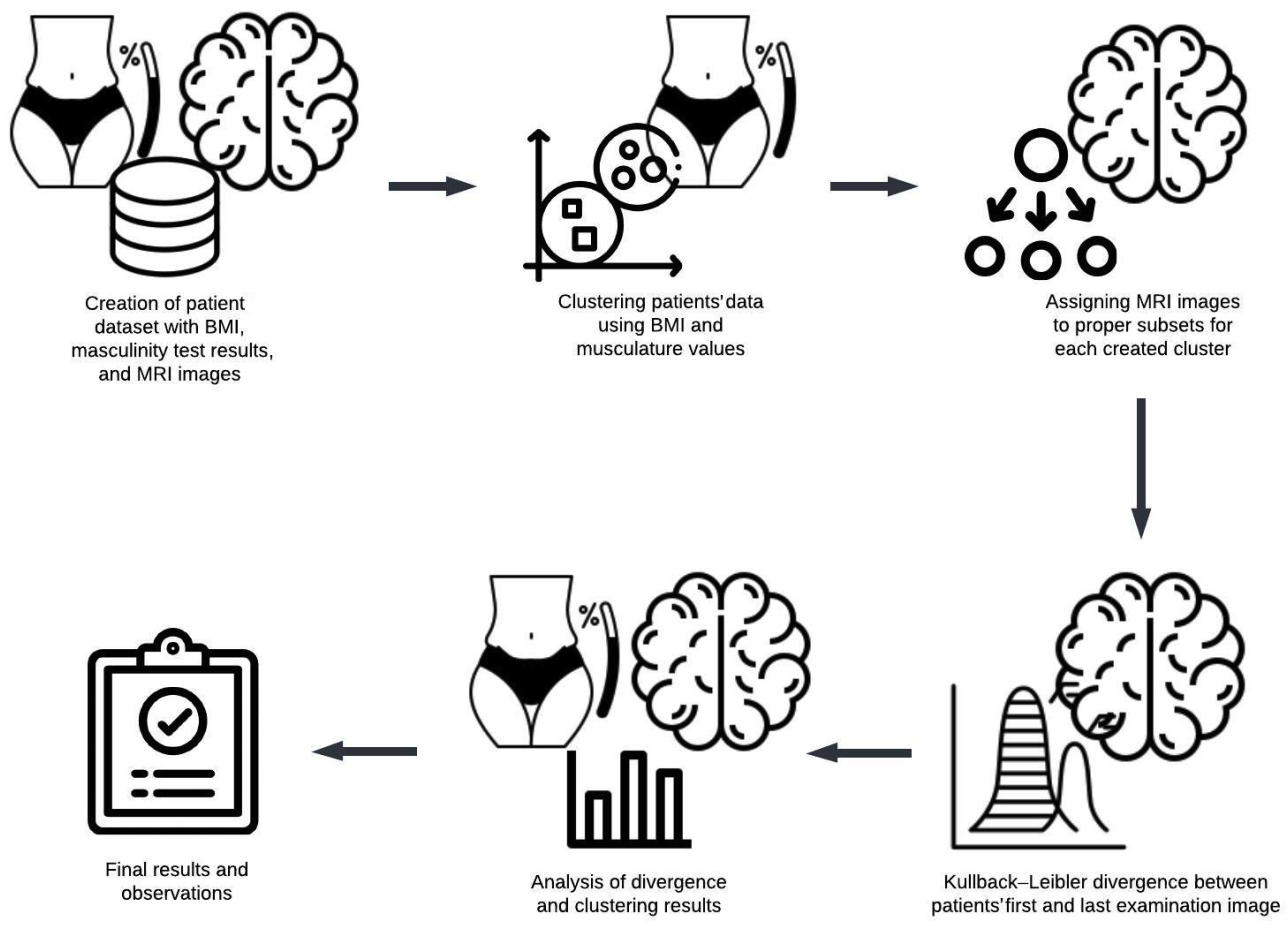
The following methodological steps (Figure 1) were carried out to examine the relationship between the body mass index (BMI) values and brain alterations observed in MRI scans:
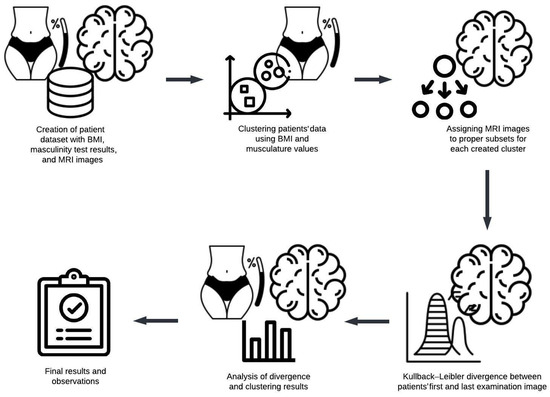
Figure 1.
An overview of the experimental research process and methodology.
- Initially, a dataset comprising patients who underwent body weight, height, and musculature measurements was curated. To be included, patients were required to have undergone multiple assessments, with the initial examination indicating minimal changes and subsequent assessments reflecting advanced stages of dementia or Alzheimer’s disease.
- Cluster analysis was performed using numerical data, including calculated BMI values and muscularity scores, forming up to five distinct clusters.
- The Kullback–Leibler divergence was computed separately for each patient’s first and final MRI images.
- The mean Kullback–Leibler divergence value within each cluster was calculated to evaluate whether participants’ physical characteristics corresponded to the progression of brain changes.
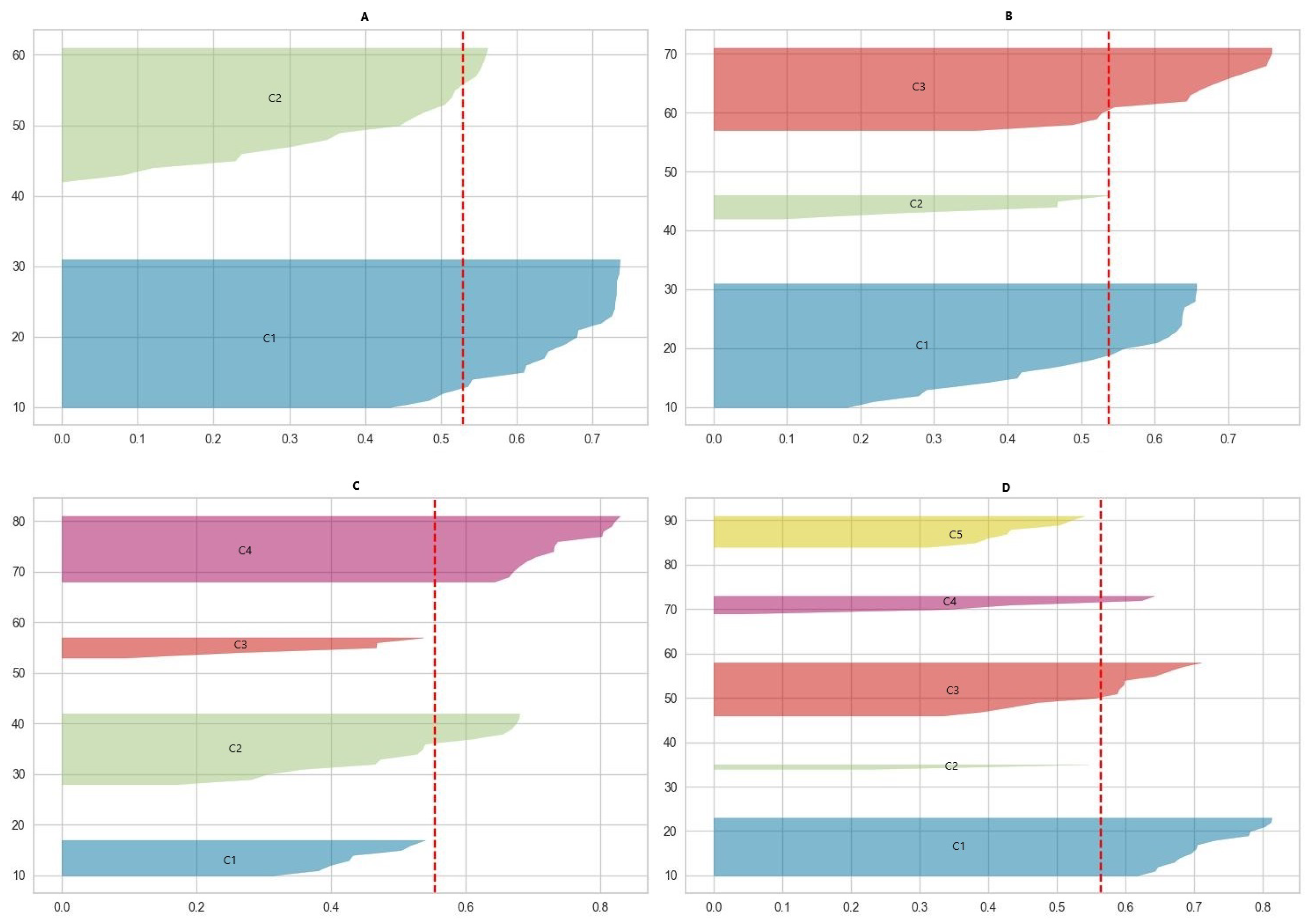
This study employed a cluster analysis on numerical data, focusing on BMI and muscle test scores, to explore patterns across different cluster configurations. The elbow method and the silhouette score were utilized to determine the optimal number of clusters. The scores were computed for clusters of two, three, four, and five. The results of the silhouette score are displayed in Figure 2 and Table 1.
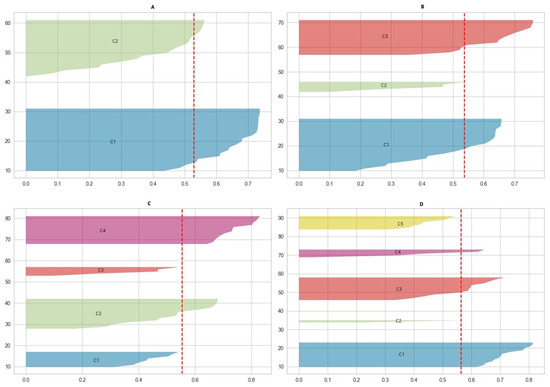
Figure 2.
The results of the silhouette score in different clustering schemas ((A)—two clusters; (B)—three clusters; (C)—four clusters; (D)—five clusters). The red dashed line represents the average silhouette score for each schema.

Table 1.
Silhouette scores for clustering schemas with two, three, four, and five clusters.
When analyzing the silhouette score in relation to the overall average score of the entire dataset, the optimal number of clusters was two, as these clusters were the only ones with a maximum score exceeding the dataset’s overall average. For three clusters, one cluster’s score was equal to the average. In the case of four clusters, the scores for two were close enough to warrant consideration. However, when examining five clusters, the scores for two were too low to be significant.
The results are presented in Table 2 for two clusters, Table 3 for three clusters, and Table 4 for four clusters. Each table details the results for individual participants, with the average values for each cluster summarized in the final row.

Table 2.
Overview of BMI, muscle test values (1—low; 2—high), and Kullback–Leibler divergence results, including average/mean values calculated across two clusters.

Table 3.
Overview of BMI, muscle test values (1—low; 2—high), and Kullback–Leibler divergence results, including average values calculated across three clusters.

Table 4.
Overview of BMI, muscle test values (1—low; 2—high), and Kullback–Leibler divergence results, including average values calculated across four clusters.
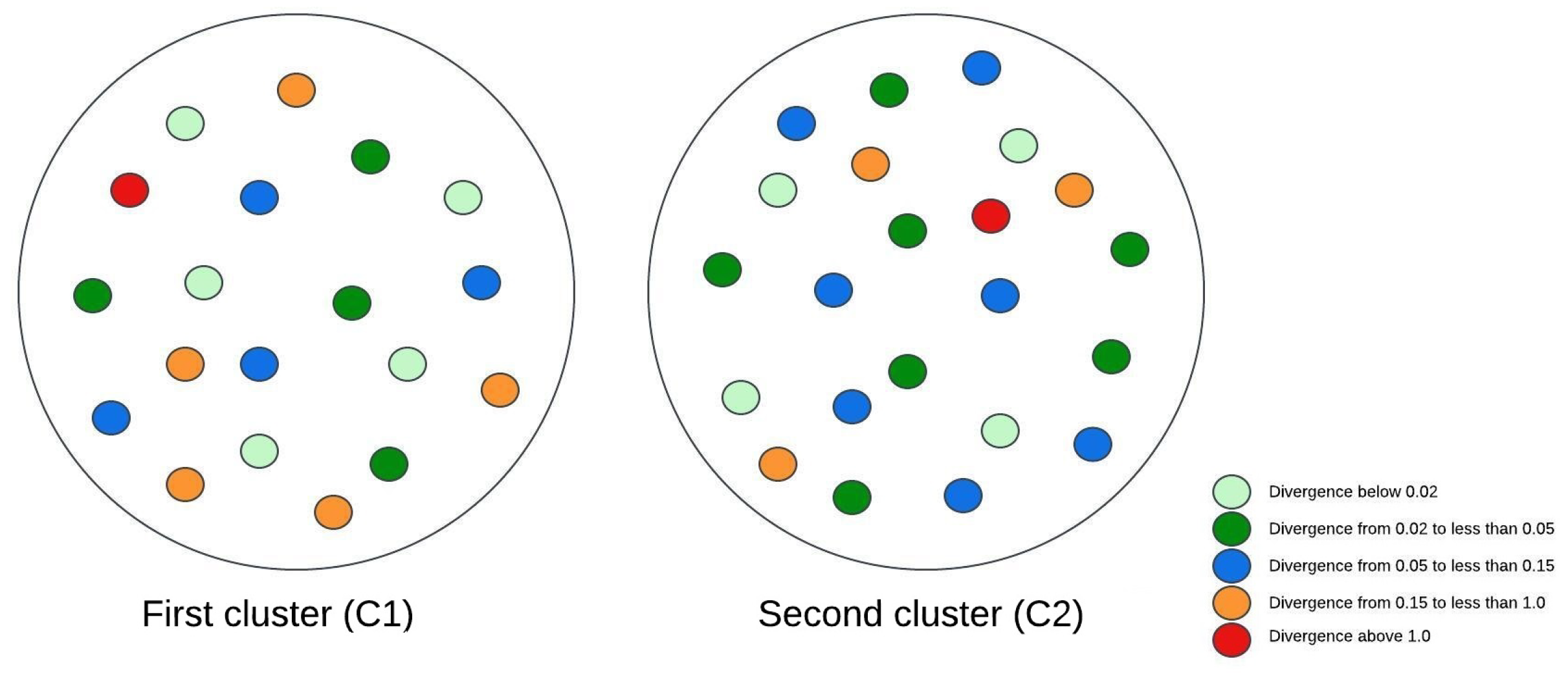
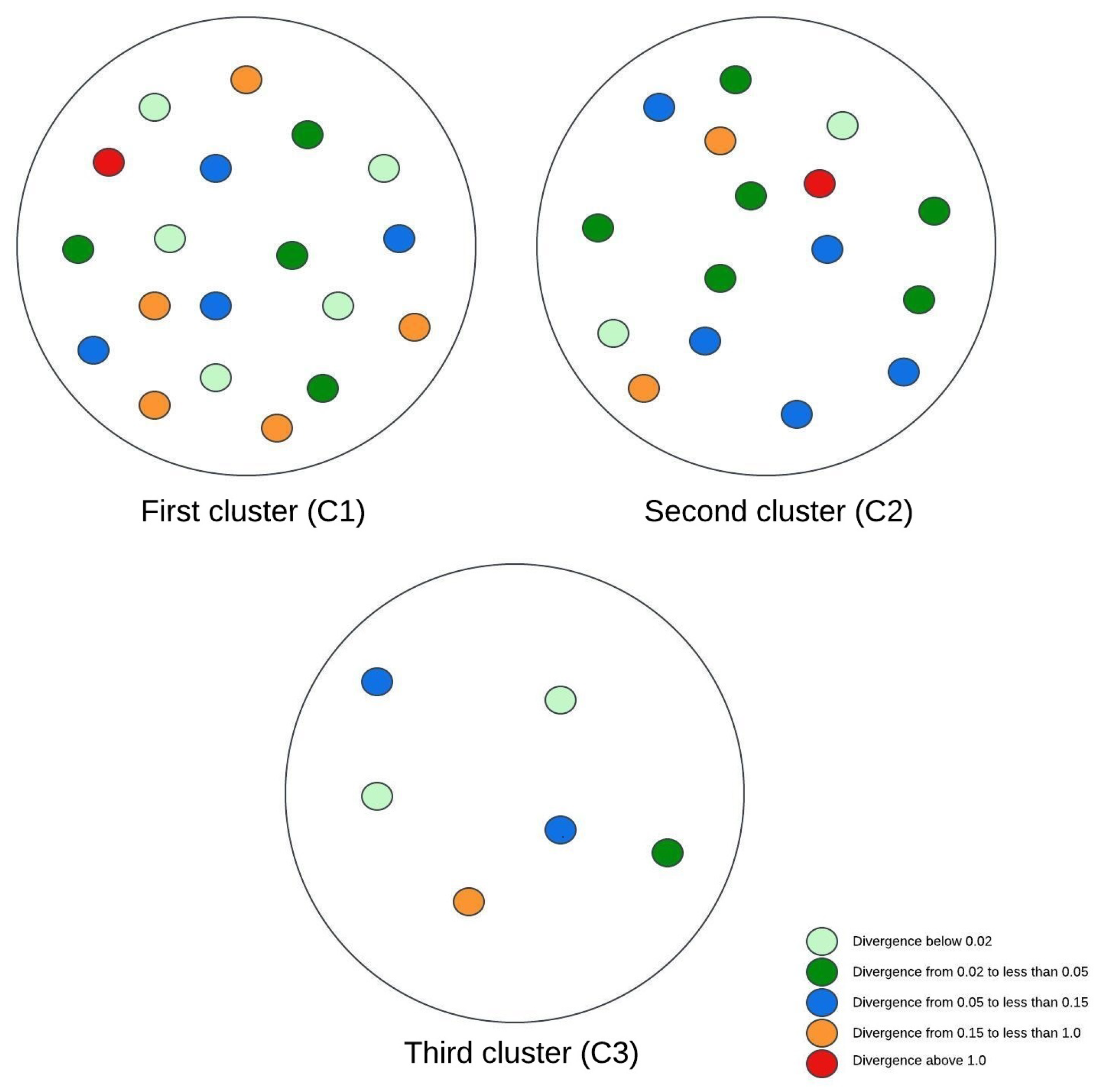
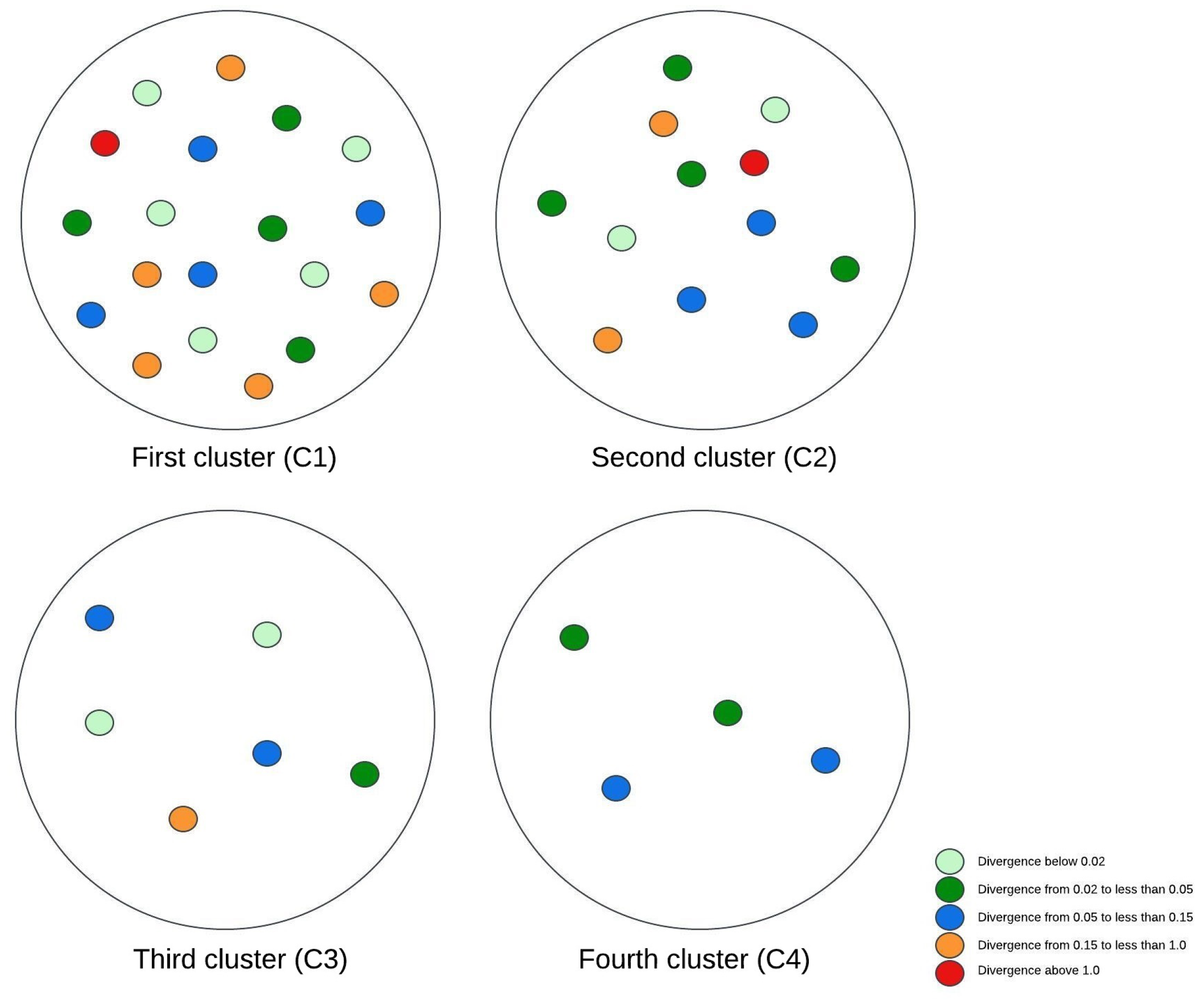
Figure 3, Figure 4 and Figure 5 illustrate visual representations of the clusters. Each point represents an individual participant, with the color intensity corresponding to the calculated Kullback–Leibler divergence value. The results were categorized into five groups based on the divergence values:
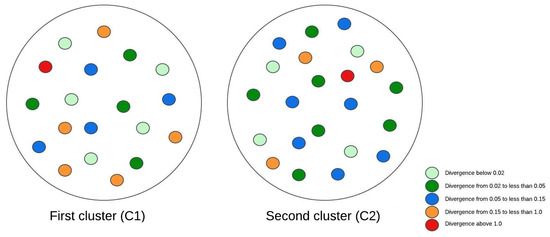
Figure 3.
The visual representation of the experimental research results for two clusters.
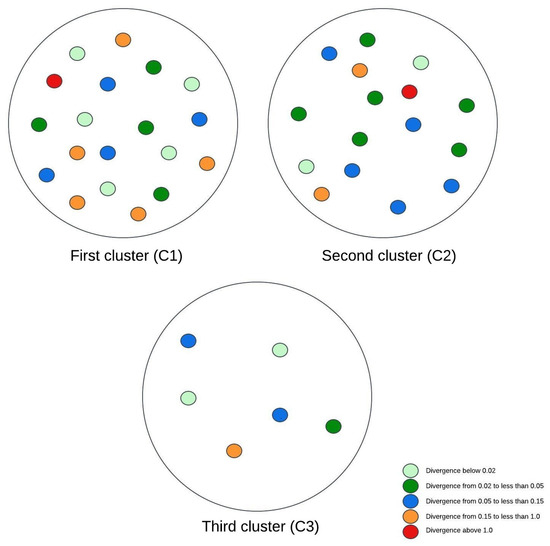
Figure 4.
The visual representation of the experimental research results for three clusters.
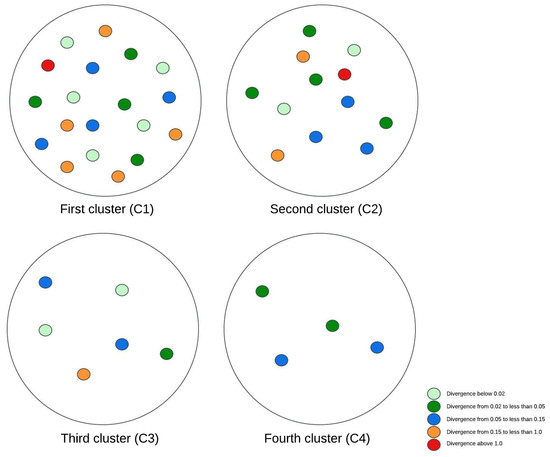
Figure 5.
The visual representation of the experimental research results for four clusters.
- -
- Divergence values below 0.02 are represented in light green;
- -
- Divergence values ranging from 0.02 to less than 0.05 are shown in dark green;
- -
- Divergence values between 0.05 and 0.15 are depicted in blue;
- -
- Divergence values from 0.15 to less than 1 are marked in orange;
- -
- Divergence values equal to or exceeding one are highlighted in red.
The comparison of patients grouped into two clusters revealed that individuals with a higher body mass index (BMI) exhibited fewer brain changes over time. Moreover, these individuals achieved higher scores on muscle strength assessments. However, the results from the two-cluster analysis provided limited insights, as dividing patients into only two groups did not allow for detailed conclusions. Nonetheless, a consistent subgroup of patients was identified from the beginning: this group exhibited the lowest average body mass index (BMI) and predominantly achieved a physical test score of 1.
When the data were analyzed using a three-cluster division, the first cluster remained consistent with the grouping from the prior two-cluster analysis. The second cluster included the patients with the highest BMI values, who also displayed elevated muscle tissue indices. Comparing the mean Kullback–Leibler divergence values between clusters revealed no statistically significant differences between the lowest and average BMI groups. In contrast, a considerable difference emerged when these two groups were compared with the third cluster, indicating that patients with higher BMI values experienced slower disease progression. To sum up, the analysis of three clusters indicated that patients within the same cluster consistently exhibited higher BMI values and superior muscle test scores. These findings suggest a negative correlation between the BMI and the degree of brain changes.
The final methodology involved extending the cluster analysis to create four distinct clusters. It is worth noticing that the subgroups of patients with the lowest BMI and low muscle scores and those with a higher BMI and superior muscle scores remained consistent with previous cluster schemas. One of the new clusters comprised patients with an average BMI of 28.59 and a muscle index of 2, while individuals with a relatively higher BMI, originally part of the second cluster, were separated to form the fourth cluster. According to the Kullback–Leibler divergence measure, this newly formed fourth cluster exhibited the most favorable outcomes, with participants showing the lowest degree of brain tissue changes. In contrast, participants assigned to the second cluster demonstrated the most unfavorable outcomes.
The classification of patients into separate subgroups showed that those with a moderate BMI and less physical activity exhibited the most significant differences in the brain structure. These observations endorse the fact that the combined state of a lower BMI and reduced muscle mass is the least beneficial condition for brain changes that occur in dementia or Alzheimer’s disease.
This observation corroborates earlier findings on having excess weight, which could reduce the mortality risk in old age through protective influences against osteoporotic fractures and cognitive decline. Furthermore, a higher body weight may serve as an energy reserve, protecting against protein–energy malnutrition [30]. The concept of “frailty” pertains to the overall health status of older adults. Frailty is theoretically characterized as a clinically identifiable condition marked by heightened vulnerability due to age-related declines in physiological reserves and function across multiple systems, which impair the ability to manage routine or acute stressors [31]. Studies have proposed that excess body weight may protect against mortality in individuals experiencing moderate or severe frailty [32].
The comparison of results across clusters indicated a positive association between higher body mass index (BMI) scores and a slower progression of structural brain changes. It should be noted that increased physical activity may lead to weight gain during specific lifestyle transitions, particularly following an athletic career, potentially resulting in elevated BMI values [33]. Considering this alongside the longitudinal trajectory of brain changes, individuals with a higher BMI in older age may have engaged in more excellent physical activity during their youth compared to those with lower BMI values. The findings suggest that older adults with higher BMI values may have been more active in physical activity during their youth. This hypothesis is reinforced by evidence that resistance training significantly influences the body composition and muscle strength in older adults [34]. Since muscle tissue is denser than fat, a higher muscle mass can result in an elevated BMI [35]. Within this framework, a higher BMI in older adults is more advantageous than a particularly low BMI.
The controversy and debate surrounding the claim that a higher BMI in late life is associated with a decreased likelihood of developing dementia among older adults is acknowledged. However, there is a paucity of studies that directly address this issue. A study involving nearly two million individuals in the UK found that the risk of dementia decreases as the body mass index (BMI) increases in older age. The findings indicated that individuals with a BMI above 40 kg/m2 exhibited a 29% lower risk of dementia compared to those with a normal BMI. Furthermore, the risk of dementia was observed to be highest in underweight individuals (BMI < 20 kg/m2), who demonstrated a 34% higher risk compared to individuals with a normal BMI. The underlying mechanisms that precipitate these observations may be associated with the energy reserve provided by a higher BMI, particularly among older adults, who are susceptible to losing muscle mass and body fat [36].
Additionally, the impact of a higher BMI on reducing the risk of malnutrition and sarcopenia, which are strongly associated with cognitive decline, could be a contributing factor. Furthermore, the BMI in older age may serve as a marker of overall metabolic health, which could offer a protective effect on the brain. This assertion is further substantiated by the findings of Qizilbash et al. [36], who conducted a retrospective cohort study encompassing two million individuals over a two-decade period. Their research, published in the Lancet Diabetes Endocrinol, revealed that the BMI is associated with a reduced risk of dementia.
As indicated by the authors of article [37], a correlation between the BMI and the risk of developing dementia has been identified, particularly in the context of weight fluctuations across various stages of life. The epidemiological studies presented demonstrate that a high BMI in middle age is associated with an increased risk of dementia, while an elevated BMI in later life is linked to a reduced risk of dementia. Conversely, weight loss or a low BMI in later life is associated with an elevated risk of developing dementia. This observation suggests a potentially protective role for a higher BMI in late life. Considering the study by Tessier et al. [38], it is noteworthy that their findings indicate that low levels of muscle mass, independent of muscle strength, are associated with an accelerated decline in executive function in elderly individuals. This association remained significant even after controlling for variables such as the body fat percentage, hand grip strength, and level of physical activity. These findings offer a potential explanation for the seemingly “contradictory” observations reported in the literature regarding the relationship between the BMI and cognitive function. A low index of muscle mass (ALM) has been associated with a decline in executive function, which encompasses abilities such as problem-solving, organization, and working memory, functions that are crucial to maintaining independence in daily activities among older adults. In the context of this study, weight loss, including the loss of muscle mass, can be a risk marker for accelerated cognitive decline. The authors identified potential mechanisms to explain the association of a high BMI with cognitive function:
- –
- Higher body fat levels may provide an energy reserve, which is particularly important for older people at a higher risk of malnutrition and sarcopenia;
- –
- Skeletal muscles secrete myokines (e.g., BDNF), which may benefit cognitive function by promoting brain health;
- –
- A higher BMI may help reduce oxidative stress and chronic inflammation, both of which are associated with sarcopenia and dementia.
Hainer and Aldhoon-Hainerová, in article [39], described the concept of the obesity paradox, which refers to the phenomenon in which a higher body mass index (BMI) in late life is associated with lower mortality and better health outcomes, even though obesity is considered a significant risk factor for cardiovascular disease, diabetes, and other metabolic conditions. The obesity paradox indicates that in the elderly, overweight and moderate obesity may have a protective effect on health and prolong survival compared to normal-weight individuals. This phenomenon has been documented in a number of chronic diseases, including coronary heart disease, heart failure, hypertension, stroke, and type 2 diabetes. Importantly, studies demonstrate that this protective effect is especially pronounced in older people, who may benefit from a higher BMI, particularly in terms of avoiding malnutrition and sarcopenia (the loss of muscle mass). These conditions are strongly associated with cognitive decline, underscoring the greater risk of malnutrition and weight loss in older people compared to moderate obesity. A lower BMI in the elderly may be an indication of underlying malnutrition, which is associated with accelerated cognitive decline and an elevated risk of dementia. However, it is essential to note that the BMI is a flawed indicator. It does not consider the fat to fat-free mass ratio, nutritional status, cardiorespiratory fitness level, and fat distribution. Research by Hainer and Aldhoon-Hainerová [39] suggests that the body composition, rather than the BMI alone, is the key protective factor. Specifically, high muscle mass and favorable fat distribution (i.e., less visceral fat and more subcutaneous fat) may reduce the risk of dementia.
As demonstrated by the preceding studies, the relationship between the BMI and cognitive health can vary according to age. While being overweight in middle age is frequently linked to an elevated risk of chronic diseases, a moderately elevated BMI in late life may offer a protective benefit, aligning with the concept of the so-called “obesity paradox”. This phenomenon posits that a higher body mass index may have a beneficial effect on older people, particularly in terms of reducing the risk of cognitive decline and dementia. However, it is crucial to acknowledge the limitations of the BMI as a metric. This index fails to differentiate between fat and muscle mass, potentially leading to erroneous conclusions regarding the health status. It is particularly salient given the findings of the Tessier et al. study [38], which identified low muscle mass, not body weight alone, as a pivotal risk factor for cognitive decline. These observations underscore the significance of preserving muscle mass during the aging process, which may serve as an effective protective mechanism against the development of cognitive impairment.
When discussing the relationship between the BMI and cognitive health, it is crucial to consider the possibility of a so-called reverse causality effect. This is a scenario in which the onset of health problems leads to decreased body weight, even before the manifestation of disease symptoms. This issue is particularly salient in studies conducted on the elderly population. Consequently, further research is necessary to control for this effect and account for variability in the body composition, as well as the dynamics of BMI changes over time, especially within an aging population. Additionally, elucidating the mechanisms that might underpin the potential protective effect of a higher BMI in older age groups is imperative.
In the current study, we analyzed data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), focusing on a subset of 42 participants who underwent multiple MRI scans. We are aware that the relatively small sample size is a limitation, as larger cohorts are generally preferred to enhance statistical power and improve the generalizability of results. However, this sample size reflects the availability of longitudinal imaging data within the ADNI cohort, and despite its limitations, it provides valuable insights into neuroimaging changes over time. However, our future studies will aim to include larger datasets to validate and extend our findings.
4. Conclusions
This study aimed to examine the impact of physical activity on the progression of chronic diseases, with a particular emphasis on brain changes related to Alzheimer’s disease.
A clustering analysis was performed to group patients into varying numbers of clusters based on data reflecting their physical condition. As the number of clusters increased, a more distinct correlation was observed between physical condition indicators and the progression of brain changes associated with Alzheimer’s disease or dementia. In summary, we observed a positive association between higher body mass index (BMI) scores and a slower progression of structural brain changes.
Future research should explore a more extensive dataset, incorporating a broader range of demographic, clinical, and lifestyle variables to validate the observed relationships between the BMI, muscle mass, and neurodegenerative processes. Longitudinal studies with repeated MRI assessments and advanced imaging techniques could provide deeper insights into the temporal dynamics of brain atrophy. Additionally, integrating genetic markers such as the APOE-4 status and more detailed physical activity records could enhance the understanding of protective factors against dementia. Developing advanced clustering algorithms and applying deep neural networks may also improve patient stratification and the disease progression prediction accuracy. These efforts could contribute to designing more personalized intervention strategies to mitigate cognitive decline in older adults.
Author Contributions
Conceptualization, A.W. and M.K.; methodology, A.W.; software, M.K.; validation, A.W., M.K. and K.Ż.; formal analysis, A.W. and M.K.; investigation, A.W. and K.Ż; resources, A.W. and K.Ż.; data curation, M.K.; writing—original draft preparation, A.W.; writing—review and editing, A.W., M.K. and K.Ż.; visualization, A.W. and M.K.; supervision, M.K.; project administration, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available online at https://adni.loni.usc.edu/data-samples/adni-data/ (accessed on 20 November 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Soleimani, R.; Guo, S.; Haley, K.L.; Jacks, A.; Lobaton, E. The Impact of Pause and Filler Word Encoding on Dementia Detection with Contrastive Learning. Appl. Sci. 2024, 14, 8879. [Google Scholar] [CrossRef]
- Schardt, D. Walk This Way, Please: 7 Reasons To Lace Up Your Sneakers. Nutr. Action Health Lett. 2015, 42, 1–6. [Google Scholar]
- Ma, Y.; Sui, D.; Yang, S.; Fang, N.; Wang, Z. Application of the (fr)AGILE Scale in the Evaluation of Multidimensional Frailty in Elderly Inpatients from Internal Medicine Wards: A Cross-Sectional Observational Study. Front. Aging Neurosci. 2024, 15, 1276250. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Vock, D.M.; Zhang, L.; Salisbury, D.; Nelson, N.W.; Chow, L.S.; Smith, G.; Barclay, T.R.; Dysken, M.; Wyman, J.F. Cognitive Effects of Aerobic Exercise in Alzheimer’s Disease: A Pilot Randomized Controlled Trial. J. Alzheimer’s Dis. 2021, 80, 233–244. Available online: https://pubmed.ncbi.nlm.nih.gov/33523004/ (accessed on 5 November 2024). [CrossRef] [PubMed]
- Schmidt-Kassow, M.; Kulka, A.; Gunter, T.C.; Rothermich, K.; Kotz, S.A. Aerobic Exercise for Alzheimer’s Disease: A Randomized Controlled Pilot Trial. Alzheimer’s Res. Ther. 2017, 9, 53–60. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5302785/ (accessed on 5 November 2024).
- Williams, C.; Brown, M. Interactive Effects of Physical Activity and APOE-4 on White Matter Tract Diffusivity in Healthy Elders. Neurobiol. Aging 2018, 45, 72–82. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4746115/ (accessed on 5 November 2024).
- Mat Ludin, A.F.; Ahmad, Z. The Relationship between Physical Activity, Body Mass Index, and Body Composition among Students at a Pre-University Centre in Malaysia. J. Health Fit. 2020. Available online: https://www.researchgate.net/publication/346450737 (accessed on 5 November 2024).
- Cardona, M.I.; Weißenborn, M.; Zöllinger, I.; Kroeber, E.S.; Bauer, A.; Luppa, M.; Pabst, A.; Czock, D.; König, H.-H.; Wiese, B.; et al. Physical Activity Determinants in Older German Adults at Increased Dementia Risk with Multimorbidity: Baseline Results of the AgeWell.de Study. Int. J. Environ. Res. Public Health 2022, 19, 3164. [Google Scholar] [CrossRef]
- Bowes, A.; Dawson, A.; Jepson, R.; McCabe, L. Physical Activity for People with Dementia: A Scoping Study. BMC Geriatr. 2013, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Green, O. Lifestyle and Neurocognition in Older Adults with Cognitive Impairments: A Randomized Trial. Neurol. J. 2019, 92, e212–e220. Available online: https://n.neurology.org/content/92/3/e212 (accessed on 5 November 2024).
- Binabaji, S.; Rahimi, M.; Rajabi, H.; Keshavarz, M.; Rahimi, R.; Ahmadi, A.; Gahreman, D. Effects of Physical Training on Coagulation Parameters, Interleukin-6, and Angiotensin-Converting Enzyme-2 in COVID-19 Survivors. Sci. Rep. 2024, 14, 18968. [Google Scholar] [CrossRef]
- Sui, S.X.; Balanta-Melo, J.; Pasco, J.A.; Plotkin, L.I. Musculoskeletal Deficits and Cognitive Impairment: Epidemiological Evidence and Biological Mechanisms. Curr. Osteoporos. Rep. 2022, 20, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Oudbier, S.J.; Goh, J.; Looijaard, S.M.L.M.; Reijnierse, E.M.; Meskers, C.G.M.; Maier, A.B. Pathophysiological mechanisms explaining the association between low skeletal muscle mass and cognitive function. J. Gerontol. Ser. A 2022, 77, 1959–1968. [Google Scholar] [CrossRef]
- Allen, M.D.; Dalton, B.H.; Gilmore, K.J.; McNeil, C.J.; Doherty, T.J.; Rice, C.L.; Power, G.A. Neuroprotective effects of exercise on the aging human neuromuscular system. Exp. Gerontol. 2021, 152, 111465. [Google Scholar] [CrossRef]
- Shalabi, K.M.; AlSharif, Z.A.; Alrowaishd, S.A.; Al Ali, R.E. Relationship between Body Mass Index and Health-Related Physical Fitness: A Cross-Sectional Study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 9540–9549. Available online: https://www.europeanreview.org/wp/wp-content/uploads/9540-9549.pdf (accessed on 5 November 2024). [PubMed]
- Tibco Statistica 13.3.0. Product Documentation. Available online: https://docs.tibco.com/products/spotfire-statistica/archive (accessed on 6 January 2025).
- Alzheimer’s Disease Neuroimaging Initiative (ADNI). Available online: https://adni.loni.usc.edu/data-samples/adni-data/ (accessed on 20 November 2024).
- Alzheimer’s Disease Neuroimaging Initiative (ADNI). ADNI Documentation. Available online: https://adni.loni.usc.edu/help-faqs/adni-documentation/ (accessed on 4 January 2025).
- Tryon, R.C. Cluster Analysis; Edwards Brothers: Ann Arbor, MI, USA, 1939. [Google Scholar]
- Murtagh, F.; Contreras, P. Algorithms for Hierarchical Clustering: An Overview. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2012, 2, 86–97. [Google Scholar] [CrossRef]
- Ester, M.; Kriegel, H.-P.; Sander, J.; Xu, X. A Density-Based Algorithm for Discovering Clusters in Large Spatial Databases with Noise. In Proceedings of the Second International Conference on Knowledge Discovery and Data Mining, Portland, OR, USA, 2–4 August 1996; AAAI Press: Portland, OR, USA, 1996; pp. 226–231. [Google Scholar]
- Fraley, C.; Raftery, A.E. Model-Based Clustering, Discriminant Analysis, and Density Estimation. J. Am. Stat. Assoc. 2002, 97, 611–631. [Google Scholar] [CrossRef]
- Arthur, D.; Vassilvitskii, S. k-Means++: The Advantages of Careful Seeding. In Proceedings of the Eighteenth Annual ACM-SIAM Symposium on Discrete Algorithms; Society for Industrial and Applied Mathematics: New Orleans, LA, USA, 2007; pp. 1027–1035. [Google Scholar]
- Dalmaijer, E.S.; Nord, C.L.; Astle, D.E. Statistical Power for Cluster Analysis. BMC Bioinform. 2022, 23, 205. [Google Scholar] [CrossRef]
- Kullback, S.; Leibler, R.A. On Information and Sufficiency. Ann. Math. Stat. 1951, 22, 79–86. [Google Scholar] [CrossRef]
- Cover, T.M.; Thomas, J.A. Elements of Information Theory, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Lee, J.; Kang, J.; Park, C.-S.; Jeong, J. Distributed Fire Classification and Localization Model Based on Federated Learning with Image Clustering. Appl. Sci. 2024, 14, 9162. [Google Scholar] [CrossRef]
- Bonnici, V. A Maximum Value for the Kullback–Leibler Divergence between Quantized Distributions. Information 2024, 15, 547. [Google Scholar] [CrossRef]
- Nawa, V.; Nadarajah, S. Exact Expressions for Kullback–Leibler Divergence for Univariate Distributions. Entropy 2024, 26, 959. [Google Scholar] [CrossRef]
- Leigh, L.; Byles, J.E.; Jagger, C. BMI and Healthy Life Expectancy in Old and Very Old Women. Br. J. Nutr. 2016, 116, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jayanama, K.; Theou, O.; Godin, J.; Mayo, A.; Cahill, L.; Rockwood, K. Relationship of Body Mass Index with Frailty and All-Cause Mortality Among Middle-Aged and Older Adults. BMC Med. 2022, 20, 404. [Google Scholar] [CrossRef]
- Buckley, G.L.; Hall, L.E.; Lassemillante, A.-C.M.; Ackerman, K.E.; Belski, R. Retired Athletes and the Intersection of Food and Body: A Systematic Literature Review Exploring Compensatory Behaviours and Body Change. Nutrients 2019, 11, 1395. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, D.; Kim, S.K. Effects of Physical Activity on Body Composition, Muscle Strength, and Physical Function in Old Age: Bibliometric and Meta-Analyses. Healthcare 2024, 12, 197. [Google Scholar] [CrossRef]
- Prior, B.M.; Modlesky, C.M.; Evans, E.M.; Sloniger, M.A.; Saunders, M.J.; Lewis, R.D.; Cureton, K.J. Muscularity and the Density of the Fat-Free Mass in Athletes. J. Appl. Physiol. 2001, 90, 1523–1531. [Google Scholar] [CrossRef]
- Qizilbash, N.; Gregson, J.; Johnson, M.E.; Pearce, N.; Douglas, I.; Wing, K.; Evans, S.J.W.; Pocock, S.J. BMI and Risk of Dementia in Two Million People Over Two Decades: A Retrospective Cohort Study. Lancet Diabetes Endocrinol. 2015, 3, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Emmerzaal, T.L.; Kiliaan, A.J.; Gustafson, D.R. 2003-2013: A Decade of Body Mass Index, Alzheimer’s Disease, and Dementia. J. Alzheimer’s Dis. 2015, 43, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Wing, S.S.; Rahme, E.; Morais, J.A.; Chevalier, S. Association of Low Muscle Mass With Cognitive Function During a 3-Year Follow-up Among Adults Aged 65 to 86 Years in the Canadian Longitudinal Study on Aging. JAMA Netw. Open 2022, 5, e2219926. [Google Scholar] [CrossRef] [PubMed]
- Hainer, V.; Aldhoon-Hainerová, I. Obesity Paradox Does Exist. Diabetes Care 2013, 36, S276–S281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).