Comprehensive Evaluation of the Nutritional Quality of Stored Watermelon Seed Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Oil Extraction from Watermelon Seeds
2.2.2. Fatty Acid Composition Analysis
2.2.3. Health Indices of Oils
2.2.4. Fatty Acid Distribution Analysis
2.2.5. Hydrolytic Stability Determination
2.2.6. Oxidative Stability Determination
2.3. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition
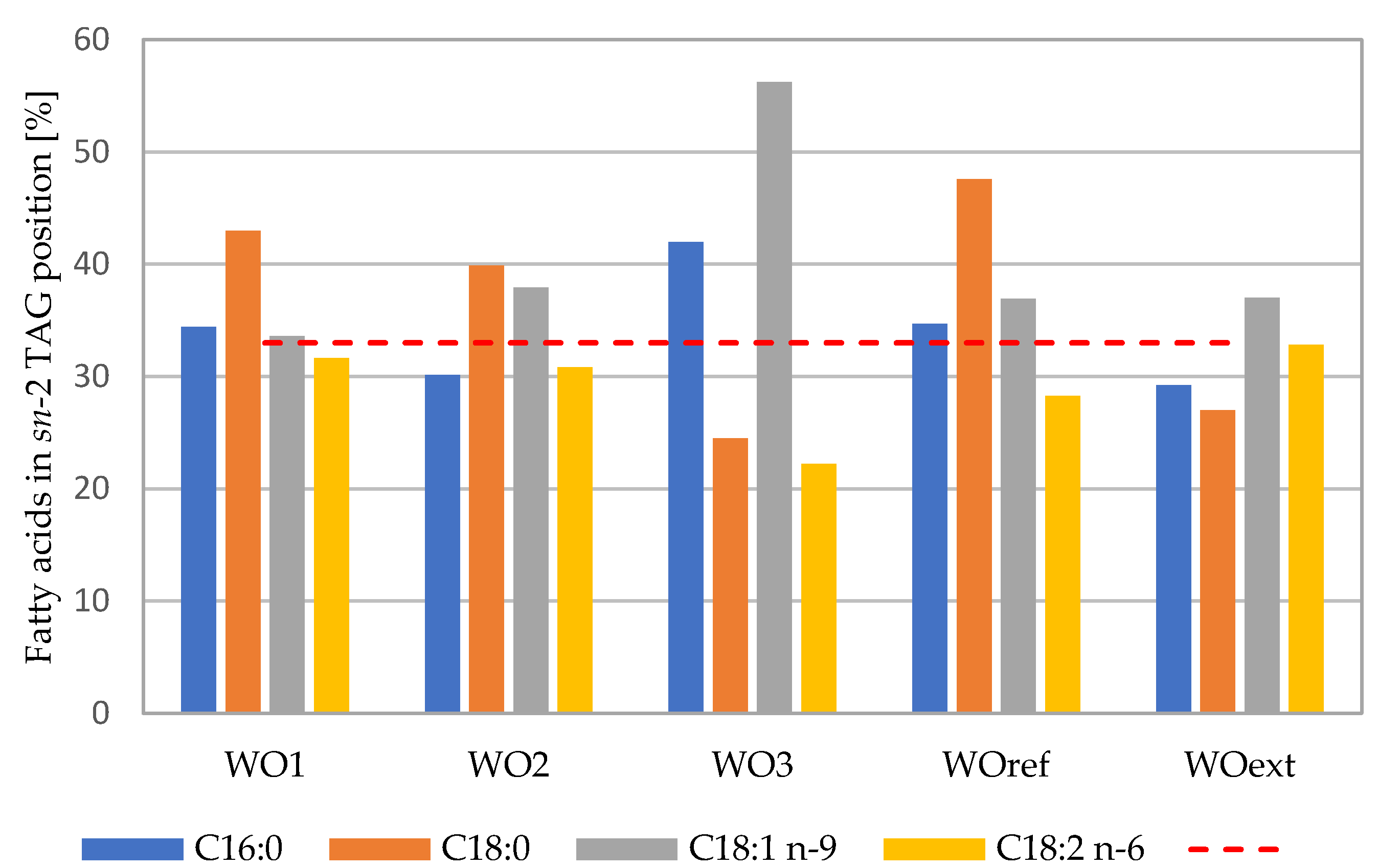
3.2. Fatty Acid Distribution
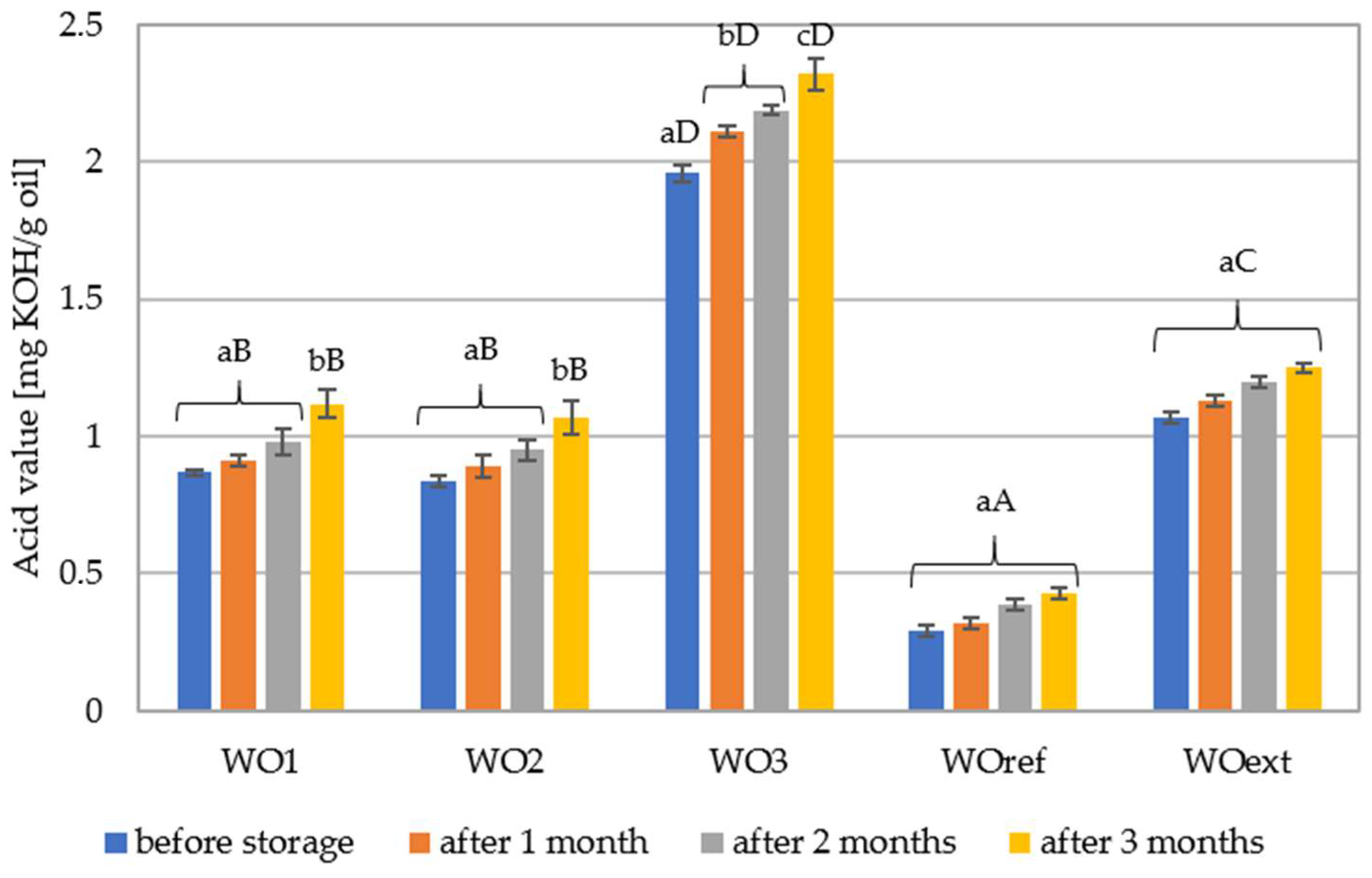
3.3. Determination of Acid Value
3.4. Determination of Peroxide Value
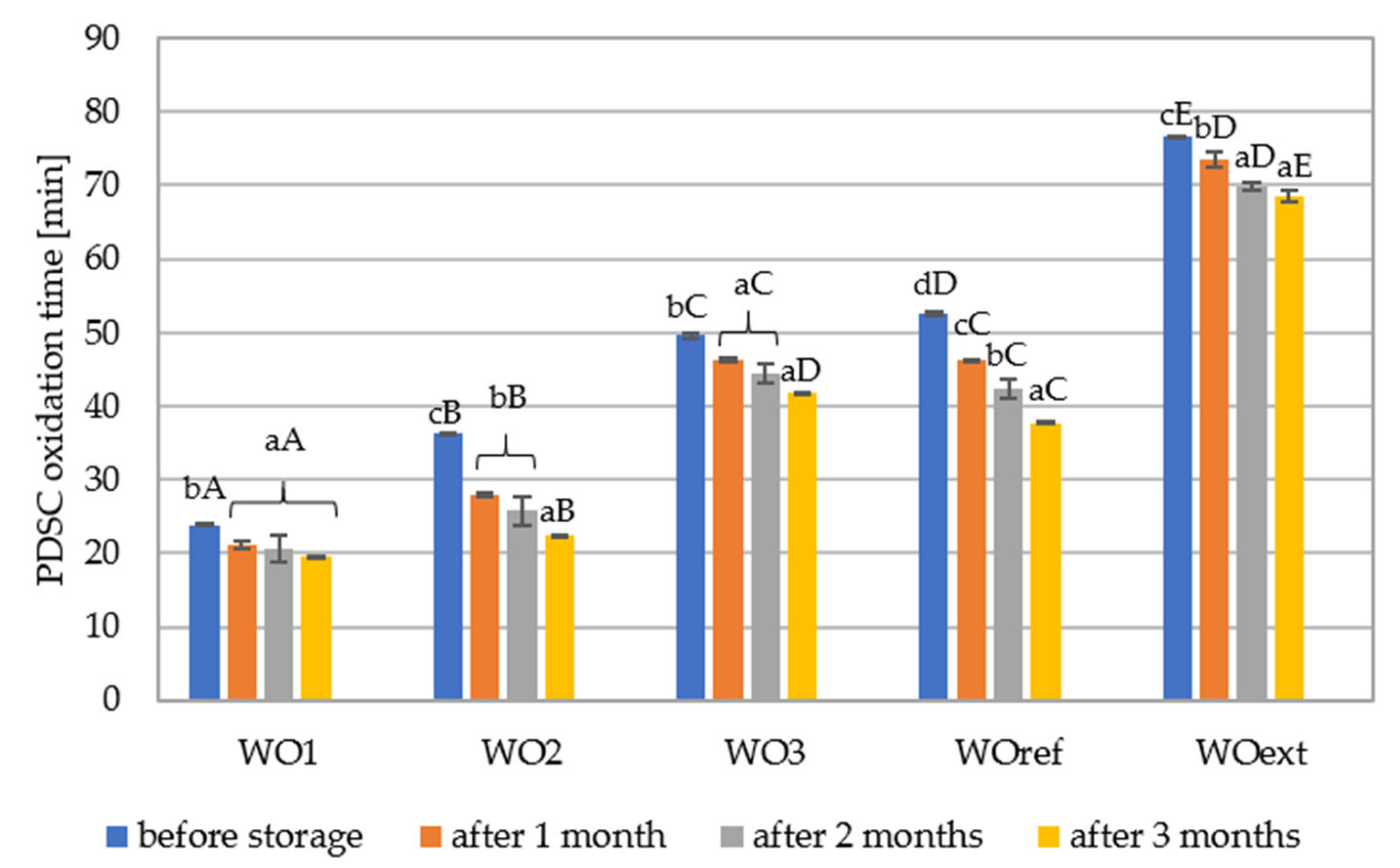
3.5. Oxidative Stability, Obtained via Pressure Differential Scanning Calorimetry (PDSC)—Isothermal Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Łopusiewicz, Ł. Antioxidant, Antibacterial Properties and the Light Barrier Assessment of Raw and Purified Melanins Isolated from Citrullus lanatus (Watermelon) Seeds. Herba Pol. 2018, 64, 25–36. [Google Scholar] [CrossRef]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of High Value-Added Compounds from Pineapple, Melon, Watermelon and Pumpkin Processing by-Products: An Overview. Food Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef]
- FAOSTAT Statistical Database; Food and Agriculture Organization of the United Nations: Italy, Rome, 2023.
- Zero Waste International Allince. 2018. Available online: https://zwia.org/zero-waste-definition (accessed on 18 September 2024).
- Tlili, I.; Hdider, C.; Lenucci, M.S.; Riadh, I.; Jebari, H.; Dalessandro, G. Bioactive Compounds and Antioxidant Activities of Different Watermelon (Citrullus lanatus (Thunb.) Mansfeld) Cultivars as Affected by Fruit Sampling Area. J. Food Compos. Anal. 2011, 24, 307–314. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.A.; Ahmed, A.R. Utilization of Watermelon Rinds and Sharlyn Melon Peels as a Natural Source of Dietary Fiber and Antioxidants in Cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Yang, L.; Gou, Q.; Han, L.; Yu, Q. Utilization of Watermelon Peel as a Pectin Source and the Effect of Ultrasound Treatment on Pectin Film Properties. LWT 2021, 147, 111569. [Google Scholar] [CrossRef]
- Braide, W.; Odiong, I.J.; Oranusi, S. Phytochemical and Antibacterial Properties of the Seed of Watermelon (Citrullus lanatus). Prime J. Microbiol. Res. 2012, 2, 99–104. [Google Scholar]
- Choudhary, B.R.; Haldhar, S.M.; Maheshwari, S.K.; Bhargava, R.; Sharma, S.K. Phytochemicals and Antioxidants in Watermelon (Citrullus lanatus) Genotypes under Hot Arid Region. Indian J. Agric. Sci. 2015, 85, 414–417. [Google Scholar] [CrossRef]
- Olamide, A.A.; Olayemi, O.O.; Demetrius, O.O.; Olatoye, O.J.; Kehinde, A.A. Effects of Methanolic Extract of Citrullus lanatus Seed on Experimentally Induced Prostatic Hyperplasia. Eur. J. Med. Plants 2011, 1, 171–179. [Google Scholar] [CrossRef]
- Hassan, L.E.A.; Sirat, H.; Yagi, S.; Koko, W.; Abdelwahab, S. In Vitro Antimicrobial Activities of Chloroformic, Hexane and Ethanolic Extracts of Citrullus lanatus var. Citroides (Wild Melon). J. Med. Plants Res. 2011, 5, 1338–1344. [Google Scholar]
- Wang, F.; Li, H.; Zhao, H.; Zhang, Y.; Qiu, P.; Li, J.; Wang, S. Antidiabetic Activity and Chemical Composition of Sanabi Melon Seed Oil. Evid. Based Complement. Altern. Med. 2018, 2018, 5434156. [Google Scholar] [CrossRef] [PubMed]
- Bazabang, S.; Monday, N.; Adebisi, S.; Makena, W.; Iliya, I. Hepatoprotective Effects of Aqueous Extract of Watermelon (Citrullus lanatus) Seeds on Ethanol-Induced Oxidative Damage in Wister Rats. Sub-Sahar. Afr. J. Med. 2018, 5, 129–137. [Google Scholar] [CrossRef]
- Wani, A.A.; Sogi, D.S.; Singh, P.; Shivhare, U.S. Characterization and Functional Properties of Watermelon (Citrullus lanatus) Seed Protein Isolates and Salt Assisted Protein Concentrates. Food Sci. Biotechnol. 2011, 20, 877–887. [Google Scholar] [CrossRef]
- Enemor, V.H.A.; Oguazu, C.E.; Odiakosa, A.U.; Okafor, S.C. Evaluation of the Medicinal Properties and Possible Nutrient Composition of Citrullus lanatus (Watermelon) Seeds. Res. J. Med. Plants 2019, 13, 129–135. [Google Scholar] [CrossRef]
- Raihana, A.R.N.; Marikkar, J.M.N.; Amin, I.; Shuhaimi, M. A Review on Food Values of Selected Tropical Fruits’ Seeds. Int. J. Food Prop. 2015, 18, 2380–2392. [Google Scholar] [CrossRef]
- Teotia, M.; Ramakrishna, P. Chemistry and Technology of Melon Seeds. J. Food Sci. Technol. 1984, 21, 332–340. [Google Scholar]
- Nwokolo, E.; Sim, J.S. Nutritional Assessment of Defatted Oil Meals of Melon (Colocynthis citrullus L.) and Flutes Pumpkin (Telfaria occidentalis Hook) by Chick Assay. J. Sci. Food Agric. 1987, 38, 237–246. [Google Scholar] [CrossRef]
- Milovanovic, M.; Picuric-Jovanovic, K. Characteristics and Composition of Melon Seed Oil. J. Agric. Sci. 2005, 50, 41–47. [Google Scholar] [CrossRef]
- Ouassor, I.; Aqil, Y.; Belmaghraoui, W.; Hajjaji, S.E. Characterization of Two Moroccan Watermelon Seeds Oil Varieties by Three Different Extraction Methods. OCL 2020, 27, 13. [Google Scholar] [CrossRef]
- Poornima, D.S.; Hanumantharaju, K.N.; Shankar, V.S.; Kumar, K.S.; Ramya, H.N. Influence of Moisture Content and Temperature on Mechanical Extraction of Oil from Watermelon (Citrullus lanatus) Seeds. J. Pharmacogn. Phytochem. 2019, 8, 275–279. [Google Scholar]
- Bryś, J.; Flores, L.F.V.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC Methods to Characterize Human Milk Fat Substitutes Obtained from Lard and Milk Thistle Oil Mixtures. J. Therm. Anal. Calorim. 2017, 130, 319–327. [Google Scholar] [CrossRef]
- PN-EN ISO 5509:2001; Vegetable and Animal Oils and Fats. Preparation of Fatty Acid Methyl Esters. Polish Committee for Standardization: Warsaw, Poland, 2001.
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Górska, A.; Brzezińska, R.; Piasecka, I. Assessment of the Nutritional Potential and Resistance to Oxidation of Sea Buckthorn and Rosehip Oils. Appl. Sci. 2024, 14, 1867. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of Genotype, Feeding System and Slaughter Weight on the Quality of Light Lambs: II. Fatty Acid Composition of Meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Bandara, R.R.; Louis-Gavet, C.; Bryś, J.; Mańko-Jurkowska, D.; Górska, A.; Brzezińska, R.; Siol, M.; Makouie, S.; Palani, B.K.; Obranović, M.; et al. Enzymatic Interesterification of Coconut and Hemp Oil Mixtures to Obtain Modified Structured Lipids. Foods 2024, 13, 2722. [Google Scholar] [CrossRef]
- AOCS Official Method Te 1a-64; Acid Value Official Methods and Recommended Practices of the AOCS. AOCS Press: Champaign, IL, USA, 2009.
- AOCS Official Method Cd 8b-90; Peroxide Value Acetic Acid-Isooctane Method Official Methods and Recommended Practices of the AOCS, 6th ed.; Firestone, D., Ed.; AOCS Press: Champaign, IL, USA, 2009. [Google Scholar]
- Siol, M.; Dudek, A.; Bryś, J.; Mańko-Jurkowska, D.; Gruczyńska-Sękowska, E.; Makouie, S.; Palani, B.K.; Obranović, M.; Koczoń, P. Chromatographic and Thermal Characteristics, and Hydrolytic and Oxidative Stability of Commercial Pomegranate Seed Oil. Foods 2024, 13, 1370. [Google Scholar] [CrossRef]
- Krygier, K.; Wroniak, M.; Grześkiewicz, S.; Obiedziński, M. Study on Effect of Destroyed Seeds Content on the Quality of Cold Pressed Rapeseed Oil. Oilseed Crops 2000, 21, 587–596. [Google Scholar]
- Bialek, A.; Bialek, M.; Jelinska, M.; Tokarz, A. Fatty Acid Composition and Oxidative Characteristics of Novel Edible Oils in Poland. CYTA-J. Food 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Yurchenko, S.; Sats, A.; Tatar, V.; Kaart, T.; Mootse, H.; Jõudu, I. Fatty Acid Profile of Milk from Saanen and Swedish Landrace Goats. Food Chem. 2018, 254, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Ying, Q.; Wojciechowska, P.; Siger, A.; Kaczmarek, A.; Rudzińska, M. Phytochemical Content, Oxidative Stability, and Nutritional Properties of Unconventional Cold-Pressed Edible Oils. J. Food Nutr. Res. 2018, 6, 476–485. [Google Scholar] [CrossRef]
- Górska, A.; Piasecka, I.; Wirkowska-Wojdyła, M.; Bryś, J.; Kienc, K.; Brzezińska, R.; Ostrowska-Ligęza, E. Berry Seeds—A By-Product of the Fruit Industry as a Source of Oils with Beneficial Nutritional Characteristics. Appl. Sci. 2023, 13, 5114. [Google Scholar] [CrossRef]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive Compounds, Nutritional Quality and Oxidative Stability of Cold-Pressed Camelina (Camelina sativa L.) Oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef]
- Indelicato, S.; Bongiorno, D.; Pitonzo, R.; Di Stefano, V.; Calabrese, V.; Idelicato, S.; Avellone, G. Triacylglycerols in Edible Oils: Determination, Characterization, Quantitation, Chemometric Approach and Evaluation of Adulterations. J. Chromatogr. A. 2017, 1515, 1–16. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, W.; Zhou, H.; Zhang, D.; Li, R.; Li, C.; Wang, S. The relations between minor components and antioxidant capacity of five fruits and vegetables seed oils in China. J. Oleo Sci. 2019, 68, 625–635. [Google Scholar] [CrossRef]
- Montesano, D.; Blasi, F.; Simonetti, M.; Santini, A.; Cossignani, L. Chemical and Nutritional Characterization of Seed Oil from Cucurbita maxima L. (var. Berrettina) Pumpkin. Foods 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Codex-ALINORM 09/32/17; Codex Alimentarius 2009. Codex Standard for Named Vegetable Oils. Codex Alimentarius Commission: Rome, Italy, 2009.
- Gharby, S.; Hajib, A.; Ibourki, M.; Sakar, E.H.; Nounah, I.; Moudden, H.E.; Elibrahimi, M.; Harhar, H. Induced Changes in Olive Oil Subjected to Various Chemical Refining Steps: A Comparative Study of Quality Indices, Fatty Acids, Bioactive Minor Components, and Oxidation Stability Kinetic Parameters. Chem. Data Coll. 2021, 33, 100702. [Google Scholar] [CrossRef]
- Popis, E.; Ratusz, K.; Krygier, K. Quality Assessment of Selected Refined and Cold-Pressed Rapeseed Oils Available on Polish Market. Apar. Badaw. Dydakt. 2014, 3, 251–258. [Google Scholar]
- Yogeesha, H.S.; Bhanuprakash, K.; Naik, L.B. Seed Storability in Three Varieties of Papaya in Relation to Seed Moisture, Packaging Material and Storage Temperature. Seed Sci. Technol. 2008, 36, 721–729. [Google Scholar] [CrossRef]
- Matthäus, B. Quality Parameters for Cold Pressed Edible Argan Oils. Nat. Prod. Commun. 2013, 8, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Symoniuk, E.; Wroniak, M.; Napiórkowska, K.; Brzezińska, R.; Ratusz, K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods 2022, 11, 1597. [Google Scholar] [CrossRef]
- Cheikhyoussef, N.; Kandawa-Schulz, M.; Böck, R.; de Koning, C.; Cheikhyoussef, A.; Hussein, A.A. Characterization of Acanthosicyos horridus and Citrullus lanatus Seed Oils: Two Melon Seed Oils from Namibia Used in Food and Cosmetics Applications. 3 Biotech 2017, 7, 297. [Google Scholar] [CrossRef]
- Athar, S.; Ghazi, A.; Chourasiya, O.; Karadbhajne, V.Y. Watermelon Seed Oil: Its Extraction, Analytical Studies, Modification and Utilization in Cosmetic Industries. Int. Res. J. Eng. Technol. 2020, 7, 2862–2865. [Google Scholar]
- Egbuonu, A.C.C.; Aguguesi, R.G.; Samuel, R.; Ojunkwu, O.; Onyenmeri, F.; Uzuegbu, U. Some Physicochemical Properties of the Petroleum Ether-Extracted Watermelon (Citrullus lanatus) Seed Oil. Asian J. Sci. Res. 2015, 8, 519–525. [Google Scholar] [CrossRef]
- Ogunwole, O. Production of Biodiesel from Watermelon (Citrullus lanatus) Seed Oil. Leonardo J. Scis. 2015, 14, 63–74. [Google Scholar]
- Machado, M.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Vegetable Oils Oxidation: Mechanisms, Consequences and Protective Strategies. Food Rev. Int. 2023, 39, 4180–4197. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 3960:2017-03; Vegetable and Animal Oils and Fats. Determination of Peroxide Number (Reference Method). Polish Committee for Standardization: Warsaw, Poland, 2017.
- Gharby, S.; Harhar, H.; Guillaume, D.; Roudani, A.; Boulbaroud, S.; Ibrahimi, M.; Ahmad, M.; Sultana, S.; Hadda, T.B.; Chafchaouni-Moussaoui, I.; et al. Chemical Investigation of Nigella sativa L. Seed Oil Produced in Morocco. J. Saudi Soc. Agric. Sci. 2015, 14, 172–177. [Google Scholar] [CrossRef]
- Oyeleke, G.O.; Olagunju, E.O.; Ojo, A. Functional and Physicochemical Properties of Watermelon (Citrullus lanatus) Seed and Seed-Oil. IOSR J. Appl. Chem. 2012, 2, 29–31. [Google Scholar] [CrossRef]
- Francis, A.K.; Nicolas, C.L.; Felix, A.E. Variety of Watermelon and Method of Drying Affect the Chemical and Functional Characteristics of Oils Extracted from Watermelon Seeds. Res. J. Food Nutr. 2019, 3, 17–24. [Google Scholar] [CrossRef]
- Górska, A.; Mańko-Jurkowska, D.; Bryś, J.; Górska, A. Lipid Fraction Properties of Homemade Raw Cat Foods and Selected Commercial Cat Foods. Appl. Sci. 2021, 11, 10905. [Google Scholar] [CrossRef]
- Gao, F.; Birch, J. Oxidative Stability, Thermal Decomposition, and Oxidation Onset Prediction of Carrot, Flax, Hemp, and Canola Seed Oils in Relation to Oil Composition and Positional Distribution of Fatty Acids. Eur. J. Lipid Sci. Technol. 2016, 118, 1042–1052. [Google Scholar] [CrossRef]
- Wijesundera, C. The Influence of Triacylglycerol Structure on the Oxidative Stability of Polyunsaturated Oils. Lipid Technol. 2008, 20, 199–202. [Google Scholar] [CrossRef]
- Piasecka, I.; Górska, A.; Ostrowska-Ligęza, E.; Kalisz, S. The Study of Thermal Properties of Blackberry, Chokeberry and Raspberry Seeds and Oils. Appl. Sci. 2021, 11, 7704. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Ostrowska-Ligęza, E.; Krygier, K. Impact of Selected Chemical Characteristics of Cold-Pressed Oils on their Oxidative Stability Determined Using the Rancimat and Pressure Differential Scanning Calorimetry Method. Food Anal. Methods 2018, 11, 1095–1104. [Google Scholar] [CrossRef]

| Composition After Opening/Extraction [%] | Composition After 3 Months of Storage After Opening/Extraction [%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid | WO1 | WO2 | WO3 | WOref | WOext | WO1 | WO2 | WO3 | WOref | WOext |
| C14:0 | 0.26 ± 0.01 | 0.25 ± 0.04 | 0.07 ± 0.01 | 0.25 ± 0.01 | 0.07 ± 0.01 | 0.27 ± 0.01 | 0.24 ± 0.01 | 0.06 ± 0.01 | 0.25 ± 0.01 | 0.07 ± 0.01 |
| C16:0 | 11.79 ± 0.06 | 11.43 ± 0.29 | 12.45 ± 0.21 | 12.18 ± 0.01 | 11.00 ± 0.05 | 11.68 ± 0.03 | 11.42 ± 0.10 | 12.06 ± 0.12 | 11.89 ± 0.07 | 11.07 ± 0.32 |
| C16:1 | 0.32 ± 0.01 | 0.15 ± 0.01 | 0.10 ± 0.01 | 0.16 ± 0.01 | 0.10 ± 0.01 | 0.32 ± 0.01 | 0.16 ± 0.01 | 0.10 ± 0.01 | 0.16 ± 0.01 | 0.10 ± 0.01 |
| C17:0 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.14 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.13 ± 0.01 |
| C17:1 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.01 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.01 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.01 |
| C18:0 | 4.42 ± 0.02 | 4.97 ± 0.07 | 12.52 ± 0.21 | 4.85 ± 0.07 | 8.54 ± 0.01 | 4.32 ± 0.01 | 5.01 ± 0.02 | 12.00 ± 0.03 | 4.76 ± 0.03 | 8.42 ± 0.09 |
| C18:1 n-9 | 27.02 ± 0.02 | 25.23 ± 0.05 | 14.20 ± 0.28 | 28.18 ± 0.02 | 16.99 ± 0.02 | 26.76 ± 0.02 | 25.22 ± 0.07 | 13.70 ± 0.04 | 28.11 ± 0.02 | 16.90 ± 0.06 |
| C18:2 n-6 | 54.58 ± 0.03 | 56.20 ± 0.27 | 58.56 ± 0.58 | 52.90 ± 0.06 | 62.21 ± 0.01 | 55.01 ± 0.02 | 56.28 ± 0.02 | 60.04 ± 0.13 | 53.23 ± 0.10 | 62.34 ± 0.14 |
| C18:3 n-3 | 0.29 ± 0.01 | 0.40 ± 0.02 | 0.38 ± 0.01 | 0.42 ± 0.01 | 0.20 ± 0.01 | 0.31 ± 0.01 | 0.40 ± 0.01 | 0.39 ± 0.01 | 0.43 ± 0.01 | 0.22 ± 0.01 |
| C20:0 | 0.36 ± 0.01 | 0.38 ± 0.02 | 0.57 ± 0.02 | 0.33 ± 0.01 | 0.43 ± 0.01 | 0.36 ± 0.01 | 0.35 ± 0.01 | 0.53 ± 0.01 | 0.34 ± 0.01 | 0.43 ± 0.02 |
| C20:1 n-9 | 0.25 ± 0.01 | 0.22 ± 0.01 | 0.10 ± 0.01 | 0.20 ± 0.01 | 0.14 ± 0.01 | 0.25 ± 0.01 | 0.22 ± 0.01 | 0.10 ± 0.01 | 0.23 ± 0.02 | 0.12 ± 0.01 |
| C20:3 n-3 | 0.25 ± 0.01 | 0.27 ± 0.02 | 0.13 ± 0.01 | 0.29 ± 0.01 | 0.10 ± 0.01 | 0.24 ± 0.01 | 0.25 ± 0.02 | 0.11 ± 0.01 | 0.31 ± 0.01 | 0.12 ± 0.01 |
| C24:1 | - | - | 0.74 ± 0.02 | - | - | - | - | 0.71 ± 0.04 | - | - |
| other | 0.35 ± 0.01 | 0.39 ± 0.07 | 0.08 ± 0.01 | 0.13 ± 0.01 | 0.06 ± 0.01 | 0.38 ± 0.02 | 0.35 ± 0.02 | 0.08 ± 0.02 | 0.17 ± 0.01 | 0.07 ± 0.01 |
| Σ SFA | 16.91 ± 0.04 aA | 17.10 ± 0.27 aAB | 25.70 ± 0.31 bD | 17.68 ± 0.07 aB | 20.19 ± 0.03 aC | 16.70 ± 0.04 aA | 17.09 ± 0.09 aAB | 24.74 ± 0.15 aD | 17.32 ± 0.10 aB | 20.12 ± 0.21 aC |
| Σ MUFA | 27.62 ± 0.02 aD | 25.64 ± 0.07 aC | 15.15 ± 0.27 aA | 28.58 ± 0.03 aE | 17.25 ± 0.02 aB | 27.37 ± 0.02 aD | 25.64 ± 0.08 aC | 14.63 ± 0.02 aA | 28.55 ± 0.01 aE | 17.13 ± 0.06 aB |
| Σ PUFA | 55.12 ± 0.03 aB | 56.87 ± 0.28 aC | 59.07 ± 0.57 aD | 53.61 ± 0.06 aA | 62.51 ± 0.02 aE | 55.56 ± 0.02 aB | 56.92 ± 0.03 aC | 60.54 ± 0.13 bD | 53.97 ± 0.11 aA | 62.67 ± 0.15 aE |
| Health indices | ||||||||||
| AI | 0.16 | 0.15 | 0.17 | 0.16 | 0.14 | 0.15 | 0.15 | 0.16 | 0.16 | 0.14 |
| TI | 0.39 | 0.39 | 0.65 | 0.40 | 0.48 | 0.38 | 0.39 | 0.62 | 0.39 | 0.48 |
| HPI | 6.45 | 6.64 | 5.83 | 6.24 | 7.07 | 6.50 | 6.67 | 6.11 | 6.40 | 7.03 |
| h/H | 6.82 | 7.03 | 5.85 | 6.58 | 7.18 | 6.89 | 7.04 | 6.13 | 6.76 | 7.14 |
| Fatty Acid | Composition [%] | |||||
|---|---|---|---|---|---|---|
| WO1 | WO2 | WO3 | WOref | WOext | ||
| C16:0 | TAG | 11.79 ± 0.04 b | 11.43 ± 0.04 b | 12.45 ± 0.04 b | 12.18 ± 0.04 b | 11.00 ± 0.04 b |
| sn-2 | 12.18 ± 0.66 ab | 10.34 ± 0.66 ab | 15.68 ± 0.66 ab | 12.68 ± 0.66 ab | 9.65 ± 0.66 ab | |
| sn-1,3 | 11.60 ± 0.33 b | 11.98 ± 0.33 b | 10.84 ± 0.33 b | 11.93 ± 0.33 b | 9.35 ± 0.33 b | |
| C18:0 | TAG | 4.42 ± 0.01 bc | 4.97 ± 0.01 bc | 12.52 ± 0.01 bc | 4.85 ± 0.01 bc | 8.54 ± 0.01 bc |
| sn-2 | 5.70 ± 0.13 a | 5.95 ± 0.13 a | 9.19 ± 0.13 a | 6.92 ± 0.13 a | 6.92 ± 0.13 a | |
| sn-1,3 | 3.78 ± 0.07 b | 4.48 ± 0.07 b | 14.19 ± 0.07 b | 3.81 ± 0.07 b | 9.35 ± 0.07 b | |
| C18:1 n-9 | TAG | 27.02 ± 0.06 c | 25.23 ± 0.06 c | 14.20 ± 0.06 c | 28.18 ± 0.06 c | 16.99 ± 0.06 c |
| sn-2 | 27.26 ± 1.29 c | 28.71 ± 1.29 c | 23.95 ± 1.29 c | 31.22 ± 1.29 c | 18.87 ± 1.29 c | |
| sn-1,3 | 26.90 ± 0.65 c | 23.49 ± 0.65 c | 9.32 ± 0.65 c | 26.66 ± 0.65 c | 16.05 ± 0.65 c | |
| C18:2 n-6 | TAG | 54.58 ± 0.06 b | 56.20 ± 0.06 b | 58.56 ± 0.06 b | 52.90 ± 0.06 b | 62.21 ± 0.06 b |
| sn-2 | 51.85 ± 1.35 b | 51.99 ± 1.35 b | 39.00 ± 1.35 b | 44.89 ± 1.35 b | 61.27 ± 1.35 b | |
| sn-1,3 | 55.95 ± 0.68 b | 58.30 ± 0.68 b | 68.34 ± 0.68 b | 56.91 ± 0.68 b | 62.68 ± 0.68 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siol, M.; Witkowska, B.; Mańko-Jurkowska, D.; Makouie, S.; Bryś, J. Comprehensive Evaluation of the Nutritional Quality of Stored Watermelon Seed Oils. Appl. Sci. 2025, 15, 830. https://doi.org/10.3390/app15020830
Siol M, Witkowska B, Mańko-Jurkowska D, Makouie S, Bryś J. Comprehensive Evaluation of the Nutritional Quality of Stored Watermelon Seed Oils. Applied Sciences. 2025; 15(2):830. https://doi.org/10.3390/app15020830
Chicago/Turabian StyleSiol, Marta, Beata Witkowska, Diana Mańko-Jurkowska, Sina Makouie, and Joanna Bryś. 2025. "Comprehensive Evaluation of the Nutritional Quality of Stored Watermelon Seed Oils" Applied Sciences 15, no. 2: 830. https://doi.org/10.3390/app15020830
APA StyleSiol, M., Witkowska, B., Mańko-Jurkowska, D., Makouie, S., & Bryś, J. (2025). Comprehensive Evaluation of the Nutritional Quality of Stored Watermelon Seed Oils. Applied Sciences, 15(2), 830. https://doi.org/10.3390/app15020830







