On the Stability of Graphene-Based Aqueous Dispersions and Their Performance in Cement Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Exfoliation Pretreatment
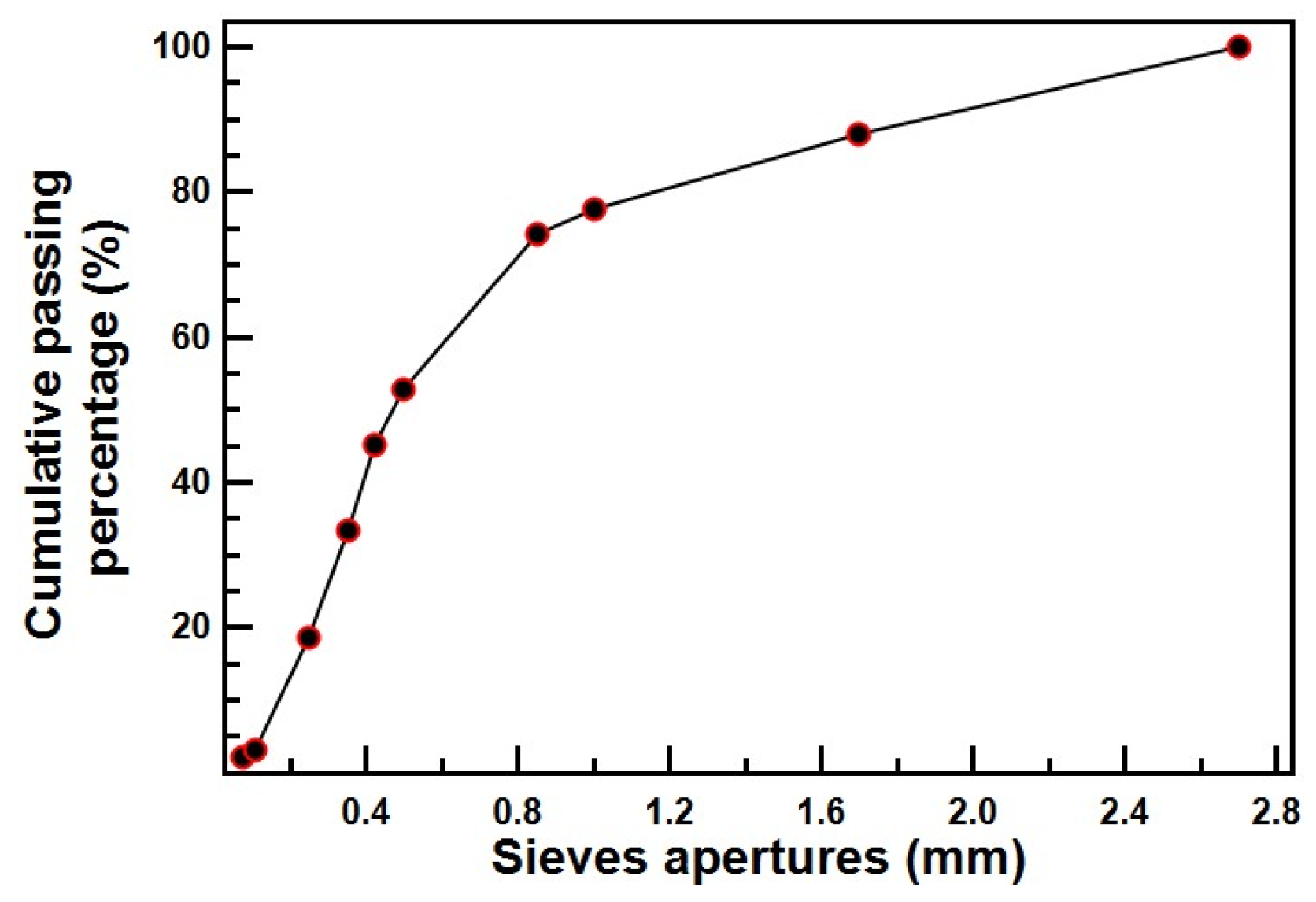
2.3. Mix Design
2.4. FESEM and Raman Spectroscopy
2.5. Suspension Stability Characterisation
2.6. Analysis of Workability
2.7. Ultrasonic and Mechanical Tests
2.8. Capillary Water Absorption Test
2.9. Nitrogen and Mercury Intrusion Porosimetry
3. Results and Discussion
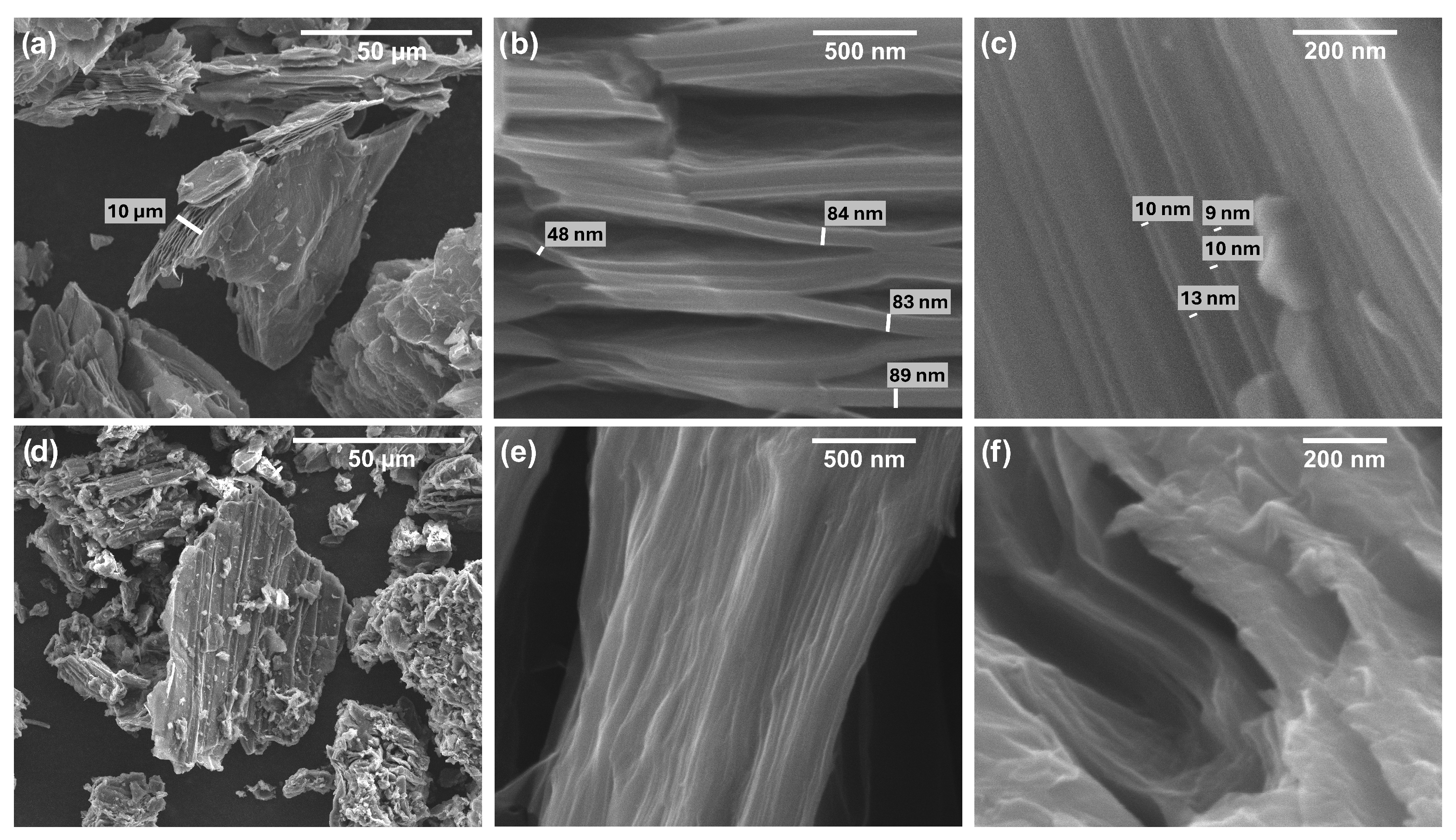
3.1. FESEM Measurements on Pristine Additives
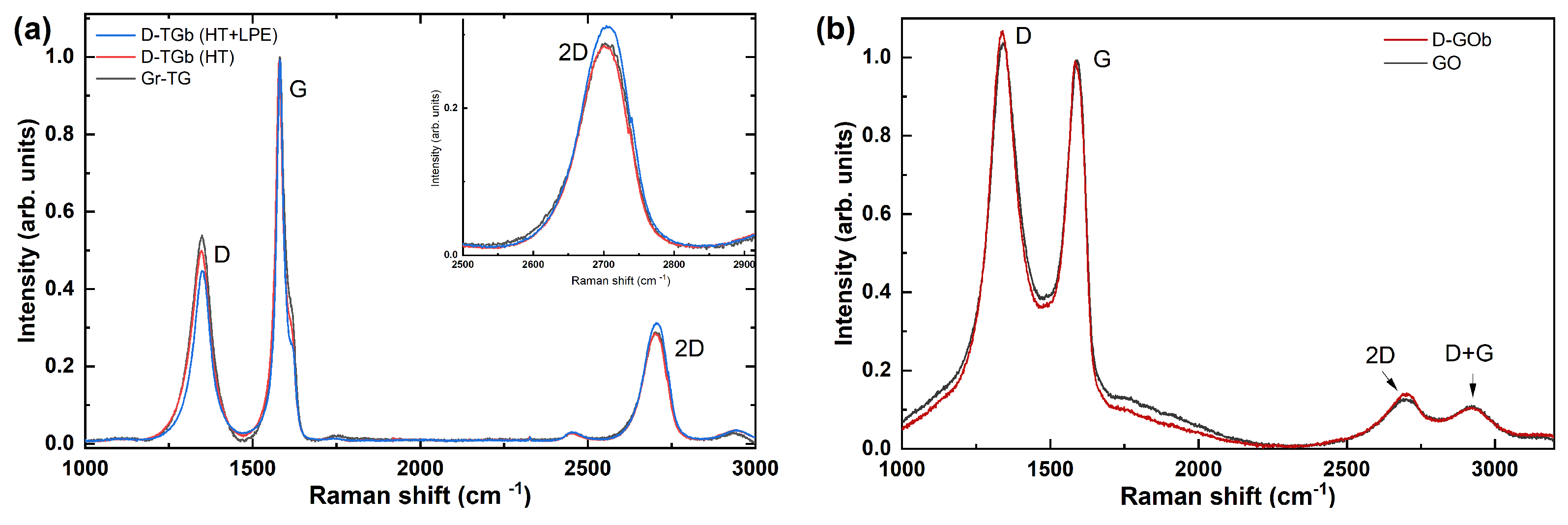
3.2. Raman Spectra
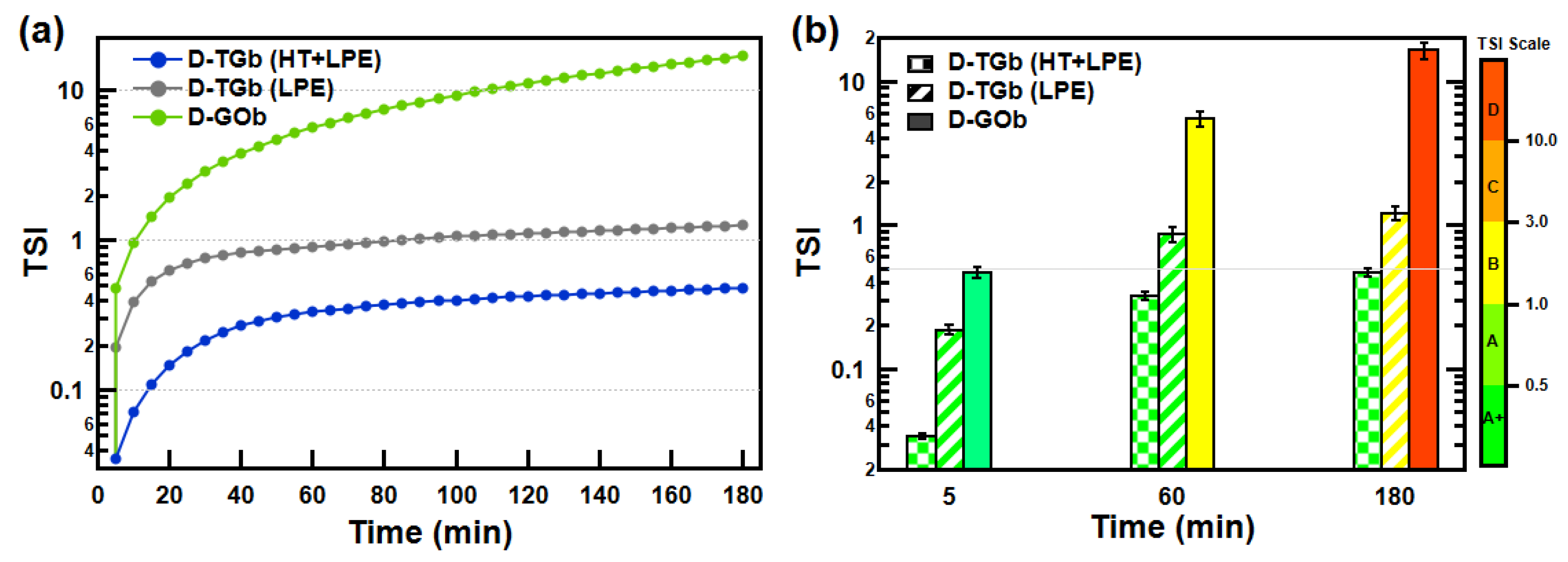
3.3. Suspension Stability Characterization
3.4. Workability Analysis
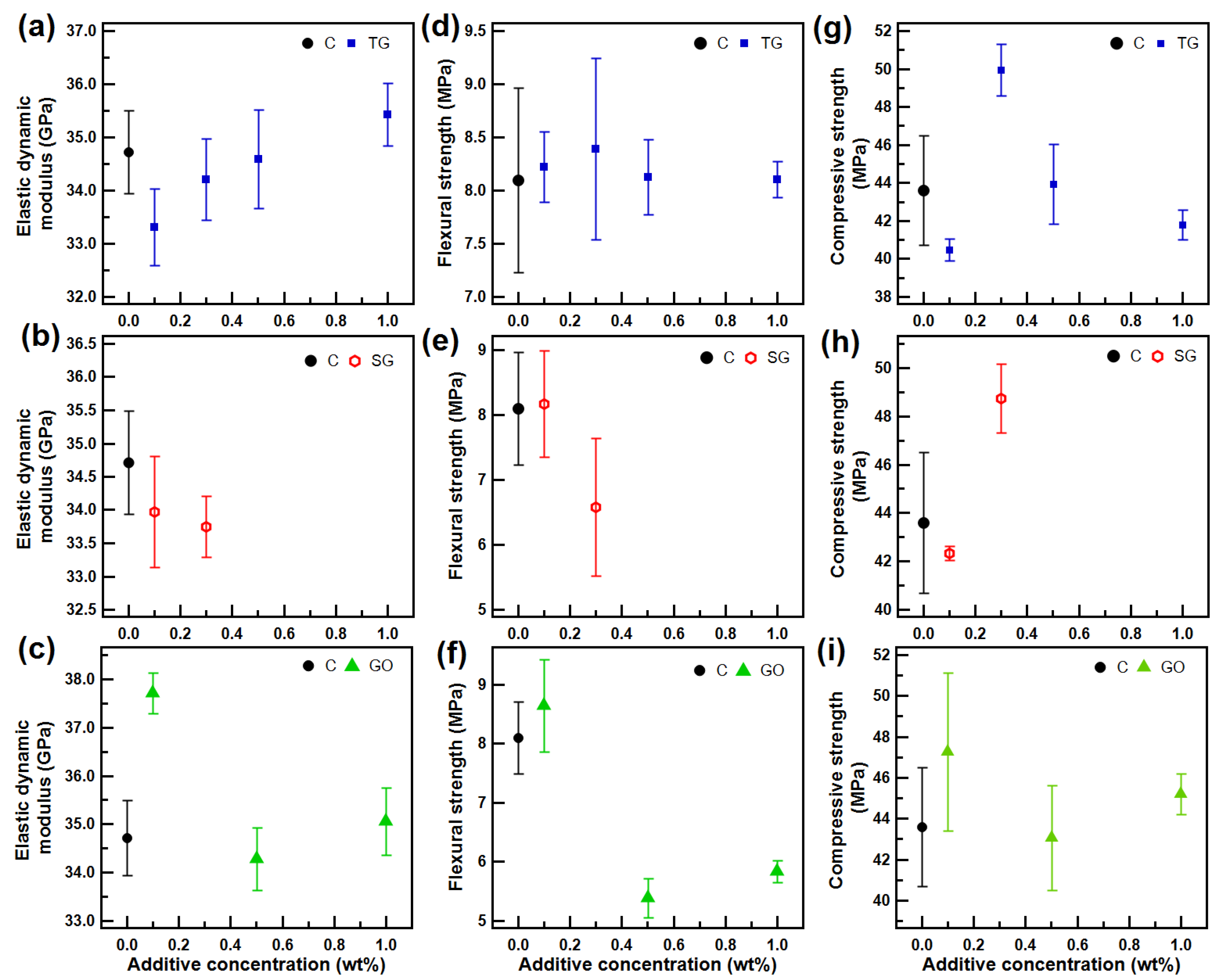
3.5. Ultrasonic and Mechanical Tests
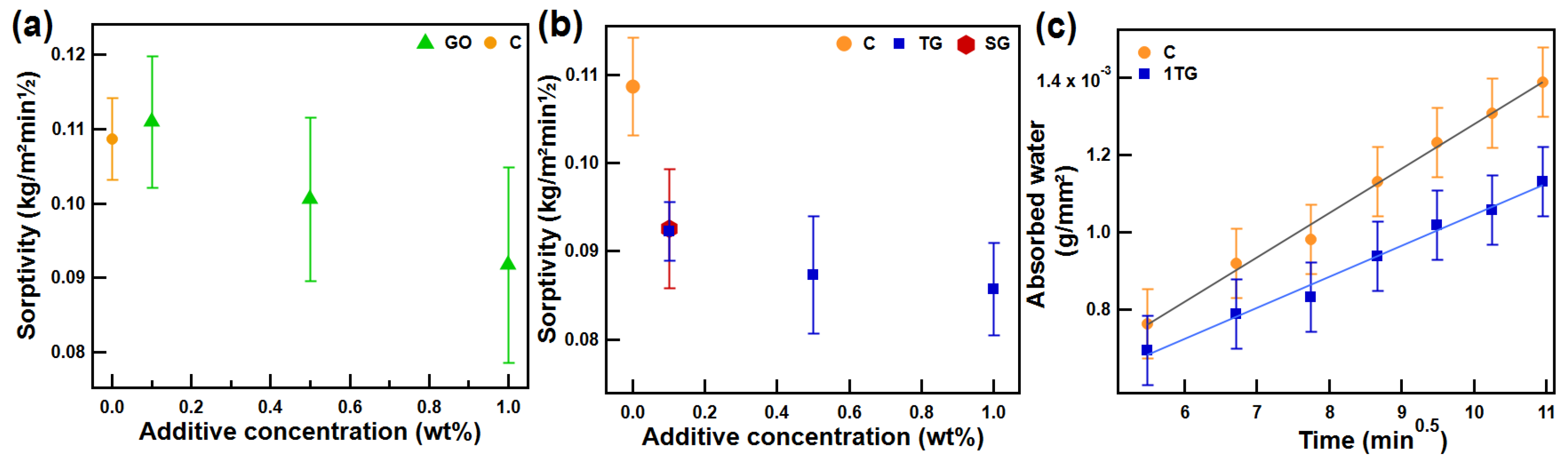
3.6. Capillary Water Absorption Test
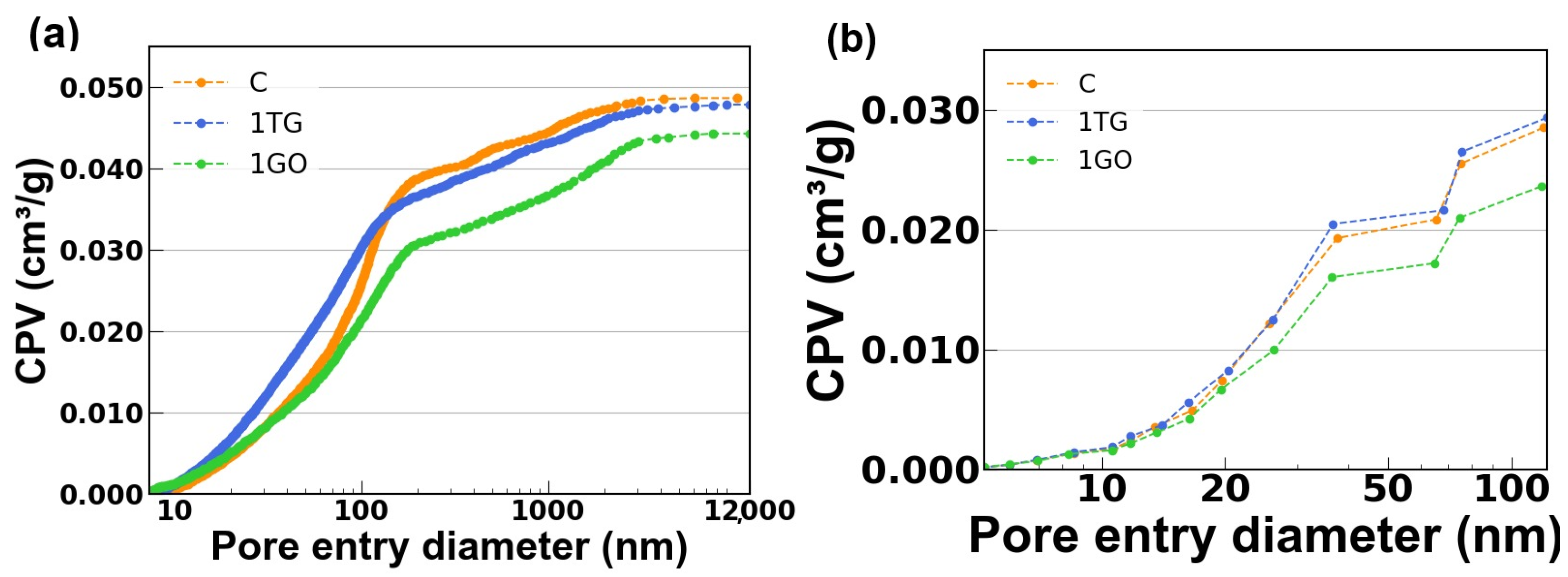
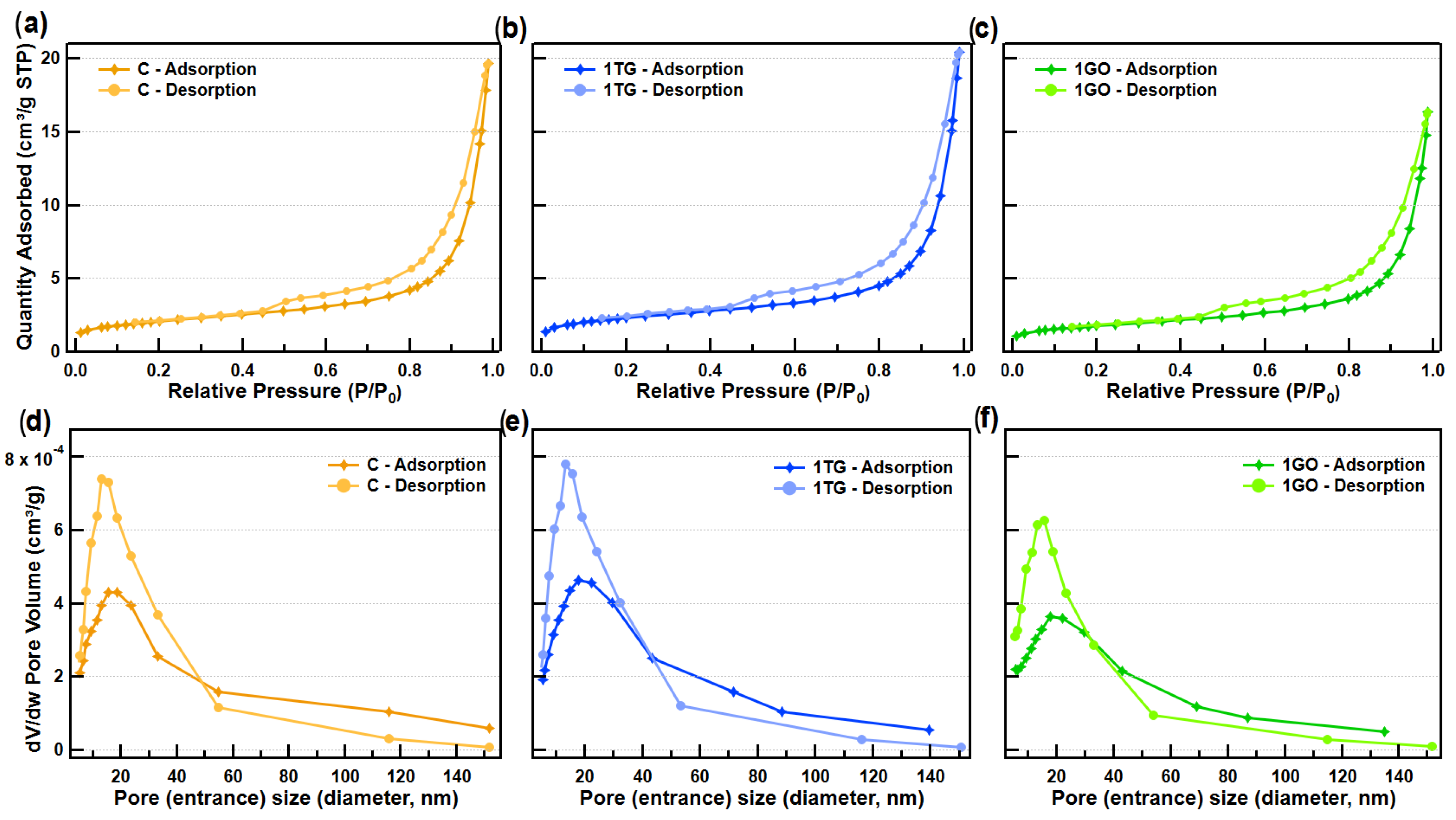
3.7. Porosimetry
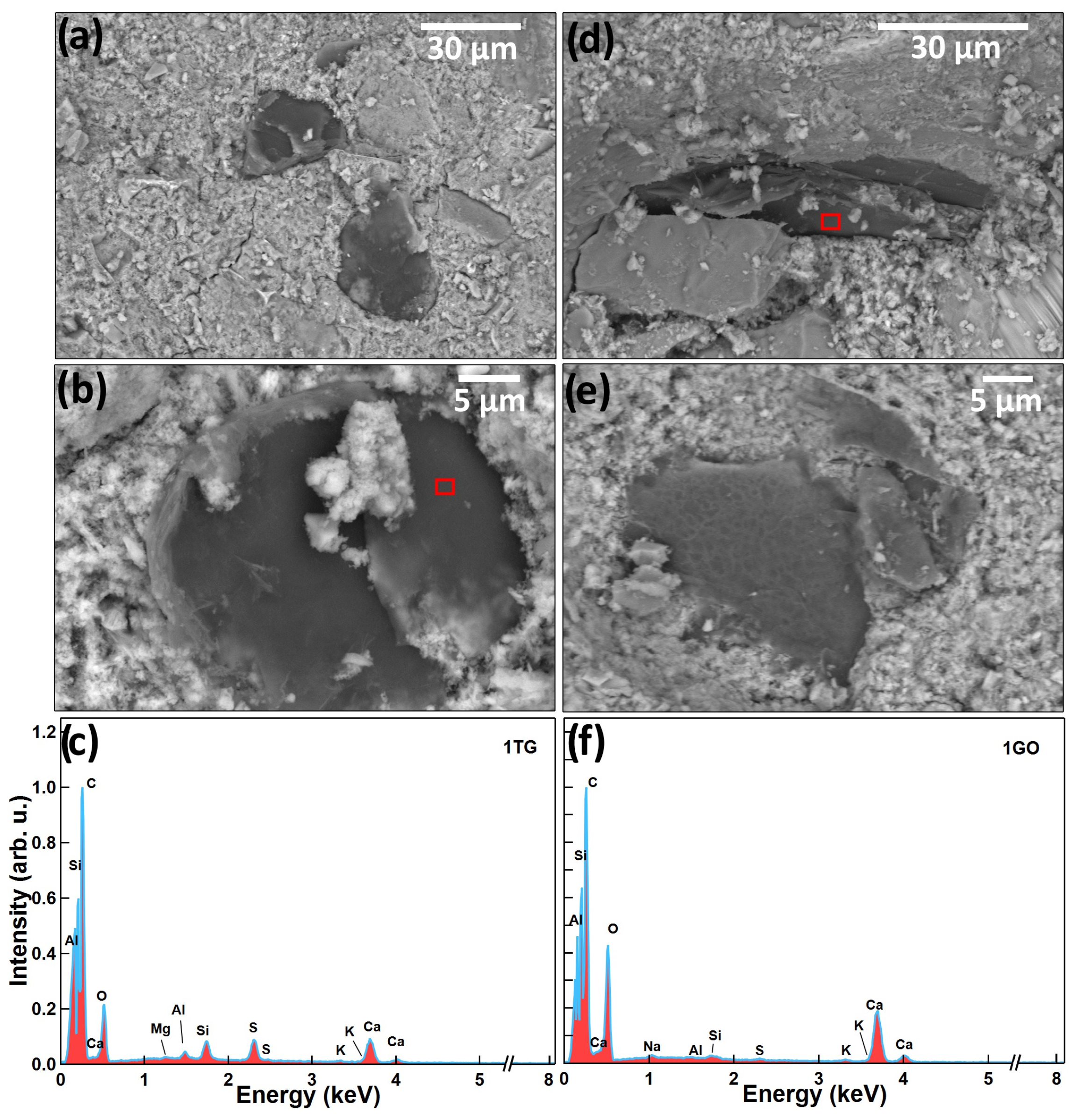
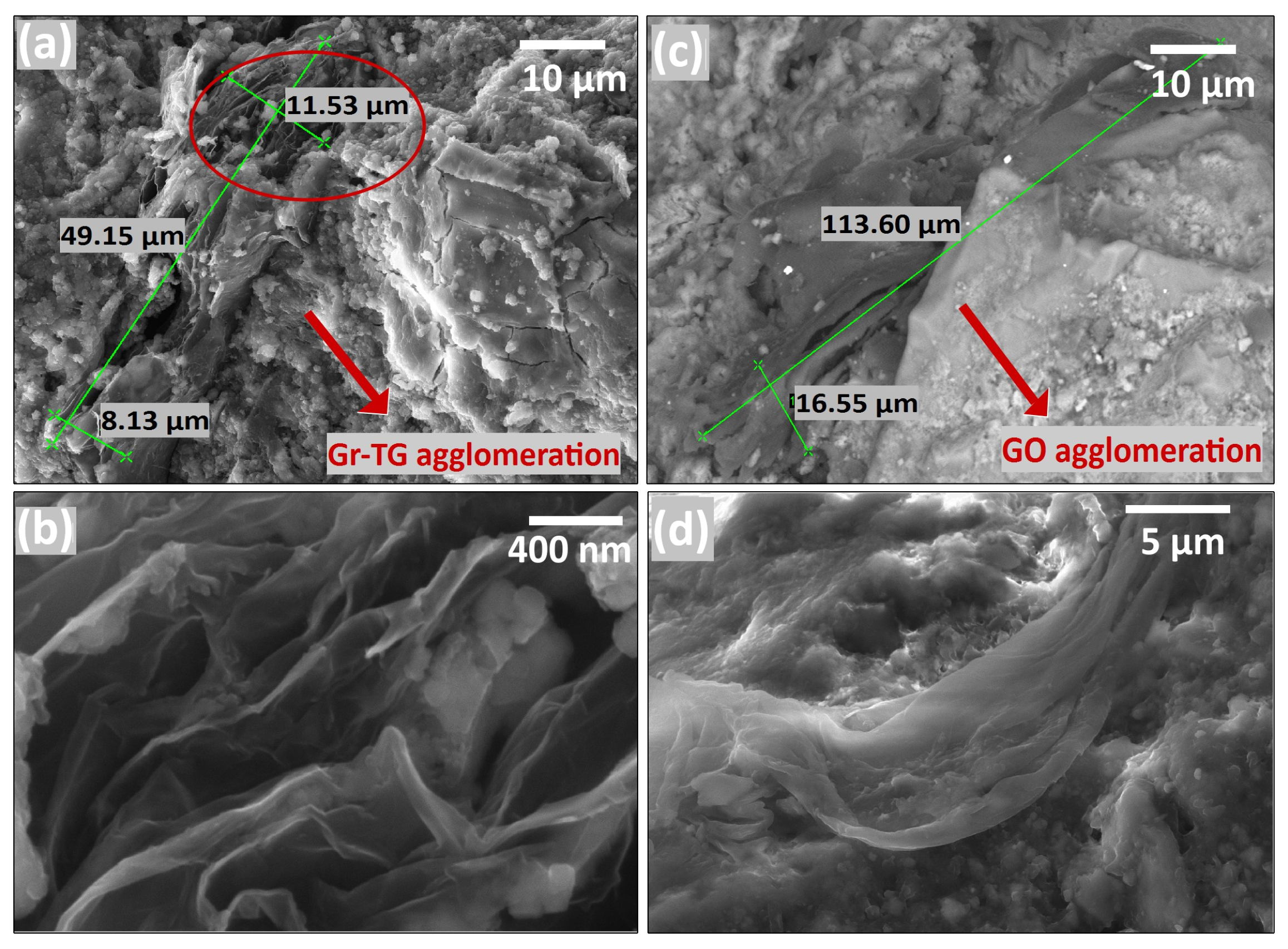
4. FESEM Measurements on Cement Mortar Specimens
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| BS | BackScattered light |
| CPV | Cumulative Pore Volume |
| CWA | Capillary Water Absorption |
| EDX | Energy Dispersive X-ray |
| FESEM | Field Emission Scanning Electron Microscopy |
| GNP | Graphene NanoPlatelet |
| GO | Graphene Oxide |
| Gr | Graphene |
| Gr-SG | Graphene Super Grade |
| Gr-TG | Graphene Technical Grade |
| HT | Hydrothermal Treatment |
| ITZ | Interfacial Transition Zone |
| LPE | Liquid Phase Exfoliation |
| MIP | Mercury Intrusion Porosimetry |
| OPC | Ordinary Portland Cement |
| PESD | Pore Entrance Size Distribution |
| PSD | Pore Size Distribution |
| PV | Pore Volume |
| S | Sorpitivity |
| SC | Sodium Cholate |
| SMLS | Static Multiple Light Scattering |
| SP | SuperPlasticizer |
| SSA | Specific Surface Area |
| T | Transmitted light |
| TOF | Time of Flight |
| TSI | Turbiscan Stability Index |
Appendix A. Raw Data for Porosimetry Study
References
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Han, B.; Cui, X.; Yu, X.; Ou, J. Graphene-engineered cementitious composites: Small makes a big impact. Nanomater. Nanotechnol. 2017, 7, 1847980417742304. [Google Scholar] [CrossRef]
- Shamsaei, E.; de Souza, F.B.; Yao, X.; Benhelal, E.; Akbari, A.; Duan, W. Graphene-based nanosheets for stronger and more durable concrete: A review. Constr. Build. Mater. 2018, 183, 642–660. [Google Scholar] [CrossRef]
- Wang, B.; Pang, B. Mechanical property and toughening mechanism of water reducing agents modified graphene nanoplatelets reinforced cement composites. Constr. Build. Mater. 2019, 226, 699–711. [Google Scholar] [CrossRef]
- Du, H.; Pang, S.D. Enhancement of barrier properties of cement mortar with graphene nanoplatelet. Cem. Concr. Res. 2015, 76, 10–19. [Google Scholar] [CrossRef]
- Liu, J.; Fu, J.; Yang, Y.; Gu, C. Study on dispersion, mechanical and microstructure properties of cement paste incorporating graphene sheets. Constr. Build. Mater. 2019, 199, 1–11. [Google Scholar] [CrossRef]
- Meng, W.; Khayat, K.H. Effect of graphite nanoplatelets and carbon nanofibers on rheology, hydration, shrinkage, mechanical properties, and microstructure of UHPC. Cem. Concr. Res. 2018, 105, 64–71. [Google Scholar] [CrossRef]
- Matalkah, F.; Soroushian, P. Graphene nanoplatelet for enhancement the mechanical properties and durability characteristics of alkali activated binder. Constr. Build. Mater. 2020, 249, 118773. [Google Scholar] [CrossRef]
- Sedaghat, A.; Ram, M.K.; Zayed, A.; Kamal, R.; Shanahan, N. Investigation of physical properties of graphene-cement composite for structural applications. Open J. Compos. Mater. 2014, 2014. [Google Scholar] [CrossRef]
- Lin, Y.; Du, H. Graphene reinforced cement composites: A review. Constr. Build. Mater. 2020, 265, 120312. [Google Scholar] [CrossRef]
- Sun, S.; Ding, S.; Han, B.; Dong, S.; Yu, X.; Zhou, D.; Ou, J. Multi-layer graphene-engineered cementitious composites with multifunctionality/intelligence. Compos. Part B Eng. 2017, 129, 221–232. [Google Scholar] [CrossRef]
- Dong, W.; Li, W.; Vessalas, K.; He, X.; Sun, Z.; Sheng, D. Piezoresistivity deterioration of smart graphene nanoplate/cement-based sensors subjected to sulphuric acid attack. Compos. Commun. 2021, 23, 100563. [Google Scholar] [CrossRef]
- Dong, W.; Li, W.; Zhu, X.; Sheng, D.; Shah, S.P. Multifunctional cementitious composites with integrated self-sensing and hydrophobic capacities toward smart structural health monitoring. Cem. Concr. Compos. 2021, 118, 103962. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Collins, F.; Lambert, J.; Duan, W.H. The influences of admixtures on the dispersion, workability, and strength of carbon nanotube–OPC paste mixtures. Cem. Concr. Compos. 2012, 34, 201–207. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, D.; Yang, C.; Liu, Y.; Liu, Y. Effect of graphene oxide on the rheological properties of cement pastes. Constr. Build. Mater. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Liu, C.; Hunag, X.; Wu, Y.Y.; Deng, X.; Zheng, Z.; Yang, B. Studies on mechanical properties and durability of steel fiber reinforced concrete incorporating graphene oxide. Cem. Concr. Compos. 2022, 130, 104508. [Google Scholar] [CrossRef]
- Polverino, S.; Del Rio Castillo, A.E.; Brencich, A.; Marasco, L.; Bonaccorso, F.; Morbiducci, R. Few-Layers Graphene-Based Cement Mortars: Production Process and Mechanical Properties. Sustainability 2022, 14, 784. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ukrainczyk, N.; Zaoui, A.; Koenders, E. Electrical conductivity of geopolymer-graphite composites: Percolation, mesostructure and analytical modeling. Constr. Build. Mater. 2024, 411, 134536. [Google Scholar] [CrossRef]
- Li, W.; Qu, F.; Dong, W.; Mishra, G.; Shah, S.P. A comprehensive review on self-sensing graphene/cementitious composites: A pathway toward next-generation smart concrete. Constr. Build. Mater. 2022, 331, 127284. [Google Scholar] [CrossRef]
- Dimov, D.; Amit, I.; Gorrie, O.; Barnes, M.D.; Townsend, N.J.; Neves, A.I.; Withers, F.; Russo, S.; Craciun, M.F. Ultrahigh performance nanoengineered graphene–concrete composites for multifunctional applications. Adv. Funct. Mater. 2018, 28, 1705183. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, R.; Wu, Z. Investigation of the Mechanical Properties and Microstructure of Graphene Nanoplatelet-Cement Composite. Nanomaterials 2016, 6, 200. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide–cement composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Yan, X.; Zheng, D.; Yang, H.; Cui, H.; Monasterio, M.; Lo, Y. Study of optimizing graphene oxide dispersion and properties of the resulting cement mortars. Constr. Build. Mater. 2020, 257, 119477. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Gao, T.; Ling, Y.; Jiang, N.; Tawfek, A.M.; Yuan, H. Optimization of Graphene Nanoplatelets Dispersion and Its Performance in Cement Mortars. Materials 2022, 15, 7308. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.; Datta, A. Disentangling the liquid phase exfoliation of two- dimensional materials: An ”in silico” perspective. Phys. Chem. Chem. Phys. 2020, 22, 22157–22179. [Google Scholar] [CrossRef]
- Han, J.; Jang, J.; Kim, H.; Hwang, J.Y.; Yoo, H.K.; Woo, J.; Choi, S.; Kim, H.; Jeong, H.; Jeong, S.; et al. Extremely Efficient Liquid Exfoliation and Dispersion of Layered Materials by Unusual Acoustic Cavitation. Sci. Rep. 2014, 4, 5133. [Google Scholar] [CrossRef]
- Paton, K.; Varrla, E.; Backes, C.; Smith, R.; Khan, U.; Gallagher, A.; Boland, C.; Lotya, M.; Istrate, O.; King, P.; et al. Scalable production of large quantities, defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Paton, K.R.; Anderson, J.; Pollard, A.J.; Sainsbury, T. Production of few-layer graphene by microfluidization. Mater. Res. Express 2017, 4, 025604. [Google Scholar] [CrossRef]
- Ramalingam, P.; Pusuluri, S.T.; Periasamy, S.; Veerabahu, R.; Kulandaivel, J. Role of deoxy group on the high concentration of graphene in surfactant/water media3. RSC Adv. 2013, 3, 2369–2378. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, M.; Yang, M.; Zhang, Z.; Yu, J.; Zhang, Y.; Cheng, W.; Li, X. High-Yield Production of Few-Layer Graphene via New-fashioned Strategy Combining Resonance Ball Milling and Hydrothermal Exfoliation. Nanomaterials 2020, 10, 667. [Google Scholar] [CrossRef]
- Wei, X.X.; Jia, Q.; Zheng, C.; Zhu, J.H.; Pei, C. Enhancement in durability and performance for cement-based materials through tailored water-based graphene nanofluid additives. Constr. Build. Mater. 2024, 457, 139455. [Google Scholar] [CrossRef]
- Nochaiya, T.; Chaipanich, A. Behavior of multi-walled carbon nanotubes on the porosity and microstructure of cement-based materials. Appl. Surf. Sci. 2011, 257, 1941–1945. [Google Scholar] [CrossRef]
- Chen, J.; Kou, S.; Poon, C.S. Hydration and properties of nano-TiO2 blended cement composites. Cem. Concr. Compos. 2012, 34, 642–649. [Google Scholar] [CrossRef]
- Jing, G.; Ye, Z.; Wu, J.; Wang, S.; Cheng, X.; Strokova, V.; Nelyubova, V. Introducing reduced graphene oxide to enhance the thermal properties of cement composites. Cem. Concr. Compos. 2020, 109, 103559. [Google Scholar] [CrossRef]
- UNI EN 1015-3:2007; Consistency of Wet Mortar. European Committee for Standardization: Brussels, Belgium, 2007.
- UNI EN 12504-4:2005; Testing Concrete—PART 4: Determination of Ultrasonic Pulse Velocity. European Committee for Standardization: Brussels, Belgium, 2005.
- Hernández, M.; Anaya, J.; Izquierdo, M.; Ullate, L. Application of micromechanics to the characterization of mortar by ultrasound. Ultrasonics 2002, 40, 217–221. [Google Scholar] [CrossRef]
- UNI EN 1015-11:2001; Methods of Test for Mortar for Masonry—Determination of Flexural and Compressive Strength of Hardened Mortar. European Committee for Standardization: Brussels, Belgium, 1999.
- UNI EN 1015-18; Methods of Test for Mortar for Masonry—Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar. European Committee for Standardization: Brussels, Belgium, 2002.
- Candamano, S.; De Luca, P.; Frontera, P.; Crea, F. Production of Geopolymeric Mortars Containing Forest Biomass Ash as Partial Replacement of Metakaolin. Environments 2017, 4, 74. [Google Scholar] [CrossRef]
- Jaroniec, M.; Kruk, M.; Sayari, A. Adsorption methods for characterization of surface and structural properties of mesoporous molecular sieves. Stud. Surf. Sci. Catal. 1998, 117, 325–332. [Google Scholar] [CrossRef]
- Chen, Z.; Qing, H.; Zhou, K.; Sun, D.; Wu, R. Metal-organic framework-derived nanocomposites for electrocatalytic hydrogen evolution reaction. Prog. Mater. Sci. 2020, 108, 100618. [Google Scholar] [CrossRef]
- Li, Z.; Kruk, M.; Jaroniec, M. Synthesis and adsorption properties of novel carbons of tailored porosity. Stud. Surf. Sci. Catal. 2002, 141, 345–352. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Davankov, V.; Tsyurupa, M. Properties of Hypercrosslinked Polystyrene. Compr. Anal. Chem. 2011, 56, 195–295. [Google Scholar] [CrossRef]
- Parveen, S.; Rana, S.; Fangueiro, R. A review on nanomaterial dispersion, microstructure, and mechanical properties of carbon nanotube and nanofiber reinforced cementitious composites. J. Nanomater. 2013, 2013, 710175. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Backes, C.; Paton, K.; Hanlon, D.; Yuan, S.; Katsnelson, M.; Houston, J.; Smith, R.; Mccloskey, D.; Donegan, J.; Coleman, J. Spectroscopic metrics allow in-situ measurement of mean size and thickness of liquid-exfoliated graphene nanosheets. Nanoscale 2016, 8, 4311–4323. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. Aip Adv. 2012, 2. [Google Scholar] [CrossRef]
- Samimi, K.; Pakan, M.; Eslami, J.; Asgharnejad, L. Investigation of two different water-dispersed graphene on the performance of graphene/cement paste: Surfactant and superplasticizer effect. Constr. Build. Mater. 2022, 349, 128756. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; An, M.; Sun, Y.; Yu, Z.; Huang, H. Factors Influencing the Capillary Water Absorption Characteristics of Concrete and Their Relationship to Pore Structure. Appl. Sci. 2022, 12, 2211. [Google Scholar] [CrossRef]
- Gao, Y.; Jing, H.; Zhou, Z.; Chen, W.; Du, M.; Du, Y. Reinforced impermeability of cementitious composites using graphene oxide-carbon nanotube hybrid under different water-to-cement ratios. Constr. Build. Mater. 2019, 222, 610–621. [Google Scholar] [CrossRef]
- Sills, I.; Aylmore, L.; Quirk, J. A comparison between mercury injection and nitrogen sorption as methods of determining pore size distributions. Soil Sci. Soc. Am. J. 1973, 37, 535–537. [Google Scholar] [CrossRef]
- Wu, Z. Discussion on the recent development direction of concrete science and technology. J. Chin. Ceram. Soc 1979, 3, 262–270. [Google Scholar]
- Brown, S.M.; Lard, E.W. A comparison of nitrogen and mercury pore size distributions of silicas of varying pore volume. Powder Technol. 1974, 9, 187–190. [Google Scholar] [CrossRef]
- Hajnos, M. Porosimetry; Springer: Dordrecht, The Netherlands, 2011; pp. 647–650. [Google Scholar] [CrossRef]
- Milburn, D.R.; Davis, B.H. Comparison of surface areas calculated from nitrogen adsorption and Mercury porosimetry. In A Collection of Papers on Engineering Aspects of Fabrication of Ceramics: Ceramic Engineering and Science Proceedings; The American Ceramics Society: Westerville, OH, USA, 1993; Volume 14, pp. 130–134. [Google Scholar] [CrossRef]
- Wang, X. Chapter 3—Lacustrine Shale Gas Reservoir in the Ordos Basin. In Lacustrine Shale Gas; Wang, X., Ed.; Gulf Professional Publishing: Houston, TX, USA, 2017; pp. 93–178. [Google Scholar] [CrossRef]
- Pastorino, D.; Canal, C.; Ginebra, M.P. Multiple characterization study on porosity and pore structure of calcium phosphate cements. Acta Biomater. 2015, 28, 205–214. [Google Scholar] [CrossRef]
- Lai, W.; Yang, S.; Jiang, Y.; Zhao, F.; Li, Z.; Zaman, B.; Fayaz, M.; Li, X.; Chen, Y. Artefact peaks of pore size distributions caused by unclosed sorption isotherm and tensile strength effect. Adsorption 2020, 26, 633–644. [Google Scholar] [CrossRef]

| Raw | Characteristics | ||||||
|---|---|---|---|---|---|---|---|
| Materials | |||||||
| Cement | Manufactured in accordance to standard UNI EN 197-1. Main Components, in terms of oxides: | ||||||
| SiO2 = 18.61 wt%; Fe2O3 = 2.87 wt%; Al2O3 = 4.38 wt%; CaO = 60.60 wt%; MgO = 3.16 wt%; | |||||||
| K2O = 0.79 wt%; Na2O = 0.35 wt%; MnO = 0.17 wt%; SO3 = 2.83 wt%; TiO2 = 0.2 wt%. | |||||||
| Particle size distribution, based on volume distribution: D10 = 8.414 µm; D50 = 19.84 µm; | |||||||
| D90 = 36.11 µm. | |||||||
| Additive | SSA | Thickness | Lateral | Bulk | Elemental composition (%) | ||
| type | (m2/g) | (nm) | size | density | C | O | S |
| (g/cm3) | |||||||
| Gr-TG | 80 | 10–20 | 10 µm | 0.25 | >99 | <1 | <1 |
| Gr-SG | >250 | 1.6 | 6–100 nm | 0.06 | >99 | 0 | 0 |
| GO | 350 | 10–20 | <10 µm | 0.35 | 74 | 25 | 0.34 |
| Sample Label | Additive Type | Additive Concentr. (wt%) | SC/Additive Ratio | HT Treat. (h–°C) | LPE Treat. (h) |
|---|---|---|---|---|---|
| D-TGa | Gr-TG | 1.53 | 1/5 | 6–180 | 6 |
| D-TGb | Gr-TG | 2.50 | 1/5 | 6–180 | 6 |
| D-SGa | Gr-SG | 1.53 | 1/5 | 6–180 | 6 |
| D-GOa | GO | 1.53 | 0 | 0 | 6 |
| D-GOb | GO | 2.50 | 0 | 0 | 6 |
| Mortar Label | Dispersion Type | Carbon Nanomaterial (wt% *) | w/c Ratio by wt. | s/c Ratio by wt. | SP/c Ratio by wt. |
|---|---|---|---|---|---|
| C | - | 0 | 0.475 | 2.5 | 0.009 |
| 01TG | D-TGa | 0.1 | 0.475 | 2.5 | 0.009 |
| 03TG | D-TGa | 0.3 | 0.475 | 2.5 | 0.009 |
| 05TG | D-TGb | 0.5 | 0.475 | 2.5 | 0.009 |
| 1TG | D-TGb | 1.0 | 0.475 | 2.5 | 0.009 |
| 01SG | D-SGa | 0.1 | 0.475 | 2.5 | 0.009 |
| 03SG | D-SGa | 0.3 | 0.475 | 2.5 | 0.009 |
| 01GO | D-GOa | 0.1 | 0.475 | 2.5 | 0.009 |
| 05GO | D-GOb | 0.5 | 0.475 | 2.5 | 0.009 |
| 1GO | D-GOb | 1.0 | 0.475 | 2.5 | 0.009 |
| Specimen | W (%) | ΔW |
|---|---|---|
| C | 119.05 | 0.05 |
| 03SG | 64.76 | 0.05 |
| 03TG | 116.19 | 0.13 |
| 01GO | 106.19 | 0.20 |
| 05GO | 108.04 | 0.08 |
| 1GO | 70.89 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerace, T.; Candamano, S.; Bartucci, S.; Poselle Bonaventura, C.; Policicchio, A.; Agostino, R.G.; Marroccoli, M.; Telesca, A.; Davoli, M.; Scarcello, A.; et al. On the Stability of Graphene-Based Aqueous Dispersions and Their Performance in Cement Mortar. Appl. Sci. 2025, 15, 835. https://doi.org/10.3390/app15020835
Gerace T, Candamano S, Bartucci S, Poselle Bonaventura C, Policicchio A, Agostino RG, Marroccoli M, Telesca A, Davoli M, Scarcello A, et al. On the Stability of Graphene-Based Aqueous Dispersions and Their Performance in Cement Mortar. Applied Sciences. 2025; 15(2):835. https://doi.org/10.3390/app15020835
Chicago/Turabian StyleGerace, Teresa, Sebastiano Candamano, Simone Bartucci, Carlo Poselle Bonaventura, Alfonso Policicchio, Raffaele Giuseppe Agostino, Milena Marroccoli, Antonio Telesca, Mariano Davoli, Andrea Scarcello, and et al. 2025. "On the Stability of Graphene-Based Aqueous Dispersions and Their Performance in Cement Mortar" Applied Sciences 15, no. 2: 835. https://doi.org/10.3390/app15020835
APA StyleGerace, T., Candamano, S., Bartucci, S., Poselle Bonaventura, C., Policicchio, A., Agostino, R. G., Marroccoli, M., Telesca, A., Davoli, M., Scarcello, A., Caputi, L. S., & Pacilè, D. (2025). On the Stability of Graphene-Based Aqueous Dispersions and Their Performance in Cement Mortar. Applied Sciences, 15(2), 835. https://doi.org/10.3390/app15020835










