Silver Nanoparticle-Infused Pullulan Films for the Inhibition of Foodborne Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Culture and Inoculum Preparation
2.3. Determination of MIC and MBC of AgNPs
2.4. Time–Kill Assay
2.5. Determination of Nucleic Acid and Protein Leakage
2.6. Bacterial Morphology Exposed to AgNPs Observed by SEM and TEM
2.7. Preparation of Pullulan Film with Colloidal Solution of AgNPs (HydroSilver 1000)
2.7.1. Antibacterial Activity of Pullulan Film with AgNPs Determined Using Disk Diffusion Methods
2.7.2. Scanning Electron Microscopy
2.7.3. Thickness
2.7.4. Opacity and Light Transmittance
2.7.5. Color Properties of Pullulan Film
2.8. Statistical Analyses
3. Results
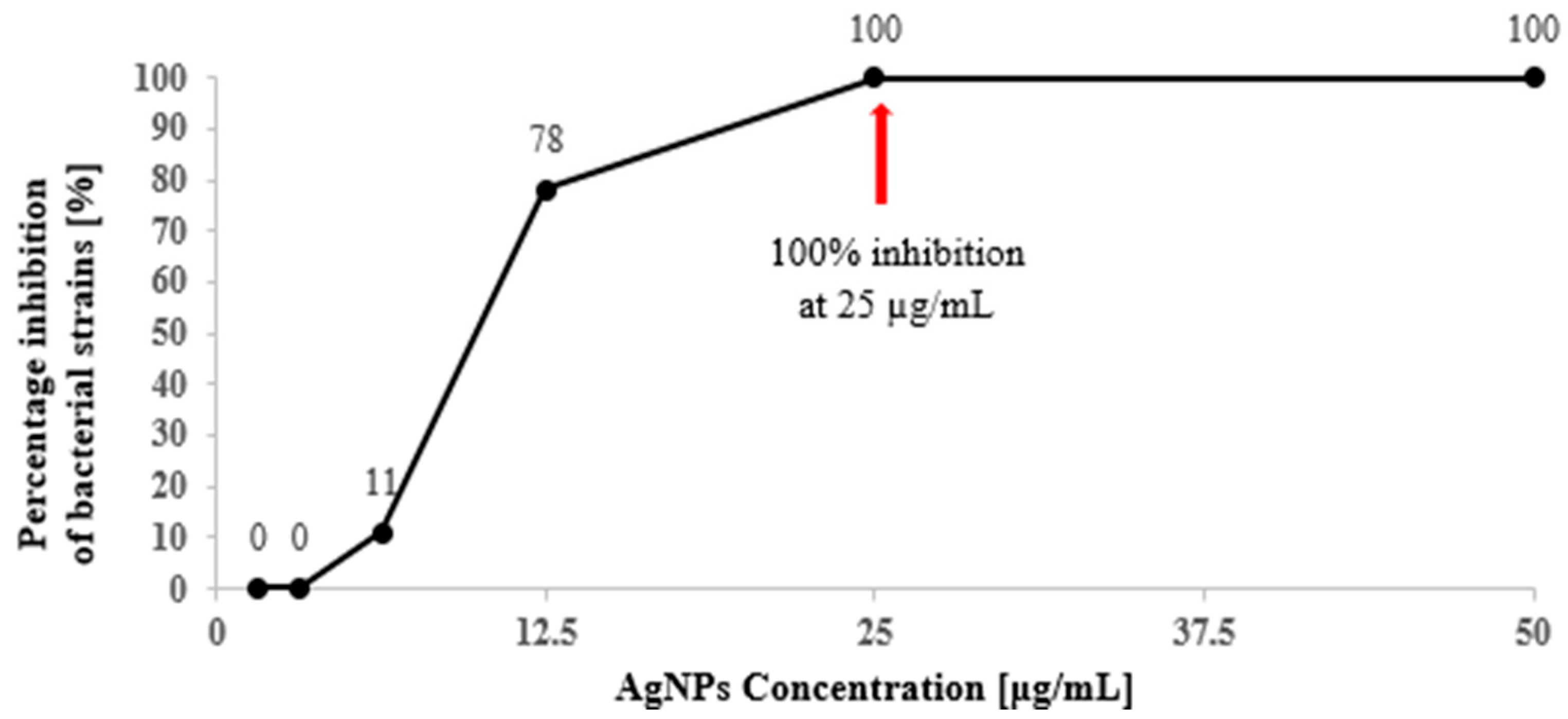
3.1. MIC and MBC of AgNPs Against Foodborne Bacteria
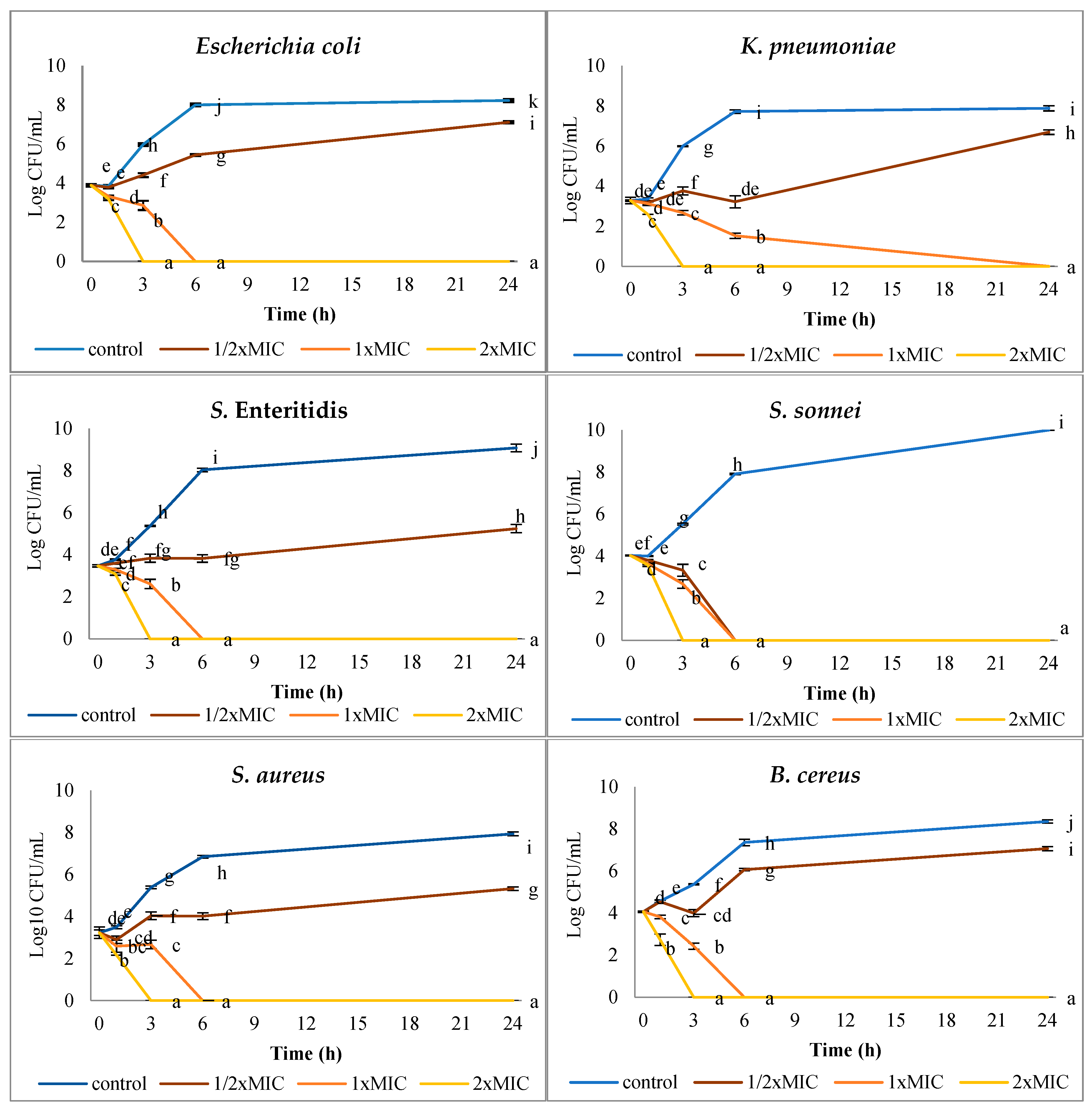
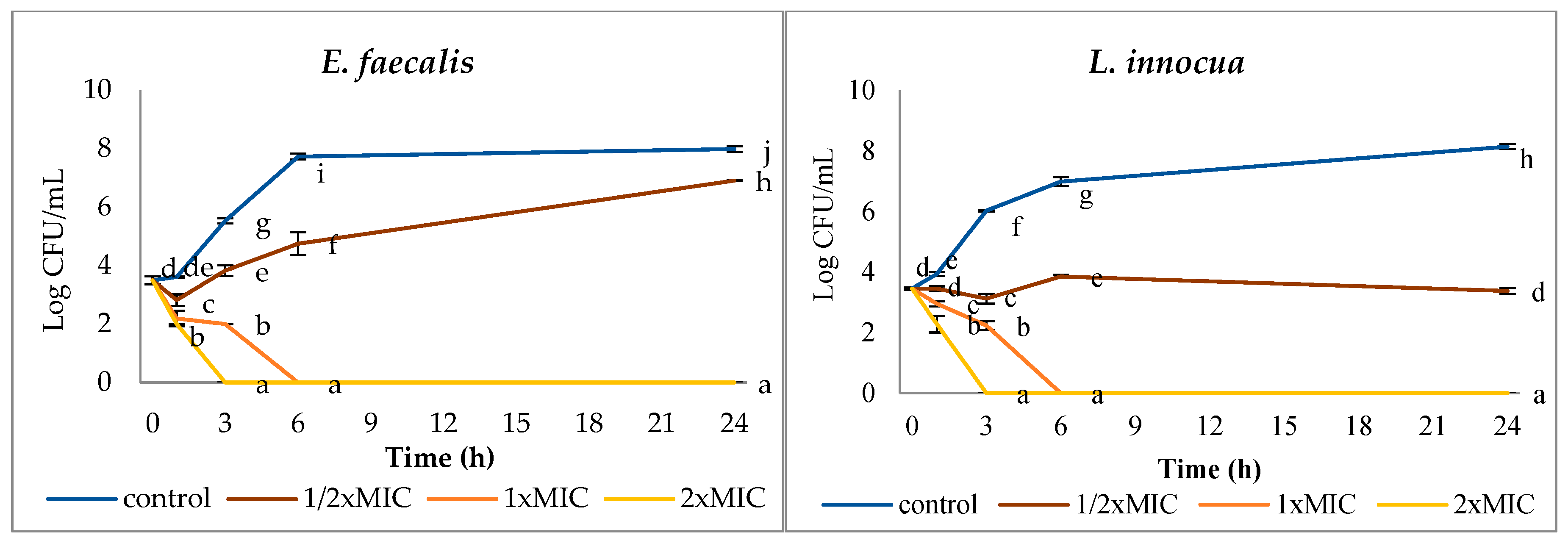
3.2. Effect of AgNPs on Bacterial Survival
3.3. Leakage of DNA and Intracellular Proteins from Cells Assessed by Spectrophotometric Measurements
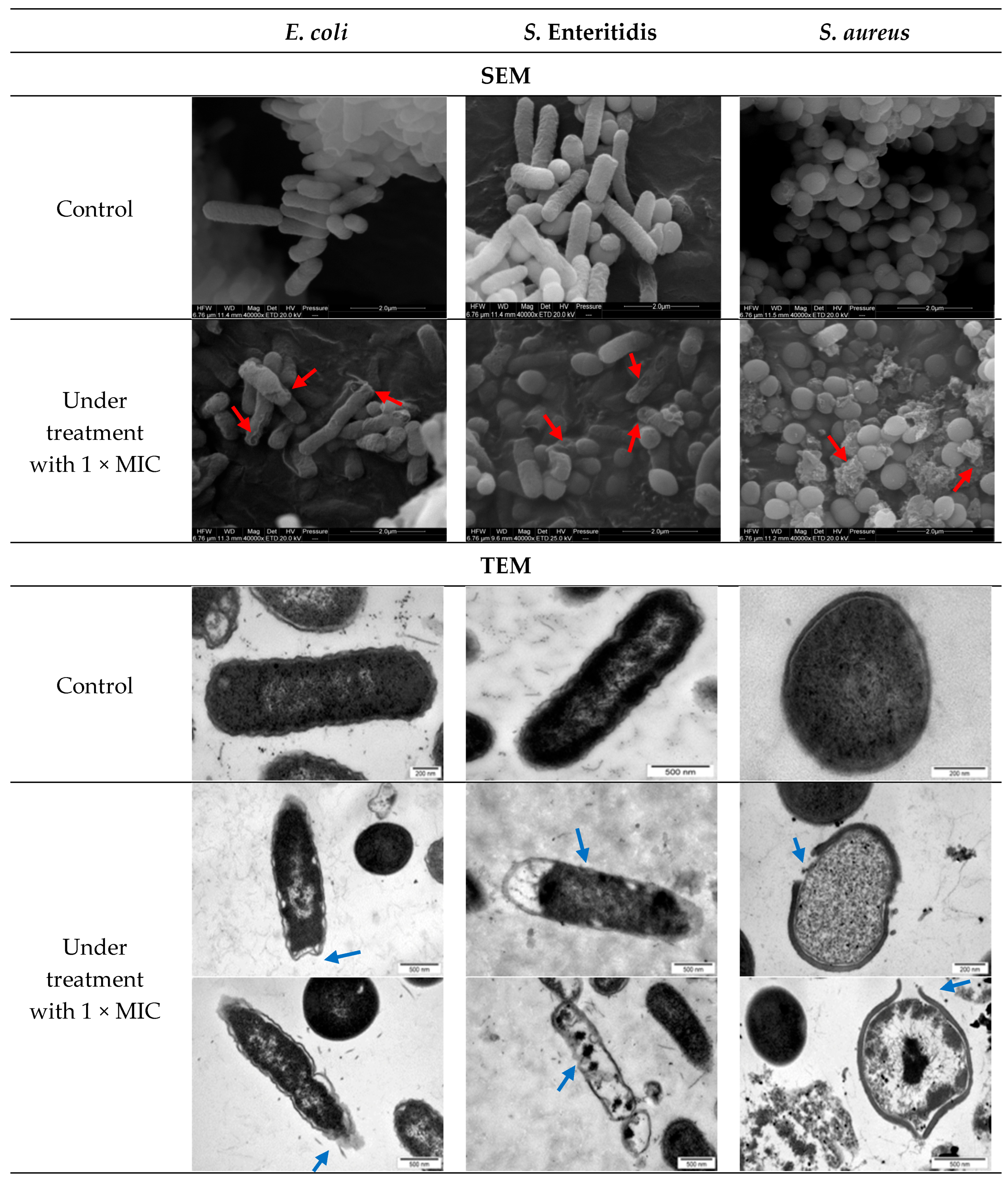
3.4. Morphological Assessment of Bacterial Cells After AgNPs Exposure
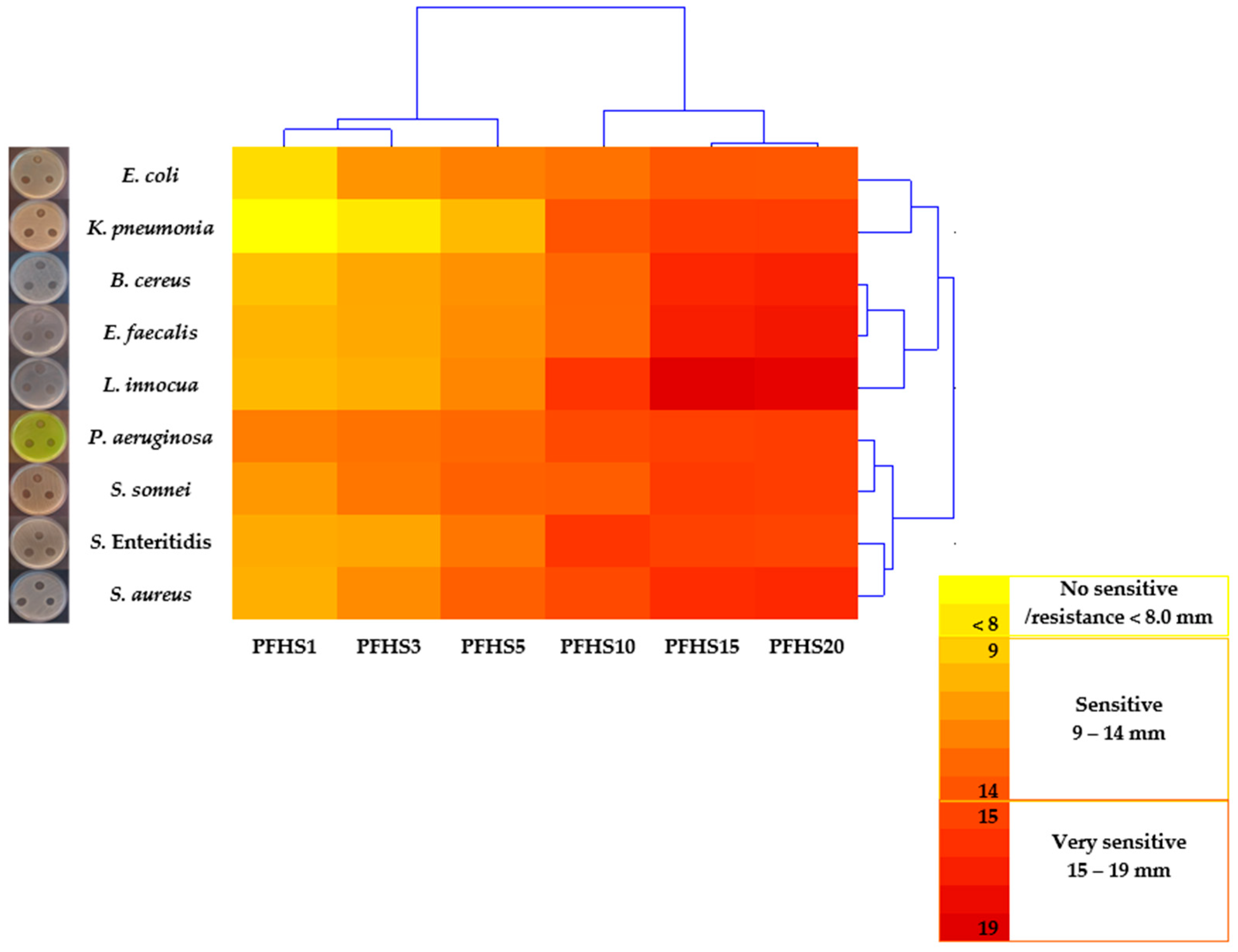
3.5. Antibacterial Activity of Pullulan Films with AgNPs
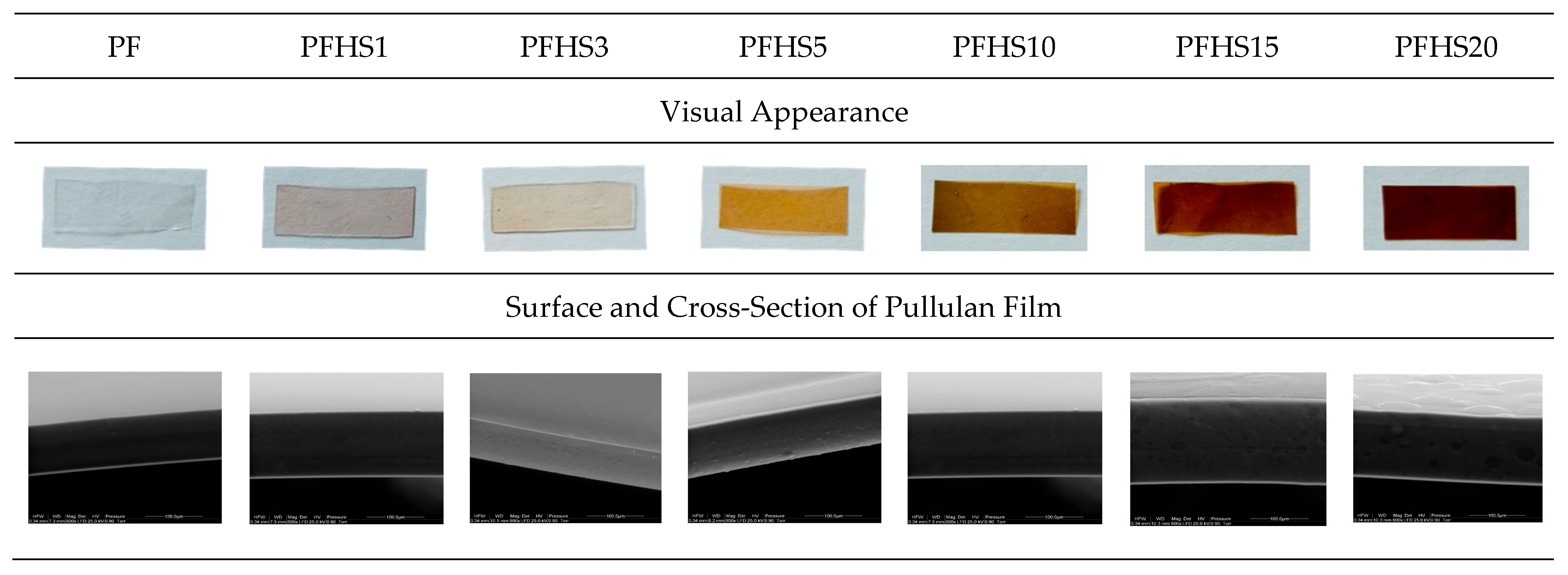
3.6. Visual Appearance and Microscopic Characterization of Pullulan Films with AgNPs
3.7. Physical Properties of Pullulan Films with AgNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slepička, P.; Kasálkova, N.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of Gold and Silver Nanoparticles Preparation. Materials 2020, 13, 1. [Google Scholar]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Kaiser, K.G.; Delattre, V.; Frost, G.W.; Buck, G.W.; Phu, J.V.; Fernandez, T.G.; Pavel, I.E. Nanosilver: An old antibacterial agent with great promise in the fight against antibiotic resistance. Antibiotics 2023, 12, 1264. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Núñez, N.V.; Ixtepan Turrent, L.D.C.; Rodríguez Padilla, C. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Stevens, S.E., Jr. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals 1998, 11, 27–32. [Google Scholar] [PubMed]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.J.; Shah, S.I. Synthesis and Antibacterial Properties of Silver Nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 244–249. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Kokura, S.; Handa, O.; Takagi, T.; Ishikawa, T.; Naito, Y.; Yoshikawa, T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 570–574. [Google Scholar] [CrossRef]
- Goa, Y.; Cranston, R. Recent advances in antimicrobial treatments of textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar] [CrossRef]
- Rao, M.M.V.; Mohammad, N.; Banerjee, S.; Khanna, P.K. Synthesis and food packaging application of silver nano-particles: A review. Hybrid Adv. 2024, 6, 100230. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications–A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
- Bin Ahmad, M.B.; Lim, J.J.; Shameli, K.; Ibrahim, N.A.; Tay, M.E.; Chieng, B.W. Antibacterial activity of silver bionanocoposites synthesized by chemical reduction rout. Chem. Cent. J. 2012, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.-C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef]
- Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Silver Bionanocomposites as Active Food Packaging: Recent Advances & Future Trends Tackling the Food Waste Crisis. Polymers 2023, 15, 4243. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abdel-Aziz, M.S.; El-Sayed, S.M. Chitosan nanocomposite films based on Ag-NP and Au-NP biosynthesis by Bacillus subtilis as packaging materials. Int. J. Biol. Macromol. 2014, 69, 185–191. [Google Scholar] [CrossRef]
- Wang, H.; Shi, T.; Ma, J.; Meng, S.; Wei, Z.; Sun, Y.; Wang, H.; Zhou, M. Chitosan-based nanocomposite films incorporated with AgNPs/porphyrinic MOFs for killing pathogenic bacteria. Int. J Biol. Macromol. 2025, 295, 139584. [Google Scholar] [CrossRef]
- Ortega, F.; Minnaard, J.; Arce, V.B.; García, M.A. Biodegradable starch-based nanocomposite films with laser-synthesized silver nanoparticles: A materials approach for packaging. Int. J. Biol. Macromol. 2025, 318, 144904. [Google Scholar] [CrossRef]
- Varsha, C.; Bajpai, S.K.; Navin, C. Investigation of water vapour permeation and anti-bacterial properties of nano silver loaded cellulose acetate film. Int. Food Res. J. 2010, 17, 623–639. [Google Scholar]
- De Moura, M.R.; Mattoso, L.H.C.; Zucolotto, V. Development of cellulose-based bactericidal nanocomposites containing silver nanoparticles and their use as active food packaging. J. Food Eng. 2012, 109, 520–524. [Google Scholar] [CrossRef]
- Maloufi, M.; Djelad, A.; Mokhtar, A.; Reguig, K.; Hasnaoui, M.A.; Kebir-Medjhouda, Z.A.; Ghamni, M.; Sassi, M. Fabrication and characterization of cellulose-based packaging film with polyethylene glycol and silver nanoparticles for enhanced antimicrobial efficacy. Int. J Biol. Macromol. 2025, 308, 142381. [Google Scholar] [CrossRef] [PubMed]
- Gniewosz, M.; Duszkiewicz-Reinhard, W. Comparative studies on pullulan synthesis, melanin synthesis and morphology of white mutant Aureobasidium pullulans B-1 and parent strain A.p.-3. Carbohydr. Polym. 2008, 72, 431–438. [Google Scholar] [CrossRef]
- European Parliament and the Council (EC). Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council Regulation (EU) No 231/2012 of the European Parliament and of the Council. Off. J. Eur. Union 2012, L81, 1–295. [Google Scholar]
- European Parliament and the Council (EC). Regulation (EC) No 1333/2008 of 16 December 2008 on food additives. Off. J. Eur. Union 2008, L354, 16–33. [Google Scholar]
- Food and Drug Administration. GRAS Notice No. GRN 000099. 2002. Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=grasnotices&id=99 (accessed on 20 July 2025).
- EFSA Panel on Food Additives and Flavourings (FAF); Castle, L.; Andreassen, M.; Aquilina, G.; Bastos, M.L.; Boon, P.; Fallico, B. Re-evaluation of pullulan (E 1204) as a food additive and new application for its extension of use. EFSA J. 2002, 23, e9267. [Google Scholar]
- Kraśniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan—Biopolymer with potential for use as food packaging. Int. J. Food Eng. 2019, 15, 20190030. [Google Scholar] [CrossRef]
- Ghosh, T.; Priyadarshi, R.; Krebs de Souza, C.; Angioletti, B.L. Advances in pullulan utilization for sustainable applications in food packaging and preservation: A mini-review. Trends Food Sci. Technol. 2022, 125, 43–53. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, F.; Chen, L.; Lyu, W.; Chi, Z.; Liu, C.; Chi, Z. An alternative hard capsule prepared with the high molecular weight pullulan and gellan: Processing, characterization, and in vitro drug release. Carbohydr. Polym. 2020, 237, 116172. [Google Scholar] [CrossRef]
- Diab, T.; Biliaderis, C.G.; Gerasopoulous, D.; Sfakiotakis, E. Physicochemical properties and applications of pullulan edible films and coatings in fruit preservation. J. Sci. Food Agric. 2001, 81, 988–1000. [Google Scholar] [CrossRef]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan films containing rockrose essential oil for potential food packaging applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef]
- Chu, Y.; Cheng, W.; Feng, X.; Gao, C.; Wu, D.; Meng, L.; Zhang, Y.; Tang, X. Fabrication, structure, and properties of pullulan-based active films incorporated with ultrasound-assisted cinnamon essential oil nanoemulsions. Food Packag. Shelf Life 2020, 25, 100547. [Google Scholar]
- Kraśniewska, K.; Gniewosz, M.; Synowiec, A.; Przybył, J.L.; Bączek, K.; Węglarz, Z. The use of pullulan coating enriched with plant extracts from Satureja hortensis L. to maintain pepper and apple quality and safety. Postharvest Biol. Technol. 2014, 90, 63–72. [Google Scholar] [CrossRef]
- Synowiec, A.; Gniewosz, M.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Węglarz, Z. Antimicrobial and antioxidant properties of pullulan film containing sweet basil extract and an evaluation of coating effectiveness in the prolongation of the shelf life of apples stored in refrigeration conditions. Innov. Food Sci. Emerg. Technol. 2014, 23, 171–181. [Google Scholar] [CrossRef]
- Gniewosz, M.; Synowiec, A.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Węglarz, Z. The antimicrobial activity of pullulan film incorporated with meadowsweet flower extracts (Filipendula ulmariae flos) on postharvest quality of apples. Food Control 2014, 37, 351–361. [Google Scholar] [CrossRef]
- Trinetta, V.; Floros, J.D.; Cutter, C.N. Sakacin A-containing pullulan film: An active packaging system to control epidemic clones of Listeria monocytogenes in ready-to-eat foods. J. Food Saf. 2010, 30, 366–381. [Google Scholar] [CrossRef]
- Kanmani, P.; Lim, S.T. Synthesis and characterization of pullulan-mediated silver nanoparticles and its antimicrobial activities. Carbohydr. Polym. 2013, 97, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Burduniuc, O.; Bostanaru, A.-C.; Mares, M.; Biliuta, G.; Coseri, S. Synthesis, Characterization, and Antifungal Activity of Silver Nanoparticles Embedded in Pullulan Matrices. Materials 2021, 14, 7041. [Google Scholar] [CrossRef]
- Ghaffarlou, M.; İlk, S.; Hammamchi, H.; Kıraç, F.; Okan, M.; Güven, O.; Barsbay, M. Green and Facile Synthesis of Pullulan-Stabilized Silver and Gold Nanoparticles for the Inhibition of Quorum Sensing. ACS Appl. Bio Mater. 2022, 5, 517–527. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Almeida, A.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Trindade, T. Antifungal activity of transparent nanocomposite thin films of pullulan and silver against Aspergillus niger. Colloids Surf. B Biointerfaces 2013, 103, 143–148. [Google Scholar] [CrossRef]
- Wypij, M.; Rai, M.; Zemljič, L.F.; Bračič, M.; Hribernik, S.; Golińska, P. Pullulan-based films impregnated with silver nanoparticles from the Fusarium culmorum strains JTW1 for potential applications in food industry and medicine. Front. Bioeng. Biotechnol. 2023, 11, 1241739. [Google Scholar] [CrossRef]
- Khalaf, H.; Sharoba, A.; El-Tanahi, H.; Morsy, M. Stability of antimicrobial activity of pullulan edible films incorporated with nanoparticles and essential oils and their impact on Turkey deli meat quality. J. Food Dairy Sci. 2013, 4, 557–573. [Google Scholar] [CrossRef]
- Morsy, M.K.; Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Cutter, C.N. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne patho-gens on meat and poultry products. J. Food Sci. 2014, 79, M675–M684. [Google Scholar] [CrossRef]
- Qi, Z.; Xie, P.; Yang, C.; Xue, X.; Chen, H.; Zhou, H.; Yuan, H.; Yang, G.; Wang, C. Developing fisetin-AgNPs incorporated in reinforced chitosan/pullulan composite-film and its application of postharvest storage in litchi fruit. Food Chem. 2023, 407, 135122. [Google Scholar] [CrossRef] [PubMed]
- Harun-Ur-Rashid, M.; Foyez, T.; Krishna, S.B.N.; Poda, S.; Imran, A.B. Recent advances of silver nanoparticle-based polymer nanocomposites for biomedical application. RSC Adv. 2025, 15, 8480. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.M.G.; Medeiros, R.J.; Maciel-Magalhães, M.; Guedes, N.C.C.; Brito, T.M.; de Souza, G.F.; Oliveira, M.L.; Pereira, R.A.; Neto, S.A.V.; Jacob, S.C.; et al. Unravelling the effects of silver nanoparticles on textiles: A comprehensive toxicological and quantitative analysis. Health Nanotechnol. 2025, 1, 6. [Google Scholar] [CrossRef]
- Shan, M.; Muhammad, K.F.; Muhammad, M.R.; Wong, Y.H.; Yoshida, M. Advancements in copper and silver sintering as interconnect materials in electronics applications: Review. J. Mater. Sci. Mater. Electron. 2025, 36, 1803. [Google Scholar] [CrossRef]
- Silva-Angulo, A.B.; Zanini, S.F.; Rosenthal, A.; Rodrigo, D.; Klein, G.; Martinez, A. Comparative study of the effects of citral on the growth and injury of Listeria innocua and Listeria monocytogenes cells. PLoS ONE 2015, 10, e0114026. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Levison, M.E. Pharmacodynamics of antimicrobial drugs. Infect. Dis. Clin. N. Am. 2004, 18, 451–465. [Google Scholar] [CrossRef]
- Petersen, P.J.; Jones, C.H.; Bradford, P.A. In vitro antibacterial activities of tigecycline and comparative agents by time-kill kinetic studies in fresh Mueller-Hinton broth. Diagn. Microbiol. Infect. Dis. 2007, 59, 347–349. [Google Scholar] [CrossRef]
- Wei, C.; Cui, P.; Liu, X. Antibacterial activity and mechanism of madecassic acid against Staphylococcus aureus. Molecules 2023, 28, 1895. [Google Scholar] [CrossRef]
- Ponce, A.G.; Fritz, R.; del Valle, C.; Roura, S.I. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT-Food Sci. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film Sheet. 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Singh, P.; Pandift, P.; Mokkapati, V.R.S.S.; Garnæs, J.; Mijakovic, I.A. Sustainable approach for the green synthesis of silver nanoparticles from Solibacillus isronensis sp. and their application in biofilm inhibition. Molecules 2020, 25, 2783. [Google Scholar] [CrossRef]
- Ram, V.P.; Yasur, J.; Abishad, P.; Ramesh, C.; Gourkhede, D.P.; Arya, P.R.; Unni, V.; Vergis, J.; Malik, S.V.S.; Kaore, M.; et al. Enhanced therapeutic efficacy of biogenic nanosilver-conjugated thymol: In vitro and in vivo evaluation against emerging multi-drug resistant microbes. J. Drug. Deliv. Sci. Technol. 2023, 86, 104741. [Google Scholar] [CrossRef]
- Pencheva, D.; Bryaskova, R.; Kantardjiev, T. Polyvinyl alcohol/silver nanoparticles (PVA/AgNps) as a model for testing the biological activity of hybrid materials with included silver nanoparticles. Mater. Sci. Eng. C 2012, 32, 2048–2051. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Barrios, A.; González, G.; Reinoso, C.; Santiana, J.; Quiroz, F.; Chango, J.I.; Costa Vera, C.; Caniglia, L.; Salazar, V.; Fernández-Delgado, M. In situ synthesis and long-term stabilization of nanosilver in poly(vinyl acetate-co-butyl acrylate-co-neodecanoate) matrix for antibacterial applications. Mater. Chem. Phys. 2020, 255, 123476. [Google Scholar] [CrossRef]
- Zein, M.A.; Asghar, B.H.; Almohyawi, A.M.; Alqahtani, N.F.; Alharbi, A.; Alkabli, J.; Elshaarawy, R.F.M.; Ismail, L.A. Multifunctional nanocomposites integrated green synthesized amphiphilic chitosan/thyme extract/nanosilver for antimicrobial and anti-biofilm applications. React. Funct. Polym. 2024, 194, 105791. [Google Scholar] [CrossRef]
- Gao, M.; Sun, L.; Wang, Z.; Zhao, Y. Controlled synthesis of Ag nanoparticles with different morphologies and their antibacterial properties. Mater. Sci. Eng. C 2013, 33, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Liu, Y.; Dai, Y.; Zhang, G.; Tong, Y. Preparation of nanosilver/polymer composites and evaluation of their antimicrobial and antitumor effect. RSC Adv. 2025, 15, 6357–6369. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Hashemirad, F.S.; Kavoosi, G.; Dadfar, S.M.M. Royal jelly: A novel eco-friendly biotemplate for the sustainable green synthesis of antibacterial silver, iron, copper, and zinc nanoparticles. LWT-Food Sci. Technol. 2025, 223, 117797. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Srisod, S.; Motina, K.; Inprasit, T.; Pisitsak, P. A green and facile approach to durable antimicrobial coating of cotton with silver nanoparticles, whey protein, and natural tannin. Prog. Org. Coat. 2018, 120, 123–131. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Silver nanoparticles as an antimicrobial agent: A case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-positive bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar]
- Gevorgyan, S.; Schubert, R.; Falke, S.; Lorenzen, K.; Trchounian, K.; Betzel, C. Structural characterization and antibacterial activity of silver nanoparticles synthesized using a low-molecular-weight Royal Jelly extract. Sci. Rep. 2022, 12, 14077. [Google Scholar] [CrossRef]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, Y.; Yu, L.; Song, G.; Ge, J.; Du, R. Characterization of nanosilver antibacterial bacterial cellulose composite membranes coated with montmorillonite and their potential application in food packaging. Int. J. Biol. Macromol. 2025, 289, 138685. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. Nanosilver-loaded and polysaccharide-based biomaterial for antibacterial activity—In combination with photobiomodulation for effective wound healing applications. Mater. Lett. 2024, 363, 136293. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Pan, R.; Han, D.; Chen, T.; Geng, Z.; Xion, Y.; Chen, Y. Electrodeposition of chitosan/gelatin/nanosilver: A new method for constructing biopolymer/nanoparticle composite films with conductivity and antibacterial activity. Mater. Sci. Eng. C 2015, 53, 222–228. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Rostamabadi, H.; Habibi, N.; Falsaf, S.R. Pullulan nanocomposites: Effect of nanoparticles and essential oil reinforcement on its performance and food packaging applications. Food Humanit. 2023, 1, 887–894. [Google Scholar] [CrossRef]
- Islam, M.d.S.; Yeum, J.H. Electrospun pullulan/poly(vinyl alcohol)/silver hybrid nanofibers: Preparation and property characterization for antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 279–286. [Google Scholar] [CrossRef]
- Vimala, K.; Mohan, Y.M.; Sivudu, K.S.; Varaprasad, K.; Ravindraa, S.; Reddy, N.N.; Padma, Y.; Sreedhar, B.; MohanaRaju, K. Fabrication of porous chitosan films impregnated with silver nanoparticles: A facile approach for superior antibacterial application. Colloids Surf. B Biointerfaces 2010, 76, 248–258. [Google Scholar] [CrossRef]
- Shah, A.; Ashames, A.A.; Buabeid, M.A.; Murtaza, G. Synthesis, in vitro characterization and antibacterial efficacy of moxifloxacin-loaded chitosan-pullulan-silver-nanocomposite films. J. Drug Deliv. Sci. Technol. 2020, 55, 101366. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of polymeric food packaging materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef]
- Eldesouky, A.; Pulido, A.F.; Mesias, F.J. The Role of Packaging and Presentation Format in Consumers’ Preferences for Food: An Application of Projective Techniques. J. Sens. Stud. 2015, 30, 360–369. [Google Scholar] [CrossRef]
- Duncan, S.E.; Chang, H.H. Implications of Light Energy on Food Quality and Packaging Selection. Adv. Food Nutr. Res. 2012, 67, 25–73. [Google Scholar] [PubMed]
- Roy, S.; Ramakrishnan, R.; Goksen, G.; Singh, S.; Łopusiewicz, Ł. Recent progress on UV-light barrier food packaging films—A systematic review. Innov. Food Sci. Emerg. Technol. 2024, 91, 103550. [Google Scholar] [CrossRef]
- Gore, A.H.; Prajapat, A.L. Biopolymer Nanocomposites for Sustainable UV Protective Packaging. Front. Mater. 2022, 9, 855727. [Google Scholar] [CrossRef]
| Films | HydroSilver 1000 [%] | AgNPs [µg/mL] | AgNPs [µg/cm2] |
| PF | 0 | - | - |
| PFHS1 | 1 | 10 | 2 |
| PFHS3 | 3 | 30 | 5 |
| PFHS5 | 5 | 50 | 8 |
| PFHS10 | 10 | 100 | 16 |
| PFHS15 | 15 | 150 | 24 |
| PFHS20 | 20 | 200 | 32 |
| Bacteria Strains | AgNPs | ||
| MIC | MBC | MBC/MIC * | |
| [µg/mL] | |||
| Bacteria Gram-negative | |||
| E. coli ATCC 25922 | 12.5 | 25 | 2 |
| P. aeruginosa ATCC 27853 | 12.5 | 25 | 2 |
| K. pneumoniae ATCC 13883 | 12.5 | 25 | 2 |
| S. Enteritidis ATCC 13076 | 12.5 | 25 | 2 |
| S. sonnei NIPH-NIH “s” | 12.5 | 25 | 2 |
| Bacteria Gram-positive | |||
| S. aureus ATCC 25923 | 12.5 | 25 | 2 |
| E. faecalis ATCC 29212 | 12.5 | 25 | 2 |
| L. innocua ATCC 33090 | 25 | 50 | 2 |
| B. cereus ATCC 11778 | 6.25 | 12.5 | 2 |
| Bacteria Strains | ∆ log CFU/mL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ½ × MIC | 1 × MIC | 2 × MIC | ||||||||||
| 1 h | 3 h | 6 h | 24 h | 1 h | 3 h | 6 h | 24 h | 1 h | 3 h | 6 h | 24 h | |
| Bacteria Gram-negative | ||||||||||||
| E. coli ATCC 25922 | 0.06 | 1.56 | 2.56 | 1.11 | 0.52 | 3.12 | 8.01 | 8.22 | 0.66 | 8.03 | 8.03 | 8.22 |
| P. aeruginosa ATCC 27853 | 0.61 | 2.69 | 4.56 | 5.78 | 0.82 | 5.99 | 7.74 | 8.98 | 1.01 | 5.99 | 7.74 | 8.98 |
| K. pneumoniae ATCC 13883 | 0.34 | 2.24 | 4.46 | 7.88 | 0.26 | 3.33 | 6.20 | 7.88 | 0.76 | 5.99 | 7.71 | 7.88 |
| S. Enteritidis ATCC 13076 | 0.16 | 1.55 | 4.21 | 3.86 | 0.45 | 2.77 | 8.03 | 9.08 | 0.66 | 5.37 | 8.03 | 9.08 |
| S. sonnei NIPH–NIH “s” | 0.20 | 2.20 | 7.91 | 9.99 | 0.37 | 2.85 | 7.91 | 9.99 | 0.43 | 5.53 | 7.91 | 9.99 |
| Bacteria Gram-positive | ||||||||||||
| S. aureus ATCC 25923 | 0.58 | 1.35 | 2.83 | 2.60 | 0.89 | 2.70 | 6.84 | 7.93 | 1.33 | 5.38 | 6.84 | 7.93 |
| E. faecalis ATCC 29212 | 0.79 | 1.71 | 2.98 | 1.09 | 1.42 | 3.53 | 7.73 | 7.99 | 1.61 | 5.53 | 7.73 | 7.99 |
| L. innocua ATCC 33090 | 0.48 | 2.90 | 3.14 | 4.75 | 0.98 | 3.79 | 6.99 | 8.14 | 1.65 | 6.02 | 6.99 | 8.14 |
| B. cereus ATCC 11778 | 0.03 | 1.37 | 1.29 | 1.29 | 0.75 | 2.94 | 7.39 | 8.36 | 1.83 | 5.37 | 7.36 | 8.36 |
| Bacteria Strains | Concentration of AgNPs | Leakage of Bacterial Cellular Material at 260 nm and 280 nm | |
|---|---|---|---|
| 260 nm | 280 nm | ||
| 6 h | 6 h | ||
| E. coli ATCC 25922 | control | 0.137 ± 0.025 a ** | 0.107 ± 0.009 a |
| ½ × MIC | 0.182 ± 0.008 b | 0.124 ± 0.006 b | |
| 1 × MIC | 0.210 ± 0.022 c | 0.157 ± 0.020 c | |
| 2 × MIC | 0.229 ± 0.012 d | 0.179 ± 0.012 d | |
| S. Enteritidis ATCC 13076 | control | 0.181 ± 0.037 a | 0.122 ± 0.039 a |
| ½ × MIC | 0.173 ± 0.004 a | 0.120 ± 0.005 a | |
| 1 × MIC | 0.233 ± 0.006 b | 0.165 ± 0.005 b | |
| 2 × MIC | 0.326 ± 0.003 c | 0.260 ± 0.003 c | |
| S. sonnei NIPH–NIH “s” | control | 0.196 ± 0.048 a | 0.155 ± 0.043 a |
| ½ × MIC | 0.354 ± 0.026 b | 0.236 ± 0.023 b | |
| 1 × MIC | 0.339 ± 0.015 b | 0.238 ± 0.014 b | |
| 2 × MIC | 0.416 ± 0.014 c | 0.330 ± 0.012 c | |
| S. aureus ATCC 25923 | control | 0.348 ± 0.011 a | 0.203 ± 0.010 a |
| ½ × MIC | 0.332 ± 0.021 a | 0.211 ± 0.020 a | |
| 1 × MIC | 0.395 ± 0.002 b | 0.258 ± 0.002 b | |
| 2 × MIC | 0.513 ± 0.001 c | 0.369 ± 0.001 c | |
| E. faecalis ATCC 29212 | control | 0.165 ± 0.004 a | 0.131 ± 0.002 a |
| ½ × MIC | 0.320 ± 0.002 b | 0.229 ± 0.003 b | |
| 1 × MIC | 0.347 ± 0.007 c | 0.238 ± 0.006 b | |
| 2 × MIC | 0.469 ± 0.005 d | 0.391 ± 0.005 c | |
| L. innocua ATCC 33090 | control | 0.071 ± 0.004 a | 0.055 ± 0.004 a |
| ½ × MIC | 0.149 ± 0.008 b | 0.106 ± 0.006 b | |
| 1 × MIC | 0.236 ± 0.013 c | 0.157 ± 0.007 c | |
| 2 × MIC | 0.454 ± 0.012 d | 0.274 ± 0.007 d | |
| Bacteria Strains | Pullulan Films | |||||||
|---|---|---|---|---|---|---|---|---|
| PF | PFHS1 | PFHS3 | PFHS5 | PFHS10 | PFHS15 | PFHS20 | AZL75 | |
| Concentration of AgNPs in film [µg/cm2] | ||||||||
| 0 | 2 | 5 | 8 | 16 | 24 | 32 | x | |
| Bacteria Gram-negative | ||||||||
| Growth inhibition zones [mm ± SD] | ||||||||
| E. coli ATCC 25922 | 0.0 a | 8.4 ± 0.2 bB ** | 11.6 ± 0.3 cC | 12.5 ± 0.4 dCD | 13.1 ± 0.3 eA | 14.5 ± 0.3 fA | 14.4 ± 0.3 fAB | 16.5 ± 0.4 gB |
| K. pneumoniae ATCC 13883 | 0.0 a | 6.8 ± 0.6 bA | 7.9 ± 0.6 cA | 9.9 ± 0.6 dA | 14.6 ± 0.5 fD | 15.8 ± 0.4 gBC | 15.9 ± 0.4 gBC | 13.1 ± 0.8 eA |
| P. aeruginosa ATCC 27853 | 0.0 a | 12.6 ± 0.9 bE | 13.0 ± 0.4 bE | 13.7 ± 0.4 cF | 15.2 ± 0.3 dE | 15.7 ± 0.4 dBC | 15.8 ± 0.2 dB | 26.9 ± 0.6 eF |
| S. Enteritidis ATCC 13076 | 0.0 a | 10.6 ± 0.7 bCD | 10.9 ± 0.3 bB | 12.9 ± 0.4 cD | 16.2 ± 0.3 dF | 15.6 ± 0.4 dB | 15.4 ± 0.5 dB | 23.5 ± 1.2 eE |
| S. sonnei NIPH–NIH “s” | 0.0 a | 11.4 ± 1.0 bD | 12.5 ± 0.5 cDE | 13.9 ± 0.3 dF | 14.1 ± 0.3 dC | 16.0 ± 0.5 eC | 15.8 ± 0.8 eB | 18.7 ± 1.0 fC |
| Bacteria Gram-positive | ||||||||
| B. cereus ATCC 11778 | 0.0 a | 9.6 ± 0.6 bC | 10.8 ± 0.7 cB | 11.7 ± 0.4 dB | 13.6 ± 0.4 eBC | 17.0 ± 0.2 fE | 17.3 ± 0.2 gD | 13.3 ± 0.8 eA |
| E. faecalis ATCC 29212 | 0.0 a | 10.2 ± 0.4 bC | 10.7 ± 0.3 bB | 12.0 ± 0.5 cBC | 13.5 ± 0.4 dAB | 17.5 ± 0.3 eF | 17.8 ± 0.1 eDE | 29.3 ± 0.6 fG |
| S. aureus ATCC 25923 | 0.0 a | 10.4 ± 0.5 bCD | 12.0 ± 0.5 cCD | 14.0 ± 0.6 dF | 15.2 ± 1.1 eE | 17.0 ± 0.6 fE | 17.0 ± 0.4 fCD | 20.0 ± 0.8 gD |
| L. innocua ATCC 33090 | 0.0 a | 9.9 ± 0.7 bC | 10.7 ± 1.1 bB | 12.2 ± 0.8 cBC | 16.3 ± 0.5 dF | 19.2 ± 0,4 eG | 18.9 ± 0.2 eE | 33.4 ± 0.3 fH |
| Film Sample | Concentration of AgNPs in Film [µg/cm2] | Thickness [mm] | Opacity [a.u./mm] | Transmittance (%) | Color | ||||
|---|---|---|---|---|---|---|---|---|---|
| 280 nm | 600 nm | L* | a* | b* | ΔE | ||||
| PF | 0 | 0.071 ± 0.005 a ** | 0.53 ± 0.04 a | 79.05 ± 1.38 a | 91.76 ± 0.40 a | 91.25 ± 0.15 a | 2.08 ± 0.04 a | −5.21 ± 0.03 a | - |
| PFHS1 | 2 | 0.080 ± 0.004 ab | 0.59 ± 0.04 a | 52.10 ± 0.79 b | 89.63 ± 1.16 a | 84.22 ± 0.20 b | 4.71 ± 0.30 b | 2.86 ± 0.12 g | 7.9 |
| PFHS3 | 5 | 0.095 ± 0.007 b | 0.61 ± 0.07 a | 51.25 ± 0.72 b | 87.57 ± 0.76 a | 79.14 ± 0.21 c | 4.38 ± 0.13 c | 25.78 ± 0.62 f | 24.0 |
| PFHS5 | 8 | 0.098 ± 0.007 b | 1.64 ± 0.06 b | 51.17 ± 1.74 b | 69.16 ± 1.20 b | 71.11 ± 0.24 d | 10.16 ± 0.34 d | 20.89 ± 0.64 e | 26.7 |
| PFHS10 | 16 | 0.115 ± 0.011 c | 2.37 ± 0.31 c | 33.59 ± 3.20 c | 53.73 ± 3.86 c | 63.67 ± 1.13 e | 16.47 ± 0.70 f | 18.59 ± 0.26 d | 33.9 |
| PFHS15 | 24 | 0.125 ± 0.002 c | 2.54 ± 0.23 c | 20.77 ± 1.50 d | 48.30 ± 3.19 c | 41.82 ± 2.54 f | 20.20 ± 0.61 g | 11.05 ± 0.56 c | 53.0 |
| PFHS20 | 32 | 0.144 ± 0.005 d | 3.83 ± 0.26 d | 15.53 ± 1.32 e | 28.24 ± 2.12 d | 41.45 ± 1.57 f | 13.50 ± 0.96 e | 0.01 ± 0.0 b | 51.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraśniewska, K.; Gniewosz, M. Silver Nanoparticle-Infused Pullulan Films for the Inhibition of Foodborne Bacteria. Appl. Sci. 2025, 15, 11297. https://doi.org/10.3390/app152011297
Kraśniewska K, Gniewosz M. Silver Nanoparticle-Infused Pullulan Films for the Inhibition of Foodborne Bacteria. Applied Sciences. 2025; 15(20):11297. https://doi.org/10.3390/app152011297
Chicago/Turabian StyleKraśniewska, Karolina, and Małgorzata Gniewosz. 2025. "Silver Nanoparticle-Infused Pullulan Films for the Inhibition of Foodborne Bacteria" Applied Sciences 15, no. 20: 11297. https://doi.org/10.3390/app152011297
APA StyleKraśniewska, K., & Gniewosz, M. (2025). Silver Nanoparticle-Infused Pullulan Films for the Inhibition of Foodborne Bacteria. Applied Sciences, 15(20), 11297. https://doi.org/10.3390/app152011297









