Abstract
Thorium has emerged as a promising alternative to uranium in nuclear energy systems due to its higher natural abundance, favorable conversion to fissile 233U, and reduced generation of long-lived transuranic waste. This review provides a comprehensive overview of advanced techniques for thorium recovery from primary ores and secondary resources. The main mineralogical carriers—including monazite, thorianite, thorite, and cheralite as well as industrial by-products such as rare-earth processing tailings—are critically examined with respect to their occurrence and processing potential. Physical enrichment methods (gravity, magnetic, and electrostatic separation) and hydrometallurgical approaches (acidic and alkaline leaching) are analyzed in detail, highlighting their efficiencies, limitations, and environmental implications. Particular emphasis is placed on modern separation strategies such as solvent extraction with organophosphorus reagents, diglycolamides, and ionic liquids, as well as extraction chromatography, nanocomposite sorbents, ion-imprinted polymers, and electrosorption on carbon-based electrodes. These techniques demonstrate significant progress in enhancing selectivity, reducing reagent consumption, and enabling recovery from low-grade and secondary feedstocks. Environmental and radiological aspects, including waste minimization, immobilization, and regulatory frameworks, are discussed as integral components of sustainable thorium management. Finally, perspectives on hybrid technologies, digital process optimization, and economic feasibility are outlined, underscoring the need for interdisciplinary approaches that combine chemistry, materials science, and environmental engineering. Collectively, the analysis highlights the transition from conventional practices to integrated, scalable, and environmentally responsible technologies for thorium recovery.
1. Introduction
Nuclear energy continues to play a key role in ensuring a stable and low-carbon energy supply to the world [1,2,3]. However, the traditional uranium fuel cycle is subject to limitations related to limited resources, the formation of long-lived transuranic waste, and the potential risks of nuclear proliferation [4,5]. In this context, thorium (Th) stands as a promising alternative. Its use can increase the sustainability of the nuclear industry, reduce the volume of highly radioactive waste, and expand the geography of available raw materials [6,7]. Next, the paper will consider the rationale for the use of thorium in modern nuclear power systems and provide an overview of the main mineral sources of this element.
1.1. Justification of the Use of Thorium in Nuclear Power Systems
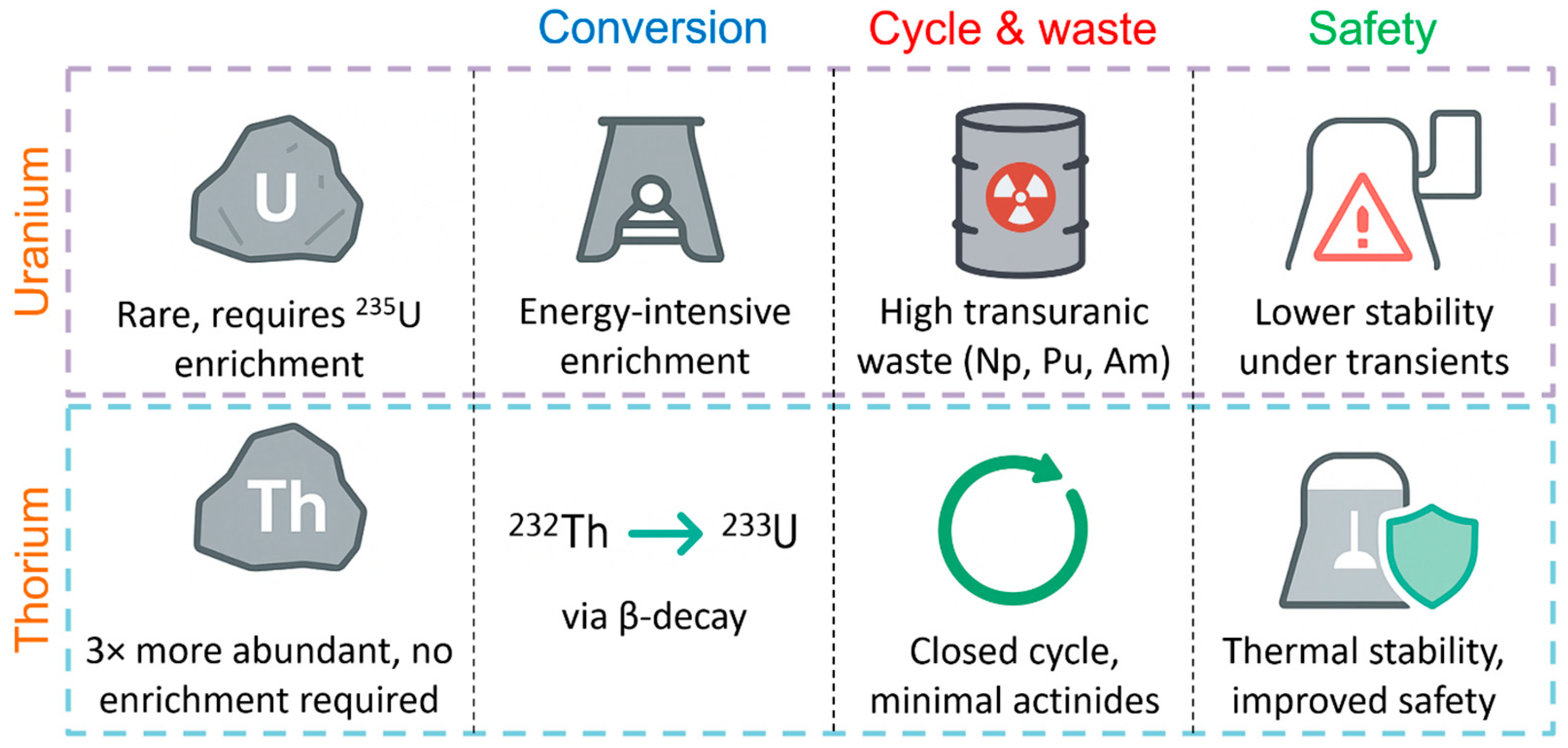
Thorium is considered as a promising replacement for uranium due to the combination of a number of distinctive advantages that contribute to increasing the stability and safety of the nuclear fuel cycle [8,9,10]. Firstly, thorium is about three times more abundant in the Earth’s crust than uranium, which provides a more reliable and long-term raw material base for nuclear energy. Secondly, when irradiated in a reactor, the stable isotope 232Th is converted into fissile 233U, practically without requiring the energy-intensive enrichment process typical of 235U. As illustrated in Figure 1, such a scheme can significantly reduce the number of technological operations and the consumption of operational resources.
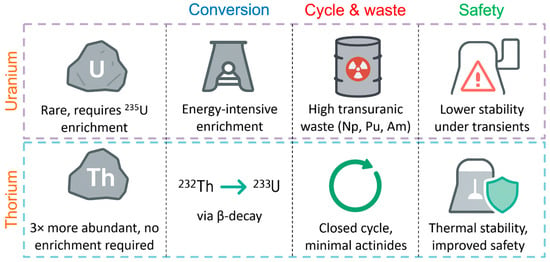
Figure 1.
Schematic advantages of thorium-based fuel cycle over uranium.
In addition, the thorium-based fuel cycle generates significantly less long-lived transuranic waste (Np, Pu, Am), which simplifies and reduces the cost of their disposal and long-term storage [11]. The high degree of conversion of 232Th to 233U makes it possible to create a closed cycle with minimal formation of new actinides. Finally, thorium technology has increased resistance to abnormal and emergency operating conditions of the reactor; fuel based on Th–233U demonstrates a more stable heat dissipation capacity when changing the parameters of the coolant [12]. All these factors make thorium an attractive element for fourth-generation reactors and stimulate the active development of relevant research in the world’s leading scientific centers.
1.2. Prevalence and Main Mineral Carriers of Thorium
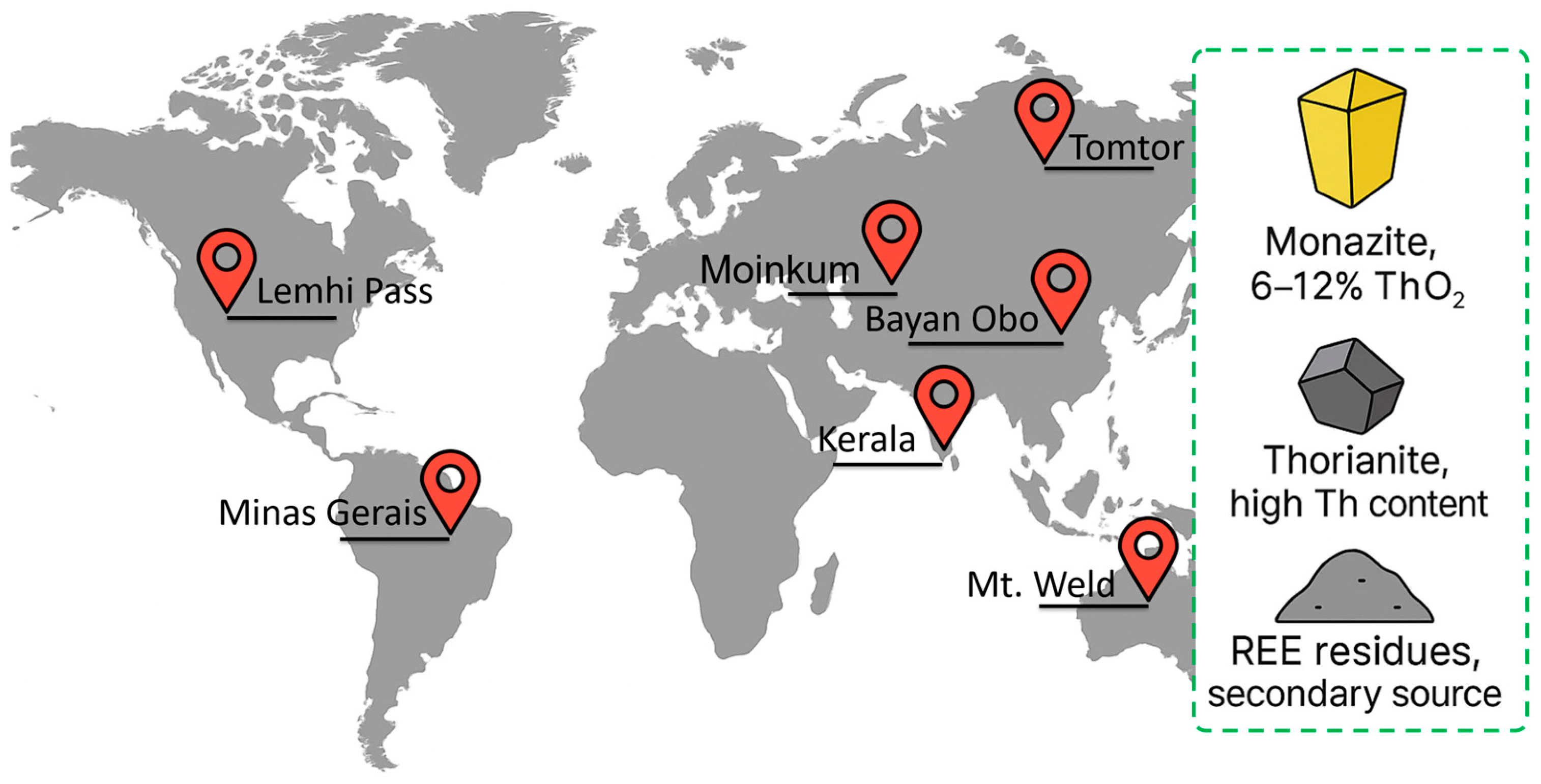
Thorium is a relatively common element; its average content in the earth’s crust is estimated at 10–12 ppm, which is comparable to some rare earth elements and about three times the concentration of uranium [13,14]. Geochemically, thorium mainly accumulates in heavy phosphate and silicate minerals, forming significant concentrations in coastal heavy sands and pegmatite veins. The world’s largest thorium deposits are concentrated in the coastal deposits of India (Kerala), Brazil (Minas-Gerais), Australia (Mt. Weld), China (Bayan Obo) and Russia (Kola Peninsula, Tomtor), as well as in Kazakhstan (Dzhezkazgan, Moinkum), where thorium is mainly present in the residues of processing rare earth ores [15].
The main mineralogical carrier of thorium is monazite ((Ce,La,Th)PO4)—a lanthanide phosphate from which up to 6–12% ThO2 is obtained in commercial concentrates after preliminary enrichment. Thorianite (ThO2), found in granite pegmatites and metamorphic rocks, has one of the highest specific concentrations of thorium and serves as an additional source of Th [16]. In addition, tailings and sludge from the processing of rare earth ores may contain noticeable residual concentrations of thorium, which makes them an attractive secondary raw material for further processing [17].
In addition to the listed large deposits, noticeable manifestations of thorium are also observed in the United States, mainly in areas of rare earth mining. Thus, the Lemhi Pass (Montana–Idaho) and Mountain Pass (California) deposits contain monazite and other minerals with high concentrations of thorium, and its presence has also been recorded in Colorado, Wyoming and Alaska [18,19]. However, unlike India, Brazil, or Australia, these resources are not industrially developed and are considered rather as a byproduct of REE processing, which explains the limited role of the United States in the global thorium resource base.
Figure 2 shows the abundance of thorium in the Earth’s crust and the main mineralogical carriers: monazite, thorianite, and REE tailings, as well as key global deposits.
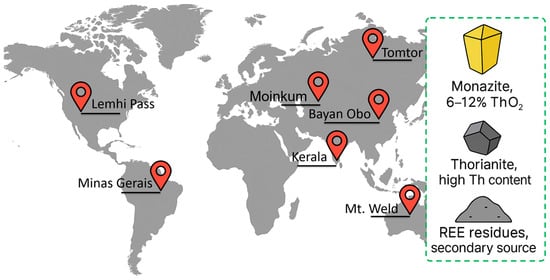
Figure 2.
Global distribution and main mineral carriers of thorium.
2. Initial Raw Materials
2.1. Monazite Ores
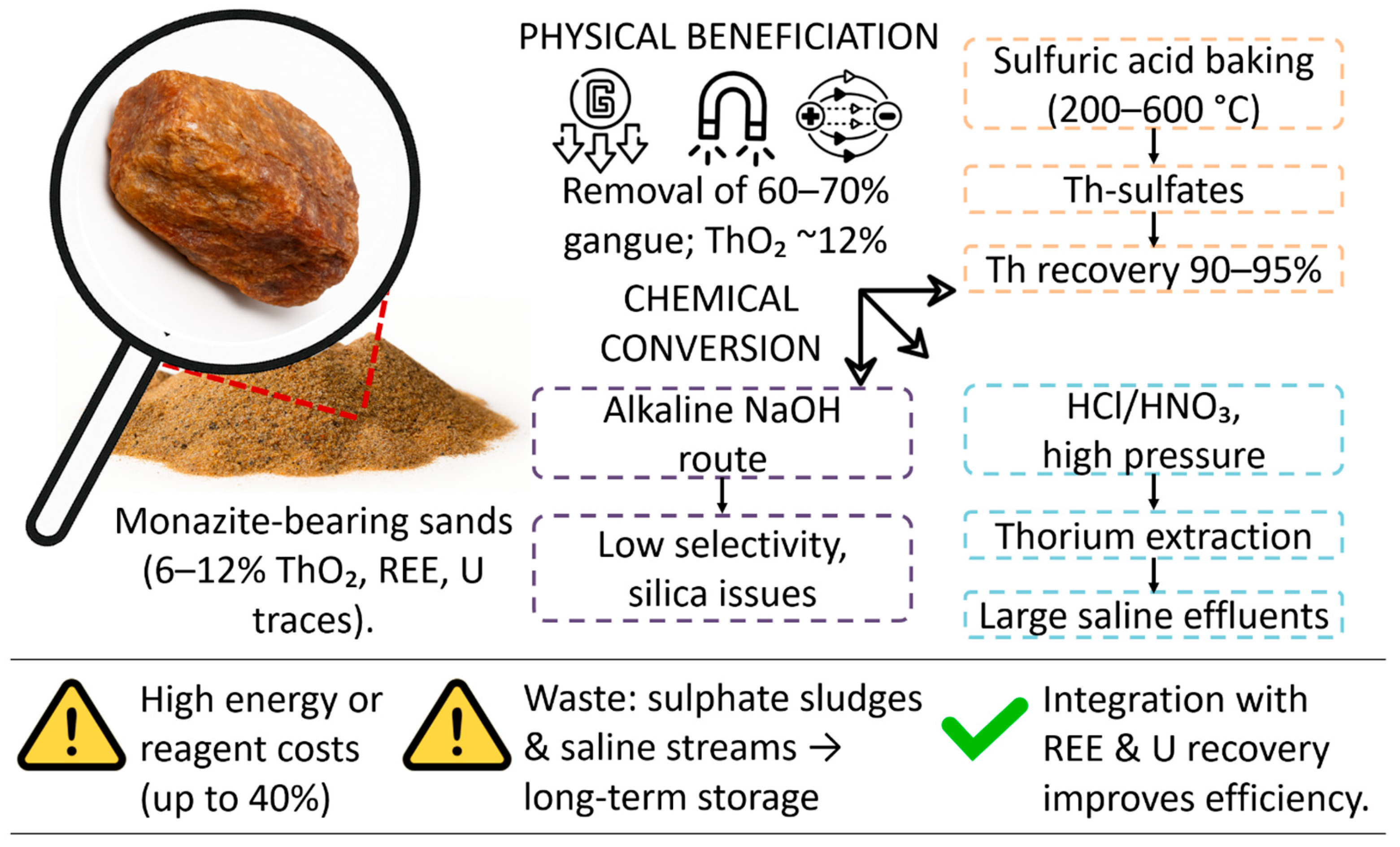
Monazite ((Ce,La,Th)PO4) is formed in heavy mineral sands of coastal and alluvial deposits as a result of weathering of the parent rocks and hydrodynamic sorting. The largest commercial deposits are located in India (Kerala, Odisha), Brazil (Minas-Gerais) and Australia (Queensland), where monazite concentrates contain from 6 to 12% ThO2 after preliminary enrichment [20,21]. Inclusions of uranium (up to 0.3%) and rare earth elements require comprehensive management of the radionuclide background and extraction of related metals.
Physical methods for the enrichment of monazite concentrates include the sequential use of gravitational, magnetic and electrostatic separation, which makes it possible to remove up to 60–70% of ballast minerals and increase the thorium content to ~12% [22]. However, the similar physicochemical properties of monazite and adjacent minerals (zircon, ilmenite) create technological limitations; multiple separation cycles increase energy consumption, and the accumulation of fine fractions complicates existing water and dust treatment systems.
The chemical conversion of concentrates is traditionally carried out by baking with sulfuric acid at 200–600 °C, which leads to the formation of thorium sulfates, which are easily leached out with water or dilute acids with a Th yield of up to 90–95% [23]. Direct acidic leaching of HCl/HNO3 at elevated pressure simplifies the technological scheme, but is accompanied by the formation of large volumes of salt effluents and requires subsequent neutralization, whereas alkaline methods (NaOH) demonstrate lower selectivity and complicated purification from silica.
Critically assessing modern practices, it should be noted that the energy and reagent costs during sulfur baking can amount to up to 40% of the cost of thorium production, especially with small amounts of processing [24]. The resulting sulfate sludge and salt runoff require reliable long-term storage, which increases capital costs and environmental risks. At the same time, the integration of uranium and rare earth element extraction can increase economic efficiency, but this requires the development of adapted combined schemes for solvex extraction and sorption processes.
The processing routes of monazite concentrates for thorium extraction are presented in Figure 3.
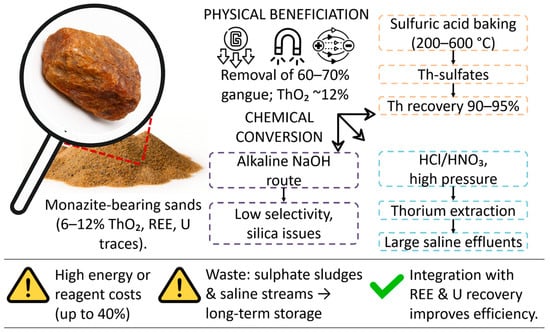
Figure 3.
Processing routes of monazite concentrates for thorium extraction.
2.2. Thorianite, Torite, and Cheralite as Key Thorium-Bearing Minerals
Thorianite (ThO2) is the most concentrated natural source of thorium, the content of which can reach 90–95% [25]. It is formed mainly under conditions of low silica activity in carbon dioxide and fluid-saturated systems, including alkaline pegmatites, carbonatites, and metasomatic veins. Due to its high melting point and resistance to weathering, thorianite is often preserved in placer deposits, especially in Sri Lanka, where it was first described in the early 20th century [26,27,28]. In such deposits, it occurs in the form of dense cubic grains containing, in addition to ThO2, UO2 and PbO in variable proportions. The geochemical specificity of Th4+, characterized by extremely low mobility, explains the formation of an independent ThO2 phase even at low concentrations in natural solutions [29].
Another important thorium mineral is thorite (ThSiO4), a structural analog of zircon formed in granite pegmatites and hydrothermal veins in conditions of silica-saturated but depleted CO2 systems. Under the influence of radioactive self-damage, the crystal lattice of thorite often amorphizes, forming thorohummite (ThSiO4·nH2O).
In evolved granites with an excess of calcium and phosphorus, part of thorium is fixed in an isomorphic series with monazite in the form of a mineral known as cheralite (CaTh(PO4)2), previously described under the name «brabantite». Cheralite is a solid solution of Ca2+ + Th4+ ↔ 2Ln3+ (lanthanides) and geochemically plays the role of an additional carrier of thorium in phosphate systems [30].
Figure 4 shows the morphology of thorianite, thorite, and cheralite, their chemical features, and the scheme of acid processing. In all three processes, thorium exists in the ionic form (Th4+), as the metal is completely dissolved in strong acid media such as HNO3, HF, or H2SO4.
Among the most significant objects containing thorianite and torite are the placer deposits of Sri Lanka (70–80% ThO2), the Lemhi Pass veins (USA) enriched in thorite and monazite with a ThO2 content of up to 0.43%, the Steenkampskraal deposit (South Africa), where venous ores include monazite with 8–10% ThO2, as well as Nolans Bore (Australia), where thorianite and secondary thorite are associated with rare earth phosphates. Most of these facilities are considered promising sources of thorium, but in real practice it remains a byproduct of processing rare earth elements [31].
The processing of thorianite and thorite is complicated by their high chemical resistance. Concentrated solutions of H2SO4 at 100–200 °C or mixtures with HF, which contribute to the destruction of the silica matrix, are used for the decomposition of thorite. Thorianite is even more stable; traditionally, it is treated with prolonged boiling in 13–16 M HNO3 with the addition of HF and aluminum salts; however, more modern methods use Trifluoromethanesulfonic (CF3SO3H), which makes it possible to achieve complete dissolution in one hour at a concentration of 65%.
Historically, ThO2 obtained from these minerals has been used in gas mantles, optical glass, argon-arc welding electrodes, and as a catalyst for oxidative processes. Today, the main focus is on its prospects as a fertile material for fourth-generation reactors. Despite low current demand and radiation restrictions, the high concentration of thorium and the presence of industrial and potential deposits indicate the importance of these minerals in shaping the global raw material base [32].
A simplified overview of the main technological routes for thorium recovery, including physical beneficiation, hydrometallurgical extraction, and sorption–purification processes, is provided in the Supplementary Information (Figure S2).
The typical chemical composition of thorium-bearing minerals and industrial residues used in this study is summarized in Table 1, which compiles data from the literature. Values may vary depending on deposit origin and beneficiation history. To provide additional insight into the morphology and texture of the raw materials, representative SEM micrographs of monazite and thorite particles are presented in the Supplementary File (Figure S1). These images reveal the characteristic angular morphology of monazite grains and the prismatic to subhedral crystal habit of thorite, consistent with previously reported microstructural features of these minerals.
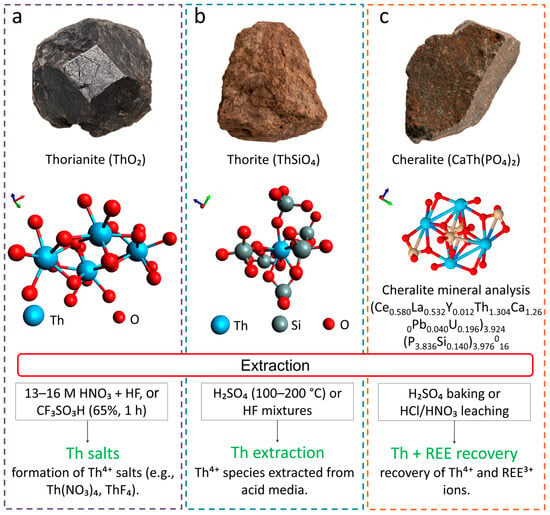
Figure 4.
Principal thorium-bearing minerals and their extraction pathways: (a) thorianite (ThO2) [33], (b) thorite (ThSiO4) [34], and (c) cheralite (CaTh(PO4)2) [35]. The molecular structures were taken from literature sources and remastered using VMD (Visual Molecular Dynamics) for clarity.
Figure 4.
Principal thorium-bearing minerals and their extraction pathways: (a) thorianite (ThO2) [33], (b) thorite (ThSiO4) [34], and (c) cheralite (CaTh(PO4)2) [35]. The molecular structures were taken from literature sources and remastered using VMD (Visual Molecular Dynamics) for clarity.

Table 1.
Typical chemical composition of thorium-bearing minerals and processing residues (wt %).
Table 1.
Typical chemical composition of thorium-bearing minerals and processing residues (wt %).
| Material/Source | REE | ThO2 | UO2/U3O8 | P2O5 | Fe2O3 | CaO | SiO2 | Al2O3 | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Typical monazite composition (literature average) | 60–70 | 4–9 | 0.2–0.4 | 25–28 | 1–3 | – | – | – | [36,37,38] |
| Monazite concentrate (Egypt) | 63.1 | 5.39 | 0.29 | 10.05 | 12.13 | – | – | 2.3 | [39] |
| Rare-earth waste residue—impurity type (China) | 6.53 | 0.44 | – | – | 0.42 | – | 2.60 | 55.42 | [40] |
| Rare-earth waste residue—dissolved type (China) | 14.57 | 1.25 | – | – | 29.52 | – | 20.45 | 5.70 | [40] |
| Rare-earth waste residue—neutralization type (China) | 17.35 | – | – | – | 3.71 | 53.28 | 5.32 | 3.14 | [40] |
| Phosphogypsum (Voskresensk, Russia) | 0.42 | 7 × 10−4 | 2 × 10−4 | – | – | – | – | – | [41] |
| Monazite concentrate (Russia, Krasnoufimsk) | 65.0 | 6.6 | 0.18 | – | – | – | – | – | [42] |
| Thorite (ThSiO4, Bayan Obo, China) | ≤5 | 38–72 | – | – | 1–20 | – | 7–12 | – | [43] |
2.3. REE Processing Tailings and Other By-Products
One of the most promising alternative sources of thorium is tailings and sludge formed during the processing of rare earth ores, primarily monazite and bastnesite. These wastes often contain residual amounts of thorium in the form of ThO2, ThSiO4 or Th-phosphates, depending on the mineralogical composition of the feedstock and the processing regime. The concentration of thorium in tailings can vary from 0.1 to 3%, which makes them an attractive object for recycling [44,45,46]. In countries with developed REE processing, such as India, Brazil, China and Malaysia, tens of thousands of tons of tailings with a high Th content have accumulated, which represents not only a potential resource, but also an environmental burden.
Mineralogically, thorium in such wastes may be present in the form of undetected monazite, in the form of amorphous thorium phosphates, or secondary forms of thorite and thorohummite formed as a result of hydrothermal or acid processing. Depending on the technology used (acid or alkaline leaching), some of the thorium can be converted to precipitation (for example, Th(OH)4 or ThPO4), and the other part is to remain in the solid phase. During acid treatment of rare earth raw materials in the presence of phosphates, especially at high pH, thorium can crystallize as insoluble precipitates, which are then concentrated in slurries and not extracted in the main stream [47].
The development of methods for extracting thorium from such by-products requires consideration of both the mineralogical composition and the phase distribution of thorium. Effective approaches include repeated acid leaching using HNO3 or HCl, as well as the use of extraction chromatographic resins capable of selectively capturing Th4+ and separating it from residual rare earths and transition metals. Low-energy technologies such as electrosorption and membrane separation are also being actively studied, allowing thorium to be extracted from liquid residues with minimal formation of secondary waste [48,49].
The tailings of REE processing and related by-products represent a strategically important reserve of thorium, especially in the context of a global shortage of uranium raw materials and interest in the thorium fuel cycle. Their processing requires modernization of existing technologies with an emphasis on selectivity, environmental friendliness and integration with existing extraction lines.
2.4. Research Methodology
The research methodology was designed to ensure a systematic and transparent review of the available literature on thorium-bearing minerals, extraction, and processing technologies. Major scientific databases—ScienceDirect, SpringerLink, Wiley Online Library, ACS Publications, and MDPI Journals—were searched for peer-reviewed publications. Additional materials such as conference papers, dissertations, and technical reports were also considered to complement recent findings. The search covered the period from 2000 to 2024, with earlier studies included when they provided essential background or historical context.
The search strategy used the following keywords and their Boolean combinations: thorium extraction, thorium recovery, monazite, thorite, rare earths separation, acid leaching, alkaline leaching, solvent extraction, hydrometallurgy, and thorium fuel cycle. The inclusion criteria prioritized studies that (i) presented experimental or technological data relevant to thorium recovery, (ii) provided quantitative results on extraction efficiency or material composition, and (iii) discussed sustainable or industrially scalable processes. Publications that lacked methodological transparency or originality were excluded.
In total, more than 150 publications were reviewed, from which the most representative and high-impact studies were selected to form the analytical basis of this review.
3. Physical Methods of Enrichment
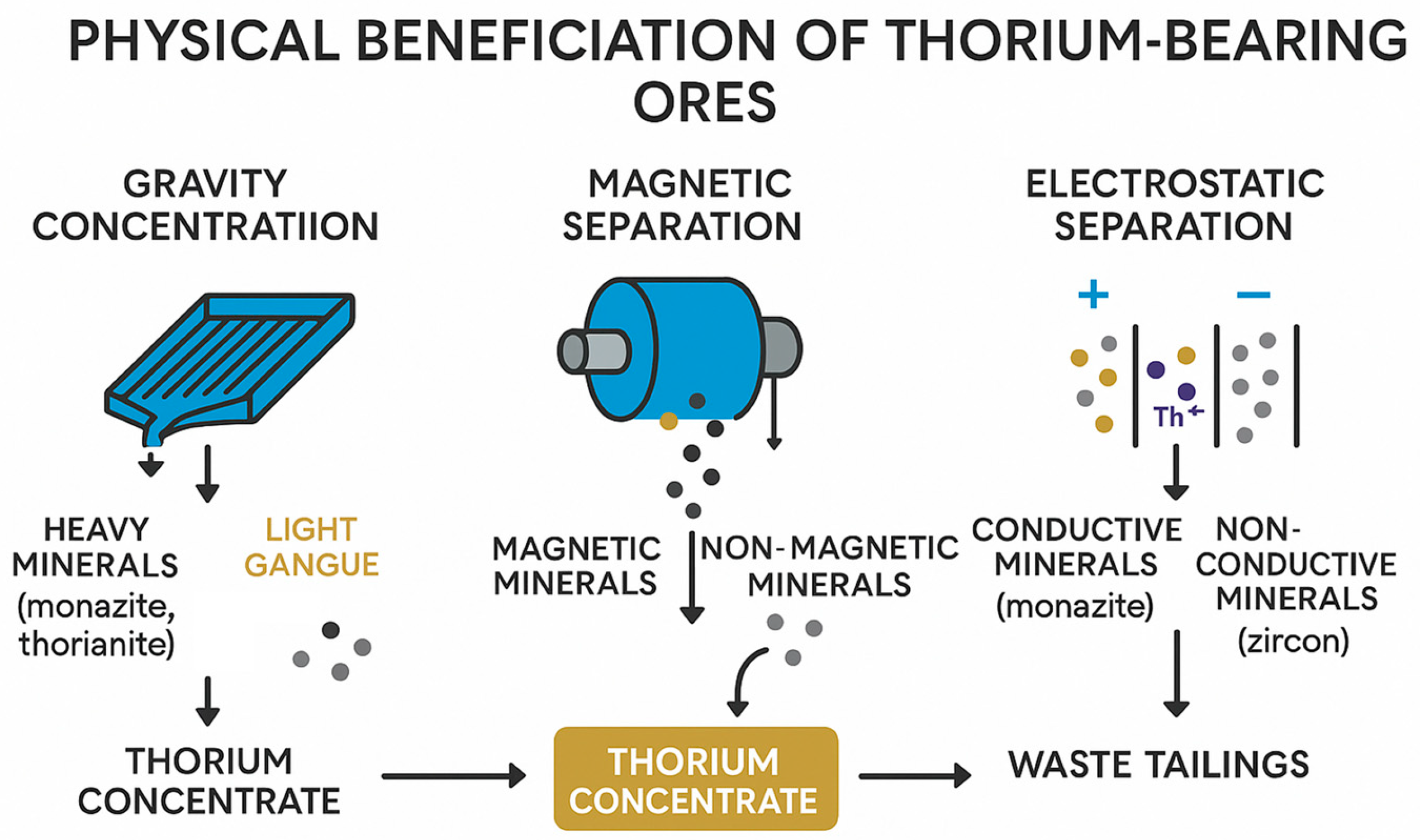
Physical methods of enrichment are the most important stage in the preparation of raw materials containing thorium before its further hydrometallurgical processing. At this stage, the raw materials are mechanically processed in order to increase the concentration of the target element (thorium) and remove waste rock and unwanted minerals. Gravity enrichment, magnetic and electrostatic separation are among the most effective and widespread methods of physical enrichment of thorium-containing materials.
3.1. Gravity Enrichment
Gravity enrichment is based on differences in the density and particle size of thorium-containing minerals and waste rock. It is the most ancient and widely used method for the primary separation of minerals such as monazite, zircon, and xenotime, which have high densities compared to quartz and other silicates. The main gravity processes include sedimentation, concentration on screw separators, Moseley tables, and hydrocyclones.
Deposition is used in the initial stage of processing, when the source material contains large particles of thorium-containing minerals. The method is based on the pulsating movement of water, which causes stratification of minerals by density. Heavier minerals (thorium phosphates, oxides) are deposited at the bottom of the layer, while lighter rocks are carried away by the flow of water. However, the jigging efficiency is significantly reduced by reducing the particle size to less than 150 microns [50,51].
Screw separators and Mosley concentration tables are widely used for the enrichment of small and medium fractions (from 20 to 150 microns). At the same time, screw separators use centrifugal forces and gravity, allowing efficient extraction of monazite and thorianite from the ore material. An important advantage of screw separators is their high productivity and simplicity of design, which makes them in demand in large-scale processing of raw materials. Moseley tables further refine the concentration of minerals due to the inclined plane and the variable movement of the surface, which contributes to the accurate separation of particles by density [52].
Despite its effectiveness, gravity enrichment does not provide complete separation of thorium-containing minerals and requires additional application of other physical methods to achieve the required concentration values.
3.2. Magnetic and Electrostatic Separation
Magnetic separation is based on the differences in the magnetic properties of minerals and allows the separation of thorium-containing minerals from impurities such as magnetite, ilmenite and other iron oxides. The method is widely used in combination with gravity enrichment to increase selectivity and reduce the content of ferrous impurities. High gradient magnetic separation (HGMS) is particularly effective in extracting weakly magnetic minerals, including monazite and xenotime, due to the presence of rare earth elements in their structure, which contribute to the weak paramagnetism of minerals [53,54,55].
The use of magnetic separation, however, has a number of limitations due to the similar magnetic characteristics of various minerals containing rare earths and thorium, which requires fine-tuning of the process parameters (magnetic field intensity, material feed rate, particle size). In practice, optimal conditions are selected empirically, depending on the composition of the feedstock and the enrichment objectives [56].
Electrostatic separation complements magnetic and gravitational separation and is based on differences in the electrical conductivity of minerals. Thorium-containing minerals such as monazite and zircon are characterized by different electrical conductivity, which allows them to be effectively separated from each other and from waste rock. It is important to note that pre-drying and cleaning of the particle surface is a prerequisite for successful electrostatic separation. At the same time, the greatest efficiency is achieved when processing particles smaller than 100 microns [57].
The combined use of magnetic and electrostatic separation demonstrates high potential in the processing of complex ores containing thorium, rare earths and uranium, ensuring high purity of the concentrate and optimizing reagent consumption at subsequent stages of hydrometallurgical extraction [58]. Figure 5 shows the scheme of physical enrichment of thorium-containing ores.
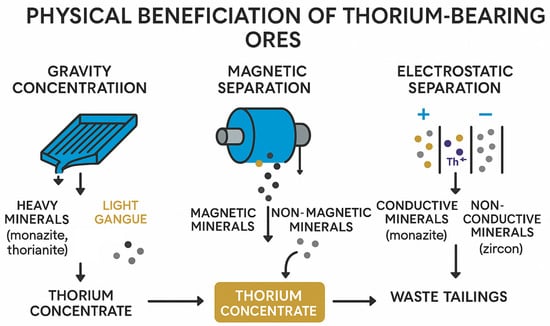
Figure 5.
Physical concentration methods for thorium recovery: gravity, magnetic, and electrostatic separation leading to thorium concentrates and waste tailings.
Thus, physical enrichment methods provide a significant reduction in the volume of raw materials, an increase in the thorium content and the removal of undesirable components, facilitating further processing of thorium-containing concentrates and increasing the efficiency of the entire technological cycle.
4. Hydrometallurgical Leaching
Hydrometallurgical leaching is a key step in the extraction of thorium from natural raw materials and secondary resources. This method is based on the dissolution of valuable components from the mineral matrix using liquid reagents (acids or alkalis), followed by the separation of insoluble residues and the conversion of thorium into a soluble form. Two main approaches are most widely used—acidic and alkaline leaching, the choice of which is determined by the mineral composition of the feedstock and the requirements for subsequent separation stages.
4.1. Acid Leaching (H2SO4, HCl, HNO3)
Acid leaching is traditionally used for most thorium-containing ores, especially monazite and complex phosphate concentrates, as well as for processing tailings and industrial waste. Sulfuric acid (H2SO4), hydrochloric acid (HCl) and nitric acid (HNO3) are used as reagents, both individually and in combination.
H2SO4 is the most common is the treatment of concentrates with sulfuric acid, often with pre-baking of raw materials at a temperature of 200–800 °C, which promotes the decomposition of the mineral matrix and increases the recoverability of thorium and related rare earth elements (REE) [59]. After baking, the product is leached with diluted H2SO4 at a temperature of 20–90 °C, maintaining a pH < 1. With this approach, thorium passes into solution mainly in the form of Th(SO4)2, and the leaching efficiency can reach 85–95%. Insoluble iron, titanium compounds and radioactive residues remain as by-products.
Hydrochloric acid leaching is used, as a rule, to obtain thorianite concentrates, oxides and enrichment tailings containing significant amounts of alkaline earth elements, or, if necessary, to minimize the dissolution of iron and other interfering components [60,61]. The process is usually carried out at a temperature of 60–90 °C and an HCl concentration of 2–6 M, achieving high selectivity for the extraction of thorium and associated uranium. This method is also effective in processing technological solutions enriched with thorium and provides a convenient matrix for subsequent extraction.
Hydrometallurgical processing of thorium-bearing minerals typically involves the decomposition of phosphate and silicate matrices through acid or alkaline leaching, followed by selective separation and purification of thorium. The main purpose of these reactions is to convert thorium into soluble sulfate, nitrate, or hydroxide forms suitable for subsequent extraction and precipitation steps.
The dissolution of monazite in concentrated sulfuric acid proceeds according to the following reaction:
2ThPO4 + 6H2SO4 → 2Th(SO4)2 + 2H3PO4 + 3H2O
Leaching with nitric acid is used in the processing of high-quality concentrates, as well as when it is necessary to separate thorium and rare earth elements [62]. In some cases, the process is carried out with simultaneous oxidation of raw materials (for example, using H2O2 or other oxidizing agents), which contributes to a more complete conversion of thorium into solution. The concentration of HNO3 varies from 2 to 8 M, and the temperature ranges from room temperature to 80 °C. The main advantage of this method is to obtain solutions suitable for the subsequent extraction of thorium by extraction with organic reagents, including TBP, D2EHPA, amines and their mixtures.
A special feature of acid leaching is its high efficiency, which makes it possible to extract thorium even from complex and difficult-to-process ores due to the destruction of the mineral matrix and the conversion of thorium into a soluble form [63]. This approach ensures the simultaneous extraction of thorium, rare earth elements and, if necessary, uranium, which significantly increases the complexity and economic feasibility of processing raw materials. However, a significant disadvantage of acid leaching is the formation of multicomponent solutions containing a wide range of impurities—iron, aluminum, calcium, titanium and other elements, which can negatively affect the subsequent stages of selective extraction and purification of target components [64]. Therefore, to ensure high purity of the final product, multi-stage solution purification processes are required, including sorption, extraction or precipitation of interfering impurities. In addition, acid leaching is accompanied by the formation of significant volumes of acid tailings containing residual toxic and radioactive substances, which requires the introduction of effective technologies for neutralization, conditioning and environmentally safe disposal of the resulting waste [65].
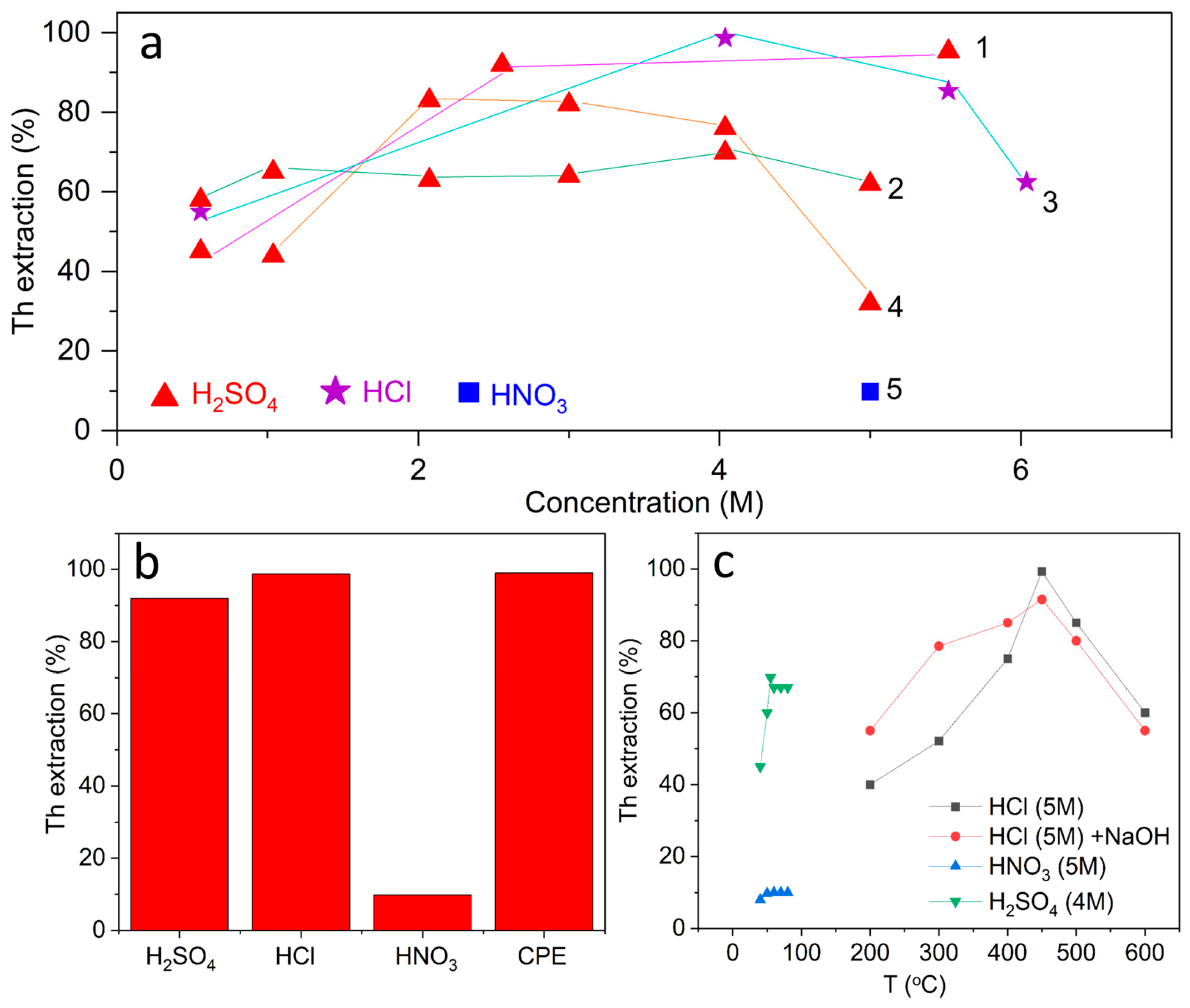
Figure 6a shows the dependence of the efficiency of acidic leaching of thorium on the concentration of the reagent, obtained on the basis of data from various studies. When using H2SO4, thorium extraction increases steadily with increasing concentration, reaching 85–95% in the range of 5–11 M [66,67]. This result is explained by the high ability of sulfate anions to form soluble complexes with Th4+ ions, which ensures almost complete decomposition of monazite and similar phosphate phases. For tailings of Ta/Nb ores, on the contrary, a more moderate efficiency is observed; at 4 M H2SO4, the degree of thorium extraction does not exceed 70% [68], which indicates a stronger embedding of thorium into the silicate oxide matrix.
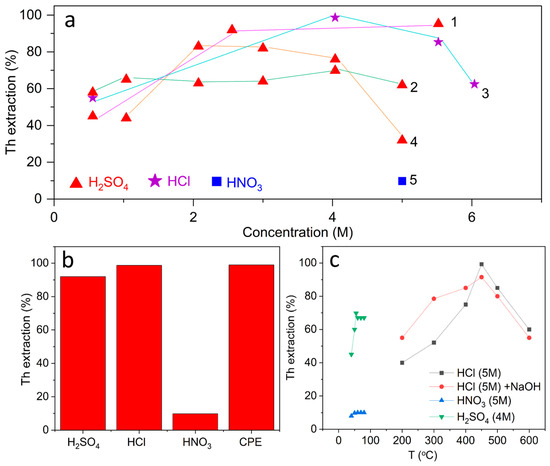
Figure 6.
Comparative analysis of thorium extraction results reported for acid leaching processes: (a) dependence of extraction efficiency on reagent concentration (1—[59,60], 2—[63], 3—[58,59], 4—[62,64], 5—[61]. (b) influence of temperature on thorium recovery from different feedstocks (mo nazite, tin slag, Ta/Nb tailings) under acidic conditions; (c) maximum thorium extraction efficiencies obtained by different acid-based processing methods, including direct leaching, roasting–leaching, and advanced acid-mediated separation techniques.
A fundamentally different picture is observed with the use of HNO3. Even at 5 M concentration and an increase in the leaching time, the proportion of extracted thorium does not exceed 10%, which confirms the weak solubility of the nitrate medium in relation to thorium phases. For HCl, the dependence is intermediate. At concentrations of 5–6 M, 90–95% recovery is achieved [69,70]; however, at lower values (0.5 M), the efficiency drops sharply to 40–50%.
Figure 6b shows the effect of temperature on the efficiency of thorium extraction from various types of raw materials under acidic conditions. For monazite concentrates, an increase in the roasting temperature from 200 to 500–600 °C is accompanied by a sharp increase in the degree of extraction from 20% to 95%, which is associated with the decomposition of the phosphate matrix and the transition of thorium into soluble sulfate forms [71]. With a further increase in temperature (>700 °C), the efficiency decreases due to the formation of insoluble phases, which indicates the presence of an optimal range of heat treatment. For tin slags, the best results are achieved at 400 °C (~50%), whereas at higher temperatures, extraction decreases, reflecting stable binding of thorium in the silicate matrix [72]. In the case of tailings of Ta/Nb ores, an increase in the temperature of the solution from 40 to 55 °C also has a positive effect on the efficiency of the process (an increase from 30 to ~70%); however, a further increase in temperature to 80 °C practically does not change the degree of extraction [73].
Figure 6c shows a comparison of the maximum degrees of thorium extraction achieved by various acidic methods. Modern approaches such as Cloud Point Extraction (CPE) and selective extraction technologies demonstrate the highest efficiency, where almost complete thorium extraction (~98–99%) is achieved with minimal co-extraction of impurities [38]. Classical acid leaching methods also show high results, but they depend on the nature of the reagent; for HCl at 5–6 M, recovery reaches 90–95%, while for H2SO4 in similar conditions, the range of 80–90% is observed [74]. The use of HNO3 proved to be ineffective (less than 10%), which is consistent with its low ability to destroy stable thorium phases. Pre-firing methods (roasting + H2SO4) provide up to 95% recovery, but require additional energy consumption and optimization of the temperature regime [75,76].
The results show that the efficiency of thorium extraction is determined by the choice of reagent, the time and temperature of treatment, as well as the methodology used.
Firstly, the dependence of extraction on acid concentration shows the obvious advantage of sulfuric acid, which provides the most versatile and reproducible result, while nitric acid remains ineffective even at high concentrations. Hydrochloric acid is highly effective only in concentrated form, which emphasizes the need for strict control of conditions.
Secondly, kinetic dependences confirm that the optimal time for acid leaching or firing is usually limited to 2–5 h, after which the process reaches a plateau or even demonstrates a decrease in efficiency due to the formation of insoluble phases.
Thirdly, the temperature factor plays a dual role; a moderate increase in temperature significantly intensifies the transition of thorium into solution, but exceeding the optimal range can lead to the opposite effect due to the stabilization of new phases.
Finally, a comparison of the maximum achievements of various approaches indicates that modern methods (for example, cloud point extraction or ion exchange/extraction technologies) provide almost quantitative isolation of thorium, whereas classical acids and combined schemes demonstrate a wide range of results—from less than 10% (HNO3) to ~95% (HCl, H2SO4, roasting–leaching).
4.2. Alkaline Leaching (NaOH)
Alkaline leaching should be considered primarily as a preparation stage, rather than as an independent method for the final extraction of thorium [77,78]. The treatment of concentrates with NaOH solutions makes it possible to effectively destroy the phosphate or silicate matrix, converting phosphorus into a solution in the form of sodium phosphates and concentrating thorium as insoluble hydroxide in the solid phase [79]. This approach provides high selectivity and facilitates the subsequent separation of thorium and rare earth elements; however, an additional acid step is required to convert thorium into a soluble form and isolate it as pure compounds [80]. Sodium hydroxide (NaOH) is most often used as a reagent, sometimes in combination with soda (Na2CO3) [81].
Typically, alkaline leaching is carried out at a temperature of 130–180 °C (in an autoclave or under pressure) using a 30–50% NaOH solution [37]. During processing, the phosphate matrix of monazite is destroyed, forming soluble sodium phosphates and insoluble thorium hydroxide. After filtration, the thorium hydroxide precipitate is transferred to a solution by further acid leaching, or separated as a semi-finished product for subsequent separation of pure ThO2 [38].
The advantages of alkaline leaching are its high selectivity, which ensures effective separation of rare earths and thorium from related impurities such as silicon, iron and aluminum [82]. This approach is especially useful for processing hard-to-decompose ores and minerals, in which a phosphate or silicate matrix makes it difficult for acidic reagents to access the target components [83]. As a result of processing, an insoluble residue enriched with thorium is formed, which can serve as a semi-finished product for the subsequent production of pure thorium compounds or be sent for additional acid decomposition, which increases the technological flexibility of the process and the convenience of further processing of the concentrate.
Similarly, when thorite (ThSiO4) is treated with alkaline media such as sodium hydroxide, thorium oxide and sodium silicate are formed:
ThSiO4 + 2NaOH → Na2SiO3 + ThO2 + H2O
However, the method has a number of significant limitations. One of the key problems is that after alkaline leaching, thorium often remains in an insoluble form, requiring subsequent acid treatment to transfer it to a solution and extract it in its pure form. In addition, the process requires high concentrations of alkali and elevated temperatures, which are often realized in autoclave conditions, which significantly increases energy costs and reagent costs. An additional problem is the formation of a large volume of alkaline wastewater that contains dissolved sodium salts and decomposition products of the mineral matrix, which requires a mandatory stage of neutralization and the introduction of water cycle systems or environmentally safe disposal [84].
In general, a comparison of acid and alkaline leaching shows that the choice of the optimal approach is determined by the mineral composition of the raw material, the purity requirements of the final product, and the waste disposal capabilities of the enterprise. Acid leaching provides a high degree of thorium recovery and ease of integration with subsequent separation stages, but requires careful solution purification and radioactive waste management. Alkaline methods provide advantages when working with hard-to-decompose or high-phosphate ores and allow efficient concentration of thorium in the solid residue; however, they are associated with additional costs and environmental risks. In modern practice, hybrid technological schemes are increasingly being implemented, combining the strengths of both approaches to achieve maximum efficiency and environmental safety of the process.
In the last decade, considerable attention has been paid to the integrated use of acid and alkaline methods, as well as the development of environmentally friendly technologies with minimal formation of radioactive and chemically aggressive waste. Research is underway on the intensification of processes (the use of ultrasound, microwaves, additives of complexing agents), as well as on the creation of closed cycles with the recycling of reagents and waters.
Figure 7a shows the distribution of mineral and man-made thorium sources by country and type of raw material. The main object of alkaline dissection remains monzite, which is widely found in India, Malaysia, Brazil, Egypt and China. Other carriers such as thorite, bastnesite, and phosphogypsum are considered along with it, but their contribution is less significant [41]. This visualization highlights the global prevalence of monzite as a key raw material for thorium extraction.
The results demonstrate differences in the efficiency of alkaline leaching depending on the mineral composition of the raw material and the applied conditions. The lowest thorium recovery (<5%) is observed during the processing of WLP waste [85], which is associated with the formation of difficult-to-dissolve thorium phases, in particular THP2O7 pyrophosphate, which practically do not degrade in an alkaline environment. For monazite concentrates, alkaline treatment provides only a partial conversion of thorium to a soluble form (≈15–20%); however, the key role of the process is the effective removal of the phosphate matrix, which facilitates further acid decomposition [86]. Significantly higher rates are achieved using thermally enhanced schemes; the alkaline fusion method allows the achievement of up to 82% Th recovery, and the combined NaOH-roasting approach followed by acid leaching provides a maximum recovery rate of ~91% [87,88].
Figure 7b shows that the efficiency of alkaline opening in most studies exceeds 90%, achieving almost complete thorium extraction at 150–210 °C and 2–4 h of processing. Lower values (~75%) are associated with low-grade raw materials and less optimal conditions [89]. Modern approaches with mechanochemical activation or combined leaching provide a stable increase in the degree of decomposition, confirming the crucial role of NaOH concentration and temperature regime.
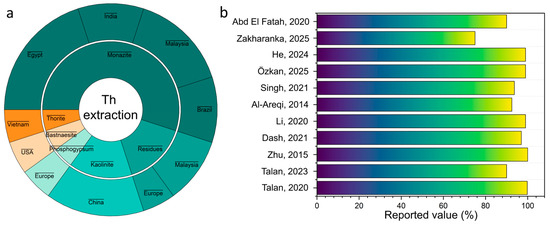
Figure 7.
Comparative overview of thorium alkaline processing: (a) feedstock distribution by mineral type and country; (b) reported recovery efficiencies from selected studies [61,79,80,82,84,85,87,90,91,92,93].
Figure 7.
Comparative overview of thorium alkaline processing: (a) feedstock distribution by mineral type and country; (b) reported recovery efficiencies from selected studies [61,79,80,82,84,85,87,90,91,92,93].
5. Extraction and Chromatographic Separation Methods
Effective separation and purification of thorium at the stages of hydrometallurgical processing require the use of selective methods to separate it from related rare earth elements, uranium and other impurities. Among such methods, liquid–liquid extraction using organic reagents and extraction chromatography, including the use of specialized resins, have become the most widespread. The choice of a suitable extractant or sorbent is determined by the composition of the solution, the purity requirements of the product, and the possibilities for subsequent reagent regeneration. Organophosphorus compounds such as tributyl phosphate (TBP) and di-2-ethylhexylphosphoric acid (D2EHPA) occupy a leading position among extractants for the separation and concentration of thorium [94,95,96].
5.1. Organophosphorus Reagentter (TBP, D2EHPA)
TBP is widely used to extract thorium from nitrate solutions formed after acid leaching and pre-cleaning [97]. The extraction mechanism is based on the formation of thorium coordination complexes with TBP, which provides high selectivity even with a significant content of rare earth elements and uranium. TBP provides convenient phase separation, is easily regenerated and compatible with industrial schemes, but requires a sufficiently high acidity of the medium (usually 4–6 m HNO3) for effective extraction [98].
D2EHPA is used primarily in sulfate and chloride media and provides selective extraction of thorium compared to many rare earth elements and iron. The advantage of D2EHPA is its high extraction capacity relative to thorium and the possibility of its subsequent effective stripping with dilute acid or salt solutions. At the same time, it is possible to carry out multi-stage processes with sequential extraction of thorium, uranium and REE. However, due to the propensity of D2EHPA to form a third phase and to extract some related elements, careful optimization of the composition of the organic phase and extraction conditions is required [99].
The combined use of TBP and D2EHPA, as well as the use of various modifiers (n-decanol, kerosene, etc.), makes it possible to increase selectivity and reduce losses of the organic phase, which makes these methods indispensable in the industrial processing of thorium-containing solutions [100].
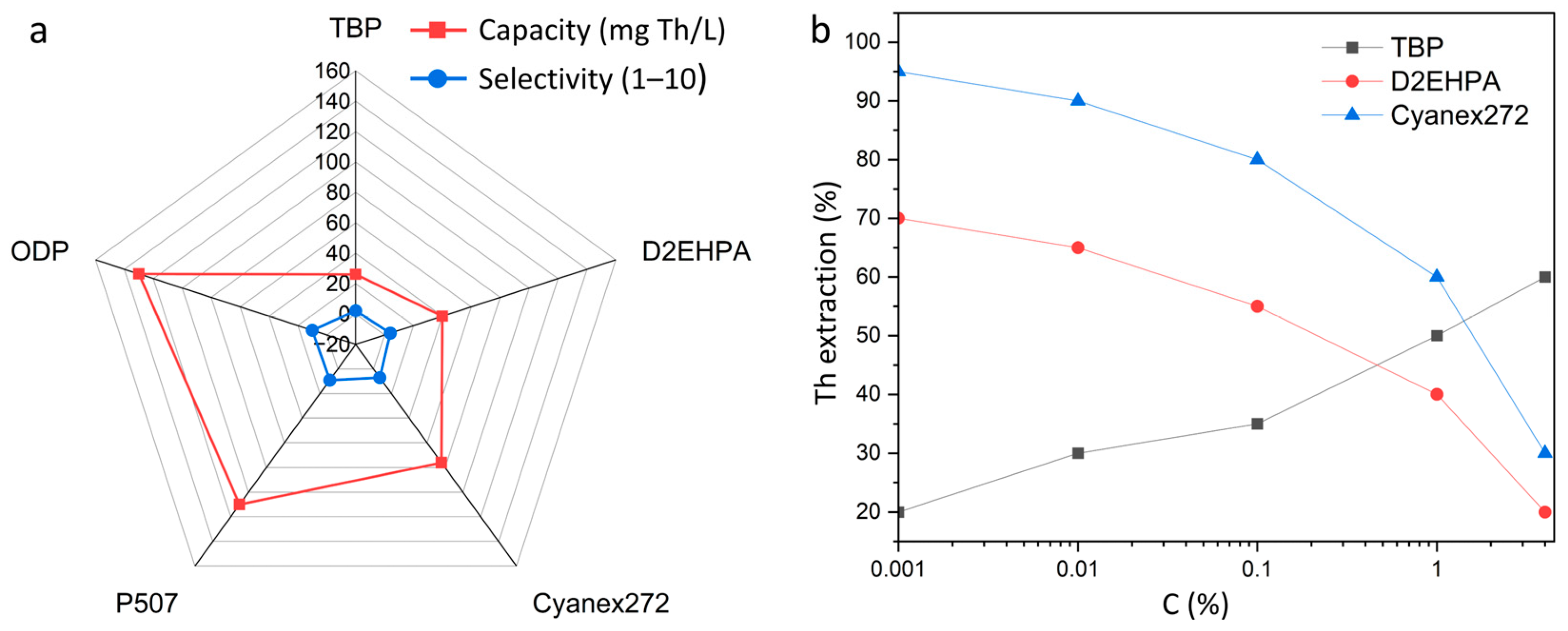
Figure 8b shows the comparative characteristics of the main thorium extractants, expressed in terms of their loading capacity and selectivity. It can be seen that traditional reagents (TBP, D2EHPA) exhibit relatively low values in both parameters, which limits their use in modern processing schemes. At the same time, Cyanex272 and P507 provide a higher balance between extraction efficiency and selectivity, and new organophosphoric compounds such as ODP have the greatest potential, combining high loading capacity (>120 mg/L) and almost complete separation of thorium from impurity elements. Thus, the choice of extractant plays a critical role in optimizing thorium extraction and purification processes [101].
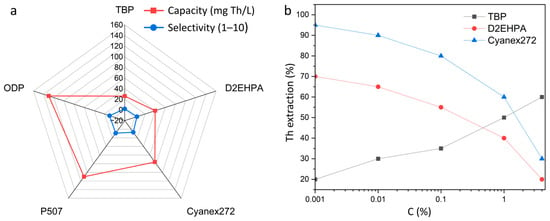
Figure 8.
Comparison of thorium extractants: (a) loading capacity and selectivity (TBP, D2EHPA, Cyanex272, P507, ODP); (b) dependence of extraction efficiency on acidity, reflecting differences in the operating modes of extractants.
Figure 8a shows the effect of the acidity of the solution on the efficiency of thorium extraction by various extractants. TBP demonstrates an increase in extraction with increasing acid concentration, which is associated with the need for high ionic strength for the formation of stable coordination complexes. On the contrary, D2EHPA works most effectively in slightly acidic environments and loses its ability to separate at high acidity. Cyanex272 provides maximum recovery at low acidity (0.001–0.01 M), which emphasizes its selectivity and technological attractiveness for mild hydrometallurgical schemes [102,103,104].
5.2. Schiff Bases, Diglycolamides, and Ionic Liquids
In recent years, much attention has been paid to the use of new classes of extractants with high selectivity for tetravalent thorium.
Schiff bases (imines and their derivatives) form strong complexes with Th(IV), which makes it possible to use them for the selective extraction of thorium from multicomponent solutions, including in the presence of large amounts of REE, iron, and other impurities. A special feature of these extractants is the possibility of fine-tuning their structure to increase selectivity to specific ions by modifying donor groups, as well as the possibility of using them in organic solvents of various natures [105].
Diglycolamides (DGA) are a relatively new field promising for the extraction and separation of actinides. DGA exhibits high extraction ability with respect to Th(IV) and other tetra- and hexa-valence elements in acidic solutions, forming stable complexes. The use of DGA is especially relevant in systems with a high content of rare earth elements and a minimal presence of Fe and Al, since maximum selectivity is achieved in such conditions [106].
Ionic liquids are modern solvents consisting of organic ions that have unique solvent properties and high thermal stability. The use of ionic liquids as a medium for thorium extraction makes it possible to increase the efficiency of the process due to the higher solubility of the target complexes, lower volatility and the possibility of reuse. They can be both independent extractants and a medium for dissolving complex ligands (Schiff bases, DGA, etc.), which provides flexibility in the development of technological schemes [107].
5.3. Extraction Chromatography and Resins
Extraction chromatography combines the advantages of liquid extraction and ion exchange sorption and is used for selective concentration and purification of thorium at deep separation stages.
Extraction resins are polymer carriers on the surface of which a layer of organic extractant is applied (for example, D2EHPA, TBP, Aliquat 336). Such resins provide selective extraction of thorium from multicomponent solutions, allow operation at low concentrations of ionic components and are resistant to aggressive media. Their use is particularly effective for aftertreatment of weakly concentrated solutions, tailings processing and capture of residual thorium during deep processing of REE products [108].
Columns with extraction resins are used in a dynamic mode; the solution is passed through a column where thorium is selectively sorbed, after which sequential elution is carried out, allowing the separation of thorium, uranium and related impurities [109]. The use of such columns reduces the amount of organic solvents used, increases process safety, and facilitates automation and scaling.
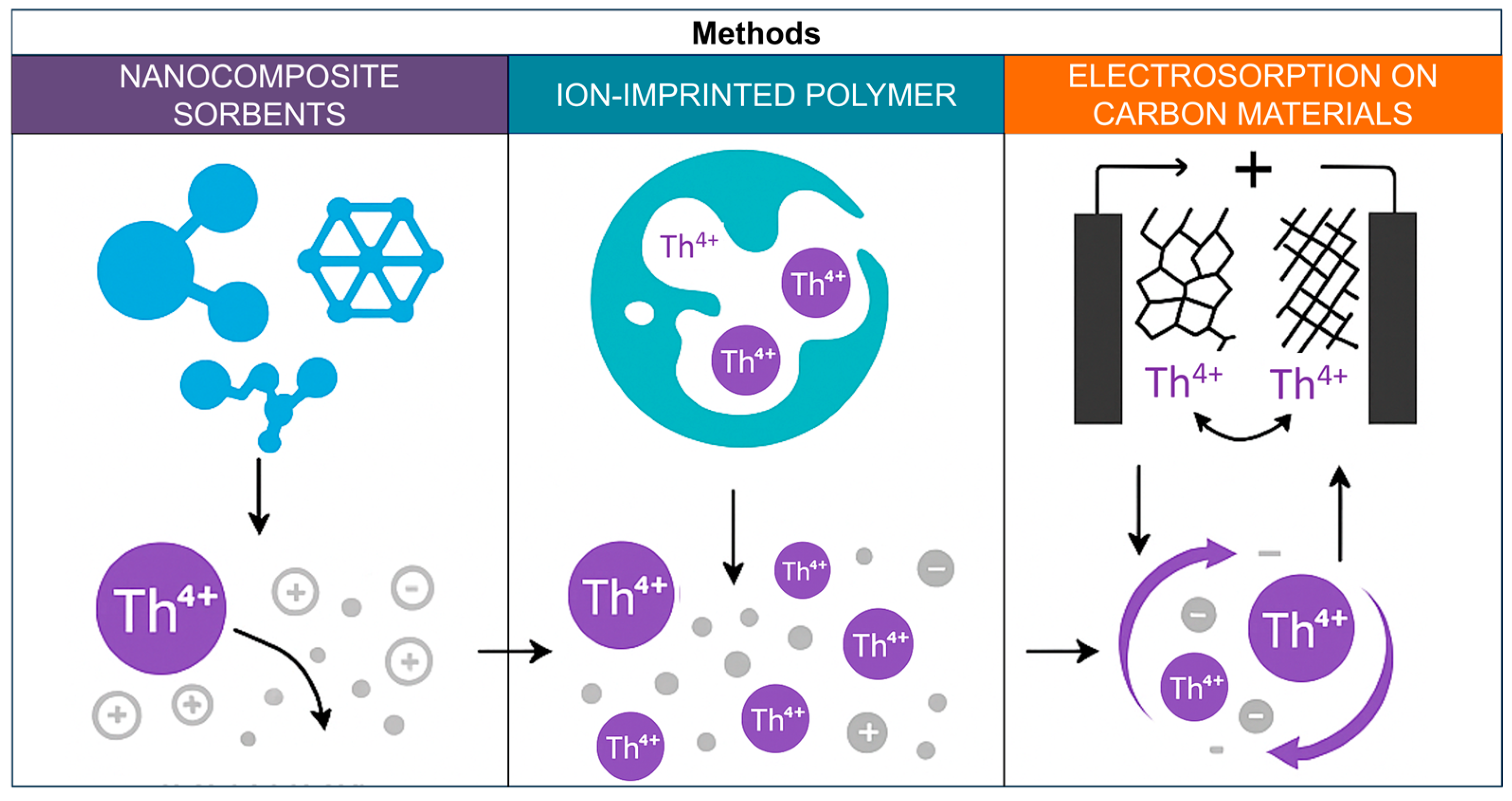
Modern research is aimed at creating new types of resins with high sorption capacity and selectivity to Th(IV), as well as combining chromatographic methods with membrane and ion exchange technologies to further improve the efficiency and environmental friendliness of processes [110]. Figure 9 shows a schematic representation of sorption and electrosorption methods for thorium separation.
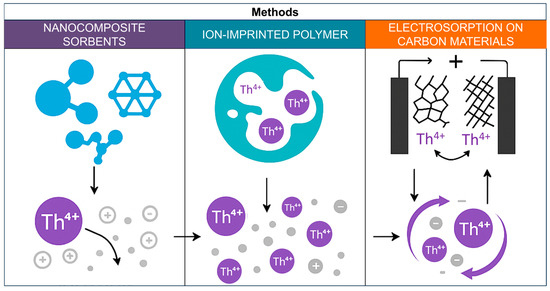
Figure 9.
Sorption and electrosorption methods for thorium separation.
6. Sorption and Electrosorption Methods
In recent years, sorption and electrosorption methods have attracted increasing attention as effective, economically and environmentally attractive approaches to the selective extraction of thorium from dilute and complex solutions [111,112,113]. These methods allow not only to achieve a high degree of purification of the target product, but also to integrate separation processes with the possibility of reuse of reagents, which reduces waste generation and increases the technological flexibility of processing.
6.1. Nanocomposite and Ion-Printed Sorbents (TIO2, MOF, Magnetic Nanocomposites)
One of the most promising areas of modern sorption separation is nanocomposite and ion-sealed sorbents, which have a high surface area, a developed porous structure, and the ability to modify the surface with functional groups with a given selectivity [114].
TiO2-based nanocomposites are widely used for the extraction of Th(IV) due to their ability to form strong complex compounds with tetravalent ions. Such materials can be additionally modified with organic ligand groups (for example, phosphonic, carboxylic), which increases the selectivity and capacity of thorium even in the presence of a large number of interfering ions [115].
Organometallic frameworks (MOF, metal–organic frameworks) are highly porous crystalline structures capable of selectively trapping actinides and rare earth elements. Particular attention is paid to MOFs containing functional groups (amine, sulfone groups) focused on the binding of Th(IV) [116]. Due to their adjustable pore structure, these materials exhibit high sorption capacity, fast adsorption kinetics, and excellent regeneration ability [117,118]. Typical sorption processes are carried out at temperatures of 25–80 °C with contact times ranging from 30 to 120 min, achieving 85–99% Th(IV) extraction efficiency. The sorption capacity of various materials depends on their surface chemistry and morphology; functionalized activated carbons exhibit 35–60 mg Th g−1, while sulfonated ion-exchange resins reach 75–120 mg Th g−1. In mixed solutions containing Fe3+, Al3+, and REE3+ ions, thorium selectivity remains high at pH 2–4.5. Functionalization with phosphonate or sulfonic groups significantly enhances both the uptake capacity and selectivity for Th(IV), enabling efficient regeneration and reuse of sorbents.
Magnetic nanocomposites (for example, based on Fe3O4 with a modified shell) can significantly simplify the stage of separation of the sorbent from the solution by using an external magnetic field [119,120,121]. Modern magnetic composites are often coated with layers of glutathione, chelated ligands, or ionogenic polymers, which provides high selectivity for thorium. The use of such materials has demonstrated effective thorium extraction (up to 99.4% under optimal conditions) with the possibility of repeated use without significant loss of sorption capacity [122].
Ion-sealed polymers (IIPs) are synthetic sorbents whose structure is formed in the presence of thorium ion matrices [123]. After removing the matrix, cavitations remain in the polymer that strictly correspond to the size, charge, and coordination sphere of Th(IV), which gives the material a unique selectivity even in complex multicomponent solutions. Such sorbents are used in both static and dynamic systems, including column processes and cartridge modules [124,125,126].
6.2. Electrosorption of Carbon Materials
Electrosorption is an innovative method of extracting ions from solutions due to their adsorption on electrodes under the action of an external electric potential [127,128]. For these purposes, electrode materials based on porous carbon (activated carbon, carbon nanotubes, graphene composites), characterized by high specific surface area, electrical conductivity, and chemical stability, are the most promising [129,130,131].
Experimental studies have shown that optimal electrosorption occurs under applied potentials of 1.0–2.5 V, electrolyte concentrations of 0.1–1 M HNO3, and contact times of 10–30 min. Under these conditions, the Th(IV) removal efficiency reaches up to 98%, depending on electrode type and surface modification. Electrodes based on nitrogen-doped graphene and carbon nanotube composites demonstrate superior ion transport and regeneration stability, maintaining performance over multiple sorption–desorption cycles. During electrosorption, a double electric layer is formed on the surface of carbon electrodes, which makes it possible to selectively concentrate thorium cations from dilute solutions [132]. Unlike classical sorption, electrosorption allows the process to be controlled by changing the potential, which makes it possible to selectively extract and subsequently release thorium ions without the use of additional reagents. This is especially important for the creation of closed technological cycles and multiple regeneration of electrodes. Studies show that the efficiency of Th(IV) electrosorption on carbon materials can exceed 90%, and the electrodes themselves can withstand multiple sorption-desorption cycles without loss of performance [133].
The use of electrosorption also allows the integration of water purification processes, the capture of radionuclides and the concentration of valuable elements in a single technological circuit. Current developments are aimed at creating hybrid systems combining nanocomposite materials with electrodes to increase selectivity, resistance to contamination and simplify operation [134,135].
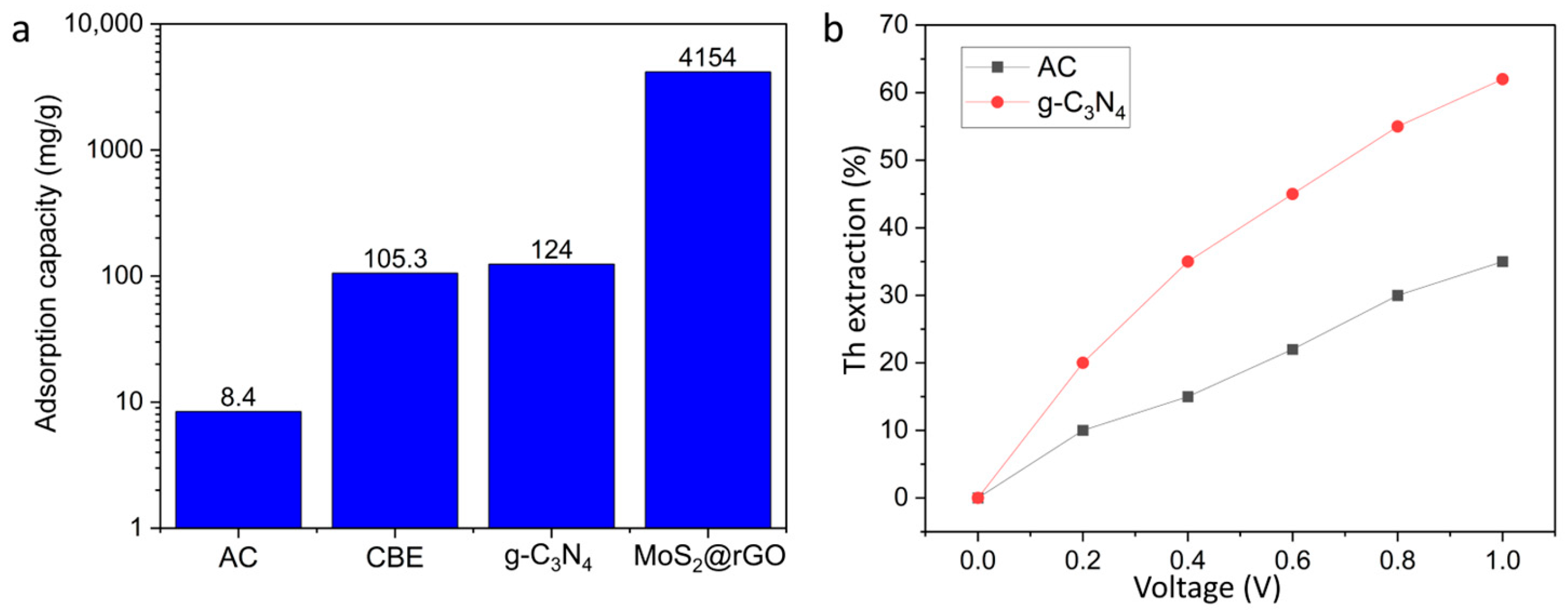
Figure 10a shows the evolution of adsorption capacity of carbon-based electrodes applied for thorium electrosorption. A clear trend can be observed; early studies with activated carbon reported very low capacities below 10 mg/g, reflecting the limited surface functionality and pore accessibility of conventional carbons. The introduction of continuous-flow systems and nanostructured g-C3N4 significantly increased the uptake to ~100–120 mg/g, indicating the role of electrical conductivity and nitrogen functional groups in enhancing ion capture [136]. The most remarkable improvement is achieved with hybrid MoS2@rGO aerogels, which exhibit capacities above 4000 mg/g, highlighting the synergistic effect of 2D/3D architectures and heteroatom doping. This progression demonstrates the shift from proof-of-concept materials to advanced nanocomposites with industrially relevant performance for thorium recovery.
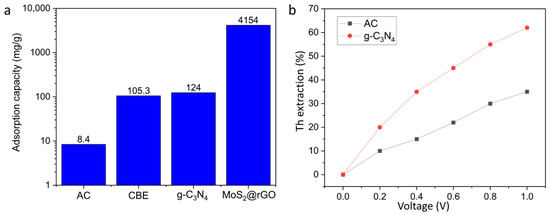
Figure 10.
Comparative performance of carbon-based electrodes for thorium electrosorption: (a) evolution of adsorption capacity from conventional activated carbon (<10 mg/g) to advanced MoS2@rGO aerogels (>4000 mg/g); (b) effect of applied voltage on removal efficiency, showing modest performance of activated carbon versus the enhanced response of g-C3N4 electrodes.
Figure 10b illustrates the influence of applied voltage on thorium electrosorption efficiency using activated carbon and g-C3N4 electrodes. Activated carbon shows only a modest increase in removal efficiency, reaching ~35% at 1.0 V, which reflects its limited surface functionality and charge storage capability. In contrast, g-C3N4 electrodes display a pronounced improvement, with efficiency rising to ~62% under the same potential. This difference highlights the importance of nitrogen-rich functional groups and higher electrical conductivity of g-C3N4 in promoting ion migration and double-layer formation. Overall, the results confirm that optimizing electrode material, in addition to applied voltage, is crucial for achieving high electrosorptive performance in thorium recovery [137].
7. Environmental and Radiological Aspects
7.1. Management of Radioactive Waste
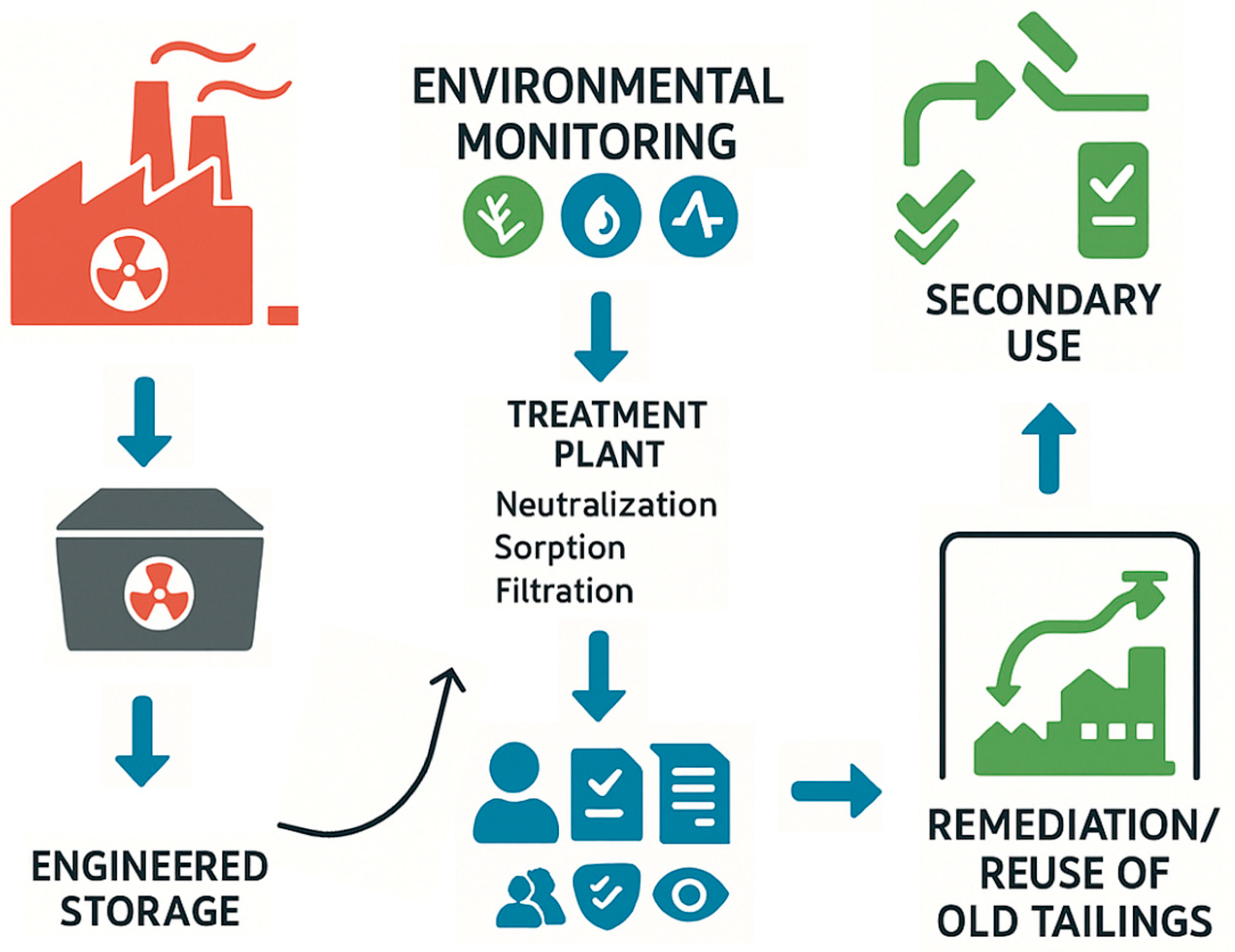
Processing of thorium-bearing ores and industrial by-products inevitably generates radioactive residues in solid, liquid, and occasionally gaseous forms. Solid wastes such as Water Leach Purification Residue (WLP), Neutralization Underflow (NUF), and Semi-Active Residual Material (SARM) contain thorium, uranium, actinium, and heavy metals, often exceeding regulatory limits. These residues require long-term isolation using engineered storage with multi-barrier systems, encapsulation, cementation, or vitrification. Experience from Malaysia, China, and India demonstrates that inadequate waste management provokes social opposition, highlighting the need for transparent handling strategies [138].
Figure 11 illustrates a simplified scheme of waste management in thorium processing, including engineered storage, treatment by neutralization and sorption, continuous monitoring, and the remediation and reuse of tailings, supported by regulatory oversight.
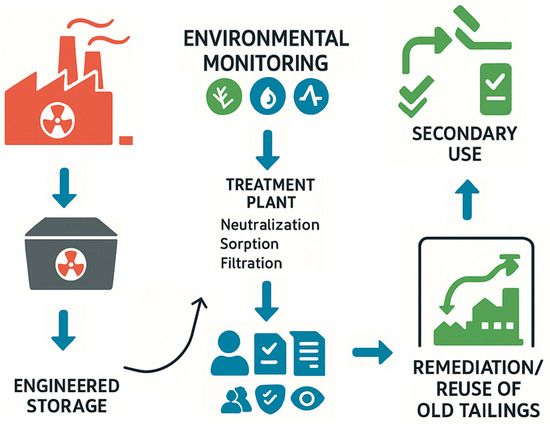
Figure 11.
The scheme of radioactive waste management during thorium processing: from the formation of solid residues to their engineering storage, neutralization and sorption purification, environmental monitoring, as well as remediation and reuse.
Liquid wastes (raffinates, wash waters, effluents) contain dissolved radionuclides and heavy metals. Their treatment involves multi-step neutralization, precipitation with sorbents or ion-exchange resins, and membrane separation, with partial recycling of purified water. Although gaseous releases are uncommon for hydrometallurgical processes, high-temperature or accidental scenarios require filtration and monitoring systems [139].
Modern approaches emphasize minimizing waste generation through comprehensive recovery of Th, REE, and U, adoption of closed water cycles, and advanced methods such as electrosorption [140]. Residual radionuclides are immobilized in stable matrices to prevent migration, while continuous environmental monitoring (dose rates, groundwater, surface water, radon migration) is mandatory under IAEA and national guidelines [141]. Remediation of legacy tailings further reduces long-term radiological risk and enables potential secondary use [142].
7.2. Environmental Safety of Technologies
Environmental safety in thorium technologies extends beyond radiological protection and includes chemical and ecological risks [143]. Reduction in emissions and discharges is achieved by optimizing flowsheets, implementing closed-loop cycles, and installing multi-stage treatment units [144]. Application of low-waste technologies-such as reusable nanocomposite sorbents, electrosorption on carbon electrodes, biosorption, and membranes-enhances both resource efficiency and waste minimization [145,146].
Integrated recovery of valuable elements from residues decreases both radiological and chemical burdens while improving economic feasibility and public acceptance. Compliance with international standards (IAEA, OECD, ISO) requires environmental impact assessment, accident scenario analysis, and monitoring programs. Transparent communication and disclosure of monitoring data are essential to maintain public trust, as social opposition has previously led to project delays [147].
Ensuring sustainability involves life-cycle assessment, independent audits, land reclamation, and the use of green chemistry, bioremediation, and phytoremediation approaches. A comprehensive framework that combines technical, regulatory, and societal measures is critical to reduce risks, support sustainable development, and align thorium technologies with international safety standards [148].
8. Future Perspectives and Recommendations
8.1. Hybrid Methods and Research Priorities
One of the most promising directions in thorium processing is the development of hybrid technologies that combine conventional hydrometallurgical approaches with advanced separation methods [149,150]. Early stages typically involve acid or alkaline leaching, while downstream purification integrates solvent extraction, sorption on functionalized nanocomposites, electrosorption on carbon electrodes, or membrane-based techniques. Such hybrid systems enable higher selectivity, lower reagent consumption, and a significant reduction in secondary waste, thereby improving both process efficiency and environmental safety. Increasingly, these systems are supported by digital process control and artificial intelligence tools, which allow real-time optimization of operating conditions and minimize operator-dependent variability.
Further research efforts are directed towards the design of novel extractants, sorbents, and membranes with enhanced selectivity and stability in aggressive media [151,152]. Particular attention is given to the separation of thorium from rare earths, uranium, and decay products, where conventional reagents show limited efficiency. The processing of secondary resources such as tailings, slags, and industrial residues is also becoming a central focus, as it addresses both resource recovery and long-term environmental liabilities. Molecular dynamics and thermodynamic modeling provide predictive insights into solubility, selectivity, and phase equilibria, offering a rational basis for optimizing leaching, extraction, and sorption conditions.
Another research priority lies in the experimental validation of closed-loop flowsheets, where reagents and water are continuously recycled and radioactive residues are immobilized in stable matrices. At the same time, remediation strategies for legacy sites contaminated by thorium processing are advancing, with emphasis on cost-effective methods that combine physical, chemical, and biological approaches. Biotechnological solutions, including the application of natural and modified biosorbents, and low-energy «green chemistry» processes further expand the scope of sustainable thorium management. Collectively, these developments highlight a strong shift from proof-of-concept studies to integrated, sustainable, and scalable technologies for thorium recovery [153].
8.2. Economic Feasibility and Optimization
The large-scale implementation of thorium processing technologies is ultimately determined by their economic viability. Reagent and energy costs remain major contributors to the overall process economy, and strategies for cost reduction are closely linked to the use of reusable materials such as regenerable sorbents, membranes, and solvent extractants. Process digitalization and automation further contribute to lowering operational expenses by improving control, reducing downtime, and enabling adaptive optimization under variable feedstock conditions [154,155].
Minimization of radioactive waste volumes is another decisive factor influencing both direct costs and long-term liabilities associated with storage and remediation. Integrated recovery of thorium, rare earth elements, and uranium from primary and secondary resources not only reduces the amount of waste requiring disposal but also creates additional revenue streams, strengthening the overall economic case. Flexibility in process design is particularly valuable, as it allows the adaptation of flowsheets to low-grade ores and industrial residues, which are increasingly relevant as alternative sources [156].
Economic assessment must also consider environmental and social dimensions. Life-cycle analysis provides a framework to account for hidden costs, including waste management, site rehabilitation, and long-term monitoring. Incorporating these factors is essential for meeting international standards of sustainability and for justifying investments in thorium-based technologies [157]. In this context, the alignment of technical innovation with regulatory compliance and social acceptance is a prerequisite for the industrial deployment of thorium processing on a competitive scale. The main advantages, limitations, and development prospects of thorium processing are systematically outlined in Table 2, which provides a SWOT analysis of the current technological landscape.

Table 2.
SWOT analysis of thorium processing technologies.
9. Conclusions
This review reflects current achievements and trends in the extraction, separation, and purification of thorium from various natural and man-made sources. The main mineral forms and types of raw materials, physical enrichment methods, hydrometallurgical approaches, modern extraction, chromatographic, and sorption technologies, as well as environmental and radiation aspects that determine the stability and safety of production processes are consistently considered.
The analysis shows that the technological chains of processing thorium-containing raw materials require the complex application of various methods, from traditional physical and chemical operations to the latest nanotechnology, hybrid circuits and intelligent control systems. Selective extractants, nanocomposite and ion-printed materials, electrosorption and membrane processes are of particular importance, which significantly increase the efficiency, selectivity and ecological purity of thorium extraction even from low-grade or secondary resources.
Radioactive waste management systems, continuous environmental and radiation monitoring, as well as the application of international standards and regulatory requirements at all stages of the material’s life cycle are an integral part of modern technology. Ensuring environmental safety, minimizing waste generation, introducing closed cycles and re-involving valuable components are becoming not only a technological, but also a public priority.
The development of hybrid and integrated circuits, integrated waste management, and the creation of sustainable and cost-effective production facilities are the main areas of further research and industrial implementation of thorium management technologies. Only a systematic interdisciplinary approach based on the achievements of chemistry, materials science, ecology and economics will ensure the safe and competitive development of the industry in the context of global challenges of energy and sustainable development.
Despite the impressive successes achieved in the field of thorium extraction and processing technologies, an analysis of the literature and experimental data revealed a number of unresolved problems and contradictions that require further scientific understanding. Many modern approaches still face fundamental technological limitations, in particular, the high energy intensity of alkaline methods, insufficient selectivity of a number of extractants, and the need for additional purification of the target product from associated radionuclides and chemical impurities. In addition, most of the published studies were conducted on model solutions or on a laboratory scale, which makes it difficult to extrapolate the data obtained to industrial conditions and real mineral raw materials with variable composition.
Environmental and radiation safety issues discussed in detail in modern publications are often addressed at the level of regulatory requirements or pilot projects, while the experience of large industrial implementations demonstrates the continuing risks of unauthorized release of radionuclides, accumulation of long-term tailings and insufficient effectiveness of long-term monitoring. The problem of public rejection of large thorium and rare earth projects remains relevant, due to historical mistakes in waste management, insufficient transparency of companies and the lack of effective communication strategies.
Thus, the further development of the industry requires not only the improvement of chemical and engineering solutions, but also the formation of integrated strategies, including early modeling of environmental impacts, constant dialog with society, multilevel regulation and interdisciplinary scientific research. A critical analysis of existing approaches highlights the need to move from narrowly focused optimizations to a comprehensive lifecycle of thorium management, where economic, environmental, and social efficiency should be considered as inextricably linked goals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app152111403/s1, Figures S1 and S2 are provided in the Supplementary File. Additional references are cited therein [158,159,160].
Author Contributions
Conceptualization, T.A., M.A. and S.T.; methodology, Z.A. and A.K.; validation, Z.A., A.K. and Z.I.; formal analysis, Z.A. and A.K.; writing—original draft preparation, T.A. and M.A.; writing—review and editing, M.A., S.T. and B.L.; visualization, Z.A. and A.K.; supervision, Z.M. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research is funded by a grant from the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant no. BR24993225).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, L.; Guo, H.; Dai, L.; Liu, M.; Xiao, Y.; Cong, T.; Gu, H. The role of nuclear energy in the carbon neutrality goal. Prog. Nucl. Energy 2023, 162, 104772. [Google Scholar] [CrossRef]
- Mathew, M.D. Nuclear energy: A pathway towards mitigation of global warming. Prog. Nucl. Energy 2022, 143, 104080. [Google Scholar] [CrossRef]
- Krūmiņš, J.; Kļaviņš, M. Investigating the Potential of Nuclear Energy in Achieving a Carbon-Free Energy Future. Energies 2023, 16, 3612. [Google Scholar] [CrossRef]
- Costa Peluzo, B.M.T.; Kraka, E. Uranium: The Nuclear Fuel Cycle and Beyond. Int. J. Mol. Sci. 2022, 23, 4655. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv, S. Analysis of the variability of low-carbon energy sources, nuclear technology and renewable energy sources, in meeting electricity demand. Energy Rep. 2023, 9, 276–283. [Google Scholar] [CrossRef]
- Rubbia, C. A Future for Thorium Power? In Proceedings of the ThEC13 Conference: Thorium Energy for the World, CERN, Globe of Science and Innovation, Geneva, Switzerland, 27–31 October 2013; Springer International Publishing: Cham, Switzerland, 2016; pp. 9–25. [Google Scholar] [CrossRef]
- Manchanda, V.K. Thorium as an Abundant Source of Nuclear Energy and Challenges in Separation Science. Radiochim. Acta 2023, 111, 243–263. [Google Scholar] [CrossRef]
- Humphrey, U.E.; Khandaker, M.U. Viability of Thorium-Based Nuclear Fuel Cycle for the Next Generation Nuclear Reactor: Issues and Prospects. Renew. Sustain. Energy Rev. 2018, 97, 259–275. [Google Scholar] [CrossRef]
- Revol, J.P.; Bourquin, M.; Kadi, Y.; Lillestol, E.; de Mestral, J.C.; Samec, K. Thorium Energy for the World. In Proceedings of the ThEC13 Conference: Thorium Energy for the World, CERN, Globe of Science and Innovation, Geneva, Switzerland, 27–31 October 2013; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–8. [Google Scholar] [CrossRef]
- Schaffer, M.B. Abundant Thorium as an Alternative Nuclear Fuel: Important Waste Disposal and Weapon Proliferation Advantages. Energy Policy 2013, 60, 4–12. [Google Scholar] [CrossRef]
- Li, D. TRU Utilization and MA Transmutation in Thorium-Based Fluorinated Molten Salt Fast Reactor. Prog. Nucl. Energy 2024, 168, 105015. [Google Scholar] [CrossRef]
- Mohsen, M.Y.; Luhaib, S.A.; Alnassar, N.; Magzoub, O.A.; Abdel-Rahman, M.A.; Sallah, M.; Galahom, A.A. Investigating the Possible Advantages of Using Different Concentrations of Transuranic Elements with Thorium–Uranium Dioxide as a Fuel for PBMR-400. Prog. Nucl. Energy 2025, 178, 105512. [Google Scholar] [CrossRef]
- Degueldre, C.; Joyce, M.J. Evidence and Uncertainty for Uranium and Thorium Abundance: A Review. Prog. Nucl. Energy 2020, 124, 103299. [Google Scholar] [CrossRef]
- Herring, J.S. Uranium and Thorium Resources. In Nuclear Energy; Springer: New York, NY, USA, 2018; pp. 65–185. [Google Scholar] [CrossRef]
- Hedrick, J.B. Scandium; Mineral Commodity Summaries 2010a; U.S. Geological Survey: Reston, VA, USA, 2010.
- Ramadan, A.; El-Metwally, A.; Abd El-Bary, A. Mineralogy and Radioactivity Evaluation of Dry Stream Sediments as Raw Materials Resources in Umm Naggat Area, Egypt. Iraqi Geol. J. 2025, 58, 239–259. [Google Scholar] [CrossRef]
- Findeiß, M.; Schäffer, A. Fate and Environmental Impact of Thorium Residues during Rare Earth Processing. J. Sustain. Metall. 2017, 3, 179–189. [Google Scholar] [CrossRef]
- Jyothi, R.K.; De Melo, L.G.T.C.; Santos, R.M.; Yoon, H.S. An Overview of Thorium as a Prospective Natural Resource for Future Energy. Front. Energy Res. 2023, 11, 1132611. [Google Scholar] [CrossRef]
- Cuney, M. Uranium and Thorium: The Extreme Diversity of the Resources of the World’s Energy Minerals. In Non-Renewable Resource Issues: Geoscientific and Societal Challenges; Sotirov, I., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 91–129. [Google Scholar] [CrossRef]
- Fesenko, S.V.; Emlutina, E.S. Thorium Concentrations in the Environment: A Review of the Global Data. Biol. Bull. 2021, 48, 2086–2097. [Google Scholar] [CrossRef]
- Raju, R.D. Critical Minerals-U-Th, Recoverable from the Placer Valuable Heavy Minerals in Mineral Sand Deposits. Int. J. Res. Innov. Appl. Sci. 2025, 10, 807–819. [Google Scholar] [CrossRef]
- Cao, M.; Bu, H.; Gao, Y. Preconcentration of Low-Grade Ta–Nb Deposit Using Physical Separation Methods. JOM 2021, 73, 1410–1418. [Google Scholar] [CrossRef]
- Williams, M.A.; Kelsey, D.E.; Rubatto, D. Thorium Zoning in Monazite: A Case Study from the Ivrea–Verbano Zone, NW Italy. J. Metamorph. Geol. 2022, 40, 1015–1042. [Google Scholar] [CrossRef]
- Corbett, M.K.; Eksteen, J.J.; Niu, X.Z.; Croue, J.P.; Watkin, E.L. Interactions of Phosphate Solubilising Microorganisms with Natural Rare-Earth Phosphate Minerals: A Study Utilizing Western Australian Monazite. Bioproc. Biosyst. Eng. 2017, 40, 929–942. [Google Scholar] [CrossRef]
- René, M.; Akitsu, T. Nature, Sources, Resources, and Production of Thorium. In Thorium: Characterization, Chemical Properties, and Applications; IntechOpen: London, UK, 2017; pp. 201–212. [Google Scholar] [CrossRef]
- UN.ESCAP. Sri Lanka: Explanatory Brochure? United Nations: New York, NY, USA, 1989. [Google Scholar]
- Sameera, K.A.G.; Wickramasinghe, W.A.G.K.; Harankahawa, S.B.; Welikanna, C.R.; De Silva, K.T.U.S. Radiometric Surveying for Th and U Mineralization in Southwestern Sri Lanka: Radiological, Mineralogical and Geochemical Characteristics of the Radioactive Anomalies. J. Geol. Soc. Sri Lanka 2020, 21, 29–44. [Google Scholar] [CrossRef]
- Allison, G.K. Fluid Inclusion Evidence for the Temperature and Composition of Ore Fluids in the Lemhi Pass and Diamond Creek REE-Th Districts, Idaho-Montana. Ph.D. Thesis, University of Missouri-Columbia, Columbia, MO, USA, 2019. [Google Scholar] [CrossRef]
- Rahmansyah, A.A.A.; Srigutomo, W. Natural Radioactivity of Rock and Potential Availability of Uranium-Thorium Minerals in Indonesia. J. Phys. Conf. Ser. 2022, 2243, 012058. [Google Scholar] [CrossRef]
- Rosianna, I.; Nugraha, E.D.; Syaeful, H.; Putra, S.; Hosoda, M.; Akata, N.; Tokonami, S. Natural Radioactivity of Laterite and Volcanic Rock Sample for Radioactive Mineral Exploration in Mamuju, Indonesia. Geosciences 2020, 10, 376. [Google Scholar] [CrossRef]
- Abdel Gawad, A.E.; Panova, E.G.; Ghoneim, M.M.; Yanson, S.Y.; Alsufyani, S.J.; Saftah, A.; Alresheedi, N.M.; Hanfi, M.Y. Radioactive Assessment and Th-, Nb-Ta-, Zr-, REE-Bearing Minerals in Alkaline Syenite: Environmental Implications for Radiological Safety. Geosciences 2025, 15, 138. [Google Scholar] [CrossRef]
- Ault, T.; Van Gosen, B.; Krahn, S.; Croff, A. Natural Thorium Resources and Recovery: Options and Impacts. Nucl. Technol. 2016, 194, 136–151. [Google Scholar] [CrossRef]
- Chidester, B.A.; Pardo, O.S.; Fischer, R.A.; Thompson, E.C.; Heinz, D.L.; Prescher, C.; Prakapenka, V.B.; Campbell, A.J. High-Pressure Phase Behavior and Equations of State of ThO2 Polymorphs. Am. Mineral. 2018, 103, 749–756. [Google Scholar] [CrossRef]
- Taylor, M.A.R.K.; Ewing, R.C. The Crystal Structures of the ThSiO4 Polymorphs: Huttonite and Thorite. Acta Crystallogr. Sect. B Struct. Sci. 1978, 34, 1074–1079. [Google Scholar] [CrossRef]
- Raison, P.E.; Heathman, S.; Wallez, G.; Zvoriste, C.E.; Bykov, D.; Ménard, G.; Caciuffo, R. Structure and Nuclear Density Distribution in the Cheralite—CaTh(PO4)2: Studies of Its Behaviour under High Pressure (36 GPa). Phys. Chem. Miner. 2012, 39, 685–692. [Google Scholar] [CrossRef]
- Amaral, J.C.; Sá, M.L.; Morais, C.A. Recovery of Uranium, Thorium and Rare Earth from Industrial Residues. Hydrometallurgy 2018, 181, 148–155. [Google Scholar] [CrossRef]
- Amer, T.E.; El-Sheikh, E.M.; Gado, M.A.; Abu-Khoziem, H.A.; Zaki, S.A. Selective recovery of lanthanides, uranium and thorium from Rosetta monazite mineral concentrate. Sep. Sci. Technol. 2018, 53, 1522–1530. [Google Scholar] [CrossRef]
- Su, J.; Xu, R.; Ni, S.; Li, F.; Sun, X. A Cost-Effective Process for Recovering Thorium and Rare Earths from Radioactive Residues. J. Clean. Prod. 2020, 254, 119931. [Google Scholar] [CrossRef]
- Shahr El-Din, A.M.; Borai, E.H.; Abd El-Ghany, M. Selective separation of thorium from rare earth elements liquor during the alkaline processing of Egyptian monazite concentrate. Main Group Chem. 2018, 17, 79–88. [Google Scholar] [CrossRef]
- Su, J.; Gao, Y.; Ni, S.; Xu, R.; Sun, X. A Safer and Cleaner Process for Recovering Thorium and Rare Earth Elements from Radioactive Waste Residue. J. Hazard. Mater. 2021, 406, 124654. [Google Scholar] [CrossRef]
- Samsonov, M.D.; Trofimov, T.I.; Kulyako, Y.M.; Malikov, D.A.; Myasoedov, B.F. Supercritical Fluid Extraction of Rare Earth Elements, Thorium and Uranium from Monazite Concentrate and Phosphogypsum Using Carbon Dioxide Containing Tributyl Phosphate and Di-(2-Ethylhexyl) Phosphoric Acid. Russ. J. Phys. Chem. B 2016, 10, 1078–1084. [Google Scholar] [CrossRef]
- Gao, J.; Chen, J.; Lv, H.; Feng, X.; Liao, S.; Yan, Y.; Ma, F. The Electrolysis-Graded Leaching Process for Rapid Resource Regeneration of Th(IV)/U(VI) from Wastewater. J. Clean. Prod. 2023, 427, 139093. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, B.; Li, Q.; Jin, H. Distribution and mineralogical features of thorite in the Bayan Obo deposit: Implications for hydrothermal metasomatic Th re-enrichment. Ore Geol. Rev. 2024, 164, 105831. [Google Scholar] [CrossRef]
- Chadirji-Martinez, K.; Grosvenor, A.P.; Crawford, A.; Chernikov, R.; Heredia, E.; Feng, R.; Pan, Y. Thorium Speciation in Synthetic Anhydrite: Implications for Remediation and Recovery of Thorium from Rare-Earth Mine Tailings. Hydrometallurgy 2022, 214, 105965. [Google Scholar] [CrossRef]
- Huang, B.; Liu, Z.; Wang, Y.; Zhou, L.; Wang, C.; Ye, T. Release Behavior and Mechanism of Uranium and Thorium from Ta-Nb Tailings under Simulated Rainfall in Jiangxi Province, China. Environ. Sci. Pollut. Res. 2022, 29, 57466–57478. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, Z.; Li, X. Phytoremediation of Rare Tailings-Contaminated Soil. J. Renew. Mater. 2022, 10, 3351–3365. [Google Scholar] [CrossRef]
- Kukkonen, E.; Virtanen, E.J.; Moilanen, J.O. α-Aminophosphonates, -Phosphinates, and -Phosphine Oxides as Extraction and Precipitation Agents for Rare Earth Metals, Thorium, and Uranium: A Review. Molecules 2022, 27, 3465. [Google Scholar] [CrossRef]
- Iskandar, B.T.; Ismail, A.F.; Aziman, E.S.; Ahmad, S. Advancing towards Technology Readiness: Continuous-Flow Electrosorption for Thorium Separation from Rare Earth Processing By-Products. Nucl. Eng. Technol. 2024, 56, 4611–4619. [Google Scholar] [CrossRef]
- Lei, P.; Gao, Y.; Zhai, R.; Zhang, H.; Tian, Z.; Sun, X.; Chai, Y. The Selective Electrosorption of Thorium from Rare Earth with Molybdenum Disulfide-Modified Reduced Graphene Oxide Aerogel (MoS2@rGA). Sep. Purif. Technol. 2025, 363, 132242. [Google Scholar] [CrossRef]
- Akbari, M.; Shafaei Tonkaboni, S.Z.; Khanchi, A. Thorium Recovery from Choghart Mining Waste by Beneficiation Processes. J. Miner. Met. Mater. Soc. 2023, 75, 1045–1058. [Google Scholar] [CrossRef]
- Nzeh, N.S.; Popoola, P.; Adeleke, A. Physicochemical Concentration of Heavy Minerals—A Treatise on Wet Process Media: Gravity, Centrifugal and Flotation Techniques. Int. J. Min. Miner. Eng. 2024, 15, 161–236. [Google Scholar] [CrossRef]
- Danilenko, E.G.; Telegin, S.V. Laboratory Separator of Bulk Materials. Sib. Aerosp. J. 2020, 21, 550–555. [Google Scholar] [CrossRef]
- Svoboda, J.; Fujita, T. Recent Developments in Magnetic Methods of Material Separation. Miner. Eng. 2003, 16, 785–792. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, S. Separation of Monazite from Placer Deposit by Magnetic Separation. Minerals 2019, 9, 149. [Google Scholar] [CrossRef]
- Nzeh, N.S.; Popoola, P.A.; Adeleke, A.; Adeosun, S. Physical Concentration of Heavy Minerals: A Brief Review on Low- and High-Intensity Magnetic Separation Process Techniques. J. Miner. Met. Mater. Soc. 2024, 76, 1329–1344. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Banerjee, P.K.; Suresh, N. Magnetic Separation Studies on Ferruginous Chromite Fine to Enhance Cr:Fe ratio. Int. J. Miner. Metall. Mater. 2016, 23, 459–469. [Google Scholar] [CrossRef]
- Dwari, R.K.; Rao, D.S. Dry Beneficiation of Coal—A Review. Miner. Process. Extr. Metall. Rev. 2009, 30, 33–49. [Google Scholar] [CrossRef]
- Stopic, S.; Friedrich, B. Advances in Understanding of the Application of Unit Operations in Metallurgy of Rare Earth Elements. Metals 2021, 11, 978. [Google Scholar] [CrossRef]
- Demol, J. The Effect of Temperature and Mineralogy on Reaction Processes during the Sulfuric Acid Baking of Monazite Ores. Ph.D. Thesis, Murdoch University, Perth, Australia, 2020. [Google Scholar]
- Shuai, G.; Zhao, L.; Wang, L.; Long, Z.; Cui, D. Aqueous Stability of Rare Earth and Thorium Elements during Hydrochloric Acid Leaching of Roasted Bastnaesite. J. Rare Earths 2017, 35, 1255–1260. [Google Scholar] [CrossRef]
- Abd El Fatah, A.I.L. Commercial Approach for Highly Pure Thorium from Egyptian Monazite Mineral Acid Process. In Proceedings of the 10th International Conference on Chemical and Environmental Engineering, Military Technical College, Cairo, Egypt, 7–9 July 2020; Volume 10, pp. 1–12. [Google Scholar] [CrossRef]
- Elatontsev, D.A.; Mukhachev, A.P. Investigation of methods for separating thorium and rare-earth elements in nitric-acid leaching solutions for loparite concentrate. At. Energy 2021, 130, 82–87. [Google Scholar] [CrossRef]
- Chowdhury, M.O.S.; Talan, D. From Waste to Wealth: A Circular Economy Approach to the Sustainable Recovery of Rare Earth Elements and Battery Metals from Mine Tailings. Separations 2025, 12, 52. [Google Scholar] [CrossRef]
- Pepper, R.A.; Couperthwaite, S.J.; Millar, G.J. Comprehensive Examination of Acid Leaching Behaviour of Mineral Phases from Red Mud: Recovery of Fe, Al, Ti, and Si. Miner. Eng. 2016, 99, 8–18. [Google Scholar] [CrossRef]
- Maltrana, V.; Morales, J. The Use of Acid Leaching to Recover Metals from Tailings: A Review. Metals 2023, 13, 1862. [Google Scholar] [CrossRef]
- Borai, E.H.; Abd El-Ghany, M.S.; Ahmed, I.M.; Hamed, M.M.; El-Din, A.S.; Aly, H.F. Modified Acidic Leaching for Selective Separation of Thorium, Phosphate and Rare Earth Concentrates from Egyptian Crude Monazite. Int. J. Miner. Process. 2016, 149, 34–41. [Google Scholar] [CrossRef]
- Chung, K.W.; Yoon, H.S.; Kim, C.J.; Lee, J.Y.; Jyothi, R.K. Solvent Extraction, Separation and Recovery of Thorium from Korean Monazite Leach Liquors for Nuclear Industry Applications. J. Ind. Eng. Chem. 2020, 83, 72–80. [Google Scholar] [CrossRef]
- Liu, Z.; Xiu, T.; Du, Y.; Wang, X.; Wang, L.; Li, J.; Zhang, J. Leaching Characteristics and Kinetics of Radioactive Element Uranium and Thorium from Ta/Nb Tailing. J. Radioanal. Nucl. Chem. 2020, 323, 1197–1206. [Google Scholar] [CrossRef]
- Lan, Q.; Li, J.; Wu, F.; Liu, G.; Xu, H.; Chen, X. Recovery of rare earth elements and thorium from acid leaching residue of ionic rare earth concentrates. J. Rare Earths 2025, 43, 794–804. [Google Scholar] [CrossRef]
- Basque, J.; Lavoie, J.; Reynier, N.; Lariviere, D. Quantitative Separation of Thorium from Rare Earth Elements and Uranium in a Rare Earth Element Sulfuric Acid Leachate Using Cloud Point Extraction. Microchem. J. 2023, 190, 108724. [Google Scholar] [CrossRef]
- Demol, J.; Ho, E.; Senanayake, G. Sulfuric Acid Baking and Leaching of Rare Earth Elements, Thorium and Phosphate from a Monazite Concentrate: Effect of Bake Temperature from 200 to 800 °C. Hydrometallurgy 2018, 179, 254–267. [Google Scholar] [CrossRef]
- Prasetyo, E.; Supriyatna, Y.I.; Bahfie, F.; Trinopiawan, K. Extraction of Thorium from Tin Slag Using Acidic Roasting and Leaching Method. In AIP Conference Proceedings, Proceedings of the 3rd International Seminar on Metallurgy and Materials (ISMM2019): Exploring New Innovation in Metallurgy and Materials, Tangerang Selatan, Indonesia, 23–24 October 2019; AIP Publishing LLC: New York, NY, USA, 2020; Volume 2232, p. 040008. [Google Scholar] [CrossRef]
- Fu, C.; Du, W.; Kuang, W.; Huang, Y.; Deng, B.; Liu, X.; Liao, W. Thorium-Selective Electrosorption with Phosphorylated PANI/AC Electrode. Chem. Eng. J. 2025, 475, 165955. [Google Scholar] [CrossRef]
- García, A.C.; Velázquez, S.; Silva, R.J.; La Fuente, J.I.A.D.; Vargas, A.; Rodríguez, M.H. Separation of radioactive elements from rare earth element-bearing minerals. Metals 2020, 10, 1524. [Google Scholar] [CrossRef]
- Brückner, L.; Elwert, T.; Schirmer, T. Extraction of rare earth elements from phospho-gypsum: Concentrate digestion, leaching, and purification. Metals 2020, 10, 131. [Google Scholar] [CrossRef]
- Huang, M.; Liu, D.; Xiu, T.; Liu, Z.; Liu, Z. Aluminum-Assisted Acid Leaching for Uranium/Thorium from High-Fluorine Niobium Slag: Occurrence States and Behaviors in Mineralogy. Sep. Purif. Technol. 2025, 330, 133503. [Google Scholar] [CrossRef]
- Aziman, E.S.; Ismail, A.F. Frontier Looking of Rare-Earth Processed Residue as Sustainable Thorium Resources: An Insight into Chemical Composition and Separation of Thorium. Prog. Nucl. Energy 2020, 128, 103471. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, X.; Yang, H.; Luo, X.; Lin, X. Highly efficient extraction of thorium from aqueous solution by fungal mycelium-based microspheres fabricated via immobilization. Chem. Eng. J. 2019, 368, 37–50. [Google Scholar] [CrossRef]
- Zakharanka, A.; Gubbels, L.; Acevedo, B.; Verwerft, M.; Tyrpekl, V. Homogeneous Precipitation of Thorium Oxalate: Structural, Kinetic, and Morphological Aspects. J. Nucl. Mater. 2025, 605, 155574. [Google Scholar] [CrossRef]
- He, X.; Tang, J.; Wang, Z.; Feng, W.; Yan, Q.; Wei, Y.; Chen, L. Method and Mechanism for Efficient Radium Isolation from Bulk Thorium Based on Anion Exchange. Chem. Eng. J. 2024, 496, 154283. [Google Scholar] [CrossRef]
- Udayakumar, S.; Baharun, N.; Rezan, S.A.; Ismail, A.F.; Takip, K.M. Economic Evaluation of Thorium Oxide Production from Monazite Using Alkaline Fusion Method. Nucl. Eng. Technol. 2021, 53, 2418–2425. [Google Scholar] [CrossRef]
- Özkan, B.; Altaş, Y.; İnan, S. Extraction and Purification of Thorium and Rare Earth Elements from Bastnaesite Mineral: A Comprehensive Leaching and Precipitation Study. J. Radioanal. Nucl. Chem. 2025, 334, 541–550. [Google Scholar] [CrossRef]
- Lan, Q.; Zhang, X.; Niu, F.; Liu, D.; Yang, Y. Recovery Process of Rare Earths, Al, U, and Th from Ionic Rare Earth Purification Residue Using Sequential Alkaline Leaching, Acid Leaching, Solvent Extraction and Stripping. Hydrometallurgy 2025, 119, 106524. [Google Scholar] [CrossRef]
- Singh, B.K.; Hafeez, M.A.; Kim, H.; Hong, S.; Kang, J.; Um, W. Inorganic Waste Forms for Efficient Immobilization of Radionuclides. ACS EST Eng. 2021, 1, 1149–1170. [Google Scholar] [CrossRef]
- Al-Areqi, W.M.; Majid, A.A.; Sarmani, S. Separation of Thorium (IV) from Lanthanide Concentrate (LC) and Water Leach Purification (WLP) Residue. In AIP Conference Proceedings, Proceedings of the 2014 Ukm Fst Postgraduate Colloquium, Universiti Kebangsaan Malaysia, Faculty of Science and Technology 2014 Postgraduate Colloquium, Selangor, Malaysia, 9–11 April 2014; American Institute of Physics: Melville, NY, USA, 2014; Volume 1614, pp. 482–485. [Google Scholar] [CrossRef]
- Lapidus, G.T.; Doyle, F.M. Selective Thorium and Uranium Extraction from Monazite: II. Approaches to Enhance the Removal of Radioactive Contaminants. Hydrometallurgy 2015, 155, 161–167. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Zhang, D.; Gao, K.; Wang, H.; Xu, W.; Ma, X. Decomposition of Mixed Rare Earth Concentrate by NaOH Roasting and Kinetics of Hydrochloric Acid Leaching Process. J. Rare Earths 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Xie, B.; Liu, C.; Wei, B.; Wang, R.; Ren, R. Recovery of Rare Earth Elements from Waste Phosphors via Alkali Fusion Roasting and Controlled Potential Reduction Leaching. Waste Manag. 2023, 163, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Medjahed, B.; Didi, M.A. Spectroscopic Study of Complexes from UO2(II), Th(IV) and Sm(III) Based on the Effects of the Characteristic TBP, TOPO and D2EHPA Bands in Various Organic Solvents. J. Radioanal. Nucl. Chem. 2018, 318, 1427–1438. [Google Scholar] [CrossRef]
- Dash, S.; Hıal, P.; Senapatı, S.; Dalai, B. A Survey on Various Methods of Extraction and Recovery of Thorium. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 1197–1210. [Google Scholar] [CrossRef]
- Zhu, Z.; Pranolo, Y.; Cheng, C.Y. Separation of Uranium and Thorium from Rare Earths for Rare Earth Production—A Review. Miner. Eng. 2015, 77, 185–196. [Google Scholar] [CrossRef]
- Talan, D.; Huang, Q.; Liang, L.; Song, X. Conceptual Process Development for the Separation of Thorium, Uranium, and Rare Earths from Coarse Coal Refuse. Miner. Process. Extr. Metall. Rev. 2023, 44, 330–345. [Google Scholar] [CrossRef]
- Talan, D.; Huang, Q. Separation of Thorium, Uranium, and Rare Earths from a Strip Solution Generated from Coarse Coal Refuse. Hydrometallurgy 2020, 197, 105446. [Google Scholar] [CrossRef]
- Salehuddin, A.H.J.M.; Ismail, A.F.; Aziman, E.S.; Mohamed, N.A.; Teridi, M.A.M.; Idris, W.M.R. Production of High-Purity ThO2 from Monazite Ores for Thorium Fuel-Based Reactor. Prog. Nucl. Energy 2021, 136, 103728. [Google Scholar] [CrossRef]
- Gao, S.; Shen, X.; Chen, Q.; Gao, H. Solvent Extraction of Thorium(IV) Using W/O Microemulsion. Sci. China Chem. 2012, 55, 1712–1718. [Google Scholar] [CrossRef]
- Arabi, H.R.; Milani, S.A.; Abolghasemi, H.; Zahakifar, F. Recovery and Transport of Thorium(IV) through Polymer Inclusion Membrane with D2EHPA from Nitric Acid Solutions. J. Radioanal. Nucl. Chem. 2021, 327, 653–665. [Google Scholar] [CrossRef]
- Eskandari Nasab, M.; Sam, A.; Alamdar Milani, S. Determination of Optimum Process Conditions for Solvent Extraction of Thorium Using Taguchi Method. J. Radioanal. Nucl. Chem. 2011, 287, 239–245. [Google Scholar] [CrossRef]
- Dong, Y.; Li, S.; Su, X.; Wang, Y.; Shen, Y.; Sun, X. Separation of Thorium from Rare Earths with High-Performance Diphenyl Phosphate Extractant. Hydrometallurgy 2017, 171, 387–393. [Google Scholar] [CrossRef]
- Pathak, P.N.; Veeraraghavan, R.; Prabhu, D.R.; Mahajan, G.R.; Manchanda, V.K. Separation Studies of Uranium and Thorium Using Di-2-Ethylhexyl Isobutyramide (D2EHIBA). Sep. Sci. Technol. 1999, 34, 2601–2614. [Google Scholar] [CrossRef]
- Shaeri, M.; Torab-Mostaedi, M.; Rahbar Kelishami, A. Solvent Extraction of Thorium from Nitrate Medium by TBP, Cyanex272 and Their Mixture. J. Radioanal. Nucl. Chem. 2015, 303, 2093–2099. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pathak, P.; Mohapatra, P.K.; Manchanda, V.K. Chemistry of Diglycolamides: Promising Extractants for Actinide Partitioning. Chem. Rev. 2012, 112, 1751–1772. [Google Scholar] [CrossRef] [PubMed]
- Al Zoubi, W. Solvent Extraction of Metal Ions by Use of Schiff Bases. J. Coord. Chem. 2013, 66, 2264–2289. [Google Scholar] [CrossRef]
- Rahman, M.S. Advancements in solvent extraction of metal ions: Mechanisms, kinetics, and efficiency with diverse extractants. Int. J. Sci. Res. Arch. 2024, 12, 2374–2396. [Google Scholar] [CrossRef]
- Yokoyama, T.; Makishima, A.; Nakamura, E. Separation of Thorium and Uranium from Silicate Rock Samples Using Two Commercial Extraction Chromatographic Resins. Anal. Chem. 1999, 71, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.M.; Nita Lazar, M.; Popa, M.; Galaon, T.; Pascu, L.F. Current Trends in Development and Use of Polymeric Ion-Exchange Resins in Wastewater Treatment. Materials 2024, 17, 5994. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Z.; Kuang, S.; Wei, H.; Li, Y.; Wu, G.; Liao, W. Removal of thorium and uranium from leach solutions of ion-adsorption rare earth ores by solvent extraction with Cextrant 230. Hydrometallurgy 2020, 194, 105343. [Google Scholar] [CrossRef]
- Wang, H.; Kuang, S.; Liao, W. Synergistic extraction and separation of thorium from rare earths in chloride media using mixture of Cextrant 230 and Cyanex 923. J. Rare Earths 2024, 42, 759–767. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, Z.; Zhang, X.; Bu, X.; Du, Y.; Shi, W.; Yuan, L. Enabling the extraction of high-purity thorium from rare earth ores by simple one-step crystallization. Chem. Eng. J. 2025, 519, 165158. [Google Scholar] [CrossRef]
- Agboola, O.; Fayomi, O.S.I.; Ayodeji, A.; Ayeni, A.O.; Alagbe, E.E.; Sanni, S.E.; Oni, B.A. A Review on Polymeric Nanocomposites and Their Effective Applications in Membranes and Adsorbents for Water Treatment and Gas Separation. Membranes 2021, 11, 139. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Hasan, I. A Review on Properties and Applications of TiO2 and Associated Nanocomposite Materials. Mater. Today Proc. 2023, 81, 1073–1078. [Google Scholar] [CrossRef]
- Moghaddam, Z.S.; Kaykhaii, M.; Khajeh, M.; Oveisi, A.R. Synthesis of UiO-66-OH zirconium metal-organic framework and its application for selective extraction and trace determination of thorium in water samples by spectrophotometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 194, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yuan, L.Y.; Guo, W.L.; Luo, S.Z.; Chai, Z.F.; Shi, W.Q. Extending the use of highly porous and functionalized MOFs to Th(IV) capture. ACS Appl. Mater. Interfaces 2017, 9, 25216–25224. [Google Scholar] [CrossRef]
- Gaidimas, M.A.; Smoljan, C.S.; Ye, Z.M.; Stern, C.L.; Malliakas, C.D.; Kirlikovali, K.O.; Farha, O.K. Thorium metal–organic framework crystallization for efficient recovery from rare earth element mixtures. Chem. Sci. 2025, 16, 3895–3903. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, P.; Yadav, L.; Janu, V.C.; Gupta, R. Sequestration and recovery of thorium ions using a recyclable, low-cost, glutathione-based magnetic nanocomposite: Experimental study and statistical modeling. Sep. Purif. Technol. 2023, 322, 124264. [Google Scholar] [CrossRef]
- Hamdy, G.; El-Sabbagh, I.A.; Taher, F.A. Highly efficient sorption of thorium(IV) onto a ternary magnetic TiO2/Fe3O4/GO nanocomposite. Mater. Today Proc. 2021, 42, 2218–2226. [Google Scholar] [CrossRef]
- Alharthi, S. Separation of thorium(IV) from aquatic media using magnetic ferrite nanoparticles. Radiochim. Acta 2021, 109, 823–833. [Google Scholar] [CrossRef]
- Özkan, B.; Altaş, Y.; İnan, S. Investigation of nanocomposite efficiency on the separation and purification processes of thorium and rare earth elements. J. Radioanal. Nucl. Chem. 2023, 332, 4677–4686. [Google Scholar] [CrossRef]
- Othman, N.A.F.; Selambakkannu, S.; Azian, H.; Ratnam, C.T.; Yamanobe, T.; Hoshina, H.; Seko, N. Synthesis of surface ion-imprinted polymer for specific detection of thorium under acidic conditions. Polym. Bull. 2021, 78, 165–183. [Google Scholar] [CrossRef]
- He, D.; Ning, S.; Liu, J.; Zhang, S.; Chen, L.; Xu, Y.; Wei, Y. Efficiently selective removal of radioactive thorium and uranium from rare earths by trialkyl phosphine oxide modified porous silica–polymer based adsorbent. Sep. Purif. Technol. 2025, 364, 132426. [Google Scholar] [CrossRef]
- Lin, C.; Wang, H.; Wang, Y.; Zhou, L.; Liang, J. Selective preconcentration of trace thorium from aqueous solutions with Th (IV)-imprinted polymers prepared by a surface-grafted technique. Int. J. Environ. Anal. Chem. 2011, 91, 1050–1061. [Google Scholar] [CrossRef]
- He, Q.; Chang, X.; Wu, Q.; Huang, X.; Hu, Z.; Zhai, Y. Synthesis and applications of surface-grafted Th (IV)-imprinted polymers for selective solid-phase extraction of thorium (IV). Anal. Chim. Acta 2007, 605, 192–197. [Google Scholar] [CrossRef]
- da Costa, J.G.D.R.; Costa, J.M.; de Almeida Neto, A.F. Recent advances and future applications in electro-adsorption technology: An updated review. J. Environ. Chem. Eng. 2021, 9, 106355. [Google Scholar] [CrossRef]
- Lissaneddine, A.; Pons, M.N.; Aziz, F.; Ouazzani, N.; Mandi, L.; Mousset, E. Electro-sorption/-desorption with bio-sourced granular activated carbon electrode for phenols recovery and combination with advanced electro-oxidation for residual olive mill wastewater treatment. J. Water Process Eng. 2025, 69, 106663. [Google Scholar] [CrossRef]
- Lesbayev, B.; Rakhymzhan, N.; Ustayeva, G.; Maral, Y.; Atamanov, M.; Auyelkhankyzy, M.; Zhamash, A. Preparation of nanoporous carbon from rice husk with improved textural characteristics for hydrogen sorption. J. Compos. Sci. 2024, 8, 74. [Google Scholar] [CrossRef]
- Taurbekov, A.; Abdisattar, A.; Atamanov, M.; Kaidar, B.; Yeleuov, M.; Joia, R.; Atamanova, T. Investigations of activated carbon from different natural sources for preparation of binder-free few-walled CNTs/activated carbon electrodes. J. Compos. Sci. 2023, 7, 452. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, M.; Chen, L.; Gong, Z.; Hu, J.; Ma, D. Electrokinetic remediation for the removal of heavy metals in soil: Limitations, solutions and prospection. Sci. Total Environ. 2023, 903, 165970. [Google Scholar] [CrossRef] [PubMed]
- Aziman, E.S.; Ismail, A.F.; Muttalib, N.A.; Hanifah, M.S. Investigation of thorium separation from rare-earth extraction residue via electrosorption with carbon based electrode toward reducing waste volume. Nucl. Eng. Technol. 2021, 53, 2926–2936. [Google Scholar] [CrossRef]
- Aziman, E.S.; Ismail, A.F. Rapid selective removal of thorium via electrosorption towards efficiently managing rare-earth extraction residue. J. Environ. Chem. Eng. 2021, 9, 105478. [Google Scholar] [CrossRef]
- Akhtar, N.; Ismail, A.F.; Hanafiah, M.M.; Fadzil, S.M.; Park, S. Evaluating environmental impacts of thorium extraction: A comparative study of solvent and electrosorption technologies using life cycle assessment (LCA). Clean. Eng. Technol. 2025, 26, 100960. [Google Scholar] [CrossRef]
- Aziman, E.S.; Ismail, A.F. Removal of thorium from industrial waste via electrosorption technique. IOP Conf. Ser. Mater. Sci. Eng. 2020, 785, 012014. [Google Scholar] [CrossRef]
- Aziman, E.S.; Mohd Salehuddin, A.H.J.; Ismail, A.F. Remediation of thorium(IV) from wastewater: Current status and way forward. Sep. Purif. Rev. 2021, 50, 177–202. [Google Scholar] [CrossRef]
- Yussuf, N.M.; Ismail, A.F.; Yasir, M.S. Revolutionizing thorium capture: G-C3N4 electrodes for enhanced thorium electrosorption. J. Saudi Chem. Soc. 2025, 29, 1. [Google Scholar] [CrossRef]
- Yussuf, N.M.; Ismail, A.F.; Rahmat, M.A.; Mohamed, N.A. Electrosorption-driven selective thorium removal from radioactive wastewater with phosphate–incorporated g-C3N4 electrode. J. Environ. Chem. Eng. 2024, 12, 113440. [Google Scholar] [CrossRef]
- Williamson, A.J.; Binet, M.; Sergeant, C. Radionuclide biogeochemistry: From bioremediation toward the treatment of aqueous radioactive effluents. Crit. Rev. Biotechnol. 2024, 44, 698–716. [Google Scholar] [CrossRef]
- Sukadana, I.G.; Sulaeman; Syaeful, H.; Indrastomo, F.D.; Adimedha, T.B.; Ciputra, R.C.; Widodo, S. High field strength element (HFSE) and rare earth element (REE) enrichment in laterite deposit of high background natural radiation area (HBNRA) of Mamuju, West Sulawesi, Indonesia. Resources 2025, 14, 84. [Google Scholar] [CrossRef]
- Karthikayini, S.; Sathish, V.; Chandrasekaran, A.; Issa, S.A.; Hanfi, M.Y.; Khandaker, M.U. Natural radioactivity and concomitant health hazards in Kovalam Beach sediments, Tamil Nadu with physico-chemical and mineralogical analysis. Radiat. Phys. Chem. 2025, 113161. [Google Scholar] [CrossRef]
- Ion, A.; Cosac, A.; Ene, V.V. Natural radioactivity in soil and radiological risk assessment in Lișava uranium mining sector, Banat Mountains, Romania. Appl. Sci. 2022, 12, 12363. [Google Scholar] [CrossRef]
- Akakçe, N.; Öztürk Atay, N.; Tüney Kizilkaya, I.; Uğur Görgün, A. Radioactivity and heavy metal accumulation in surface sediment from the West Aegean Coast. Soil Sediment Contam. Int. J. 2024, 33, 560–577. [Google Scholar] [CrossRef]
- El-Shlemy, E.S.; Gad, A.; El Feky, M.G.; Mahmoud, A.M.A.; El-Sayed, O.; Abed, N.S. Potentially toxic elements and natural radioactivity in Nasser Lake sediments: Environmental risks in a key Egyptian freshwater lake. Toxics 2025, 13, 745. [Google Scholar] [CrossRef]
- Seif, R.A.; Ene, A.; Zakaly, H.M.; Sallam, A.M.; Taalab, S.A.; Fnais, M.S.; Awad, H.A. Distribution of heavy metals along the Mediterranean shoreline from Baltim to El-Burullus (Egypt): Consequences for possible contamination. Minerals 2024, 14, 931. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Yu, K.; Luo, M.; Luo, F. Constructing a cationic porous organic polymer with [PF6]−-charge-balanced anion for a rapid, selective and high-capacity thorium capture. Sep. Purif. Technol. 2025, 362, 131814. [Google Scholar] [CrossRef]
- Wimmers, A.; Böse, F.; Beppler, J.; Morawe, P.; Weber, M.; von Hirschhausen, C. (Re)integrating radioactive materials and waste into a global sustainable development context. Radiat. Environ. Biophys. 2024, 63, 519–536. [Google Scholar] [CrossRef]
- Bhatt, D.; Kumar, R.; Kumar, R.; Prakash, O. Health effects and toxicity mechanism of thorium: Knowledge gaps and research prospects. In Hazardous Chemicals; Academic Press: Cambridge, MA, USA, 2025; pp. 729–740. [Google Scholar] [CrossRef]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Sandu, A.V.; Vizureanu, P.; Razak, R.A.; Imjai, T. Solidification/stabilization technology for radioactive wastes using cement: An appraisal. Materials 2023, 16, 954. [Google Scholar] [CrossRef] [PubMed]
- Eddy, N.O.; Igwe, O.; Eze, I.S.; Garg, R.; Akpomie, K.; Timothy, C.; Paktin, H. Environmental and public health risk management, remediation and rehabilitation options for impacts of radionuclide mining. Discov. Sustain. 2025, 6, 209. [Google Scholar] [CrossRef]
- Akbari, R.; Nasr, M.A.; D’Auria, F.; Cammi, A.; Maiorino, J.R.; de Stefani, G.L. Analysis of thorium–transuranic fuel deployment in a LW-SMR: A solution toward sustainable fuel supply for the future plants. Nucl. Eng. Des. 2024, 421, 113090. [Google Scholar] [CrossRef]
- Rehm, T.E. Advanced nuclear energy: The safest and most renewable clean energy. Curr. Opin. Chem. Eng. 2023, 39, 100878. [Google Scholar] [CrossRef]
- Titarenko, Y.E.; Ananev, S.S.; Batyaev, V.F.; Belousov, V.I.; Blandinskiy, V.Y.; Chernov, K.G.; Konobeyev, A.Y. Radiation and nuclear physics aspects of the use of the thorium fuel cycle in a hybrid fusion facility. Fusion Sci. Technol. 2023, 79, 117–134. [Google Scholar] [CrossRef]
- Man, G.T.; Albu, P.C.; Nechifor, A.C.; Grosu, A.R.; Popescu, D.I.; Grosu, V.A.; Marinescu, V.E.; Nechifor, G. Simultaneously Recovery of Thorium and Tungsten Through Hybrid Electrolysis–Nanofiltration Processes. Toxics 2024, 12, 103. [Google Scholar] [CrossRef]
- Man, G.T.; Albu, P.C.; Nechifor, A.C.; Grosu, A.R.; Tanczos, S.K.; Grosu, V.A.; Ioan, M.-R.; Nechifor, G. Thorium removal, recovery and recycling: A membrane challenge for urban mining. Membranes 2023, 13, 765. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, X.; Tan, S.; Wang, H.; Kuang, S.; Liu, X.; Liao, W. Ultra-selective removal of thorium from rare earths by aminophosphonic acid-modified porous silica. Sep. Purif. Technol. 2024, 341, 126952. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Tong, J.; Hu, K.; Li, B.; Chang, B.; Shi, K.; Yang, J.; Hou, X. A novel strategy for sequential separation of Th(IV) from highly acidic wastewater based on solid phase extraction. Chem. Eng. J. 2024, 499, 155892. [Google Scholar] [CrossRef]
- Ghosh, B.; Vapnik, H.; Kim, H.-E.; Kim, Y.; Birawat, R.; Lu, Y.; Su, X.; Yang, H. Electrochemical separation and clean energy applications of rare earth elements. Chem. Rev. 2025, 125, 16. [Google Scholar] [CrossRef]
- Lin, Z.; Ooi, J.K.; Woon, K.S. An integrated life cycle multi-objective optimization model for health–environment–economic nexus in food waste management sector. Sci. Total Environ. 2022, 816, 151541. [Google Scholar] [CrossRef]
- Sharma, B.K.; Chandel, M.K. Life cycle cost analysis of municipal solid waste management scenarios for Mumbai, India. Waste Manag. 2021, 124, 293–302. [Google Scholar] [CrossRef]
- Aryan, Y.; Kumar, A.; Samadder, S.R. Environmental and economic assessment of waste collection and transportation using LCA: A case study. Environ. Res. 2023, 231, 116108. [Google Scholar] [CrossRef] [PubMed]
- Salehuddin, A.H.J.M.; Ismail, A.F.; Bahri, C.N.A.C.Z.; Aziman, E.S. Economic analysis of thorium extraction from monazite. Nucl. Eng. Technol. 2019, 51, 631–640. [Google Scholar] [CrossRef]
- Li, X.C.; Yang, K.F.; Spandler, C.; Fan, H.R.; Zhou, M.F.; Hao, J.L.; Yang, Y.H. The Effect of Fluid-Aided Modification on the Sm-Nd and Th-Pb Geochronology of Monazite and Bastnäsite: Implication for Resolving Complex Isotopic Age Data in REE Ore Systems. Geochim. Cosmochim. Acta 2021, 300, 1–24. [Google Scholar] [CrossRef]
- Anitha, J.K.; Joseph, S.; Rejith, R.G.; Sundararajan, M. Monazite Chemistry and its Distribution along the Coast of Neendakara–Kayamkulam Belt, Kerala, India. SN Appl. Sci. 2020, 2, 812. [Google Scholar] [CrossRef]
- Colombo, F.; Simmons, W.; Falster, A.U.; Lira, R. Occurrence, Crystal Chemistry and Alteration of Thorite from the NYF-Type Miarolitic Pegmatites of the El Portezuelo Granite, Papachacra (Catamarca, NW Argentina). Asoc. Geol. Argent. Ser. D Publ. Espec. 2011, 14, 65–67. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).