Abstract
Carboxytherapy is a promising treatment modality with multidirectional effects on the skin; yet, its impact on hydration and viscoelasticity in the delicate periorbital area requires further exploration. This study aimed to evaluate the effects of carboxytherapy as monotherapy and in combination with selected acids on the hydration and viscoelasticity of the skin around the eyes. Thirty-nine participants underwent five weekly treatments, with carboxytherapy applied to the right eye area and carboxytherapy combined with acids (ferulic acid 14% with L-ascorbic acid 12% or lactobionic acid 20%) administered to the left eye area. Skin hydration and viscoelasticity were measured using Corneometer and Cutometer probes, respectively. Statistically significant improvements (p < 0.0001) in viscoelasticity were observed in both treated areas. The combination of carboxytherapy with lactobionic acid led to a modest but statistically insignificant increase in skin hydration. These findings indicate that carboxytherapy, particularly in combination with tested acids, is effective for improving skin viscoelasticity. While its effect on hydration is more pronounced in dry skin, the therapy significantly enhances skin elasticity, supporting its use as a preventative and corrective treatment for age-related changes, including progressive loss of skin density and firmness.
1. Introduction
The impact of carboxytherapy on angiogenesis (the formation of new blood vessels), neovascularization (widening and restoration of narrowed blood vessels), lipolysis, and neocollagenesis is well-documented. This therapy has high potential and exerts multidirectional effects on the skin; however, its effects on skin hydration and viscoelasticity require further investigation.
Under physiological conditions, carbon dioxide, generated as a product of cellular metabolism, diffuses through the cell membrane. Upon reaching the vascular bed, it undergoes a series of physical and biochemical reactions. A higher concentration of carbon dioxide in the blood induces the physiological Bohr effect. This phenomenon involves a decrease in hemoglobin’s affinity for oxygen due to a reduction in blood pH (increased hydrogen ion concentration). Consequently, the dissociation curve of oxyhemoglobin (the unstable combination of hemoglobin and oxygen) shifts to the right, allowing oxygen to be more easily released from hemoglobin into the tissues.
This mechanism forms the basis of carboxytherapy, an innovative and minimally invasive procedure involving the subcutaneous injection of CO2. Originating in 1932 at the Royal Spas in France, it was initially employed to treat obliterating arteriopathies. Over the years, its application has expanded to aesthetic medicine, particularly in the treatment of periorbital concerns such as fine lines and hyperpigmentation. Subcutaneous injections of CO2, administered weekly over seven weeks, have demonstrated significant improvements in these parameters. Carboxytherapy’s physiological effects include inducing a controlled oxygen deficit, which triggers an increase in blood flow and the release of growth factors such as vascular endothelial growth factor (VEGF). This stimulation promotes angiogenesis, enhancing cutaneous microcirculation and vasomotion [1].
Local hypoxia caused by the administration of carbon dioxide during carboxytherapy is a strong stimulus for angiogenesis, triggered by the release of angiogenic factors from hypoxic tissues [2,3,4]. As a result, tissue perfusion and lymphatic circulation increase, improving tissue drainage. The rise in blood pressure and dilation of blood vessels following carbon dioxide administration enhances nourishment, oxygenation, and regeneration in the treated area, translating into a general improvement in skin function [1,3]. Carboxytherapy appears to improve hydration and skin viscoelasticity by increasing the water content of the skin. Tissue trauma caused by the rapid flow of carbon dioxide induces localized, controlled inflammation, facilitating connective tissue remodeling. Old collagen fibers are degraded, while the synthesis of new fibers is enhanced, leading to a more organized and compact structure. This process increases skin thickness and elasticity. Furthermore, carboxytherapy promotes an increase in the number of collagen fibers and improves their structure, including increased diameter [5].
Studies have demonstrated that carboxytherapy can thicken the epidermis and improve skin structure. For instance, El-Domyati et al. observed epidermal thickening through histopathological analysis [6,7,8], while Moftah et al. reported significant epidermal thickening after six sessions of carboxytherapy for acne scars [9]. These findings suggest that carboxytherapy-induced epidermal remodeling may also enhance skin hydration and reduce transepidermal water loss (TEWL). The relationship between transepidermal water loss (TEWL) and the structural integrity of the skin barrier is well-documented, with TEWL playing a critical role in the development of skin dryness. It is hypothesized that the epidermal thickening induced by carboxytherapy could improve water retention and consequently reduce TEWL [10].
Importantly, carboxytherapy is considered a safe procedure for cosmetic and dermatological applications, though contraindications exist for patients with severe respiratory, renal, or cardiovascular conditions, as well as during pregnancy or breastfeeding. Despite these limitations, the therapy has gained significant recognition as a minimally invasive approach for treating cosmetic concerns such as periorbital pigmentation, loss of skin firmness, and other age-related changes, highlighting its versatility and clinical relevance [11].
Carboxytherapy is a widely used procedure recommended for various skin problems, including skin dryness, elasticity loss, dark circles under the eyes, and stretch marks. The growing number of studies on this topic highlights the need for further research to develop effective and safe treatment protocols, including combining carboxytherapy with other therapeutic methods.
Currently, no studies have specifically evaluated the effects of injection-based carboxytherapy on hydration and viscoelasticity in the periorbital area. This study combines carboxytherapy with chemical peels (lactobionic acid or ferulic acid with ascorbic acid), which are known to enhance skin function and exert anti-aging effects. The combination aims to evaluate their synergistic impact on improving hydration and viscoelasticity in this delicate region.
2. Materials and Methods
2.1. Material
Thirty-nine Caucasian participants aged 25–55 years (35 women and 4 men) with Fitzpatrick skin phototypes II and III were enrolled in this study. Each participant underwent five sessions of carboxytherapy, combined with chemical peels selected based on indications (ferulic acid 14% with L-ascorbic acid 12% or lactobionic acid 20%) applied to the skin around the left eye. Carboxytherapy alone was performed in the area of the right eye. There were no contraindications to this therapy in the participants, and they did not receive any additional treatments in the examined areas. Participants were advised not to make changes to their current eye-area skincare routines, as this could affect the study results.
All participants were informed about the sensations that might occur during and after the procedure, possible complications, and post-treatment recommendations. They signed informed consent forms agreeing to the procedures, Corneometer measurements, and the use of their results and photos for study purposes.
2.2. Methods
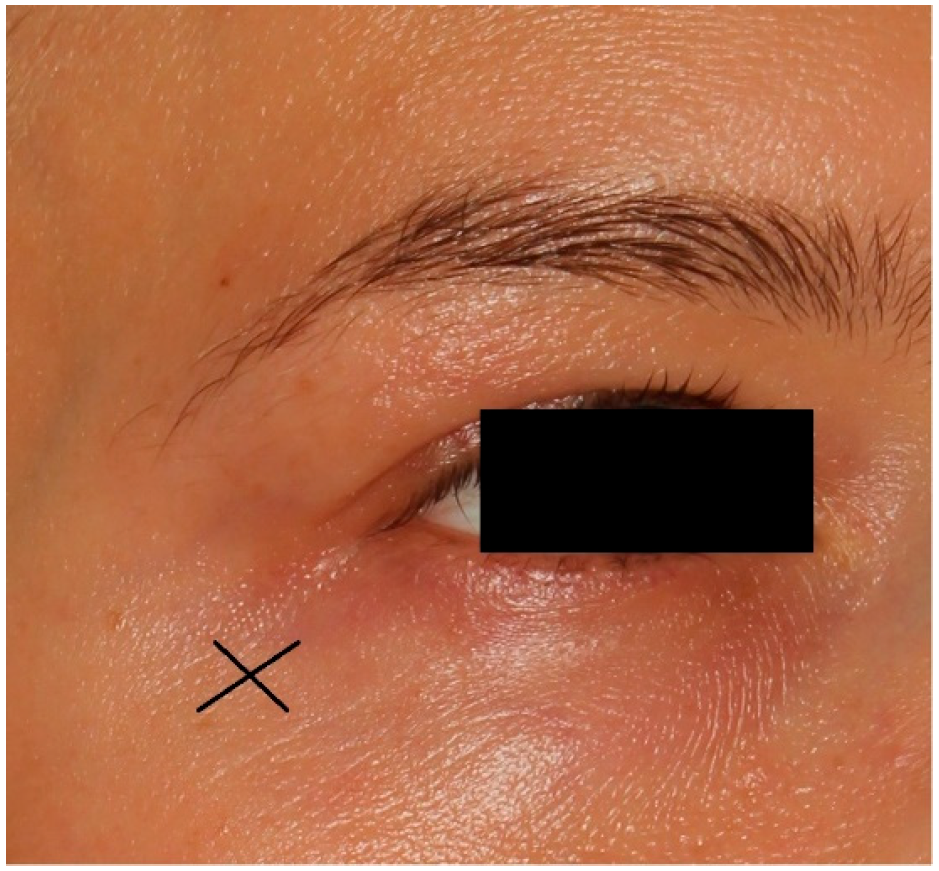
The carboxytherapy procedure involved intradermal, controlled injections of purified carbon dioxide (CO2) using a 32 G needle inserted at a 30° angle to a depth of 1 mm near both the left and right eyes (Figure 1). The parameters were as follows: flow rate of 10 cc/min and a total dose of 3 cc per eye. Each session lasted a few minutes.
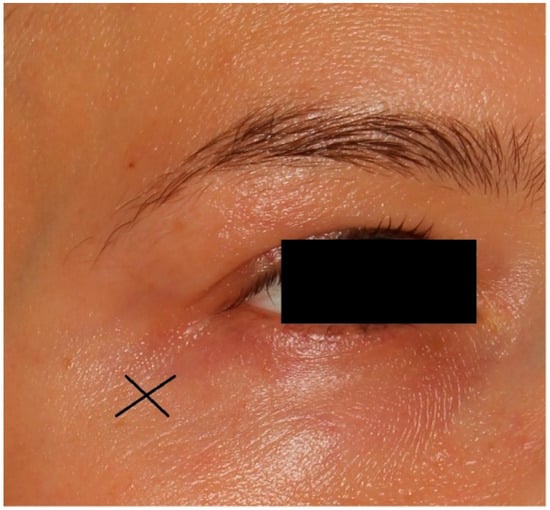
Figure 1.
X is the place of injection in carboxytherapy treatment.
2.3. Treatment Procedures
The area around the left eye was treated based on individual indications using the following protocols:
Twenty percent lactobionic acid: applied for 10 min in a group of 25 participants.
Ferulic acid (14%, pH 4.0–5.0, base: propylene glycol) with ascorbic acid (12%, base: propylene glycol): applied in a group of 14 participants until fully absorbed and left on the skin for 5 h.
This study was non-randomized, and the choice of chemical peels was determined by the observed symptoms and specific treatment indications.
The series consisted of five treatments performed at weekly intervals.
The research project and procedures were approved by the Bioethics Committee of the Medical University of Lodz (Protocol No RNN/105/22/KE) on 10 May 2022.
2.4. Measurement of Skin Parameters
2.4.1. Skin Hydration
Skin hydration was assessed using the CM 825 probe of the Corneometer, which measures the capacitive resistance of the stratum corneum, reflecting its degree of hydration. The device operates at low frequencies (40–75 Hz) and detects changes in the dielectric properties of the skin. Since the stratum corneum functions as a dielectric conductor, the speed of current flow increases with higher water content, indicating better hydration levels.
The Corneometer probe enables indirect measurements of hydration in the superficial skin layers, approximately 10–20 μm deep. Measurements were taken at several points (7–9) under the left eye, with the mean value used for statistical analysis. The first measurement was conducted before the treatment series, and the second was performed two weeks after the final treatment. The procedure was conducted in a controlled environment. Results were expressed in arbitrary units ranging from 0 to 130, where one unit corresponds to 0.02 mg of water per cm3 of the stratum corneum. Higher values indicate better hydration of the epidermis.
2.4.2. Skin Viscoelasticity
Skin viscoelasticity was assessed using the Cutometer MPA 580 probe, which measures the elasticity of the upper skin layers by applying a vacuum that mechanically deforms the skin. The degree of skin deformation correlates inversely with elasticity: the less the skin is deformed, the more elastic it is. Studies have demonstrated that hydrated skin is more elastic than dry skin.
The R6 parameter (ratio of delayed distension to immediate deformation, Uv/Ue) was used to evaluate the relative contribution of viscoelastic and viscous deformation to elastic deformation. A decrease in the R6 value indicates an increase in skin elasticity. This parameter is strongly correlated with water content in the skin and reflects the skin’s ability to return to its original state after deformation. From a physical perspective, viscoelasticity encompasses the skin’s plastic deformation, representing its capacity to recover after being stretched by the probe.
2.5. Statistical Analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) and median with interquartile range (median (25%; 75%)). For parameters following a normal distribution (verified using the Shapiro–Wilk test) and showing homogeneity of variance, the standard t-test was used to compare groups. For data that did not meet these assumptions, the Mann–Whitney U test was applied. For repeated measures, paired t-tests were used for normally distributed data, while the Wilcoxon signed-rank test was applied for non-parametric comparisons. Categorical variables were analyzed using the chi-square test, or Fisher’s exact test where appropriate. A p-value of less than 0.05 was considered statistically significant.
3. Results
The results of measurements before and after the series of treatments were compared to evaluate procedure effectiveness (Table 1). A statistically significant (p < 0.0001) improvement in skin viscoelasticity (parameter R6 cutometer) was demonstrated for both the left and right eyes. The improvement was observed in 32 of 39 subjects (82.1% of subjects) for the right eye (carboxytherapy) and in 34 of 39 subjects (87.2% of subjects) for the left eye—a combined procedure. The changes in Corneometer measurements were statistically insignificant; however, skin hydration was improved in 59% of subjects in the right-eye area and in 74.4% of subjects in the left-eye area (Table 2).

Table 1.
The results of Corneometer and Cutometer measurements performed before and after a series of five treatments.

Table 2.
The efficacy of applied treatment.
The analysis of the efficacy of carboxytherapy (right) in monotherapy and the treatment including carboxytherapy and acids (left) revealed that both treatments were effective. There were no statistically significant differences in treatment results, assessed with the use of the Corneometer and the Cutometer, between both therapies (Table 2).
The combination of carboxytherapy and lactobionic acid was used in 25 individuals. The improvement in hydration was observed in 80% of them following the therapy. Carboxytherapy in combination with ferulic acid and ascorbic acid was performed in 14 patients and the improvement was observed in 64% of cases. This shows that lactobionic acid has stronger moisturizing properties compared to ferulic and ascorbic acids. In 92.9% of individuals treated with ferulic and ascorbic acid, an improvement in viscoelasticity was demonstrated (R6). It cannot be ruled out that carboxytherapy improves hydration since its higher values were found in 59% of study participants after applying therapy. In turn, the improvement in the hydration within the area of the left eye was observed in 74.4% of individuals after combined therapy. Slight differences in viscoelasticity (R6) were demonstrated between the right and left sides (82.1% vs. 87.2%, respectively).
The analysis of changes in studied parameters in age groups (25–40 years vs. 41–55 years) is presented in Table 3. This analysis demonstrated that, in both age groups, applied treatments were associated with similar improvement.

Table 3.
Effects of applied treatment in age groups.
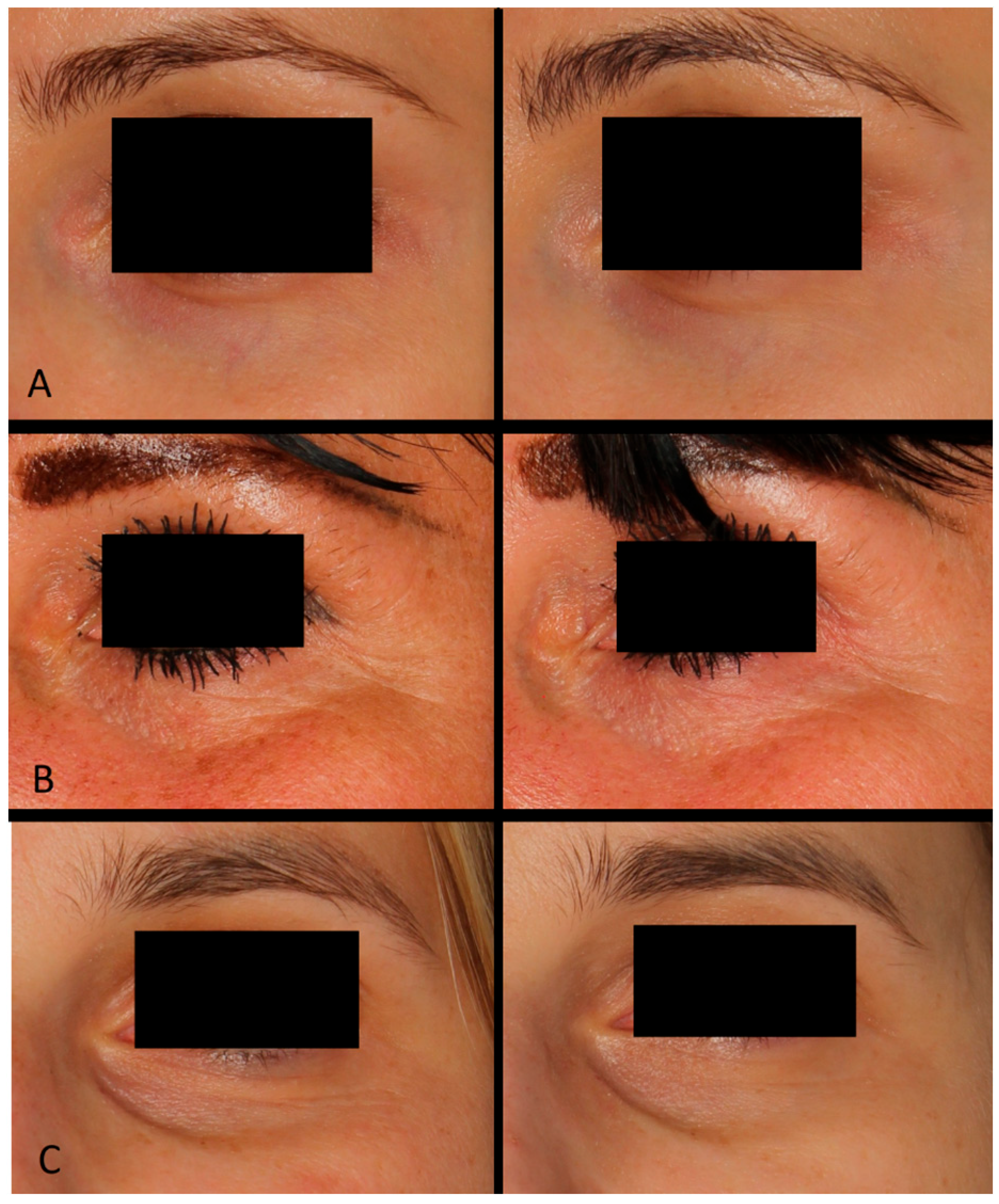
Based on photographic documentation and the case descriptions presented in Figure 2, generalized and simplified outcomes of the treatments were assessed. A series of carboxytherapy treatments combined with selected acids demonstrated diverse benefits in improving the appearance of the periorbital area across different patients. Treatments with lactobionic acid resulted in noticeable smoothing of the skin, reduction in fine wrinkles associated with dryness, brightening of the skin, and diminishing vascular shadows. In contrast, the combination of carboxytherapy with ferulic and ascorbic acid led to an improvement in skin texture, reduction in the depth of wrinkles, and lightening of hyperpigmentation. These results highlight the versatility of carboxytherapy combined with active acids in addressing various cosmetic concerns in the delicate periorbital region.
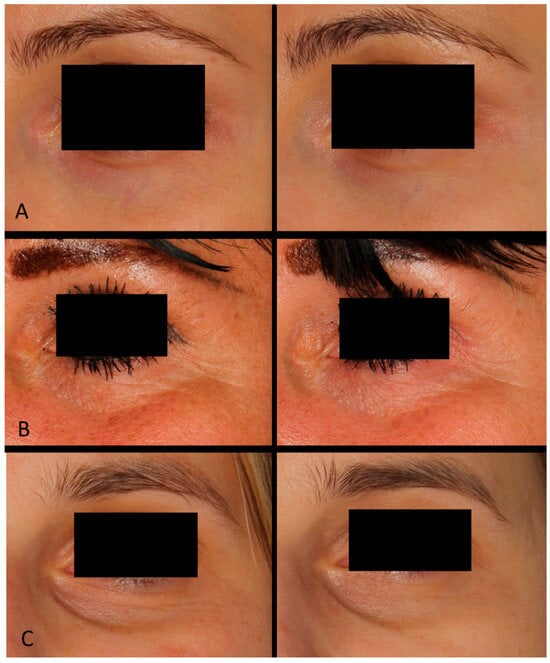
Figure 2.
Patient (A): woman, 32 years old—carboxytherapy combined with lactobionic acid; patient (B): woman, 44 years old—carboxytherapy combined with ferulic and ascorbic acid; patient (C): woman, 37 years old—carboxytherapy combined with lactobionic acid.
4. Discussion
The aim of this study was to determine whether a series of five carboxytherapy sessions affects the hydration and viscoelasticity of the skin around the eyes (Figure 2). This topic was undertaken in response to reports suggesting that carboxytherapy may also improve other functional parameters of the skin, such as hydration and epidermal thickness, including the improvement of skin hydration [7,8,9].
Interestingly, recent research by El-Domyati et al. found that carboxytherapy significantly increases epidermal thickness. Histological evaluation and histometric analysis conducted prior to striae distensae treatment revealed a thin and flattened epidermis with attenuated rete ridges in most skin biopsies. Following treatment, there was a noticeable morphological and architectural improvement in the epidermis, including the development of rete ridges (marked undulations of the dermoepidermal junction) [6].
While our study did not directly measure epidermal thickness, the observed improvements in hydration and viscoelasticity, as indicated by Corneometric and Cutometric assessments, suggest an indirect enhancement of epidermal structure. These findings align with trends reported in previous research, where functional improvements often correspond to structural changes. For instance, a study evaluating a carboxytherapy gel mask demonstrated significant improvement in skin quality, including hydration and reduced erythema, dryness, and crusting, following a nanofractional radiofrequency treatment. Although this method does not involve injectable carboxytherapy, its ability to accelerate wound healing and improve epidermal structure supports the potential multifaceted benefits of carbon dioxide treatments in skin rejuvenation [12,13]. For instance, a clinical study by Leibaschoff et al. demonstrated that the transcutaneous application of a CO2 gel led to significant improvements in microcirculation, including an increase in vertical and horizontal capillary density and a reduction in areas of ischemia. These dermal changes were found to be comparable to those achieved with subcutaneous CO2 injections. These results further support the potential of carboxytherapy, both topical and injectable, in enhancing the structural and functional properties of the skin. Improvements in tissue oxygenation, microcirculation, and vascular density underline the multifaceted benefits of CO2-based therapies in promoting skin rejuvenation and repair [14].
In the present study, the evaluation of skin hydration using the Corneometer did not yield statistically significant results for either the right side (p = 0.101) or the left side (p = 0.052). However, a general trend of improved hydration was observed, with 59% of participants in the carboxytherapy group and 74.4% in the combined treatment group showing improvement. The overall mean increase in hydration across the study group was 2.47 units. Notably, the obtained results can be explained by the fact that only 8 out of 39 individuals in our study had dry skin in the area of the eyes (Corneometer readings below 60) before the start of the treatment series. In the case of these patients, the increase in skin hydration amounted to 9.351 units (mean value). In our opinion, the impact of applied therapies would be more significant if more people with dry skin were enrolled in this study.
Our results of the R6 parameter measurements using the Cutometer indicate that carboxytherapy significantly enhances skin viscoelasticity, as evidenced by statistically significant outcomes for both the right (<0.0001) and left (<0.0001) sides of the face. Kapoor and Saraf have previously established a link between skin hydration and viscoelasticity, highlighting that increased water content influences the viscosity of interstitial fluid and the structural integrity of proteoglycans [12,15]. This relationship is critical, as it provides insight into how hydration contributes to improved skin biomechanical properties. Additionally, other studies confirm that the R6 parameter is directly dependent on hydration levels in the skin [16,17,18].
Kruger et al. identified a strong correlation between R2 (gross skin elasticity) and age, whereas R6 (“viscoelasticity”, focusing on the ratio of elastic to viscous deformation) demonstrated only a weak association with aging. This distinction suggests that the R2 parameter is more reliable for assessing skin aging compared to R6, providing a clearer representation of age-related changes in skin biomechanical properties [19]. Our findings are consistent with these observations, as no correlation was detected between skin viscoelasticity (R6) and participant age in our study. Additionally, previous research has noted that changes in the R2 parameter tend to be more pronounced with aging than those in R6, further supporting our results [20].
In our study, carboxytherapy alone and in combination with acids resulted in significant improvements in viscoelasticity, supporting existing research on the positive effects of this treatment modality on skin biomechanical properties [7,8,9]. Building on these findings, it is essential to explore the nuanced relationships between different skin biomechanical parameters and aging. These results underscore carboxytherapy’s potential as an effective intervention for enhancing skin resilience and elasticity, further amplified when integrated with complementary therapies.
The ability of carboxytherapy to stimulate collagen synthesis has been confirmed in studies by Oliveira et al., who utilized histological and morphometric analyses and demonstrated a significant increase in collagen fibers (41.44 ± 4.50%) compared to the control group (37.44 ± 3.87%) after a single application of carboxytherapy with observations conducted 60 days post-treatment. These findings highlight the capacity of carboxytherapy to induce the synthesis of dermal components, even after a single session. Additionally, demonstrated significant differences in the morphometry for collagen fiber. Interestingly, the study also observed that higher infusion rates and greater gas volumes may enhance collagen synthesis due to barotrauma. Further research is needed to establish standardized protocols and investigate the impact of individual patient characteristics on treatment efficacy [5].
El-Domyati et al. demonstrated carboxytherapy to significantly improve skin structure in various conditions of striae distensae (SD). Carboxytherapy alone or in combination with fractional CO2 laser therapy effectively reduced the length, width, and pigmentation of SD lesions while enhancing skin texture. The study highlighted that while combined therapies showed slightly better clinical improvement, the difference was not statistically significant, suggesting that carboxytherapy alone is a highly effective modality for dermal remodeling. Histological analysis revealed marked remodeling of the dermis, with compact, dense, and well-organized collagen fibers accompanied by increased elastic fibers [6].
In the study by Bagherani et al., Orcein Giemsa staining demonstrated a statistically significant increase in elastin synthesis. Additionally, the impairment of cutaneous vascular networks, characterized by reduced microvasculature, is a hallmark of skin aging. In the same study, a significant increase in VEGF expression levels observed in the IHC analysis confirmed the effectiveness of carboxytherapy in treating and partially reversing skin aging, primarily through the promotion of angiogenesis. These findings collectively reinforce the role of carboxytherapy as a safe and minimally invasive method for skin rejuvenation. Its ability to stimulate collagen and elastin synthesis, enhance epidermal and dermal thickness, and promote angiogenesis positions carboxytherapy as a versatile and effective treatment for managing skin aging [21].
A recent scoping review by Prazeres et al. highlighted the therapeutic potential of carboxytherapy in enhancing wound healing, emphasizing its ability to improve blood flow, increase local oxygenation, and stimulate collagen production. These mechanisms not only accelerate wound repair but also reduce inflammation, contributing to the restoration of skin integrity. While primarily studied in the context of chronic wounds, these findings underscore the broader applicability of carboxytherapy in dermatology and aesthetics. The demonstrated capacity of CO2 therapy to promote collagen synthesis and improve skin quality aligns with its established role in cosmetic procedures, such as rejuvenation and addressing skin laxity. These results further support the growing use of carboxytherapy as a versatile and minimally invasive modality in aesthetic practice [22].
The weakening of the epidermal barrier due to aging is a key factor contributing to increased skin dehydration. External environmental factors, including UV radiation and pollutants, further compromise the water-lipid bilayer, leading to a rise in transepidermal water loss (TEWL) and triggering inflammatory processes within the skin. To effectively address aesthetic concerns in the periorbital region, it is vital to integrate antioxidant and moisturizing agents into skincare routines. Compounds such as ferulic acid, ascorbic acid, and lactobionic acid have demonstrated significant efficacy in reducing inflammation, enhancing hydration, and repairing the skin barrier.
Although our study did not yield statistically significant differences between monotherapy and combined treatments, the combination of carboxytherapy with acids showed modest yet consistent improvements across all parameters. Interestingly, these benefits were more evident on the left side of the face, highlighting the potential of integrated treatment strategies for achieving superior results.
Lactobionic acid possesses moisturizing (hygroscopic), anti-inflammatory, and antioxidant properties. It is an inhibitor of metalloproteinases, exerting an indirect effect on the dermis and vasculature, and it supports the repair of the epidermal barrier. Consequently, lactobionic acid was selected for participants presenting symptoms of chronological aging, vascular shadows, and dry skin. Among the most notable properties of lactobionic acid are its chelating abilities, which contribute to its antioxidant effects, and its remarkable capacity to bind substantial amounts of water. As a potent humectant, it enhances skin hydration, smoothing the skin’s surface and diminishing the appearance of fine wrinkles [23,24].
Tasic-Kostov et al. evaluated the effects of formulations containing lactobionic acid versus those with glycolic acid on several skin parameters, including skin tone (measured with Mexameter), transepidermal water loss (Tewameter), electrical capacitance (Corneometer), and pH (pHmeter). Their findings demonstrated that 6% lactobionic acid was significantly more effective in moisturizing the skin compared to glycolic acid, without causing irritation or compromising the epidermal barrier [23].
Algiert-Zielinska B. investigated the effects of a 20% lactobionic acid peel on skin hydration, elasticity, and TEWL. Corneometer readings revealed a statistically significant increase in skin hydration following the procedure. Although Cutometer measurements for the R6 parameter were not statistically significant, they still indicated a positive trend toward improved skin viscoelasticity [24].
In our study, the combination of carboxytherapy with lactobionic acid resulted in greater improvements compared to the combination of carboxytherapy with ferulic and ascorbic acids, although these differences were not statistically significant. The most pronounced differences in Corneometer results were noted in the group treated with lactobionic acid, where 80% of individuals showed improvement, compared to 59% in the carboxytherapy-only group and 64.3% in the group treated with ferulic and ascorbic acids. Skin viscoelasticity demonstrated the greatest improvement in participants receiving carboxytherapy combined with ferulic and ascorbic acids, with 92.9% of individuals showing positive outcomes. These findings underscore the potential efficacy of combining carboxytherapy with active ingredients tailored to specific skin needs, particularly in anti-aging and photoaging therapies where the synergistic effects of acids and carboxytherapy can be leveraged.
Ferulic acid exhibits antioxidant and anti-inflammatory properties, enhances angiogenesis, accelerates healing, inhibits tyrosinase production, and improves hydration. Ascorbic acid promotes collagen synthesis, acts as an antioxidant, inhibits tyrosinase activity, and has moisturizing, anti-inflammatory, and exfoliating properties (when used at concentrations above 20%). This combination was chosen for participants with signs of photoaging and/or pigmented eye shadows. Ferulic acid, frequently utilized in chemical peels at concentrations ranging from 12% to 20%, is known for its multi-functional properties. These peels are gentle on the skin, do not cause visible exfoliation, and are suitable for year-round use. Ferulic acid acts as a suppressor of metalloproteinases (MMP-2 and MMP-9). Additionally, it absorbs UV radiation (290–320 nm), exhibits anti-inflammatory properties, and promotes skin regeneration and epithelialization. These attributes contribute to improved microcirculation and overall skin health [25,26,27,28]. Zdunska K. et al. evaluated the combination of ferulic acid and vitamin C on various skin parameters, including hydration, melanin levels, erythema intensity, and skin topography. Their study revealed a statistically significant improvement in hydration (p < 0.0001) and observed initial positive changes in elasticity (R2 parameter) after eight weeks [29]. Additional research by Zdunska demonstrated that ferulic acid enhances hydration in the stratum corneum, creating a smoother skin surface, as evidenced by Visioscan imaging [30]. Chiu et al. further confirmed that the topical application of ferulic acid supports the hydrolipid barrier, reduces TEWL, and increases water content in both the epidermis and dermis [31].
In the context of our findings, the combination of carboxytherapy with ferulic acid and ascorbic acid demonstrated a significant improvement in skin viscoelasticity. In 92.9% of individuals treated with ferulic and ascorbic acid, an improvement in viscoelasticity was demonstrated (R6). Although differences in hydration between treatment groups were not statistically significant, carboxytherapy in combination with ferulic acid and ascorbic acid (14 patients) additionally contributed to a 64% of cases improvement in hydration within the treated area. The synergistic effect observed in our study underscores the utility of incorporating ferulic acid into combination treatments for improved anti-aging and skin-repair outcomes.
5. Conclusions
This study demonstrated that carboxytherapy significantly improves skin elasticity in the periorbital area, particularly when combined with active acids such as ferulic and ascorbic acids. While hydration benefits were limited, they may be more pronounced in individuals with initially dehydrated skin. These findings support carboxytherapy as an effective treatment for enhancing skin elasticity and mitigating age-related changes in skin firmness.
6. Limitations
The study faced several limitations that may affect the generalizability of its findings. First, the relatively small sample size, especially within subgroups treated with different chemical peels, limits the statistical power and the ability to draw definitive conclusions about the differences between treatment modalities. Second, the study was not randomized, which may introduce selection bias as treatment allocation was based on the observed symptoms and indications. Additionally, the lack of a placebo-controlled group makes it challenging to fully isolate the effects of carboxytherapy and combined treatments from natural variability or placebo effects.
Moreover, while the study evaluated hydration and viscoelasticity using objective measurements, it did not directly assess other parameters such as epidermal thickness or biochemical markers, which could have provided further insights into the mechanisms underlying the observed changes. Finally, the study participants were limited to Caucasian individuals with Fitzpatrick phototypes II and III, which restricts the applicability of the findings to individuals of other skin types and ethnicities. Future research should aim to address these limitations by incorporating larger, more diverse cohorts and employing randomized controlled designs.
Author Contributions
Conceptualization, A.K.; methodology, A.K.; formal analysis, H.R.; investigation, A.K. and A.R.; data curation, A.K. and A.R.; writing—original draft preparation, A.K. and A.R.; writing—review and editing, A.K.; supervision, H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Medical University of Lodz, grant No. 503/3-066-02/503-31-001-19-00.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Bioethics Committee of the Medical University of Lodz (Protocol No RNN/105/22/KE) on 10 May 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This study was supported by statutory research activity Department of Cosmetology and Aesthetic Dermatology, Faculty of Pharmacy, Medical University of Lodz no.: 503/3-066-01/503-31-001-19-00.
Conflicts of Interest
The authors declare no conflicts of interest. The authors themselves are responsible for the content and writing of the paper.
References
- Seirafianpour, F.; Atefi, N.; Amin, N.G.; Namazi, M.R.; Behrangi, E.; Shafiei, A.; Ghassemi, M.; Mozafarpoor, S.; Goodarzi, A. Effectiveness, safety, and patient satisfaction of carboxy-therapy as an adjunctive treatment for periorbital hyperpigmentation. Skin Res. Technol. 2024, 30, e13651. [Google Scholar] [CrossRef] [PubMed]
- Barańska-Rybak, W.; Mehrholz, D.M. Carboxytherapy in the light of the latest reports. Erythema multiforme-like eruption as a side effect of carboxytherapy. Dermatol. Rev. 2019, 106, 46–51. [Google Scholar] [CrossRef]
- Varlaro, V.; Manzo, G.; Mugnaini, F.; Bisacci, C.; Fiorucci, P.; De Rango, P.; Bisacci, R. Carboxytherapy: Effects on microcirculation and its use in the treatment of severe lymphedema. Acta Phlebol. 2007, 8, 79–91. [Google Scholar]
- Doghaim, N.N.; El-Tatawy, R.A.; Neinaa, Y.M.E.; Abd El-Samd, M.M. Study of the efficacy of carboxytherapy in alopecia. J. Cosmet. Dermatol. 2018, 17, 1275–1285. [Google Scholar] [CrossRef]
- Oliveira, S.M.D.; Rocha, L.B.; da Cunha, M.T.R.; Cintra, M.M.M.; Pinheiro, N.M.; Mendonça, A.C. Effects of carboxytherapy on skin laxity. J. Cosmet. Dermatol. 2020, 19, 3007–3013. [Google Scholar] [CrossRef]
- El-Domyati, M.; El-Din, W.H.; Medhat, W.; Khaled, Y.; Ibrahim, M.R. Carboxytherapy versus its Combination with Fractional CO2 Laser for the Treatment of Striae Distensae: An Objective, Right-to-left, Comparative Study. J. Clin. Aesthet. Dermatol. 2024, 17, E69–E75. [Google Scholar]
- El-Domyati, M.; Hosam El-Din, W.; Medhat, W.; Ibrahim, M.R.; Khaled, Y. The use of Carboxytherapy alone or in combination with fractional CO2 laser for facial rejuvenation: A split-face comparative study. J. Cosmet. Dermatol. 2020, 19, 1648–1655. [Google Scholar] [CrossRef]
- El-Domyati, M.; Hosam El-Din, W.; Medhat, W.; Ibrahim, M.R.; Khaled, Y. Carboxytherapy for striae distensae: A promising modality. J. Cosmet. Dermatol. 2021, 20, 546–553. [Google Scholar] [CrossRef]
- Moftah, N.H.; El Khayyat, M.A.; Ragai, M.H.; Alaa, H. Carboxytherapy Versus Skin Microneedling in Treatment of Atrophic Postacne Scars: A Comparative Clinical, Histopathological, and Histometrical Study. Dermatol. Surg. 2018, 44, 1332–1341. [Google Scholar] [CrossRef]
- Nikolovski, J.; Stamatas, G.N.; Kollias, N.; Wiegand, B.C. Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. J. Investig. Dermatol. 2008, 128, 1728–1736. [Google Scholar] [CrossRef]
- Tabaie, S.M.; Shirkavand, A.; Mansouri, P.; Mehrizi, A.A.H.; Farshi, S. Evaluation of the ef-fects of carboxytherapy in the treatment of periorbital dark circles. J. Cosmet. Dermatol. 2024, 23, 2711–2715. [Google Scholar] [CrossRef] [PubMed]
- Medrano, K.; Arruda, S.; Oza, N.; Sadick, N. Carboxytherapy Mask as Post Nanofractional Radiofrequency Treatment for Improvement of Facial Skin Quality and Photoaging. J. Drugs Dermatol. 2021, 20, 461–465. [Google Scholar] [CrossRef]
- Shamban, A.; Roberts, W.E.; Bucay, V.; Chilukuri, S.; Simmons-O’Brien, E.; Orlinsky, D. Topical Carboxytherapy for Skin Rejuvenation. J. Clin. Aesthet. Dermatol. 2025, 18, 55–57. [Google Scholar] [PubMed]
- Leibaschoff, G.H.; Coll, L.; Roberts, W.E. A Prospective Clinical and Instrumental Study on the Effects of a Transcutaneous Cosmeceutical Gel that is Claimed to Produce CO₂. Surg. Technol. Int. 2018, 32, 33–45. [Google Scholar]
- Kapoor, S.; Saraf, S. Assessment of viscoelasticity and hydration effect of herbal moisturizers using bioengineering techniques. Pharmacogn. Mag. 2010, 6, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Wickett, R.R. Stretching the skin surface: Skin elasticity. Cosmet. Toilet. 2001, 116, 47–54. [Google Scholar]
- Pierard, G.E.; Nikkels-Tassoudji, N.; Pierard-Franchimont, C. Influence of the test area on the mechanical properties of the skin. Dermatology 1995, 191, 9–15. [Google Scholar] [CrossRef]
- Dobrev, H. Use of Cutometer to assess epidermal hydration. Skin Res. Technol. 2000, 6, 239–244. [Google Scholar] [CrossRef]
- Krueger, N.; Luebberding, S.; Oltmer, M.; Streker, M.; Kerscher, M. Age-related changes in skin mechanical properties: A quantitative evaluation of 120 female subjects. Skin Res. Technol. 2011, 17, 141–148. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, S.; Lee, H.; Moon, S.; Chang, I. Correlation between a Cutometer and quantitative evaluation using Moire topography in age-related skin elasticity. Skin Res. Technol. 2007, 13, 280–284. [Google Scholar] [CrossRef]
- Bagherani, N.; Ghanadan, A.; Mirmomeni, G.; Firooz, A.; Smoller, B.R.; Shojaei, R.; Rafipour, H.; Bagherani, N.; Abdolhosseini, M.; Tavoosidana, G. Pathological and Immunohistochemical Assessment of Aging of the Abdominal Skin Treated with Carboxytherapy: A Randomized, Split-body Trial. J. Clin. Aesthet. Dermatol. 2024, 17, 62–69. [Google Scholar] [PubMed]
- Prazeres, J.; Lima, A.; Ribeiro, G. Effects of Carbon Dioxide Therapy on Skin Wound Healing. Biomedicines 2025, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Tasic-Kostov, M.; Savic, S.; Lukic, M.; Tamburic, S.; Pavlovic, M.; Vuleta, G. Lactobionic acid in a natural alkylpolyglucoside-based vehicle: Assessing safety and efficacy aspects in comparison to glycolic acid. Post. Derm. Alerg. 2003, 2, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Algiert-Zielińska, B.; Mucha, P.; Rotsztejn, H. Effects of lactobionic acid peel, aluminum oxide crystal microdermabrasion, and both procedures on skin hydration, elasticity, and transepidermal water loss. J. Cosmet. Dermatol. 2019, 18, 1463–1474. [Google Scholar] [CrossRef]
- Kiewlicz, J.; Szymusiak, H.; Zieliński, R. Thermal stability and antioxidant activity of long-chain alkyl esters od ferulic acid. Zywn. Nauk. Technol. Ja. 2015, 4, 188–200. [Google Scholar]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic acid and ferulic acid inhibit UVA-induced matrix metalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef]
- He, S.; Guo, Y.; Zhao, J.; Xu, X.; Song, J.; Wang, N.; Liu, Q. Ferulic acid protects against heat stress-induced intestinal epithelial barrier dysfunction in IEC-6 cells via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Int. J. Hyperth. 2018, 35, 112–121. [Google Scholar] [CrossRef]
- Lin, C.; Chiu, J.; Wu, I.; Wang, B.; Pan, C.; Chen, Y. Ferulic acid augments angiogenesis via VEGF, PDGF and HIF-1 alpha. J. Nutr. Biochem. 2010, 21, 627–633. [Google Scholar] [CrossRef]
- Zduńska-Pęciak, K.; Kołodziejczak, A.; Rotsztejn, H. Two superior antioxidants: Ferulic acid and ascorbic acid in reducing signs of photoaging—A split-face comparative study. Dermatol. Ther. 2022, 35, e15254. [Google Scholar] [CrossRef]
- Zduńska-Pęciak, K.; Dębowska, R.; Kołodziejczak, A.; Rotsztejn, H. Ferulic acid—A novel topical agent in reducing signs of photoaging. Dermatol. Ther. 2022, 35, e15543. [Google Scholar] [CrossRef]
- Chiu, A.; Kimball, A.B. Topical vitamins, minerals and botanical ingredients as modulators of environmental and chronological skin damage. Br. J. Dermatol. 2003, 149, 681–691. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).