Bioelectrical Impedance Versus Air-Displacement Plethysmography for Body Fat Measurements in Subjects with Abdominal Obesity: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Measurements
2.3. Determination of Body Composition by AP
2.4. Determination of Body Composition by BIA
2.5. Statistical Analysis
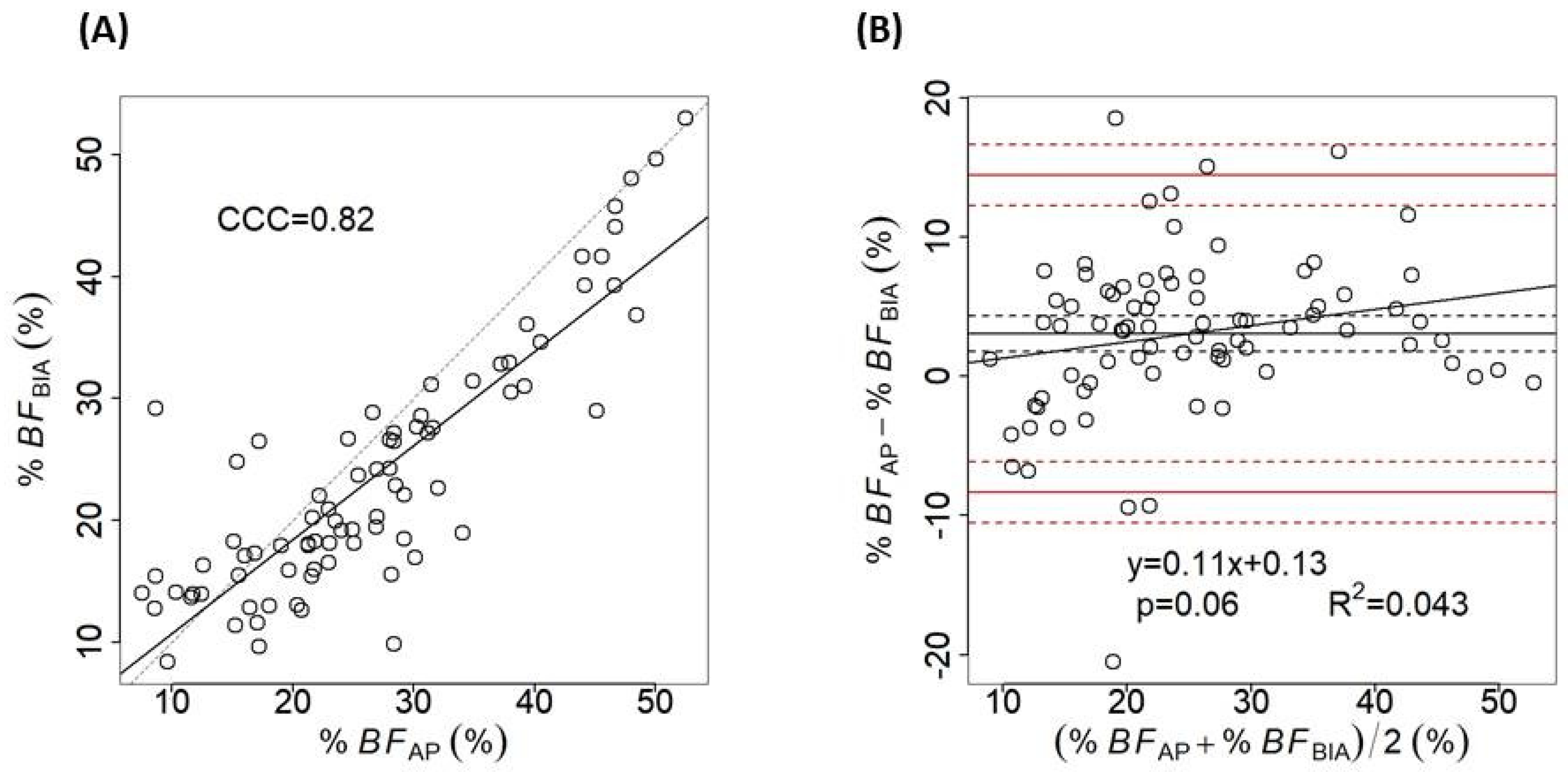
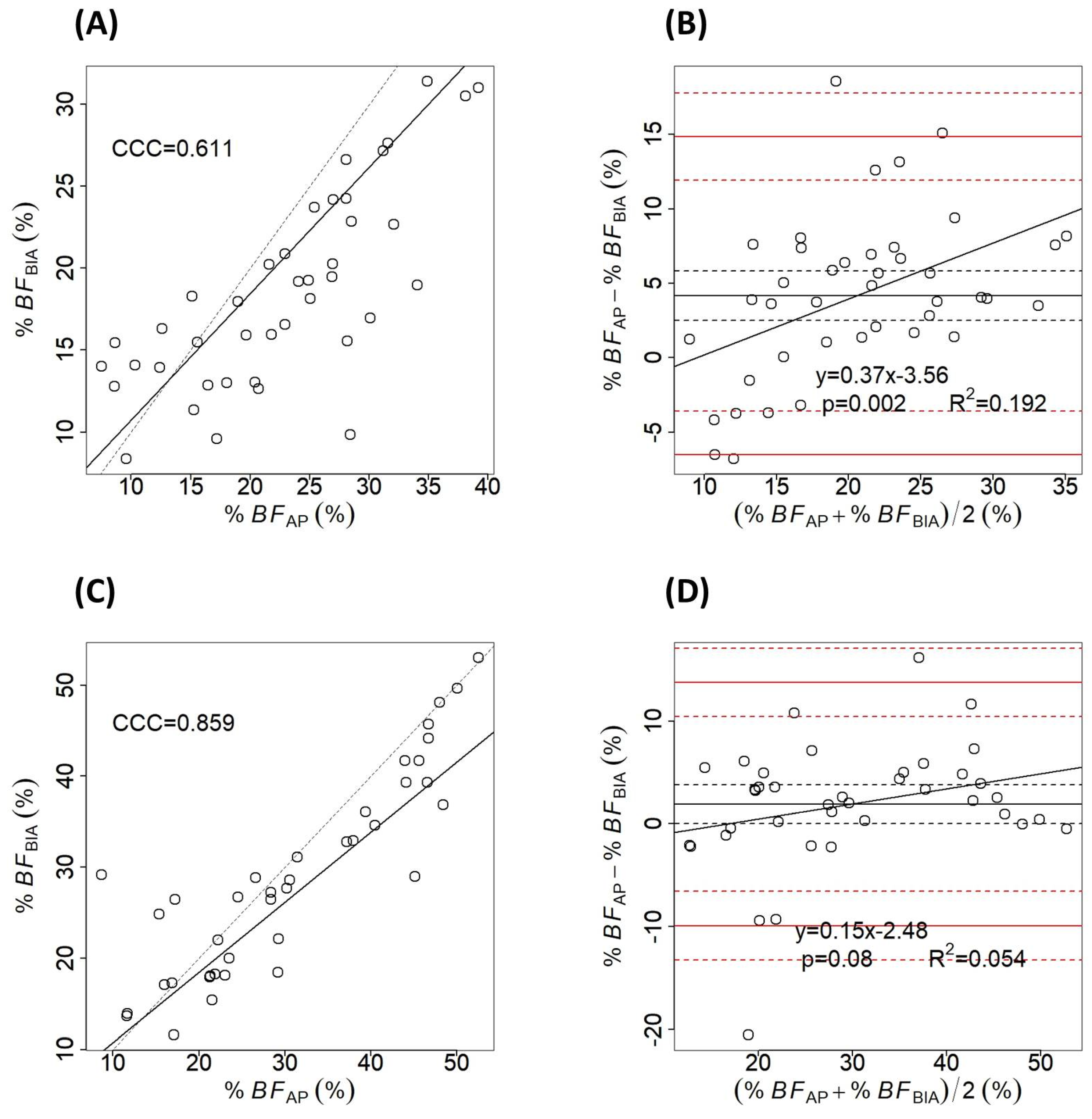
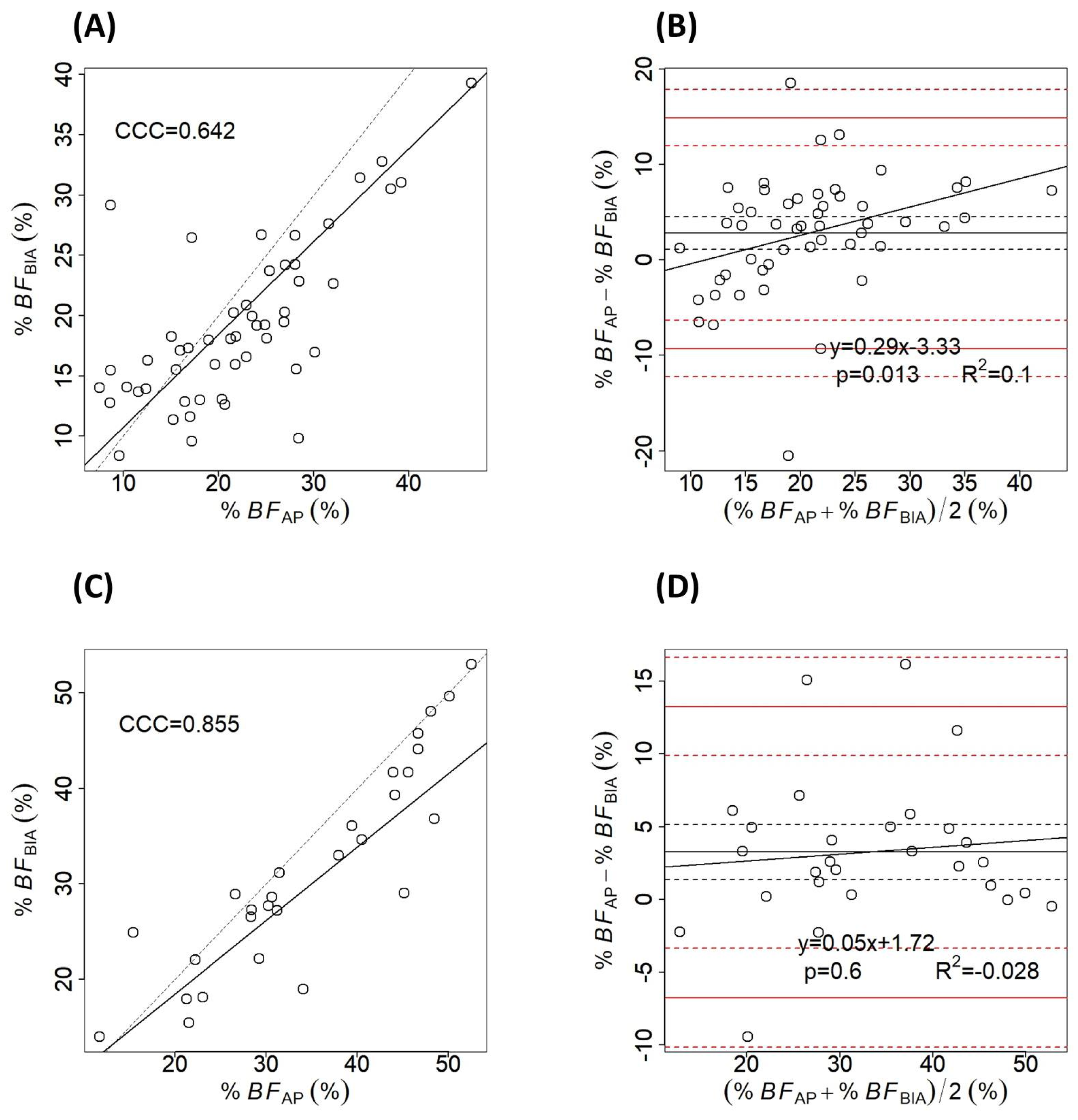
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| %BF | Body fat percentage |
| WC | Waist circumference |
| WHtR | Waist to height ratio |
| WHO | World Health Organization |
| ABSI | The A body shape index |
| WWI | Weight-adjusted-waist index |
| AP | Air-displacement plethysmography |
| BIA | Bioelectrical impedance |
| DEXA | Dual-X absorptiometry |
| MRI | Magnetic resonance imaging |
| CT | Computer tomography |
| CCC | Concordance correlation coefficient |
| SD | Standard deviation |
| CI | Confidence interval |
References
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salmón-Gómez, L.; Catalán, V.; Frühbeck, G.; Gómez-Ambrosi, J. Relevance of body composition in phenotyping the obesities. Rev. Endocr. Metab. Disord. 2023, 24, 809–823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frühbeck, G.; Busetto, L.; Dicker, D.; Yumuk, V.; Goossens, G.H.; Hebebrand, J.; Halford, J.G.C.; Farpour-Lambert, N.J.; Blaak, E.E.; Woodward, E.; et al. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes. Facts 2019, 12, 131–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gutin, I. In BMI We Trust: Reframing the Body Mass Index as a Measure of Health. Soc. Theory Health 2018, 16, 256–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Okorodudu, D.O.; Jumean, M.F.; Montori, V.M.; Romero-Corral, A.; Somers, V.K.; Erwin, P.J.; Lopez-Jimenez, F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int. J. Obes. 2010, 34, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Muntner, P.; Wildman, R.P.; Reynolds, K.; He, J. Measures of adiposity and cardiovascular disease risk factors. Obesity 2007, 15, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Pugliese, G.; Blasetti Fantauzzi, C.; Federici, M.; Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. 2019, 92, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, M.; Li, Y. Adult obesity diagnostic tool: A narrative review. Medicine 2024, 103, e37946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bashir, M.A.; Yahaya, A.I.; Muhammad, M.; Yusuf, A.H.; Mukhtar, I.G. Prevalence of central obesity in Nigeria: A systematic review and meta-analysis. Public Health 2022, 206, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bosomworth, N.J. Normal-weight central obesity: Unique hazard of the toxic waist. Can. Fam. Physician 2019, 65, 399–408. [Google Scholar] [PubMed] [PubMed Central]
- Tewari, A.; Kumar, G.; Maheshwari, A.; Tewari, V.; Tewari, J. Comparative Evaluation of Waist-to-Height Ratio and BMI in Predicting Adverse Cardiovascular Outcome in People with Diabetes: A Systematic Review. Cureus 2023, 15, e38801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, Y.; Kim, N.H.; Kwon, T.Y.; Kim, S.G. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci. Rep. 2018, 8, 16753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Yu, J.; Wang, L.; Zhang, X.; Wang, F.; Zhu, Y. Weight-adjusted waist index as a practical predictor for diabetes, cardiovascular disease, and non-accidental mortality risk. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 2498–2510. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, X.; Zhan, T.; Huang, M.; Tian, X.; Tian, X.; Huang, X. Weight-adjusted waist index is positively and linearly associated with all-cause and cardiovascular mortality in metabolic dysfunction-associated steatotic liver disease: Findings from NHANES 1999-2018. Front. Endocrinol. 2024, 15, 1457869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macek, P.; Biskup, M.; Terek-Derszniak, M.; Krol, H.; Smok-Kalwat, J.; Gozdz, S.; Zak, M. Optimal cut-off values for anthropometric measures of obesity in screening for cardiometabolic disorders in adults. Sci. Rep. 2020, 10, 11253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suwała, S.; Junik, R. Body Mass Index and Waist Circumference as Predictors of Above-Average Increased Cardiovascular Risk Assessed by the SCORE2 and SCORE2-OP Calculators and the Proposition of New Optimal Cut-Off Values: Cross-Sectional Single-Center Study. J. Clin. Med. 2024, 13, 1931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tucker, L.A.; Lecheminant, J.D.; Bailey, B.W. Test-retest reliability of the Bod Pod: The effect of multiple assessments. Percept. Mot. Ski. 2014, 118, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.C. Bioelectrical impedance analysis for body composition assessment: Reflections on accuracy, clinical utility, and standardisation. Eur. J. Clin. Nutr. 2019, 73, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Macias, N.; Alemán-Mateo, H.; Esparza-Romero, J.; Valencia, M.E. Body fat measurement by bioelectrical impedance and air displacement plethysmography: A cross-validation study to design bioelectrical impedance equations in Mexican adults. Nutr. J. 2007, 6, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okosun, I.S.; Tedders, S.H.; Choi, S.; Dever, G.E. Abdominal adiposity values associated with established body mass indexes in white, black and hispanic Americans. A study from the Third National Health and Nutrition Examination Survey. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- von Hurst, P.R.; Walsh, D.C.I.; Conlon, C.A.; Ingram, M.; Kruger, R.; Stonehouse, W. Validity and reliability of bioelectrical impedance analysis to estimate body fat percentage against air displacement plethysmography and dual-energy X-ray absorptiometry. Nutr. Diet. 2016, 73, 197–204. [Google Scholar] [CrossRef]
- Biaggi, R.R.; Vollman, M.W.; Nies, M.A.; Brener, C.E.; Flakoll, P.J.; Levenhagen, D.K.; Sun, M.; Karabulut, Z.; Chen, K.Y. Comparison of air-displacement plethysmography with hydrostatic weighing and bioelectrical impedance analysis for the assessment of body composition in healthy adults. Am. J. Clin. Nutr. 1999, 69, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Vasold, K.L.; Parks, A.C.; Phelan, D.M.L.; Pontifex, M.B.; Pivarnik, J.M. Reliability and Validity of Commercially Available Low-Cost Bioelectrical Impedance Analysis. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.E.; Beck, L.; Petropoulou, A.; Clegg, M.E. A comparison of body composition measurement techniques. J. Hum. Nutr. Diet. 2014, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.J.; Swan, P.D.; Schlairet, M.C.; Sanderson, S. Comparison of Body Composition Measurement with Whole Body Multifrequency Bioelectrical Impedance and Air Displacement Plethysmography in Healthy Middle-Aged Women. Health Care Women Int. 2011, 32, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Boneva-Asiova, Z.; Boyanov, M.A. Body composition analysis by leg-to-leg bioelectrical impedance and dual-energy X-ray absorptiometry in non-obese and obese individuals. Diabetes Obes. Metab. 2008, 10, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Pateyjohns, I.R.; Brinkworth, G.D.; Buckley, J.D.; Noakes, M.; Clifton, P.M. Comparison of three bioelectrical impedance methods with DXA in overweight and obese men. Obesity 2006, 14, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Fakhrawi, D.H.; Beeson, L.; Libanati, C.; Feleke, D.; Kim, H.; Quansah, A.; Darnell, A.; Lammi-Keefe, C.J.; Cordero-MacIntyre, Z. Comparison of body composition by bioelectrical impedance and dual-energy x-ray absorptiometry in overweight/obese postmenopausal women. J. Clin. Densitom. 2009, 12, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Marín, D.; Escribano, J.; Closa-Monasterolo, R.; Ferré, N.; Venables, M.; Singh, P.; Wells, J.C.; Muñoz-Hernando, J.; Zaragoza-Jordana, M.; Gispert-Llauradó, M.; et al. Validation of bioelectrical impedance analysis for body composition assessment in children with obesity aged 8–14y. Clin. Nutr. 2021, 40, 4132–4139. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wu, L.; Chen, Y.; Wang, J.; Liu, J.; Huang, G.; Hou, D.; Liao, Z.; Zhang, T.; Xie, X.; et al. A comparison of bioelectrical impedance analysis and air displacement plethysmography to assess body composition in children. Front. Public Health 2023, 11, 1164556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- COSMED. BOD POD Gold Standard Body Composition Tracking System Operator’s Manual—P/N 210-2400 Rev. M—DCO 1765; COSMED USA, Inc.: Concord, CA, USA, 2015. [Google Scholar]
- Silveira, E.A.; Barbosa, L.S.; Rodrigues, A.P.S.; Noll, M.; De Oliveira, C. Body fat percentage assessment by skinfold equation, bioimpedance and densitometry in older adults. Arch. Public Health 2020, 78, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Henche, S.A.; Torres, R.R.; Pellico, L.G. An evaluation of patterns of change in total and regional body fat mass in healthy Spanish subjects using dual-energy X-ray absorptiometry (DXA). Eur. J. Clin. Nutr. 2008, 62, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.H.; de Craen, A.J.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.; Westendorp, R.G.; Maier, A.B. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, M.C.; Barros, A.J. Bioelectrical impedance analysis in clinical practice: A new perspective on its use beyond body composition equations. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Coratella, G.; Cerullo, G.; Noriega, Z.; Francisco, R.; Charrier, D.; Irurtia, A.; Lukaski, H.; Silva, A.M.; Paoli, A. High-standard predictive equations for estimating body composition using bioelectrical impedance analysis: A systematic review. J. Transl. Med. 2024, 22, 515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ward, L.C.; Isenring, E.; Dyer, J.M.; Kagawa, M.; Essex, T. Resistivity coefficients for body composition analysis using bioimpedance spectroscopy: Effects of body dominance and mixture theory algorithm. Physiol. Meas. 2015, 36, 1529–1549. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]

| Exclusion Criteria | Disease/Condition |
|---|---|
| Chronic diseases | Cardiac chronic diseases (coronary artery disease, congestive heart failure, symptomatic dysrhythmia) Chronic obstructive pulmonary disease Renal failure Hepatic failure Diabetes mellitus Thyroid dysfunction Chronic neurological disorders (e.g., Parkinson disease, tremor, paresis, etc.) Cancer Chronic infections (e.g., hepatitis, tuberculosis) Significant mental impairment |
| Acute infections | Acute respiratory infections Gastroenteritis Enterocolitis |
| Other conditions | Pregnancy Claustrophobia Treatment with diuretics or other medications influencing body composition |
| All (n = 80) | Female (n = 39) | Male (n = 41) | p-Value | |
|---|---|---|---|---|
| Age (yrs) | 36.31 ± 11.73 (18–71) | 38.36 ± 12.74 (18–71) | 34.97 ± 11.63 (18–56) | 0.15 |
| Height (cm) | 170.38 ± 10.3 (148–192) | 162.38 ± 7.61 (148–179.5) | 180 ± 5.71 (168–192) | <0.001 |
| Waist circumference (cm) | 82.65 ± 14.2 (59–131) | 76.46 ± 13.88 (59–118) | 88.54 ± 11.94 (69.5–131) | <0.001 |
| Body mass (kg) | 73.571 ± 18.457 (39.894–148.8) | 62.501 ± 15.267 (39.894–114.681) | 84.102 ± 14.771 (58.323–148.8) | <0.001 |
| BMI (kg/m2) | 25.23 ± 5.59 (14.98–49.43) | 23.84 ± 6.16 (14.98–44.15) | 26.55 ± 4.7 (19.49–49.43) | 0.03 |
| %BFAP (%) | %BFBIA (%) | %BFAP − %BFBIA (%) | p-Value | |
|---|---|---|---|---|
| All (n = 80) | 26.62 ± 11.35 (7.5–52.55) | 23.55 ± 10.18 (8.36–53.03) | 3.07 ± 5.81 | <0.001 |
| Sex | ||||
| -male (n = 41) | 21.73 ± 9.66 (7.5–46.7) | 20.45 ± 7.57 (11.36–45.74) | 1.28 ± 6.57 | 0.2 |
| -female (n = 39) | 31.77 ± 10.8 (9.6–52.55) | 26.81 ± 11.58 (8.36–53.03.18) | 4.95 ± 4.23 | <0.001 |
| BMI (kg/m2) | ||||
| <18.5 (n = 5) | 18.66 ± 5.83 (9.6–25.05) | 13.59 ± 4.33 (8.36–18.13) | 5.07 ± 2.6 | 0.012 |
| 18.5–24.9 (n = 36) | 23.21 ± 8.5 (7.5–39.2) | 19.17 ± 5.86 (9.83–31.41) | 4.04 ± 5.74 | <0.001 |
| 25–29.9 (n = 29) | 26.66 ± 11.15 (8.65–46.6) | 24.98 ± 8.79 (11.58–41.7) | 1.68 ± 6.64 | 0.18 |
| >30 (n = 10) | 42.79 ± 8.74 (26.6–52.55) | 40.16 ± 9.08 (27.67–53.03) | 2.63 ± 4.02 | 0.07 |
| Central obesity * | ||||
| Absent (n = 52) | 22.21 ± 8.67 (7.5–46.6) | 19.25 ± 6.67 (8.36–39.3) | 2.97 ± 6.21 | <0.001 |
| Present (n = 28) | 34.81 ± 11.32 (11.7–52.55) | 31.54 ± 10.84 (13.93–53.03) | 3.26 ± 5.1 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cretescu, I.; Horhat, R.; Mocanu, V.; Munteanu, O. Bioelectrical Impedance Versus Air-Displacement Plethysmography for Body Fat Measurements in Subjects with Abdominal Obesity: A Comparative Study. Appl. Sci. 2025, 15, 2056. https://doi.org/10.3390/app15042056
Cretescu I, Horhat R, Mocanu V, Munteanu O. Bioelectrical Impedance Versus Air-Displacement Plethysmography for Body Fat Measurements in Subjects with Abdominal Obesity: A Comparative Study. Applied Sciences. 2025; 15(4):2056. https://doi.org/10.3390/app15042056
Chicago/Turabian StyleCretescu, Iuliana, Raluca Horhat, Valeria Mocanu, and Oana Munteanu. 2025. "Bioelectrical Impedance Versus Air-Displacement Plethysmography for Body Fat Measurements in Subjects with Abdominal Obesity: A Comparative Study" Applied Sciences 15, no. 4: 2056. https://doi.org/10.3390/app15042056
APA StyleCretescu, I., Horhat, R., Mocanu, V., & Munteanu, O. (2025). Bioelectrical Impedance Versus Air-Displacement Plethysmography for Body Fat Measurements in Subjects with Abdominal Obesity: A Comparative Study. Applied Sciences, 15(4), 2056. https://doi.org/10.3390/app15042056






