Enhancing Continuum Robotics Accuracy Using a Particle Swarm Optimization Algorithm and Closed-Loop Wire Transmission Model for Minimally Invasive Thyroid Surgery
Abstract
1. Introduction
2. Methods
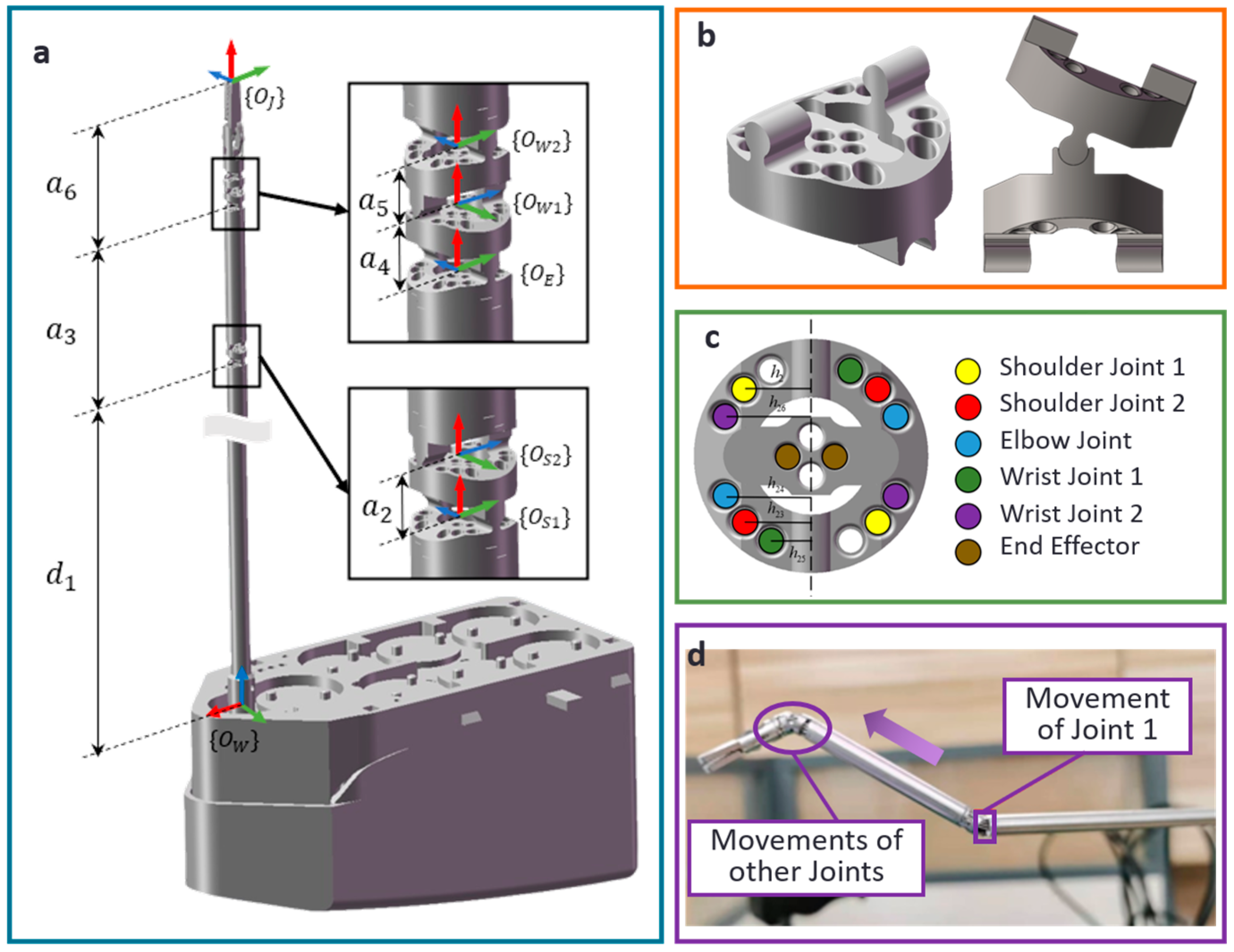
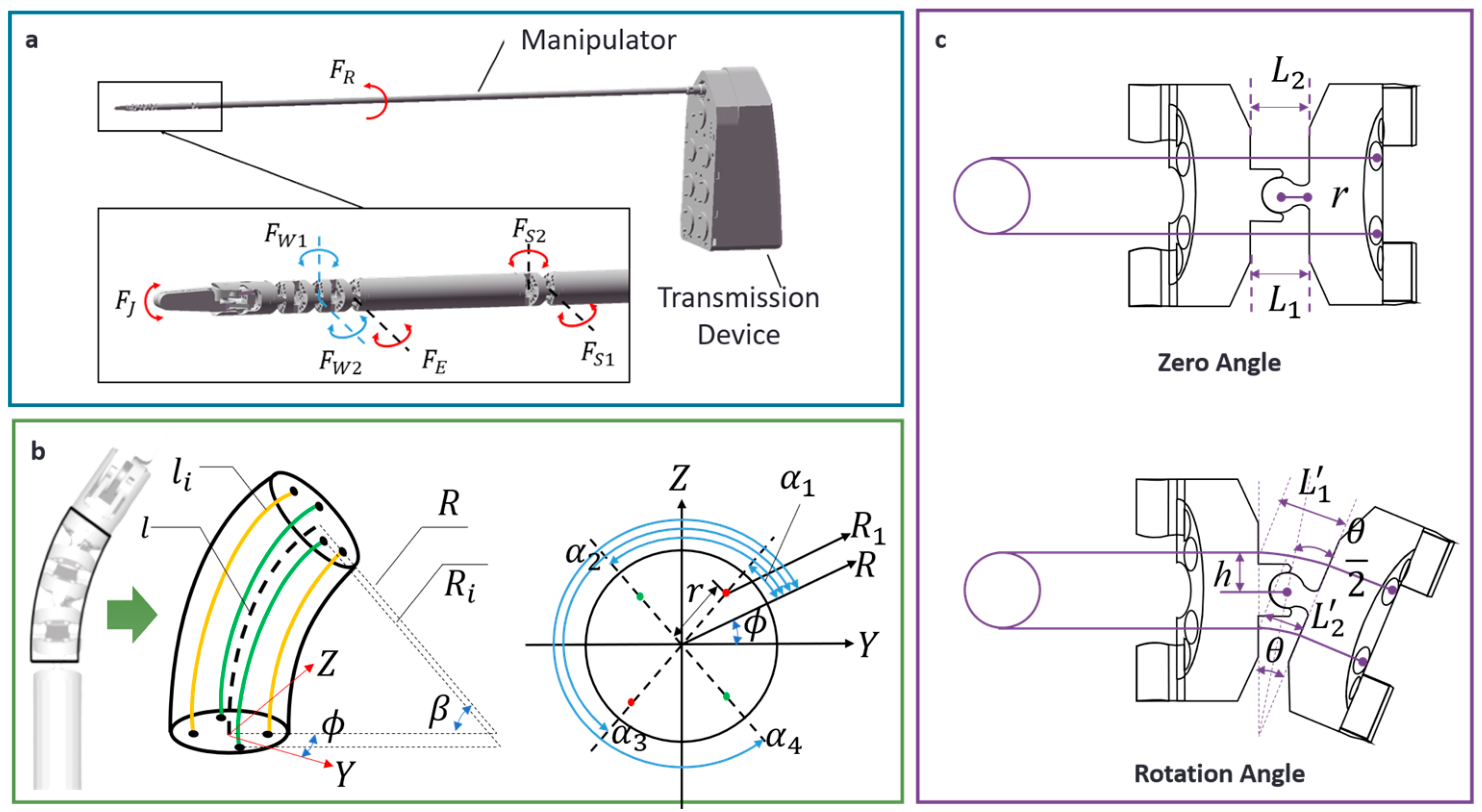
2.1. Decoupling Model for Motion of Multi-Joints
2.2. Inverse Kinematics Solution Based on SOP
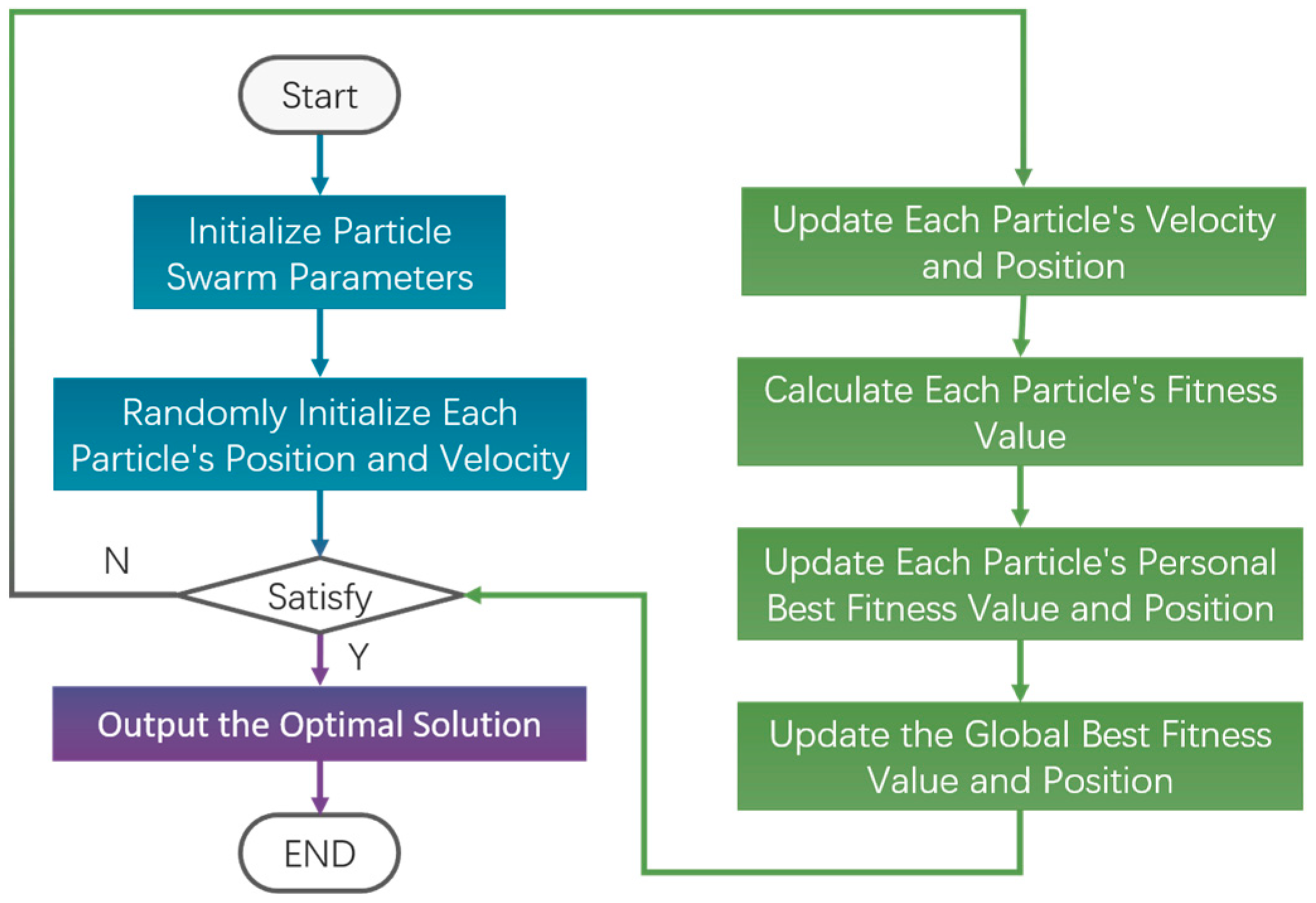
2.3. Particle Swarm Optimization Algorithm for Parameters Identification of Continuum Robots
- (1)
- The position of the i-th particle and its update formulae are:
- (2)
- The velocity of the i-th particle and its update formulae are:
- (3)
- The individual optimal solution for the d-th dimension of the i-th particle at the k-th iteration, denoted is
- (4)
- The group optimal solution for the d-th dimension at the k-th iteration is
- (5)
- The fitness value of the optimal position found by the i-th particle is .
- (6)
- The fitness value of the optimal position found by the swarm is .
2.4. Robotic System for Thyroid Surgery
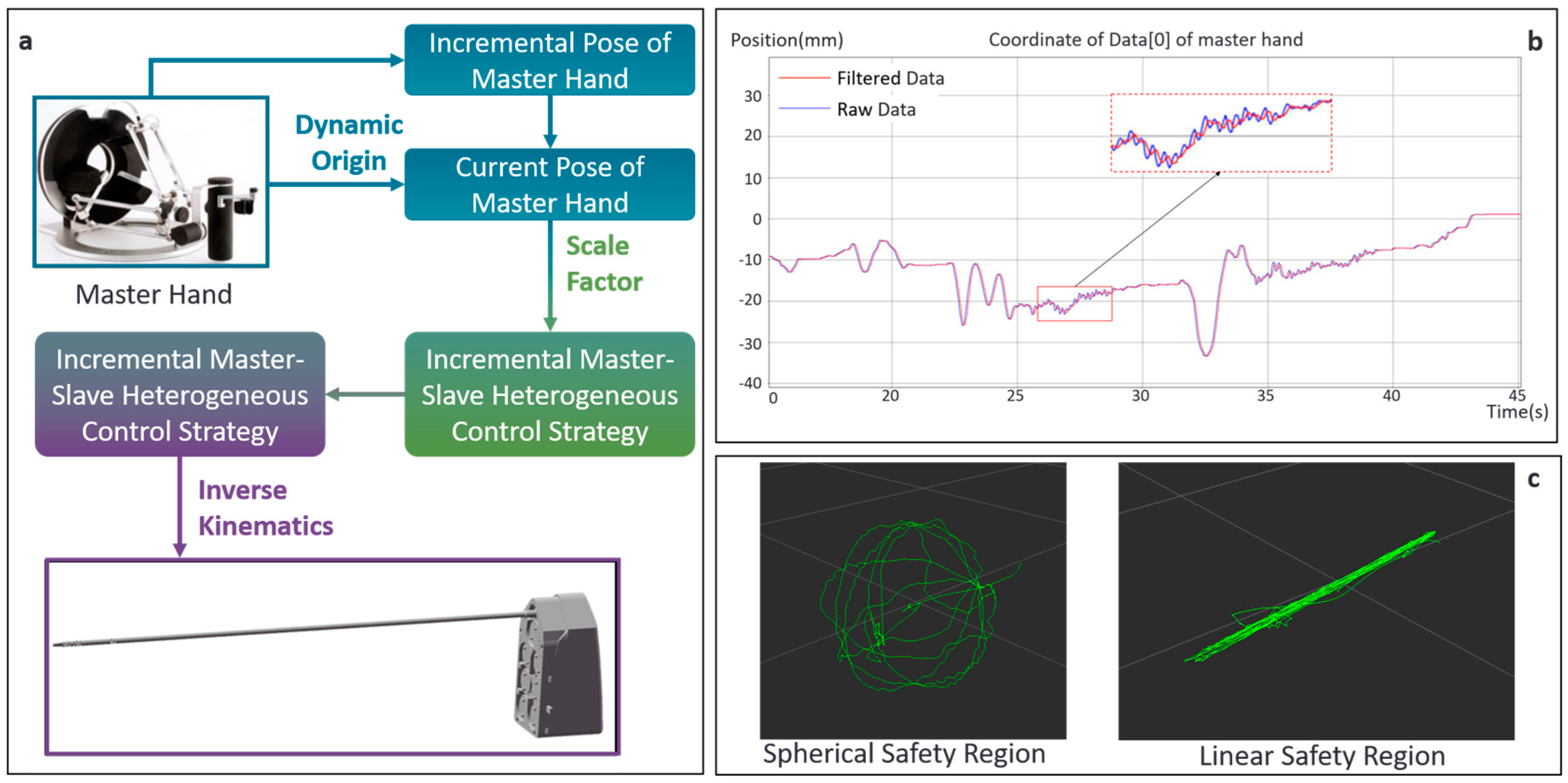
2.4.1. Master–Slave Control System for Thyroid Surgery
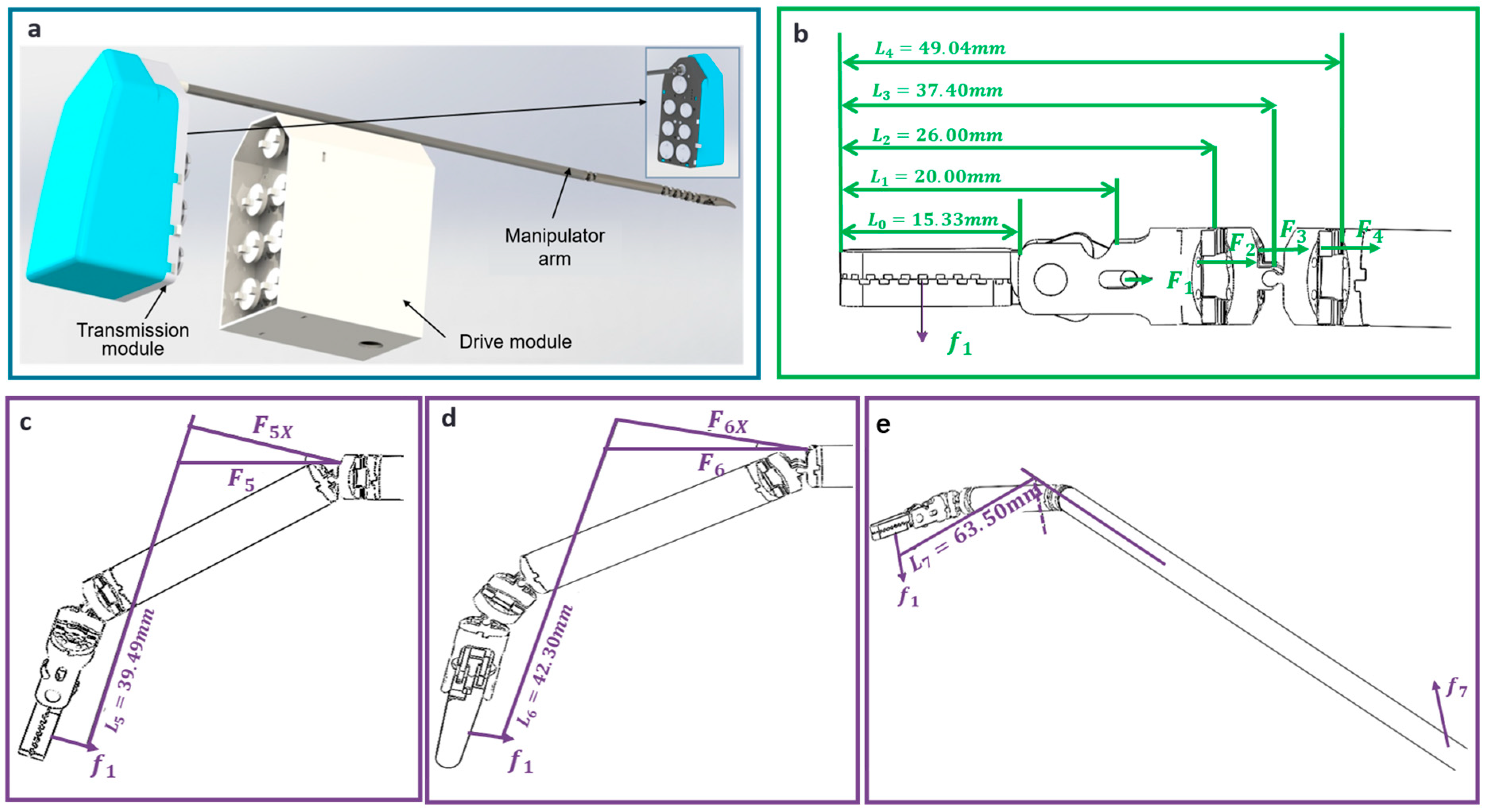
2.4.2. Drive System for the Thyroid Surgical Robot
2.4.3. Master–Slave Safety Constraints Based on Safety Zones
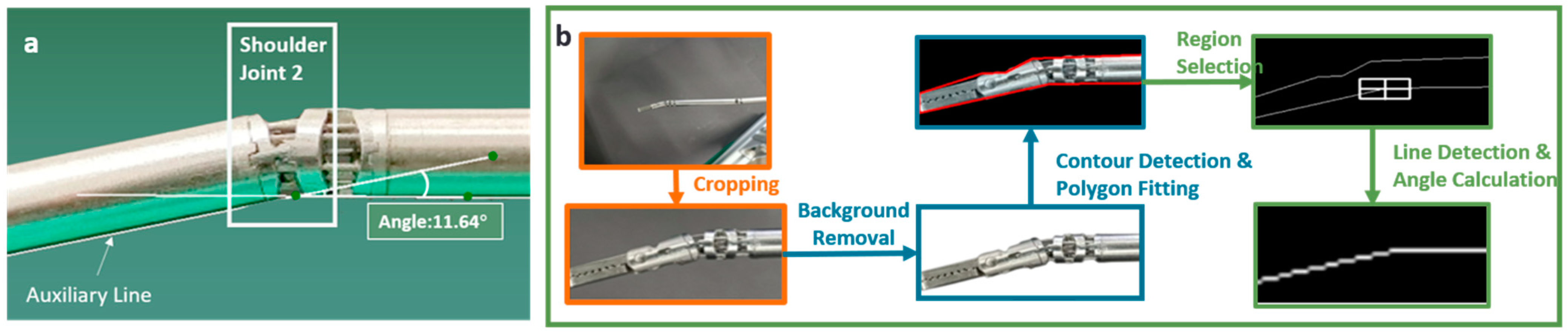
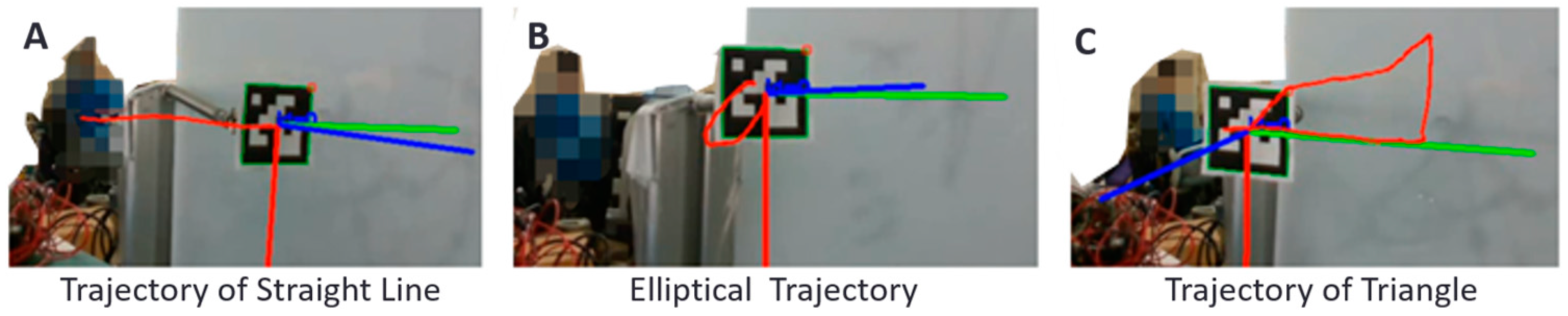
2.5. Vision-Based Measurement Technique for Angles of Joints
3. Experiments
3.1. Verification of Kinematic Model of Surgical Robot
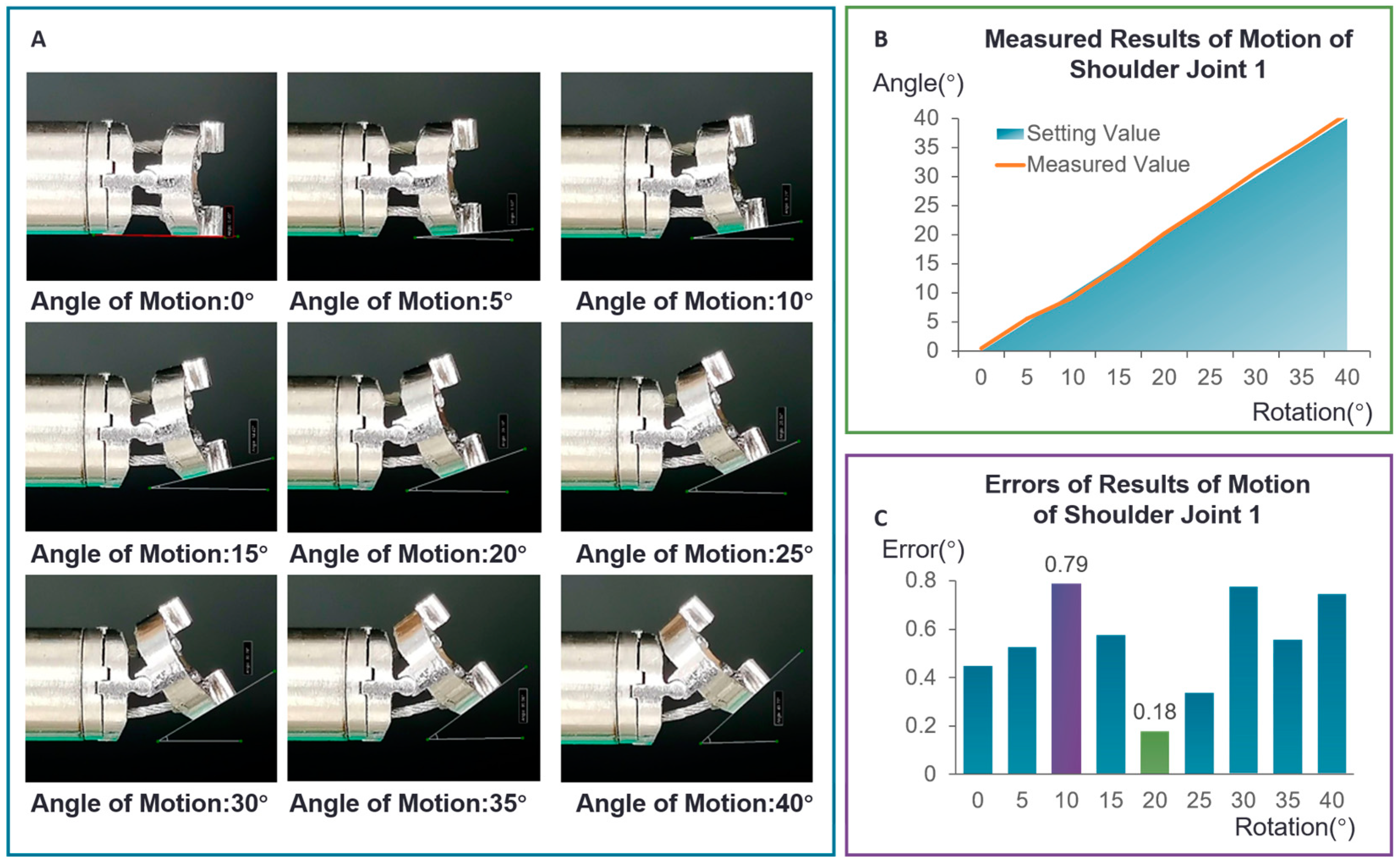
3.1.1. Accuracy Measurement Experiment for Single Joint
- The joint was controlled to move in 5-degree increments within a range of 0 to 40 degrees.
- Camera images were captured at each increment to record joint positions.
- Angle measurements were obtained using the IC Measure software.
3.1.2. Experiment for Decoupling Model for Multi-Joints
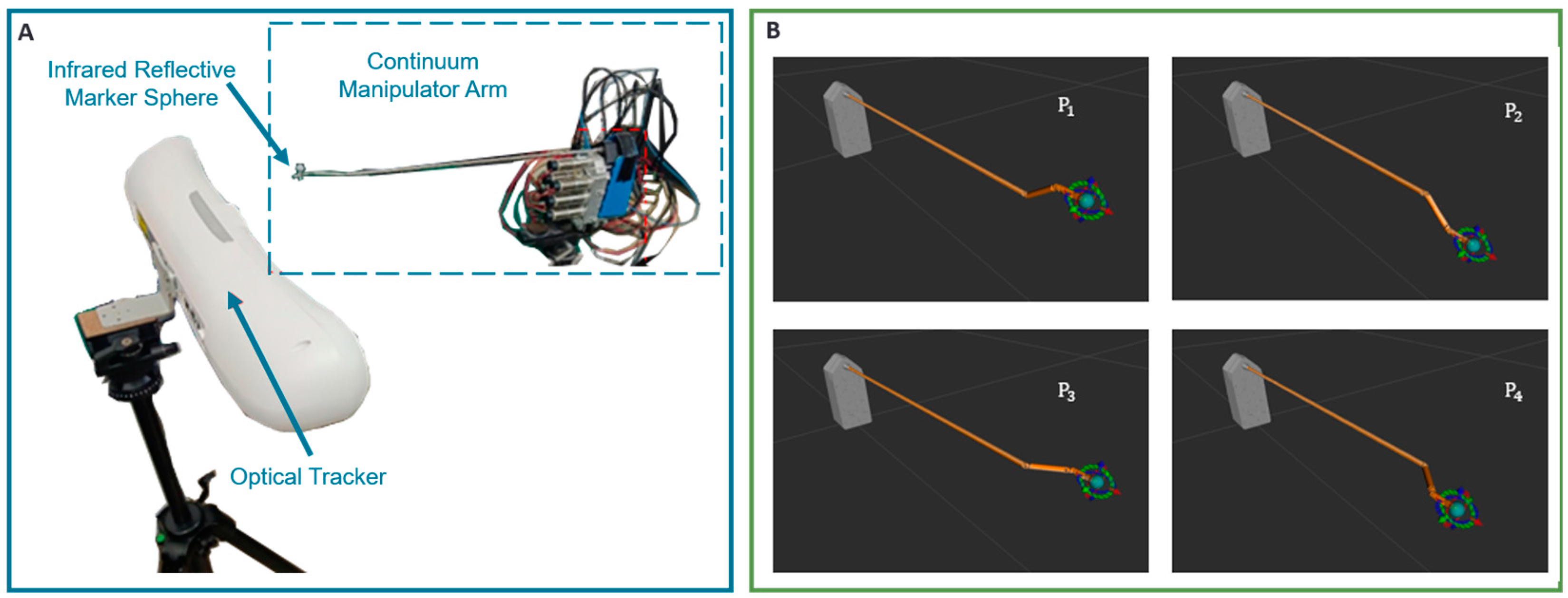
3.2. Implementation of Parameter Identification and Experiments of Repetitive Positioning Accuracy of End-Effector
3.2.1. Accuracy of Vision-Based Measurements
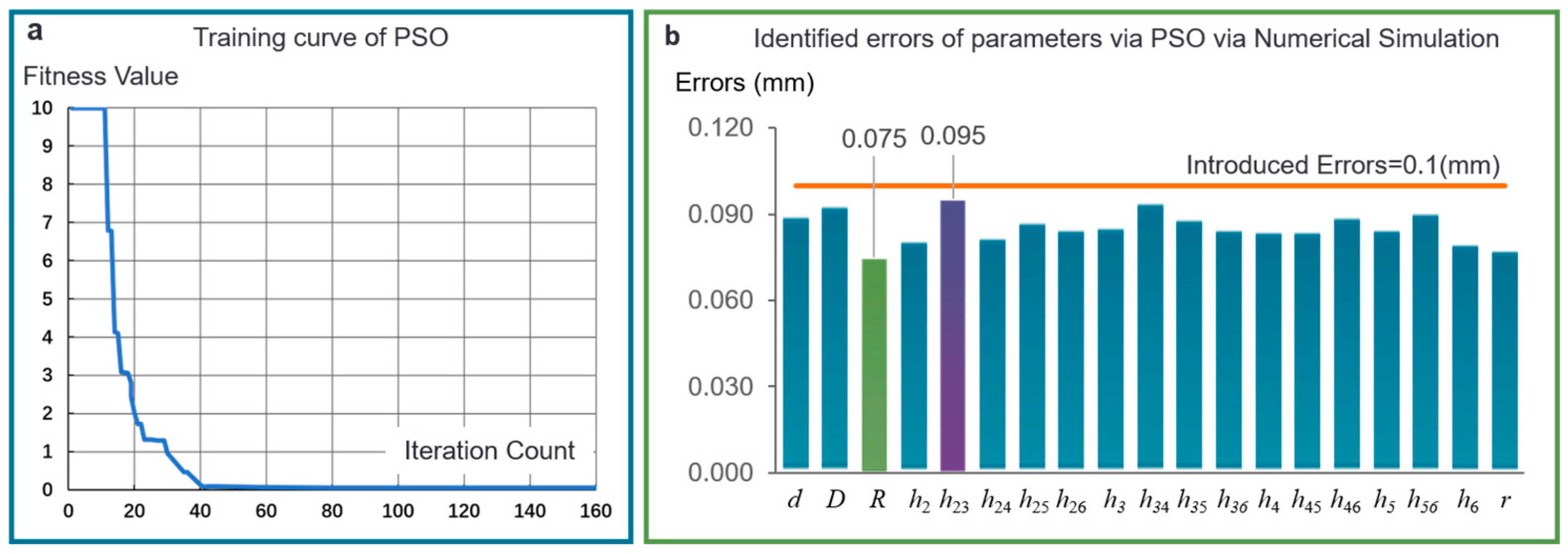
3.2.2. Verification of Parameter Identification Algorithm
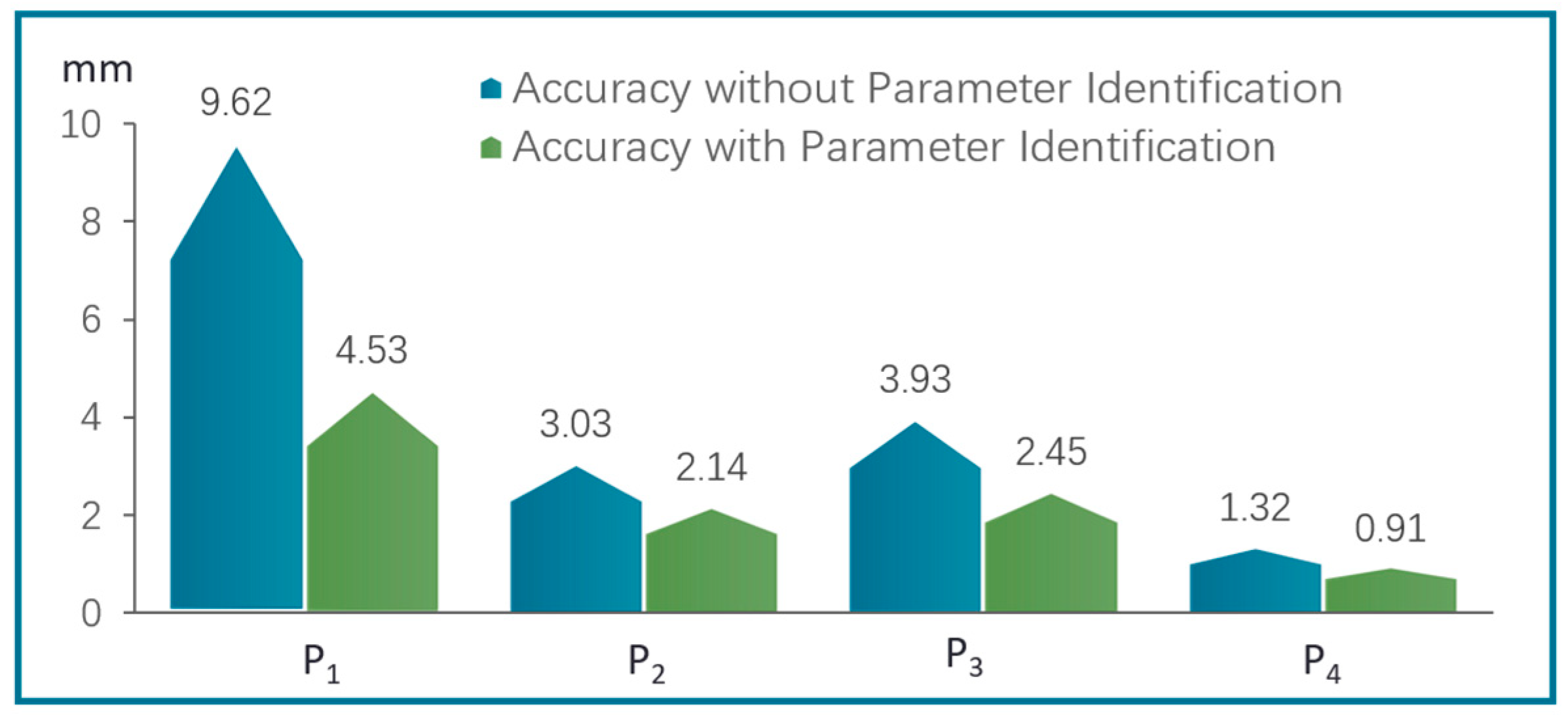
3.2.3. Experiments of Repetitive Positioning Accuracy of Continuum Robots
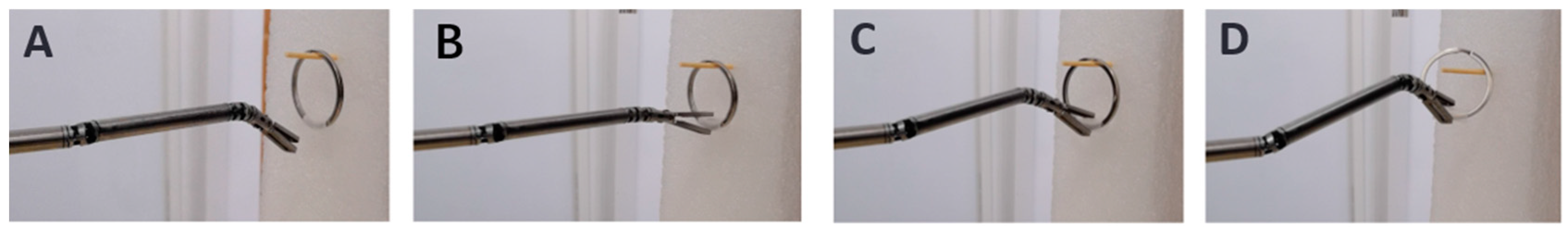
3.3. Experiments of Master–Slave Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, F.; Chen, B.; Gao, S.; She, S. Dynamic model and control for a cable-driven continuum manipulator used for minimally invasive surgery. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2234. [Google Scholar] [CrossRef] [PubMed]
- Douissard, J.; Hagen, M.E.; Morel, P. The da Vinci Surgical System. In Bariatric Robotic Surgery, 2nd ed.; Domene, C., Kim, K., Vilallonga Puy, R., Volpe, P., Eds.; Springer: Cham, Switzerland, 2019; Volume 3, pp. 154–196. [Google Scholar] [CrossRef]
- Matippa, P.; Tyagaraj, K.; Krishnappa, P. Clinical implementation of Versius surgical robotic system in urology. Int. J. Adv. Robotic Innov. Surg. 2023, 1, 7–9. [Google Scholar]
- Prata, F.; Ragusa, A.; Anceschi, U.; Iannuzzi, A.; Tedesco, F.; Cacciatore, L.; Civitella, A.; Tuzzolo, P.; Cirillo, R.; Callè, P.; et al. MP073—Three-arms off-clamp robot-assisted partial nephrectomy with the Hugo RAS system: Introducing a novel technology for advanced robotic renal surgery. Eur. Urol. Open Sci. 2024, 59, S101. [Google Scholar] [CrossRef]
- Ozgur, I.; Cheong, J.Y.; Liska, D.; Holubar, S.; Valante, M.A.; Steele, S.R.; Gorgun, E. Endorobotic submucosal dissection of rectal lesions using the single-port robot da Vinci-SP: Initial experience of the first 10 cases. ANZ J. Surg. 2024, 94, 691–696. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Jing, T.; Wei, Y.; Xiao, C.; Wang, Y.; Fang, Y.; Wu, X.; Tang, S.; Xu, H.; et al. A novel single-port robotic system in urology: A prospective multicenter single-arm clinical trial evaluating feasibility and efficacy of first 50 cases. Asian J. Urol. 2024; in press. [Google Scholar] [CrossRef]
- Cowan, B.; Gomes, C.; Morris, P.; Fryrear, R., II; Petraiuolo, W.; Walton, M.; Alseidi, A.; Horgan, S.; Hagen, M. Robotic technology in surgery; a classification system of soft tissue surgical robotic devices. Surg. Endosc. Other Interv. Technol. 2024, 38, 3645–3653. [Google Scholar] [CrossRef]
- Montanaro, F.; Bertolo, R.; Costantino, S.; De Maria, N.; Veccia, A.; Migliorini, F.; Caliò, A.; Brunelli, M.; Montemezzi, S.; Cerruto, M.A.; et al. Robot-assisted excision of hemangioma of the right renal vein. Urol. Case Rep. 2024, 53, 102651. [Google Scholar] [CrossRef] [PubMed]
- Takemasa, I.; Hamabe, A.; Takenaka, A.; Kobayashi, H.; Mandai, M.; Kinugasa, Y.; Saika, T.; Shimbo, M.; Morizane, S.; Sekiyama, K.; et al. Standardization of robot-assisted pelvic lymph node dissection—Development of a common understanding of regional anatomy and surgical technique based on cross-disciplinary discussion among colorectal surgery, urology, and gynecology. Asian J. Endosc. Surg. 2024, 17, e13274. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.T.; Clanahan, J.M.; Kiani, A.; Vachharajani, N.; Yu, J.; Martens, G.R.; Cullinan, D.R.; Hill, A.L.; Olumba, F.; Matson, S.C.; et al. Robotic performance metrics model fellow proficiency in living donor nephrectomy. J. Robotic Surg. 2024, 18, 271. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, T.; Yokota, K.; Munekage, M.; Maeda, H.; Kitagawa, H.; Okamoto, K.; Uchida, K.; Sato, T.; Kobayashi, M.; Hanazaki, K.; et al. Robot-assisted surgery for gastrointestinal cancer using indocyanine green conjugated endoscopic marking clip under firefly fluorescence imaging. Anticancer Res. 2024, 44, 3937–3943. [Google Scholar] [CrossRef] [PubMed]
- Padrez, Y.; Golubewa, L.; Timoshchenko, I.; Enache, A.; Eftimie, L.G.; Hristu, R.; Rutkauskas, D. Machine learning-based diagnostics of capsular invasion in thyroid nodules with wide-field second harmonic generation microscopy. Comput. Med. Imaging Graph. 2024, 117, 102440. [Google Scholar] [CrossRef] [PubMed]
- Viduetsky, A.; Herrejon, C.L. Sonographic evaluation of thyroid size: A review of important measurement parameters. J. Diagn. Med. Sonogr. 2019, 35, 206–210. [Google Scholar] [CrossRef]
- Sun, L.; Chen, X. Flexible continuum robot system for minimally invasive endoluminal gastrointestinal endoscopy. Machines 2024, 12, 370. [Google Scholar] [CrossRef]
- Craig, J.J. Introduction to Robotics: Mechanics and Control; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 1986. [Google Scholar]
- Harrison, H.R. Quaternions and Rotation Sequences: A Primer with Applications to Orbits, Aerospace and Virtual Reality; Kuipers, J.B., Ed.; Princeton University Press: Princeton, NJ, USA, 1999; p. 372. [Google Scholar]
- Warner, F.W. Foundations of Differentiable Manifolds and Lie Groups; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Crane, C.D., III; Griffis, M.; Duffy, J. Screw Theory and Its Application to Spatial Robot Manipulators; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Jin, X.; Zhang, H.; Wang, L.; Li, Q. Review on control strategies for cable-driven parallel robots with model uncertainties. Chin. J. Mech. Eng. 2024, 37, 1–17. [Google Scholar] [CrossRef]
- Zhang, G.; Su, J.; Du, F.; Zhang, X.; Li, Y.; Song, R. Composite continuum robots: Accurate modeling and model reduction. Int. J. Mech. Sci. 2024, 276, 109342. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Li, H.; Liu, B.; He, Q. Kinematics modeling and simulation of a flexible surgical robotic instrument with a multi-joint wrist. In Proceedings of the 12th International Conference on CYBER Technology in Automation, Control, and Intelligent Systems (CYBER), Changbaishan, China, 27–31 July 2022; pp. 821–826. [Google Scholar] [CrossRef]
- Sciavicco, L.; Siciliano, B. Modelling and Control of Robot Manipulators; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Nocedal, J.; Wright, S.J. (Eds.) Numerical Optimization; Springer: New York, NY, USA, 1999. [Google Scholar]
- Spong, M.W.; Hutchinson, S.; Vidyasagar, M. Robot Modeling and Control; John Wiley & Sons: New York, NY, USA, 2020. [Google Scholar]
- Murphy, K.P. Machine Learning: A Probabilistic Perspective; MIT Press: Cambridge, MA, USA, 2012. [Google Scholar]
- LaValle, S.M. Planning Algorithms; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Knežević, N.; Petrović, M.; Jovanović, K. Cartesian stiffness shaping of compliant robots—Incremental learning and optimization based on sequential quadratic programming. Actuators 2024, 13, 32. [Google Scholar] [CrossRef]
- Veluvolu, K.C.; Tan, U.X.; Latt, W.T.; Shee, C.Y.; Ang, W.T. Adaptive filtering of physiological tremor for real-time compensation. In Proceedings of the 2008 IEEE International Conference on Robotics and Biomimetics, Bangkok, Thailand, 22–25 February 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 524–529. [Google Scholar]
- Guo, N.; Wang, T.; Yang, B.; Hu, L.; Liu, H.; Wang, Y. An online calibration method for Microsoft HoloLens. IEEE Access 2019, 7, 101795–101803. [Google Scholar] [CrossRef]
- Thielmann, S.; Seibold, U.; Haslinger, R.; Passig, G.; Bahls, T.; Jörg, S.; Nickl, M.; Nothhelfer, A.; Hagn, U.; Hirzinger, G. MICA—A new generation of versatile instruments in robotic surgery. In Proceedings of the IROS 2010, IEEE International Conference on Intelligent Robots and Systems, Taipei, Taiwan, 18–22 October 2010. [Google Scholar]
- Smith, T.J.; Smith, T.R.; Faruk, F.; Bendea, M.; Kumara, S.T.; Capadona, J.R.; Hernandez-Reynoso, A.G.; Pancrazio, J.J. Real-time assessment of rodent engagement using ArUco markers: A scalable and accessible approach for scoring behavior in a nose-poking Go/No-Go task. eNeuro 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Ding, J. Research on Dynamic Parameter Identification of Robot. Modul. Mach. Tool Autom. Manuf. Tech. 2023, 5, 10–13. [Google Scholar]
- Zhong, P.; Wang, X.; Zhang, C.; Zhang, Z.; Wang, X.; Liu, J. Robot Dynamic Parameter Identification Based on Chaotic Particle Swarm Optimization Algorithm. Instrum. Tech. Sens. 2023, 8, 107–113. [Google Scholar]
- Leboutet, Q.; Roux, J.; Janot, A.; Guadarrama-Olvera, J.R.; Cheng, G. Inertial Parameter Identification in Robotics: A Survey. Appl. Sci. 2021, 11, 4303. [Google Scholar] [CrossRef]
- Qi, F.; Zhang, H.; Pei, H.; Chen, B.; Wu, H. Drive Error Compensation of Continuum Manipulator Based on Force Transfer Model. Trans. Chin. Soc. Agric. Mach. 2023, 54, 402–411. [Google Scholar]



| Index | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| Name | Rotary Joint | Shoulder Joint 1 | Shoulder Joint 2 | Elbow Joint | Wrist Joint 1 | Wrist Joint 2 | End Fixture | |
| 1 | Rotary joint | ◎ | × | × | × | × | × | × |
| 2 | Shoulder joint 1 | × | ◎ | √ | √ | √ | √ | × |
| 3 | Shoulder joint 2 | × | × | ◎ | √ | √ | √ | √ |
| 4 | Elbow joint | × | × | × | ◎ | √ | √ | × |
| 5 | Wrist joint 1 | × | × | × | × | ◎ | √ | √ |
| 6 | Wrist joint 2 | × | × | × | × | × | ◎ | × |
| Index of Joint | Name | Required Force (N) | ) | Reduction Ratios | ||
|---|---|---|---|---|---|---|
| 1 | Effector Ender | 20.0 | 0.81 | 274.2 | 822.6 | 1:89 |
| 2 | Wrist joint 2 | 26.00 | 2.35 | 140.5 | 421.5 | 1:72 |
| 3 | Wrist joint 1 | 37.40 | 2 | 267.7 | 803.1 | 1:72 |
| 4 | Elbow joint | 49.04 | 2.46 | 307.2 | 921.6 | 1:89 |
| 5 | Shoulder joint 2 | 38.49 | 2.46 | 299.6 | 898.8 | 1:72 |
| 6 | Shoulder joint 1 | 19.09 | 1.97 | 411.2 | 1233.6 | 1:89 |
| 7 | Rotary joint | 63.5 | 3 | 381 | 762 | 1:72 |
| Measuring Times | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Theoretical angle (°) | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 |
| Measuring angle (°) | 0.45 | 5.53 | 9.21 | 14.42 | 20.18 | 25.34 | 30.78 | 35.56 | 40.75 |
| Absolute error (°) | 0.45 | 0.53 | 0.79 | 0.58 | 0.18 | 0.34 | 0.78 | 0.56 | 0.75 |
| Parameter Name | Inertia Weight | Individual Learning Factor | Social Learning Factor | Random Number | Random Number | Number of Particles | Search Space Dimension | Number of Iterations |
|---|---|---|---|---|---|---|---|---|
| Parameter Value | 0.5 | 2 | 2 | 0.8 | 0.6 | 100 | 19 | 160 |
| Parameter Name | Value of Parameters in Numerical Simulation (mm) | Value of Parameters in Real Experiment (mm) | ||||
|---|---|---|---|---|---|---|
| Theoretical Value | Introduced Error | Errors of Identification | Theoretical Value | Errors of Identification | Actual Value | |
| d | 14 | 0.1 | 0.0903 | 14 | 0.006 | 13.994 |
| D | 28 | 0.1 | 0.0938 | 28 | 0.005 | 27.995 |
| R | 3 | 0.1 | 0.0749 | 3 | 0.005 | 2.995 |
| h2 | 2.18 | 0.1 | 0.0814 | 2.18 | 0.007 | 2.173 |
| h23 | 1.01 | 0.1 | 0.0952 | 1.01 | 0.007 | 1.003 |
| h24 | 2.18 | 0.1 | 0.0826 | 2.18 | 0.006 | 2.174 |
| h25 | 1.7 | 0.1 | 0.088 | 1.7 | 0.008 | 1.692 |
| h26 | 1.7 | 0.1 | 0.0855 | 1.7 | 0.008 | 1.692 |
| h3 | 2.18 | 0.1 | 0.0863 | 2.18 | 0.006 | 2.174 |
| h34 | 1.01 | 0.1 | 0.0949 | 1.01 | 0.007 | 1.003 |
| h35 | 1.7 | 0.1 | 0.089 | 1.7 | 0.007 | 1.693 |
| h36 | 1.7 | 0.1 | 0.0853 | 1.7 | 0.009 | 1.692 |
| h4 | 2.18 | 0.1 | 0.0848 | 2.18 | 0.006 | 2.174 |
| h45 | 1.7 | 0.1 | 0.0846 | 1.7 | 0.006 | 1.694 |
| h46 | 1.7 | 0.1 | 0.0897 | 1.7 | 0.006 | 1.694 |
| h5 | 1.7 | 0.1 | 0.0856 | 1.7 | 0.007 | 1.693 |
| h56 | 1.7 | 0.1 | 0.0914 | 1.7 | 0.005 | 1.695 |
| h6 | 1.7 | 0.1 | 0.0803 | 1.7 | 0.007 | 1.693 |
| r | 0.8 | 0.1 | 0.078 | 0.8 | 0.007 | 0.793 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, N.; Zhang, H.; Li, X.; Cui, X.; Liu, Y.; Pan, J.; Song, Y.; Zhang, Q. Enhancing Continuum Robotics Accuracy Using a Particle Swarm Optimization Algorithm and Closed-Loop Wire Transmission Model for Minimally Invasive Thyroid Surgery. Appl. Sci. 2025, 15, 2170. https://doi.org/10.3390/app15042170
Guo N, Zhang H, Li X, Cui X, Liu Y, Pan J, Song Y, Zhang Q. Enhancing Continuum Robotics Accuracy Using a Particle Swarm Optimization Algorithm and Closed-Loop Wire Transmission Model for Minimally Invasive Thyroid Surgery. Applied Sciences. 2025; 15(4):2170. https://doi.org/10.3390/app15042170
Chicago/Turabian StyleGuo, Na, Haoyun Zhang, Xingshuai Li, Xinnan Cui, Yang Liu, Jiachen Pan, Yajuan Song, and Qinjian Zhang. 2025. "Enhancing Continuum Robotics Accuracy Using a Particle Swarm Optimization Algorithm and Closed-Loop Wire Transmission Model for Minimally Invasive Thyroid Surgery" Applied Sciences 15, no. 4: 2170. https://doi.org/10.3390/app15042170
APA StyleGuo, N., Zhang, H., Li, X., Cui, X., Liu, Y., Pan, J., Song, Y., & Zhang, Q. (2025). Enhancing Continuum Robotics Accuracy Using a Particle Swarm Optimization Algorithm and Closed-Loop Wire Transmission Model for Minimally Invasive Thyroid Surgery. Applied Sciences, 15(4), 2170. https://doi.org/10.3390/app15042170






