Abstract
Background: The aquaculture sector is vital for food supply and marine species conservation. However, vibriosis poses significant threats, affecting fish and invertebrates. Vibrio harveyi outbreaks are increasing due to global warming-driven expansion of virulent strains. Methods: This study investigates the bactericidal potential of in situ-generated electrolyzed water (EW) as a complementary strategy to antibiotic therapy. Strains of V. harveyi isolated from diseased fish were exposed to EW under varying conditions of salinity (1.5 and 3%), pH (5, 6.5, and 7.5), and free available chlorine (FAC) (5, 20, 25, and 125 ppm) to reduce bacterial concentrations. Results: EW demonstrated high bactericidal potential at 20–25 ppm FAC and non-acidic pH, reducing bacterial populations by over four logarithmic units within 15 min. Efficacy diminished when EW was stored for days. Conclusions: EW shows a promising disinfection method during vibriosis outbreaks and as a preventive measure during stress in aquaculture. Its advantages include on-site production and avoidance of concentrated chemicals, which minimize health risks.
1. Introduction
The aquaculture sector has undergone exponential growth since the 1980s, becoming a crucial component in ensuring global food security [1], especially in low-income and food-scarce nations [2]. However, this aspect is threatened by the presence of pathogenic microorganisms affecting fish crops, coupled with the limitation of treatments that do not generate resistance or have negative effects on aquatic animals.
To combat bacterial diseases, various treatments like antibiotics or heat treatments are commonly used, as well as other solutions with a broad spectrum of action, including viruses and parasites [2], although preventive measures such as vaccination are desirable [3]. The overuse of antibiotics has led to resistance in numerous pathogenic bacteria, presenting significant challenges in controlling diseases. Meanwhile, employing heat treatments leads to increased energy and economic costs. Thus, the use of disinfection technologies must also be considered. The selection of the appropriate disinfectant must consider multiple aspects, mainly regarding safety and toxicity for cultured species [4].
Faced with these challenges, electrolyzed water (EW) emerges as a sustainable and safe alternative with remarkable bactericidal capacity [5]. Electrolyzed water is a solution produced through the process of electrolysis, which involves passing an electric current through water containing salts or minerals. This process separates the water into acidic and alkaline components. It is used in various industries, including food production, healthcare, and agriculture, for cleaning surfaces, disinfecting water, and reducing microbial contamination. EW offers a sustainable, eco-friendly, and advantageous alternative to traditional chemical disinfectants [6,7,8].
In contrast to conventional methods like antibiotics or vaccines, EW avoids the need for additional chemicals, making it environmentally friendly and suitable for human application and for scenarios where the use of hazardous chemicals is not recommendable [8]. Other chlorine species, like commercial bleach and chlorine, are used in pool water to maintain healthiness and in odontology, respectively. Moreover, the elimination of reliance on disinfectant chemicals leads to a reduction in the production of toxic waste, which during EW procedures is negligible, thereby contributing to the establishment of a more pristine and sustainable environment. Additionally, it is cost-effective to generate [7,9,10], compared to methodologies like ozone or UV [11]. This advantage is one of the most important, along with respect for the environment as it degrades over time. Finally, on-site production capability and ease and safety in its application in environments involving living organisms transform EW into a potent weapon against pathogens [12].
In terms of its impact on bacteria, it is emphasized that hypochlorous acid (HOCl), an essential component of electrolyzed water, generates hydroxyl radicals, thus triggering a relevant bactericidal effect with significant relevance [5]. The optimization of operational conditions becomes a crucial goal, mainly regarding the conditions under which further disinfection will be achieved. The main parameters are pH, recommended between 2.5 and 9 [12], and the concentration of free available chlorine (FAC).
The disinfection of bacteria using hypochlorous acid, or free chlorine, has been studied for decades [13]. Following the introduction of EW to a bacterial culture, the noticeable effects are the morphological alteration in the cell surface and the permeabilization of the cytoplasmic membrane [14]. At low chlorine concentrations, surviving microorganisms experience damage rather than inactivation. Moreover, chlorine targets and disrupts the bacterial protective barrier, comprising the cell wall and cell membrane [15,16]. This disinfectant has even been tested in the fight against COVID-19 and is recommended by the United States Environmental Protection Agency [17]. Its most developed application has focused on the disinfection and elimination of bacteria and viruses, although the effect is strongly dependent on the nature of the microorganism. Different trials evidenced that in the presence of EW, Escherichia coli population underwent a significant reduction (4.83 log) in colony-forming units (CFUs) at FAC of 43 ppm in only 1 min [18], and even the E. coli O157:H7 strain had a notable reduction in 3 min with FAC of 1.5 ppm [1]. Salmonella spp. also showed high susceptibility to EW, but Listeria innocua needed FAC of 89 ppm to achieve the same reduction (5 log) [12,19].
Additionally, research on efficacy against viruses, including the avian flu virus, has yielded highly promising results. In fact, a reduction of up to 99% was achieved using a sample of EW with FAC of 10 ppm [20].
Although it is a less-explored area, EW has demonstrated its promising application in aquaculture. Hypochlorous acid at 25 ppm reduced the concentration of the bacterial pathogen Vibrio vulnificus, which causes warm water vibriosis in fish, by 5 log units [21]. These results suggest that EW could find versatile applications in various industrial processes, clinical environments, surface cleaning, among others.
V. harveyi, a Gram-negative marine bacterium, stands out as a significant pathogen for different marine organisms of economic relevance, such as fish, mollusks, and crustaceans [22,23]. Due to climate change, it has become an aquaculture-emerging pathogen causing economic and biological losses. Moreover, in the last three decades, coinciding with the rapid development of shrimp aquaculture, V. harveyi has risen as a significant factor in the occurrence of diseases, causing luminous vibriosis and other syndromes with massive mortality in larvae and adult shrimps, especially in regions of South America and Asia [22,24]. The abundance of V. harveyi in water and aquatic biofilms is another factor that can exacerbate the development of the disease in different cultured fish species such as seabass [25,26].
Building on previous studies, we hypothesize that electrolyzed water (EW) could serve as an effective disinfection tool in aquaculture systems to control significant diseases like vibriosis caused by V. harveyi, a major threat to both invertebrate and vertebrate marine animals. Our aim is to optimize chlorine concentrations, reducing them to levels suitable for future in vivo applications, ensuring the effective elimination of bacteria without negatively impacting marine species.
2. Materials and Methods
2.1. Generation of Electrolyzed Water
The electrolyzed water was produced using a LAMI-50 electrolyzed water generator, supplied by Aquactiva Solutions (Valencia, Spain). This generator is equipped with a membrane electrolysis cell. A solution of saturated NaCl and deionized water was employed, eliminating the need for pre-treatment of the inlet water.
For the characterization of the electrolyzed water, parameters such as pH, oxidation–reduction potential (ORP), and conductivity were measured using the pH/Ion/DO Multimeter SG68 provided by Mettler Toledo (Columbus, OH, USA). The FAC concentration was determined using the Handheld Colorimeter Chlorine UHR, supplied by Hanna Instruments (RI Woonsocket, Providence County, RI, USA). Each sample was prepared at the desired FAC level by adjusting the intensity value applied to the cell and by dilution. The characterization values are given in Table 1. If the desired FAC level was not obtained, a dilution was carried out to obtain the exact concentration. The %NaCl was adjusted by adding salt and measuring the conductivity of the sample until the value corresponding to this percentage in deionized water was obtained.

Table 1.
Physico-chemical parameters of the electrolyzed water samples used.
2.2. Bacterial Strains and Cultures
In this study, strains of V. harveyi isolated from diseased cultured sea bass (Dicentrarchus labrax) in Spain have been used (Table 2). Strains were isolated from the internal organs of the fish, characterized by using phenotypic and genetic methods in our laboratory, and stored in our culture bank. Strains were routinely grown in Tryptic soy agar with 1% NaCl (TSA-1) plates at 28 °C for 24 h and maintained in frozen stocks at −80 °C in LB-1 with 20% of glycerol until use. Overnight, Luria Bertani broth with 1% NaCl (LB-1) cultures at 28 °C were used for the assays with EW. The C2 strain, highly virulent for fish, was selected to optimize experimental conditions in some parts of this study.

Table 2.
Origin of the Vibrio harveyi strains used in this study.
2.3. Bactericidal Assay
Overnight cultures (at 28 °C) in LB-1 were diluted with the same culture medium to achieve a concentration of 107 CFU/mL. Subsequently, 10 mL of the bacterial suspension was mixed with 90 mL of the electrolyzed water sample (Table 1) to achieve a starting concentration around 106 CFU/mL, and the bacterial viability was measured at 1, 5, 10, and 15 min using drop plate counting on TSA-1 plates [27]. Three 10 uL drops for each dilution were quickly placed at plates in the selected time points. All assays were performed at room temperature by triplicate. To determine the evolution of the free available chlorine (FAC) along the assay, mixtures were sampled at 1, 5, 10, and 15 min, and the FAC was measured using the Handheld Colorimeter Chlorine UHR (Hanna Instruments). In addition, a test using PBS instead of LB-1 was performed to demonstrate the effect of organic matter on the electrolyzed water.
2.4. Statistical Analysis
Experimental data were analyzed by IBM SPSS Statistics package version 28.0.0.1. The variance of the results was assessed using one-way ANOVA, and the significance of the differences was examined through Duncan’s multiple comparison test. The values were presented as the mean ± SD (standard deviation) of replicates. Statistical significance was tested at a significance level of p < 0.05. Furthermore, to optimize the experimental conditions and reveal the relevance of the independent variables, the biocidal activity together with the standardized values of the chemical characteristics of the solutions and time were fitted using a multivariate general linear model.
3. Results
3.1. Evaluation of the Effect of the Main Operational Labels in the Biocidal Activity
To evaluate the operational parameters involved in the use of EW, key variables such as FAC, time, pH, salinity, and the C2 strain were selected (Table 2). Specifically, pH values of 5, 6.5, and 7.5 were tested due to their similarity to real conditions in various aquaculture facilities [12]. Moreover, FAC solutions of 5, 25, and 125 ppm were assessed to cover a broad range of potential applications. Salinities of 1.5% and 3% NaCl were chosen to simulate brackish and marine water conditions, where the bacterium thrives. Importantly, time was limited to 15 min to minimize potential animal exposure to EW.
Regarding the initial trials, survival percentages exceeding 100% were considered outliers since differences in colony counts were negligible. The outcomes of these trials are summarized in Table 3.

Table 3.
Survival rate (%) of Vibrio harveyi C2 obtained during the evaluation of the operational labels.
In terms of FAC concentration, the highest level tested (125 ppm) demonstrated an almost immediate effectiveness in bacterial reduction (less than 1 min), irrespective of pH. While no significant differences were observed between salinities, a slightly higher reduction was noted at 3%. Conversely, at low FAC concentrations (5 ppm), the effect of EW was minimal. At 25 ppm, the bacterial population decreased significantly, with survival percentages varying according to disinfection time (Table 3).
Additional trials conducted with bacterial suspensions in PBS instead of LB-1 medium revealed rapid reductions in bacterial populations at 25 ppm of free chlorine (SAEW, NEW, and SBEW). Remarkably, within the first minute, survival rates dropped below 0.01% in all cases. suggesting that organic matter in the medium influences EW’s antibacterial activity.
Building on these findings, further studies were conducted with FAC concentrations ranging from 15 to 25 ppm to determine the minimum effective disinfection concentration. These solutions were prepared at pH 7.5 and salinities of 1.5% or 3% (SBEW15, SBEW20, and SBEW25), conditions under which V. harveyi thrives. The results at 5, 10, and 15 min are shown in Table 3. While SBEW15 was ineffective (survival rate > 65%), SBEW20 and SBEW25 exhibited strong bactericidal effects. Notably, higher salinity (3%) enhanced disinfection. The optimal conditions identified were SBEW25 for 10 to 15 min, although extended exposure to SBEW20 also achieved satisfactory results.
To further understand the impact of the variables, a multivariate general linear model analysis was performed. This analysis included independent variables such as FAC concentration, pH, salinity, time, and their first-order combinations (Table 1). The results revealed significant contributions (p < 0.05) from FAC, time, and pH–salinity interactions to bacterial reduction (Table 4). Moreover, combinations such as FAC–time and pH–FAC were significant, indicating that free chlorine’s effectiveness is influenced by environmental pH and salinity.

Table 4.
Results on statistical analysis applying a multivariate general linear model.
Finally, standard beta coefficients highlighted FAC, time, and pH–salinity as the main drivers of biocidal activity, with FAC and time being the most influential variables. These findings align with expectations, as higher concentrations and longer treatments enhance effectiveness. However, no synergies between variables were observed (Table 5).

Table 5.
Results of the linear regression analyses of beta coefficients.
3.2. Evaluation of Residual Chlorine
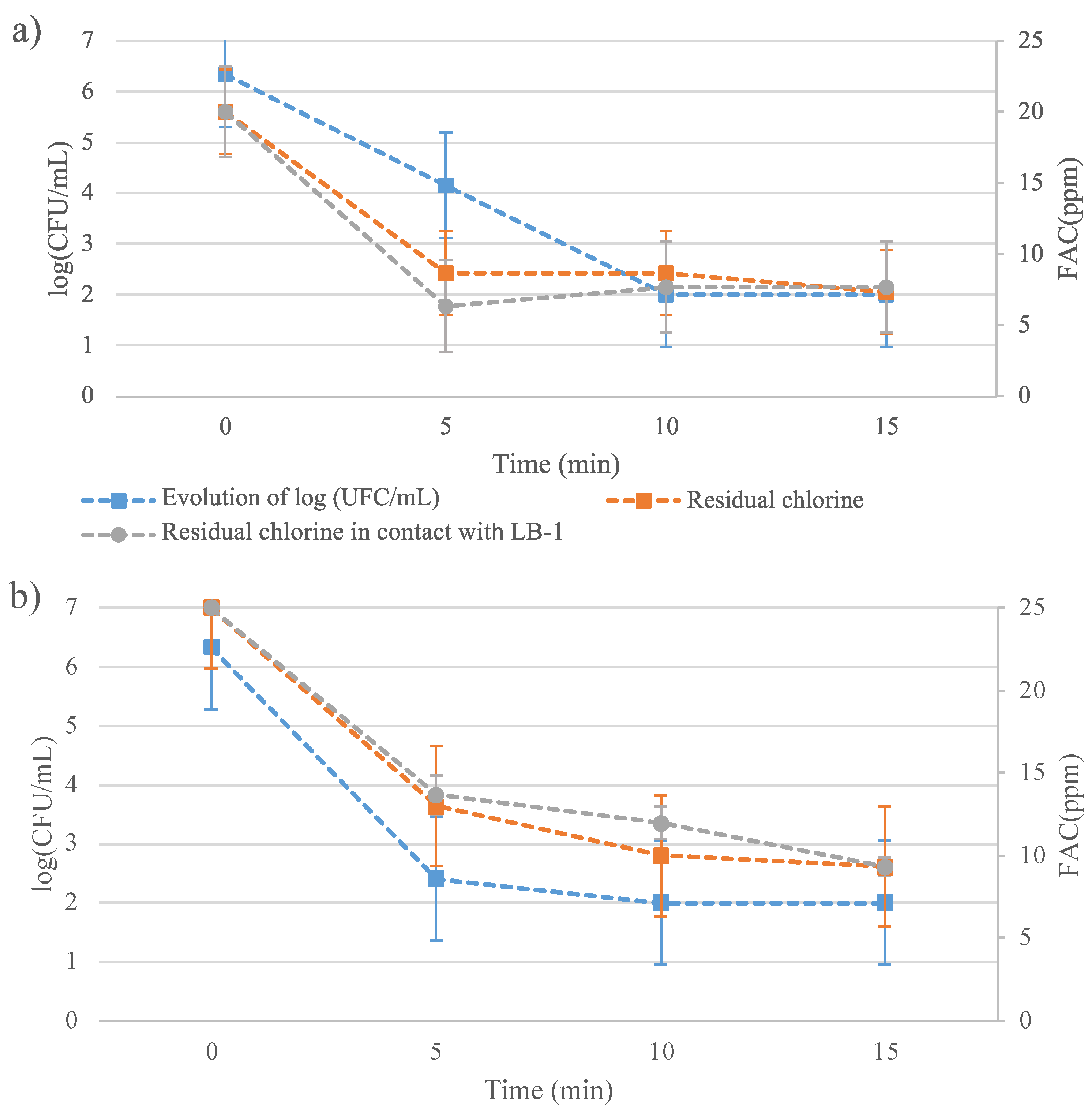
The primary objective of this study was to evaluate the effectiveness of a low-concentration EW solution in destroying bacteria while minimizing residual chlorine levels. To achieve this, a comprehensive trial was conducted in which bacterial population data were collected simultaneously with chlorine concentration measurements. The outcomes of the experiment are graphically represented in Figure 1, which visually demonstrates the relationship between chlorine concentration and bacterial population reduction. This dual data collection allows for a holistic assessment of the disinfectant’s efficacy, ensuring that the chlorine concentration used is sufficiently low to maintain animal health while still achieving significant bacterial reduction.
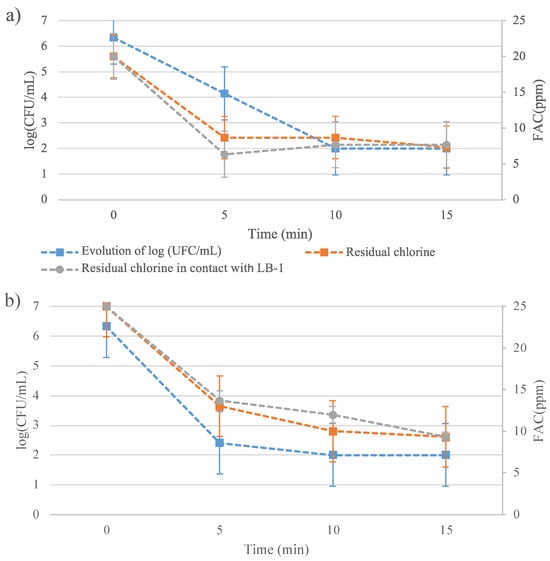
Figure 1.
Evaluation of residual chlorine (free available chlorine, FAC) and Vibrio harveyi C2 population (CFU/mL) using slightly basic electrolyzed water (SBEW) with 20 ppm (a) or 25 ppm (b) of FAC.
The results revealed an almost total bacterial reduction, reaching levels below the detection limit of the counting method within 10 min (Figure 1). Furthermore, the chlorine concentration decreased to values below 10 ppm within 5 to 10 min, which is an encouraging result for practical applications.
3.3. Effect of Electrolyzed Water on Vibrio harveyi Strains
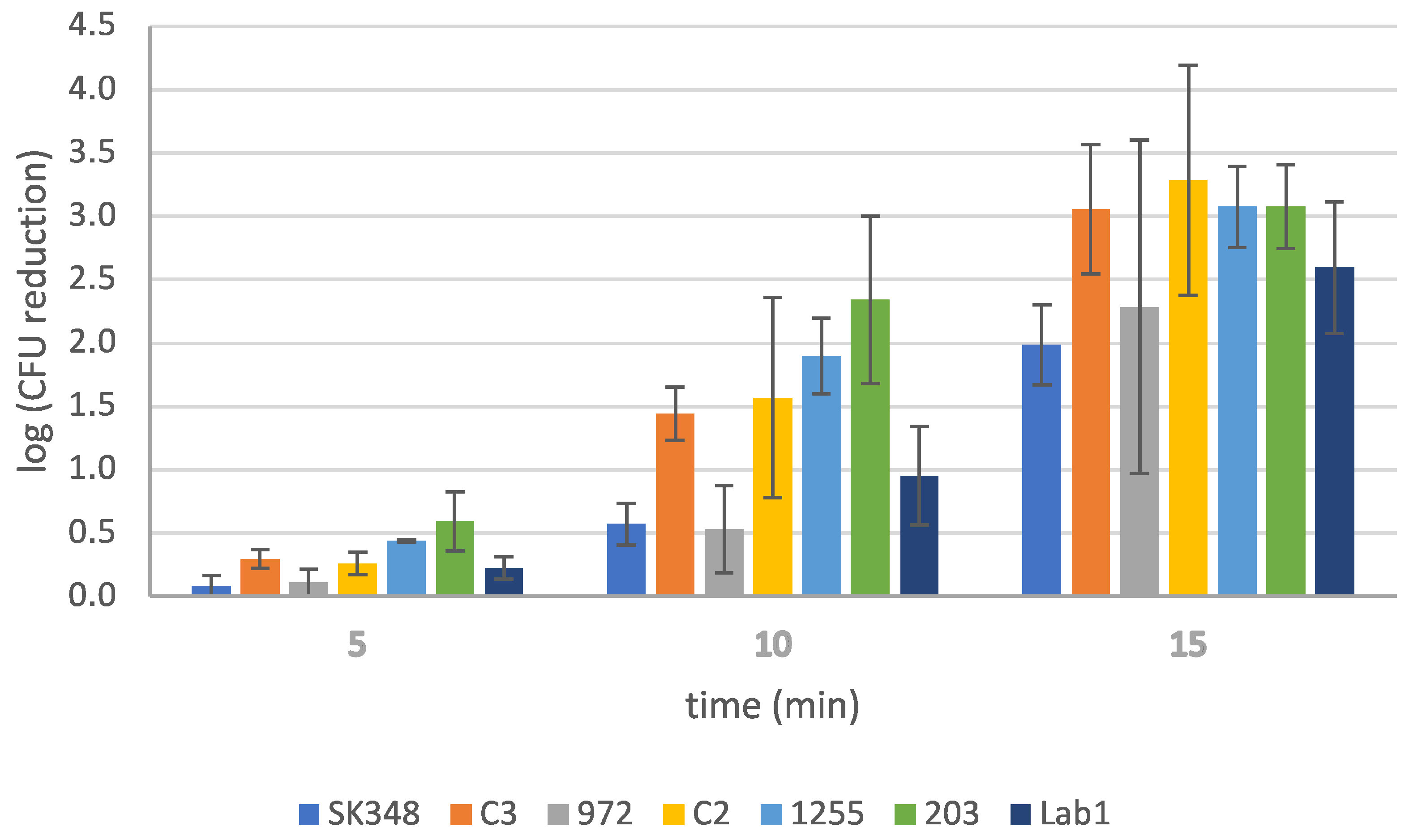
Additional trials were performed using different V. harveyi strains (Table 2) under the previously identified optimal conditions (SBEW25, 3% NaCl). These strains, detailed in Table 2, were subjected to the same experimental setup to evaluate the disinfectant’s performance across a broader range of pathogens. The outcomes are visually depicted in Figure 2, offering a practical and focused evaluation of the efficacy of EW under ideal conditions. By expanding the assessment to multiple strains, this study provides a more comprehensive understanding of EW’s effectiveness against a diverse group of V. harveyi strains. Although data showed varying levels of susceptibility among the bacterial strains tested, the reduction in populations was notable, by 2 to 3.5 log units.
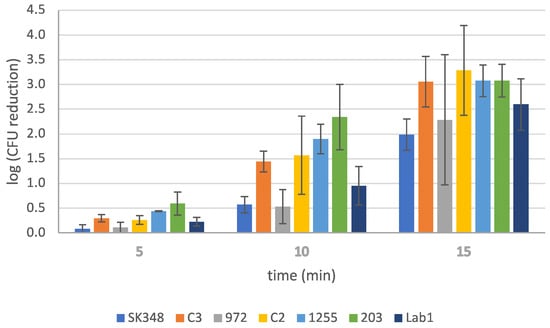
Figure 2.
Effect of slightly basic electrolyzed water (SBEW) with 25 ppm of free available chlorine on different Vibrio harveyi strains.
3.4. Recently Generated vs. Stored Electrolyzed Water
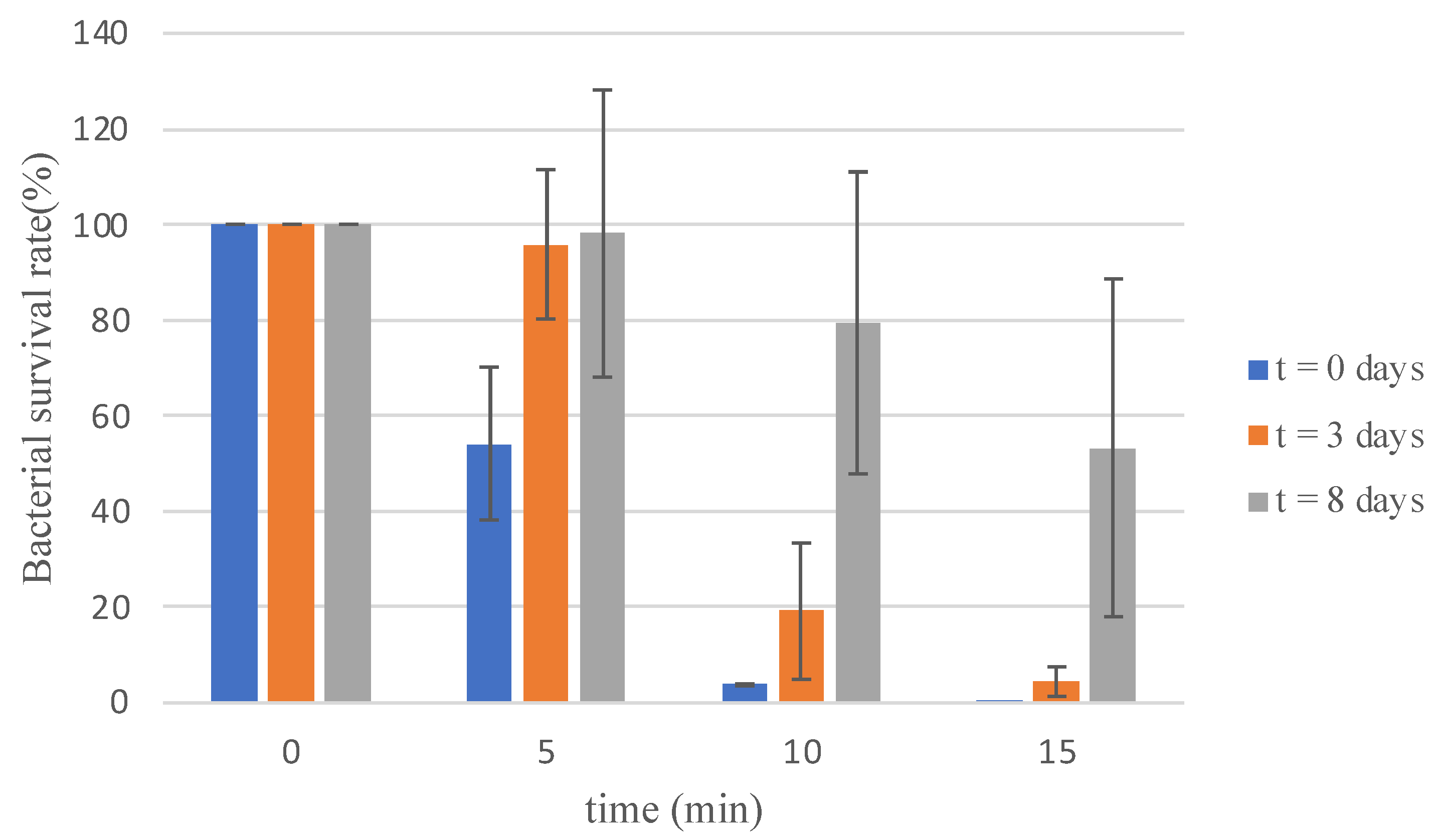
One of the key advantages of electrolyzed water (EW), as highlighted in the introduction, is its capability for on-site production [28]. To evaluate the effect of storage time on biocidal activity, SBEW25 was used as a model. Samples were tested immediately after preparation, as well as after three and eight days of storage in a closed package under dark conditions at 5 °C. The survival rates observed over different time intervals are presented in Figure 3.
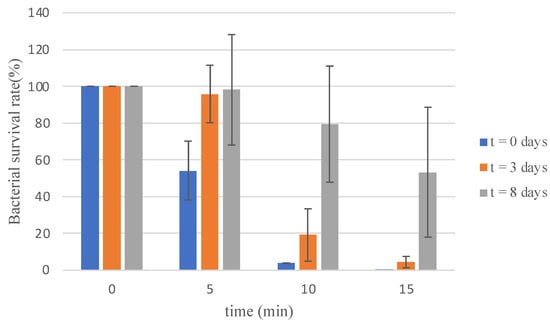
Figure 3.
Survival of Vibrio harveyi C2 strain in electrolyzed water with 25 ppm of free available chlorine recently generated and stored for 3 and 8 days at 5 °C.
Importantly, the evolution of FAC showed the same pattern in all experiments, ensuring consistency and reliability in the findings. The results demonstrated the swift action of EW shortly after production, with a 46% reduction in bacterial load within 5 min of exposure. This effect intensified over time, achieving a 96% reduction after 10 min and an almost complete elimination (0.1% survival) after 15 min.
For samples stored for three days, a notable bactericidal effect was still observed, albeit with a more gradual reduction. After 15 min of exposure, bacterial load decreased by 95%, indicating persistent antimicrobial action despite a slight reduction in efficacy compared to freshly prepared samples (Figure 3). The sample stored for 8 days showed a non-effect in the reduction in V. harveyi population. Although further studies would be necessary, this effect could be attributed to the presence of reactive species of oxygen, which are generated in the electrolysis process of EW [5].
4. Discussion
The biocidal effect of electrolyzed water against the aquatic pathogen V. harveyi has been evaluated. The quick effectiveness of 125 ppm FAC in reducing bacterial populations in less than 1 min underscores its potential utility for surface and facility disinfection. However, this high concentration may not be suitable for applications involving living organisms [29]. In contrast, lower concentrations (25 ppm) achieved significant reductions over time, demonstrating their feasibility for safer applications. This aligns with findings from previous studies, which have shown the high efficacy of hypochlorous acid (HOCl) in similar concentrations under moderately acidic conditions, emphasizing its potency compared to other chlorine species [17]. The variability in bacterial concentrations observed within the experiments is due to the differential growth (or yield rate) of the bacteria among replicates. As they are living organisms, their growth can be affected by any environmental parameter.
Additionally, the drastic effect observed in the PBS trials (in absence of organic matter) highlights the medium’s influence on EW’s antibacterial activity. These findings align with previous studies that emphasize the critical role of the medium in determining disinfectant efficacy [30]. For instance, research has shown that organic matter can react with available chlorine, reducing its effectiveness and necessitating adjustments in disinfection protocols [31].
The additional assays further underscored the importance of balancing FAC concentration and treatment duration. Although SBEW20 and SBEW25 proved effective, the enhanced performance observed at higher salinity (3%) suggests that environmental salinity plays a crucial role in improving disinfection. These results corroborate findings from similar studies, which indicate that increasing salinity during EW production can enhance antimicrobial efficacy by improving the electrical conductivity and the generation of active chlorine species [32].
When compared to prior studies, such as Huang et al. [28], which required highly acidic conditions and higher chlorine concentrations, this study demonstrates a significant advancement. Equivalent bacterial reductions were achieved at moderate pH and FAC concentrations, reducing the risks associated with extreme conditions. Furthermore, the rapid and substantial bacterial reductions observed in this study, especially at 125 ppm FAC, highlight the superior efficacy of EW compared to findings from Quan et al. [21].
The multivariate analysis provided additional insights, emphasizing FAC, time, and pH–salinity interactions as the primary variables influencing disinfection. Although no synergies were found between these factors, their independent contributions offer opportunities for targeted optimizations in disinfection protocols. The role of salinity as a facilitator for optimal bacterial survival conditions aligns with prior research suggesting that salinity can enhance the production and stability of hypochlorous acid (HOCl) under certain conditions [32]. Lastly, the emphasis on treatment duration as a critical factor reinforces the need to carefully consider both chemical and application variables when developing protocols across industries such as food safety and environmental management.
About the development of chlorine residuals, the findings underscore the potential of low-concentration EW solutions to effectively reduce bacterial populations while limiting residual chlorine levels. The almost complete reduction in bacteria within 10 min highlights the solution’s efficiency, making it a promising option for disinfection protocols where minimal residues are required. Moreover, the continued decrease in chlorine concentration even after the bacteria were eliminated, as observed after 5 min, suggests that organic matter may play a fundamental role in reducing residual chlorine. This phenomenon could be critical for practical applications, as it demonstrates the possibility of achieving effective disinfection without leaving harmful chlorine residues. These observations are consistent with studies that emphasize the interaction of organic matter with disinfectants, which can modulate their effectiveness and residual levels [31].
In conclusion, these results provide a strong foundation for further studies aimed at applying this disinfection strategy in real-world conditions. By addressing the interplay between organic matter and chlorine reduction, future research could refine the use of EW solutions to optimize both safety and effectiveness in various applications. The findings also reveal that strains C3, C2, and SK348 were the most susceptible to EW, with survival rates of 0.11%, 0.15%, and 1.1%, respectively, after 15 min of treatment (Figure 2). Furthermore, strains 203 and 1255 exhibited comparable reductions, while strain 972 demonstrated higher survival at 2% (Figure 2). These results highlight the significant effectiveness of EW as a disinfection strategy against all strains tested, confirming its broad-spectrum activity. Despite this, it is worth noting the variability in susceptibility, which underscores the need for future trials. Specifically, further studies should evaluate these strains using EW samples with higher FAC and different times of contact or pH to determine whether similar outcomes could be achieved. Such efforts would enhance the understanding of EW’s robustness and reliability as a pathogen-control measure.
When comparing the bactericidal activity of other biocides, such as benzalkonium chloride, our results indicate improved efficacy due to the concentration of this agent. Several studies have reported that the minimum inhibitory concentration (MIC) of benzalkonium chloride for Gram-negative bacteria like E. coli is established at 40 ppm [33]. In addition, our results show potential in comparison with chlorine because this disinfectant with a FAC range between 0.1 and 10 ppm requires a contact time of minutes to hours to achieve a reduction in Legionella population [34]. In our experiments, we achieved a comparable bacterial reduction using lower chlorine concentrations. Futures studies could explore the synergetic effect of different agents. Concerning storage, the findings highlight the immediate and sustained antimicrobial efficacy of EW, particularly when used shortly after production. As the reduction in the bacteria is below 4 log, we cannot consider a total biocidal activity. However, the product shows the capacity to contain the proliferation of the bacterial population within 10 min of contact. Such rapid activity is especially critical in environments where bacterial contamination poses immediate risks. The sustained efficacy observed over 15 min further emphasizes EW’s potential for comprehensive pathogen control. For samples stored for three days, the results indicate that although the bactericidal activity remains notable, the reduction rate becomes more gradual compared to freshly prepared EW. A 95% reduction in bacterial load after 15 min demonstrates that EW retains much of its effectiveness even after storage, supporting its practicality for use in applied settings. However, the slightly diminished rate of action reinforces the importance of preparing EW on-site and promptly using it for optimal results. This aligns with findings from previous studies, which advocate against storing disinfectants and emphasize the benefits of producing EW immediately before use [35,36]. Overall, these results validate EW’s practicality and efficacy as a versatile and reliable disinfectant for diverse applications.
5. Conclusions
The bactericidal potential of electrolyzed water has been thoroughly evaluated and confirmed as a highly effective method for reducing in vitro bacterial population using the pathogen V. harveyi as a model. The rapid and strong bactericidal effect of FAC at 125 ppm within less than one minute underscores its potential in disinfection. A significant reduction in the concentration of V. harveyi strains was achieved at 20 and 25 ppm of FAC, a pH of 7.5, and an oxidation–reduction potential (ORP) value close to 950 mV. This phenomenal bactericidal power positions EW as a promising alternative for controlling pathogenic bacteria in aquaculture facilities. This study also highlights the critical role of the environment to determine the EW’s antibacterial activity. In fact, the effect of organic matter suggests that medium composition influences disinfectant efficacy. This aligns with prior research indicating that organic matter can react with available chlorine, reducing its effectiveness and necessitating adjustments in disinfection protocols.
The outstanding ability of EW to eliminate bacteria suggests its direct applicability in the aquaculture industry. The on-site generation of electrolyzed water using only common salt (NaCl) and water not only facilitates its immediate application but also reduces health risks associated with consumers and workers. This innovative approach eliminates the need for thermal inactivation treatments and also avoids difficult-to-obtain or handle chemicals.
In terms of environmental footprint, it is relevant to highlight that these chlorine-based disinfectants offer a relatively short lifetime in water and degrade upon contact with the organic matter, thus minimizing their environmental impact. The application of electrolyzed water emerges as a promising technology, opening doors to ongoing research and development in this field, with significant implications for improving healthy and sustainable practices in aquaculture and beyond. However, the application of the EW into aquaculture facilities will require experiments related to the toxicity for the species of interest. Further experiments are being considered for testing the toxicity of EW for some marine fish and invertebrate species. In fact, the concentration used in this study is below the limit reported by Katayose et al. [11] as adverse for marine species, set at 27.2 ppm.
Author Contributions
All authors contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
This study forms part of the ThinkInAzul programme and was supported by MICIU with funding from European Union NextGenerationEU (PRTR-C17.I1) and by Generalitat Valenciana (GVA-THINKINAZUL/2021/028; principle investigator: J.V. Ros-Lis, Universitat de València (UV)) and (GVA-THINKINAZUL/2021/027; principle investigators: C. Amaro and B. Fouz, Universitat de València (UV)); PID2020-120619RB-I00 funded by MCIN/AEI/10.13039/501100011033 and CIAICO/2021/293 by “Conselleria de Innovacion, Universidades, Ciencia y Sociedad Digital” (GV, Spain). P. Ibáñez received funds from the grant MRR-GVA Programa Investigo 2022.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Culmarex S.A.U. and Skretting Spain companies for providing strains for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nan, S.; Li, Y.; Li, B.; Wang, C.; Cui, X.; Cao, W. Effect of Slightly Acidic Electrolyzed Water for Inactivating Escherichia coli O157:H7 and Staphylococcus aureus Analyzed by Transmission Electron Microscopy. J. Food Prot. 2010, 73, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.; Phu, T.M.; Satapornvanit, K.; Min, J.; Shahabuddin, A.M.; Henriksson, P.J.G. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 2013, 412–413, 231–243. [Google Scholar] [CrossRef]
- Zeng, Q.; Sun, Y.; Lai, P.; Chen, Q.; Wang, H. Advancements in Vibrio vaccines for aquaculture. Aquacult Int. 2024, 32, 3331–3356. [Google Scholar] [CrossRef]
- Boyd, C.E.; Massaut, L. Risks associated with the use of chemicals in pond aquaculture. Aquac. Eng. 1999, 20, 113–132. [Google Scholar] [CrossRef]
- Huang, Y.R.; Hung, Y.C.; Hsu, S.Y.; Huang, Y.W.; Hwang, D.F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Al-Haq, M.I.; Sugiyama, J.; Isobe, S. Applications of Electrolyzed Water in Agriculture & Food Industries. Food Sci. Technol. Res. 2005, 11, 135–150. [Google Scholar]
- Chen, B.K.; Wang, C.K. Electrolyzed Water and Its Pharmacological Activities: A Mini-Review. Molecules 2022, 27, 1222. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.F.; Ming, L.C.; Wong, L.C. Dermatologic reactions to disinfectant use during the COVID-19 pandemic. Clin. Dermatol. 2021, 39, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Das, P.; Buschmann, M.; Gilbert, J.A. The Future of Microbiome-Based Therapeutics in Clinical Applications. Clin. Pharma Ther. 2020, 107, 123–128. [Google Scholar] [CrossRef]
- Stoica, M. Sustainable Sanitation in the Food Industry. In Sustainable Food Systems from Agriculture to Industry; Academic Press: Cambridge, MA, USA, 2018; pp. 309–339. [Google Scholar]
- Katayose, M.; Yoshida, K.; Achiwa, N.; Eguchi, M. Safety of electrolyzed seawater for use in aquaculture. Aquaculture 2007, 264, 119–129. [Google Scholar] [CrossRef]
- Rahman, S.; Khan, I.; Oh, D. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Comp. Rev. Food Sci. Food Safe 2016, 15, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Venkobachar, C.; Iyengar, L.; Prabhakara Rao, A.V.S. Mechanism of disinfection: Effect of chlorine on cell membrane functions. Water Res. 1977, 11, 727–729. [Google Scholar] [CrossRef]
- Virto, R.; Mañas, P.; Álvarez, I.; Condon, S.; Raso, J. Membrane Damage and Microbial Inactivation by Chlorine in the Absence and Presence of a Chlorine-Demanding Substrate. Appl. Environ. Microbiol. 2005, 71, 5022–5028. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tian, Y.; Zhao, C.; Qu, T.; Ma, C.; Liu, X. Bactericidal Effect of Strong Acid Electrolyzed Water against Flow Enterococcus faecalis Biofilms. J. Endod. 2016, 42, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, S.; Chen, T.; Gao, W.; Zhu, S.; He, J. Inactivation Mechanism of Escherichia coli Induced by Slightly Acidic Electrolyzed Water. Sci. Rep. 2017, 7, 6279. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Daliri, E.B.M.; Oh, D.H. New Clinical Applications of Electrolyzed Water: A Review. Microorganisms 2021, 9, 136. [Google Scholar] [CrossRef]
- Issa-Zacharia, A.; Kamitani, Y.; Tiisekwa, A.; Morita, K.; Iwasaki, K. In vitro inactivation of Escherichia coli, Staphylococcus aureus and Salmonella spp. using slightly acidic electrolyzed water. J. Biosci. Bioeng. 2010, 110, 308–313. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Oliveira, M.; Alegre, I.; Viñas, I. Efficacy of neutral electrolyzed water (NEW) for reducing microbial contamination on minimally processed vegetables. Int. J. Food Microbiol. 2008, 123, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Tabernero de Paz, M.J.; Bodas, R.; Bartolomé, D. Electrolyzed water as a cleaning agent in animal production: Effects on health and performance. Zootecnia 2013, 62, 13–23. [Google Scholar] [CrossRef]
- Ibányez-Payá, P.; Blasco, A.; Ros-Lis, J.V.; Fouz, B.; Amaro, C. Electrolyzed water treatment for the control of the zoonotic pathogen Vibrio vulnificus in Aquaculture: A one healthe perspective. Microorganisms 2024, 12, 1992. [Google Scholar] [CrossRef]
- Austin, B.; Zhang, X.H. Vibrio harveyi: A significant pathogen of marine vertebrates and invertebrates. Lett. Appl. Microbiol. 2006, 43, 119–124. [Google Scholar] [CrossRef]
- Zhang, X.H.; He, X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.; Gomez-Gil, B.; Lozano, R. ‘Bright-red’ syndrome in Pacific white shrimp Litopenaeus vannamei is caused by Vibrio harveyi. Dis. Aquat. Org. 2010, 92, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mougin, J.; Roquigny, R.; Flahaut, C.; Bonnin-Jusserand, M.; Grard, T.; Le Bris, C. Abundance and spatial patterns over time of Vibrionaceae and Vibrio harveyi in water and biofilm from a seabass aquaculture facility. Aquaculture 2021, 542, 736862. [Google Scholar] [CrossRef]
- Amaro, C.; Fouz, B.; Sanjuán, E.; Romalde, J.L. Vibriosis. Climate Change and Infectious Fish Diseases; CABI: Wallingford, UK, 2020; pp. 182–210. [Google Scholar]
- Hoben, H.J.; Somasegaran, P. Comparison of the Pour, Spread, and Drop Plate Methods for Enumeration of Rhizobium spp. in Inoculants Made from Presterilized Peat. Appl. Environ. Microbiol. 1985, 44, 1246–1247. [Google Scholar] [CrossRef] [PubMed]
- Nisola, G.M.; Yang, X.; Cho, E.; Han, M.; Lee, C.; Chung, W.J. Disinfection performances of stored acidic and neutral electrolyzed waters generated from brine solution. J. Environ. Sci. Health Part A 2011, 46, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Chowdhury, S.; Goel, S. Environmental impacts of the widespread use of chlorine-based disinfectants during the COVID-19 pandemic. Environ. Sci. Pollut. Res. 2022, 29, 85742–85760. [Google Scholar] [CrossRef] [PubMed]
- Rebezov, M.; Saeed, K.; Khaliq, A.; Rahman, S.J.U.; Sameed, N.; Semenova, A. Application of Electrolyzed Water in the Food Industry: A Review. Appl. Sci. 2022, 12, 6639. [Google Scholar] [CrossRef]
- Oomori, T.; Oka, T.; Inuta, T.; Arata, Y. The efficency of disinfection of acidic electrolyzed water in the presence of organic materials. Anal. Sci. 2000, 16, 365–369. [Google Scholar] [CrossRef]
- Hsu, S.Y. Effects of flow rate, temperature and salt concentration on chemical and physical properties of electrolyzed oxidizing water. J. Food Eng. 2005, 66, 171–176. [Google Scholar] [CrossRef]
- Fazlara, A.; Ekehtelat, M. The disinfectant effects of benzalkonium chloride on some important foodborne pathogens. Am.-Eurasian J. Agric. Environ. Sci. 2012, 12, 23–29. [Google Scholar]
- Kim, B.R.; Anderson, J.E.; Mueller, S.A.; Gaines, W.A.; Kendall, A.M. Literature review—Efficacy of various disinfectants against Legionella in water systems. Water Res. 2002, 36, 4433–4444. [Google Scholar] [CrossRef] [PubMed]
- Block, Z.; Eyles, A.; Corkrey, R.; Stanley, R.; Ross, T.; Kocharunchitt, C. Effect of Storage Conditions on Shelf Stability of Undiluted Neutral Electrolyzed Water. J. Food Prot. 2020, 83, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.T.; Wang, M.M.; Ahn, J.; Ma, Y.N.; Chen, S.G.; Ye, X.Q. Storage Stability of Slightly Acidic Electrolyzed Water and Circulating Electrolyzed Water and Their Property Changes after Application. J. Food Sci. 2016, 81, E610–E617. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).